Abstract
Short-term, high-altitude (HA) exposure raises pulmonary artery systolic pressure (PASP) and decreases left-ventricular (LV) volumes. However, relatively little is known of the long-term cardiac consequences of prolonged exposure in Sherpa, a highly adapted HA population. To investigate short-term adaptation and potential long-term cardiac remodeling, we studied ventricular structure and function in Sherpa at 5,050 m (n = 11; 31 ± 13 yr; mass 68 ± 10 kg; height 169 ± 6 cm) and lowlanders at sea level (SL) and following 10 ± 3 days at 5,050 m (n = 9; 34 ± 7 yr; mass 82 ± 10 kg; height 177 ± 6 cm) using conventional and speckle-tracking echocardiography. At HA, PASP was higher in Sherpa and lowlanders compared with lowlanders at SL (both P < 0.05). Sherpa had smaller right-ventricular (RV) and LV stroke volumes than lowlanders at SL with lower RV systolic strain (P < 0.05) but similar LV systolic mechanics. In contrast to LV systolic mechanics, LV diastolic, untwisting velocity was significantly lower in Sherpa compared with lowlanders at both SL and HA. After partial acclimatization, lowlanders demonstrated no change in the RV end-diastolic area; however, both RV strain and LV end-diastolic volume were reduced. In conclusion, short-term hypoxia induced a reduction in RV systolic function that was also evident in Sherpa following chronic exposure. We propose that this was consequent to a persistently higher PASP. In contrast to the RV, remodeling of LV volumes and normalization of systolic mechanics indicate structural and functional adaptation to HA. However, altered LV diastolic relaxation after chronic hypoxic exposure may reflect differential remodeling of systolic and diastolic LV function.
Keywords: hypoxia, cardiac remodeling, ventricular mechanics, Sherpa
exposure to high altitude (HA) challenges the cardiovascular system to meet the metabolic demand for oxygen (O2) in an environment where arterial O2 content is markedly reduced. The drop in arterial O2 has both direct and indirect consequences for the heart, including depressed inotropy of cardiac muscle (40, 44), changes in blood volume and viscosity, and vasoconstriction of the pulmonary arteries (33). Despite these broad physiological changes, which have been reviewed previously (28, 49), there is evidence that the heart copes relatively well at HA (29, 34).
Short-term HA exposure in lowland natives is characterized by a decreased plasma volume (PV), an increased sympathetic nerve activity, and pulmonary vasoconstriction (17, 30, 37), all of which have considerable impact on cardiac function and in time, could stimulate cardiac remodeling. Himalayan native Sherpa, who are of Tibetan lineage and have resided at HA for ∼25,000 yr (2), are well adapted to life at HA, demonstrating greater lung-diffusing capacity (11) and an absence of polycythemia compared with acclimatized lowlanders (4). Previous studies have also reported Sherpa to have higher maximal heart rates (HRs) and only moderate pulmonary hypertension compared with lowlanders at HA (11, 25). Due to their longevity at HA, Sherpa provide an excellent model to investigate the effects of chronic hypoxic exposure. Despite this, neither the acute nor lifelong effects of HA on right- and left-ventricular (RV and LV, respectively) structure and function have been fully assessed in lowlanders or the unique Sherpa population.
Due to the unique arrangement of myofibers, cardiac form and function are intrinsically linked, as reflected in the cardiac mechanics (LV twist and rotation and ventricular strain) that underpin ventricular function. In response to altered physiological demand, ventricular mechanics acutely change (16, 41) and chronically remodel (31, 42) to reduce myofiber stress and achieve efficient ejection (5, 47). Therefore, concomitant examination of myocardial mechanics and ventricular structure in both the acute and chronic HA setting will provide novel insight into human adaptation to hypoxia.
To investigate the effects of chronic hypoxic exposure, we compared ventricular volumes and mechanics in Sherpa at 5,050 m with lowlanders at sea level (SL). In addition, to reveal potential stimuli for remodeling and to examine the time course of adaptation, we compared Sherpa with lowlanders after short-term HA exposure.
We hypothesized that: 1) Sherpa would exhibit smaller LV volumes and a higher RV/LV ratio than lowlanders at SL, 2) LV mechanics in Sherpa will closely resemble those of lowlanders at SL, and 3) following partial acclimatization to HA, LV volumes would be reduced in lowlanders and LV mechanics acutely increased.
METHODS
Study Participants and Design
All experimental procedures and protocols were approved by the Clinical Research Ethics Board at the University of British Columbia and the Nepal Health Medical Research Council and conformed with the Declaration of Helsinki. Eleven Caucasian, male lowlanders (34 ± 7 yr) and 11 Nepalese male highland Sherpa (31 ± 13 yr) provided informed consent and volunteered to participate in the study. Four weeks before departure, Caucasian participants underwent a thorough transthoracic echocardiographic assessment (TTE) close to SL (344 m in Kelowna, Canada) and then after 10 ± 3 days at the Ev-K2-CNR Pyramid Laboratory (Lobuche, Nepal; at 5,050 m). One lowlander was excluded due to poor acoustic windows and a second due to significant nonaltitude-related illness. Sherpa were assessed at 5,050 m only. All participants were free from respiratory and cardiovascular disease and were not taking any prescription medications. The native Sherpa participants originated from and were residents of the Khumbu Valley at an altitude >3,000 m and self-identified to be of Sherpa ethnicity. None of the Sherpa had traveled <3,000 m for at least 6 mo before testing. Although it was not possible to assess physical activity thoroughly, it is our belief that Sherpa and lowlander participants were relatively comparable in this regard, with Sherpa making their living through expedition trekking and lowlanders engaging in frequent recreational activity.
Stature, mass, blood pressure, and O2 saturation (SaO2) were recorded before each TTE. Venous blood samples were taken from lowlanders to assess total hemoglobin (HemoCue, Ängelholm, Sweden) concentration and hematocrit (Micro Hematocrit Reader) to approximate changes in PV (9), assuming that erythropoiesis would have only minor effects in the timeframe of our study (37). After travel to Nepal and four nights in Kathmandu (1,400 m), the participants flew to Lukla (2,800 m) and began a 10-day ascent to the Pyramid Research Centre (5,050 m). During the following 9 days, a cautious ascent profile was adopted with no more than 300 m net gain in altitude/day. To aid acclimatization, 3 full rest days with no net change in altitude were included in the 10-day ascent.
Transthoracic Echocardiography
All echocardiographic images were recorded on a commercially available, portable ultrasound system (Vivid q; GE Medical Systems Israel, Tirat Carmel, Israel) using a 1.5- to 4-MHz-phased array transducer. Images were captured by the same highly trained cardiac sonographer, with the participant lying in the left lateral decubitus position. Following 10 min of supine rest, parasternal long- and short-axis images and apical four-chamber views were recorded at end-expiration, and three consecutive cardiac cycles were stored for offline analysis (EchoPAC; GE Healthcare, Horten, Norway). HR was recorded via ECG.
