Abstract
Defects in mitochondrial dynamics, the processes of fission, fusion, and mitochondrial autophagy, may contribute to metabolic disease including type 2 diabetes. Dynamin-related protein-1 (Drp1) is a GTPase protein that plays a central role in mitochondrial fission. We hypothesized that aerobic exercise training would decrease Drp1 Ser616 phosphorylation and increase fat oxidation and insulin sensitivity in obese (body mass index: 34.6 ± 0.8 kg/m2) insulin-resistant adults. Seventeen subjects performed supervised exercise for 60 min/day, 5 days/wk at 80–85% of maximal heart rate for 12 wk. Insulin sensitivity was measured by hyperinsulinemic-euglycemic clamp, and fat oxidation was determined by indirect calorimetry. Skeletal muscle biopsies were obtained from the vastus lateralis muscle before and after the 12-wk program. The exercise intervention increased insulin sensitivity 2.1 ± 0.2-fold (P < 0.01) and fat oxidation 1.3 ± 0.3-fold (P < 0.01). Phosphorylation of Drp1 at Ser616 was decreased (pre vs. post: 0.81 ± 0.15 vs. 0.58 ± 0.14 arbitrary units; P < 0.05) following the intervention. Furthermore, reductions in Drp1 Ser616 phosphorylation were negatively correlated with increases in fat oxidation (r = −0.58; P < 0.05) and insulin sensitivity (rho = −0.52; P < 0.05). We also examined expression of genes related to mitochondrial dynamics. Dynamin1-like protein (DNM1L; P < 0.01), the gene that codes for Drp1, and Optic atrophy 1 (OPA1; P = 0.05) were significantly upregulated following the intervention, while there was a trend towards an increase in expression of both mitofusin protein MFN1 (P = 0.08) and MFN2 (P = 0.07). These are the first data to suggest that lifestyle-mediated improvements in substrate metabolism and insulin sensitivity in obese insulin-resistant adults may be regulated through decreased activation of the mitochondrial fission protein Drp1.
Keywords: mitochondrial fission, mitochondrial dynamics, insulin sensitivity, aerobic exercise, fat oxidation
increased intramyocellular lipids (27, 41) and lipid intermediates, including diacylglycerols (DAGs) and ceramides (36), combined with impaired fat oxidation (43) suggests the presence of mitochondrial dysfunction in the pathogenesis of insulin resistance. However, despite significant attention, no consensus has thus far emerged regarding a mitochondrial defect antecedent to the development of insulin resistance (for review, see Refs. 10 and 15).
One aspect of mitochondrial physiology that has heretofore received comparatively less attention is the potential role of altered mitochondrial dynamics in the pathogenesis of insulin resistance. Mitochondria are highly dynamic organelles that continually fuse and divide. In skeletal muscle, mitochondria form as a highly reticular branched network (23) with morphological characteristics that differ between muscle fiber type (39) and muscle mitochondrial subpopulations (45). It is the balance among the processes of fusion, fission, and mitophagy that define the reticular nature of the mitochondrial network (4, 54). Recently, greater knowledge has emerged regarding the protein machinery that regulates these processes. Outer mitochondrial membrane fusion is regulated by the activity of the mitofusin proteins (Mfn1 and Mfn2) (17, 53) while fusion of the inner mitochondrial membrane is coordinated by the activity of Optic atrophy 1 (OPA1) (53). For fission to occur, evidence suggests that Fission-1 (Fis1) (19, 62) binds to the outer mitochondrial membrane and recruits the dynamin-like GTPase protein dynamin-related protein-1 (Drp1) (62), which, when activated, pinches off a portion of the mitochondrial network (28, 49, 50) The excised mitochondrial fragment may then be tagged for autophagy by the recruitment of PTEN-induced putative kinase 1 (PINK1) and Parkin or may, after a period, rejoin the mitochondrial network by the action of the fusion proteins (59). Crucially, Drp1-mediated fission events are associated with a transient loss of mitochondrial membrane potential in the excised mitochondrial portion (59).
Dysregulation of mitochondrial dynamics has been implicated in an increasing number of disease states (4, 18, 57, 65), including type 2 diabetes (1, 16). However, data linking alterations in mitochondrial dynamics and skeletal muscle insulin resistance are limited. Muscle mitochondria from patients with type 2 diabetes, observed by electron microscopy, are smaller in size compared with nondiabetic controls (22), a morphological characteristic that is partially rescued by exercise training (58), while protein expression studies indicate a profragmentary environment in muscle from obese individuals (2) and in type 2 diabetes (21). Furthermore, Bach et al. (2) reported a 25% reduction in the mitochondrial network in skeletal muscle of obese Zucker rats, compared with wild type, in the absence of differences in mitochondrial mass. In the same study, fibroblasts treated with an Mfn2 antisense sequence exhibited decreased glucose oxidation and oxygen consumption. Mfn2 repression is also associated with decreased rates of pyruvate and palmitate oxidation in L6E9 muscle cells (46). A recent report by Jheng et al. (20) provides evidence that Drp1-mediated mitochondrial fission results in mitochondrial fragmentation, loss of mitochondrial membrane potential, increased oxidative stress, decreased ATP content, and insulin-mediated glucose uptake in C2C12 cells preincubated with palmitic acid. Moreover, Drp1 knockdown experiments reversed these alterations. These findings were duplicated in ob/ob mice. In this case, mice treated with Mdivi-1, a Drp1 inhibitor, recovered tubular mitochondrial percentage and membrane potential, decreased oxidative stress to baseline levels, and increased insulin-mediated glucose uptake. Collectively, these data provide strong evidence implicating Drp1-mediated mitochondrial fission as a likely contributing factor to insulin resistance. However, there are currently no data that link this process to insulin resistance in humans.
