Abstract
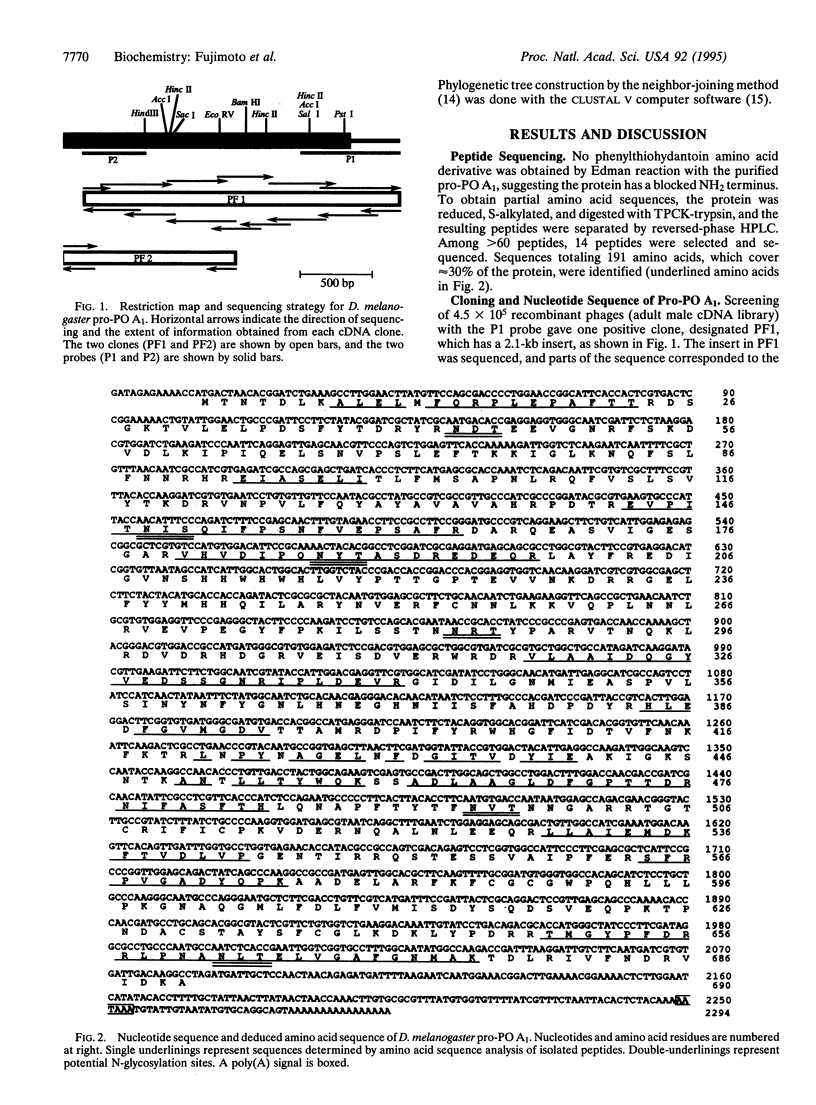
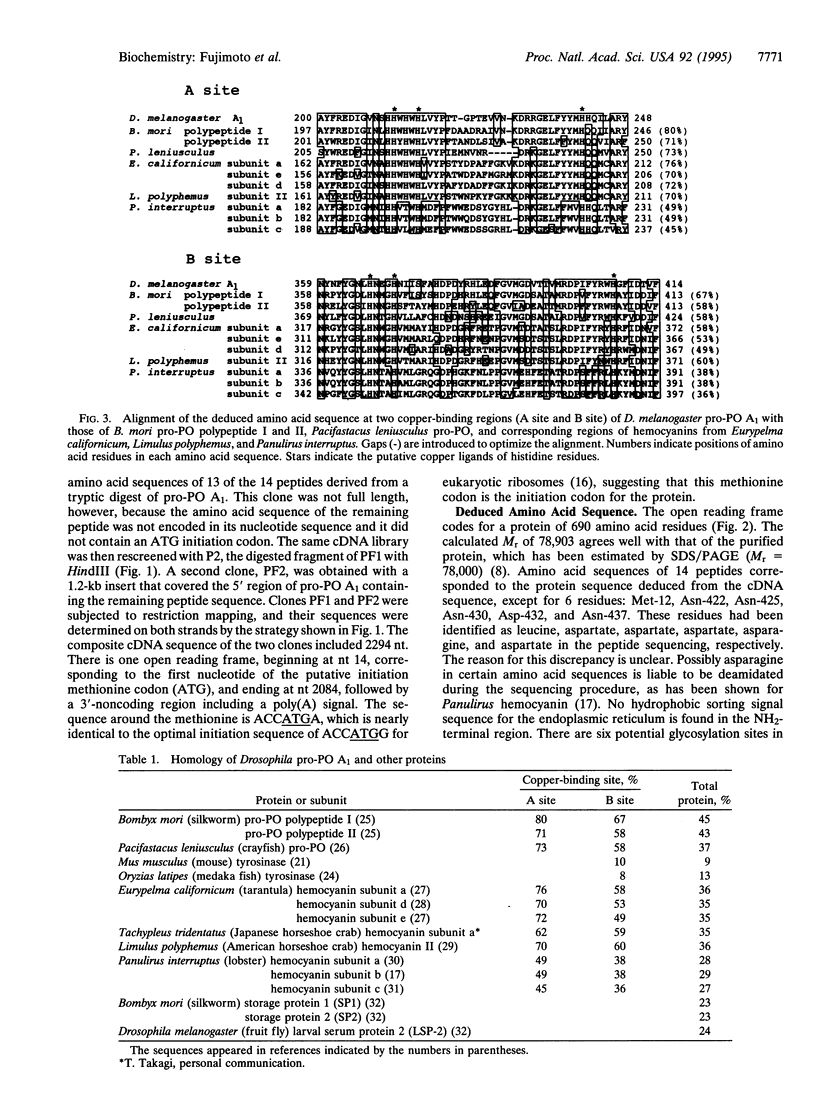
Clones encoding pro-phenol oxidase [pro-PO; zymogen of phenol oxidase (monophenol, L-dopa:oxygen oxidoreductase, EC 1.14.18.1)] A1 were isolated from a lambda gt10 library that originated from Drosophila melanogaster strain Oregon-R male adults. The 2294 bp of the cDNA included a 13-bp 5'-noncoding region, a 2070-bp encoding open reading frame of 690 amino acids, and a 211-bp 3'-noncoding region. A hydrophobic NH2-terminal sequence for a signal peptide is absent in the protein. Furthermore, there are six potential N-glycosylation sites in the sequence, but no amino sugar was detected in the purified protein by amino acid analysis, indicating the lack of an N-linked sugar chain. The potential copper-binding sites, amino acids 200-248 and 359-414, are highly homologous to the corresponding sites of hemocyanin of the tarantula Eurypelma californicum, the horseshoe crab Limulus polyphemus, and the spiny lobster Panulirus interruptus. On the basis of the phylogenetic tree constructed by the neighbor-joining method, vertebrate tyrosinases and molluscan hemocyanins constitute one family, whereas pro-POs and arthropod hemocyanins group with another family. It seems, therefore, likely that pro-PO originates from a common ancestor with arthropod hemocyanins, independently to the vertebrate and microbial tyrosinases.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asada N., Fujimoto K., Tanaka M., Ohnishi E. Genetic polymorphism of prophenoloxidase A1 in Drosophila melanogaster. Jpn J Genet. 1993 Jun;68(3):219–227. [PubMed] [Google Scholar]
- Ashida M. Purification and characterization of pre-phenoloxidase from hemolymph of the silkworm Bombyx mori. Arch Biochem Biophys. 1971 Jun;144(2):749–762. doi: 10.1016/0003-9861(71)90383-3. [DOI] [PubMed] [Google Scholar]
- Aspán A., Huang T. S., Cerenius L., Söderhäll K. cDNA cloning of prophenoloxidase from the freshwater crayfish Pacifastacus leniusculus and its activation. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):939–943. doi: 10.1073/pnas.92.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beintema J. J., Stam W. T., Hazes B., Smidt M. P. Evolution of arthropod hemocyanins and insect storage proteins (hexamerins). Mol Biol Evol. 1994 May;11(3):493–503. doi: 10.1093/oxfordjournals.molbev.a040129. [DOI] [PubMed] [Google Scholar]
