Abstract
Cutaneous hyperemia in response to rapid skin local heating to 42°C has been used extensively to assess microvascular function. However, the response is dependent on both nitric oxide (NO) and endothelial-derived hyperpolarizing factors (EDHFs), and increases cutaneous vascular conductance (CVC) to ∼90–95% maximum in healthy subjects, preventing the study of potential means to improve cutaneous function. We sought to identify an improved protocol for isolating NO-dependent dilation. We compared nine heating protocols (combinations of three target temperatures: 36°C, 39°C, and 42°C, and three rates of heating: 0.1°C/s, 0.1°C/10 s, 0.1°C/min) in order to select two protocols to study in more depth (protocol 1; N = 6). Then, CVC was measured at four microdialysis sites receiving: 1) lactated Ringer solution (Control), 2) 50-mM tetraethylammonium (TEA) to inhibit EDHFs, 3) 20-mM nitro-L-arginine methyl ester (L-NAME) to inhibit NO synthase, and 4) TEA+L-NAME, in response to local heating either to 39°C at 0.1°C/s (protocol 2; N = 10) or 42°C at 0.1°C/min (protocol 3; N = 8). Rapid heating to 39°C increased CVC to 43.1 ± 5.2%CVCmax (Control), which was attenuated by L-NAME (11.4 ± 2.8%CVCmax; P < 0.001) such that 82.8 ± 4.2% of the plateau was attributable to NO. During gradual heating, 81.5 ± 3.3% of vasodilation was attributable to NO at 40°C, but at 42°C only 32.7 ± 7.8% of vasodilation was attributable to NO. TEA+L-NAME attenuated CVC beyond L-NAME at temperatures >40°C (43.4 ± 4.5%CVCmax at 42°C, P < 0.001 vs. L-NAME), suggesting a role of EDHFs at higher temperatures. Our findings suggest local heating to 39°C offers an improved approach for isolating NO-dependent dilation and/or assessing perturbations that may improve microvascular function.
Keywords: laser-doppler flowmetry, endothelial function, axon reflex, nitric oxide, endothelial-derived hyperpolarizing factors
local heating of the skin produces a profound vasodilation, also known as cutaneous thermal hyperemia, which has commonly been assessed by heating the skin to a temperature of 42°C at a rate of 0.1°C/s (12, 18, 34, 36, 43). This method of heating elicits an initial peak in skin blood flow (SkBF) within the first 5 min of heating, followed by a prolonged secondary plateau which is reached ∼20–30 min into heating. A brief nadir is typically observed between the two phases. Many studies have explored the molecular mechanisms behind this response. Of note, Minson et al. (34) demonstrated the initial peak was predominantly the result of a sensory nerve axon reflex, which appears to elicit vasodilation via both nitric oxide (NO) (34) and endothelium-derived hyperpolarizing factors (12). Wong et al. (43) also reported a large role of transient receptor potential vanilloid type-1 (TRPV-1) channels, which are predominantly located on the sensory nerves and may be involved in the release of neurotransmitter(s), possibly calcitonin gene-related peptide (CGRP) and/or substance P (23, 41). The plateau phase is ∼50–60% dependent on NO (26, 34). TRPV-1 channels (43), adenosine receptors (18), and reactive oxygen species (33) are known to modulate NO bioavailability during this phase. The remaining ∼40–50% of the plateau has been attributed to EDHFs that stimulate calcium-activated potassium (KCa) channels on the endothelium and smooth muscle, of which the predominant type of EDHF involved is epoxyeicosatrienoic acid (EET) (12). Neither prostanoids via the cyclooxygenase (COX) pathway (32) nor histamine receptors (45) contribute significantly to cutaneous thermal hyperemia.
Cutaneous thermal hyperemia has commonly been utilized to assess cutaneous microvascular function, and has been demonstrated to be impaired under a variety of disease states (2, 6, 29, 35, 39). As such, the response has been suggested to be reflective of generalized microvascular health (11, 21, 36), providing a simple, noninvasive means of assessing microvascular health across a variety of health conditions (14, 36). Nevertheless, some limitations exist with the currently accepted protocol for cutaneous thermal hyperemia. For example, in young, healthy subjects, the plateau commonly reaches ∼90–95% of maximal cutaneous vascular conductance (CVC), making it difficult to evaluate the effects of potentially beneficial interventions due to a ceiling effect. Furthermore, as the plateau is substantially dependent on both NO and EDHF, it is not possible to attribute impairments and/or improvements to either pathway without the use of pharmacological techniques, such as microdialysis or iontophoresis.
Therefore, our overall purpose was to identify new protocol(s) for local heating which would 1) evoke a plateau at a lower percentage of maximal CVC (CVCmax), thus avoiding the ceiling effect, and/or 2) have greater dependence on NO, to allow for the detection of impairments or improvements in NO-dependent dilation without the use of pharmacological approaches. In protocol 1, we compared nine different local heating protocols using noninvasive approaches by varying the rate of heating (0.1°C/s, 0.1°C/10 s, and 0.1°C/min) and the target temperature reached (36°C, 39°C, and 42°C). Based on these data, we selected two heating protocols to study in more depth through using pharmacological intervention and the microdialysis technique.