It has been postulated that alterations in energy balance may contribute to changes in mitochondrial network structure (44) such that increased energy demand/starvation leads to a more highly reticular mitochondrial network (13, 47), while increased energy supply results in a more highly fragmented network (20, 35, 60, 61). Since aerobic exercise interventions are an effective strategy for increasing energy demand and simultaneously enhancing insulin sensitivity and fat oxidation in humans, it is possible that a mitochondrial mechanism for improvement in insulin sensitivity may reside in alterations in the mitochondrial fission pathway. However, no studies have, hitherto, reported on the effect of exercise on activation of Drp1-mediated mitochondrial fission in insulin-resistant humans. Indeed, if Drp1-mediated mitochondrial fission is implicated in insulin resistance, we would expect to see reductions in Drp1 activation following an aerobic exercise intervention. Further, alterations in Drp1 activation would likely be related to changes in fat oxidation and insulin sensitivity. To test this hypothesis, we examined the effect of a 12-wk aerobic exercise intervention on skeletal muscle Drp1 activation at Ser616 phosphorylation (the form required for mitochondrial fission activity) (56), insulin sensitivity, and fat oxidation in obese insulin-resistant individuals.
METHODS
Participants.
Seventeen (male/female: 10/7) older (age; 66 ± 1 yr), previously sedentary (individuals exercising for 20 min or more at least 2 times per wk were excluded), nonsmoking, obese (body mass index: 34.6 ± 0.8 kg/m2) adults were recruited from the Greater Cleveland community to undergo a 12 wk aerobic exercise intervention. Medical screenings excluded individuals with heart, kidney, liver, thyroid, intestinal, and pulmonary diseases as well as individuals taking medications known to affect the outcome variables of the study. Resting 12-lead electrocardiograms and submaximal exercise stress tests excluded individuals with any contraindication to physical activity. All women were postmenopausal and not using hormone replacement therapy. Participants had also been weight stable for at least the previous 6 mo. The Cleveland Clinic Institutional Review Board approved the study, and all subjects provided informed consent in accordance with guidelines on the protection of human subjects.
Intervention.
Participants performed 60 min (20 min cycle ergometry and 40 min treadmill walking) of supervised aerobic exercise at ∼80–85% maximum heart rate on 5 days/wk for 12 wk. Compliance with exercise intensity was monitored using a heart rate monitor (Polar Electro, Woodbury, NY). Caloric intake was monitored weekly, and average energy intake pre and post was 1984.1 ± 169.7 and 1,898.7 ± 175.7 kcal/day, respectively.
Inpatient control period.
Pre- and postintervention measures were controlled during a 3-day in-patient stay in the Clinical Research Unit at the Cleveland Clinic. During the inpatient control periods, participants were provided with a weight maintenance isocaloric diet (total kcal/day = resting metabolic rate × 1.25; 55% carbohydrate, 35% fat, and 10% protein) derived from indirect calorimetry measures conducted at the beginning of the inpatient control period. All metabolic measurements were conducted during the inpatient control period and within 24 h of the last exercise bout.
Body composition.
Height and body weight were measured using a stadiometer and digital scale. Whole body adiposity was measured by dual energy X-ray absorptiometry (model iDXA; Lunar, Madison, WI).
Aerobic fitness.
Each participant performed an incremental graded treadmill exercise test to determine maximal oxygen consumption (V̇o2max), as previously described (38). Exhaled air was continuously sampled online with the use of an automated system (Jaeger Oxycon Pro; Viasys, Yorba Linda, CA).
Insulin sensitivity and substrate metabolism.
Insulin sensitivity measurements were obtained after an overnight fast using a 2-h euglycemic-hyperinsulinemic clamp (90 mg/dl, 40 mU·m−2·min−1), as previously described (24). Briefly, a primed (3.28 mg/kg) continuous (0.036 mg·kg−1·min−1) infusion of [6,6-2H2]glucose began at t = −120 min and continued throughout the procedure for estimation of endogenous glucose production. At t = 0, the insulin infusion commenced and glucose was infused at a variable rate. Arterialized venous blood was sampled at 5-min intervals (YSI 2300; STAT Plus, Yellow Springs, OH), and glucose infusion was adjusted according to the calculations of DeFronzo et al. (9). Insulin sensitivity was calculated as insulin-stimulated glucose disposal rate (GDR; mg·kg−1·min−1) divided by plasma insulin (μU/ml) during the last 40 min of the clamp (24). Homeostatic model assessment of insulin resistance (HOMA-IR) {[fasting plasma glucose (FPG) × fasting plasma insulin (FPI)] ÷ 405} (34) was also measured as an estimate of insulin resistance. Indirect calorimetry (Vmax Encore; Viasys) measures were performed before the clamp procedure for determination of respiratory exchange rate (RER) and substrate metabolism. Furthermore, protein metabolism was estimated from overnight, timed measurements of urinary nitrogen excretion as previously described (52). Protein corrected fat oxidation was calculated using the Frayn equation (1.67 V̇o2 − 1.67 V̇co2 − 1.92 n) (12). Plasma for glucose kinetics analyses was deproteinized, extracted and derivatized before analysis by gas chromatography-mass spectrometry. First, 1 ml 70% methanol was added to 200 μl plasma and centrifuged at 1,000 rpm for 10 min. The supernatant fluid was collected, dried under air, and reconstituted with 200 μl double-distilled H2O. The sample was then applied to a glass column containing a cation exchange resin (AG50W-X8 200–400 mesh; Bio-Rad, Hercules, CA), eluted with 5 ml double-distilled H2O (pH 8.0), and dried (Labconco, Kansas City, MO). Thirty microliters of pyridine and 15 μl acetic anhydride were added to the dried sample and incubated for 2 h at room temperature. Finally, 400 μl H2O and 400 μl ethyl acetate were added. The sample was centrifuged for 5 min at 1,000 rpm, and the upper layer was collected for injection into a Hewlett-Packard 5985A gas chromatograph-mass spectrometer (Hewlett-Packard, Palo Alto, CA). Ion mass-to-charge ratios (m/z) 200 and 202 were selectively monitored. The isotopic enrichment (mol percent excess) of the samples were obtained by comparing their peak area percentage (m/z 202)/(m/z 202 + m/z 200) with that of a standard curve (51).