- Bernan V., Filpula D., Herber W., Bibb M., Katz E. The nucleotide sequence of the tyrosinase gene from Streptomyces antibioticus and characterization of the gene product. Gene. 1985;37(1-3):101–110. doi: 10.1016/0378-1119(85)90262-8. [DOI] [PubMed] [Google Scholar]
- Brenowitz M., Bonaventura C., Bonaventura J. Self-association and oxygen-binding characteristics of the isolated subunits of Limulus polyphemus hemocyanin. Arch Biochem Biophys. 1984 Apr;230(1):238–249. doi: 10.1016/0003-9861(84)90105-x. [DOI] [PubMed] [Google Scholar]
- Drexel R., Siegmund S., Schneider H. J., Linzen B., Gielens C., Préaux G., Lontie R., Kellermann J., Lottspeich F. Complete amino-acid sequence of a functional unit from a molluscan hemocyanin (Helix pomatia). Biol Chem Hoppe Seyler. 1987 Jun;368(6):617–635. doi: 10.1515/bchm3.1987.368.1.617. [DOI] [PubMed] [Google Scholar]
- Fujimoto K., Masuda K., Asada N., Ohnishi E. Purification and characterization of prophenoloxidases from pupae of Drosophila melanogaster. J Biochem. 1993 Mar;113(3):285–291. doi: 10.1093/oxfordjournals.jbchem.a124040. [DOI] [PubMed] [Google Scholar]
- Giebel L. B., Strunk K. M., Spritz R. A. Organization and nucleotide sequences of the human tyrosinase gene and a truncated tyrosinase-related segment. Genomics. 1991 Mar;9(3):435–445. doi: 10.1016/0888-7543(91)90409-8. [DOI] [PubMed] [Google Scholar]
- Hall M., Scott T., Sugumaran M., Söderhäll K., Law J. H. Proenzyme of Manduca sexta phenol oxidase: purification, activation, substrate specificity of the active enzyme, and molecular cloning. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7764–7768. doi: 10.1073/pnas.92.17.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T., Miyoshi T., Tsukamoto T. Comparative studies of larval and pupal phenoloxidase of the housefly, Musca domestica L. Comp Biochem Physiol B. 1993 Oct;106(2):287–292. doi: 10.1016/0305-0491(93)90302-l. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Bleasby A. J., Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992 Apr;8(2):189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- Huber M., Hintermann G., Lerch K. Primary structure of tyrosinase from Streptomyces glaucescens. Biochemistry. 1985 Oct 22;24(22):6038–6044. doi: 10.1021/bi00343a003. [DOI] [PubMed] [Google Scholar]
- Inagaki H., Bessho Y., Koga A., Hori H. Expression of the tyrosinase-encoding gene in a colorless melanophore mutant of the medaka fish, Oryzias latipes. Gene. 1994 Dec 15;150(2):319–324. doi: 10.1016/0378-1119(94)90445-6. [DOI] [PubMed] [Google Scholar]
- Jekel P. A., Bak H. J., Soeter N. M., Vereijken J. M., Beintema J. J. Panulirus interruptus hemocyanin. The amino acid sequence of subunit b and anomalous behaviour of subunits a and b on polyacrylamide gel electrophoresis in the presence of SDS. Eur J Biochem. 1988 Dec 15;178(2):403–412. doi: 10.1111/j.1432-1033.1988.tb14464.x. [DOI] [PubMed] [Google Scholar]
- Kawabata S., Nakagawa K., Muta T., Iwanaga S., Davie E. W. Rabbit liver microsomal endopeptidase with substrate specificity for processing proproteins is structurally related to rat testes metalloendopeptidase 24.15. J Biol Chem. 1993 Jun 15;268(17):12498–12503. [PubMed] [Google Scholar]
- Kawabata T., Yasuhara Y., Ochiai M., Matsuura S., Ashida M. Molecular cloning of insect pro-phenol oxidase: a copper-containing protein homologous to arthropod hemocyanin. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7774–7778. doi: 10.1073/pnas.92.17.7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Lang W. H., van Holde K. E. Cloning and sequencing of Octopus dofleini hemocyanin cDNA: derived sequences of functional units Ode and Odf. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):244–248. doi: 10.1073/pnas.88.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch K. Primary structure of tyrosinase from Neurospora crassa. II. Complete amino acid sequence and chemical structure of a tripeptide containing an unusual thioether. J Biol Chem. 1982 Jun 10;257(11):6414–6419. [PubMed] [Google Scholar]