Ventricular Structure
LV wall thickness and internal diameter were measured from the two-dimensional (2D) parasternal long-axis view. LV mass was calculated using the current American Society of Echocardiography Guidelines, and relative wall thickness was defined as (2 × LV posterior wall thickness)/LV internal diameter (26). The systolic and diastolic eccentricity index was calculated from the parasternal short-axis view at the mitral-valve level to assess the impact of RV pressure increase on LV shape (39). LV end-systolic volume (ESV), end-diastolic volume (EDV), and LV ejection fraction were calculated from planar tracings of the LV endocardial border in the apical four- and two-chamber views (Simpson's biplane approach) (26). LV end-diastolic length was also measured using an apical four-chamber view and defined as the distance from the mitral-valve hinge-point plane to the most distal endocardium at the apex of the LV. RV end-diastolic area (EDA) was calculated by tracing the endocardial border from a modified, apical four-chamber orientation. RV basal diameter was also recorded from an apical four-chamber view (38) and divided by LV basal diameter to obtain the ratio of RV/LV diameter (RV/LV).
Scaling of Cardiac Parameters
To account for the potential influence of body size, cardiac parameters were scaled allometrically for height. The data were tested for the appropriateness of ratiometric scaling (3) and discounted if the coefficient of variation for height divided by the coefficient of variation for the cardiac parameter was not equal to the Pearson's product moment correlation between the two variables (43). To determine whether the data could be grouped to derive a single exponent, an analysis of covariance was performed. As the exponents for lowlanders and Sherpa were similar, a common exponent was calculated for each parameter and used to scale structural and volume parameters.
Systolic Function
LV stroke volume (SV) was calculated as EDV-ESV and multiplied by HR for cardiac output. RV SV was obtained by placing a sample volume in the RV outflow tract (RVOT) from a parasternal short axis to obtain the velocity time integral. This was then multiplied by the cross-sectional area of the RVOT measured from the same view. Tissue Doppler imaging (TDI) was used to assess peak LV and RV myocardial velocity during systole, with the sample volume placed in the basal septum and RV free wall, respectively. M-Mode echocardiography was used to assess the tricuspid annular plane systolic excursion (TAPSE) (22). The pulmonary vascular response was quantified as the peak systolic tricuspid regurgitation jet velocity (V), recorded in an apical four-chamber view using continuous wave Doppler, and the RV to right-atrium (RA) pressure gradient was calculated using the simplified Bernoulli equation (4V2). With the addition of RA pressure, estimated using the collapsibility index of the inferior vena cava, pulmonary artery systolic pressure (PASP) was also calculated (38).
Diastolic Function
Pulsed-wave Doppler recordings were obtained to assess transmitral early (E) and late (A) diastolic filling velocities from an apical four-chamber view, with the sample volume placed between the tips of the open mitral valve. From the TDI traces described above, peak early diastole (E′) and late diastole (A′) were identified, and isovolumic relaxation time (IVRT) was assessed as described previously (1).
Ventricular Mechanics: Strain, Rotation, and Twist
LV circumferential strain, LV rotation, and their time-derivative strain rate and rotational velocity were assessed from parasternal short-axis views obtained from the LV base at the level of the mitral valve and the LV apex. The LV apex was defined as the point just above end-systolic luminal obliteration and obtained by moving the transducer one to two intercostal spaces caudally from the basal position to align with the apical short axis (46), keeping the LV cross-section as circular as possible. LV and RV longitudinal strain and strain rate were analyzed from an apical four-chamber view. Images were acquired with the highest possible frame rate (>70 frames/s) and kept constant for repeat examinations. All images were analyzed offline using 2D speckle-tracking analysis to assess global rotation, rotational velocity, strain, and strain rate and to calculate LV twist and untwist (“LV mechanics”; EchoPAC, V110.1.1; GE Healthcare). To time align and adjust for inter- and intraindividual variability of HR and frame rate, postprocessing was completed as described previously (41, 42). Briefly, raw, frame-by-frame data were exported to bespoke software (2D Strain Analysis Tool; Stuttgart, Germany), and cubic spline interpolation was applied. Twist variables were calculated by subtracting the apical, frame-by-frame data from the basal data. The time it took to achieve peak twist, twisting velocity, rotation, rotational velocity, strain, and strain rate from the onset of systole was expressed as a percentage of the cardiac cycle. Peak basal rotation during isovolumic contraction (IVC) was defined as the peak counter-clockwise basal rotation during early systole. For analysis and interpretation of diastolic mechanics, untwist was expressed as the percentage of peak twist to normalize for differences in absolute peak twist (32). Peak untwisting velocity has previously been shown to provide an accurate and reproducible measure of diastolic function and has been validated against invasive measures of LV chamber stiffness (50). Untwist data were analyzed up to 50% diastole, as reported previously (45). To account for differences in absolute (milliseconds) and relative (percent diastole) differences in IVRT, percentage untwist was expressed relative to IVRT.
Statistical Analyses
Comparison of lowlander and Sherpa was performed using independent-samples t-test. The two lowlander conditions were analyzed using paired-samples t-tests. For detailed analysis of untwisting mechanics, a mixed-design repeated-measures ANOVA was used. Alpha was set a priori to 0.05. All statistical analyses were performed using the Statistical Package for Social Science (SPSS) software for Windows 19.0 (SPSS, Chicago, IL).
RESULTS
Hemodynamics
Sherpa exhibited higher systemic and pulmonary systolic pressure and a lower SaO2 compared with lowlanders at SL (Table 1). HR was higher in Sherpa than lowlanders at SL and HA. Once lowlanders had partially acclimatized to HA, differences in hemodynamics and SaO2 were no longer evident (Table 1). HA exposure in lowlanders was associated with a significant increase in hematocrit (47 ± 2 vs. 59 ± 5%, P < 0.01) and hemoglobin concentration (15.1 ± 0.7 vs. 15.9 ± 0.6, P < 0.05), from which a 20 ± 7% decrease in PV was estimated.
Table 1.
Anthropometric and cardiovascular measurements in lowlanders at sea level and 5,050 m and Sherpa at 5,050 m
| Altitude, m |
|||
|---|---|---|---|
| SL | 5,050 m | Sherpa 5,050 m | |
| Mass, kg | 82 ± 10 | 78 ± 10 | 68 ± 10*† |
| SaO2, % | 98 ± 2 | 82 ± 3* | 83 ± 3* |
| Systolic BP, mmHg | 113 ± 8 | 127 ± 6* | 120 ± 10 |
| Diastolic BP, mmHg | 59 ± 5 | 79 ± 6* | 79 ± 8* |
| MAP, mmHg | 77 ± 4 | 93 ± 8* | 89 ± 9* |
| Heart rate, beats/min | 54 ± 6 | 61 ± 16 | 76 ± 14*† |
| PASP, mmHg | 19.7 ± 3.0 | 28.1 ± 4.7* | 28.8 ± 4.8* |
Data presented are mean ± SD;
P < 0.05 vs. sea level (SL);
P < 0.05 vs. 5,050-m lowlander. SaO2, oxygen saturation; BP, blood pressure; MAP, mean arterial pressure; PASP, pulmonary artery systolic pressure.
Ventricular Structure
Following scaling, Sherpa demonstrated smaller wall thicknesses, LV mass, and ventricular volumes compared with lowlanders at SL, with no between-group differences in relative wall thickness observed (Table 2). Sherpa had a similar eccentricity index to lowlanders at HA; however, both were moderately higher than lowlanders at SL (P < 0.05). After exposure to HA, lowlanders reported a reduced LV EDV and LV mass, meaning differences observed between lowlanders at SL and Sherpa were no longer evident. Despite a reduction in LV EDV and PV, lowlanders reported no change in RV EDA.
Table 2.