In protocol 2, we investigated the contributions of NO and EDHF to the vasodilator response during local heating to 39°C at a rate of 0.1°C/s, which was selected as the protocol which most consistently reached a plateau of ∼50% of CVCmax, based on protocol 1. We hypothesized that rapid local heating to this lower target temperature would produce a plateau which would be more dependent on NO than EDHF compared with the more commonly used protocol (42°C at a rate of 0.1°C/s).
In protocol 3, we investigated the contributions of NO and EDHF to the vasodilator response during heating to 42°C at a rate of 0.1°C/min. Others have utilized slower rates of heating, with the intention of better isolating NO-dependent dilation (15, 40); however, the mechanisms have not been fully elucidated. The specific mechanisms activated appear to depend on the target temperature. For example, gradual heating to 40°C elicits vasodilation which can be largely attenuated by either NO synthase (NOS) inhibition or blockade of the adrenergic nerves with bretylium (19, 22). Gradual heating from 40–42°C appears to still be NO-dependent, but only by ∼50% (5), with the remaining ∼50% of dilation unknown. No one has studied the contribution of EDHF to slow local heating, but it may be that EDHFs account for the remaining ∼50% of dilation when heating to temperatures greater than 40°C. Therefore, we hypothesized that gradual heating would be more dependent on NO than EDHF at temperatures below 40°C, but would display a greater reliance on EDHFs at temperatures >40°C.
MATERIALS AND METHODS
Subjects.
Twenty-four (12 male, 12 female) young (22 ± 1 years of age), healthy (body mass index 23.9 ± 0.6 kg/m2) subjects participated in the study. All subjects were nonsmokers with no history of cardiovascular disease, and were not taking any medications, except for oral contraceptives. Before participating in the study, all subjects refrained from alcohol and caffeine for 12 h, and from over-the-counter medications, including dietary supplements, for 24 h. Subjects fasted for at least 4 h prior to the study. All female subjects participated in the study during menses in order to reduce the effects of female sex hormones (10, 13). All protocols were approved by the Institutional Review Board at the University of Oregon, in accordance with the guidelines set forth by the Declaration of Helsinki. Each subject gave oral and written informed consent before participating in the study.
Six subjects participated in protocol 1. These subjects reported to the laboratory on three occasions within a 7-day time period. All three study days were held at the same time of day. All nine local heating protocols were performed on each subject, divided up into three protocols per study day. The days on which each protocol was performed were randomized across subjects. Subjects in protocols 2 and 3 reported to the laboratory on just one occasion.
Instrumentation.
Throughout the studies, subjects sat in a semirecumbent position with the left arm positioned at the level of the heart. For protocols 2 and 3, four microdialysis fibers (MD 2000; Bioanalytical Systems, West Lafeyette, IN; 10-mm membrane, 30-kDa cutoff membrane) were placed in the skin on the ventral surface of the left forearm at least 4 cm apart, with entry and exit point ∼2.5 cm apart. Fibers were introduced using aseptic technique with a 25-gauge needle, which was inserted into the dermal layer of the skin. Fibers were threaded through the lumen of the needle, after which the needle was removed, leaving the fiber in place in the skin. Fibers were secured with tape and perfused with lactated Ringer solution at a rate of 2 μl/min (CMA 102 Syringe Pump; CMA Microdialysis AB, Kista, Sweden) until the start of drug infusions (see “Pharmacological agents” below). A period of 60–90 min was allowed after fiber placement before the start of local heating in order for the trauma associated with needle insertion to subside.
Local skin heaters (SH02 Skin Heater/Temperature Monitor; Moor Instruments, Axminster, UK) covering an area of ∼0.78 cm2 were placed over each site (three sites for protocol 1, four sites for protocols 2 and 3). Red blood cell flux, an index of skin blood flow (SkBF), was measured at each site using single-point laser-Doppler flowmetry probes (DRT-4 and moorLab; Moor Instruments), which were seated in the center of each local heater. Arterial blood pressure was measured throughout the study on the nonexperimental arm via brachial artery oscillation (Dinamap ProCare 100; GEMedical Systems, Tampa, FL).
Experimental protocol.
Baseline SkBF was recorded for at least 10 min with skin temperature held constant at 33°C, after which the local skin temperature was increased as follows. For subjects enrolled in protocol 1 (N = 6; 3 male, 3 female), local skin temperature at each site was raised to either 36°C, 39°C, or 42°C at a rate of either 0.1°C/s, 0.1°C/10 s, or 0.1°C/min, for a total of nine combinations which were completed across the three study days (one site per combination). For subjects enrolled in protocol 2 (N = 10; 5 female, 5 male), local skin temperature was raised to 39°C at a rate of 0.1°C/s. For subjects enrolled in protocol 3 (N = 8; 4 female, 4 male; all different subjects from protocol 1), local skin temperature was raised to 42°C at a rate of 0.1°C/min. Once reached, these target temperatures were maintained for at least 30–40 min, until SkBF had reached a stable plateau for at least 10 min. Lastly, maximal SkBF was attained by increasing the local skin temperature to 43.5°C at a rate of 0.1°C/s. For protocols 2 and 3, 56 mM sodium nitroprusside (SNP) (Nitropress, Ciba Pharmaceuticals, East Hanover, NJ) was simultaneously infused during maximal heating.