Skeletal muscle biopsy.
Basal muscle specimens were obtained from the vastus lateralis before and after the intervention. The muscle was dissected free from visible fat and connective tissue. The tissue was immediately frozen and stored in liquid nitrogen until subsequent analysis. Biopsies and indirect calorimetry measurements were taken immediately before initiation of insulin infusion.
RNA extraction.
RNA was extracted from human muscle with TRI Reagent (Sigma, St. Louis, MO). Briefly, 10–20 mg of muscle tissue were homogenized in 1 ml of TRI Reagent at 4°C with repetitive short bursts. Homogenized tissue was incubated at room temperature for 5–10 min, followed by centrifugation at 12,000 g for 10 min at 4°C. RNA was separated into an aqueous phase using 0.1 ml of 1-bromo-3-chloropropane and precipitated with 0.5 ml of isopropanol. Isolated RNA was washed with 1 ml of 75% ethanol, air dried, and dissolved in 40 μl nuclease free water. RNA concentration and purity were determined using a NanoDrop ND-1,000 Spectrophotometer (Thermo Scientific, Wilmington, DE). RNA integrity was also randomly assessed using an Agilent bioanalyzer (Agilent, Santa Clara, CA). Isolated RNA was aliquoted and stored at −80°C until further analysis.
cDNA synthesis.
Before cDNA synthesis, RNA samples were treated with DNase I for 15 min at room temperature to remove any contaminating DNA (Invitrogen, Carlsbad, CA). One microgram of total RNA was reverse transcribed into cDNA (iScript cDNA synthesis kit; Bio-Rad) using a PX2 Thermal Cycler (Thermo Scientific). The reaction volume was 20 μl, and synthesis was performed at 25°C for 5 min, 42°C for 30 min, and 85°C for 5 min, respectively. cDNA samples were stored at −20°C until later analysis.
Quantitative RT-PCR primer pairs.
Primer pairs for target genes were obtained from PrimerBank database (pga.mgh.harvard.edu/primerbank/; see Table 1). All primers were checked for specificity to the genes of interest by Blast analysis.
Table 1.
Primers
| Gene | Forward | Reverse | GenBank Accession No. |
|---|---|---|---|
| DNM1L | AGGTTGCCCGTGACAAATGA | ATCAGCAAAGTCGGGGTGTT | NM_012063 |
| FIS1 | GTCCAAGAGCACGCAGTTTG | ATGCCTTTACGGATGTCATCATT | NM_016068 |
| PARK2 | GTGTTTGTCAGGTTCAACTCCA | GAAAATCACACGCAACTGGTC | NM_152410 |
| MFN1 | ATGACCTGGTGTTAGTAGACAGT | AGACATCAGCATCTAGGCAAAAC | NM_033540 |
| MFN2 | CACATGGAGCGTTGTACCAG | TTGAGCACCTCCTTAGCAGAC | NM_001127660 |
| PINK1 | AGTGATTGACTACAGCAAGGCTGAT | ATCTTGTCTAACTTCAGATTCTTCAGG | NM_032409 |
| OPA1 | AGCCTCGCAATTTTTGG | AGCCGATCCTAGTATGAGATAGC | NM_130837 |
Primer pairs for genes involved in mitochondrial dynamics. MFN1, Mitofusin 1; MFN2, Mitofusin 2; OPA1, Optic atrophy 1; FIS1, Fission 1; DNM1L, Dynamin1-like protein; PINK1, PTEN-induced putative kinase 1; PARK2, Parkin 2.
Semiquantitative real-time PCR analysis.
Determination of relative mRNA expression was performed in duplicate on an MX3000P QPCR system (Agilent Technologies/Stratagene, La Jolla, CA) using 10 ng of cDNA as the template and the Brilliant II SYBR Green QPCR Master Mix (Stratagene). The human GAPDH gene was used as an internal standard (31). Relative change in mRNA abundance was calculated using the comparative ΔΔCt method (29). Briefly, the threshold cycle (Ct) for GAPDH was subtracted from the Ct for the gene of interest to adjust for variations in mRNA/cDNA generation efficacy to generate the ΔCt value. Preintervention was used as the baseline and the fold induction of the target gene at postintervention was calculated as an exponential of the negative value of the subtraction of ΔCt at preintervention from ΔCt at postintervention (2−ΔΔCt) (29).
Tissue homogenization and Western blot analysis.
