Abstract
The endogenous neuroactive steroid allopregnanolone (ALLO) has previously been shown to induce reinstatement of ethanol seeking in rodents. ALLO is a positive allosteric modulator at both synaptic and extrasynaptic GABAA receptors. The contribution of each class of GABAA receptors in mediating reinstatement of ethanol seeking is unknown. The first aim of the present study was to determine whether ganaxolone (GAN), a longer-acting synthetic analog of ALLO, also promotes reinstatement of ethanol seeking. The second aim was to examine whether preferentially activating extrasynaptic GABAA receptors with the selective agonist gaboxadol (THIP) was sufficient to reinstate responding for ethanol in mice. Male C57BL/6J mice were trained to lever press for access to a 10% ethanol (v/v) solution (10E), using a sucrose fading procedure. Following extinction of the lever pressing behavior, systemic THIP (0, 4 and 6 mg/kg) and GAN (0, 10, and 15 mg/kg) were tested for their ability to reinstate ethanol-appropriate responding in the absence of 10E access. GAN significantly increased lever pressing on the previously active lever, while THIP did not alter lever pressing behavior. The results of this study suggest that direct activation of extrasynaptic GABAA receptors at the GABA site is not sufficient to induce ethanol seeking in the reinstatement procedure. Future studies are necessary to elucidate the mechanisms and brain areas by which differences in the pharmacological activity of GAN and THIP at the GABAA receptor contribute to the dissimilarity in their effect on the reinstatement of ethanol seeking. Nonetheless, based on the increased use of these drugs in clinical trials across multiple disease states, the effects of GAN or THIP on alcohol seeking may be an important consideration if these drugs are to be used clinically in a population with a co-occurring alcohol use disorder.
Keywords: THIP, GABAA receptor, alcohol, relapse, allopregnanolone, extrasynaptic
The National Institute on Alcohol Abuse and Alcoholism estimates that nearly 18 million Americans suffer from an alcohol use disorder (AUD). AUDs are considered chronic relapsing disorders (Leshner, 1997; Volkow and Li, 2005). Nearly 90% of dependent individuals relapse at least once in a 4-year span (Polich et al., 1980), highlighting the need for a better understanding of the mechanisms underlying craving and relapse. Although relapse is difficult to model in animals, the reinstatement model provides a measure of drug seeking during abstinence from the drug (de Wit and Stewart, 1981; Shaham, 2003). The model has predictive validity in that re-exposure to drugs, drug-related cues, and stressors, all of which can provoke craving and possibly relapse in humans (de Wit, 1996; Childress et al., 1993; Sinha, 2001), also promote reinstatement of drug-seeking in animals (Epstein et al., 2006).
Allopregnanolone (ALLO) is an endogenous neuroactive steroid that can be increased in the brain and plasma by stress, estrus, pregnancy, and ethanol (Purdy et al., 1991; Concas et al., 1998; VanDoren et al., 2000; Barbaccia et al., 2001; Finn et al., 2004). Due to its potent positive modulation at GABAA receptors, ALLO shares many behavioral properties with ethanol, such as anxiolysis, sedation, and anticonvulsant properties (Kumar et al., 2009). ALLO substitutes for ethanol in drug discrimination procedures, indicating that it shares subjective stimulus properties with ethanol (Bowen et al., 1999; Grant et al., 1996, 2008). Exogenous ALLO alters ethanol intake in a biphasic manner and reinstates ethanol seeking in rodents (Finn et al., 2008; Ford et al., 2005; Sinnott et al., 2002; Janak et al., 1998; Janak and Gill, 2003; Nie and Janak, 2003), suggesting that it plays a role in both ethanol seeking and consumption.
Although ALLO may have clinical benefit in multiple disease states (epilepsy, premenstrual dysphoric disorder, depression, traumatic brain injury; clinicaltrial.gov), the therapeutic potential of ALLO is limited by its short half-life (Timby et al., 2006). Ganaxolone (GAN) is a synthetic analog of ALLO with an added methyl group that renders it more resistant to metabolism (Nohria and Giller, 2007). Although its primary pharmacological and behavioral properties are unaltered, when compared to ALLO (Carter et al., 1997; Ungard et al., 2000), the half-life of GAN is 3–4 times that of ALLO (Reddy and Rogawski, 2000). The use of GAN in clinical trials has broadened in the last decade, to include potential treatment of epilepsy, post-traumatic stress disorder, and smoking cessation (clinicaltrial.gov).
GABAA receptors are chloride channels composed of 5 subunits from a pool of at least 16 possible subunits: α1–6, β1–3, γ1–3, δ, ε, π, and θ. Importantly, inclusion of the δ subunit limits the location of the receptor to the extrasynaptic space, where it is postulated to contribute exclusively to tonic inhibition (Farrant and Nusser, 2005). In vitro work using recombinant receptors suggests that δ subunit-containing GABAA receptors may be particularly sensitive to the GABA-modulatory effects of ALLO (Belelli et al., 2002). Similarly, δ subunit-containing GABAA receptors have been proposed to be a more sensitive target of physiologically-relevant doses of ethanol than non-δ subunit-containing GABAA receptors (Olsen et al., 2007; Mody et al., 2007; but also Borghese and Harris, 2007). Consistent with the importance of the δ subunit in the actions of neuroactive steroids and ethanol, δ subunit knockout mice showed reduced sensitivity to some of the behavioral effects of both neuroactive steroids and ethanol, and the knockout mice self-administered less ethanol than their wild-type littermates (Mihalek et al., 1999, 2001). Although GAN and ALLO can act at both synaptic and extrasynaptic GABAA receptors (Belelli and Herd, 2003), the contribution of each class of receptors to the reinstatement of ethanol seeking has not been examined.
