Abstract
Certain retroviruses induce progressive spongiform motor neuron disease with features resembling prion diseases and amyotrophic lateral sclerosis. With the neurovirulent murine leukemia virus (MLV) FrCasE, Env protein expression within glia leads to postsynaptic vacuolation, cellular effacement, and neuronal loss in the absence of neuroinflammation. To understand the physiological changes associated with MLV-induced spongiosis, and its neuronal specificity, we employed patch-clamp recordings and voltage-sensitive dye imaging in brain slices of the mouse inferior colliculus (IC), a midbrain nucleus that undergoes extensive spongiosis. IC neurons characterized by postinhibitory rebound firing (PIR) were selectively affected in FrCasE-infected mice. Coincident with Env expression in microglia and in glia characterized by NG2 proteoglycan expression (NG2 cells), rebound neurons (RNs) lost PIR, became hyperexcitable, and were reduced in number. PIR loss and hyperexcitability were reversed by raising internal calcium buffer concentrations in RNs. PIR-initiated rhythmic circuits were disrupted, and spontaneous synchronized bursting and prolonged depolarizations were widespread. Other IC neuron cell types and circuits within the same degenerative environment were unaffected. Antagonists of NMDA and/or AMPA receptors reduced burst firing in the IC but did not affect prolonged depolarizations. Antagonists of L-type calcium channels abolished both bursts and slow depolarizations. IC infection by the nonneurovirulent isogenic virus Friend 57E (Fr57E), whose Env protein is structurally similar to FrCasE, showed no RN hyperactivity or cell loss; however, PIR latency increased. These findings suggest that spongiform neurodegeneration arises from the unique excitability of RNs, their local regulation by glia, and the disruption of this relationship by glial expression of abnormal protein.
Keywords: auditory midbrain, inferior colliculus, postinhibitory rebound neurons, retrovirus, voltage-sensitive dyes
certain retroviruses are capable of causing progressive paralytic spongiform neurodegenerative diseases, with features reminiscent of prion diseases and amyotrophic lateral sclerosis (ALS). In particular, the chimeric murine leukemia virus (MLV) FrCasE induces rapid, stereotypic spongiform motor neuron disease upon CNS infection (Czub et al. 1994; Lynch et al. 1991; Portis et al. 1990). The viral Env protein is necessary and sufficient for disease, acts by first targeting virions within the CNS, and induces vacuolation through expression within nonneuronal cells (Li et al. 2011). Upon peripheral neonatal inoculation, FrCasE infects hematogenous cells, spreads to the CNS vasculature, and is broadly disseminated to CNS parenchymal cells. Nevertheless, spongiosis is restricted to motor/premotor areas from the cortex through the spinal cord, including auditory and vestibular areas (Czub et al. 1994). Because vacuolation first arises within postsynaptic neuronal processes (Lynch et al. 1991; Nagra et al. 1993) and can be limited spatially by focal transplant of virus-infected cells (Lynch et al. 1995, 1996), it is thought that intracellular Env expression disrupts local intrinsic homeostatic signaling mechanisms.
Infection of CNS glia most closely correlates with the appearance of degenerative changes (Baszler and Zachary 1991; Clase et al. 2006; Hansen et al. 2000; Kay et al. 1991; Lynch et al. 1991; Morey and Wiley 1990; Robertson et al. 1997); however, which glial subtype(s) is/are involved and their pathogenic mechanism(s) remain unclear (cf. Dimcheff et al. 2006; Li et al. 2011). Glial fibrillary acidic protein (GFAP)-positive astrocytes and NG2 glia appear to be two distinct glial cell populations. Both glial populations are abundant in the CNS and make close contact with neurons in white and gray matter. Glial filaments are not found in NG2 glia, however, and NG2 glia do not express glutamine synthetase or S-100β, which are present in GFAP-positive astrocytes (Nishiyama et al. 2005). Because neurovirulent and nonneurovirulent viruses infect the same cell populations (Askovic et al. 2000; Li et al. 2011), disease is believed to be mediated by target cell expression of a unique Env conformation (Renszel et al. 2013).
To understand the neurophysiological consequences of Env-induced neurodegeneration, we examined changes in neuronal and circuit behavior in the inferior colliculus (IC), a susceptible auditory midbrain nucleus possessing well-defined neuronal cell types (Peruzzi et al. 2000; Sivaramakrishnan and Oliver 2001) and circuits (Chandrasekaran et al. 2013; Grimsley et al. 2013). IC neuron subtypes are distinguished by their intrinsic firing patterns and voltage-dependent currents (Sivaramakrishnan and Oliver 2001) and by the calcium dependence of their synaptic efficacy (Wu et al. 2002). Moreover, rodent IC neurons acquire their unique properties during the second postnatal week (Sivaramakrishnan and Oliver 2001), a time coinciding with the onset of hearing (Zheng et al. 1999) and CNS susceptibility to retroviral spongiogenesis (Lynch and Portis 1993). Thus this brain region provides a system in which clearly defined neuronal subtypes and local circuits can be examined for selective degenerative mechanisms.
Here we show that FrCasE infection of the IC selectively affects rebound neurons (RNs), which lose postinhibitory rebound firing (PIR), become hyperexcitable, show dendritic alteration, and are lost, coincident with elevated intracellular calcium. RNs exhibit widespread bursting, and local circuits that require PIR become nonfunctional. In the same degenerative environment, other IC neuron types and circuits remain normal. Neurophysiological alterations arise coincident with Env expression in NG2 and microglia, and with vacuolar pathology, suggesting that neurodegeneration results from Env-induced perturbation of neuron-glia interactions, where glia affect the excitability of RNs by modulating resting calcium levels.
MATERIALS AND METHODS
Mice, viruses, and infection with murine leukemia viruses.
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Northeast Ohio Medical University and were performed in accordance with guidelines of the National Institutes of Health. Inbred Rocky Mountain White (IRW) mice of either sex were used for these experiments because they are highly susceptible to infection and neurodegeneration induced by neurovirulent ecotropic MLVs (Portis et al. 1990). IRW mice were intraperitoneally inoculated on the day of birth (P0; <24 h after parturition) with 2–5 × 104 infectious FrCasE (Portis et al. 1990) or Friend 57E (Fr57E) (Dimcheff et al. 2006) virions or were not inoculated. Animals were killed at P7–P16 for histological, immunohistochemical, and physiological analysis. The viruses, FrCasE and Fr57E, were prepared in NIH 3T3 cells, yielding stocks of 1–2 × 106 focus forming units per milliliter (FFU/ml) of tissue culture medium [Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, penicillin (50 IU/ml), and streptomycin (50 μg/ml)].
Experimental mice were observed daily for overt signs of disease beginning at P10, a time at which spongiform changes can first be detected within the CNS (Czub et al. 1994). Clinical neurological signs, which included unkempt fur, wasting (body weight difference of <80% of that in normal control mice), kyphosis, tremor, hind- and or forelimb adduction upon elevation by the tail, imbalance, and/or paralysis, are not observed until P16, consistent with previous reports for the FrCasE virus (Portis et al. 1990). Mice were killed by decapitation under deep isoflurane anesthesia prior to processing for histological and immunohistochemical assessment.
Molecular modeling.
Molecular modeling of the CasBrE Env receptor binding domain (RBD) was performed with I-TASSER (Roy et al. 2010) and was based on the known crystal structure coordinates of the Friend Env RBD (clone 57) (Fass et al. 1997) (GenBank accession no. J02192) and the CasBrE Env RBD sequence (GenBank accession no. X57540).
Histopathology and immunocytochemistry.
Brains from infected and control mice were harvested at P10, P12, P14, and P16, immersion fixed in phosphate-buffered 10% Formalin overnight, paraffin embedded, sectioned, and stained with hematoxylin and eosin for neuropathological assessment. Pathological severity was determined blind by a single investigator experienced in grading spongiform pathological severity with multiple periodic sections from the IC from each animal. Scores (0–4+) were determined by assessing the size of the vacuoles and area occupied in the section at low power (×4) combined with an evaluation of the percentage of a microscope field occupied by vacuoles at high magnification (×40), as described previously by Czub et al. for this and other brain regions (Czub et al. 1994). In parallel, paraffin sections were processed for immunohistochemistry and/or immunofluorescence for cell type and viral antigens after antigen retrieval, as described previously (Lynch et al. 1996, 1999). FrCasE-infected cells were detected with either the mouse monoclonal antibody 697, which is specific for the CasBrE envelope protein (McAtee and Portis 1985), or a more broadly reactive goat anti-Friend Env-specific polyclonal antibody (a gift from R. Friedrich) diluted 1:8,000. Fr57E-infected cells were detected with a mixture of two Friend Env-specific mouse monoclonal antibodies, 720 (Robertson et al. 1991) and 48 (Chesebro et al. 1983), or the Friend Env-specific polyclonal antibody noted above. Microglia were detected with a rabbit polyclonal antibody directed to Iba-1 (WAKO) diluted 1:2,000. NG2 cells were detected with a rabbit anti-NG2 antibody (Chemicon) diluted 1:500. Astrocytes were identified with rabbit anti-GFAP polyclonal antisera diluted 1:2,000 (Dako). Oligodendrocytes were detected with a mouse monoclonal antibody directed toward 2′,3′-cyclic nucleotide 3′-phosphohydrolase (CNPase; Chemicon), while neurons were detected with mouse monoclonals directed toward HuC/D (Invitrogen) or NeuN as outlined previously (Li et al. 2011). Species-specific secondary antibodies were used to selectively label the primary antibodies and included biotinylated horse anti-mouse IgG (Vector), biotinylated donkey anti-goat IgG (Jackson Immunoresearch), biotinylated donkey anti-rat IgG (Jackson Immunoresearch), biotinylated donkey anti-rabbit IgG (Jackson Immunoresearch), biotinylated goat anti-rabbit IgG, Alexa Fluor 488 goat anti-mouse IgG, Alexa Fluor 594 donkey anti-mouse IgG, and Alexa Fluor 594 donkey anti-rabbit IgG. For detection of biotinylated secondary antibodies Alexa Fluor 488 streptavidin and Alexa Fluor 594 streptavidin were used (1:2000 dilution), and for cytochemistry HRP- and alkaline phosphatase-coupled streptavidin (Biogenex) were used. Images were collected with an Olympus BX51 fitted with epifluorescence and differential interference contrast optics or an Olympus FV1000 confocal microscope. All immunostaining was performed on at least three separate brains for each time point with each antibody, and representative images from the IC are shown.
Brain slice recordings.
Techniques for making transverse slices through the IC have been described previously (Chandrasekaran et al. 2013; Sivaramakrishnan and Oliver 2001) and are briefly outlined here. Animals were anesthetized with isoflurane and decapitated. A block containing the IC was removed from the brain with two transverse cuts and glued to the cutting stage of a vibratome (Dosaka), and slices were made parallel to the surface of the brain block. Slices were cut, stored, and recorded from at 35°C in oxygenated (95% O2-5% CO2) artificial cerebrospinal fluid (ACSF) (in mM: 120 NaCl, 3 KCl, 2 CaCl2, 1.3 MgSO4, 1 NaH2PO4, 20 NaHCO3, 25 glucose, pH 7). Slices were transferred to a temperature-regulated recording chamber and superfused with ACSF at 2 ml/min during recordings. Antagonists of the AMPA receptor (AMPAR) (NBQX, 10 μM) and the NMDA receptor (NMDAR) (D-APV, 100 μM), tetrodotoxin (1 μM), a blocker of voltage-gated sodium channels, and an antagonist of L-type calcium channels (nimodipine, 10 μM) were bath applied at the same flow rates as ACSF. Chemicals were obtained from Sigma-Aldrich. Data are reported from 234 cells.
Electrophysiological recordings.
Whole cell patch-clamp recordings were made from the central nucleus of the IC (ICC) in 300-μm-thick slices as described previously (Sivaramakrishnan et al. 2013; Sivaramakrishnan and Oliver 2001). Recordings were made under visual control with an upright microscope (Zeiss Axioskop) fitted with a water immersion objective (×40/NA 0.75) and differential interference optics. Patch pipettes were made from borosilicate glass (Kimax, 1.5-mm OD), with resistances of 5–7 MΩ when filled with a recording solution containing (in mM) 120 K gluconate, 10 KCl, 0.2 EGTA, 0.1 CaCl2, 4 Mg-ATP, 0.3 Na-GTP, 10 HEPES, and 10 phosphocreatine, pH 7.3. Some pipette recording solutions contained an altered Ca-to-EGTA ratio (11 mM EGTA-5 mM Ca vs. 0.2 mM EGTA-0.1 mM Ca). Calculated free Ca2+ values were as follows: 11 mM EGTA-5 mM Ca2+: free Ca2+ = 70 nM; 0.2 mM EGTA-0.1 mM Ca2+: free Ca2+ = 100 nM (maxchelator.stanford.edu). Series resistances were generally <12 MΩ and compensated by 75–80%. A junction potential correction of −11 mV was applied to all voltages; reported resting membrane potentials include this correction. An EPC-10 amplifier and Patchmaster/Fitmaster software (HEKA Elektroniks/Instrutech) were used respectively for recordings, data collection, and analyses. Origin software (OriginLab) was used for statistical analysis and graphing data.
