Abstract
Regulation of feeding behavior involves the integration of multiple physiological and neurological pathways that control both nutrient-seeking and consummatory behaviors. The consummatory phase of ingestion includes stereotyped oromotor movements of the tongue and jaw that are controlled through brain stem pathways. These pathways encompass not only cranial nerve sensory and motor nuclei for processing feeding-related afferent signals and supplying the oromotor musculature but also reticular neurons for orchestrating ingestion and coordinating it with other behaviors that utilize the same musculature. Based on decerebrate studies, this circuit should be sensitive to satiety mechanisms mediated centrally by A2 noradrenergic neurons in the caudal nucleus of the solitary tract (cNST) that are potently activated during satiety. Because the first observable phase of satiety is inhibition of oromotor movements, we hypothesized that norepinephrine (NE) would act to inhibit prehypoglossal neurons in the medullary reticular formation. Using patch-clamp electrophysiology of retrogradely labeled prehypoglossal neurons and calcium imaging to test this hypothesis, we demonstrate that norepinephrine can influence both pre- and postsynaptic properties of reticular neurons through both α1- and α2-adrenoreceptors. The α1-adrenoreceptor agonist phenylephrine (PE) activated an inward current in the presence of TTX and increased the frequency of both inhibitory and excitatory miniature postsynaptic currents. The α2-adrenoreceptor agonist dexmedetomidine (DMT) inhibited cNST-evoked excitatory currents as well as spontaneous and miniature excitatory currents through presynaptic mechanisms. The diversity of adrenoreceptor modulation of these prehypoglossal neurons may reflect their role in a multifunctional circuit coordinating both ingestive and respiratory lingual function.
Keywords: norepinephrine, adrenoreceptor, feeding, nucleus of the solitary tract
regulation of feeding behavior involves a complex network of brain regions and pathways that include both forebrain and hindbrain structures. Forebrain structures such as the hypothalamus regulate the appetitive aspect of the feeding, while, as demonstrated in decerebrate rat studies, the hindbrain controls the consummatory phase (Grill 2010; Grill and Smith 1988; Schneider et al. 2013; Seeley et al. 1994). Neurons in the caudal nucleus of the solitary tract (cNST) play a critical role in the control of the consummatory response. During the course of a meal, satiety signals, like those arising from gastric stretch and the release of gut peptides, activate neurons located in the cNST including the A2 noradrenergic group (Smith et al. 1981; Willing and Berthoud 1997). These neurons receive direct input from the gastrointestinal tract via vagal afferents, and transection of the vagus nerve attenuates the effects of satiety factors such as CCK (Rinaman et al. 1998; Smith et al. 1985). While much attention has focused on the influence of norepinephrine in regulating the dorsal vagal complex (Appleyard et al. 2007; Fukuda et al. 1987; Martinez-Peña y Valenzuela et al. 2004; Rogers et al. 2003) and gastric reflexes (Hermann et al. 2005; Hikasa et al. 1992), little research has explored the neural pathways through which visceral cNST neurons influence the first observable consequence of satiety, i.e., the inhibition of ingestive oromotor activity that defines meal termination.
In the decerebrate rat, normal ingestive mechanisms such as suppression of feeding by CCK (Grill and Smith 1988) persist, indicating that the neuronal substrate controlling this behavior resides in the brain stem. However, because ingestion and respiration share a common lingual musculature (Miller 2002), ingestive oromotor behaviors including swallowing must also be coordinated with respiratory activity to protect and maintain the patency of the airway. The neuronal architecture controlling this orolingual coordination may derive from a multifunctional substrate that is housed in the brain stem, as respiratory inhibition during a swallow is also evident in the decerebrate rat (Saito et al. 2003). The reticular formation lateral to the hypoglossal nucleus may be involved in this coordination. Neurons in this region of the reticular formation project to the hypoglossal nucleus and are active during licking, swallowing, and respiration (Ono et al. 1994, 1998; Travers et al. 2000, 2005). Furthermore, pharmacological manipulation of this area modulates hypoglossal inspiratory-phase activity (Chamberlin et al. 2007; Okabe et al. 1994).