The primary aim of the present studies was to determine whether GAN reinstates ethanol seeking in mice, as previously demonstrated with ALLO (Finn et al., 2008). The second aim of the study was to examine whether preferentially activating extrasynaptic GABAA receptors with gaboxadol (THIP), a GABAA receptor agonist with selectivity for δ subunit-containing GABAA receptors (Meera et al., 2011; Wafford et al., 2009), was sufficient to induce reinstatement of ethanol seeking. Locomotor tests were performed with each drug to elucidate whether changes in lever pressing behavior during extinction were accounted for by changes in general locomotor activity. Information regarding the ability of GAN or THIP to promote alcohol seeking may be of significant value considering the increasing use of these drugs in clinical trials across multiple disease states, such as smoking (GAN), post-traumatic stress disorder (GAN), and depression (THIP) (clinicaltrials.gov). Particularly in diseases that have a high comorbidity with AUDs, the effects of GAN or THIP on alcohol seeking may be an important consideration if these drugs are to be used in a clinical setting in a population with a co- occurring AUD.
1. Experimental Procedures
1.1 Animals
Male C57BL/6J mice, approximately 8 weeks of age at the start of experiments, were purchased from The Jackson Laboratory-West (Sacramento, CA). Mice were pair-housed for the reinstatement study or group-housed for the locomotor study in Biofresh bedding (Ferndale, WA), except during individual testing. Mice were provided ad libitum access to rodent chow (LabDiet, St. Louis, MO) and water (except where noted). Mice were maintained on a 12-h light-dark cycle (lights on at 0600); all experiments were conducted during the light cycle and were carried out Monday through Friday. All efforts were made to minimize animal suffering and to reduce the number of mice used, and all procedures were approved by the local Institutional Animal Care and Used Committee and complied with NIH guidelines.
1.2 Drugs
Ethanol (200 proof; Pharmco Products, Brookfield, CT) and sucrose (Sigma-Aldrich Company, St. Louis, MO) solutions were prepared by dilution in tap water. GAN was purchased from Tocris Bioscience (Ellisville, MO) and was solubilized in 20% (w/v) 2-hydroxypropyl-beta-cyclodextrin (β-CD; Cargill Inc., Cedar Rapids, IA) in Millipore water. THIP (4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridine-3-ol hydrochloride) was purchased from Tocris Bioscience and dissolved in saline. For all experiments, drugs were injected intraperitoneally (i.p.) in a volume of 0.01 ml/g body weight and with a 30 minute pretreatment time. The pretreatment times were chosen to match our previous work with GAN and THIP (Ramaker et al., 2011, 2012).
1.3 Apparatus
Ethanol self-administration acquisition, extinction, and reinstatement sessions were carried out in eight operant conditioning boxes consisting of a 21″ L x 13.75″ W x 5″ H inner chamber located inside a sound and light-attenuating chamber, as described previously (Ford et al., 2007a, 2007b). Each conditioning box contained a house light, 2 retractable levers with a stimulus light above each one, a retractable sipper apparatus, and a stainless steel grid floor. The retractable sipper was made from a 10-ml graduated pipette with a double ball-bearing metal sipper tube. Each metal sipper was connected to a lickometer circuit, which interfaced to a computer operating with MED-PC software (Med-Associates Inc., St. Albans, VT). The computer recorded time-stamped licks on the sipper throughout the acquisition phase as well as lever presses throughout all phases of the experiment.
Locomotor chambers have been described in detail previously (Gubner et al., 2013). Briefly, sixteen automated locomotor chambers (40 x 40 x 30 cm; AccuScan Instruments Inc., Columbus, OH) were used, which were equipped with pairs of 8 photocell beams and detectors located 2 cm above the floor. VERSADAT software (AccuScan Instruments Inc.) was used to convert beam interruptions into horizontal distance traveled (cm). Each chamber contained a fan to provide ventilation and background noise, and each chamber contained a 3.3 W incandescent light bulb during activity testing.
1.4 Acquisition
Self-administration was acquired with the “sipper” procedure (Ford et al., 2007a, 2007b; Samson et al., 1998). For 12 days, mice (n = 12) were fluid restricted overnight and were trained to respond for access to a 10% (w/v) sucrose solution (10S) on a fixed ratio (FR)1 schedule. Sessions were 60 minutes long, and every time the animal made a response on the “active” lever, both levers retracted, the house light turned off, the respective stimulus light turned on for 5 seconds, and the sipper extended into the chamber for 60 seconds. Responses on the “inactive” lever were recorded but had no scheduled consequence. The position of the active lever was counterbalanced between chambers. Sipper access was reduced from 60 to 30 seconds, and session length was decreased from 60 to 30 minutes over successive training sessions. Fluid restriction was lifted and then the FR was increased by 1 every 3–4 sessions until mice were on an FR4 schedule. At this point, 10% (v/v) ethanol (10E) was added to the solution. The sucrose was then faded out in a step-wise manner (3 sessions each with 10S/10E, 5S/10E, and 2.5S/10E solutions) to eventually yield a 10E solution with no sucrose present. The FR schedule was then increased in a step-wise fashion (3–4 sessions each at FR4, FR6, FR8), and then a Response Requirement (RR)8 schedule was instituted. On the RR schedule, each mouse had 20 minutes to make its 8 lever presses on the “active” lever; upon completion of the requirement, the levers retracted, the stimulus light illuminated for 5 seconds, and the sipper came into the cage, allowing 30 minutes of continuous 10E access. The RR schedule was used as previous work has shown that mice consumed approximately twice as much on an RR versus an FR schedule (Ford et al., 2007b). Mice were maintained on the RR8 schedule for approximately three weeks. At that time, because intake was still low (~0.5 g/kg/30-minutes), mice were exposed to a week of continuous access to 10E and water in the homecage. Intake during this time was 11.5 ± 0.4 g/kg per day for 5 days. Then, mice resumed operant sessions on the RR8 schedule for 8 more weeks, where intake substantially increased. In total, mice self-administered 10E (in the absence of sucrose) for 16 weeks prior to the initiation of extinction sessions.