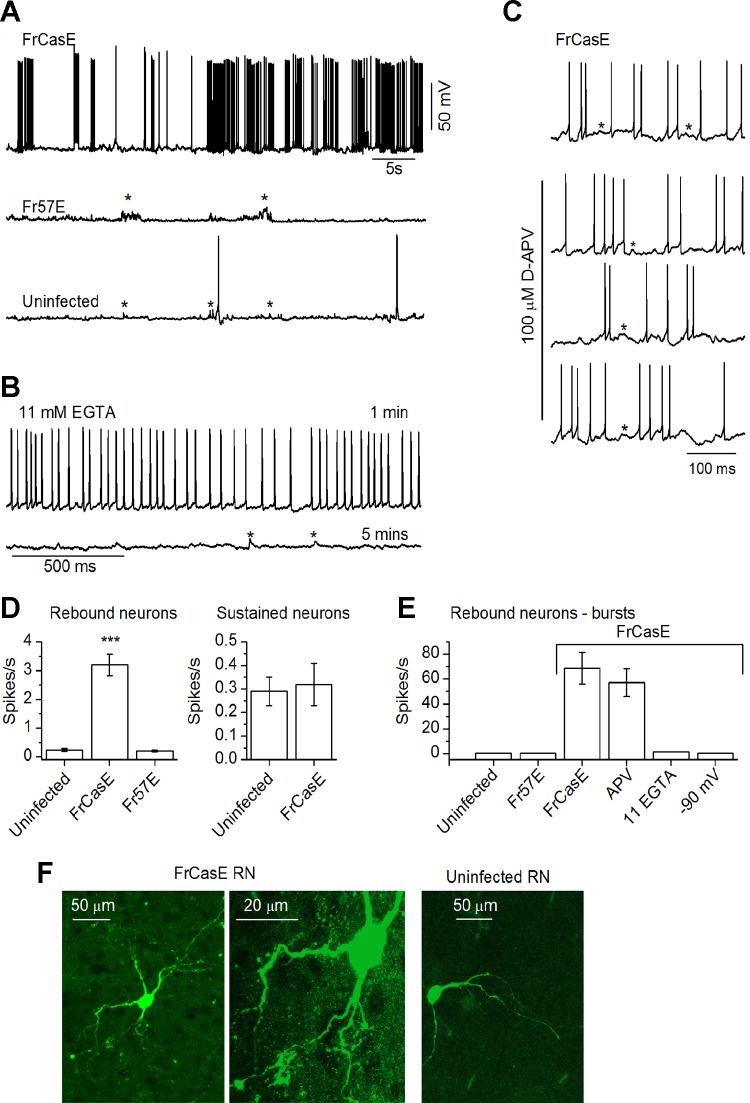
Spontaneous activity was recorded at normal resting potentials or, in some cases, during injection of hyperpolarizing DC current. Membrane potential values were constantly monitored during recordings, and holding currents were periodically checked. Spontaneous activity is reported from cells with stable membrane potentials. In normal IC brain slices, spontaneous firing occurs at a rate of ∼0.2–0.5 spikes/s, with no noticeable spike clustering (Sivaramakrishnan and Oliver 2006). While uninfected mice in this study also exhibited similarly low spontaneous rates, FrCasE-infected mice exhibited periods of comparatively high rates of spontaneous activity (>3 spikes/s), followed by periods of quiescence. Periods of high firing and quiescence alternated. We therefore defined a high-firing period as a “burst.” Bursts were high-firing periods that lasted for 2 s, with >5 spikes within the time window of 2 s, and were followed by at least 1 s of quiescence. Interspike intervals within any given burst were not similar to interspike intervals in any other burst; thus we did not use either interspike interval or a fixed number of spikes to define a burst. For the purposes of this study, a single spike in a 2-s time window was not considered a burst.
Optical imaging with voltage-sensitive dyes.
Voltage-sensitive dye (VSD) recordings in IC slices have been described previously (Chandrasekaran et al. 2013) and are briefly outlined here. One hundred fifty-micrometer-thick slices were incorporated with the oxonol VSD NK3630 (or RH482; obtained from Nippon Kankoh-Shikiso Kenkyusho) dissolved in ACSF to a final concentration of 5–10 μg/ml for 90–120 min to produce even staining in the slice. Slices were washed in dye-free ACSF for at least 30 min to remove excess dye. For recordings, slices were transilluminated by light from a 100-W tungsten-halogen bulb passing through a 705-nm (BW 15 nm) filter to measure peak absorbance or a 640-nm (BW 15 nm) filter (Chroma) as a control. Slices were exposed to 705 nm only during the recording to prevent photodynamic damage and bleaching. Images were collected with a 464-element photodiode array of optical detectors and 8 additional analog channels (WuTech Instruments, Gaithersburg, MD). The diode array was mounted on an Olympus microscope fitted with a ×5 (dry, NA 0.1) objective lens. The optical signal from each detector was individually amplified 200 times, low-pass filtered at 333 Hz, and then multiplexed and digitized with 12 bit at 1,600 samples·s−1·channel−1. Frame rates with this array are ∼1,600 frames/s (interframe interval ∼0.614 ms). Data were collected and analyzed with NeuroPlex software (RedShirtImaging, Fairfield, CT) and Origin (OriginLab) and displayed as traces for numerical analysis and pseudocolor images for still time-lapse and real-time activity patterns. A digital camera fitted on to a second port was used to photograph the slice, on which VSD images were superimposed. Field potentials were used as an indicator of the stimulus current and were measured with a tungsten electrode placed in the dorsal or external cortices of the IC. Signals from the field electrode were passed through a differential amplifier (A-M Systems) and fed into an auxiliary (BNC) channel of the data-acquisition interface board. The resting light intensity (RLI; the background absorbance in the slice) was used to assess the uniformity of dye penetration in the slice and to control for changes in signal magnitude with bleaching. The RLI was subtracted from all data. Analysis of the VSD signal was limited to the region covering the IC. Normalizing VSD data to the RLI would take into consideration any differences in dye uptake by ICC neurons between uninfected and infected animals as well as between different slices. Subjectively, we did not notice differences between intensity of dye staining in infected animals. Infected animals do not appear to show toxic effects (slice degradation, poor response amplitudes), suggesting no additional toxicity.
Activity was measured in single pixels and plotted as traces. Recordings of spontaneous activity were collected for durations of 10–15 s. Traces illustrated are portions of these responses. Spontaneous activity occurred at random in the slice, so that the same pixel did not necessarily demonstrate activity during successive recordings. This random movement of activity made it difficult to quantify how many pixels demonstrated a certain specific feature, such as burst firing or slow depolarizations. Thus we collected spontaneous recordings several times in control conditions and with each drug and analyzed the recording by freezing it in 5-s intervals and counting the number of pixels showing either rapid bursts or slow depolarizations. We defined a burst as composed primarily of rapid spikelike events and slow depolarizations as events that gradually increased in amplitude or remained elevated for >100 ms. We plotted the average number of pixels showing rapid bursts, slow depolarizations, or no activity as a percentage of the total number of pixels covering the ICC. To illustrate spontaneous patterns, we picked pixels in which the activity was representative of the predominant pattern. Conclusions of a complete lack of activity were made if there were fewer than 5% of the pixels showing above-noise (S/N > 5) responses.
Stimulation of afferent input ascending through lateral lemniscus.
To evoke synaptically driven responses for both electrophysiological and VSD recordings, the lateral lemniscal (LL) nerve bundle was stimulated with an extracellular concentric bipolar electrode made from tungsten/platinum wire. The stimulus electrode was placed on the LL tract before it entered the IC. The location of the LL stimulus electrode allowed stimulation of LL fibers of passage from lower brain stem nuclei as well as those from the dorsal nucleus of the LL. Stimulation was performed with a multichannel stimulator (AMPI) through a constant-current isolation unit (WPI A365). Stimulus parameters were modified as needed to evoke specific response patterns.
Data acquisition and analysis.
Results are expressed as means ± standard error. Standard deviation, when used, is indicated in the text. Significance was determined with Student's t-test or ANOVA where pertinent: P < 0.05 with the Bonferroni correction factor applied. Trials were repeated several times (4–10 trials) on a single slice, and the average of that data was taken as the data for that particular slice. This was done for each slice in our sample. The averaged data from each slice were further averaged across all the slices. Means and standard errors, significance, and ANOVA were performed both on data within a slice and on the pooled data from the sample.
Measurement of auditory brain stem responses.
Auditory brain stem responses (ABRs) were evoked monaurally with pure tones and recorded with two tungsten wires (0.005-in. diameter, etched to ∼0.001-in. diameter) inserted under the skin, one behind an ear and the other on top of the head. ABRs were recorded under intramuscular anesthesia with ketamine (20–40 mg/kg) and xylazine (2.5 mg/kg). This anesthetic dose creates very “light” anesthesia and is critical to obtaining detectable ABRs at the higher frequencies in our testing protocol (Grimsley and Sivaramakrishnan 2014).
Custom software (Batlab; Dr. D. Gans, NEOMED) was used to generate tone bursts and record ABRs. Sound was delivered through a loudspeaker placed 10 cm in front of the animal at 15° to the midline. Acoustic stimuli were digitally synthesized and downloaded onto a digital signal processing card, converted to analog signals, filtered, attenuated, summed, amplified, and sent to a loudspeaker (Tucker-Davis Technologies). Acoustic output was calibrated over 10–120 kHz with a condenser microphone placed in a position normally occupied by the animal's head. At different sound frequencies, 0 dB attenuation corresponded to sound pressure levels as follows: 109 dB at 4 kHz, 101 dB at 40 kHz, 93 dB at 50 kHz, and 69 dB at 80 kHz. We used a maximum tone frequency of 64 kHz. Sound pressure levels were corrected for speaker drop-off. Harmonic distortion was not detectable 60 dB below the signal intensity with a fast Fourier analysis of the digitized microphone signal.
Tones (10–96 dB SPL in 5- or 10-dB steps) were used to evoke ABRs. Tones were 5 ms long, with a 0.5-ms cosine rise and a 15-ms intertone interval. Tones were sequentially delivered from 4 to 64 kHz and repeated 300 times at 4/s at each sound level. If the same pup was used more than once, we allowed 4–5 days between recordings. ABR protocols were repeated at least twice during each recording session. Responses were identified as ABRs if they were ≥4 SD of baseline noise. Waveform morphology was examined using the response evoked by the 16-kHz tone, which had the lowest threshold and was present at all sound intensities. Increases in intensity decreased the latencies of the different waves in the ABR and increased wave amplitude. Positive peaks were used in identifying the origin of each wave. We did not observe recording artifacts and therefore did not perform artifact rejection.
RESULTS
Env-dependent retroviral spongiform neuropathology in the IC shows rapid, stereotypic kinetics and regional specificity.
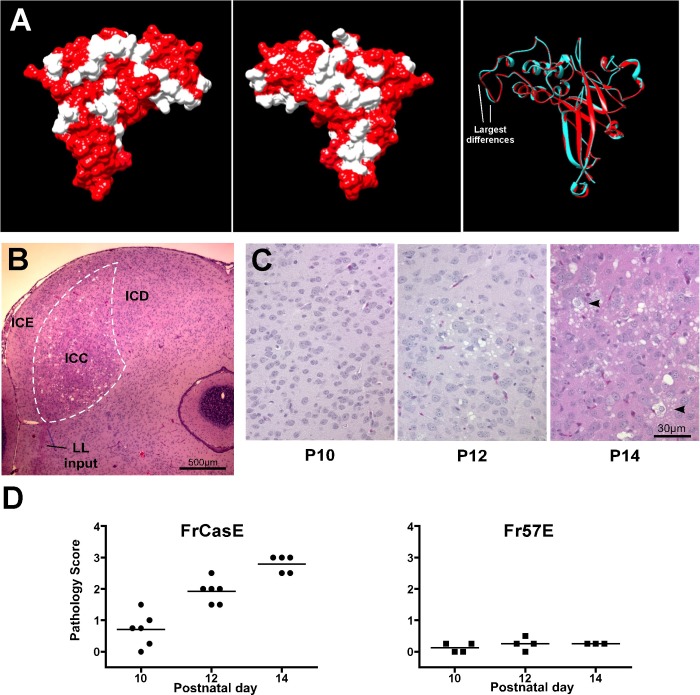
To understand the physiological basis for how abnormal proteins induce spongiform neurodegeneration, we focused our analysis on changes arising within the IC in response to infection by either FrCasE or the isogenic nonneurovirulent virus Fr57E (Dimcheff et al. 2006; Li et al. 2011). These two ecotropic viruses differ only within their respective env genes, with the most significant Env protein amino acid changes residing within the RBD (cf. Renszel et al. 2013). When mapped onto the crystal structure of Fr57 RBD (Fass et al. 1997) with I-TASSER (Roy et al. 2010), the sequence differences for the CasBrE RBD reside primarily in patches on the RBD surface (Fig. 1A). Despite these amino acid differences, the underlying backbone of the modeled CasBrE RBD is predicted to have only minor folding differences from that of the Fr57 RBD (Fig. 1B).
Fig. 1.
Structural comparison of murine leukemia virus (MLV) Env receptor binding domains (RBDs) and the associated spongiform neurodegeneration arising within the inferior colliculus (IC) with time. A, left: 2 views of the RBD crystal structure for the nonneurovirulent Fr57E Env (red; Fass et al. 1997) onto which has been projected the amino acid differences found in the neurovirulent CasBrE Env (white; DesGroseillers et al. 1984). The RBD constitutes the Env domain possessing the majority of amino acid differences and where genetic mapping studies suggest the primary neurovirulence determinants reside (cf. Murphy et al. 2004). Right: ribbon diagram alignment of the polypeptide backbone for Fr57 (red) with the backbone for CasBrE (blue) modeled with I-TASSER (Roy et al. 2010), indicating the likelihood of high underlying structural homology. The largest structural differences between loops (<0.5 Å) are indicated. B: histological assessment of the IC at the onset of clinical disease (P16) showing vacuolation concentrated in the central nucleus (ICC, white outline), with more limited changes in the external (ICE) and dorsal (ICD) cortices. The lateral lemniscal (LL) fiber tract provides the major ascending input to the IC. C: kinetic analysis of FrCasE-induced ICC spongiosis shows pathology arising as small vacuoles within the neuropil beginning at P10 (10 dpi) and progressing in severity with time to include cells with cytoplasmic effacement (arrowheads) by P14 (14 dpi). D: summary of IC pathology scores for mice infected with FrCasE (left) or the isogenic control virus, Fr57E (right). The stereotypic pathology progression for FrCasE in the IC is consistent with what has been reported for other brain regions (Czub et al. 1994). There was no ostensible IC vacuolation in mice infected with Fr57E.
Given that FrCasE is highly neurovirulent, while Fr57E is nonneurovirulent (Askovic et al. 2000; Dimcheff et al. 2006; Li et al. 2011), the question arises as to how two functionally and apparently structurally similar proteins could have such differing neurodegenerative effects within the CNS. To address this question, as well as to explore the basis for neuron subtype-specific pathogenesis, we initially asked whether the IC might be suitable for addressing these questions. When examined histopathologically at the time of clinical disease onset (P16), severe spongiosis (3–4+) was noted to be concentrated within the ICC (Fig. 1B) in FrCasE-infected animals (n = 3). Subsequent analysis of earlier time points showed that neuropil vacuolation initiated as early as P10, which progressed and came to include cells showing cytoplasmic effacement by P14 (Fig. 1C) consistent with other brain areas (Czub et al. 1994; Lynch et al. 1991; Portis et al. 1990). In contrast, Fr57E infection of IRW mice did not induce overt spongiosis during this period, in either the IC or other brain regions, as previously reported (Dimcheff et al. 2006; Li et al. 2011).
FrCasE and Fr57E infect NG2 glia and microglia in the IC.