Absolute and relative ventricular structural parameters in lowlanders at sea level and 5,050 m and Sherpa at 5,050 m
| Altitude, m |
|||
|---|---|---|---|
| SL | 5,050 m | Sherpa 5,050 m | |
| Absolute LV structural parameters | |||
| IVSd, cm | 1.21 ± 0.08 | 1.19 ± 0.14 | 1.00 ± 0.20*† |
| LVIDd, cm | 4.74 ± 0.30 | 4.57 ± 0.26* | 4.15 ± 0.24*† |
| LVPWd, cm | 1.18 ± 0.11 | 1.10 ± 0.11 | 1.02 ± 0.09* |
| LV mass, g | 211 ± 22 | 190 ± 29* | 139 ± 31*† |
| EDV, ml | 129 ± 15 | 107 ± 16* | 82 ± 13*† |
| ESV, ml | 54 ± 8 | 44 ± 8* | 33 ± 7*† |
| SV, ml | 75 ± 8 | 63 ± 10* | 49 ± 8*† |
| Q̇, liter/min | 4.0 ± 0.6 | 3.9 ± 0.7 | 3.5 ± 0.7 |
| Ejection fraction, % | 55 ± 3 | 58 ± 5 | 57 ± 4 |
| LV eccentricity index (systole) | 1.03 ± 0.06 | 1.08 ± 0.06* | 1.08 ± 0.08* |
| LV eccentricity index (diastole) | 1.06 ± 0.05 | 1.13 ± 0.09* | 1.13 ± 0.09* |
| Relative wall thickness | 0.51 ± 0.06 | 0.50 ± 0.05 | 0.49 ± 0.04 |
| Relative LV structural parameters | |||
| IVSd/height, mm/m0.83 | 7.55 ± 0.58 | 7.41 ± 0.92 | 6.50 ± 1.32* |
| LVIDd/height, mm/m1.21 | 23.71 ± 1.77 | 22.81 ± 1.52* | 21.90 ± 1.24* |
| LVPWd/height, mm/m1.11 | 6.25 ± 0.70 | 5.84 ± 0.71 | 5.67 ± 0.43* |
| LV mass/height, g/m3.27 | 33.27 ± 5.70 | 29.61 ± 6.33* | 24.83 ± 5.62* |
| EDV/height, ml/m3.79 | 14.87 ± 2.67 | 12.41 ± 2.81* | 11.10 ± 1.79* |
| ESV/height, ml/m1.51 | 22.90 ± 3.42 | 18.54 ± 3.60* | 14.94 ± 2.85*† |
| SV/height, ml/m3.68 | 9.17 ± 1.65 | 7.79 ± 1.92* | 6.96 ± 1.13* |
| Q̇/height, liter · min−1 · m2.95 | 0.74 ± 0.11 | 0.72 ± 0.10 | 0.74 ± 0.11 |
| Absolute RV structural parameters | |||
| EDA, cm3 | 23.3 ± 3.6 | 23.6 ± 3.1 | 19.0 ± 2.5*† |
| ESA, cm3 | 13.9 ± 2.5 | 14.8 ± 3.2 | 11.7 ± 1.9*† |
| SV, ml | 77 ± 13 | 63 ± 16* | 50 ± 10*† |
| Relative RV structural parameters | |||
| EDA, cm3/m1.05 | 12.80 ± 2.30 | 12.96 ± 2.04 | 10.92 ± 1.41*† |
| ESA, cm3/m0.79 | 8.88 ± 1.74 | 9.45 ± 2.11 | 7.72 ± 1.26† |
| SV, ml/m3.31 | 11.52 ± 1.90 | 9.51 ± 2.46* | 8.85 ± 2.05* |
| RV–LV proportional measurements | |||
| RV–LV basal diameter ratio | 1.05 ± 0.20 | 0.97 ± 0.12 | 1.11 ± 0.13† |
| RV–LV area ratio | 0.67 ± 0.10 | 0.75 ± 0.07* | 0.72 ± 0.10 |
Data presented are mean ± SD;
P < 0.05 vs. SL;
P < 0.05 vs. 5,050-m lowlander. LV, left ventricle; IVSd, interventricular septum diameter diastole; LVIDd, LV internal diameter diastole; LVPWd, LV posterior wall diastole; EDV, end-diastolic volume; ESV, end-systolic volume; SV, stroke volume; Q̇, cardiac output; RV, right ventricle; EDA, end-diastolic area; ESA, end-systolic area.
Systolic Function
When compared with lowlanders at SL, Sherpa demonstrated a lower SV (P < 0.05); however, there were no significant differences in ejection fraction or cardiac output. RV systolic performance, as measured by TAPSE, was lower in Sherpa compared with lowlanders at SL. There were no differences in RV or LV SV between Sherpa and lowlanders at 5,050 m.
Diastolic Function
Sherpa exhibited a lower early transmitral velocity, elevated atrial contribution to LV filling, and lower E′ compared with lowlanders at SL. Additionally, both LV and RV IVRTs were longer in Sherpa and lowlanders at 5,050 m (Table 3) compared with lowlanders at SL. Ascent to HA reduced the ratio of early-to-late transmitral filling (E/A ratio) and tissue (E′/A′) velocities in lowlanders.
Table 3.
Left and right ventricular function from Doppler, tissue Doppler, and M-mode echocardiography
| Altitude, m |
|||
|---|---|---|---|
| SL | 5,050 m | Sherpa 5,050 m | |
| Doppler and Tissue Doppler Parameters | |||
| Transmitral E velocity | 0.90 ± 0.14 | 0.77 ± 0.14* | 0.76 ± 0.20* |
| Transmitral A velocity | 0.44 ± 0.08 | 0.47 ± 0.08 | 0.53 ± 0.09* |
| E/A ratio | 2.05 ± 0.31 | 1.65 ± 0.22* | 1.47 ± 0.48* |
| Septal S′ | 0.09 ± 0.01 | 0.09 ± 0.02 | 0.08 ± 0.01 |
| Septal E′ | 0.14 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.03* |
| Septal A′ | 0.08 ± 0.02 | 0.09 ± 0.02 | 0.08 ± 0.01 |
| Septal E′/A′ ratio | 1.68 ± 0.27 | 1.27 ± 0.36* | 1.39 ± 0.48 |
| RV S′ | 0.14 ± 0.02 | 0.14 ± 0.03 | 0.13 ± 0.01 |
| RV E′ | 0.16 ± 0.02 | 0.15 ± 0.03 | 0.15 ± 0.05 |
| RV A′ | 0.12 ± 0.0.3 | 0.12 ± 0.02 | 0.11 ± 0.04 |
| LV IVRT, ms | 55 ± 9 | 69 ± 14* | 68 ± 11* |
| LV IVRT as percentage of diastole, % | 8 ± 1 | 11 ± 2* | 16 ± 4*† |
| RV IVRT, ms | 41 ± 11 | 78 ± 14* | 64 ± 20* |
| TAPSE | 2.9 ± 0.3 | 2.3 ± 0.3* | 2.2 ± 0.4* |
Data presented are mean ± SD;
P < 0.05 vs. SL;
P < 0.05 vs. 5,050-m lowlander. E, early; A, late; S′, peak systolic tissue velocity; E′, peak E diastolic tissue velocity; A′, peak A diastolic tissue velocity; IVRT, isovolumic relaxation time; TAPSE, tricuspid annular plane systolic excursion.