1.5 Extinction and reinstatement
Extinction consisted of a 30-minute session in the same chambers, where presses on both the previously active and inactive lever were recorded but there was no scheduled consequence. Mice underwent 16 days of extinction, and during the last 4 extinction sessions, mice were pretreated with saline 30 minutes prior to the session start in order to habituate them to injections. When presses on the previously active lever were consistently below 25% of the previous criterion of 8, mice were then tested for the ability of 4 or 6 mg/kg THIP to reinstate lever pressing. The reinstatement tests were performed in an identical manner to extinction sessions in that lever pressing did not result in any scheduled consequence. Doses were given in ascending order in a within-subject design with baseline re-established between doses (at least 2–3 days between injections). Vehicle was given on all intervening days, and the “0 mg/kg” value was calculated by averaging the 2 days that immediately preceded a drug day; there were no significant differences between active or inactive lever presses on these 2 vehicle days. Mice received 4 days off following the last THIP injection, and then extinction sessions were resumed with pretreatment of 20% β-CD given 30 minutes prior to the session start. Beginning ten days after the last THIP injection, mice were tested with 10 and 15 mg/kg GAN in ascending dose order in a within-subject design, with baseline re-established between doses. Again, there were no statistical differences between active or inactive lever presses on the 2 vehicle days that immediately preceded a drug day, so these values were collapsed to give the vehicle “0 mg/kg” value.
1.6 Blood ethanol concentrations (BECs)
During the final week of 10E self-administration, a 20 μL blood sample was taken from the orbital sinus of each mouse at the conclusion of a 30-minute self-administration session and analyzed for ethanol content, as previously described (Finn et al., 2007). Each sample was added to 500 μL of a solution that contained 4 mM n-propanol (internal standard) in deionized water. The solution was vortexed and analyzed using head-space gas chromatography. Concentrations of samples were interpolated from a standard curve, which was constructed using 6 pairs of external standards with known ethanol concentrations (from 0.5 to 3.0 mg/ml).
1.7 Locomotor activity
A separate group of drug and experimental naïve mice (n = 29) were used to test the locomotor effects of THIP and GAN. On days 1 (habituation) and 2 (baseline), all mice received an i.p. injection of 20% β-CD. Thirty minutes later, they were placed in the locomotor chambers and horizontal activity was measured for 30 minutes. On day 3, these mice were separated into groups that received an injection of 0 (n = 10), 10 (n = 10), or 15 (n = 9) mg/kg GAN 30 minutes prior to testing, and activity was recorded for 30 minutes. Drug-induced locomotor activity for each animal was calculated as a difference score by subtracting its individual baseline (day 2) activity from its activity following drug injection (day 3). A week later, mice were again tested in the locomotor chambers after a 30 minute pretreatment with saline (days 1 and 2). On day 3, mice were balanced on previous GAN dose treatment, and separate groups were tested following a 30 minute pre-treatment with 0 (n = 10), 4 (n = 10), or 6 (n = 9) mg/kg THIP. Drug-induced locomotor activity was again calculated by subtracting each individual animal’s baseline activity (day 2) from activity following drug injection (day 3).
1.8 Statistical analysis
To examine extinction, a Repeated Measures ANOVA was conducted for each lever type across sessions. Based on previous results with ALLO where ALLO significantly affected pressing on both levers, but post-hocs were only significant for the active lever (Finn et al., 2008), a priori planned comparisons analyzed active and inactive lever presses separately using one-way Repeated Measures ANOVAs to analyze the reinstatement tests. In the event of a significant main effect of dose, paired t-tests versus vehicle were performed using Fisher’s Least Significant Difference test. For locomotor activity data, between-subjects ANOVAs assessed the effect of dose on overall activity. Activity data were analyzed in 5-minute bins using a 2-way ANOVA, with dose as a between-groups measure and time as the repeated measure. Post-hoc t-tests were performed only in the event of a significant result (time x dose interaction and then main effect of dose). The significance criterion was set at p ≤ 0.050 for all experiments. A single outlier was removed from the analysis of active lever presses following reinstatement with 10 mg/kg GAN; this outlier was greater than 2 standard deviations above the group mean.
2. Results
2.1 Self-administration and extinction
Prior to the initiation of extinction sessions, mice self-administered 10E for approximately 16 weeks. Collapsed across the final week of self-administration, mice consumed 0.87 ± 0.09 g/kg/30-minutes. Following a drinking session during the final week of self-administration, BECs measured in 9 mice were 29 ± 9 mg/dl (intake: 0.79 ± 0.13 g/kg); there was a significant correlation between intake and BEC (r = 0.76, p = 0.017; data not shown). Throughout extinction, there was a significant main effect of session when analyzing presses on the active lever [F(15,165) = 14.220; p < 0.001; Fig. 1]. There was no effect of session on inactive lever presses.
Fig. 1.
Lever presses during extinction are shown for both the previously active and inactive levers. Values represent mean ± SEM of 12 mice. Baseline (B) represents the average of the last week in which mice were being reinforced.
2.2 Reinstatement tests
During the reinstatement tests, there was no significant effect of THIP on lever pressing for either the previously active or the previously inactive lever (Fig. 2). In contrast, pretreatment with GAN significantly increased presses on the previously active lever [F(2,20) = 3.575, p = 0.047; Fig. 3], with significant increases following both 10 mg/kg (p = 0.025) and 15 mg/kg (p = 0.008) GAN when compared to vehicle treatment. There also was a trend for an effect of GAN on inactive lever presses [F(2,22) = 2.973, p = 0.07; Fig. 3). To examine the pattern of lever pressing throughout the 30-minute session, total lever presses were examined split into 5-minute bins. However, there was no interaction between treatment dose and time (data not shown). With both GAN and THIP treatment, latency to first lever press was extremely variable both within and between subjects and did not result in any significant effects (data not shown).
Fig. 2.