To understand the relationship between neuropathology and viral Env expression, we performed immunostaining analysis on paraffin sections of the IC obtained from virus-infected mice between P10 and P14. FrCasE first infects vascular cells and spreads to ramified cells in the IC parenchyma by P12 (Fig. 2A), consistent with what has been previously reported for other brain areas (Czub et al. 1994). The ramified cells were identified as microglia and NG2 cells based on colocalization of Env with Iba-1 and the NG2 proteoglycan, respectively. Because infection of NG2 cells has only been examined previously in neural stem cell (NSC)-based brain chimeras, where the transplanted NSCs were NG2+ (Li et al. 2011), we examined whether both FrCasE and Fr57E viruses targeted these cells in the IC. Env expression was observed in NG2 cells for both viruses (Fig. 2B), a neurotropism consistent with their similar RBDs (Fig. 1A). As noted previously (Li et al. 2011; Lynch et al. 1991), neither FrCasE nor Fr57E Env proteins were observed in cells expressing CNPase or GFAP (not shown), despite the reported potential for NG2+ cells to differentiate into oligodendroglia or gray matter astrocytes (Zhu et al. 2008). Moreover, no Env expression was observed in IC cells expressing the neuronal markers HuC/D and NeuN (not shown), consistent with previous findings (Li et al. 2011), indicating that mitotic but not postmitotic neurons are infected by MLVs (Gravel et al. 1993; Lynch et al. 1991; Morey and Wiley 1990). To examine whether there was a spatial correlation between NG2 cell Env expression and spongiosis, Env/NG2 double-label immunohistochemistry was performed on IC sections in order to visualize coincident spongiosis. Env+/NG2+ cells were often found in close proximity to vacuoles (Fig. 2C). Whether this coincidence reflects direct degeneration of infected NG2 cells, as postulated for oligodendroglial progenitor cells (Clase et al. 2006), postsynaptic degeneration of NG2 cells, given documented synaptic input (Karadottir et al. 2008), or simple juxtaposition to ongoing neuronal degeneration could not be resolved by this light microscopic analysis.
Fig. 2.
Neuropathology correlates with Env expression in NG2 cells and microglia. A: epifluorescence examination of FrCasE infection in the IC during the window of neuropathology development. Vascular infection is evident by P10, whereas infection of microglia (Env+/Iba-1+; top) and NG2 cells (Env+/NG2+; bottom) becomes apparent only by P12, paralleling the overt appearance of spongiosis (Fig. 1). Arrowheads indicate ramified extravascular cells that coexpress Env (green) and the cell type-specific markers (red). B: examples of double-label immunostaining for NG2 (red) and MLV Env (green) at P14 demonstrate that both FrCasE and Fr57E infect NG2 glia in the IC. C: double-label immunohistochemistry of FrCasE-infected NG2 cells at P14 shows the close apposition of Env+/NG2+ (purple/brown) cells (black arrowheads) and vacuoles (white arrowheads). The Env+/NG2+ cell to right of the asterisk appears to be undergoing cytoplasmic effacement.
Intrinsic properties of rebound neurons change during pathology.
To identify the physiological changes accompanying the onset and development of pathology, we examined neuronal firing patterns in acutely isolated IC slices from FrCasE-infected, Fr57E-infected, and uninfected mice between P7 and P15. We first established that IC neurons in IRW mice could be distinguished by their intrinsic firing patterns before P11 by using previously published protocols in the rodent IC (Sivaramakrishnan and Oliver 2001). Intrinsic membrane properties were characterized by measuring the voltage responses to injected depolarizing and hyperpolarizing current pulses.
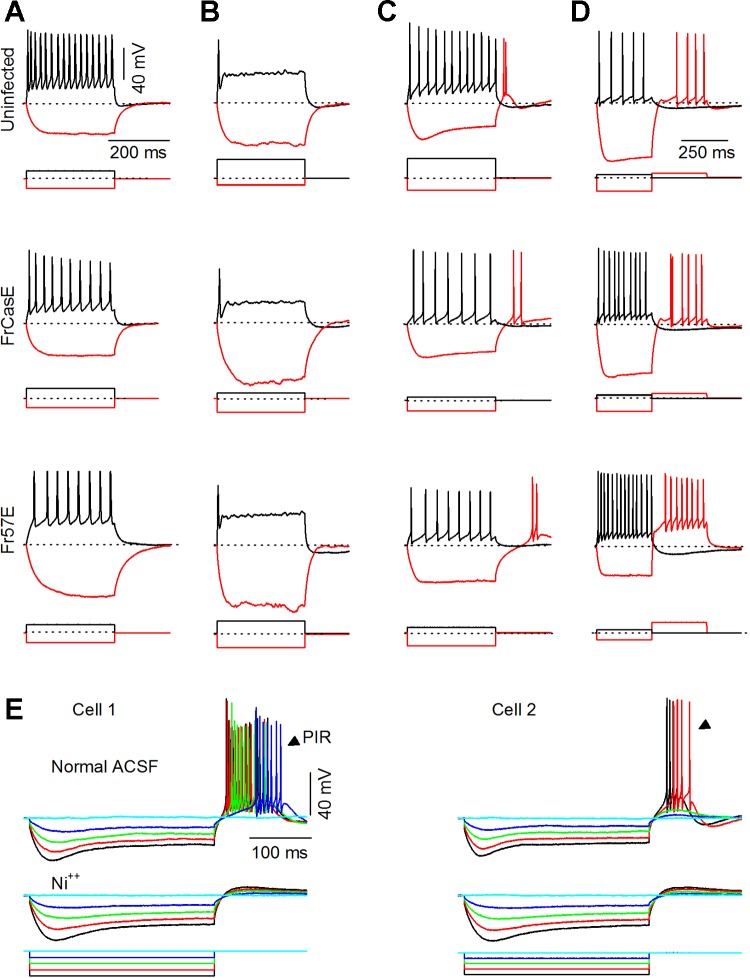
In both infected and uninfected mice, ICC neurons exhibited normal spiking by P9, and between P9 and P11, before the onset of spongiosis, cell types were distinguishable by recognizable firing patterns (uninfected, 19 cells; FrCasE, 28 cells; Fr57E, 12 cells). We placed cells into four broad groups based on their responses to a combination of depolarizing and hyperpolarizing current steps. Sustained-regular cells (28% of the total population) fired throughout the depolarizing step and rarely exhibited anode-break spikes upon recovery from hyperpolarization (Fig. 3A). Onset cells (6% of the population) responded with a single spike at the onset of depolarization and did not exhibit anode-break spikes upon recovery from hyperpolarization (Fig. 3B). Cells categorized as RNs (49% of the total population) exhibited PIR upon recovery from hyperpolarization (Fig. 3C). In response to depolarizing current steps, RNs displayed the expected variations in firing patterns (Sivaramakrishnan and Oliver 2001) and showed sustained firing (e.g., Fig. 3C) or transient firing (data not illustrated). For the purposes of this study, we pooled the different subtypes of RNs into a single category. The fourth category of cells exhibited a pause in firing followed by a buildup of depolarization and firing upon recovery from hyperpolarization. These pause-build cells (20% of the population) also exhibited sustained firing in response to depolarizing current steps (Fig. 3D). The use of both depolarizing and hyperpolarizing current steps to measure intrinsic firing patterns indicated that the subset of voltage-gated conductances that distinguish different ICC cell types (Sivaramakrishnan and Oliver 2001) appeared to function normally before the onset of pathology.
Fig. 3.
Intrinsic firing patterns are normal before spongiosis onset: intrinsic firing patterns evoked by somatic current steps between P9 and P10. Depolarizing and hyperpolarizing current steps used to identify physiological cell types in the ICC in uninfected mice (top) and after infection with FrCasE (middle) and Fr57E (bottom) are shown. Previously established cell type nomenclature: sustained-regular (A), onset (B), rebound (C), pause-build (D) (Sivaramakrishnan and Oliver 2001). Depolarizing steps evoke sustained (A, C, D) or onset (B) firing patterns. Hyperpolarizing currents evoke postinhibitory rebound firing at the offset of the current step in rebound cells (C) and no rebound firing in sustained-regular or onset cells (A and B). A depolarizing step that follows hyperpolarizing current evokes a pause and buildup in membrane potential before firing onset (D). E: dependence of postinhibitory rebound firing (PIR) on nickel: 2 examples of PIR (cell 1 and cell 2) from an uninfected IRW mouse (P40). Responses are shown to hyperpolarizing current injected into the soma (bottom). In addition to zero current (light blue trace), 4 hyperpolarizing steps of increasing magnitude were used to evoke rebound firing. Top: responses in normal ACSF show robust PIR (arrowheads). Middle: in 300 μM NiCl2 hyperpolarizing responses remain, but PIR is abolished.
To distinguish between anode-break spiking and PIR due to activation of T-type Ca2+ channels (Huguenard 1996), as occurs in RNs in the IC dorsal cortex (ICD) (Sun and Wu 2008), we tested the effects of nickel chloride (NiCl2), an antagonist of T-type Ca2+ channels (Ryu and Randic 1990), on RNs in older uninfected animals, where PIR is robust (n = 5 cells). Figure 3E illustrates recordings from two RNs in the ICC of 40-day-old uninfected IRW mice. PIR consisted of several spikes following recovery from hyperpolarization. Three hundred micromolar NiCl2 abolished PIR spiking completely, leaving a small rebound depolarization (5/5 cells), suggesting that PIR in ICC RNs may be dependent on multiple T-type Ca2+ subtypes.
During the appearance and progression of spongiosis (P11–P15), the fraction of the total sample of ICC neurons that comprised sustained-regular and pause-build cells did not change. Resting potentials in these cell types were unaffected, as were spike widths and outward current magnitudes (Table 1). Onset cells were not included in the analysis because they comprised a very small percentage (∼6%) of the total ICC population in the IRW mouse.
Table 1.
Properties of neuronal cell types in ICCs of IRW mice infected with FrCasE or Fr57E or uninfected
| No. of Cells, % of total sample |
Rm, mV |
Spike Width, ms |
Ri, MΩ |
τ, ms |
Maximum Steady-State Amplitude of Voltage-Gated Outward Currents, nA |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell Type | IRW* | FrCasE | Fr57E | IRW | FrCasE | Fr57E | IRW | FrCasE | Fr57E | IRW | FrCasE | Fr57E | IRW | FrCasE | Fr57E | IRW | FrCasE | Fr57E |
| Sustained-regular | 28% (14/48 cells) | 31% (39/127 cells) | 26% (10/39 cells) | −67 ± 5 | −65 ± 8 | −67 ± 5 | 2.1 ± 0.4 | 1.99 ± 0.6 | 1.8 ± 0.65 | 204 ± 28 | 189 ± 30 | 201 ± 23 | 33.6 ± 6 | 36 ± 8 | 38 ± 9 | 3.3 ± 0.4 | 2.9 ± 0.7 | 3.8 ± 0.8 |
| Pause-build | 20% (10/48 cells) | 23% (29/127 cells) | 18% (7/39 cells) | −64 ± 8 | −68 ± 4 | −64 ± 9 | 1.7 ± 0.3 | 1.9 ± 0.4 | 2.1 ± 0.3 | 162 ± 35 | 179 ± 42 | 158 ± 36 | 26 ± 5 | 28 ± 7 | 33 ± 6 | 3.9 ± 0.9 | 3.2 ± 0.9 | 3.6 ± 0.6 |
| Rebound | 49% (34/48 cells) | 26% (33/127 cells) | 46% (18/39 cells) | −55 ± 5 | −56 ± 7 | −60 ± 6 | 1.8 ± 0.3 | 2 ± 0.2 | 2.0 ± 0.5 | 116 ± 15 | 122 ± 38 | 125 ± 20 | 17.3 ± 8 | 15.6 ± 6 | 21 ± 6 | 2.8 ± 0.6 | 3.2 ± 0.6 | 3.4 ± 0.5 |
Values are means ± SE. ICC, central nucleus of inferior colliculus; Rm, membrane resistance; Ri, input resistance; τ, membrane time constant.
Inbred Rocky Mountain White strain mice not inoculated with virus.
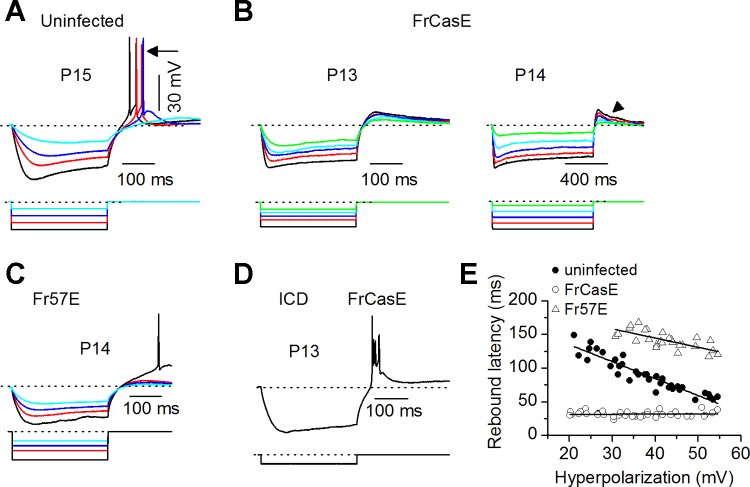
Beginning at P11 and persisting through P15 (the latest day on which recordings were made), however, specific changes occurred in the pattern of PIR in both the FrCasE- and Fr57E-infected ICC. The spiking following rebound from hyperpolarization, seen in uninfected mice (Fig. 4A), was absent in FrCasE-infected mice (Fig. 4B, left). Prolonging the hyperpolarizing current, which normally evokes PIR at lower current strengths (Sivaramakrishnan and Oliver 2001), did not evoke rebound spiking in the FrCasE-infected ICC (Fig. 4B, right). PIR loss occurred in ∼75% of RNs (23/33 cells) through the caudal to rostral extent of the ICC (n = 26 slices). Although PIR spiking was absent, RNs were still identifiable by a subthreshold rebound depolarization (Fig. 4B), which was evident through P15, the latest postnatal day on which recordings were made. In comparison, in uninfected mice subthreshold rebound depolarization without PIR spiking was observed in 5% of RNs between P9 and P11, when rebound spiking is still in the developing stages, a fraction similar to infected ICC. Between P11 and P15, however, PIR spiking in control animals became increasingly robust and purely subthreshold rebound depolarization without PIR spiking occurred in <1% of RNs. In Fr57E-infected mice, changes in RNs were less severe (Fig. 4C); PIR was not abolished, but spikes were delayed and required stronger hyperpolarization to elicit. RNs in the FrCasE ICD, however, showed normal PIR (Fig. 4D). ICD recordings from 5 RNs in uninfected, 12 in FrCasE, and 5 in Fr57E mice all showed similarly strong rebound. This result appears to be in accord with the reduced degree of spongiosis in the ICD (Fig. 1A), suggesting a differential susceptibility of ICD RNs to degeneration or, alternatively, lower metabolic rates due to less dense ICD vasculature compared with the central nucleus (Andrew and Paterson 1989; Faye-Lund and Osen 1985).
Fig. 4.