Norepinephrine is also implicated in coordinating these disparate oromotor behaviors. Norepinephrine microinjection or stimulation of noradrenergic centers, including the A2 noradrenergic group, inhibits the swallow reflex induced by superior laryngeal nerve stimulation and prolongs the time between inspiratory activity following a swallow (Kessler and Jean 1986a, 1986b; Yamanishi et al. 2010). Since noradrenergic neurons are potently activated during normal satiety (Rinaman 2011), we predicted that norepinephrine, acting through one or more of its known receptors, would either inhibit prehypoglossal neurons in the medullary reticular formation directly or inhibit the release of excitatory neurotransmitter from the presynaptic fibers that drive them.
The mechanisms by which norepinephrine operates within this multifunctional network are unclear. Norepinephrine binds to multiple adrenoreceptor subtypes and leads to multiple complex intracellular signaling pathways that can influence both postsynaptic membrane properties and presynaptic signaling mechanisms (Boychuk et al. 2011; Hermann et al. 2005; Inyushin et al. 2010; Jiménez-Rivera et al. 2012; Moore and Guyenet 1983). The present study tests the hypothesis that multiple adrenoreceptors modulate prehypoglossal neurons in the medullary reticular formation.
METHODS
Animals
Sprague-Dawley rat pups with dam (Harlan Industries, Indianapolis, IN) were maintained on a 12:12-h light-dark cycle with constant temperature and humidity control. The dam was given ad libitum access to both food and water. Pups remained with the dam until the time of each experimental procedure. All experimental procedures were conducted in accordance with National Institutes of Health guidelines and were approved by The Ohio State University Institutional Animal Care and Use Committee.
Retrograde Tracer Injections
Neonatal pups (P6–P9) were initially anesthetized with 5% isoflurane and then placed into a stereotaxic device. Once the animal was in the stereotaxic holder, a surgical level of anesthesia was maintained with 1–3% isoflurane. A midline incision was made over the back of the skull, and neck musculature was retracted to expose the dura covering the foramen magnum. A small incision was made through the dura with a 26-gauge subcutaneous needle and subsequently expanded in the caudal direction with Vanna scissors to expose the area postrema and obex. Retrograde tracer injections (30–50 nl, carboxylated polysytrene beads, 100-nm diameter; Life Technologies, Grand Island, NY) into the hypoglossal nucleus were made at the level of obex, 0.2 mm off the midline and 1.0 mm from the brain surface, with a pulled glass pipette beveled to a nominal tip diameter of 22–50 μm. These injection coordinates were chosen to be just caudal of the area postrema where patch-clamp recordings were conducted to minimize the potential for the bright fluorescent signal from the injection site to bleed over into the recording area. Injections were made at a rate of 10 nl/min, and the injection pipette was left in place for 5 min before being slowly removed. The wound was closed with 6.0 silk sutures, and topical antibiotic was applied to the wound site. The pups were given 0.15 ml of lactated Ringer solution and returned to the dam after recovery from anesthesia.
Brain Slices
Twenty-four to seventy-two hours after retrograde tracer injection, pups were anesthetized with ethyl carbamate (urethane; Sigma-Aldrich, St. Louis, MO; 2 g/kg, dissolved in sterile distilled H2O) by intraperitoneal injection and euthanized by rapid decapitation. The brain was rapidly removed from the skull and cooled to 4°C in a modified artificial cerebrospinal fluid containing (in mM) 110 choline chloride, 25 NaHCO3, 2.5 KCl, 7 MgSO4·7H2O, 1.5 NaH2PO4, 10 d-glucose, and 0.5 CaCl2·2H2O. The cerebellum was removed and the brain stem blocked rostrally at the ponto-medullary junction and caudally at the spino-medullary junction. The brain was sectioned in the coronal plane with a sapphire blade (Delaware Diamond Knives, Wilmington, DE) on a vibrating tissue slicer (Vibratome classic-1000) at a nominal thickness of 350 μm. After sectioning, slices were transferred to warmed (32°C) carboxygenated normal artificial cerebrospinal fluid (ACSF) containing (in mM) 124 NaCl, 25 NaHCO3, 3 KCl, 1 MgSO4·7H2O, 1.5 NaH2PO4, 10 d-glucose, and 1.5 CaCl2·2H2O and allowed to equilibrate for 30–60 min. Slices were then held at room temperature until being used for individual experiments.