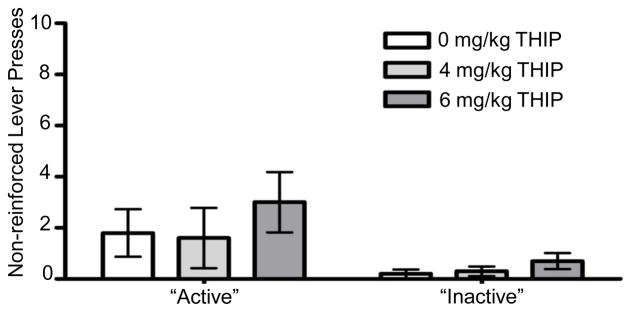
Effect of gaboxadol (THIP) on responding on the previously active and inactive levers during reinstatement tests. Values represent mean ± SEM for 12 mice.
Fig. 3.
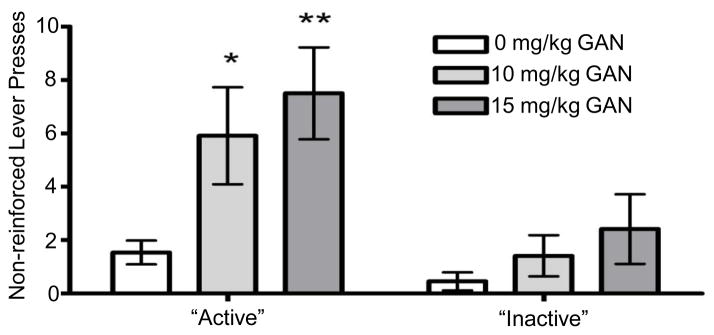
Effect of ganaxolone (GAN) on responding on the previously active and inactive levers during reinstatement tests. Values represent mean ± SEM for 12 mice. * p ≤ 0.05, ** p ≤ 0.01 versus 0 mg/kg.
2.3 Locomotor activity
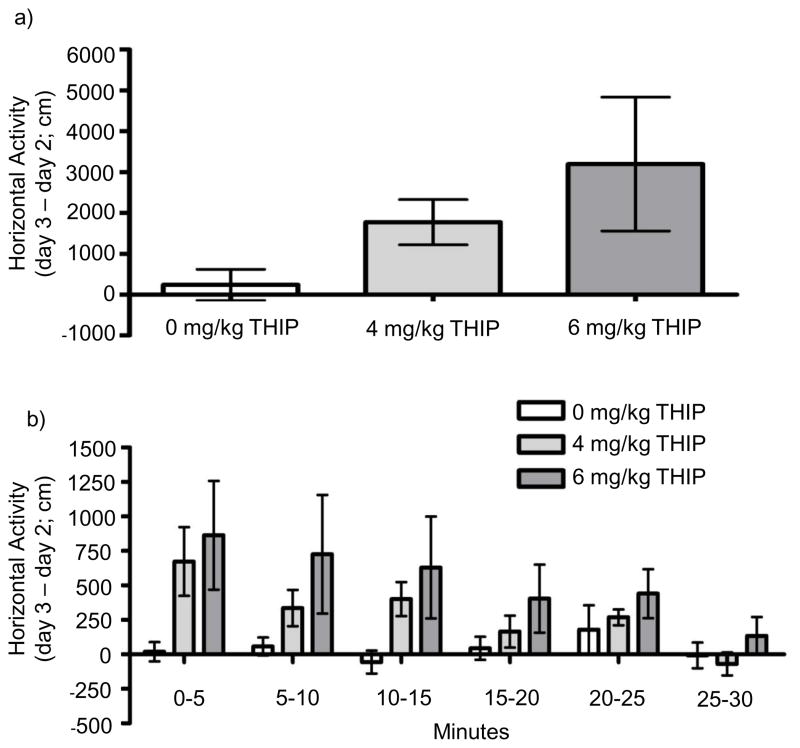
There was no difference in baseline (day 2) activity for mice that subsequently received the different doses of THIP. THIP did not significantly alter horizontal activity when data were accumulated across the 30-minute session (Fig. 4a). When examined by 5-minute bins, there was a trend for a bin by dose interaction [F(2,26) = 2.306; p = 0.097; Fig. 4b].
Fig. 4.
Locomotor effects of gaboxadol (THIP) a) across the total 30-minute session and b) split into 5-minute bins. Data depict drug-induced locomotor activity for each animal (treatment day 3 minus baseline day 2) and represent mean ± SEM for 9 – 10 mice per group.