PIR is absent in FrCasE-infected mice. A: PIR evoked by somatic hyperpolarizing current steps. In uninfected rebound neurons (RNs), subthreshold rebound depolarization at low hyperpolarizing currents (light blue trace) becomes suprathreshold as hyperpolarization increases (dark blue, red, black traces). B: in FrCasE RNs, rebound depolarization remains subthreshold (left) even with prolonged hyperpolarization (right). C: in Fr57E RNs, PIR is delayed and requires strong hyperpolarization. D: PIR in ICD in FrCasE-infected mice is robust. E: rebound latency as a function of hyperpolarization magnitude. Latencies were measured from the end of the current pulse to the peak of the first rebound spike or the subthreshold depolarization. Black lines, linear regression. Slopes and r2 values: uninfected: −2.52, 0.89731; FrCasE:, −0.03, 0.59734; Fr57E, −1.32, 0.68392.
Of the total number of neurons patched in FrCasE ICCs, only 33 of 127 (26%) were identifiably RNs, constituting a ∼50% loss compared with uninfected mice (24/48 cells). Since there was no loss in the percentage of other cell types, these data implied that FrCasE infection resulted in either a net loss of 25% of ICC neurons or a loss in the functionality of 25% of the ICC neuron population. In contrast, 18 of 39 (46%) patched neurons in Fr57E ICCs were RNs, indicating no obvious loss and consistent with the lack of overt spongiosis associated with this virus (e.g., Fig. 1) (Askovic et al. 2000; Dimcheff et al. 2006).
PIR is typically evoked tens to a few hundreds of milliseconds after the hyperpolarizing input (Bertrand and Cazalets 1998; Felix et al. 2011; Harris-Warrick et al. 1995; Sivaramakrishnan and Oliver 2001). In IC slices, PIR latency, measured from the end of the hyperpolarizing current pulse to the peak rebound depolarization, varies with the magnitude and duration of the preceding hyperpolarization (Sivaramakrishnan and Oliver 2001). Normal intrinsic PIR mechanisms in the uninfected ICC were indicated by a decrease in rebound latency with hyperpolarizing strength, with a shift of ∼100 ms between the smallest hyperpolarization that evoked a rebound and the largest hyperpolarization (Fig. 4E). In the FrCasE-infected ICC, however, rebound latency did not change with increasing magnitude of hyperpolarization. The absolute value of the latency, ∼30 ms, was similar to the shortest control rebound latency (6 cells in each group analyzed; t11 = 0.95; P = 0.36). Thus FrCasE infection must affect a slow or delayed component of rebound depolarization but spare a rapid component. At the other end of the spectrum, rebound latency in the Fr57E ICC was greatly delayed, remaining above 120 ms even with large hyperpolarizations, and with a shallower change with the magnitude of hyperpolarization compared with the uninfected state. Since the timing of PIR results from a close coupling between two or more intrinsic mechanisms, involving either multiple calcium channels or calcium channels and hyperpolarization-activated cation conductances (Felix et al. 2011; Sekirnjak and du Lac 2002; Wang et al. 2011), the unchanging, very short, PIR latency following FrCasE infection suggests an uncoupling of the intrinsic components that underlie PIR or the loss of one or more of the components. The delayed PIR in Fr57E-infected mice, also indicative of aberrant timing, is suggestive of an intermediate step between the normal PIR in uninfected mice and its complete loss with FrCasE infection, and might indicate that Env differences (Fig. 1) differentially affect one or more components of the PIR machinery.
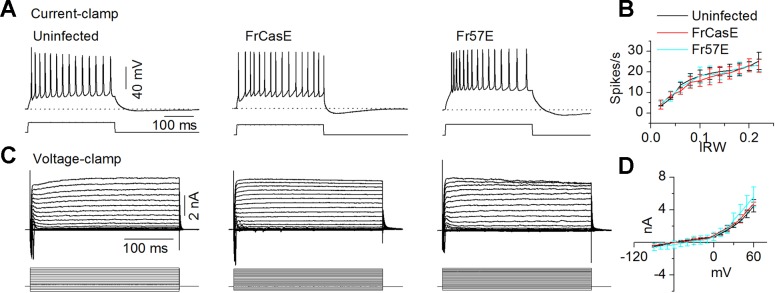
If the lack of rebound spiking following FrCasE infection was due to alterations in voltage-gated Na+ and K+ channels that underlie fast spiking, firing frequency during depolarizing currents should have been affected. Responses to depolarizing current pulses, as well as voltage-clamp recordings, suggested that FrCasE-induced PIR spike loss was not due to alterations in fast voltage-gated Na+ and K+ channels. Subtypes of RNs exhibit sustained responses to depolarizing current pulses (Sivaramakrishnan and Oliver 2001). These sustained responses were observed in uninfected as well as FrCasE- and Fr57E-infected mice (Fig. 5A). Spike frequencies increased with current strength, with no significant differences between uninfected and infected RNs (Fig. 5B; ANOVA, P = 0.37). Resting potentials, spike heights, and widths were also normal (Table 1). Steady-state amplitudes of outward currents were normal (Table 1), and outward currents rectified as in control RNs (Fig. 5, C and D, slope between 20 and 50 mV; ANOVA, P = 0.29). In addition, passive membrane time constants and input resistances were normal (Table 1), which suggested somatic membrane integrity.
Fig. 5.
Virus infection does not affect voltage-gated fast sodium and potassium currents. A: spikes in RNs evoked by depolarizing steps. Current magnitudes are approximately similar in the 3 recordings. Resting potential (RP): uninfected −62 mV, FrCasE −58 mV, Fr57E −59 mV. B: spike frequency increases with injected current, with no significant difference between uninfected and infected mice. Means and SD. C: currents recorded in RNs under voltage clamp in normal ACSF. Voltage steps from −90 mV to +100 mV. Holding potential was −60 mV. Same 3 cells as in A: IRW (P13), FrCasE (P13), Fr57E (P14). The small randomly occurring inward currents (center) are incompletely clamped sodium currents. D: steady-state current-voltage curves. Currents are measured 10 ms before the end of the voltage step. Outward currents rectify in all 3 mice. Data pooled during P11–P15. Number of cells: 8 IRW; 15 FrCasE; 7 Fr57E. Means and SD.
RNs are targets of FrCasE-induced PIR loss due to intrinsically low calcium buffering.
Because PIR spike loss was not directly due to loss of spike-generating conductances, a more likely feature affected by virus infection was the subthreshold rebound depolarization that underlies PIR in the ICC (Sivaramakrishnan and Oliver 2001). We tested the effects of virus infection on subthreshold rebound depolarization in the presence of 1 μM TTX, which blocks the sodium-dependent spike component of PIR. Subthreshold rebound depolarization in uninfected mice had two components (Fig. 6A, left). The first component was graded, could be evoked by small hyperpolarizations, and increased linearly with hyperpolarization magnitude (Fig. 6A, right). The second component was regenerative and evoked by larger hyperpolarizations, and its peak amplitude remained constant with changes in hyperpolarization magnitude (34.6 ± 3.2 mV; F5,50 = 0.39, P = 0.85; n = 11; n = 5 hyperpolarizations). In contrast, FrCasE RNs exhibited only the graded component regardless of the magnitude of hyperpolarization, reaching 13.5 ± 2.8 mV at large hyperpolarizations (n = 15) (Fig. 6B). Taken together with the lack of FrCasE effects on normal spike-generating currents, the loss of PIR in the FrCasE-infected ICC appears to occur through the reduction of the regenerative component of postinhibitory rebound depolarization.
Fig. 6.
Rescue of PIR in FrCasE-infected RNs by elevated calcium buffering. A: responses to hyperpolarizing currents in 1 μM TTX illustrate the loss of the regenerative rebound component (colored traces) in FrCasE RNs. B: peak rebound depolarization as a function of hyperpolarizing current. Bracketed region, regenerative component, which appears to be absent for FrCasE. Uninfected, 11 cells; FrCasE, 15 cells. Means and SE. C: recordings with raised EGTA in the internal solution. Left: 11 mM EGTA-5 mM Ca in the recording pipette. The 2 voltage traces in each panel (top to bottom, 1st to 4th panels) were evoked by the 2 hyperpolarizing current pulses (bottom). Recordings are shown over a 5-min period (top to bottom). The rebound depolarization is regenerative, with the same magnitude for both hyperpolarizing currents in the top trace and fails for the smaller current in the middle trace and for both current steps in the bottom trace. D, top: FrCasE RN recordings with 11 mM EGTA-5 mM Ca in the patch pipette (P14). Recordings are shown <1 min and ∼4 min after establishing whole cell access. For clarity, only a single voltage response is shown at <1 min. Bottom: spikes evoked by depolarizing current steps in FrCasE RNs appear normal over time in high EGTA. E: effect of 11 mM EGTA-5 mM Ca on a sustained-regular cell (P14) in the FrCasE ICC immediately (left) and 35 min (right) after establishing the whole cell configuration. The RP of the cell was changed to −40 mV with DC injection and the hyperpolarizing current strength adjusted until a rebound depolarization (arrowhead) was evoked. The response at right is evoked by a larger hyperpolarization than at left, to illustrate that the rebound does not become suprathreshold. F: summary of change in rebound depolarization in uninfected and FrCasE-infected RNs and FrCasE-sustained regular (SR) cells: 5 uninfected RNs, 8 FrCasE RNs, 3 FrCasE SRs. Means and SD.
Since the regenerative depolarization underlying PIR is calcium mediated in the ICC (Sivaramakrishnan and Oliver 2001), we tested the effect of intracellular calcium concentration on PIR. We recorded from RNs with pipette solutions containing elevated calcium buffering and slightly reduced free Ca2+ (11 mM EGTA-5 mM Ca: 70 nM free Ca2+ vs. 0.2 mM EGTA-0.1 mM Ca: 100 nM free Ca2+; see materials and methods). In uninfected mice, rebound depolarization decreased with time after break-in and, by 5–8 min, became subthreshold (Fig. 6C; n = 5 cells; peak amplitude of 9.5 ± 2.6 mV with a 30-mV hyperpolarization). This suggested that raising internal calcium buffering abolished the regenerative calcium response that underlay PIR. Normal PIR thus appears to depend on a level of internal Ca2+ that creates a balance between the free Ca2+ available to generate regenerative Ca2+ spikes and the high Ca2+ concentrations that inactivate the (presumably) L-type voltage-gated Ca2+ channels that provide the regenerative current required for PIR (Wang et al. 2011). In FrCasE RNs, recording with the 11 EGTA-5 Ca pipette solution had the reverse effect to the uninfected PIR and resulted in an increase in the amplitude of rebound depolarization. With a hyperpolarization of 30 mV from the resting potential, the amplitude of the rebound depolarization increased by 12.6 ± 4.3 mV (n = 8 cells; t7 = 4.2, P = 0.002), and rebound spikes reappeared within 3–8 min of break-in (Fig. 6D, top). The opposing effects of lowering the internal Ca2+ concentration in uninfected RNs (decrease in rebound depolarization) and in FrCasE-infected RNs (increasing the rebound depolarization) occurred to the same extent (10.8 ± 2.9; uninfected, FrCasE: t12 = 0.3; P = 0.77) (Fig. 6F), suggesting that changing internal calcium did not produce nonlinear effects. This similarity implies that the rebound depolarization remaining in both uninfected and FrCasE-infected RNs was resistant to high internal Ca2+ concentrations, and was likely due to T-type Ca2+ channels that are less likely to undergo Ca2+-dependent inactivation (Iftinca and Zamponi 2009). The restoration of PIR in FrCasE RNs by high EGTA therefore suggests that free Ca2+ levels are elevated in these cells, potentially inactivating L-type Ca2+ channels. When averaged across the population, the slight reduction in internal free Ca2+ with high buffering did not change spike rates during depolarization (Fig. 6D; bottom; t15 = 1.11; P = 0.28). We cannot, however, rule out small changes in the large- or small-conductance Ca2+-activated K+ channels in RNs (Sivaramakrishnan and Oliver 2001).
We examined whether 11 EGTA-5 Ca pipette solutions increased the likelihood of rebound firing in other cell types in the FrCasE ICC. Sustained-regular cells, for example, can generate small subthreshold rebound depolarizations when the resting membrane potential is depolarized by ∼25 mV, and cells are hyperpolarized beyond normal levels (to > −100 mV). The small rebound depolarization generated in sustained-regular cells was not significantly affected by 11 EGTA-5 Ca recording solutions (Fig. 6, D and F; n = 3 cells; P = 0.48). PIR in ICC RNs thus appears to depend on maintaining a delicate balance of internal calcium, whose modulation would be facilitated by an inherently low capacity to buffer calcium. This property likely explains the increased vulnerability of RNs to degeneration, an idea in accord with the selective vulnerability of motor neurons that possess low intrinsic calcium buffering in ALS (Appel et al. 2001; Hegedus et al. 2007; Pun et al. 2006; Vanselow and Keller 2000). Low calcium buffering might be a ubiquitous aspect of neuronal vulnerability in proteopathies.
FrCasE IC infection induces spontaneous bursting in RNs.
Previous ultrastructural studies on FrCasE-induced neurodegeneration suggest an excitotoxic etiology based on postsynaptic vacuolation (Lynch et al. 1991). To test for spontaneous hyperexcitability, we recorded activity in the absence of stimulation. A subset of RNs in FrCasE-infected ICCs (13/33 cells) exhibited high levels of spontaneous firing, characterized by periods of silence and bursts (Fig. 7A, top, D, and E). Bursting lasted as long as the recording remained viable (>60 min), with minimal rundown. Sustained-regular neurons did not exhibit changes in spontaneous activity in FrCasE ICCs (Fig. 7D, right), suggesting that other ICC cell types were resistant to virus-induced hyperexcitability. In Fr57E-infected mice, spontaneous activity was sparse and consisted mainly of prolonged accumulation of subthreshold depolarization and occasional spiking similar to uninfected mice (Fig. 7, A, middle, and D). Recordings with 11 EGTA-5 Ca in the patch pipette abolished FrCasE-associated bursting (Fig. 7, B and E). Bursting was also suppressed by holding cells at −90 mV (Fig. 7E), suggesting abnormally high internal calcium levels at rest. FrCasE-induced RN bursting did not appear to be due to calcium entry through NMDARs, which are activated at resting potentials in some ICC neurons (Ma et al. 2002; Sivaramakrishnan and Oliver 2006). Bath application of the NMDAR antagonist D-APV (100 μM) did not significantly alter burst rates (Fig. 7, C and E; n = 3). NMDARs were functionally active in FrCasE RNs, however, as synaptic responses evoked by stimulating the LL nerve tract were reduced by 100 μM D-APV (t5 = 3.51, P = 0.02; data not shown).
Fig. 7.