Drugs
All drugs were administered through the perfusion system at a rate of 2 ml/min. The concentrations of antagonists for the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor and the N-methyl-d-aspartate (NMDA) receptor were 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX; 10 μM) and 6,7-dinitroquinoxaline-2,3-dione (DNQX; 10 μM) and (5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate (MK-801; 10 μM) (AMPA and NMDA, respectively) and were chosen to be consistent with our previous study in the rostral reticular formation and with previously published concentrations shown to be effective (Nasse et al. 2008). Norepinephrine (Sigma-Aldrich) was delivered at a concentration of 20 μM. Dexmedetomidine (DMT) was chosen as the α2-adrenoreceptor agonist for its greater selectivity over more common agonists such as clonidine or UK14-304 (Scheinin et al. 1989; Virtanen et al. 1988) and delivered at a concentration of 100 nM. This concentration is ∼100 times greater than the binding affinity for the α2-adrenoreceptor and 10 times lower than the binding affinity for the α1-adrenoreceptor (Virtanen et al. 1988) and was selected in order to achieve potent, yet selective activation of only the α2-adrenoreceptor. DMT was perfused for 5 min prior to taking any measurements to provide adequate time to reach an equilibration state. Phenylephrine was delivered at a concentration of 20 μM, which is twofold greater than the EC50 for rat α1-adrenoreceptor activation (Minneman et al. 1994). Phenylephrine has been shown to increase intracellular calcium concentrations and depolarize neuronal membrane potentials (Hermann et al. 2005; Martinez-Peña y Valenzuela et al. 2004); therefore we limited the exposure time in each experiment to prevent potential excitotoxic effects on the recorded neurons. In calcium imaging experiments a 30-s exposure was chosen to provide an adequate signal-to-noise ratio while minimizing exposure times to the excitation laser. This protocol allowed us to repeatedly stimulate the slices with minimal photobleaching of the fluorescent indicator dye and without causing phototoxicity in the tissue. For patch-clamp experiments, we increased the exposure time to 90 s in order to characterize the maximum increase of induced currents and allow adequate time to characterize miniature and spontaneous synaptic currents. TTX concentration (1 μM) was based on previous experiments performed in our lab (Boxwell et al. 2013) and shown to be effective at blocking voltage-gated Na+ currents. TTX was perfused for 5 min prior to any measurements being taken in order to ensure complete blocking of the channels and equilibration throughout the tissue. All drugs were purchased from Tocris Biosciences (Bristol, UK) unless otherwise specified.
Patch-Clamp Electrophysiology
For patch-clamp experiments, slices were transferred to a custom-made polycarbonate recording chamber attached to a fixed-stage upright microscope (Nikon EF-600) and superfused with warm (32°C) ACSF at a rate of 2 ml/min. Slices were held in place with a custom-made gold wire “harp” strung with elastic nylon strings. The strings of the harp were oriented so that they did not overlie the NST or underlying reticular formation. A bipolar twisted wire stimulating electrode (formvar coated, silver wire, 75-μm OD; A-M Systems, Sequim, WA) was placed in the cNST and used to deliver short (0.1 ms) current pulses to the nucleus. Patch electrodes were pulled from 1.5-mm-OD borosilicate glass (A-M Systems, Carlsborg, WA) on a Narashige P-83 pipette puller (Narishige International, Tokyo, Japan) to a nominal resistance of 3–5 MΩ. Electrophysiological recordings were performed with an A-M Systems 2400 patch amplifier and recorded with a Digidata 1300 and pCLAMP 9 software (Molecular Devices, Sunnyvale, CA). Signals were sampled at 20 kHz and low-pass filtered at 3 kHz. Patch pipettes were directed toward retrogradely labeled neurons in the medullary reticular formation at the level of the area postrema. After the formation of a gigaseal, a brief pulse of suction was applied with a 5-ml syringe to rupture the cell membrane and gain electrical access to the cell. The pipette solution consisted of (in mM) 130 K-gluconate, 10 EGTA, 10 HEPES, 1 CaCl2, 1 MgCl2, and 2 ATP, at pH 7.2–7.3 and osmolality 290–295 mosmol/kgH2O. Lucifer yellow (0.1%) was included in the internal pipette solution to mark recorded neurons. Series resistance and cellular capacitance were compensated and monitored throughout the experiment. Cells were included in the study if the input resistance was >100 MΩ and compensated series resistance was <40 MΩ. Short paired-pulse (interstimulus interval = 50 ms) stimulation was delivered to the cNST with a Grass model S88 stimulator equipped with a stimulus isolation unit. Stimulus current amplitude ranged from 30 to 150 μA and was determined empirically for each cell by increasing the stimulating current until consistent postsynaptic currents were elicited. Excitatory postsynaptic currents (EPSCs) were recorded in voltage clamp at a holding potential of −60 to −70 mV. Holding potential was increased to −40 mV in a subset of cells in order to potentiate and record inhibitory postsynaptic currents (IPSCs). Membrane resistance was monitored throughout recordings using norepinephrine and phenylephrine with a short (50 ms) hyperpolarizing (−70 mV) pulse. For DMT experiments the membrane resistance was determined in current clamp using injected current steps from −200 nA to +200 nA and plotting an I-V curve from the linear portion of the response. The chloride reversal potential was −91.2 mV. Voltages reported in the text are not corrected for the liquid junction potential, calculated to be −13.9 mV.