Muscle homogenates were prepared by grinding muscle tissue with ice-cold lysis buffer (Invitrogen) in the presence of protease inhibitor cocktail, 5 mM phenylmethylsulfonyl fluoride (Sigma), and Phos-STOP (Roche Applied Sciences, Indianapolis, IN). Samples for Western blot were prepared from supernatants after centrifugation of homogenates for 10 min at 14,000 g. Protein concentrations were measured using a BCA protein assay kit (Pierce Biotechnology, Rockford, IL). Fifty micrograms of muscle homogenate were solubilized in Laemmli sample buffer containing 5% β-mercaptoethanol and boiled for 5 min. Proteins were separated by 4–20% Novex Tris Glycine SDS-PAGE Electrophoresis (Invitrogen), and transferred to polyvinylidene fluoride membrane (Bio-Rad), and blocked with 5% bovine serum albumin in phosphate-buffered saline with 0.1% Tween-20 (PBST) for 1 h. Membranes were then incubated overnight with anti-phospho Drp1 (Cell Signaling Technology; Danvers, MA, catalog no. 3455), anti-Drp1 (Cell Signaling Technology; catalog no. 8570), and anti-actin (Santa Cruz Biotechnologies, Dallas, TX) antibodies. Membranes were washed in PBST and incubated with anti-rabbit horseradish peroxidase-conjugated antibodies (GE Healthcare, Piscataway, NJ; catalog no. NA931). Immunoreactive proteins were visualized by enhanced chemiluminescence reagent (ECL Prime; GE Healthcare) and quantified by densitometric analysis using Image Quant TL software (GE Healthcare).
Statistical analyses.
Values were tested for normality using the D'Agostino and Pearson omnibus normality test on GraphPad Prism 4.0 (Graphpad Software, San Diego CA). Pre- to postintervention changes were assessed using repeated-measures ANOVA for normally distributed samples. A Wilcoxon signed-rank test was used to determine changes in gene expression. Linear regression analysis was used to determine associations between normally distributed data. In addition, Spearman's rank correlation analyses were used to identify relationships between variables that failed the normality test [Δinsulin sensitivity]. Statistical significance was accepted when P < 0.05. These analyses were carried out using StatView for Windows 5.0.1 (SAS Institute), and all data are expressed as means ± SE.
RESULTS
Participant characteristics.
Subject characteristics are summarized in Table 2. The intervention resulted in a significant 10.4 ± 1.1% decrease in body weight. This was primarily due to a decrease in fat mass (20.9 ± 3.4% reduction), while fat-free mass was largely preserved (1.9 ± 0.8% reduction). V̇o2max was also increased following the intervention (P < 0.001).
Table 2.
Pre- and postintervention subject characteristics
| Subject Characteristics | Preintervention | Postintervention | P Value |
|---|---|---|---|
| n (m/f) | 17 (10/7) | — | |
| Age, yr | 66 ± 1 | — | |
| Weight, kg | 101.5 ± 3.6 | 90.4 ± 3.1 | <0.001 |
| BMI, kg/m2 | 34.6 ± 0.8 | 31.0 ± 0.8 | <0.001 |
| FM, kg | 43.8 ± 1.9 | 35.0 ± 2.3 | <0.001 |
| FFM, kg | 55.7 ± 3.6 | 54.5 ± 3.5 | <0.05 |
| FPG, mg/dl | 98.3 ± 1.9 | 94.4 ± 1.6 | <0.01 |
| FPI, μU/ml | 13.3 ± 0.9 | 11.5 ± 0.9 | 0.06 |
| HOMA-IR | 3.3 ± 0.3 | 2.7 ± 0.2 | <0.05 |
| Insulin sensitivity, mg·kg−1·min−1·μU·ml−1 | 0.025 ± 0.005 | 0.047 ± 0.005 | <0.001 |
| Basal fat oxidation, mg·kg−1·min−1 | 0.35 ± 0.05 | 0.56 ± 0.07 | <0.01 |
| V̇o2max, ml·kg−1·min−1 | 22.4 ± 0.89 | 28.9 ± 1.68 | <0.001 |
Data are means ± SE. BMI, body mass index; FM, fat mass; FFM, fat-free mass; FPG, fasting plasma glucose; FPI, fasting plasma insulin; HOMA-IR, homeostasis model assessment of insulin resistance; V̇o2max, maximal oxygen consumption.
Insulin sensitivity and substrate metabolism.
Fasting glucose was decreased following the intervention (P < 0.01) while fasting insulin approached a significant decrease (P = 0.06). This translated into a significant reduction in HOMA-IR (P < 0.05). Insulin sensitivity derived from the clamp was also increased following the intervention (P < 0.001), as was basal fat oxidation (P < 0.01; Table 2).
Protein expression.
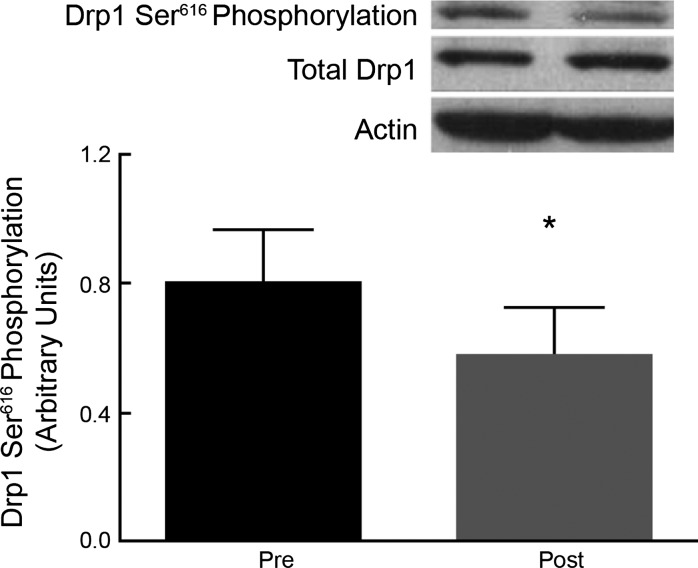
Exercise training resulted in a significant reduction in Drp1 phosphorylation at Ser616 (ratio of phosphorylated Drp1 to total Drp1; P = 0.01; Fig. 1). Total Drp1 (ratio of Drp1 to actin) protein expression was not increased by the exercise intervention (pre vs. post: 0.91 ± 0.22 vs. 1.02 ± 0.13, P = 0.63).