Ventricular Mechanics: Strain, Rotation, and Twist
Systolic mechanics.
LEFT VENTRICULAR.
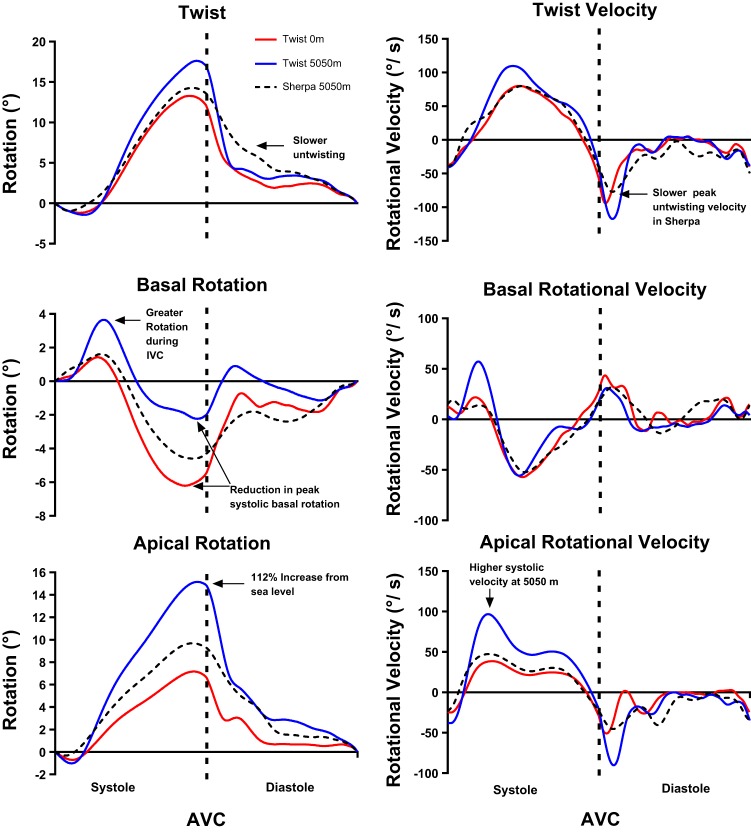
For simplicity, we report LV twist and not LV torsion, as normalizing for LV length did not alter the results. When Sherpa were compared with lowlanders at SL, the pattern of LV mechanics was similar, showing no statistical differences other than a longer time-to-peak LV systolic longitudinal strain in Sherpa (Fig. 1 and Table 4). However, at 5,050 m, basal rotation was greater and apical rotation lower in the Sherpa, but there was no difference in peak twist. This difference in basal and apical rotation between Sherpa and lowlanders at 5,050 m can be explained by acute changes in lowlander mechanics following short-term HA exposure. Peak systolic basal rotation was approximately halved, and rotation during IVC doubled after partial acclimatization (Fig. 1 and Table 4). In contrast to the base, apical rotation, systolic rotational velocity, circumferential strain, and strain rate were all increased (Fig. 1 and Table 4).
Fig. 1.
Temporal representation of twist, basal, and apical rotation and their respective velocities in lowlanders at sea level (SL) and 5,050 m and Sherpa at 5,050 m. Annotations indicate key findings. For clarity, statistical differences have not been identified; please refer to Table 3. AVC, aortic valve closure; IVC, isovolumic contraction.
Table 4.
Myocardial mechanics in lowlanders at sea level and 5,050 m and Sherpa at 5,050 m
| Altitude, m |
|||
|---|---|---|---|
| SL | 5,050 m | Sherpa 5,050 m | |
| Left ventricular twist parameters | |||
| Twist, ° | 13.6 ± 2.6 | 18.1 ± 5.6 | 15.0 ± 5.6 |
| Systolic twist velocity, °/s | 88 ± 24 | 125 ± 48 | 93 ± 25 |
| Untwisting velocity, °/s | −123 ± 30 | −153 ± 38 | −93 ± 31*† |
| Left ventricular basal parameters | |||
| Basal IVC rotation, ° | 1.6 ± 1.3 | 3.9 ± 1.9* | 1.9 ± 1.0† |
| Basal rotation, ° | −6.7 ± 1.3 | −2.9 ± 1.9* | −5.2 ± 2.4† |
| Basal systolic rotational velocity, °/s | −63 ± 22 | −67 ± 28 | −55 ± 25 |
| Basal diastolic rotational velocity, °/s | 63 ± 27 | 51 ± 24 | 53 ± 26 |
| Basal circumferential strain, % | 17.8 ± 2.5 | 18.9 ± 3 | 17.8 ± 2.5 |
| Basal circumferential strain rate, %/s | 1.1 ± 0.1 | 1.2 ± 0.3 | 1.1 ± 0.2 |
| Left ventricular apical parameters | |||
| Apical rotation, ° | 7.3 ± 2.2 | 15.5 ± 4.8* | 10.5 ± 4.3† |
| Apical systolic rotational velocity, °/s | 46 ± 13 | 101 ± 40* | 66 ± 20*† |
| Apical diastolic rotational velocity, °/s | −60 ± 18 | −125 ± 30* | −69 ± 18† |
| Apical circumferential strain, % | 25.0 ± 4.9 | 29.2 ± 6.4* | 23.8 ± 3.8 |
| Apical circumferential strain rate, %/s | 1.4 ± 0.3 | 2.1 ± 0.7* | 1.60 ± 0.3 |
| Left ventricular longitudinal parameters | |||
| Longitudinal strain peak, % | 19.1 ± 2.7 | 18.4 ± 2.1 | 18.5 ± 1.1 |
| Longitudinal strain time to peak, % | 98 ± 2 | 103 ± 5* | 102 ± 4* |
| Longitudinal strain-rate peak, %/s | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.1 |
| Longitudinal strain-rate time to peak, % | 43 ± 10 | 42 ± 10 | 42 ± 9 |
| Longitudinal diastolic strain-rate peak, % | 1.5 ± 3 | 1.2 ± 0.2 | 1.4 ± 0.3 |
| Longitudinal diastolic strain-rate time to peak, % | 118 ± 2 | 122 ± 7 | 128 ± 9* |
| Right ventricular longitudinal parameters | |||
| Longitudinal strain peak, % | 24.7 ± 3.2 | 21.8 ± 2.7* | 18.9 ± 2.5*† |
| Longitudinal strain time to peak, % | 99 ± 3 | 103 ± 3 | 104 ± 5* |
| Longitudinal systolic strain-rate peak, %/s | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.1 ± 0.1 |
| Longitudinal systolic strain-rate time to peak, % | 53 ± 16 | 40 ± 8* | 41 ± 18* |
| Longitudinal diastolic strain-rate peak, % | 1.7 ± 0.4 | 1.3 ± 0.2 | 1.5 ± 0.3 |
| Longitudinal diastolic strain-rate time to peak, % | 117 ± 2 | 123 ± 7* | 128 ± 8* |
Data presented are mean ± SD;
P < 0.05 vs. SL;
P < 0.05 vs. 5,050-m lowlander. Time to peak is expressed as a percentage, with 0–100% for systole and 101–200% representing diastole. IVC, isovolumic contraction.
RIGHT VENTRICULAR.