Calcium Imaging
Neonatal pups were injected in the hypoglossal nucleus, and coronal sections were made as described above. After a 30-min recovery at 32°C, slices were incubated in 16 μM fluo-8 AM (AAT Bioquest, Sunnyvale, CA) in a 22-mm petri dish containing ∼3 ml of ACSF continuously bubbled with 95% O2-5% CO2 for 15–20 min. Fluo-8 (50 μg) was first dissolved in 40 μl of 10% Pluronic F-120 in DMSO and then further diluted with 200 μl of ACSF. The diluted solution was pipetted over the slices with gentle agitation to ensure complete mixing of the dye and medium. After an incubation time of 15–20 min, the slices were rinsed twice with carboxygenated ACSF at 32°C for 30 min each and then held at room temperature until being used for individual experiments. Images were collected on an Olympus fixed-stage upright microscope equipped with an Infinity-3 live-cell confocal imaging system (Visitech, Sunderland, UK). The calcium indicator dye was excited with a 491-nm LED laser. Typical exposure time was 110 ms, and images were acquired at the maximum frame rate for ∼2.5 min to allow adequate time for fluorescent signals to return to baseline after drug application. Phenylephrine (20 μM) was applied though the perfusion system for 30 s, and a 5-min washout period was given between drug applications. Data from an individual cell were excluded if it did not respond with at least a 5% increase in fluorescent signal from baseline values after the first exposure to phenylephrine.
Data and Analysis
For evoked currents, a minimum of 10 sweeps were averaged for each treatment to determine amplitude, latency, and jitter values. Spontaneous and miniature postsynaptic currents were quantified with Mini Analysis software (Synaptosoft, Decatur, GA). Detection thresholds were set at three times the root mean square of the noise and then manually verified. For cases without TTX in the medium, we quantitated currents for 10 s just prior to the drug entering the chamber and either for 10 s after the equilibration period (5 min, DMT) or when the drug had reached peak concentration in the bath (phenylephrine) at ∼2 min. TTX substantially reduced the frequency of spontaneous postsynaptic currents. We therefore quantitated miniature postsynaptic currents over a period of 60 s to adequately measure changes in response to each drug condition. Action potential-independent activity was converted to frequency by dividing the total number of currents measured per given time and reported as the average number of currents per second. In most cases, a one-way ANOVA with Dunnett's post hoc tests were used to determine statistical significance for drug effects that included washout data. We report the overall ANOVA value. All significant ANOVAs were accompanied by significant post hoc tests for drug effects. However, since drug effects were not always reversible, we specifically indicate instances where washout occurred. Note that because a Dunnett test compares a treatment to the control value (ACSF baseline), a nonsignificant effect for the washout period indicates a return to baseline and is denoted as such in the figures. When sufficient washout data were not available, we conducted t-tests between baseline and drug conditions as indicated. For the analysis of spontaneous and miniature postsynaptic currents, we report both group statistics (ANOVA) and the results of a Kolmogorov-Smirnov two-sample test (KS) on individual cells to determine statistical significance between control and drug conditions. A significance level was set at P < 0.05.
For calcium imaging experiments, regions of interest were drawn over individual cells with Metamorph Imaging software (Molecular Devices) and changes in fluorescent intensity were measured. We calculated the changes in intensity (ΔF/F) by subtracting the baseline values from the peak change during the drug application and then dividing by the baseline value. Statistical analysis was performed with a one-way ANOVA with a Dunnett's multiple-comparison post hoc test; significance was set at P ≤ 0.05. Oscillations in fluorescent intensity were manually counted for each condition and compared with a Student's t-test.