Pharmacological agents (protocols 2 and 3).
Microdialysis sites were randomly assigned to receive one of the following: 1) lactated Ringer solution (Control), 2) 20 mM NG-nitro-L-arginine methyl ester (L-NAME) (Tocris Bioscience, Minneapolis, MN) to nonselectively inhibit NOS and to determine the contribution of NO to the local heating response, 3) 50 mM tetraethylammonium (TEA)(Sigma-Aldrich, St. Louis, MO) to inhibit KCa channels and determine the contribution of EDHFs, or 4) 20 mM L-NAME + 50 mM TEA to explore the combined effects of NOS and KCa channel inhibition. All drugs were dissolved in lactated Ringer solution. The concentrations of L-NAME and TEA were selected as the minimum concentrations capable of fully inhibiting NOS and KCa channels, respectively, based on previous studies (12, 19, 30). Furthermore, we have previously shown TEA at this concentration to be specific to KCa channels in the skin and to not affect other types of potassium channels (9). Specific inhibitors of large and/or small conductance KCa channels, such charybdotoxin and apamin, were not used as these are considered toxic to humans (38). All drugs were infused for at least 60 min prior to the start of local heating in order to ensure maximal efficacy (12).
Data and statistical analysis.
Data were digitized, recorded on a computer at 20 Hz, and analyzed offline using Windaq data acquisition software (Dataq Instruments, Akron, OH). SkBF was expressed as CVC (calculated as red blood cell flux divided by mean arterial pressure) and presented as a percentage of CVCmax, as determined by heating to 43.5°C plus SNP infusion. Baseline, plateau in CVC at the end of heating, and maximal CVC values were averaged over at least 5-min time periods for all three protocols. For protocol 2 (rapid heating), CVC values during the initial peak and nadir were averaged over 30-s time periods. For protocol 3 (gradual heating), CVC values at each 1.0°C increase in local heater temperature were averaged over 2-min time periods. Additionally for protocol 3, multiple peaks were observed throughout the heating period. A “peak” was defined as any immediate rise in SkBF greater than 10 laser-Doppler flux units (mV) (22, 34). Sudden increases in flux due to subject movement were excluded (noted by investigator observation and simultaneous flux changes in all sites). The temperature threshold at which a peak was first observed at each site was determined as the local heater temperature at the onset of the increase in SkBF up to a peak.
All values are presented as means ± standard error (SE). For protocol 1, plateau CVC was compared across local heating protocols using one-way repeated measures analysis of variance (ANOVA). For protocols 2 and 3, baseline, initial peak (protocol 2 only), nadir CVC (protocol 2 only), plateau, and maximal CVC, and temperature threshold (protocol 3 only) were compared across microdialysis drug sites using one-way repeated measures ANOVA. For protocol 3, CVC values at each 0.1°C increment in temperature were compared using two-way repeated measures ANOVA, with factors of local temperature and drug site. For all statistical analyses, when significant main effects were detected, significant differences between drug sites were determined using Student Newman-Keul's post hoc test. The level of significance was set at α = 0.05.
RESULTS
Protocol 1.
The plateau in CVC at each of the nine different local heating protocols is displayed in Fig. 1. Heating to 39°C at a rate of 0.1°C/s elicited a plateau of 57.7 ± 6.4% CVCmax (N = 6; P < 0.01 from the standard protocol, rapid heating to 42°C), which was the closest to our target of ∼50% of CVCmax. There were no differences in baseline CVC or maximal CVC across local heating protocols.
Fig. 1.
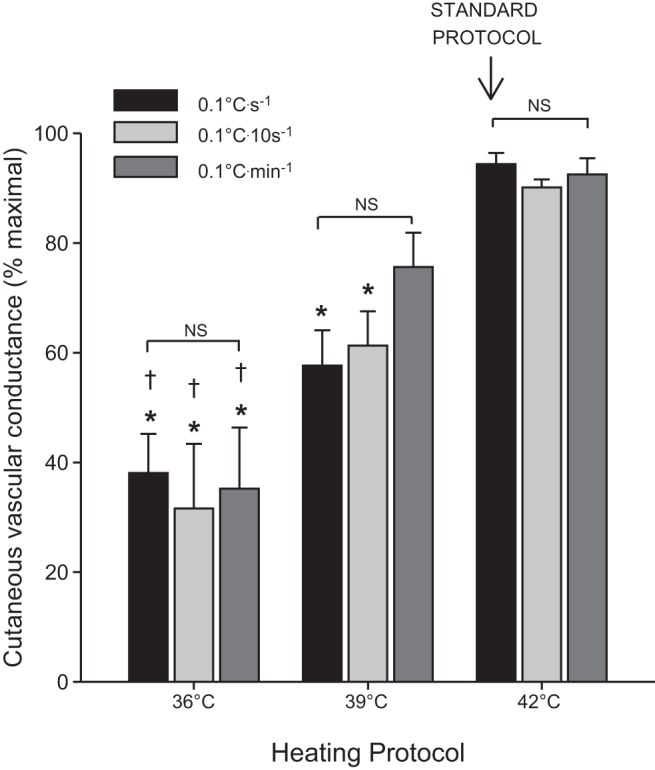
Plateau cutaneous vascular conductance across all nine local heating protocols. Protocols are combinations of three different rates of heating (0.1°C/s, 0.1°C/10 s, and 0.1°C/min) and three different target temperatures (36°C, 39°C, and 42°C). The heating protocol that has been used most commonly is indicated as the “standard protocol” (heating to 42°C at a rate of 0.1°C/s). Data are means ± SE. *P < 0.05 from all three protocols reaching 42°C, including the standard protocol; †P < 0.05 from all three protocols reaching 36°C. There were no significant differences between protocols at the same target temperature.