- Linzen B., Soeter N. M., Riggs A. F., Schneider H. J., Schartau W., Moore M. D., Yokota E., Behrens P. Q., Nakashima H., Takagi T. The structure of arthropod hemocyanins. Science. 1985 Aug 9;229(4713):519–524. doi: 10.1126/science.4023698. [DOI] [PubMed] [Google Scholar]
- MITCHELL H. K., WEBER U. M. DROSOPHILA PHENOL OXIDASES. Science. 1965 May 14;148(3672):964–965. doi: 10.1126/science.148.3672.964. [DOI] [PubMed] [Google Scholar]
- Nakashima H., Behrens P. Q., Moore M. D., Yokota E., Riggs A. F. Structure of hemocyanin II from the horseshoe crab, Limulus polyphemus. Sequences of the overlapping peptides, ordering the CNBr fragments, and the complete amino acid sequence. J Biol Chem. 1986 Aug 15;261(23):10526–10533. [PubMed] [Google Scholar]
- Neuteboom B., Jekel P. A., Beintema J. J. Primary structure of hemocyanin subunit c from Panulirus interruptus. Eur J Biochem. 1992 May 15;206(1):243–249. doi: 10.1111/j.1432-1033.1992.tb16922.x. [DOI] [PubMed] [Google Scholar]
- Pentz E. S., Wright T. R. Drosophila melanogaster diphenol oxidase A2: gene structure and homology with the mouse mast-cell tum- transplantation antigen, P91A. Gene. 1991 Jul 22;103(2):239–242. doi: 10.1016/0378-1119(91)90279-k. [DOI] [PubMed] [Google Scholar]
- Poole S. J., Kauvar L. M., Drees B., Kornberg T. The engrailed locus of Drosophila: structural analysis of an embryonic transcript. Cell. 1985 Jan;40(1):37–43. doi: 10.1016/0092-8674(85)90306-x. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schartau W., Eyerle F., Reisinger P., Geisert H., Storz H., Linzen B. Hemocyanins in spiders, XIX. Complete amino-acid sequence of subunit d from Eurypelma californicum hemocyanin, and comparison to chain e. Hoppe Seylers Z Physiol Chem. 1983 Oct;364(10):1383–1409. doi: 10.1515/bchm2.1983.364.2.1383. [DOI] [PubMed] [Google Scholar]
- Takase M., Miura I., Nakata A., Takeuchi T., Nishioka M. Cloning and sequencing of the cDNA encoding tyrosinase of the Japanese pond frog, Rana nigromaculata. Gene. 1992 Nov 16;121(2):359–363. doi: 10.1016/0378-1119(92)90144-e. [DOI] [PubMed] [Google Scholar]
- Voit R., Feldmaier-Fuchs G. Arthropod hemocyanins. Molecular cloning and sequencing of cDNAs encoding the tarantula hemocyanin subunits a and e. J Biol Chem. 1990 Nov 15;265(32):19447–19452. [PubMed] [Google Scholar]