RESULTS
Synaptic Connectivity
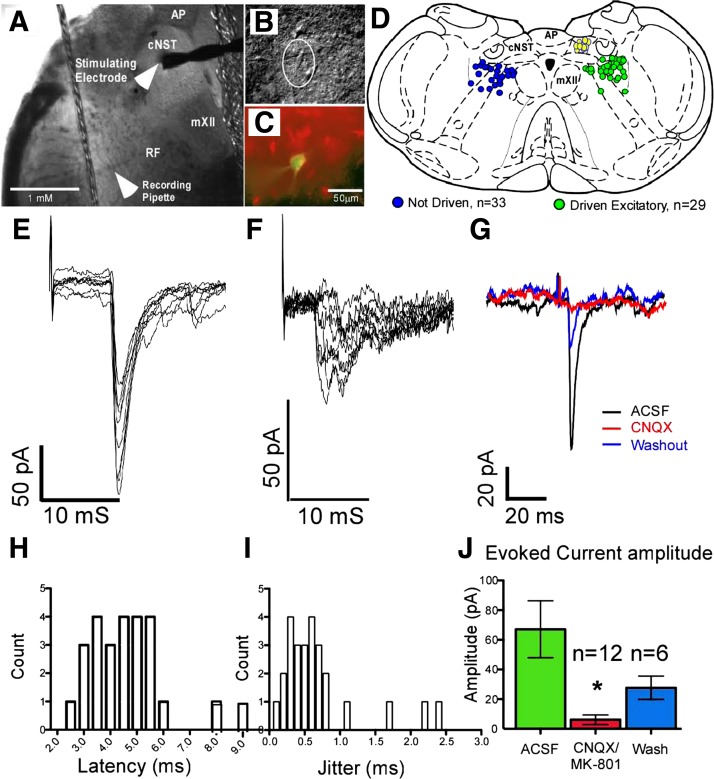
Anterograde tracing studies have demonstrated that cNST fibers traverse the medullary reticular formation in which prehypoglossal neurons are located (Cunningham and Sawchenko 1989, 2000), but physiological connectivity has not been well established. Thus our first experiment focused on demonstrating synaptic connections between the cNST and prehypoglossal neurons (Fig. 1). cNST time-locked evoked postsynaptic currents were recorded in 29 neurons, 21 of which were labeled by retrograde tracer injection into the hypoglossal nucleus. No differences in synaptic connectivity or membrane properties were observed between retrogradely traced and untraced neurons. Hence, all data were pooled for statistical analysis. EPSCs recorded in 27 neurons had an average latency of 4.6 ± 0.30 ms, with a range of 2.3–9.1 ms (Fig. 1H). This range is similar to those obtained from a previous study in which stimulation of the rostral NST (rNST) evoked excitatory currents in prehypoglossal neurons further rostral in the medullary reticular formation (Nasse et al. 2008). The jitter of the elicited currents (standard deviation of the latencies) ranged from 0.12 to 2.41 ms (Fig. 1I) and was used to classify mono- versus polysynaptic responses (Doyle and Andresen 2001). Previous recordings in the rostral reticular formation study showed a bimodal distribution of jitter with a clear separation at 0.5 ms. When we used these criteria to classify mono- and polysynaptic responses in the present data set, our results demonstrated that 55% of neurons received polysynaptic input (15 of 27 neurons) and 45% (12 of 27) received monosynaptic input from the cNST. Unlike the rNST, there was no correlation between latency and jitter. In 12 neurons, we blocked ionotropic glutamate AMPA and NMDA receptors with CNQX (10 μM) and/or MK-801 (10 μM), respectively (Fig. 1, G and J). No differences in the level of suppression were observed in response to CNQX either alone (n = 5) or in combination with MK-801 (n = 7); therefore, the data were pooled for statistical analysis. Blocking AMPA and/or NMDA receptors suppressed the evoked excitatory current amplitude by an average of 74%, with 8 of the 12 cases showing complete suppression of the evoked current (Fig. 1J; ACSF: 67.2 ± 19.2 pA, CNQX/MK-801: 6.2 ± 3.3 pA, paired t-test, P ≤ 0.01). For six neurons for which washout data were available (4 of which showed complete suppression), there was a significant drug effect (ANOVA: P ≤ 0.002) followed by washout.