Calcium-dependent spontaneous hyperexcitability in FrCasE RNs. A: records of spontaneous firing in RNs. Top: spontaneous firing in FrCasE RNs characterized by bursts and silent periods. Bursts were defined as continuous firing (≥2.5 Hz) for at least 2 s, followed by a quiescent period ≥ 1 s. Middle: Fr57E RN with low levels of spontaneous spiking and regions of accumulating depolarization (asterisks) but no suprathreshold bursts. Bottom: uninfected RN with subthreshold synaptic potentials (asterisks) without accumulating depolarization and low spontaneous spiking. Postnatal ages and RPs: FrCasE: P13, −62 mV; Fr57E: P14, −59 mV; uninfected, P14, −58 mV. B: spontaneous firing in a P13 FrCasE RN with 11 mM EGTA-5 mM Ca in the internal recording solution at <1 min (top) and 5 min (bottom) after whole cell access. Note that spontaneous firing is lost but subthreshold synaptic potentials remain (asterisks). C: D-APV (100 μM) does not reduce spontaneous firing or the prolonged depolarizations observed between spikes (asterisks) in a FrCasE RN. P14; RP −59 mV. Top: recording prior to bath application of D-APV. D, left: spontaneous firing in ICC RNs averaged over the whole recording window. Data pooled over P11–P16. No. of cells: 9 FrCasE, 8 uninfected, 7 Fr57E. Means and SD. ANOVA, ***P < 0.05. Right: spontaneous spike rates in uninfected and FrCasE sustained-regular cells are shown for comparison (t7 = 0.68; P = 0.52; n = 4 uninfected, 4 FrCasE). E: burst spike frequency in ICC RNs under the conditions outlined in A–C; effects at −90 mV membrane potential are included. Data pooled over P11–P16. Means and SD. No. of cells: ACSF: 9 FrCasE, 8 uninfected, 7 Fr57E; 4 FrCasE APV (100 μM); 6 FrCasE EGTA; 5 FrCasE. ANOVA: uninfected vs. Fr57E, P = 0.36; uninfected vs. FrCasE, P < 0.00001; FrCasE APV vs. control, P = 0.13; FrCasE EGTA vs. control, P = 0.00002; FrCasE normal RP vs. −90 mV, P < 0.000001. F, left and center: confocal image stack of a P13 FrCasE RN that showed spontaneous firing and PIR loss filled with biocytin during recording and detected with Alexa Fluor 488 conjugated-streptavidin postfixation. Arrows, areas of dendritic swelling. Right: biocytin-filled RN from an uninfected ICC showing normal morphology.
Labeling patched cells with Alexa Fluor 488 conjugated with neurobiotin indicated no apparent damage to the somatic region in RNs in the FrCasE ICC (Fig. 7F). This somatic integrity is in accord with the normal input resistances (Table 1). However, distal dendritic processes occasionally exhibited focal regions of swelling, with areas that appeared broken or thinned and then reformed (Fig. 7F, left and center). RN dendrites in uninfected mice appeared more homogeneous with few irregularities (Fig. 7F, right). Thus hyperactive RNs appear to comprise at least one component of the progressive neuronal vacuolation induced by FrCasE infection.
RN-based local circuits are specifically disrupted with FrCasE IC infection.
Since RNs constitute almost half the ICC neuronal population, PIR disruption and decreases in neuron number would be expected to affect local circuits. To examine the spatiotemporal effects of PIR loss, we used VSDs in uninfected and FrCasE mice to image responses over the entire central nucleus on one side of the IC in brain slices. We activated afferent lemniscal inputs with long (>200 ms) stimulus trains, a stimulus pattern that evokes synaptically driven PIR in single ICC neurons recorded under current clamp (S. Sivaramakrishnan, unpublished observations) and isolates RN-dependent circuits from those encoding information along frequency laminae in ICC slices (Chandrasekaran et al. 2013).
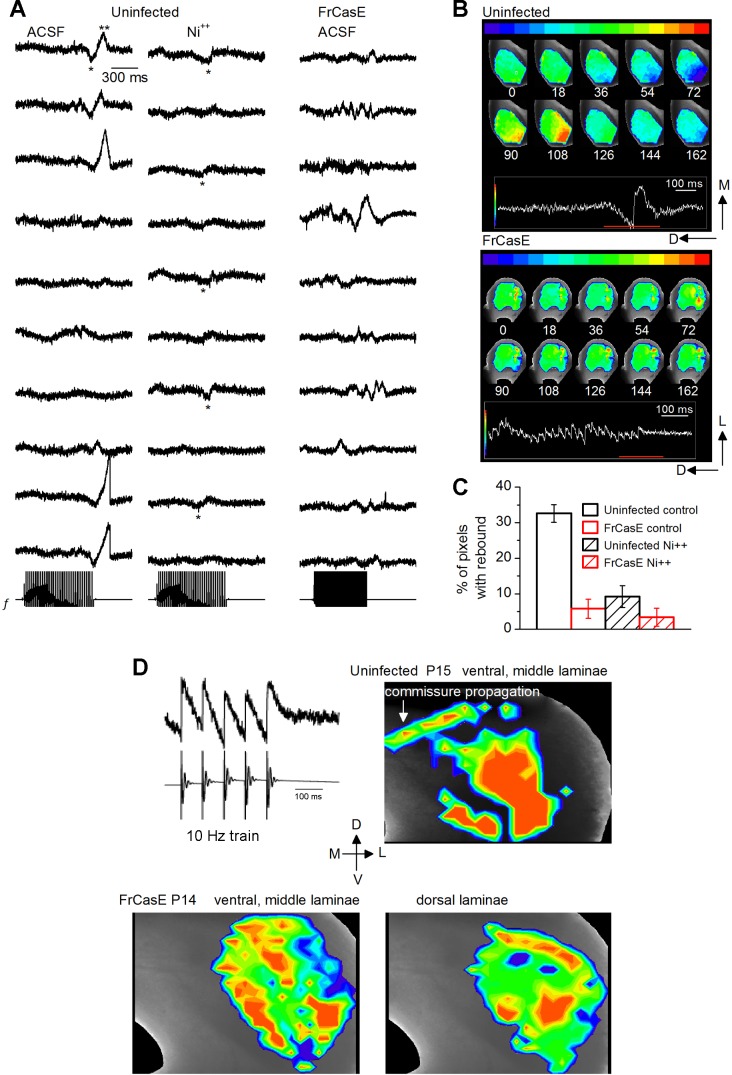
In uninfected mice, long stimulus trains evoked robust PIR. Stimulus current strengths were adjusted to keep responses during the train subthreshold. VSD traces of synaptically evoked PIR, examined in single pixels, exhibited a subthreshold response during the train, with an accumulation of inhibition or hyperpolarization that resulted in rebound spiking (n = 14 slices). In normal ACSF, responses in different pixels illustrated these rebound motifs as consisting of an inhibition followed by a rebound excitation (Fig. 8A; left, e.g., top 3 traces and bottom 2 traces). Not all pixels exhibited a rebound; pixels in which a rebound was absent exhibited primarily subthreshold responses, with little sign of oscillatory behavior (Fig. 8A, left, e.g., 5th–8th traces from top). To verify that the inhibition-excitation motif at the end of the stimulus train was due to PIR, NiCl2 was routinely applied in all recordings. In the presence of 300 μM Ni2+, the positive peak of the rebound motif was either absent or greatly reduced (Fig. 8A, center). Inhibitory potentials that normally precede rebound excitation were present in some response areas (Fig. 8A, center), indicating that the effect of Ni2+ was primarily on PIR rather than on its inhibitory drive. In the FrCasE-infected ICC, the majority of pixels did not exhibit a clear rebound motif (Fig. 8A, right). Rebound motifs were observed occasionally (Fig. 8A, right, e.g., 4th trace from top), and responses during the stimulus train were varied, with some oscillatory activity (Fig. 8A, right, e.g., 2nd trace from top). NiCl2 did not affect the oscillations (traces not illustrated; summary data in Fig. 8C).
Fig. 8.
FrCasE infection causes specific loss of PIR-driven IC circuits. A–C: rebound firing evoked by lemniscal stimulation with a 40-Hz train. A: voltage-sensitive dye (VSD) traces of rebound activity in individual pixels in the uninfected ICC (P15) (left and center) and in an FrCasE-infected ICC slice (right). Bottom: field recording of the stimulus (f). Uninfected ICC: the rebound motif consists of inhibition (single asterisks) followed by PIR spiking (double asterisks). B: time-lapse images of rebound activity visualized over the whole slice in the uninfected (top) and FrCasE-infected (bottom) ICC slice. Frames between 0 and 162 ms in top and bottom panels span the regions corresponding to the red line in insets attached to each panel. Times 0 and 162 ms (first and last frames) correspond to the frames at the start and end of the red line, respectively. Images are shown at 18-ms intervals (30 frames between successive images; 0.6-ms frame rate). Times at which frames are displayed are indicated below each image. In the uninfected ICC, rebound firing is marked by an inhibitory area (dark blue region, e.g., frames at 54 and 72 ms) that switches to an excitatory area (red/yellow, e.g., frames at 90 and 108 ms). In the FrCasE ICC, the PIR motif was absent at the end of the train. Uninfected, P15; FrCasE, P14. C: fraction of pixels exhibiting rebound. Stimulus trains were repeated 3 or 4 times, and for each repetition the number of pixels showing rebound was divided by the total number of pixels covered by the ×5 objective. This fraction was then averaged over the number of repetitions. Uninfected, 14 slices; FrCasE, 12 slices. Means and SD. D: lemniscal stimulation evokes responses in different frequency regions of the IC. Top left: VSD traces of responses to activation of LL input with a 10-Hz train of 5 pulses shows a depolarizing response that locks to each stimulus in the train. VSD panels: recordings of activity in different frequency regions of the IC as indicated by each panel. Top right: uninfected ICC. Response bands along the direction of fibrodendritic laminae in the ventral and middle to dorsal regions and propagation through the commissure. Bottom: FrCasE ICC. Images are taken at 2 time points to illustrate ventral and middle laminae (left) and dorsal laminae (right).
In a 150-μm-thick slice, with a ×5 objective each photodetector (pixel) covers a 150-μm cube (which includes the surface area of 150 × 150 μm2 and the 150 μm depth/thickness of the slice) and records responses from ∼260 soma and neuropil (Chandrasekaran et al. 2013). Activity in one direction (e.g., excitatory or inhibitory) within a specific pixel implies a strongly synchronous activation of the neuronal clusters within that pixel. The appearance of a rebound motif within single pixels suggests that there may be small regions in the ICC, defined by the volume covered by a single pixel, that have a predominant population of rebound cells. This possible clustering of RNs appears to give rise to the excitatory-inhibitory feedback loops generated by synaptically evoked rebound firing in the ICC (Chandrasekaran et al. 2013).
During the PIR phase of the response, VSD responses exhibited a characteristic switch between excitation and inhibition in overlapping regions of the slice (Fig. 8B, top right, inset trace region corresponding to the red line; Supplemental Movie S1, inset: stimuli 7–10 of the train).1 VSD images showed responses to the rebound excitation (red; frames between 90 and 108 ms) and the preceding hyperpolarization (blue; frames between 36 and 72 ms) overlapping similar areas of the slice. These responses began in the ventral ICC where the lemniscal fibers enter and spread outward toward the medial ICC. The extent of spread is unique to the strength of the lemniscal shock, and the lack of a banding pattern that would coincide with the 45° alignment of the fibrodendritic laminae is characteristic of the spread of rebound-driven activity in the normal ICC (Chandrasekaran et al. 2013). Since the VSD technique used here records absorbance changes in both the columns and rows of cells covered by each pixel (see materials and methods), responses from the blue to red spectrum imply changes from net inhibitory to net excitatory activity. Synchronized inhibition (e.g., frame at 72 ms, blue) preceded synchronized excitation (frame at 108 ms, red), which then reverted to a more quiescent state (frames at 126–144 ms). This switch between excitation and inhibition characterizes rebound-driven circuits in the normal ICC slice (Chandrasekaran et al. 2013) and here took ∼12 ms (n = 12 slices). In P14 FrCasE-infected mice, the same stimulus was unable to elicit synchronous rebound activity, or a clear rhythmic switch between excitation and inhibition (Fig. 8B, bottom right; Supplemental Movie S2). Lemniscally driven synaptic responses were still evident (red areas, e.g., frame at 72 ms; Supplemental Movie S2, inset trace), but the rebound motif was absent.
In the uninfected ICC, rebound spikes occurred in 32.6 ± 6.6% of the total pixels in the slice (n = 14 slices; ages P13–P16) (Fig. 8C). This area is less than would be expected from the proportion of RNs in the ICC (∼50%) (Sivaramakrishnan and Oliver 2001) and thus underestimates the total rebound population. Since a clear rebound motif would be observed only in pixels with a dominant density of rebound cells, pixels with fewer RNs compared with other ICC cell types would be excluded from the total number of pixels showing rebound activity. In the FrCasE ICC, the number of pixels exhibiting a rebound motif dropped to 5.8 ± 2.7% of the total number of pixels (n = 12 slices; P13–P16). It was unclear whether the loss of the rebound motif and the rhythmic circuit resulted from just the loss of PIR spiking or the loss of RNs themselves.
To examine whether FrCasE infection affected activity in ICC frequency laminae, we stimulated lemniscal inputs with 10-Hz trains designed to evoke activity in topographically distinct areas without generating rebound-driven rhythmic activity (Chandrasekaran et al. 2013). This stimulus pattern evokes a 1-to-1 stimulus-response pattern (Fig. 8B, top left), with VSD images showing banded responses both in the uninfected ICC (Fig. 8B, top right; n = 6 slices) and in the FrCasE ICC even at P12–P14 (Fig. 8B, bottom; n = 11 slices). These results suggested that the lower-frequency dorsal as well as higher-frequency ventral ICC were functional in P14–P15 FrCasE-infected mice (Fig. 8B, bottom). These banding patterns correspond to anatomically defined ICC frequency laminae, thought to be controlled by the distribution of ascending inputs (Brown et al. 1997; Morest and Oliver 1984). Thus lemniscal input segregation appears to be relatively intact in the FrCasE-infected ICC. The loss of rhythmic circuits is therefore less likely due to a loss of lemniscal input. Regardless of the precise mechanism, these data indicate that FrCasE glial infection causes a widespread functional loss of ICC circuits driven by PIR, while sparing some subset of neuronal connections along frequency laminae.
Widespread spontaneous bursting in the FrCasE ICC is blocked by antagonists of L-type calcium channels.