Protocol 2 (rapid heating).
Rapid local heating of the skin to 39°C at a rate of 0.1°C/s resulted in an initial peak in blood flow within 5 min into heating followed by a secondary plateau that was observed by ∼30–40 min into heating. Figure 2A shows a representative response from one subject at all four sites. Average initial peak CVC is displayed in Fig. 2B. Both TEA and L-NAME significantly attenuated initial peak CVC compared with the Control site, and the combination of TEA+L-NAME further attenuated initial peak CVC (P = 0.02 from the L-NAME site). The drugs affected nadir CVC in the same manner as initial peak (Table 1). Average plateau CVC is displayed in Fig. 2C. TEA attenuated plateau CVC compared with the Control site (P = 0.02). L-NAME greatly attenuated plateau CVC, such that NO accounted for 82.8 ± 4.2% of the vasodilation from baseline. TEA+L-NAME also significantly attenuated plateau CVC compared with the Control site, but no further attenuation was observed compared with the L-NAME site (P = 0.44).
Fig. 2.

A: Representative response from one subject to rapid local heating to 39°C at a rate of 0.1°C/s; B: Average initial peak; and C: plateau cutaneous vascular conductance across the four microdialysis sites. Drugs include tetraethylammonium (TEA) and nitro-L-arginine methyl ester (L-NAME). Average baseline pooled across all four sites is represented by the dotted line. Data are means ± SE. *P < 0.05 from the Control site; †P < 0.05 from the TEA site; ‡P < 0.05 from the L-NAME site.
Table 1.
Baseline, nadir, and maximal cutaneous vascular conductance
| Baseline CVC, %max |
Nadir CVC, %max | Maximal CVC, mV/MAP·100 |
|||||
|---|---|---|---|---|---|---|---|
| Protocol 1 | Protocol 2 | Protocol 3 | Protocol 2 | Protocol 1 | Protocol 2 | Protocol 3 | |
| Control | 6.9 ± 2.1 | 8.7 ± 1.4 | 7.7 ± 1.1 | 24.4 ± 3.3 | 192.6 ± 7.9 | 220 ± 20 | 294 ± 37 |
| TEA | 5.5 ± 0.9* | 3.9 ± 0.4* | 11.3 ± 2.1* | 213 ± 18 | 351 ± 43 | ||
| L-NAME | 4.9 ± 0.6* | 5.9 ± 0.7* | 8.1 ± 1.2* | 209 ± 23 | 269 ± 28 | ||
| TEA+L-NAME | 3.3 ± 0.4* | 4.4 ± 1.0* | 5.0 ± 0.6*† | 200 ± 16 | 285 ± 33 | ||
Drugs include tetraethylammonium (TEA) and nitro-L-arginine methyl ester (L-NAME); CVC, cutaneous vascular conductance. Data are means ± SE. Data for protocol 1 are pooled data across all nine combinations of rates of local heating target temperatures.
P < 0.05 compared with the Control site within each protocol;
P < 0.05 compared with the TEA site within each Protocol.
Protocol 3 (gradual heating).
Figure 3A displays a representative tracing from one subject for all four microdialysis sites. Gradual local heating of the skin to 42°C at a rate of 0.1°C/min resulted in a gradual increase in CVC up to a plateau. Multiple peaks in CVC were observed throughout heating, particularly at the Control site. At the Control site, the first peak was observed at a local heater temperature of 36.5 ± 0.3°C. The onset of peaks was significantly delayed compared with Control site to a higher local heater temperature at the TEA site (38.7 ± 0.8, P = 0.003), and to an even higher local heater temperature at the L-NAME (40.9 ± 0.3, P = 0.002 vs. TEA) and TEA+L-NAME (41.1 ± 0.5, P = 0.003 vs. TEA) sites. The local heater temperature threshold was not different between the L-NAME and TEA+L-NAME sites (P = 0.83). As such, fewer peaks were seen in the TEA, L-NAME, and TEA+L-NAME sites compared with the Control site. Average CVC at each 1°C increment in local heater temperature is displayed in Fig. 3B. TEA significantly attenuated CVC at 38–40°C, but this effect was not observed at temperatures >40°C. CVC was significantly attenuated by L-NAME at temperatures >36°C, but the extent of attenuation was diminished at higher temperatures, such that NO accounted for 81.5 ± 3.2% of the rise in CVC at 40°C, but only 32.7 ± 7.8% of the rise in CVC after prolonged heating at 42°C. CVC was further attenuated by the combination of TEA+L-NAME at temperatures >40°C, such that CVC at the TEA+L-NAME site did not become significantly elevated from preheating baseline values until local temperature had reached 42°C. Interestingly, TEA not only attenuated the vasodilator response, but also delayed the temperature at which vasodilation above baseline values was observed (39°C vs. 38°C at the TEA and Ringer's sites, and 42°C vs. 41°C at the L-NAME+TEA and L-NAME sites).