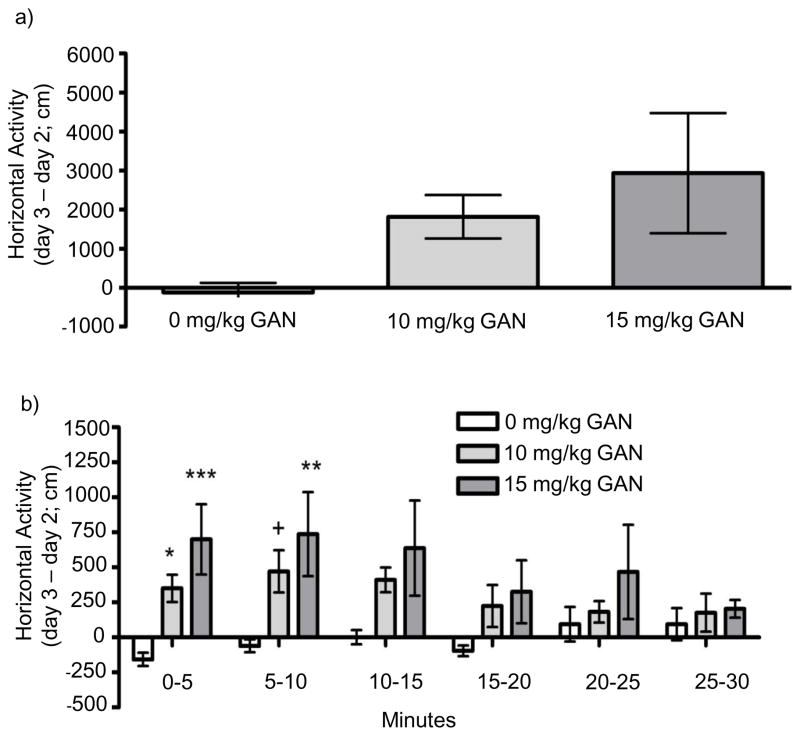
There was also no difference in baseline (day 2) activity for mice that subsequently received the different doses of GAN. There was a trend for an effect of GAN to increase locomotor activity for data accumulated across the entire 30-minute session [F(2,26) = 2.923; p = 0.072; Fig. 5a]. Analyzing by 5-minute bins revealed a significant bin by dose interaction [F(10,130) = 3.152; p < 0.001; Fig 5b], which appeared due to the fact that GAN increased activity to a greater extent at the earlier versus the later parts of the session. During minutes 0 – 5, there was a main effect of dose [F(2,26) = 8.218 p = 0.002]; both 10 mg/kg (p = 0.022) and 15 mg/kg (p < 0.001) GAN significantly increased activity during this time. There also was an effect of dose during minutes 5 – 10 [F(2,26) = 4.722 p = 0.018], with a trend for an increase following 10 mg/kg (p = 0.051) and a significant increase following 15 mg/kg (p = 0.006). There was a trend for an increase in activity during minutes 10 – 15 [F(2,26) = 2.923; p = 0.072].
Fig. 5.
Locomotor effects of ganaxolone (GAN) a) across the total 30-minute session and b) split into 5-minute bins. Data depict drug-induced locomotor activity for each animal (treatment day 3 minus baseline day 2) and represent mean ± SEM for 9 – 10 mice per group. + p ≤ 0.10, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 versus 0 mg/kg.
3. Discussion
In the present study, mice had a history of more than 4 months of lever pressing for 10E access. Even after more than 3 weeks of abstinence, during which time lever pressing did not result in 10E access or conditioned cue exposure, GAN increased ethanol seeking behavior, as measured by the number of presses on the previously active lever. Interestingly, the number of ethanol-appropriate lever presses following the largest dose of GAN was elevated to a level similar to the previous requirement of 8 presses. An increase in ethanol seeking with GAN is consistent with previous data showing that GAN increased ethanol-reinforced lever pressing (Ramaker et al., 2012) and that ALLO increased ethanol seeking in a reinstatement procedure (Nie and Janak, 2003; Finn et al., 2008).
Contrary to GAN, THIP did not reinstate ethanol seeking, suggesting that activation of extrasynaptic GABAA receptors with a direct agonist is not sufficient to promote the reinstatement of ethanol seeking. The 4 mg/kg dose of THIP was chosen based on data showing that it decreased both the appetitive and consummatory phases of self-administration in an operant procedure (Ramaker et al., 2012). Importantly, the 4 and 6 mg/kg doses of THIP correspond to low μM concentrations in the CNS, concentrations thought to act selectively on tonic inhibition (Cremers and Ebert, 2007). Although it is possible that increasing the dose may have uncovered an effect of THIP to promote reinstatement, use of 8 mg/kg prevented 11 of 12 mice from attaining an ethanol reinforcer in a prior study (Ramaker et al., 2012), consistent with the idea that THIP may decrease ethanol seeking. Future studies should test the effect of THIP to decrease an oral ethanol prime- or light cue-induced reinstatement, as a floor effect in the current procedure limited the ability to detect a decrease if one existed.
One interpretation of the differential effect between GAN and THIP is that activation of extrasynaptic GABAA receptors alone is not sufficient to promote ethanol reinstatement. It is possible therefore that GAN’s activity at synaptic GABAA receptors, or the combined actions at synaptic and extrasynaptic GABAA receptors, is necessary to promote ethanol seeking. Another not mutually-exclusive interpretation is that GAN’s action as a positive allosteric modulator versus THIP’s ability to act at the GABA binding site and directly gate the channel contributed to the differential effect of these two drugs on ethanol seeking. Examination of reinstatement of ethanol seeking with other GABAA receptor-acting drugs such as muscimol, which acts at the GABA binding site at both synaptic and extrasynaptic GABAA receptors, could help elucidate whether it is the action at synaptic versus extrasynaptic receptors or the action at the neuroactive steroid site (Hosie et al., 2006) versus the GABA site that differentiates the effects of these two drugs on measures of ethanol seeking.
One potential explanation for an increase in ethanol seeking following GAN injection is that, due to shared stimulus properties between ALLO and ethanol (Bowen et al., 1999; Grant et al., 1996, 2008), GAN may have acted similar to a drug (ethanol) prime. This is in line with studies showing that non-contingent priming injections of a drug, or agents with similar pharmacological or stimulus properties of a drug, reinstated seeking for that drug (de Wit and Stewart, 1981; de Wit and Stewart, 1983; Stewart, 1983). However, THIP did not substitute for ethanol (1.5 g/kg) in a drug discrimination study (Shelton and Grant, 2002), suggesting that, unlike GAN, THIP does not share discriminative stimulus effects with ethanol. Additionally, mice lacking the δ subunit of the GABAA receptor could discriminate between ethanol and saline (Shannon et al., 2004), indicating that the δ subunit is not necessary for the discriminative properties of ethanol. Thus it is possible that GAN’s actions at the neuroactive steroid binding site of non-δ subunit containing GABAA receptors contributed to a shared stimulus effect with ethanol, and this stimulus effect reinstated ethanol seeking. On the other hand, there is not always a direct relationship between drug discrimination and reinstatement (Shaham et al., 2003), and there could be alternative explanations as to why GAN, but not THIP, promoted reinstatement.