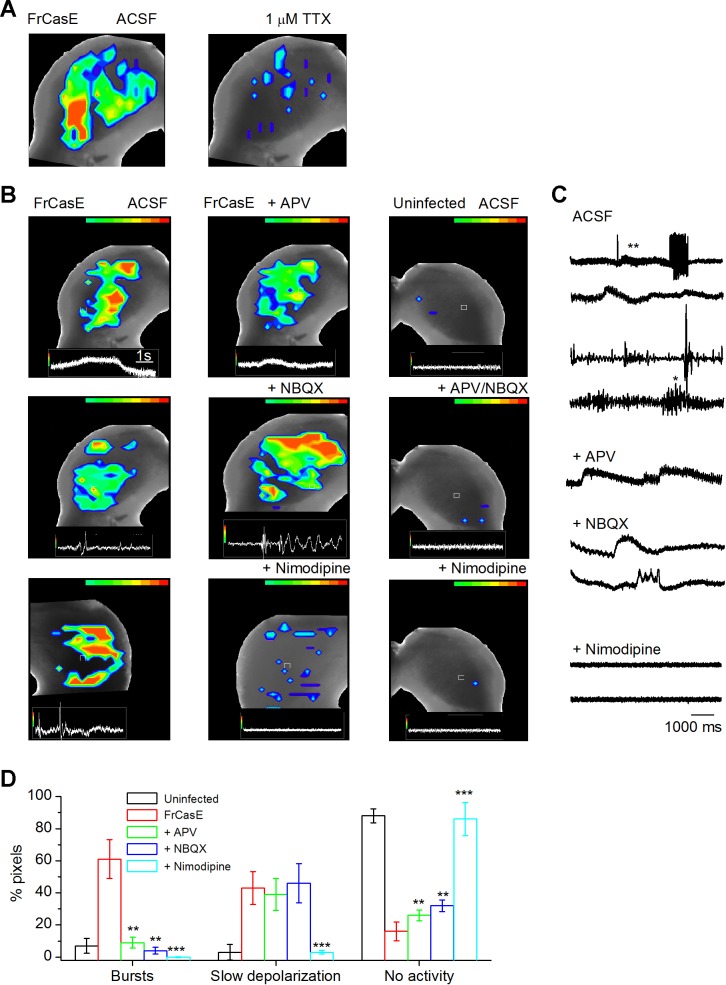
To examine whether the spontaneous hyperexcitability observed in single RNs were isolated events, we used VSD imaging to examine activity over the ICC without stimulating lemniscal inputs (8 uninfected, 17 FrCasE-infected slices). In FrCasE-infected mice, spontaneous activity was widespread in the ICC and occurred randomly within and across the different frequency regions (dorsal to ventral) (Fig. 9A, left; 13/17 slices for P14–P16 showed spontaneous activity; 4 slices between P9 and P12 did not show spontaneous activity). Bath-applied TTX eliminated spontaneous activity (Fig. 9A, right; 4/4 slices tested), suggesting the involvement of intrinsic or synaptically evoked sodium spikes.
Fig. 9.
Spread of spontaneous activity in the FrCasE-infected ICC. A, left: VSD recording of spontaneous activity in the FrCasE-infected ICC above background (S/N > 5) (P14). Right: TTX abolished spontaneous activity. B: single representative frames chosen to illustrate activity in the FrCasE-infected ICC (left and center) and uninfected ICC (right). Left: 3 examples (top to bottom) of FrCasE-induced spontaneous activity in normal ACSF; top and middle panels show activity in the same slice taken during different time windows to illustrate variations in the types of spontaneous activity patterns, and bottom panel is a different slice. Center: effects of added D-APV (100 μM), NBQX (10 μM), or nimodipine (10 μM). Right: drug treatment does not cause oscillations in the ICC of uninfected mice. Insets at bottom of each image illustrate the responses in the pixel depicted by the white square in the image. Scale bar in top left image applies to all VSD panels in B. C: VSD recordings from individual pixels chosen to illustrate different activity patterns: burst firing (single asterisk) and slow depolarizations (double asterisk) in the FrCasE ICC in normal ACSF (top). D-APV 100 μM and NBQX 10 μM abolish much of the short duration bursting seen in normal ACSF; however, slow depolarizations remain. Nimodipine 10 μM abolishes most spontaneous activity. D: summary of spontaneous activity in the FrCasE-infected ICC in the conditions indicated. Each drug, D-APV, NBQX, and nimodipine, was applied separately to different slices; n = 3 for each drug. Means and SD. Statistics are illustrated for comparisons between FrCasE and each drug (asterisks). Paired t-test with Bonferroni correction, P < 0.0166 (0.05/3) used as significance. **P < 0.00001; ***P < 10−5. Differences between uninfected and nimodipine were statistically insignificant (bursts: P = 0.63; slow depolarizations: P = 0.72; no activity; P = 0.78).
Spontaneous activity exhibited diverse temporal and spatial patterns between slices and between areas of a particular slice. In the slice shown in Supplemental Movie S3, for example, bursts of activity (Supplemental Movie S3 inset, initial part of trace) that occurred over most of the ICC were followed by sustained hot spots of depolarizations in the ventral, lateral, medial, and dorsal regions of the ICC (Fig. 9B, left, and C). Sporadic bursts of activity were observed in different areas at different times, sometimes synchronizing into large responses (Supplemental Movie S3). Activity was not constricted to fibrodendritic laminae; thus much of the spontaneous activity likely occurred in ICC neurons or their local circuits, rather than hyperactive input lemniscal axons. In contrast, the signal-to-noise ratio of optical activity in control slices was not resolvable (Fig. 9B, right; Supplemental Movie S4), consistent with the low spontaneous activity noted during patch recordings.
To determine whether glutamatergic transmission played a role in spontaneous activity, we examined VSD images after bath application of NMDAR and AMPAR antagonists (100 μM D-APV and 10 μM NBQX, respectively; n = 3 slices; P14/P15). Both antagonists, applied in isolation, reduced short-duration bursting; however, prolonged depolarizations were still evident (Fig. 9B, center; Supplemental Movies S5 and S6). With D-APV, for example, while disparate hot spots were evident, responses sometimes included more synchronous activity that continued over several seconds (Supplemental Movie S5; white and gray pixels and inset trace). Addition of NBQX did not eliminate hot spots, and depolarizations remained prolonged (Supplemental Movie S6). A separate analysis of bursts and prolonged depolarizations (see materials and methods) indicated that bursting was reduced by both D-APV and NBQX; however, prolonged depolarizing episodes remained (Fig. 9D). Neither antagonist altered background activity in uninfected mice (Fig. 9B, right). Glutamatergic transmission thus appears to give rise to bursts in the FrCasE-infected ICC. Prolonged depolarizations unaffected by glutamate receptor antagonists are likely to originate from intrinsic neuronal firing of different cell types within a pixel. Such firing may be desynchronized, as expected from different cell types with unique membrane properties (Sivaramakrishnan and Oliver 2001), and the signal within the pixel would be temporally dispersed and could manifest as a net prolonged depolarization.
Since prolonged depolarizations remained in glutamate receptor antagonists, we examined the effects of an antagonist of L-type calcium channels (nimodipine, 10 μM) on spontaneous activity (n = 6 slices). Nimodipine abolished all activity in the FrCasE ICC (6/6 slices), both bursts and prolonged depolarizations being reduced to baseline levels similar to the uninfected ICC (Fig. 9, B, bottom center and right, C, and D; Supplemental Movie S7). FrCasE-induced hyperexcitability was therefore dependent at least in part on calcium entry through L-type calcium channels. This effect of nimodipine was observed whether it was applied in isolation (Fig. 9B, bottom, C, and D) or after application of NBQX and D-APV (n = 5 slices; data not shown), suggesting that the activation of L-type calcium channels that contributed to hyperexcitability occurred independently of the plateau potential evoked by NMDAR activation (Sivaramakrishnan and Oliver 2006; Wang et al. 2013).
Auditory brain stem thresholds are raised after FrCasE infection.
FrCasE-infected animals express widespread brain stem spongiosis during the preclinical period; in addition to the IC, auditory brain stem regions affected include the dorsal cochlear nucleus (Czub et al. 1994). Thus it is unclear whether hearing thresholds would be affected. To assess whether FrCasE infection resulted in detectable changes in hearing, we measured ABRs through the preclinical period, using pure tones over a wide range of sound frequencies (4–64 kHz) (Grimsley and Sivaramakrishnan 2014) that covers a large fraction of the frequencies represented in the IC of the normal mouse (Grimsley et al. 2013).
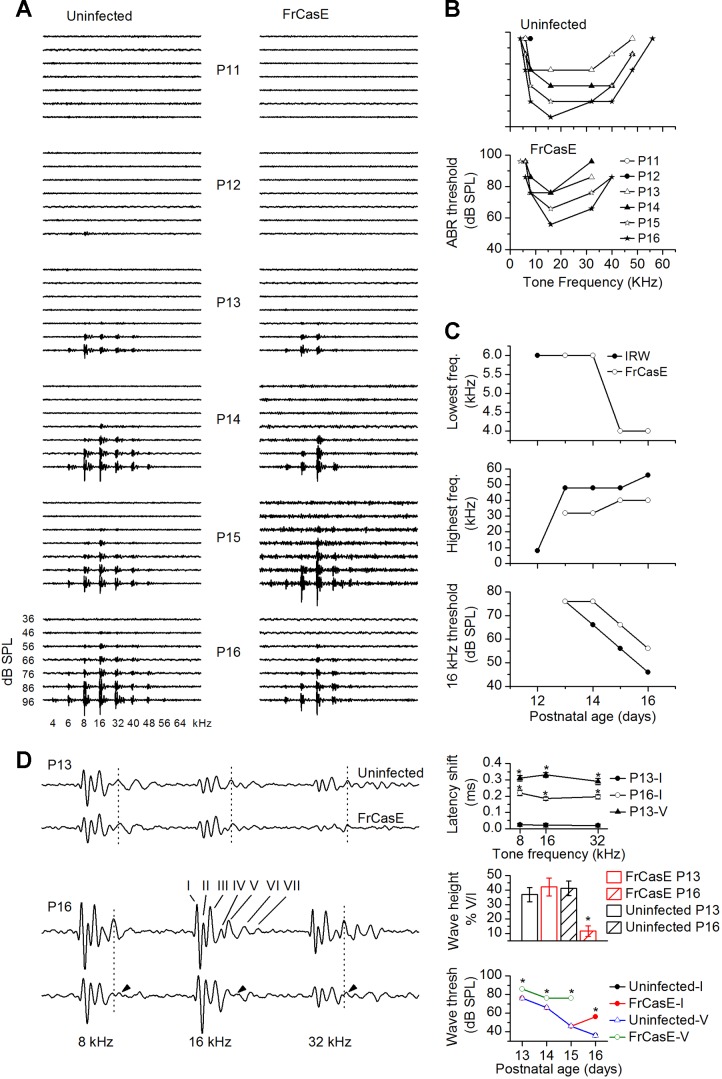
ABRs were recorded each day between P9 and P16 (3 mice at each postnatal age). In uninfected mice, ABRs were first evident between P12 and P13, with a response at 16 kHz occurring at 96 dB SPL. The frequency range increased between P13 and P15 (Fig. 10A). Responses to the lowest frequency tested, 4 kHz, first occurred at P15, which is a postnatal onset similar to that in the normal-hearing CBA/Ca mouse (Grimsley and Sivaramakrishnan 2014). Uninfected mice continued to develop a normal ABR profile, with a response to 54 kHz becoming evident at P16 (Fig. 10A, left bottom, B, and C, middle) and a response to 64 kHz by P19 (data not shown). ABR thresholds decreased with age, reaching the lowest values (e.g., 46 dB SPL to the 16-kHz tone) at P16 (Fig. 10C, bottom).
Fig. 10.
FrCasE-infected mice exhibit early-onset, high-frequency hearing loss. A: auditory brain stem responses (ABRs) measured at postnatal ages indicated in uninfected (left) and FrCasE (right) mice; 4-ms tones were presented at 36–96 dB SPL at the frequencies indicated. A lack of responses at frequencies >40 kHz is apparent at P13. B: ABR thresholds plotted as a function of postnatal age. Early-onset high-frequency hearing loss is evident in FrCasE-infected mice; 3 mice at each postnatal age. C: lowest (top) and highest (middle) frequencies observed at any sound level. Bottom: ABR threshold at 16 kHz as a function of postnatal age is higher in FrCasE mice. D, left: ABR traces at 96 dB SPL at P13 and P16 for uninfected (top trace of each pair) and FrCasE-infected (bottom trace of each pair) mice. Traces are expanded to illustrate responses at 8, 16, and 32 kHz. Waves I–VII are marked for the response to 16 kHz at P16 in uninfected mice. Vertical dotted lines, wave V corresponding to the IC response. Right: latencies of waves I and V, ratio of heights of wave V to wave I, and thresholds of waves I and V. Latencies of the peaks of waves I and V were corrected for differences in the onset times of the ABR. Average of 3 mice for each data point. Means and SD. *P < 0.05.
Hearing onset in FrCasE-infected mice occurred at normal developmental ages (P12–P13). However, we did not observe responses developing at the higher frequencies. At P14, responses above 32 kHz were absent (Fig. 10, A and C, middle). Responses to the 40-kHz tone had developed by P15 and remained at P16; however, the frequency response range did not widen further to include the responses to 48 kHz and 56 kHz observed in uninfected mice at the same postnatal ages. ABR thresholds, measured at 16 kHz, were raised at postnatal ages > P13.
We examined ABR waves I and V, corresponding to the auditory nerve and the IC/lemniscal input, respectively (Melcher et al. 1996; Wada and Starr 1983) at P13 and P16, ages reflecting the onset and late stage of pathology. For analyses, we used ABRs at 96 dB SPL, between 8 and 32 kHz, when the waves were most pronounced (Fig. 10D, left). At P13, waves I–V were still clearly discernible in FrCasE-infected mice (Fig. 10D, top). At P16, wave heights decreased in infected mice, and FrCasE wave V (Fig. 10D, bottom) could not be consistently resolved above background at different tone frequencies or in different animals. FrCasE wave V was therefore greatly reduced or absent. These results suggested that FrCasE infection affected ABRs slightly from the auditory nerve to the LL but had a very strong effect on the IC. Latencies, heights, and thresholds of ABR waves I and V confirmed that FrCasE infection had a stronger effect on the IC than on the auditory nerve (n = 3 mice each for uninfected and FrCasE-infected groups). At P13, the latency of FrCasE wave I did not shift relative to the uninfected wave I at either of the three frequencies (8, 16, 32 kHz) tested (ANOVA, P = 0.56). By P16, the FrCasE wave I was delayed by ∼0.2 ms from the uninfected wave I (P < 0.05 at each frequency), and this delay was similar across tone frequencies (ANOVA, P = 0.23). At P13, the FrCasE wave V, with a shift of 0.3 ms from the uninfected wave V, was delayed more than wave I (P < 0.02) (Fig. 10D, top left). Since FrCasE wave V was absent at P16, we did not get an estimate of its delay.