Fig. 3.

A: Representative response from one subject to gradual local heating to 42°C at a rate of 0.1°C/min. B: Average cutaneous vascular conductance at each 1°C increment in local temperature throughout the time period of gradual heating and during the prolonged plateau. Drugs include TEA and L-NAME. Data are means ± SE. *P < 0.05 from baseline (33°C) within sites.
Drug effects on baseline and maximal cutaneous vascular conductance.
Average data for baseline and maximal CVC are displayed in Table 1. There were no significant differences in maximal CVC across drug sites for either protocol. TEA, L-NAME, and TEA+L-NAME all significantly attenuated baseline compared with the Control site, as previously reported (12).
DISCUSSION
The primary goal of this study was to identify an improved protocol for local heating for use in the assessment of endothelial function that 1) would reach a plateau at a lower percentage of maximal CVC, allowing for the study of interventions that may potentially improve cutaneous microvascular function, and 2) is more dependent on NO, allowing for the assessment of NO-dependent dilation without the use of pharmacological techniques. Secondarily, we sought to determine the contribution of EDHF to slow local heating. The major findings of this study are that 1) rapid heating to 39°C at 0.1°C/s elicits an increase in CVC to ∼50% of maximal CVC, which is predominantly NO-dependent (≥80% contribution), and that 2) gradual heating to 42°C at a rate of 0.1°C/min elicits an increase in CVC to ∼85% of maximal, which is dependent on both NO and EDHFs, but also has a large NO- and EDHF-independent component (which is not present with rapid heating to 42°C).
Protocol 1.
We compared nine different protocols to identify one which consistently reached a lower percentage of maximal CVC. Heating to 39°C (at all three rates of heating) elicited plateau values in a range which would allow the assessment of both improvements and impairments in the response (57.7 ± 6.4%, 61.3 ± 6.3%, and 75.6 ± 6.3% of CVCmax when heating at a rate of 0.1°C/s, 0.1°C/10 s, and 0.1°C/min, respectively). Of these three protocols, heating to 39°C at a rate of 0.1°C/s elicited the most consistent response in plateau across subjects, although it should be noted that there was still marked variability (discussed in more depth in “limitations”).
Interestingly, heating to both 36°C and 42°C elicited consistent plateau values regardless of the rate of heating (range in average plateau values: 31.5–38.0%max and 90.0–94.4%max, respectively), such that the extent of vasodilation to both mild and high levels of local heating appear to be dependent on target temperature, and not rate of heating. When heating to 39°C, there was a larger splay in the plateau values across rates of heating, although these were not statistically different. This effect is likely because basement and ceiling effects limited the variability in the 36°C and 42°C protocols, respectively, but this was not the case when heating to 39°C.
Endothelial-dependent dilation.
Rapid heating to 39°C (protocol 2) produced a plateau which was much more dependent on NO than the standard protocol (80% NO contribution vs. ∼50–70% contribution). Combined blockade of NOS and KCa channels nearly abolished the plateau, similarly to the standard protocol (12). Thus the same mechanisms are activated with rapid heating to both 39°C and 42°C, but the contribution of NO and KCa channels to the overall response varies.
In protocol 3, CVC gradually increased throughout the heating time period up to similar levels as in the standard rapid heating protocol (86% CVCmax vs. ∼90–95%). NOS inhibition abolished much of the response up to ∼40°C, consistent with previous studies (22). However, when heating from 40°C to 42°C, NOS inhibition was only able to block ∼50% of the CVC response, consistent with the findings of Black et al. (5) and with rapid local heating to 42°C (10, 13, 34). Furthermore, combined inhibition of NOS and KCa channels significantly attenuated CVC beyond that observed in the L-NAME site when heating above 40°C. No effect of TEA alone was observed on the CVC response to gradual heating between 40–42°C; however, cross talk is known to exist between the NO and EDHF pathways, such that the roles of EDHFs are not always observed unless NOS is simultaneously blocked (4, 7, 9, 12, 30).
Taken together, these data indicate a shift in the mechanisms responsible for hyperemia around 40°C, specifically from a primary reliance on NO to a shared reliance on both NO and EDHFs. It is possible the extent to which NOS is capable of eliciting dilation reaches a maximum around ∼50% of CVCmax, and thus other mechanisms are activated to produce further vasodilation. In fact, there are no other known vasodilator responses in the skin that elicit NOS-dependent dilation to as great an extent as thermal hyperemia (25, 27, 38). It is logical that EDHFs are responsible for dilation beyond what can be produced via NOS for a few reasons. Firstly, EDHFs have often been described as a “back-up” system to the NO pathway, sharing many common signaling pathways (8, 12, 46). Secondly, EDHFs, including EETs, stimulate KCa channels by first acting on TRPV-4 channels (17). TRPV-4 channels are heat-sensitive at physiological temperatures, displaying increasing current as temperature increases (42), and thus dilation via KCa channels may occur due to direct activation of these channels by the heat and to a greater extent at higher temperatures. Lastly, the primary type of EDHF involved in thermal hyperemia is EETs (12), which are converted from arachadonic acid by cytochrome P450. Perhaps this conversion occurs more readily at higher temperatures.