Fig. 1.
A: low-magnification photomicrograph of brain stem slice indicating location of the stimulating electrode and recording pipette. Reticular formation neurons were recorded from the area subjacent to the caudal nucleus of the solitary tract (cNST). B: infrared-differential interference contrast (IR-DIC) image of recorded cell. C: fluorescent photomicrograph of recorded cell (green) showing label from the retrograde tracer (red). D: summary diagram of recorded neurons showing the location of a subset of neurons recorded in this study. On left are neurons not driven by cNST stimulation; on right are neurons driven by cNST stimulation. Stimulation sites are shown in the cNST (yellow). E and F: representative examples of monosynaptic (E) and polysynaptic (F) excitatory currents evoked by electrical stimulation of the cNST. G: electrical stimulation of cNST-evoked short-latency excitatory postsynaptic currents (EPSCs) (black trace) in a prehypoglossal neuron that was blocked with ionotropic glutamate receptor antagonists (CNQX/MK-801, 10 μM) (red trace) showed some recovery with washout (blue trace). H: evoked responses demonstrated short latencies ranging from 2 to 9 ms. I: the standard deviation of evoked current onset (jitter) indicates that the majority (15 of 27) of the EPSCs were polysynaptic. Jitter values >0.5 ms were classified as polysynaptic according to criteria from a previous study in the rostral reticular formation (Nasse et al. 2008). J: excitatory currents were significantly suppressed by a mean of 74% (*P ≤ 0.01, paired t-test, n = 12) after CNQX/MK-801, with 8 of 12 neurons being completely suppressed. AP, area postrema; RF, medullary reticular formation; mXII, hypoglossal motor nucleus.
Evoked inhibitory currents were observed in a small number of recorded neurons (5 of 29). The mean latency of inhibitory currents was 4.9 ± 0.19 ms with a mean jitter of 0.37 ± 0.06 ms (data not shown). Jitter analysis of evoked inhibitory currents showed that three of the five were monosynaptic and the remaining two were polysynaptic by the same criteria as for excitatory currents. Because evoked inhibitory currents were often observed in conjunction with excitatory currents (3/5), obtaining reliable amplitude values was not possible.
Norepinephrine
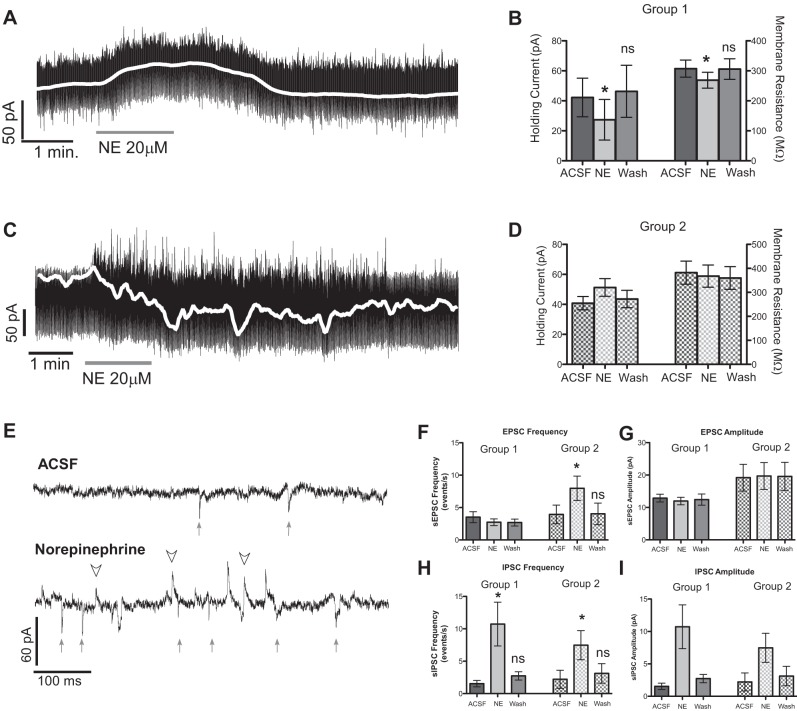
The endogenous adrenoreceptor agonist norepinephrine (20 μM) was bath applied while recording from 12 reticular formation neurons. In three cases where cNST stimulation evoked time-locked excitatory currents norepinephrine completely suppressed these responses, and in two instances these responses recovered after a 10-min washout (Fig. 2, A and B). In nine neurons that were not driven, we measured holding current, membrane resistance, and changes in the frequency and amplitude of spontaneous EPSCs (sEPSCs) and spontaneous IPSCs (sIPSCs). Norepinephrine significantly decreased the holding current (classified as group 1; Fig. 3, A and B) by ∼35% in four cells (ACSF: −42.2 ± 12.9 pA, NE: −27.4 ± 13.5 pA, P ≤ 0.015) followed by washout, and there was a concomitant decrease in membrane resistance (ACSF: 307.3 ± 28.5 MΩ, NE: 268.8 ± 26.4 MΩ, P ≤ 0.003) (Fig. 3B) that also recovered with washout. Although these data are suggestive of a postsynaptic inhibitory effect, this was subsequently not borne out by the use of selective adrenoreceptor agonists in the presence of TTX (below).