Although the overall 30 minute locomotor effect of GAN was not significant, due to an increase in activity in the first 10 minutes of the session, we cannot rule out that an increase in activity following GAN contributed to an increase in lever pressing. However, an increase in general activity does not appear to be a sufficient explanation to fully account for the increase in lever pressing for a number of reasons. Firstly, the effects of GAN on lever pressing were only significant with regard to the previously active lever, indicating that an increase in locomotor activity did not induce an indiscriminate increase in lever pressing behavior. Secondly, with regard to the lever pressing across time, there was not an interaction between lever presses and 5-minute bins that would support a similar temporal relationship between the lever pressing and the locomotor activity. In support of a separation between locomotor and lever pressing behavior, Besheer et al. (2010) showed that 1 mg/kg GAN in rats increased ethanol-reinforced responding in the absence of a general effect on locomotor activity, indicating that GAN may have effects on ethanol-reinforced responding separable from its general locomotor effects. Lastly, although the effects of THIP on activity were not statistically significant, visual comparison between the locomotor data for THIP and GAN shows that THIP induced similar quantitative increases in locomotor activity as GAN, suggesting that the magnitude of locomotor activity induced by GAN was not sufficient to produce an increase in lever pressing.
An important consideration is that mice in the present study had an extended history of ethanol intake followed by a period of ethanol abstinence. Removal of ethanol following chronic exposure can lead to alterations in GABAA receptor subunit expression, specifically a down-regulation in δ subunit surface expression (Liang et al., 2007) and a subsequent decrease in THIP-mediated tonic current (Liang et al., 2006). Although there are major differences between the present study and that used by Liang and colleagues (2006, 2007) (60 exposures to 5 or 6 g/kg ethanol injections in Liang et al. versus 4 months of self-administered ethanol of < 1 g/kg), a possible reduction in δ subunit expression across certain brain regions pertinent to reinstatement may have limited the ability of THIP to induce effects, while maintaining synaptic GABAA receptor targets of GAN. Future studies are necessary to examine which brain areas may be mediating the effect of GAN on reinstatement, and if δ subunit expression is in fact altered in these brain regions in the present procedure.
Because these studies all utilized systemic injections, they do not provide insight into the brain areas underlying the ability of GAN to promote reinstatement of ethanol seeking. In human studies, the ventromedial prefrontal cortex and anterior cingulate are both hypoactive in abstinent, recovering alcoholics, and this hypofunction is related to increased craving and possibly relapse (Seo et al., 2013). Although speculative, it is possible that GAN could have acted in the prefrontal cortex or interacting brain areas, and that this hypofunction could have contributed to increased ethanol seeking. Another brain region that may mediate the effects of GAN on the reinstatement of ethanol seeking, particularly if there is a link between drug discrimination and reinstatement, may be the nucleus accumbens core; local infusions of ALLO into the nucleus accumbens core have been shown to share subjective stimulus effects with ethanol (Hodge et al., 2001). Future studies using site-specific infusions or employing c-Fos immunoreactivity could help elucidate some of the underlying brain regions contributing to the reinstatement of ethanol seeking.
Although the specificity of GAN to enhance the reinstatement of ethanol seeking was not tested in the present study, ALLO promoted reinstatement of sucrose seeking in mice, but not in rats (Finn et al., 2008; Nie and Janak, 2003). Given that ALLO also decreased latency to feed in rats (Holmberg et al., 2013), it is possible that ALLO and GAN may have more general effects on seeking behavior, particularly for calorie-containing substances. On the other hand, ALLO decreased drug-induced reinstatement of cocaine and methamphetamine seeking, respectively, in rats, at doses that did not induce sedation (Holtz et al., 2012; Anker et al., 2009), indicating that ALLO or GAN-induced enhancement of ethanol seeking does not generalize to all drugs of abuse or reinforcers. It should be noted that in the case of reinstatement of cocaine and methamphetamine seeking, the effect of ALLO was only present in female mice (Holtz et al., 2012; Anker et al., 2009), highlighting important potential interactions with sex hormones that remain to be elucidated in the case of the reinstatement of ethanol seeking.
The present studies were performed in male mice due to data showing that female rodents were insensitive to manipulations of ALLO levels on subsequent ethanol intake (reviewed in Finn et al., 2010). Additionally, expression of the δ subunit varies across the estrous cycle (Maguire et al., 2005), as does the ability of THIP to reduce ethanol intake in female rodents (Melón et al., 2012 abstract), which can complicate interpretation of studies examining the effectiveness of THIP on reinstatement of ethanol seeking in females. Future studies are aimed at examining the effect of GAN and THIP on ethanol seeking in female mice, as well as the potential interaction of these effects with the estrous cycle.
4. Conclusions
In conclusion, GAN promoted the reinstatement of ethanol seeking, consistent with data showing that GAN increased ethanol seeking in an operant self-administration procedure (Ramaker et al., 2012) and that ALLO promoted ethanol seeking in a reinstatement procedure (Finn et al. 2008; Nie and Janak, 2003). On the other hand, THIP did not promote reinstatement of ethanol seeking in the present study, consistent with data showing that THIP decreased ethanol-reinforced responding in an operant procedure (Ramaker et al., 2012). These data suggest that direct activation of extrasynaptic GABAA receptors at the GABA site is not sufficient to induce ethanol seeking in the reinstatement procedure.
Highlights.
A GABAA receptor-active neurosteroid analog, ganaxolone, reinstated ethanol seeking in mice.
An extrasynaptic GABAA receptor agonist, gaboxadol (THIP), did not reinstate ethanol seeking.
Direct extrasynaptic GABAA receptor activation is not sufficient to reinstate ethanol seeking.
Acknowledgments
Funding
Funding was provided by grants R01 AA16981 and R01 AA12439 and the Department of Veteran Affairs (DAF). MJR was supported by F31 AA020716, MMF was supported by KO1 AA016849, and TJP was supported by the Department of Veterans Affairs, P60 AA010760, R24 AA020245 and P50 DA018165.