Peak heights of waves I and V decreased by similar amounts at P13 in FrCasE-infected mice (9.2 ± 3.1% and 8.7 ± 5.3%; t5 = 0.27; P = 0.77). At P16, the peak height of FrCasE wave I decreased by 26.4 ± 5.1%. FrCasE waves II–IV were still present and showed a decrease similar to FrCasE wave I (21 ± 4.8%) (Fig. 10D, left). Wave V, however, decreased by >93% (t5 = 17.9; P < 10−5). When wave V was plotted as a fraction of wave I, a significant drop in the peak amplitude of wave V was observed only at P16 (Fig. 10D, right middle). Waves I and V were evoked at identical thresholds in uninfected mice (76–36 dB SPL from P13 to P16); FrCasE wave I had an elevated threshold at P16 (56 dB SPL), and FrCasE wave V had an elevated threshold at all ages (86, 76, 76 dB SPL at P13, P14, P15; P < 0.05). A stronger effect on wave V compared with wave I could conceivably arise from the increase in input convergence between the periphery and CNS auditory nuclei, and a greater reduction in wave V could reflect the effects of increased convergence in amplifying peripheral degeneration, rather than greater degeneration in the IC per se.
FrCasE infection thus resulted in a failure to acquire the high-frequency components of hearing, raised hearing thresholds at midsound frequencies, and had a stronger effect on the IC than on lower brain stem regions of the auditory pathway.
DISCUSSION
In this study we demonstrate the neurophysiological consequences of abnormal protein expression within the CNS in a rapid retroviral model of spongiform motor neuron disease. Central to our understanding is the concept that PIR converts tonic inhibitory input to excitatory drive and generates pattern generation in the brain (Chandrasekaran et al. 2013; Sohal et al. 2006; Soltesz and Deschenes 1993; Ylinen et al. 1995) and spinal cord (Kiehn 2006). PIR strongly influences the timing of spikes on rebound from an inhibitory input and allows neurons to adjust their excitability to the level of inhibition (Goaillard et al. 2010). Here we show that neurons capable of PIR are selectively affected after neuroglial expression of MLV Env proteins, with the specific effects being Env conformer dependent. Our data imply that RN excitability and its modulation by inhibitory input are regulated by glia. Glial regulation appears to stem from the low inherent calcium buffering capacity of RNs, a feature that also appears to be required for PIR. These data support previous findings of glial regulation of rhythmic locomotor activity, which occurs through pathways that involve glial vesicle trafficking, ionic gradients, and calcium levels (Ng et al. 2011). Our data indicate that, in the presence of neurovirulent Env-expressing glia, RN calcium becomes elevated, leading to loss of PIR, hyperexcitability, burst firing, dendritic swelling, and RN loss. Single-neuron dysfunction is transferred to local rhythmic circuits, while sparing other neuronal cell types and microcircuits in the same degenerative environment. Dye injections into IC RNs, which show degenerative signs primarily in the distal dendrites and normal somatic input resistances and membrane time constants, suggest that vacuolation does not reach the somatic region until late in the development of the disease when clinical signs are manifest.
Consistent with their highly congruous RBD structures, both CasBrE and Fr57 Envs were observed to affect RN activity, with both having effects on PIR latency. However, the RN responses to these Envs were at opposite ends of the spectrum, with Fr57 Env increasing latency and FrCasE Env decreasing it. These divergent effects of two structurally similar proteins on hyperpolarization-rebound coupling suggests that modulation of temporal precision in rebound circuits in the auditory system (Felix et al. 2011; Kuwada and Batra 1999) and elsewhere (Selverston and Moulins 1985) could involve glial regulation. RNs in the ICD were unaffected by either virus, and this region showed almost no spongiosis. In addition to the possibility that glia in the dorsal cortex may be refractory to neurotoxic Env, or the demonstrated sparser vascularization of the dorsal cortex compared with the central nucleus (Andrew and Paterson 1989; Faye-Lund and Osen 1985), differences in endogenous calcium buffers in the mouse IC, primarily calbindin and calretinin in the ICD and parvalbumin in the ICC (Idrizbegovic et al. 1999; Zettel et al. 1997), might be a contributing factor to the differential intrinsic vulnerability of RNs to degeneration. Like the ICC, neurons in the deep cerebellar nucleus, which also undergo FrCasE-mediated degeneration (Czub et al. 1994), express parvalbumin (Baurle et al. 1998). Neurons in the cerebellar cortex that display infection, but resist degeneration (Lynch et al. 1991), express parvalbumin in addition to calbindin and calretinin (Bastianelli 2003). It seems probable that the absolute calcium buffering capacity of a neuron determines its susceptibility to viral infection. However, cobuffers within a single neuron might confer resistance to degeneration.
The opposing effect of high EGTA/low internal Ca2+ on PIR in uninfected and infected RNs is a key finding that suggests a possible mechanism for RN vulnerability. These data suggest that RN vulnerability arises from an endogenously low calcium buffering capacity rather than altered calcium homeostasis. Spontaneous bursting modes have components that depend on calcium homeostasis (Roussel et al. 2006), and a subpopulation of RNs that lose PIR are also hyperexcitable, supporting the view that the decreased rebound depolarization is due to altered intracellular calcium levels. EGTA is a slow calcium buffer and does not affect the rapid calcium transients accompanying calcium influx; thus its effect in restoring rebound depolarization in infected RNs and quieting hyperexcitability must be due to prevention of calcium channel inactivation by raised intracellular calcium levels. The inability of RNs to generate PIR in FrCasE-infected mice would suggest that the virus caused a functional decrease in the T- or L-type calcium channels that underlie the rebound depolarization (Fan et al. 2000; Liu and Shipley 2008). The small rebound hump that remains in both IRW and FrCasE RNs suggests normal calcium entry through T-type calcium channels, which are not subject to calcium-dependent inactivation (Iftinca and Zamponi 2009). The vulnerability of RNs likely arises from an inherently low calcium buffering capacity and the requirement for two successively evoked calcium currents underlying PIR generation. VSD recordings indicate that the L-type calcium channel blocker nimodipine quiets spontaneous bursting in FrCasE IC RNs. This suggests that an indirect target of glial infection may be due to calcium-dependent inactivation of L-type calcium channels in RNs. Hyperactivity in the ICC, for example in genetically epilepsy-prone rats, can arise through elevation in α1D- and α1E-subunits of L- and R-type calcium channels (N'Gouemo et al. 2010). While the majority of dihydropyridine-sensitive L-type calcium channels activate at relatively depolarized potentials, a significant class of nimodipine-sensitive, noninactivating, L-type calcium channels are low voltage activated (Avery and Johnston 1996). Low-voltage-activated nimodipine-sensitive L-type calcium channels would activate in the range of depolarizations generated by calcium entry through T-type calcium channels (∼10–20 mV from the resting potential); thus it is conceivable that ICC RNs use low-voltage-activated T- and L-type calcium channels to generate PIR.
Activation of calcium channels and low levels of spontaneous hyperexcitability in remote dendritic regions may not be detectable with our whole cell recordings in ICC soma, and population recordings with VSDs would provide a more realistic assessment of global spontaneous hyperexcitability than recordings from the soma of single neurons. Thus other cell types in the ICC may be exhibiting low levels of spontaneous activity that are quieted by nimodipine. Nonetheless, our data indicate that RNs show a much higher degree of hyperexcitability than other ICC cell types.
An interesting aspect of FrCasE effects is the preservation of activity along fibrodendritic laminae in the ICC. Lemniscal input is apparently at least partly intact. Since we recorded mainly intrinsic effects in this study, the effect of FrCasE infection on the excitatory-inhibitory input balance and local synaptic connections within and across laminae remains untested. The ABRs suggest that wave IV, corresponding to the LL, is not greatly affected by P16. Thus ascending information continues to be carried into the IC during extreme pathology. Wave V, which corresponds to the IC and/or the lemniscal tract as it enters the IC, is greatly reduced, out of proportion to the reduction in wave I. Since intrinsic membrane firing patterns in most cell types are not affected by FrCasE, the loss of wave V could include loss of synaptic connections within the ICC, in addition to a decrease in the net acoustic input. The increase in spontaneous activity in the FrCasE ICC was partly reduced by antagonists of both NMDARs and AMPARs, suggesting that excitatory input remains at least partially intact. Thus, while our data indicate that FrCasE infection targets rebound circuits and spares activity along fibrodendritic laminae, we cannot rule out further effects on local synaptic connections or on the excitatory-inhibitory balance.
The vulnerability of RNs, through their low intrinsic calcium buffering, is supported by studies in ALS and genetic mouse models, where motor neuron vulnerability correlates with low levels of endogenous calcium buffers (Hegedus et al. 2007; Pun et al. 2006). Sporadic ALS is associated with the presence of high levels of serum antibodies to L-type calcium channels that are capable of modeling motor neuron disease in vitro (Li et al. 2004; Li and Bennett 2003), suggesting that alterations in L-type channels may underlie multiple diseases with motor neuron phenotypes. Although glutamate-mediated excitotoxicity has been implicated in ALS, attempts to treat disease with glutamate receptor antagonists have met with only modest success to date. NMDAR-mediated calcium entry does not appear to be responsible for FrCasE-induced hyperexcitability, despite the fact that ICC NMDARs are active at resting membrane potentials (Sivaramakrishnan and Oliver 2006; Wu et al. 2004).
Our results support the idea that local glial infection alters neuronal properties. Much prior work has been directed toward evaluating the role of microglia in disease, especially given their potential to release neurotoxic factors when activated. However, FrCasE-infected microglia do not show an activated phenotype in vitro (Dimcheff et al. 2006; Lynch et al. 1994) or in vivo (Lynch et al. 1991), and conditions that activate these cells appear to prevent spongiosis (Lynch et al. 1995). Gene array analyses of MLV-infected microglia fail to reveal virus-induced changes that account for CNS neuropathology (Dimcheff et al. 2006). Thus it is still unclear whether infection of these cells is a proximal causative factor. The infection of oligodendroglia has also been correlated with spongiosis (Clase et al. 2006; Morey and Wiley 1990). However, neurovirulent virus expression in oligodendroglia and/or microglia is not sufficient for disease (Li et al. 2011). In the present analysis we did not observe oligodendroglia Env expression. However, we did observe infection of NG2 glia, cells capable of differentiating into oligodendroglia (Zhu et al. 2008). In addition to acting as progenitor cells, NG2 cells persist through adulthood and tile the CNS in a fashion analogous to astrocytes, and have been proposed to constitute the fourth glial element (Nishiyama 2007).
Infection of NG2 glia was observed temporally coincident with electrophysiological changes and spatially coincident with spongiosis, suggesting that functional alteration of this cell type could play a role in compromising RN activity. Understanding how NG2 cells could affect RNs is complicated by their potential to differentiate into oligodendroglia, gray matter astrocytes (Zhu et al. 2008), and/or possibly neurons (Dimou et al. 2008), but it is of particular interest that as NG2 cells they receive synaptic input (Bergles et al. 2000) and fire action potentials (Karadottir et al. 2008) (reviewed in Nishiyama et al. 2009). A functional role of NG2 glia in the mature CNS has yet to be defined, and it is unknown how Env expression in these cells might contribute to disease. Virus infection may disrupt NG2 cell differentiation, or cells may degenerate as a result of neurovirulent virus infection (Clase et al. 2006). The lack of detectable infection in astrocytes and oligodendroglia, which arise from NG2 cells, could simply reflect downregulation of viral gene expression upon their differentiation. More sensitive detection methods will be needed to resolve this issue.
In summary, the current neurophysiological analysis of the rapid FrCasE retroviral model of spongiosis has provided novel insight into how abnormal proteins induce selective neurodegeneration within the CNS. The findings suggest that regulation of PIR and excitability in neurons characterized by low inherent calcium buffering are being specifically disrupted by glial expression of a unique Env conformer. Because both PIR and bursting behavior are required in functioning rhythmic circuits and motor systems, small calcium changes at the single-neuron level are translated into loss of function and abnormal circuit properties. The specific signaling mechanisms by which glia regulate RNs and their circuits, and how Env proteins disrupt this regulatory system, remain open questions. Our findings suggest this involves L-type calcium channels in addition to excitatory synaptic pathways. Given the robust neurophysiological changes revealed here, further exploitation of this retroviral model should provide more detailed insight into normal neuron-glia interactions and the potential glial origin of neurodegenerative disorders. Because of their ability to selectively affect RNs and their circuits, and to become manifest as unique behavioral and cognitive phenotypes characteristic of human neurodegenerative diseases, Env proteins, small-molecule inhibitors, and mimics may serve as novel tools for drug discovery and therapeutic intervention.
GRANTS
This work was supported by National Institutes of Health Grants R01 DC-008120 (S. Sivaramakrishnan) and R01 NS-37614 (W. P. Lynch) and a State of Ohio research incentive award (W. P. Lynch and S. Sivaramakrishnan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.L., R.A.D., S.S., and W.P.L. performed experiments; Y.L., S.S., and W.P.L. analyzed data; Y.L., R.A.D., S.S., and W.P.L. interpreted results of experiments; Y.L., S.S., and W.P.L. approved final version of manuscript; S.S. and W.P.L. conception and design of research; S.S. and W.P.L. prepared figures; S.S. and W.P.L. drafted manuscript; S.S. and W.P.L. edited and revised manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank J. Stenger, J. Shivers, and S. Cardona for technical help and C. Grimsley for auditory brain stem recordings.
Present address of Y. Li: Johns Hopkins University, Baltimore, MD.