In contrast to rapid local heating, during gradual heating to 42°C, a significant portion of the plateau remains unexplained by combined NOS and KCa channel inhibition. As the contributions of NO and EDHFs are different at different temperatures during gradual heating, it is plausible that other vasodilatory pathways may only be stimulated above 40°C, and that these pathways may be different from those activated during rapid heating. For example, the COX pathway has been shown to have no role in rapid local heating (32) but may contribute to the vasodilation during gradual heating. As we did not test the role of the COX pathway in the present study, it cannot be ruled out. Additionally, pathways which are known to contribute to thermal hyperemia via NOS-dependent mechanisms, e.g., TRPV-1 receptors (43), adenosine receptors (18), and/or substance P (44), may be capable of having NOS-independent effects when the heating stimulus is gradual. Lastly, combined inhibition of NOS and KCa channels may result in upregulation of vasodilatory pathways that would otherwise have been quiescent. It is interesting that this is not observed during rapid heating, but perhaps the prolonged nature of the gradual heating protocol necessitates other vasodilatory mechanisms. For example, the production of vasodilators is thought to diminish following extended periods of local heating at constant high temperatures, known as the die-away phenomenon (3). Perhaps with gradual heating, as the primary vasodilators diminish, others are produced instead.
Axon reflex(es).
In the present study, rapid heating to 39°C produced an initial peak which reached a lower CVC value than in the standard protocol (44%CVCmax vs. ∼60–75%) (12, 18, 34, 43), but which was dependent on NO and EDHFs to similar extents. These findings suggest that vasodilation via NO and EDHFs in response to local warming (whether directly or via neurotransmitter release) may be activated by more mild temperatures above normal resting skin temperature, but that the final target temperature determines the magnitude of the vasodilator response.
In protocol 3, NOS inhibition significantly delayed the temperature threshold for the onset of axon reflexes, consistent with what was reported by Houghton et al. (22). The temperature threshold was also delayed by TEA, and further delayed by combined NOS and KCa channel inhibition, such that some subjects did not exhibit peaks in the combined L-NAME+TEA site until after the local temperature had reached 42°C. Houghton et al. (22) speculated the delay in peaks caused by L-NAME could be caused by desensitization of the sensory nerves, which are thought to release CGRP and/or substance P (23, 41). NO may play a role in triggering release of CGRP and/or substance P, thus reduced NO production would result in reduced neurotransmitter release (28). TEA may have a similar desensitizing effect on the sensory nerves, as 50 mM TEA has also been shown to inhibit some types of voltage-gated potassium channels in neurons (31). Although this has never been shown to occur in human skin, it would be consistent with reduced neurotransmitter release and thus a reduced initial peak.
Limitations and considerations.
There was a substantial amount of variability in the plateau CVC values during rapid heating to 39°C (protocols 1 and 2) compared with what is typically observed when rapidly heating to 42°C. Although the majority of plateau values (10 out of 16 subjects) across the two protocols fell in the range of 40–60% CVCmax, the responses ranged from 20–77% CVCmax. We assume this is due to the nature of the heating stimulus. Rapid heating elicits a near maximal vasodilation, thus there will be a “crowding” of values at the high end of the spectrum. More moderate heating should be expected to produce a greater range of responses. Regardless, the response was nearly abolished by L-NAME in all subjects. Thus despite a range in the extent of dilation, rapid heating to 39°C consistently provided a test of NO-dependent dilation.
There was also a fair amount of variability in the effects of TEA on plateau CVC during protocol 3. For example, some subjects exhibited an augmented plateau in protocol 3 at the TEA site compared with the Control site. This finding may reflect variability in the response itself or inconsistent pharmacological effects of the drug across subjects. In other vascular beds, 50 mM TEA has been shown to act on other types of potassium channels (16, 37). Although this does not appear to occur in the skin (9), we cannot rule out the possibility of TEA acting differently across subjects. However, variability in the effects of TEA does not alter our conclusions, particularly on the utility of heating to lower temperatures for assessing NO-dependent dilation.
Hodges and Sparks (20) recently showed that eNOS inhibition with NG-amino-L-Arginine (L-NAA) attenuated plateau during rapid local heating to 42°C to a greater extent when administered after a plateau had already been established (35 ± 4%CVCmax) vs. prior to the start of local heating (53 ± 4%CVCmax). Minson et al. (34) showed similar results using L-NAME (26 ± 3%CVCmax when infused after establishing plateau vs. 40 ± 5%CVCmax prior to heating), although these experiments were performed on different subjects and were not compared statistically by the authors. Taken together, these studies suggest the timing of when drugs are infused may alter the mechanisms at play, or at least the contribution of the mechanisms involved. In the present study, we infused all drugs at least 60 min prior to the start of heating, so it is possible we may have found different results had we waited to administer drugs until after a plateau had been established. However, if the pattern observed by Hodges and Sparks and Minson et al. were also true during the heating protocols utilized in our study, infusing L-NAME after establishing plateau would have attenuated CVC to an even greater extent, suggesting an even larger contribution of NO to the response. Thus our conclusion that rapid heating to 39°C is >80% NO-dependent would still be true.