We would like to thank Michelle Tanchuck, Chris Snelling, and Dr. Debra Cozzoli for assistance.
Footnotes
Disclosure
There are no conflicts of interest or competing financial interests in relation to the work described.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anker JJ, Holtz NA, Zlebnik N, Carroll ME. Effects of allopregnanolone on the reinstatement of cocaine-seeking behavior in male and female rats. Psychopharmacology (Berl) 2009;203:63–72. doi: 10.1007/s00213-008-1371-9. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Serra M, Purdy RH, Biggio G. Stress and neuroactive steroids. Int Rev Neurobiol. 2001;46:243–272. doi: 10.1016/s0074-7742(01)46065-x. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Belelli D, Herd MB. The contraceptive agent Provera enhances GABAA receptor-mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids? J Neurosci. 2003;23:10013–10020. doi: 10.1523/JNEUROSCI.23-31-10013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Lindsay TG, O’Buckley TK, Hodge CW, Morrow AL. Pregnenolone and ganaxolone reduce operant ethanol self-administration in alcohol-preferring P rats. Alcohol Clin Exp Res. 2010;34:2044–2052. doi: 10.1111/j.1530-0277.2010.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Harris RA. Studies of ethanol actions on recombinant δ-containing γ-aminobutyric acid type A receptors yield contradictory results. Alcohol. 2007;41:155–162. doi: 10.1016/j.alcohol.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen CA, Purdy RH, Grant KA. Ethanol-like discriminative stimulus effects of endogenous neuroactive steroids: effect of ethanol training dose and dosing procedure. J Pharmacol Exp Ther. 1999;289:405–411. [PubMed] [Google Scholar]
- Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, Bolger MB, Lan NC, Gee KW. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3α-hydroxy-3β-methyl-5α-pregnan-20-one), a selective, high-affinity, steroid modulator of the γ-aminobutyric acidA receptor. J Pharmacol Exp Ther. 1997;280:1284–1295. [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- ClinicalTrials.gov. [Accessed January 2014]; http://www.clinicaltrial.gov/ct2/results?term=allopregnanolone&Search=Search.
- ClinicalTrials.gov. [Accessed January 2014]; http://www.clinicaltrial.gov/ct2/results?term=ganaxolone&Search=Search.
- ClinicalTrials.gov. [Accessed January 2014]; http://www.clinicaltrial.gov/ct2/results?term=thip&Search=Search.
- Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of γ-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers T, Ebert B. Plasma and CNS concentrations of gaboxadol in rats following subcutaneous administration. Eur J Pharmacol. 2007;562:47–52. doi: 10.1016/j.ejphar.2007.01.017. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Drug reinstatement of heroin-reinforced responding in the rat. Psychopharmacology (Berl) 1983;79:29–31. doi: 10.1007/BF00433012. [DOI] [PubMed] [Google Scholar]
- de Wit H. Priming effects with drugs and other reinforcers. Exp Clin Psychopharmacol. 1996;4:5–10. [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck MA, Phillips TJ. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004;123:813–819. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Finn DA, Mark GP, Fretwell AM, Gililland-Kaufman KR, Strong MN, Ford MM. Reinstatement of ethanol and sucrose seeking by the neurosteroid allopregnanolone in C57BL/6 mice. Psychopharmacology (Berl) 2008;201:423–433. doi: 10.1007/s00213-008-1303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Beckley EH, Kaufman KR, Ford MM. Manipulation of GABAergic steroids: Sex differences in the effects on alcohol drinking- and withdrawal-related behaviors. Horm Behav. 2010;57:12–22. doi: 10.1016/j.yhbeh.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, Finn DA. Neurosteroid modulators of GABAA receptors differentially modulate ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res. 2005;29:1630–1640. doi: 10.1097/01.alc.0000179413.82308.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Mark GP, Nickel JD, Phillips TJ, Finn DA. Allopregnanolone influences the consummatory processes that govern ethanol drinking in C57BL/6J mice. Behav Brain Res. 2007a;179:265–272. doi: 10.1016/j.bbr.2007.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Fretwell AM, Mark GP, Finn DA. Influence of reinforcement schedule on ethanol consumption patterns in non-food restricted male C57BL/6J mice. Alcohol. 2007b;41:21–29. doi: 10.1016/j.alcohol.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Azarov A, Bowen CA, Mirkis S, Purdy RH. Ethanol-like discriminative stimulus effects of the neurosteroid 3α-hydroxy-5α-pregnan-20-one in female Macaca fascicularis monkeys. Psychopharmacology (Berl) 1996;124:340–346. doi: 10.1007/BF02247439. [DOI] [PubMed] [Google Scholar]
- Grant KA, Helms CM, Rogers LSM, Purdy RH. Neuroactive steroid stereospecificity of ethanol-like discriminative stimulus effects in monkeys. J Pharmacol Exp Ther. 2008;326:354–361. doi: 10.1124/jpet.108.137315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubner NR, McKinnon CS, Reed C, Phillips TJ. Accentuating effects of nicotine on ethanol response in mice with high genetic predisposition to ethanol-induced locomotor stimulation. Drug Alcohol Depend. 2013;127:108–114. doi: 10.1016/j.drugalcdep.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Nannini MA, Olive MF, Kelley SP, Mehmert KK. Allopregnanolone and pentobarbital infused into the nucleus accumbens substitute for the discriminative stimulus effects of ethanol. Alcohol Clin Exp Res. 2001;25:1441–1447. doi: 10.1097/00000374-200110000-00006. [DOI] [PubMed] [Google Scholar]
- Holmberg E, Bäckström T, Johansson M, Löfgren M, Haage D. Allopregnanolone induces a diurnally dependent hyperphagic effect and alters feeding latency and duration in male Wistar rats. Acta Physiol. 2013;208:400–409. doi: 10.1111/apha.12100. [DOI] [PubMed] [Google Scholar]