Fig. 1.
A: representative blots of phosphorylated-Drp1 at Ser616, total Drp1, and actin. B: densitometric analysis of phosphorylated Drp1 (pDrp1), total Drp1 (Drp1), and actin. Exercise resulted in a reduction in Drp1 Ser616 phosphorylation (P = 0.01). Data are means ± SE. *P < 0.05.
Gene expression.
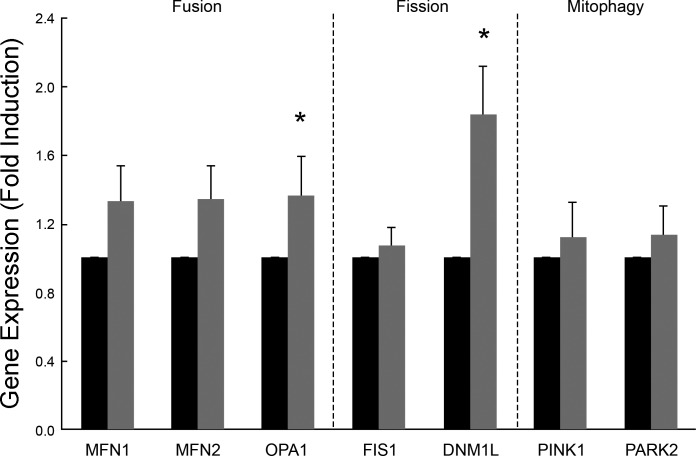
Dynamin1-like protein (DNM1L) is the gene that codes for Drp1, and its expression was also significantly upregulated (P < 0.01) following the intervention. Further, exercise training increased OPA1 gene expression (P = 0.05), while there was a trend towards an increase in expression of both MFN1 (P = 0.08) and MFN2 (P = 0.07) genes. The expression of FIS1 was unchanged and no change was observed in the expression of PINK1 or PARK2 genes (Fig. 2).
Fig. 2.
Expression of genes related to mitochondrial dynamics. Exercise resulted in increased expression of both OPA1 (*P < 0.05) and DNM1L (*P < 0.01) but no change in the expression of the other genes. MFN1, Mitofusin 1; MFN2, Mitofusin 2; OPA1, Optic atrophy 1; FIS1, Fission 1; DNM1L, Dynamin1-like protein; PINK1, PTEN-induced putative kinase 1; PARK2, Parkin 2.
Correlations.
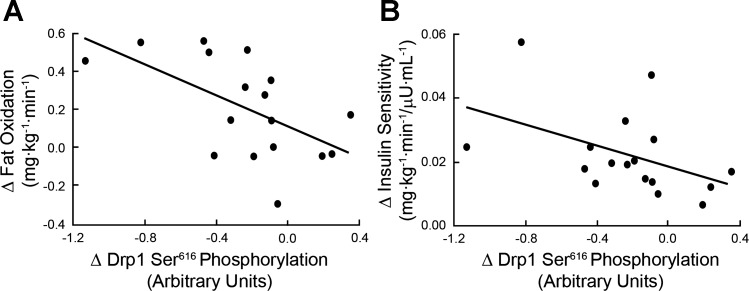
There was a significant correlation between changes in Drp1 Ser616 phosphorylation and fat oxidation (r = −0.58; P < 0.05; Fig. 3A), and also changes in Drp1 Ser616 phosphorylation and insulin sensitivity (rho = −0.52; P < 0.05; Fig. 3B).
Fig. 3.
A: correlation between change in Drp1 Ser616 phosphorylation and whole body fat oxidation following the exercise intervention (r = −0.58; P < 0.05). B: correlation between changes in the ratio of phosphorylated Drp1 Ser616 to total Drp1 and insulin sensitivity following the exercise intervention (rho = −0.52; P < 0.05).
DISCUSSION
Our data show that a 12-wk aerobic exercise intervention that induced significant weight loss in obese adults improved peripheral insulin sensitivity and fat oxidation in parallel with alterations in basal Drp1 activation. Since phosphorylation of Drp1 at Ser616 increases mitochondrial fission activity (56), our data suggest that Drp1-mediated mitochondrial fission may be decreased following exercise training. Furthermore, this downregulation of Drp1 activity was significantly correlated with improvements in fat oxidation and insulin sensitivity. These data are novel because they provide the first in vivo evidence in humans that supports the findings of recent research using cell and animal models, which suggest that Drp1-mediated mitochondrial fission may be an important determinant of skeletal muscle insulin resistance.
Much has been made in recent years of the potential role of mitochondria in the development of diabetes. Despite this, no mechanism has thus far emerged linking impairments in mitochondrial function to insulin resistance. The hypothesis that mitochondrial fission may result in mitochondrial dysfunction and insulin resistance is intriguing, as this mechanism would require no intrinsic mitochondrial respiratory chain defect and can also account for how mitochondrial dysfunction could be induced without reductions in mitochondrial mass or content.