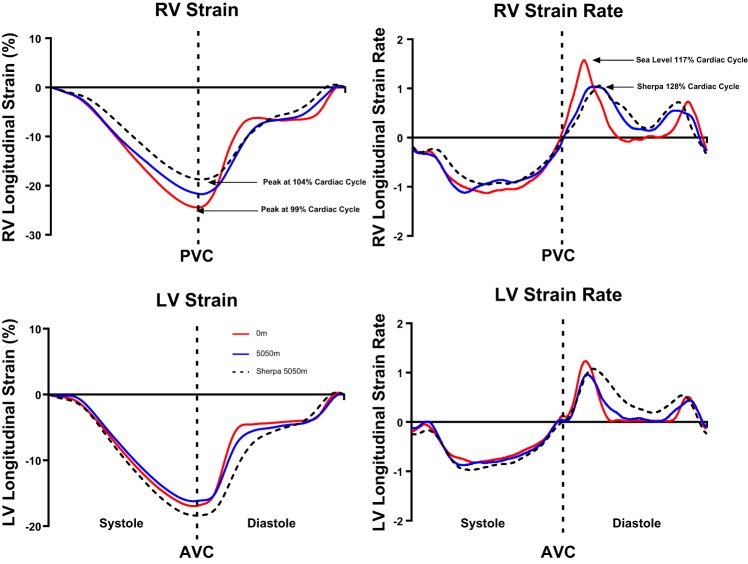
In Sherpa, peak RV longitudinal strain was lower and occurred later in the cardiac cycle compared with lowlanders at SL (P < 0.05). Following short-term HA exposure, lowlanders reported a reduction in peak RV longitudinal strain, meaning the difference between lowlanders and Sherpa observed at SL was no longer evident (Table 4 and Fig. 2).
Fig. 2.
Temporal representation of right ventricle (RV) and left ventricle (LV) strain and strain rate in lowlanders at SL and 5,050 m and Sherpa at 5,050 m. Annotations indicate key findings. For clarity, statistical differences have not been identified; please refer to Table 3. PVC, pulmonary valve closure.
Diastolic Mechanics
Despite the same peak twist, Sherpa showed a lower peak-untwisting velocity than lowlanders at both SL and 5,050 m (Fig. 1; see annotation). Relative to peak twist, Sherpa achieved significantly less untwisting during the first 45% of diastole than either of the lowlander conditions (Fig. 3). However, when considered relative to the longer IVRT observed in Sherpa, no differences in the percentage of untwist before mitral-valve opening were evident. Time-to-peak LV diastolic strain rate was longer in Sherpa than lowlanders at SL but not different at 5,050 m. Additionally, time-to-peak RV diastolic strain rate was longer in Sherpa and lowlanders at 5,050 m compared with lowlanders at SL (Fig. 2).
Fig. 3.
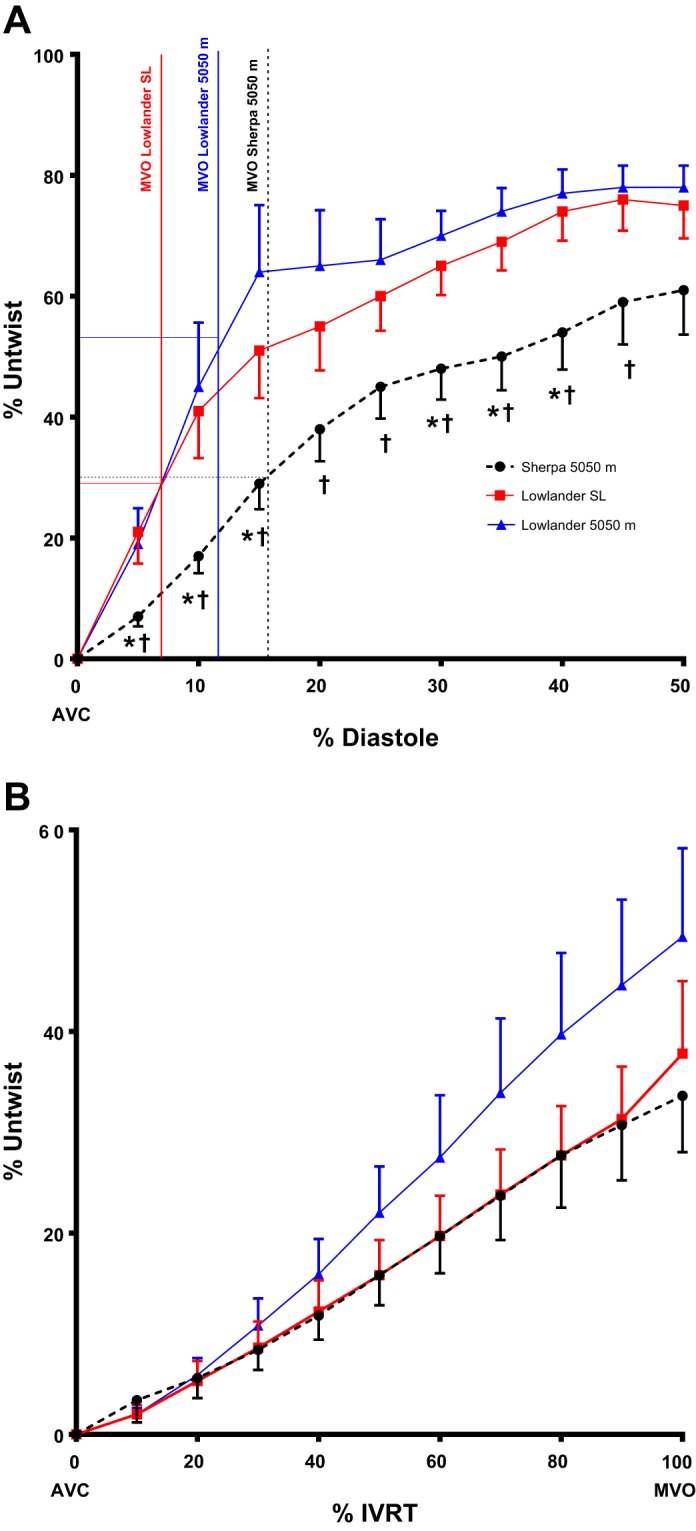
A: the slower untwisting in Sherpa, expressed relative to peak systolic twist up to 50% diastole. Sherpa isovolumic relaxation time (IVRT) is double that of lowlanders at SL when expressed as a percent of diastole. Vertical lines indicate mitral-valve opening (MVO) for each condition, and horizontal lines indicate the percentage of untwist preceding MVO. B: percent untwist in 3 conditions against percent IVRT. No statistical differences were observed when untwisting was normalized for IVRT duration. Data presented are mean ± SE; *P < 0.05 vs. lowlander SL; †P < 0.05 vs. 5,050-m lowlander.
DISCUSSION
The purpose of this study was to assess the impact of chronic hypoxic exposure on cardiac structure and function in HA Sherpa residents through comparison with lowlanders at SL and after short-term HA exposure. The main findings were: 1) Sherpa have smaller relative LV size compared with lowlanders at SL yet no difference in the RV/LV area ratio; 2) Sherpa exhibited slower diastolic relaxation and similar systolic mechanics compared with lowlanders at SL; 3) in lowlanders, short-term HA exposure resulted in increased PASP, reduced RV strain and SV, and a mismatch between RV and LV filling; and 4) acute changes in loading conditions and an increase in PASP led to a differential response in LV mechanics at the base and apex.