Fig. 2.
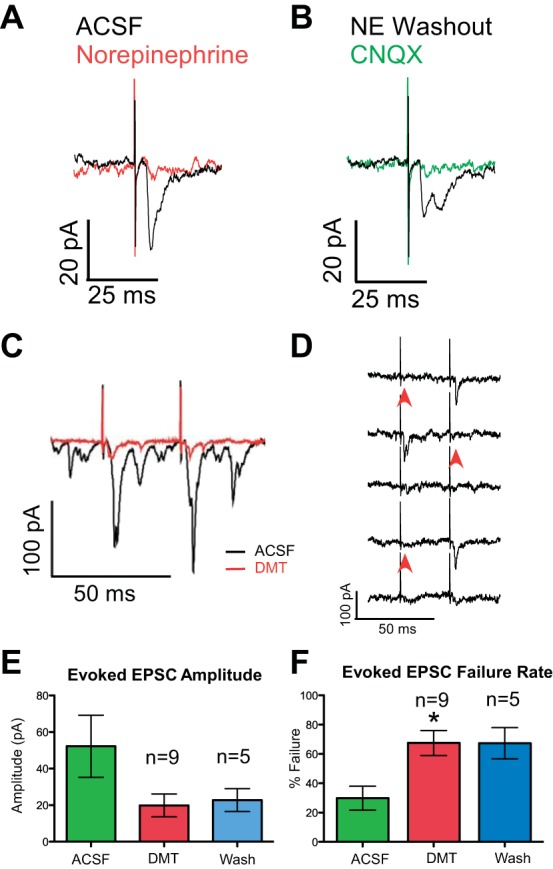
A: example of a neuron in which excitatory currents elicited by electrical stimulation of the cNST (black trace) were inhibited by 20 μM norepinephrine (red trace). B: in the same neuron after washout (black trace), application of the ionotropic glutamate receptor antagonist CNQX blocked the excitatory current (green trace). C: cNST-evoked EPSCs (black trace) were attenuated by bath application of dexmedetomidine (DMT, red trace). D: representative traces showing failures to evoke an EPSC (arrowheads). E: with each neuron serving as its own control, there was a significant decrease after DMT in 7 of 9 neurons (all P ≤ 0.05) for an overall reduction of 53.5%. F: DMT significantly increased the failure rate of evoked currents by 37.6% (*P ≤ 0.05, paired t-test).
Fig. 3.
A: representative decrease in holding current in response to norepinephrine (NE). The apparent high noise level is actually short hyperpolarizing currents injected to measure membrane resistance and is not noise per se; a moving average of the holding current is indicted in white. B: these group 1 neurons (n = 4) showed a significant 35% decrease (*P ≤ 0.015) in mean holding current compared with baseline (ACSF) and a significant decrease (*P ≤ 0.003) in membrane resistance. During the washout period, neither of these measures was significantly different from the predrug ACSF period (ns), indicating a reversal of the drug effect. C: other neurons showed an increase in holding current in response to NE. D: these group 2 neurons (n = 5) had a nonsignificant (P = 0.11) 26% increase in holding current compared with baseline and no change in membrane resistance. E: representative traces exhibiting an increase in both spontaneous EPSCs (sEPSCs, arrows) and spontaneous inhibitory postsynaptic currents (sIPSCs, arrowheads) in response to NE (bottom) compared with ACSF (top). F: NE significantly increased the frequency of sEPSCs in group 2 cells (those showing an increase in holding current, *P ≤ 0.002) but not in group 1 cells (those showing a decrease in holding current). G: the amplitude of EPSCs was not changed in either group. H and I: the frequency of IPSCs was significantly increased (H) by NE in both groups (group 1: *P ≤ 0.03, group 2: *P < 0.03), but significant changes in amplitude (I) were only observed when the data from both groups were combined (P ≤ 0.02).