Conclusions and perspectives: cutaneous thermal hyperemia for assessing NO-dependent dilation and microvascular health.
Increasingly, more studies are showing that endothelial dysfunction in the microvasculature may precede dysfunction in the conduit arteries (1, 24). Furthermore, endothelial dysfunction occurs predominantly due to reduced NO bioavailability in many disease states; thus developing a test of microvascular function that is primarily NO-dependent has significant clinical utility. In the present study, we have discovered that reducing the target temperature during rapid local heating from 42°C to 39°C produces a hyperemic response which is consistently >80% dependent on NO. This is the most robust test of NO-dependent dilation that can be performed in humans, as there is a multifold increase in conductance and vessel radius from baseline that is almost entirely mediated by NO. Due to the noninvasiveness and ease of performing the test, rapid local heating to 39°C offers a powerful clinical tool for assessing NO-dependent dilation and microvascular function. Furthermore, rapid local heating to 39°C elicits a plateau which reaches ∼50% of CVCmax, which allows ample room to assess pharmacological and behavioral therapies that may be capable of improving microvascular function. Previous studies which have been unable to show full benefits of such interventions may have been limited by a ceiling effect with the standard protocol (10). As such, heating to a lower target temperature may be preferable to the standard heating protocol when assessing NO production and/or interventions that could potentially improve microvascular function.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL081671.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.J.C., V.E.B., and C.T.M. conception and design of research; P.J.C., V.E.B., and N.F. performed experiments; P.J.C. and V.E.B. analyzed data; P.J.C., V.E.B., N.F., and C.T.M. interpreted results of experiments; P.J.C. drafted manuscript; P.J.C., V.E.B., N.F., and C.T.M. approved final version of manuscript; V.E.B. prepared figures; V.E.B., N.F., and C.T.M. edited and revised manuscript.
ACKNOWLEDGMENTS
The authors graciously thank the subjects for their participation.
REFERENCES
- 1.Abularrage CJ, Sidawy AN, Aidinian G, Singh N, Weiswasser JM, Arora S. Evaluation of the microcirculation in vascular disease. J Vasc Surg 42: 574–581, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Agarwal SC, Allen J, Murray A, Purcell IF. Laser Doppler assessment of dermal circulatory changes in people with coronary artery disease. Microvasc Res 84: 55–59, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Barcroft H, Edholm OG. The effect of temperature on blood flow and deep temperature in the human forearm. J Physiol 102: 5–20, 1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauersachs J, Popp R, Hecker M, Sauer E, Fleming I, Busse R. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation 94: 3341–3347, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Black MA, Green DJ, Cable NT. Exercise prevents age-related decline in nitric-oxide-mediated vasodilator function in cutaneous microvessels. J Physiol 586: 3511–3524, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boignard A, Salvat-Melis M, Carpentier PH, Minson CT, Grange L, Duc C, Sarrot-Reynauld F, Cracowski JL. Local hyperemia to heating is impaired in secondary Raynaud's phenomenon. Arthritis Res Ther 7: R1103–R1112, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature 368: 850–853, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Brandes RP, Schmitz-Winnenthal FH, Félétou M, Gödecke A, Huang PL, Vanhoutte PM, Fleming I, Busse R. An endothelium-derived hyperpolarizing factor distinct from NO and prostacyclin is a major endothelium-dependent vasodilator in resistance vessels of wild-type and endothelial NO synthase knockout mice. Proc Natl Acad Sci USA 97: 9747–9752, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunt VE, Fujii N, Minson CT. No independent, but an interactive, role of calcium-activated potassium channels in human cutaneous active vasodilation. J Appl Physiol 115: 1290–1296, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunt VE, Miner JA, Meendering JR, Kaplan PF, Minson CT. 17β-estradiol and progesterone independently augment cutaneous thermal hyperemia but not reactive hyperemia. Microcirculation 18: 347–355, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunt VE, Minson CT. Cutaneous thermal hyperemia: more than skin deep. J Appl Physiol 111: 5–7, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Brunt VE, Minson CT. KCa channels and epoxyeicosatrienoic acids: major contributors to thermal hyperaemia in human skin. J Physiol 590: 3523–3534, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charkoudian N, Johnson JM. Female reproductive hormones and thermoregulatory control of skin blood flow. Exerc Sport Sci Rev 28: 108–112, 2000 [PubMed] [Google Scholar]