- Holtz NA, Lozama A, Prisinzano TE, Carroll ME. Reinstatement of methamphetamine seeking in male and female rats treated with modafinil and allopregnanolone. Drug Alcohol Depend. 2012;120:233–237. doi: 10.1016/j.drugalcdep.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HMA, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Janak PH, Redfern JE, Samson HH. The reinforcing effects of ethanol are altered by the endogenous neurosteroid, allopregnanolone. Alcohol Clin Exp Res. 1998;22:1106–1112. [PubMed] [Google Scholar]
- Janak PH, Gill TM. Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol. 2003;30:1–7. doi: 10.1016/s0741-8329(03)00068-5. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Suryanarayanan A, Abriam A, Snyder B, Olsen RW, Spigelman I. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci. 2007;27:12367–12377. doi: 10.1523/JNEUROSCI.2786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Meera P, Wallner M, Otis TS. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABAA receptors. J Neurophysiol. 2011;106:2057–2064. doi: 10.1152/jn.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melón LC, Nolan ZT, Bohem SL., II Activation of GABAA receptors and inhibition of neurosteroid synthesis have separable estrous-dependent effects on binge drinking in female mice. Alcohol Clin Exp Res. 2012;36:184A. [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone L, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalek RM, Bowers BJ, Wehner JM, Kralic JE, VanDoren MJ, Morrow AL, Homanics GE. GABAA receptor δ subunit knockout mice have multiple defects in behavioral responses to ethanol. Alcohol Clin Exp Res. 2001;25:1708–1718. [PubMed] [Google Scholar]
- Mody I, Glykys J, Wei W. A new meaning for “Gin & Tonic”: tonic inhibition as the target for ethanol action in the brain. Alcohol. 2007;41:145–153. doi: 10.1016/j.alcohol.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Janak PH. Comparison of reinstatement of ethanol- and sucrose-seeking by conditioned stimuli and priming injections of allopregnanolone after extinction in rats. Psychopharmacology (Berl) 2003;168:222–228. doi: 10.1007/s00213-003-1468-0. [DOI] [PubMed] [Google Scholar]
- Nohria V, Giller E. Ganaxolone. Neurotherapeutics. 2007;4:102–105. doi: 10.1016/j.nurt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Hanchar HJ, Meera P, Wallner M. GABAA receptor subtypes: the “one glass of wine” receptors. Alcohol. 2007;41:201–209. doi: 10.1016/j.alcohol.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich JM, Armor DJ, Braiker HB. Patterns of alcoholism over four years. J Stud Alcohol. 1980;41:397–416. doi: 10.15288/jsa.1980.41.397. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaker MJ, Ford MM, Fretwell AM, Finn DA. Alteration of ethanol drinking in mice via modulation of the GABAA receptor with ganaxolone, finasteride, and gaboxadol. Alcohol Clin Exp Res. 2011;35:1994–2007. doi: 10.1111/j.1530-0277.2011.01551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaker MJ, Strong MN, Ford MM, Finn DA. Effect of ganaxolone and THIP on operant and limited-access ethanol self-administration. Neuropharmacology. 2012;63:555–564. doi: 10.1016/j.neuropharm.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J Pharmacol Exp Ther. 2000;295:1241–1248. [PubMed] [Google Scholar]
- Samson HH, Slawecki CJ, Sharpe AL, Chappell A. Appetitive and consummatory behaviors in the control of ethanol consumption: a measure of ethanol seeking behavior. Alcohol Clin Exp Res. 1998;22:1783–1787. [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA psychiatry. 2013;70:727–739. doi: 10.1001/jamapsychiatry.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shannon EE, Shelton KL, Vivian JA, Yount I, Morgan AR, Homanics GE, Grant KA. Discriminative stimulus effects of ethanol in mice lacking the γ-aminobutyric acid type A receptor δ subunit. Alcohol Clin Exp Res. 2004;28:906–913. doi: 10.1097/01.alc.0000128227.28794.42. [DOI] [PubMed] [Google Scholar]
- Shelton KL, Grant KA. Discriminative stimulus effects of ethanol in C57BL/6J and DBA/2J inbred mice. Alcohol Clin Exp Res. 2002;26:747–757. [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinnott RS, Phillips TJ, Finn DA. Alteration of voluntary ethanol and saccharin consumption by the neurosteroid allopregnanolone in mice. Psychopharmacology (Berl) 2002;162:438–447. doi: 10.1007/s00213-002-1123-1. [DOI] [PubMed] [Google Scholar]
- Stewart J. Conditioned and unconditioned drug effects in relapse to opiate and stimulant drug self-adminstration. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7:591–597. doi: 10.1016/0278-5846(83)90030-1. [DOI] [PubMed] [Google Scholar]
- Timby E, Ballard M, Nyberg S, Spigset O, Andersson A, Porankiewicz-Asplund J, Purdy RH, Zhu D, Bäckström T. Pharmacokinetic and behavioral effects of allopregnanolone in healthy women. Psychopharmacology (Berl) 2006;186:414–424. doi: 10.1007/s00213-005-0148-7. [DOI] [PubMed] [Google Scholar]
- Ungard JT, Beekman M, Gasior M, Carter RB, Dijkstra D, Witkin JM. Modification of behavioral effects of drugs in mice by neuroactive steroids. Psychopharmacology (Berl) 2000;148:336–343. doi: 10.1007/s002130050060. [DOI] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Li TK. Drugs and alcohol: treating and preventing abuse, addiction and their medical consequences. Pharmacol Ther. 2005;108:3–17. doi: 10.1016/j.pharmthera.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Wafford KA, van Niel MB, Ma QP, Horridge E, Herd MB, Peden DR, Belelli D, Lambert JJ. Novel compounds selectively enhance δ subunit containing GABAA receptors and increase tonic currents in thalamus. Neuropharmacology. 2009;56:182–189. doi: 10.1016/j.neuropharm.2008.08.004. [DOI] [PubMed] [Google Scholar]