The precise mechanism by which Drp1 activation may mediate improvements in insulin resistance and fat oxidation is unclear. However, multiple studies have reported that Drp1-mediated mitochondrial fission is associated with a transient loss of mitochondrial membrane potential in the excised mitochondrial fragment (3, 32, 33, 56). Dispersal of mitochondrial membrane potential may result in accelerated respiration, but this necessarily requires suppression of ATP production (11). Consequently, higher basal rates of mitochondrial fission may result in increased uncoupling and a corresponding reduction in overall mitochondrial network efficiency. Interestingly, data from Conley et al. (7) suggest that sedentary individuals indeed exhibit higher mitochondrial respiration and uncoupling compared with active subjects, while Chavez et al. (5) demonstrated decreased mitochondrial membrane potential in the presence of decreased insulin sensitivity in healthy subjects following lipid infusion. Furthermore, increased uncoupling is evident in several obesity-related pathologies (6, 8). Therefore, it is conceivable that Drp1 activation may result in loss of membrane potential through sensitization of the mitochondrial permeability transition pore (mPTP) (25, 64). Importantly, activation of the mPTP in this case would potentially result in increased reactive oxygen species production despite a loss of membrane potential (40, 48, 64, 66) rather than membrane hyperpolarization as has previously been suggested (63). Furthermore, such an event would likely result in sufficient permeabilization of the mitochondrial membrane to factors such as fatty-acyl CoAs and acylcarnitines, which under normal circumstances would be sequestered in the mitochondria and have the ability to interfere with the insulin signaling pathway (26). This postulation is supported by recent work of Taddeo et al. (55), which suggests that opening of the mPTP may indeed be an important novel mediator of insulin resistance. In that study, inhibition of the mPTP restored glucose uptake in muscle cells, although the increase in GLUT-4 expression on the cell membrane appeared to be independent of the classical insulin signaling pathway. Although we did not directly measure membrane potential in vivo in the present study, it is reasonable to infer that the exercise training-mediated reductions in basal Drp1 activation are likely accompanied by increased mitochondrial membrane potential, decreased mitochondrial permeability within the mitochondrial population, and improved mitochondrial efficiency and reduced insulin resistance.
Further to this hypothesis, Jheng et al. (20) recently reported that in C2C12 cells preincubation with palmitic acid induced Drp1-mediated mitochondrial fragmentation, higher levels of oxidative stress, reduced ATP production, and reduced insulin-mediated glucose uptake. Knockdown of Drp1 prevented these palmitate-induced effects. Furthermore, ob/ob mice treated with Mdivi-1, a Drp1 inhibitor, recovered tubular mitochondrial percentage and membrane potential, decreased oxidative stress to baseline levels, and increased insulin-stimulated glucose uptake. The data presented herein indicate that exercise training may also reduce Drp1-mediated mitochondrial fission. Additionally, these alterations correlate with changes in fat oxidation and insulin sensitivity. Taken together, these data corroborate the hypothesis that mitochondrial fission may be a determinant of insulin resistance in obesity. However, further research is required to determine whether Drp1 activation and its downstream effects, either directly or indirectly, regulate insulin sensitivity in human skeletal muscle.
Our data are also consistent with previous research suggesting that energy balance may be a key determinant of mitochondrial dynamics activity (13, 20, 35, 47, 60, 61). The weight loss observed in the current study confirms that individuals were placed in energy deficit. The increased expression of fusion related genes combined with total Drp1 protein expression suggests that either the exercise training itself, or the energy deficit, initiated a profusion state within the skeletal muscle. However, this finding cannot be confirmed without imaging the mitochondrial network, something that was outside the scope of the current investigation.
Interestingly, DNM1L, the gene that codes for Drp1, was significantly upregulated following the intervention. It is possible that the increase in DNM1L gene expression is related to the last exercise bout. It was previously reported that total Drp1 protein content was increased in skeletal muscle of lean healthy males 24 h after a single bout of high-intensity interval training and peaked 24 h after the third exercise session; however, total Drp1 protein expression trended downward thereafter for up to 2 wk of training (42). Nevertheless, we cannot discount the possibility that the effects observed in the current study are in part due to an acute rather than chronic effect of exercise on gene expression. Similarly, previous reports have demonstrated that both fat oxidation (14, 30) and insulin sensitivity (37) are increased following a single bout of exercise. Further research will be required to determine whether the exercise effects observed here are driven by short-term gains following a single exercise bout or chronic adaptations to exercise training. Interestingly, the increase in DNM1L gene expression in the current study was not reflected in total Drp1 protein expression, suggesting that Drp1 in skeletal muscle may undergo posttranscriptional regulation.
In summary, this study provides new in vivo evidence that altering energy balance through an aerobic exercise intervention may result in decreased Drp1 activity and a profusion environment in skeletal muscle of older obese individuals with insulin resistance and that these alterations are associated with improvements in fat oxidation and insulin sensitivity. These findings support the hypothesis that elevated mitochondrial fission may result in mitochondrial dysfunction and insulin resistance and that exercise training may cause improvements in insulin sensitivity through inhibition of this pathway. The data presented here also suggest that a more complete investigation into the role of Drp1 activation and its downstream effects on mitochondrial morphology, mitochondrial function, and insulin sensitivity is warranted and may result in novel approaches for therapeutic treatment of insulin resistance and type 2 diabetes.
GRANTS
This research was supported by National Institute on Aging Grants RO1-AG-12834 (to J. P. Kirwan) and National Institutes of Health National Center for Research Resources Grant 1UL1RR024989 (Cleveland, OH).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.E.F. and J.P.K. conception and design of research; C.E.F. and A.M. performed experiments; C.E.F., A.M., and J.P.K. analyzed data; C.E.F., A.M., N.L., and J.P.K. interpreted results of experiments; C.E.F. prepared figures; C.E.F. drafted manuscript; C.E.F., A.M., N.L., and J.P.K. edited and revised manuscript; C.E.F., A.M., N.L., and J.P.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the research volunteers for outstanding dedication and effort, the staff of the Clinical Research Unit, and the technical staff and students who helped with the implementation of the study and assisted with data collection. We also thank Charles L. Hoppel for insight and suggestions during the many discussions on this topic.