- 14.Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci 27: 503–508, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Dahmus JD, Bruning RS, Larry Kenney W, Alexander LM. Oral clopidogrel improves cutaneous microvascular function through EDHF-dependent mechanisms in middle-aged humans. Am J Physiol Regul Integr Comp Physiol 305: R452–R458, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies NW, Spruce AE, Standen NB, Stanfield PR. Multiple blocking mechanisms of ATP-sensitive potassium channels of frog skeletal muscle by tetraethylammonium ions. J Physiol 413: 31–48, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Earley S. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res 97: 1270–1279, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Fieger SM, Wong BJ. Adenosine receptor inhibition with theophylline attenuates the skin blood flow response to local heating in humans. Exp Physiol 95: 946–954, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Hodges GJ, Kosiba WA, Zhao K, Johnson JM. The involvement of norepinephrine, neuropeptide Y, and nitric oxide in the cutaneous vasodilator response to local heating in humans. J Appl Physiol 105: 233–240, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodges GJ, Sparks PA. Noradrenaline and neuropeptide Y contribute to initial, but not sustained, vasodilation in response to local skin warming in humans. Exp Physiol 99: 381–392, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol 105: 370–372, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Houghton BL, Meendering JR, Wong BJ, Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol 572: 811–820, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes SR, Brain SD. Nitric oxide-dependent release of vasodilator quantities of calcitonin gene-related peptide from capsaicin-sensitive nerves in rabbit skin. Br J Pharmacol 111: 425–430, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joannides R, Bellien J, Thuillez C. Clinical methods for the evaluation of endothelial function—a focus on resistance arteries. Fundam Clin Pharmacol 20: 311–320, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Kellogg DL, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol 85: 824–829, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Kellogg DL, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol 86: 1185–1190, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Kellogg DL, Zhao JL, Coey U, Green JV. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J Appl Physiol 98: 629–632, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Klede M, Clough G, Lischetzki G, Schmelz M. The effect of the nitric oxide synthase inhibitor N-nitro-L-arginine-methyl ester on neuropeptide-induced vasodilation and protein extravasation in human skin. J Vasc Res 40: 105–114, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Kruger A, Stewart J, Sahityani R, O'Riordan E, Thompson C, Adler S, Garrick R, Vallance P, Goligorsky MS. Laser Doppler flowmetry detection of endothelial dysfunction in end-stage renal disease patients: correlation with cardiovascular risk. Kidney Int 70: 157–164, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Lorenzo S, Minson CT. Human cutaneous reactive hyperaemia: role of BKCa channels and sensory nerves. J Physiol 585: 295–303, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathie A, Wooltorton JR, Watkins CS. Voltage-activated potassium channels in mammalian neurons and their block by novel pharmacological agents. Gen Pharmacol 30: 13–24, 1998 [DOI] [PubMed] [Google Scholar]
- 32.McCord GR, Cracowski JL, Minson CT. Prostanoids contribute to cutaneous active vasodilation in humans. Am J Physiol Regul Integr Comp Physiol 291: R596–R602, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Medow MS, Bamji N, Clarke D, Ocon AJ, Stewart JM. Reactive oxygen species (ROS) from NADPH and xanthine oxidase modulate the cutaneous local heating response in healthy humans. J Appl Physiol 111: 20–26, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 91: 1619–1626, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol 93: 1644–1649, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Minson CT. Thermal provocation to evaluate microvascular reactivity in human skin. J Appl Physiol 109: 1239–1246, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol Heart Circ Physiol 259: H3–H18, 1990 [DOI] [PubMed] [Google Scholar]
- 38.Pickkers P, Hughes AD, Russel FG, Thien T, Smits P. Thiazide-induced vasodilation in humans is mediated by potassium channel activation. Hypertension 32: 1071–1076, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Smith CJ, Santhanam L, Bruning RS, Stanhewicz A, Berkowitz DE, Holowatz LA. Upregulation of inducible nitric oxide synthase contributes to attenuated cutaneous vasodilation in essential hypertensive humans. Hypertension 58: 935–942, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sprung VS, Cuthbertson DJ, Pugh CJA, Daousi C, Atkinson G, Aziz NF, Kemp GJ, Green DJ, Cable NT, Jones H. Nitric oxide-mediated cutaneous microvascular function is impaired in polycystic ovary syndrome but can be improved by exercise training. J Physiol 591: 1475–1487, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallengren J, Ekman R, Sundler F. Occurrence and distribution of neuropeptides in the human skin. An immunocytochemical and immunochemical study on normal skin and blister fluid from inflamed skin. Acta Derm Venereol 67: 185–192, 1987 [PubMed] [Google Scholar]
- 42.Watanabe H. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem 277: 47044–47051, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Wong BJ, Fieger SM. Transient receptor potential vanilloid type-1 (TRPV-1) channels contribute to cutaneous thermal hyperaemia in humans. J Physiol 588: 4317–4326, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong BJ, Minson CT. Altered thermal hyperaemia in human skin by prior desensitization of neurokinin-1 receptors. Exp Physiol 96: 599–609, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong BJ, Williams SJ, Minson CT. Minimal role for H1 and H2 histamine receptors in cutaneous thermal hyperemia to local heating in humans. J Appl Physiol 100: 535–540, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Zhou MS, Raij L. Cross-talk between nitric oxide and endothelium-derived hyperpolarizing factor: synergistic interaction? J Hypertens 21: 1449–1451, 2003 [DOI] [PubMed] [Google Scholar]