Comparison of Cardiac Structure and Function in Sherpa and Lowlanders
Sherpa are renowned for their superior exercise and mountaineering performance (12). In SL athletes, higher levels of aerobic fitness normally coincide with a large LV EDV, thus enabling a larger SV (24). However, cross-sectional comparison in the present study revealed smaller absolute and relative LV size in Sherpa compared with lowlanders at SL. Whereas cross-sectional comparisons cannot establish cause and effect, it is tempting to speculate that the lower RV systolic function observed may result in decreased LV filling and act as a stimulus for structural remodeling and could determine cardiac development in HA natives. This hypothesis is partially supported by findings in pulmonary hypertension patients, where a reduced RV function has been shown to decrease LV filling (27), which ultimately results in ventricular remodeling and a smaller LV (7). It should be noted, however, that despite a decrease in our load-dependent measures of RV systolic function, intrinsic contractility is often preserved in HA populations, even in patients with chronic mountain sickness (34). This suggests that the alterations in RV longitudinal function observed previously (21) and in the current study likely reflect altered loading conditions rather than pathological dysfunction.
To generate the required cardiac output with a smaller LV and hence, SV, HR needs to be higher. In agreement with this, previous authors have shown a greater maximal HR in Sherpa compared with lowlanders at HA (25). Therefore, whereas cardiac output may be similar between Sherpa and lowlanders, the way in which it is achieved could differ. Although we observed a smaller LV EDV in Sherpa compared with lowlanders, Sherpa did not demonstrate a statistically, significantly larger RV/LV area ratio (P = 0.2). This finding is in contrast to the short-term HA response in lowlanders and also Andean HA natives (21). Although this contradicts our hypothesis and may be related to limited statistical power, it is also possible that it reflects genetic differences among ethnic groups. Tibetans have been shown to exhibit a lower incidence of RV hypertrophy than other ethnic groups who have migrated to and reside at HA (15). As such, it is possible that Sherpa do not demonstrate the disproportionate increase in RV size seen in other populations.
Impaired Diastolic Relaxation and Comparable LV Systolic Mechanics in Sherpa Compared with Lowlanders at SL
Modification of diastolic function at HA has been widely reported in the literature, with a decrease in both the LV and RV E/A ratio as the most common finding (8, 21). Researchers have speculated that either changes in intrinsic properties, such as calcium handling or ATP availability, or loading conditions modify diastolic function (19-21, 23). In the present study, we examined myocardial mechanics to assess the impact of HA exposure on diastolic function. Temporal analysis of our data shows that in Sherpa, a peak RV and LV longitudinal systolic strain occurred during early diastole. This is in contrast to lowlanders at SL, where peak longitudinal strain immediately precedes pulmonary and aortic valve closure. Our results support the work of Gibbs (14), who suggested that increased pulmonary pressures at HA impact LV filling by prolonging the systolic ejection time.
Whereas Sherpa achieved less untwisting during early diastole compared with lowlanders at SL or HA, IVRT was significantly longer, and the percentage of untwist preceding mitral-valve opening was not different (Fig. 3; see annotation). Lower untwist during early diastole, as seen in healthy aging (45), and prolongation of IVRT may reflect a smaller, stiffer LV in Sherpa. In combination, delayed systolic and diastolic longitudinal strain, prolongation of IVRT, and slower untwist velocity suggest altered diastology. Interestingly, despite a longer IVRT in lowlanders at HA, greater untwisting during early diastole was achieved compared with Sherpa. This may represent an acute response to the lower LV filling pressure and greater systolic apical rotational velocity, which over time, may act as a stimulus for chronic remodeling.
As shown in Fig. 1, lowlanders demonstrate rapid changes in systolic mechanics after ascent to HA. It is known that LV mechanics adjust in response to altered hemodynamics to optimize efficiency and equalize fiber stress across the myocardium (47). The profile of systolic LV mechanics in Sherpa, however, is more comparable with lowlanders at SL than at HA. As mechanical stress is linearly related to myocardial O2 demand (6), changes in LV mechanics could represent altered myocardial efficiency. In this context, the heart of lowlanders at HA may be inefficient initially. However, prolonged exposure, as experienced by Sherpa, may result in remodeling of the ventricular wall, normalization of mechanics, and improved myocardial efficiency. Moreover, as there are no differences in relative wall thickness between lowlanders and Sherpa, it would appear that the Sherpa LV is not exposed to a greater stress than that of lowlanders. Previously, we have shown the importance of a mechanical reserve in response to exercise in healthy lowlanders at SL (41). The “normalized” systolic mechanics in Sherpa may facilitate this reserve, which is likely absent during acute HA exposure, due to higher resting levels of twist, rotation, and strain. Whereas systolic mechanics appear to normalize, diastolic mechanics suggest impaired relaxation. However, it is interesting that the higher untwisting velocity observed in lowlanders at 5,050 m is not able to facilitate LV filling and increase EDV, suggesting that other factors independent of myocardial relaxation reduce LV EDV. Whether the altered diastolic mechanics in Sherpa represent positive, long-term adaptation or an inability to remodel is not known, but it appears from our data that systolic function has a greater capacity to adapt to residence at HA.
Ventricular Mismatch: Preserved RV EDA and Decreased LV Volume after Acute HA Exposure
Short-term HA exposure increased PASP and reduced PV in lowlanders, as has been reported previously (21, 35, 37). However, despite the reduction in PV, there was no change in RV EDA, indicating that either RV filling was maintained, or due to a reduced RV SV, the same EDA was achieved with a lower filling pressure (36). There was, however, a reduction in LV EDV, a finding previously thought to be partly related to the lower blood volume observed with short-term HA exposure (8). Our data indicate that the reduction in LV filling may be independent of changes in blood volume and more likely related to the decreased RV SV observed. The reduction in RV SV at HA coincided with a reduction in systolic performance, as quantified by RV longitudinal strain and TAPSE. It is likely that in response to increased PASP and therefore, RV afterload, RV systolic performance is impaired, and SV is reduced. This, in turn, impacts on LV diastolic function, resulting in modified LV filling, as evidenced by the change in E/A and ultimately, decreased LV EDV.
Differential Response in LV Basal and Apical Mechanics in Lowlanders following Ascent to 5,050 m
Following ascent to HA, lowlanders demonstrated a reduction in peak LV basal systolic rotation and an increase in LV apical circumferential strain and rotation. It is likely that the reduction in LV EDV, increase in PASP, and subsequent changes in LV geometry, as indicated by an increased LV eccentricity index, play a significant role in the differential response of the base and apex. Increased PASP and altered LV geometry have been shown to lower peak LV basal rotation in pulmonary hypertension (10), whereas a reduction in LV preload has been associated with increased apical rotation (13, 18). In addition to the decrease in peak LV basal systolic rotation, basal rotation during IVC was elevated in lowlanders at HA, as described previously, where PASP is increased or LV preload reduced (10, 48). The increase in rotation during IVC alters the starting position of clockwise systolic basal rotation. However, the net change in rotation between peak IVC and peak systole remains relatively constant at SL and HA, with no change in circumferential deformation. As this modification of basal rotation was not evident in Sherpa, who exhibited a similar PASP, it seems more likely that the decrease in basal rotation was due to decreased LV filling rather than significant LV–RV interaction.