REFERENCES
- 1.Anello M, Lupi R, Spampinato D, Piro S, Masini M, Boggi U, Del Prato S, Rabuazzo AM, Purrello F, Marchetti P. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia 48: 282–289, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, Daugaard JR, Lloberas J, Camps M, Zierath JR, Rabasa-Lhoret R, Wallberg-Henriksson H, Laville M, Palacin M, Vidal H, Rivera F, Brand M, Zorzano A. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem 278: 17190–17197, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva YE, Graber S, Kovacs I, Lee WD, Waggoner J, Cui JK, White AD, Bossy B, Martinou JC, Youle RJ, Lipton SA, Ellisman MH, Perkins GA, Bossy-Wetzel E. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J 25: 3900–3911, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell 125: 1241–1252, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Chavez AO, Kamath S, Jani R, Sharma LK, Monroy A, Abdul-Ghani MA, Centonze VE, Sathyanarayana P, Coletta DK, Jenkinson CP, Bai Y, Folli F, Defronzo RA, Tripathy D. Effect of short-term free Fatty acids elevation on mitochondrial function in skeletal muscle of healthy individuals. J Clin Endocrinol Metab 95: 422–429, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chavin KD, Yang S, Lin HZ, Chatham J, Chacko VP, Hoek JB, Walajtys-Rode E, Rashid A, Chen CH, Huang CC, Wu TC, Lane MD, Diehl AM. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J Biol Chem 274: 5692–5700, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Conley KE, Amara CE, Bajpeyi S, Costford SR, Murray K, Jubrias SA, Arakaki L, Marcinek DJ, Smith SR. Higher mitochondrial respiration and uncoupling with reduced electron transport chain content in vivo in muscle of sedentary vs. active subjects. J Clin Endocrinol Metab 98: 129–136, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dedukhova VI, Mokhova EN, Skulachev VP, Starkov AA, Arrigoni-Martelli E, Bobyleva VA. Uncoupling effect of fatty acids on heart muscle mitochondria and submitochondrial particles. FEBS Lett 295: 51–54, 1991 [DOI] [PubMed] [Google Scholar]
- 9.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E214–E223, 1979 [DOI] [PubMed] [Google Scholar]
- 10.Dela F, Helge JW. Insulin resistance and mitochondrial function in skeletal muscle. Int J Biochem Cell Biol 45: 11–15, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Dimroth P, Kaim G, Matthey U. Crucial role of the membrane potential for ATP synthesis by F(1)F(o) ATP synthases. J Exp Biol 203: 51–59, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55: 628–634, 1983 [DOI] [PubMed] [Google Scholar]
- 13.Gomes LC, Di Benedetto G, Scorrano L. During autophagy mitochondria elongate are spared from degradation and sustain cell viability. Nat Cell Biol 13: 589–598, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson GC, Fattor JA, Horning MA, Faghihnia N, Johnson ML, Mau TL, Luke-Zeitoun M, Brooks GA. Lipolysis and fatty acid metabolism in men and women during the postexercise recovery period. J Physiol 584: 963–981, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoeks J, Schrauwen P. Muscle mitochondria and insulin resistance: a human perspective. Trends Endocrinol Metab 23: 444–450, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Holmstrom MH, Iglesias-Gutierrez E, Zierath JR, Garcia-Roves PM. Tissue-specific control of mitochondrial respiration in obesity-related insulin resistance and diabetes. Am J Physiol Endocrinol Metab 302: E731–E739, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Ishihara N, Fujita Y, Oka T, Mihara K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J 25: 2966–2977, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itoh K, Nakamura K, Iijima M, Sesaki H. Mitochondrial dynamics in neurodegeneration. Trends Cell Biol 23: 64–71, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James DI, Parone PA, Mattenberger Y, Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem 278: 36373–36379, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Jheng HF, Tsai PJ, Guo SM, Kuo LH, Chang CS, Su IJ, Chang CR, Tsai YS. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol 32: 309–319, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joseph AM, Joanisse DR, Baillot RG, Hood DA. Mitochondrial dysregulation in the pathogenesis of diabetes: potential for mitochondrial biogenesis-mediated interventions. Exp Diabetes Res 2012: 642038, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51: 2944–2950, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Kirkwood SP, Munn EA, Brooks GA. Mitochondrial reticulum in limb skeletal muscle. Am J Physiol Cell Physiol 251: C395–C402, 1986 [DOI] [PubMed] [Google Scholar]
- 24.Kirwan JP, Huston-Presley L, Kalhan SC, Catalano PM. Clinically useful estimates of insulin sensitivity during pregnancy: validation studies in women with normal glucose tolerance and gestational diabetes mellitus. Diabetes Care 24: 1602–1607, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Kong D, Xu L, Yu Y, Zhu W, Andrews DW, Yoon Y, Kuo TH. Regulation of Ca2+-induced permeability transition by Bcl-2 is antagonized by Drpl and hFis1. Mol Cell Biochem 272: 187–199, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45–56, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Krssak M, Brehm A, Bernroider E, Anderwald C, Nowotny P, Dalla Man C, Cobelli C, Cline GW, Shulman GI, Waldhausl W, Roden M. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes 53: 3048–3056, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Labrousse AM, Zappaterra MD, Rube DA, van der Bliek AM. Elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol Cell 4: 815–826, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-delta delta C) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Magkos F, Wright DC, Patterson BW, Mohammed BS, Mittendorfer B. Lipid metabolism response to a single, prolonged bout of endurance exercise in healthy young men. Am J Physiol Endocrinol Metab 290: E355–E362, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Mahoney DJ, Carey K, Fu MH, Snow R, Cameron-Smith D, Parise G, Tarnopolsky MA. Real-time RT-PCR analysis of housekeeping genes in human skeletal muscle following acute exercise. Physiol Genomics 18: 226–231, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Mannella CA. Structure and dynamics of the mitochondrial inner membrane cristae. Biochim Biophys Acta 1763: 542–548, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, Kimura M, Komatsu M, Hattori N, Tanaka K. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol 189: 211–221, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetes 28: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 35.Molina AJ, Wikstrom JD, Stiles L, Las G, Mohamed H, Elorza A, Walzer G, Twig G, Katz S, Corkey BE, Shirihai OS. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes 58: 2303–2315, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moro C, Galgani JE, Luu L, Pasarica M, Mairal A, Bajpeyi S, Schmitz G, Langin D, Liebisch G, Smith SR. Influence of gender, obesity, and muscle lipase activity on intramyocellular lipids in sedentary individuals. J Clin Endocrinol Metab 94: 3440–3447, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newsom SA, Everett AC, Hinko A, Horowitz JF. A single session of low-intensity exercise is sufficient to enhance insulin sensitivity into the next day in obese adults. Diabetes Care 36: 2516–2522, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Leary VB, Marchetti CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol 100: 1584–1589, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogata T, Yamasaki Y. Ultra-high-resolution scanning electron microscopy of mitochondria and sarcoplasmic reticulum arrangement in human red, white, and intermediate muscle fibers. Anat Rec 248: 214–223, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 121: 2012–2022, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 46: 983–988, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol 588: 4795–4810, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perseghin G, Scifo P, Danna M, Battezzati A, Benedini S, Meneghini E, del Maschio A, Luzi L. Normal insulin sensitivity and IMCL content in overweight humans are associated with higher fasting lipid oxidation. Am J Physiol Endocrinol Metab 283: E556–E564, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Picard M, Turnbull DM. Linking the metabolic state and mitochondrial DNA in chronic disease, health, and aging. Diabetes 62: 672–678, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Picard M, White K, Turnbull DM. Mitochondrial morphology, topology, and membrane interactions in skeletal muscle: a quantitative three-dimensional electron microscopy study. J Appl Physiol (1985) 114: 161–171, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pich S, Bach D, Briones P, Liesa M, Camps M, Testar X, Palacin M, Zorzano A. The Charcot-Marie-Tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through expression of OXPHOS system. Hum Mol Genet 14: 1405–1415, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci USA 108: 10190–10195, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russell JW, Golovoy D, Vincent AM, Mahendru P, Olzmann JA, Mentzer A, Feldman EL. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J 16: 1738–1748, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12: 2245–2256, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smirnova E, Shurland DL, Ryazantsev SN, van der Bliek AM. A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol 143: 351–358, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solomon TP, Haus JM, Kelly KR, Cook MD, Riccardi M, Rocco M, Kashyap SR, Barkoukis H, Kirwan JP. Randomized trial on the effects of a 7-d low-glycemic diet and exercise intervention on insulin resistance in older obese humans. Am J Clin Nutr 90: 1222–1229, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Solomon TP, Haus JM, Marchetti CM, Stanley WC, Kirwan JP. Effects of exercise training and diet on lipid kinetics during free fatty acid-induced insulin resistance in older obese humans with impaired glucose tolerance. Am J Physiol Endocrinol Metab 297: E552–E559, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell 20: 3525–3532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev 22: 1577–1590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taddeo EP, Laker RC, Breen DS, Akhtar YN, Kenwood BM, Liao JA, Zhang M, Fazakerley DJ, Tomsig JL, Harris TE, Keller SR, Chow JD, Lynch KR, Chokki M, Molkentin JD, Turner N, James DE, Yan Z, Hoehn KL. Opening of the mitochondrial permeability transition pore links mitochondrial dysfunction to insulin resistance in skeletal muscle. Mol Metab 3: 124–134, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem 282: 11521–11529, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Thomas KJ, Jacobson MR. Defects in mitochondrial fission protein dynamin-related protein 1 are linked to apoptotic resistance and autophagy in a lung cancer model. PLoS One 7: e45319, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Toledo FG, Menshikova EV, Ritov VB, Azuma K, Radikova Z, DeLany J, Kelley DE. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes 56: 2142–2147, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27: 433–446, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wikstrom JD, Katzman SM, Mohamed H, Twig G, Graf SA, Heart E, Molina AJ, Corkey BE, de Vargas LM, Danial NN, Collins S, Shirihai OS. beta-Cell mitochondria exhibit membrane potential heterogeneity that can be altered by stimulatory or toxic fuel levels. Diabetes 56: 2569–2578, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Wikstrom JD, Mahdaviani K, Liesa M, Sereda SB, Si Y, Las G, Twig G, Petrovic N, Zingaretti C, Graham A, Cinti S, Corkey BE, Cannon B, Nedergaard J, Shirihai OS. Hormone-induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. EMBO J 33: 418–436, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol 23: 5409–5420, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA 103: 2653–2658, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res 79: 341–351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao J, Zhang J, Yu M, Xie Y, Huang Y, Wolff DW, Abel PW, Tu Y. Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene 32: 4814–4824, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta 1757: 509–517, 2006 [DOI] [PubMed] [Google Scholar]