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- Andrew DL, Paterson JA. Postnatal development of vascularity in the inferior colliculus of the young rat. Am J Anat 186: 389–396, 1989. [DOI] [PubMed] [Google Scholar]
- Appel SH, Beers D, Siklos L, Engelhardt JI, Mosier DR. Calcium: the Darth Vader of ALS. Amyotroph Lateral Scler Other Motor Neuron Disord 2, Suppl 1: S47–S54, 2001. [PubMed] [Google Scholar]
- Askovic S, McAtee FJ, Favara C, Portis JL. Brain infection by neuroinvasive but avirulent murine oncornaviruses. J Virol 74: 465–473, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery RB, Johnston D. Multiple channel types contribute to the low-voltage-activated calcium current in hippocampal CA3 pyramidal neurons. J Neurosci 16: 5567–5582, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastianelli E. Distribution of calcium-binding proteins in the cerebellum. Cerebellum 2: 242–262, 2003. [DOI] [PubMed] [Google Scholar]
- Baszler TV, Zachary JF. Murine retroviral neurovirulence correlates with an enhanced ability of virus to infect selectively, replicate in, and activate resident microglial cells Am J Pathol 138: 655–671, 1991. [Erratum. Am J Pathol 138 (May): 1058, 1991]. [PMC free article] [PubMed] [Google Scholar]
- Baurle J, Hoshi M, Grusser-Cornehls U. Dependence of parvalbumin expression on Purkinje cell input in the deep cerebellar nuclei. J Comp Neurol 392: 499–514, 1998. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405: 187–191, 2000. [DOI] [PubMed] [Google Scholar]
- Bertrand S, Cazalets JR. Postinhibitory rebound during locomotor-like activity in neonatal rat motoneurons in vitro. J Neurophysiol 79: 342–351, 1998. [DOI] [PubMed] [Google Scholar]
- Brown M, Webster WR, Martin RL. The three-dimensional frequency organization of the inferior colliculus of the cat: a 2-deoxyglucose study. Hear Res 104: 57–72, 1997. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran L, Xiao Y, Sivaramakrishnan S. Functional architecture of the inferior colliculus revealed with voltage-sensitive dyes. Front Neural Circuits 7: 41, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B, Britt W, Evans L, Wehrly K, Nishio J, Cloyd M. Characterization of monoclonal antibodies reactive with murine leukemia viruses: use in analysis of strains of Friend MCF and Friend ecotropic murine leukemia virus. Virology 127: 134–148, 1983. [DOI] [PubMed] [Google Scholar]
- Clase AC, Dimcheff DE, Favara C, Dorward D, McAtee FJ, Parrie LE, Ron D, Portis JL. Oligodendrocytes are a major target of the toxicity of spongiogenic murine retroviruses. Am J Pathol 169: 1026–1038, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czub S, Lynch WP, Czub M, Portis JL. Kinetic analysis of spongiform neurodegenerative disease induced by a highly virulent murine retrovirus. Lab Invest 70: 711–723, 1994. [PubMed] [Google Scholar]
- DesGroseillers L, Barrette M, Jolicoeur P. Physical mapping of the paralysis-inducing determinant of a wild mouse ecotropic neurotropic retrovirus. J Virol 52: 356–363, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimcheff DE, Volkert LG, Li Y, DeLucia AL, Lynch WP. Gene expression profiling of microglia infected by a highly neurovirulent murine leukemia virus: implications for neuropathogenesis. Retrovirology 3: 26, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou L, Simon C, Kirchhoff F, Takebayashi H, Gotz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci 28: 10434–10442, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YP, Horn EM, Waldrop TG. Biophysical characterization of rat caudal hypothalamic neurons: calcium channel contribution to excitability. J Neurophysiol 84: 2896–2903, 2000. [DOI] [PubMed] [Google Scholar]
- Fass D, Davey RA, Hamson CA, Kim PS, Cunningham JM, Berger JM. Structure of a murine leukemia virus receptor-binding glycoprotein at 2.0 angstrom resolution. Nature 277: 1662–1666, 1997. [DOI] [PubMed] [Google Scholar]
- Faye-Lund H, Osen KK. Anatomy of the inferior colliculus in rat. Anat Embryol 171: 1–20, 1985. [DOI] [PubMed] [Google Scholar]
- Felix RA, 2nd, Fridberger A, Leijon S, Berrebi AS, Magnusson AK. Sound rhythms are encoded by postinhibitory rebound spiking in the superior paraolivary nucleus. J Neurosci 31: 12566–12578, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goaillard JM, Taylor AL, Pulver SR, Marder E. Slow and persistent postinhibitory rebound acts as an intrinsic short-term memory mechanism. J Neurosci 30: 4687–4692, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravel C, Kay DG, Jolicoeur P. Identification of the infected target cell type in spongiform myeloencephalopathy induced by the neurotropic Cas-Br-E murine leukemia virus. J Virol 67: 6648–6658, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsley CA, Sanchez JT, Sivaramakrishnan S. Midbrain local circuits shape sound intensity codes. Front Neural Circuits 7: 174, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsley CA, Sivaramakrishnan S. Postnatal developmental changes in the medial nucleus of the trapezoid body in a mouse model of auditory pathology. Neurosci Lett 559: 152–157, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen R, Czub S, Werder E, Herold J, Gosztonyi G, Gelderblom H, Schimmer S, Mazgareanu S, ter Meulen V, Czub M. Abundant defective viral particles budding from microglia in the course of retroviral spongiform encephalopathy. J Virol 74: 1775–1780, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM, Coniglio LM, Barazangi N, Guckenheimer J, Gueron S. Dopamine modulation of transient potassium current evokes phase shifts in a central pattern generator network. J Neurosci 15: 342–358, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegedus J, Putman CT, Gordon T. Time course of preferential motor unit loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 28: 154–164, 2007. [DOI] [PubMed] [Google Scholar]
- Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol 58: 329–348, 1996. [DOI] [PubMed] [Google Scholar]
- Idrizbegovic E, Bogdanovic N, Canlon B. Sound stimulation increases calcium-binding protein immunoreactivity in the inferior colliculus in mice. Neurosci Lett 259: 49–52, 1999. [DOI] [PubMed] [Google Scholar]
- Iftinca MC, Zamponi GW. Regulation of neuronal T-type calcium channels. Trends Pharmacol Sci 30: 32–40, 2009. [DOI] [PubMed] [Google Scholar]
- Karadottir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci 11: 450–456, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay DG, Gravel C, Robitaille Y, Jolicoeur P. Retrovirus-induced spongiform myeloencephalopathy in mice: regional distribution of infected target cells and neuronal loss occurring in the absence of viral expression in neurons. Proc Natl Acad Sci USA 88: 1281–1285, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci 29: 279–306, 2006. [DOI] [PubMed] [Google Scholar]
- Kuwada S, Batra R. Coding of sound envelopes by inhibitory rebound in neurons of the superior olivary complex in the unanesthetized rabbit. J Neurosci 19: 2273–2287, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol 90: 857–869, 2003. [DOI] [PubMed] [Google Scholar]
- Li Y, Cardona SM, Traister RS, Lynch WP. Retrovirus-induced spongiform neurodegeneration is mediated by unique central nervous system viral targeting and expression of env alone. J Virol 85: 2060–2078, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91: 767–783, 2004. [DOI] [PubMed] [Google Scholar]
- Liu S, Shipley MT. Multiple conductances cooperatively regulate spontaneous bursting in mouse olfactory bulb external tufted cells. J Neurosci 28: 1625–1639, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WP, Brown WJ, Spangrude GJ, Portis JL. Microglial infection by a neurovirulent murine retrovirus results in defective processing of envelope protein and intracellular budding of virus particles. J Virol 68: 3401–3409, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WP, Czub S, McAtee FJ, Hayes SF, Portis JL. Murine retrovirus-induced spongiform encephalopathy: productive infection of microglia and cerebellar neurons in accelerated CNS disease. Neuron 7: 365–379, 1991. [DOI] [PubMed] [Google Scholar]
- Lynch WP, Portis JL. Murine retrovirus-induced spongiform encephalopathy: disease expression is dependent on postnatal development of the central nervous system. J Virol 67: 2601–2610, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WP, Robertson SJ, Portis JL. Induction of focal spongiform neurodegeneration in developmentally resistant mice by implantation of murine retrovirus-infected microglia. J Virol 69: 1408–1419, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WP, Sharpe AH, Snyder EY. Neural stem cells as engraftable packaging lines can mediate gene delivery to microglia: evidence from studying retroviral env-related neurodegeneration. J Virol 73: 6841–6851, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WP, Snyder EY, Qualtiere L, Portis JL, Sharpe AH. Late virus replication events in microglia are required for neurovirulent retrovirus-induced spongiform neurodegeneration: evidence from neural progenitor-derived chimeric mouse brains. J Virol 70: 8896–8907, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CL, Kelly JB, Wu SH. AMPA and NMDA receptors mediate synaptic excitation in the rat's inferior colliculus. Hear Res 168: 25–34, 2002. [DOI] [PubMed] [Google Scholar]
- McAtee FJ, Portis JL. Monoclonal antibodies specific for wild mouse neurotropic retrovirus: detection of comparable levels of virus replication in mouse strains susceptible and resistant to paralytic disease. J Virol 56: 1010–1022, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher JR, Knudson IM, Fullerton BC, Guinan JJ, Jr, Norris BE, Kiang NY. Generators of the brainstem auditory evoked potential in cat. I. An experimental approach to their identification. Hear Res 93: 1–27, 1996. [DOI] [PubMed] [Google Scholar]
- Morest DK, Oliver DL. The neuronal architecture of the inferior colliculus in the cat: defining the functional anatomy of the auditory midbrain. J Comp Neurol 222: 209–236, 1984. [DOI] [PubMed] [Google Scholar]
- Morey MK, Wiley CA. Immunohistochemical localization of the neurotropic ecotropic murine leukemia virus in moribund mice. Virology 178: 104–112, 1990. [DOI] [PubMed] [Google Scholar]
- Murphy SL, Honczarenko MJ, Dugger NV, Hoffman PM, Gaulton GN. Disparate regions of envelope protein regulate syncytium formation versus spongiform encephalopathy in neurological disease induced by murine leukemia virus TR. J Virol 78: 8392–8399, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N'Gouemo P, Yasuda R, Faingold CL. Seizure susceptibility is associated with altered protein expression of voltage-gated calcium channel subunits in inferior colliculus neurons of the genetically epilepsy-prone rat. Brain Res 1308: 153–157, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagra RM, Masliah E, Wiley CA. Synaptic and dendritic pathology in murine retroviral encephalitis. Exp Neurol 124: 283–288, 1993. [DOI] [PubMed] [Google Scholar]
- Ng FS, Tangredi MM, Jackson FR. Glial cells physiologically modulate clock neurons and circadian behavior in a calcium-dependent manner. Curr Biol 21: 625–634, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A. Polydendrocytes: NG2 cells with many roles in development and repair of the CNS. Neuroscientist 13: 62–76, 2007. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci 10: 9–22, 2009. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Yang Z, Butt A. Astrocytes and NG2-glia: what's in a name? J Anat 207: 687–693, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peruzzi D, Sivaramakrishnan S, Oliver DL. Identification of cell types in brain slices of the inferior colliculus. Neuroscience 101: 403–416, 2000. [DOI] [PubMed] [Google Scholar]
- Portis JL, Czub S, Garon CF, McAtee FJ. Neurodegenerative disease induced by the wild mouse ecotropic retrovirus is markedly accelerated by long terminal repeat and gag-pol sequences from nondefective Friend murine leukemia virus. J Virol 64: 1648–1656, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun S, Santos AF, Saxena S, Xu L, Caroni P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci 9: 408–419, 2006. [DOI] [PubMed] [Google Scholar]
- Renszel KM, Traister RS, Lynch WP. Unique N-linked glycosylation of CasBrE Env influences its stability, processing, and viral infectivity but not its neurotoxicity. J Virol 87: 8372–8387, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MN, Miyazawa M, Mori S, Caughey B, Evans LH, Hayes SF, Chesebro B. Production of monoclonal antibodies reactive with a denatured form of Friend murine leukemia virus gp70 envelope protein: use in a focal infectivity assay, immunohistochemical studies, electron microscopy and Western blotting. J Virol Methods 34: 255–271, 1991. [DOI] [PubMed] [Google Scholar]
- Robertson SJ, Hasenkrug KJ, Chesebro B, Portis JL. Neurologic disease induced by polytropic murine retroviruses: neurovirulence determined by efficiency of spread to microglial cells. J Virol 71: 5287–5294, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel C, Erneux T, Schiffmann SN, Gall D. Modulation of neuronal excitability by intracellular calcium buffering: from spiking to bursting. Cell Calcium 39: 455–466, 2006. [DOI] [PubMed] [Google Scholar]
- Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 5: 725–738, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu PD, Randic M. Low- and high-voltage-activated calcium currents in rat spinal dorsal horn neurons. J Neurophysiol 63: 273–285, 1990. [DOI] [PubMed] [Google Scholar]
- Sekirnjak C, du Lac S. Intrinsic firing dynamics of vestibular nucleus neurons. J Neurosci 22: 2083–2095, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selverston AI, Moulins M. Oscillatory neural networks. Annu Rev Physiol 47: 29–48, 1985. [DOI] [PubMed] [Google Scholar]
- Sivaramakrishnan S, Oliver DL. Distinct K currents result in physiologically distinct cell types in the inferior colliculus of the rat. J Neurosci 21: 2861–2877, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaramakrishnan S, Oliver DL. Neuronal responses to lemniscal stimulation in laminar brain slices of the inferior colliculus. J Assoc Res Otolaryngol 7: 1–14, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaramakrishnan S, Sanchez JT, Grimsley CA. High concentrations of divalent cations isolate monosynaptic inputs from local circuits in the auditory midbrain. Front Neural Circuits 7: 175, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Pangratz-Fuehrer S, Rudolph U, Huguenard JR. Intrinsic and synaptic dynamics interact to generate emergent patterns of rhythmic bursting in thalamocortical neurons. J Neurosci 26: 4247–4255, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz I, Deschenes M. Low- and high-frequency membrane potential oscillations during theta activity in CA1 and CA3 pyramidal neurons of the rat hippocampus under ketamine-xylazine anesthesia. J Neurophysiol 70: 97–116, 1993. [DOI] [PubMed] [Google Scholar]
- Sun H, Wu SH. Physiological characteristics of postinhibitory rebound depolarization in neurons of the rat's dorsal cortex of the inferior colliculus studied in vitro. Brain Res 1226: 70–81, 2008. [DOI] [PubMed] [Google Scholar]
- Vanselow BK, Keller BU. Calcium dynamics and buffering in oculomotor neurones from mouse that are particularly resistant during amyotrophic lateral sclerosis (ALS)-related motoneurone disease. J Physiol 525: 433–445, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada SI, Starr A. Generation of auditory brain stem responses (ABRs). III. Effects of lesions of the superior olive, lateral lemniscus and inferior colliculus on the ABR in guinea pig. Electroencephalogr Clin Neurophysiol 56: 352–366, 1983. [DOI] [PubMed] [Google Scholar]
- Wang D, Grillner S, Wallen P. 5-HT and dopamine modulates CaV1.3 calcium channels involved in postinhibitory rebound in the spinal network for locomotion in lamprey. J Neurophysiol 105: 1212–1224, 2011. [DOI] [PubMed] [Google Scholar]
- Wang D, Grillner S, Wallen P. Calcium dynamics during NMDA-induced membrane potential oscillations in lamprey spinal neurons—contribution of L-type calcium channels (CaV1.3). J Physiol 591: 2509–2521, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SH, Ma CL, Kelly JB. Contribution of AMPA, NMDA, and GABAA receptors to temporal pattern of postsynaptic responses in the inferior colliculus of the rat. J Neurosci 24: 4625–4634, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SH, Ma CL, Sivaramakrishnan S, Oliver DL. Synaptic modification in neurons of the central nucleus of the inferior colliculus. Hear Res 168: 43–54, 2002. [DOI] [PubMed] [Google Scholar]
- Ylinen A, Soltesz I, Bragin A, Penttonen M, Sik A, Buzsaki G. Intracellular correlates of hippocampal theta rhythm in identified pyramidal cells, granule cells, and basket cells. Hippocampus 5: 78–90, 1995. [DOI] [PubMed] [Google Scholar]
- Zettel ML, Frisina RD, Haider SE, O'Neill WE. Age-related changes in calbindin D-28k and calretinin immunoreactivity in the inferior colliculus of CBA/CaJ and C57Bl/6 mice. J Comp Neurol 386: 92–110, 1997. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res 130: 94–107, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 135: 145–157, 2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.