In contrast to basal mechanics and in agreement with our hypothesis, systolic apical rotation and circumferential strain were increased significantly at HA compared with SL. This is likely related to the decreased LV EDV and increased sympathetic drive, previously reported at HA (17). Although the importance and functional significance of changes in apical mechanics are yet to be determined, such changes likely signify enhanced systolic function at HA. For example, increased apical rotation and deformation could help to maintain ejection fraction and prevent further decline in SV in the presence of decreased LV filling.
Limitations and Future Directions
We acknowledge the limitations associated with small, cross-sectional studies; however, due to logistical difficulties and expense associated with work of this nature, large longitudinal studies are less practicable. Due to the anatomy of the heart, imaging of the RV with ultrasound is not ideal; however, the guidelines published by the American Society of Echocardiography were followed (38), and MRI was not available. Whereas all participants were physically active and matched for age, we were unable to quantify physical-activity patterns and therefore, cannot rule out the influence of training status. Lastly, we acknowledge the confounding nature of drawing comparisons between two diverse ethnic groups; it is possible that Sherpa may exhibit different cardiac phenotypes, irrespective of HA exposure. However, to address our primary research question, it was not possible to avoid the comparison of different ethnic groups. Future research should attempt to investigate the combined influence of chronic altitude exposure and healthy aging, the reversible nature of long-term cardiac adaptation to HA, and the consequences for exercise capacity in Sherpa.
Conclusions
Life-long HA exposure resulted in structural and functional remodeling of the Sherpa heart. Altered biventricular loading conditions are likely the cause for the physiological adaptation observed. Despite a higher RV afterload, there was no evidence of disproportionate RV structural enlargement in Sherpa, which may be a consequence of environmental or genetic adaptation. Normalization of LV systolic mechanics in Sherpa but slower diastolic relaxation indicate differential, functional remodeling that has not been observed previously in HA populations, and its functional relevance remains to be confirmed. Lowlanders also demonstrated increased RV afterload and consequently, altered RV function, which may impair LV filling. Decreased LV filling is accompanied by an increase in apical systolic mechanics, likely helping to prevent a further decline in SV. Persistent underfilling of the LV and elevated apical mechanics may restrict cardiac reserve during exercise and be the precursor to the chronic LV structural and functional remodeling observed in a well-adapted Sherpa population.
GRANTS
Support for this study was provided, in part, by the Natural Sciences and Engineering Research Council of Canada and a Canada Research Chair (to P. N. Ainslie), as well as contributions from the Italian National Research Council.
DISCLOSURES
No conflicts of interest are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.S., P.N.A., and R.S. conception and design of research; M.S., P.N.A., and J.D.C. performed experiments; M.S., M.G.H., E.J.S., J.D.C., and A.Q.X.N. analyzed data; M.S., P.N.A., M.G.H., E.J.S., J.D.C., A.Q.X.N., and R.S. interpreted results of experiments; M.S. prepared figures; M.S. drafted manuscript; M.S., P.N.A., M.G.H., E.J.S., J.D.C., A.Q.X.N., and R.S. edited and revised manuscript; M.S., P.N.A., M.G.H., E.J.S., J.D.C., A.Q.X.N., and R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
This study was carried out within the framework of the Ev-K2-CNR Project in collaboration with the Nepal Academy of Science and Technology, as foreseen by the Memorandum of Understanding between Nepal and Italy. The authors are grateful to the other members of this international research expedition for assistance with the organization of this project. We also thank Dr. David Oxborough for his initial input related to the RV methodology.
REFERENCES
- 1.Alam M, Wardell J, Andersson E, Samad BA, Nordlander R. Characteristics of mitral and tricuspid annular velocities determined by pulsed wave Doppler tissue imaging in healthy subjects. J Am Soc Echocardiogr 12: 618–628, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Aldenderfer M. Peopling the Tibetan plateau: insights from archaeology. High Alt Med Biol 12: 141–147, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Batterham AM, George KP, Mullineaux DR. Allometric scaling of left ventricular mass by body dimensions in males and females. Med Sci Sports Exerc 29: 181–186, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Beall CM, Brittenham GM, Strohl KP, Blangero J, Williams-Blangero S, Goldstein MC, Decker MJ, Vargas E, Villena M, Soria R, Alarcon AM, Gonzales C. Hemoglobin concentration of high-altitude Tibetans and Bolivian Aymara. Am J Phys Anthropol 106: 385–400, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Beyar R, Sideman S. A computer study of the left ventricular performance based on fiber structure, sarcomere dynamics, and transmural electrical propagation velocity. Circ Res 55: 358–375, 1984 [DOI] [PubMed] [Google Scholar]
- 6.Beyar R, Sideman S. Left ventricular mechanics related to the local distribution of oxygen demand throughout the wall. Circ Res 58: 664–677, 1986 [DOI] [PubMed] [Google Scholar]
- 7.Bossone E, Duong-Wagner TH, Paciocco G, Oral H, Ricciardi M, Bach DS, Rubenfire M, Armstrong WF. Echocardiographic features of primary pulmonary hypertension. J Am Soc Echocardiogr 12: 655–662, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Boussuges A, Molenat F, Burnet H, Cauchy E, Gardette B, Sainty JM, Jammes Y, Richalet JP. Operation Everest III (Comex' 97): modifications of cardiac function secondary to altitude-induced hypoxia. An echocardiographic and Doppler study. Am J Respir Crit Care Med 161: 264–270, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37: 247–248, 1974 [DOI] [PubMed] [Google Scholar]
- 10.Dong L, Zhang F, Shu X, Guan L, Chen H. Left ventricular torsion in patients with secundum atrial septal defect. Circ J 73: 1308–1314, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Faoro V, Huez S, Vanderpool RR, Groepenhoff H, de Bisschop C, Martinot JB, Lamotte M, Pavelescu A, Guenard H, Naeije R. Pulmonary circulation and gas exchange at exercise in Sherpas at high altitude. J Appl Physiol (1985) 116: 919–926, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Garrido E, Rodas G, Javierre C, Segura R, Estruch A, Ventura JL. Cardiorespiratory response to exercise in elite Sherpa climbers transferred to sea level. Med Sci Sports Exerc 29: 937–942, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Gibbons Kroeker CA, Tyberg JV, Beyar R. Effects of load manipulations, heart rate, and contractility on left ventricular apical rotation. An experimental study in anesthetized dogs. Circulation 92: 130–141, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Gibbs JS. Biventricular function at high altitude: implications for regulation of stroke volume in chronic hypoxia. Adv Exp Med Biol 618: 13–24, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Halperin BD, Sun S, Zhuang J, Droma T, Moore LG. ECG observations in Tibetan and Han residents of Lhasa. J Electrocardiol 31: 237–243, 1998 [PubMed] [Google Scholar]
- 16.Hansen DE, Daughters GT, 2nd, Alderman EL, Ingels NB, Stinson EB, Miller DC. Effect of volume loading, pressure loading, and inotropic stimulation on left ventricular torsion in humans. Circulation 83: 1315–1326, 1991 [DOI] [PubMed] [Google Scholar]
- 17.Hansen J, Sander M. Sympathetic neural overactivity in healthy humans after prolonged exposure to hypobaric hypoxia. J Physiol 546: 921–929, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodt A, Hisdal J, Stugaard M, Stranden E, Atar D, Steine K. Reduced preload elicits increased LV twist in healthy humans: an echocardiographic speckle-tracking study during lower body negative pressure. Clin Physiol Funct Imaging 31: 382–389, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Holloway C, Cochlin L, Codreanu I, Bloch E, Fatemian M, Szmigielski C, Atherton H, Heather L, Francis J, Neubauer S, Robbins P, Montgomery H, Clarke K. Normobaric hypoxia impairs human cardiac energetics. FASEB J 25: 3130–3135, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Holloway CJ, Montgomery HE, Murray AJ, Cochlin LE, Codreanu I, Hopwood N, Johnson AW, Rider OJ, Levett DZ, Tyler DJ, Francis JM, Neubauer S, Grocott MP, Clarke K. Cardiac response to hypobaric hypoxia: persistent changes in cardiac mass, function, and energy metabolism after a trek to Mt. Everest Base Camp. FASEB J 25: 792–796, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Huez S, Faoro V, Guenard H, Martinot JB, Naeije R. Echocardiographic and tissue Doppler imaging of cardiac adaptation to high altitude in native highlanders versus acclimatized lowlanders. Am J Cardiol 103: 1605–1609, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Kaul S, Tei C, Hopkins JM, Shah PM. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J 107: 526–531, 1984 [DOI] [PubMed] [Google Scholar]
- 23.Kihara Y, Grossman W, Morgan JP. Direct measurement of changes in intracellular calcium transients during hypoxia, ischemia, and reperfusion of the intact mammalian heart. Circ Res 65: 1029–1044, 1989 [DOI] [PubMed] [Google Scholar]
- 24.La Gerche A, Burns AT, Taylor AJ, Macisaac AI, Heidbuchel H, Prior DL. Maximal oxygen consumption is best predicted by measures of cardiac size rather than function in healthy adults. Eur J Appl Physiol 112: 2139–2147, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Lahiri S, Milledge JS, Chattopadhyay HP, Bhattacharyya AK, Sinha AK. Respiration and heart rate of Sherpa highlanders during exercise. J Appl Physiol 23: 545–554, 1967 [DOI] [PubMed] [Google Scholar]
- 26.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Lumens J, Blanchard DG, Arts T, Mahmud E, Delhaas T. Left ventricular underfilling and not septal bulging dominates abnormal left ventricular filling hemodynamics in chronic thromboembolic pulmonary hypertension. Am J Physiol Heart Circ Physiol 299: H1083–H1091, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Naeije R. Physiological adaptation of the cardiovascular system to high altitude. Prog Cardiovasc Dis 52: 456–466, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Naeije R. RV is doing well at high altitudes!—always? JACC Cardiovasc Imaging 6: 1298–1300, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Naeije R, Huez S, Lamotte M, Retailleau K, Neupane S, Abramowicz D, Faoro V. Pulmonary artery pressure limits exercise capacity at high altitude. Eur Respir J 36: 1049–1055, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Nottin S, Doucende G, Schuster-Beck I, Dauzat M, Obert P. Alteration in left ventricular normal and shear strains evaluated by 2D-strain echocardiography in the athlete's heart. J Physiol 586: 4721–4733, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Opdahl A, Remme EW, Helle-Valle T, Edvardsen T, Smiseth OA. Myocardial relaxation, restoring forces, and early-diastolic load are independent determinants of left ventricular untwisting rate. Circulation 126: 1441–1451, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Penaloza D, Arias-Stella J. The heart and pulmonary circulation at high altitudes: healthy highlanders and chronic mountain sickness. Circulation 115: 1132–1146, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Pratali L, Allemann Y, Rimoldi SF, Faita F, Hutter D, Rexhaj E, Brenner R, Bailey DM, Sartori C, Salmon CS, Villena M, Scherrer U, Picano E, Sicari R. RV contractility and exercise-induced pulmonary hypertension in chronic mountain sickness: a stress echocardiographic and tissue Doppler imaging study. JACC Cardiovasc Imaging 6: 1287–1297, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Pugh LG. Blood volume and haemoglobin concentration at altitudes above 18,000 ft. (5500 M). J Physiol 170: 344–354, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reeves JT, Groves BM, Sutton JR, Wagner PD, Cymerman A, Malconian MK, Rock PB, Young PM, Houston CS. Operation Everest II: preservation of cardiac function at extreme altitude. J Appl Physiol 63: 531–539, 1987 [DOI] [PubMed] [Google Scholar]
- 37.Robach P, Dechaux M, Jarrot S, Vaysse J, Schneider JC, Mason NP, Herry JP, Gardette B, Richalet JP. Operation Everest III: role of plasma volume expansion on VO(2)(max) during prolonged high-altitude exposure. J Appl Physiol 89: 29–37, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23: 685–713, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Ryan T, Petrovic O, Dillon JC, Feigenbaum H, Conley MJ, Armstrong WF. An echocardiographic index for separation of right ventricular volume and pressure overload. J Am Coll Cardiol 5: 918–927, 1985 [DOI] [PubMed] [Google Scholar]
- 40.Silverman HS, Wei S, Haigney MC, Ocampo CJ, Stern MD. Myocyte adaptation to chronic hypoxia and development of tolerance to subsequent acute severe hypoxia. Circ Res 80: 699–707, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Stohr EJ, Gonzalez-Alonso J, Shave R. Left ventricular mechanical limitations to stroke volume in healthy humans during incremental exercise. Am J Physiol Heart Circ Physiol 301: H478–H487, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Stohr EJ, McDonnell B, Thompson J, Stone K, Bull T, Houston R, Cockcroft J, Shave R. Left ventricular mechanics in humans with high aerobic fitness: adaptation independent of structural remodelling, arterial haemodynamics and heart rate. J Physiol 590: 2107–2119, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanner JM. Fallacy of per-weight and per-surface area standards, and their relation to spurious correlation. J Appl Physiol 2: 1–15, 1949 [DOI] [PubMed] [Google Scholar]
- 44.Tucker R, Kayser B, Rae E, Raunch L, Bosch A, Noakes T. Hyperoxia improves 20 km cycling time trial performance by increasing muscle activation levels while perceived exertion stays the same. Eur J Appl Physiol 101: 771–781, 2007 [DOI] [PubMed] [Google Scholar]
- 45.van Dalen BM, Soliman OI, Kauer F, Vletter WB, Zwaan HB, Cate FJ, Geleijnse ML. Alterations in left ventricular untwisting with ageing. Circ J 74: 101–108, 2010 [DOI] [PubMed] [Google Scholar]
- 46.van Dalen BM, Vletter WB, Soliman OI, ten Cate FJ, Geleijnse ML. Importance of transducer position in the assessment of apical rotation by speckle tracking echocardiography. J Am Soc Echocardiogr 21: 895–898, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Vendelin M, Bovendeerd PH, Engelbrecht J, Arts T. Optimizing ventricular fibers: uniform strain or stress, but not ATP consumption, leads to high efficiency. Am J Physiol Heart Circ Physiol 283: H1072–H1081, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Vogel M, Cheung MM, Li J, Kristiansen SB, Schmidt MR, White PA, Sorensen K, Redington AN. Noninvasive assessment of left ventricular force-frequency relationships using tissue Doppler-derived isovolumic acceleration: validation in an animal model. Circulation 107: 1647–1652, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Wagner PD. Reduced maximal cardiac output at altitude—mechanisms and significance. Respir Physiol 120: 1–11, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Zhou W, Benharash P, Ho J, Ko Y, Patel NA, Mahajan A. Left ventricular twist and untwist rate provide reliable measures of ventricular function in myocardial ischemia and a wide range of hemodynamic states. Physiol Rep 1: e00110, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]