There were also significant excitatory effects with norepinephrine application. Five cells showed a nonsignificant increase in holding current (classified as group 2; Fig. 3, C and D) by ∼26% (ACSF: −40.8 ± 4.48, NE: −51.3 ± 6.0, P = 0.11) suggestive of depolarization, and there was a significant increase in the frequency of sEPSCs from 3.9 ± 1.4 EPSC/s to 8.0 ± 1.9 EPSC/s (P ≤ 0.002) (Fig. 3, E and F). Although only the neurons with an increase in holding current had a significant increase in the frequency of sEPSCs, both group 1 and group 2 neurons had significant increases in sIPSC frequency after norepinephrine (Fig. 3H; both P ≤ 0.03). When analyzed separately group 1 and group 2 neurons did not show significant changes in sIPSC amplitude (Fig. 3I); however, when neurons from both groups were combined there was a significant (47%) increase in IPSC amplitude (ACSF: 13.0 ± 1.1 pA, NE: 19.1 ± 2.8 pA, P ≤ 0.02). Additional statistical testing showed significant differences in the distribution of IPSC amplitudes after drug infusion in all cells (KS: all P < 0.03). These data suggest that multiple, or different, adrenoreceptors are involved in the oromotor pathway. Thus we conducted experiments with selective α-adrenoreceptor agonists to determine which receptors may contribute to the contrasting effects observed with norepinephrine and where they may be expressed.
α2-Adrenoreceptor
Evoked synaptic currents.
The selective α2-adrenoreceptor agonist DMT (100 nM) was applied through the perfusion system while recording from 11 prehypoglossal neurons. cNST stimulation-evoked EPSCs were seen in 9 of the 11 neurons (Fig. 2, C and D). When slices were exposed to DMT, on average, the amplitude of evoked EPSCs was diminished by 53.5 ± 11.15% of control values (Fig. 2E; ACSF: 52.2 ± 17.0 pA, DMT: 19.8 ± 6.2 pA), which trended toward but did not reach significance (P < 0.09, paired t-test). However, individual paired t-tests showed that DMT induced suppression of the excitatory current in seven of the nine cases (all P ≤ 0.05). In conjunction with a decrease in amplitude, DMT also caused a significant increase in the failure rate of evoked currents (Fig. 2, D and F; DMT: 67.5%, ACSF: 29.9%, P ≤ 0.05, paired t-test). Even with up to 35 min of continuous perfusion with ACSF, we observed washout in only two of five neurons. DMT binds with high affinity to the α2-adrenoreceptor, and others have reported only partial washout after even longer (1–2 h) time frames (Chiu et al. 1995; Shirasaka et al. 2007).
Spontaneous synaptic currents.
In this same group of neurons, the application of DMT significantly reduced the frequency of sEPSCs by 72%, compared with control values (Fig. 4B; ACSF: 13.4 ± 2.4 EPSCs/s, DMT: 4.1 ± 0.79 EPSCs/s, P = 0.0002, n = 11) and there was a trend toward a decrease in amplitude (47% decrease; ACSF: 32.0 ± 2.0 pA, DMT: 16.9 ± 0.6 pA, P = 0.18). Consistent with this trend were significant reductions in the cumulative distributions of the amplitudes of the sEPSCs in 8 of the 11 neurons (KS: all P < 0.03). In contrast to sEPSCs, sIPSCs showed no significant changes in either frequency (ACSF: 9.9 ± 4.8 sIPSCs/s, DMT: 8.5 ± 4.4 sIPSCs/s) or amplitude (ACSF: 21.5 ± 3.5 pA, DMT: 18.6 ± 3.6 pA, n = 5, data not shown). This suggests that α2-adrenoreceptors may be expressed selectively on excitatory neurons or terminals but not on inhibitory neurons.
Fig. 4.
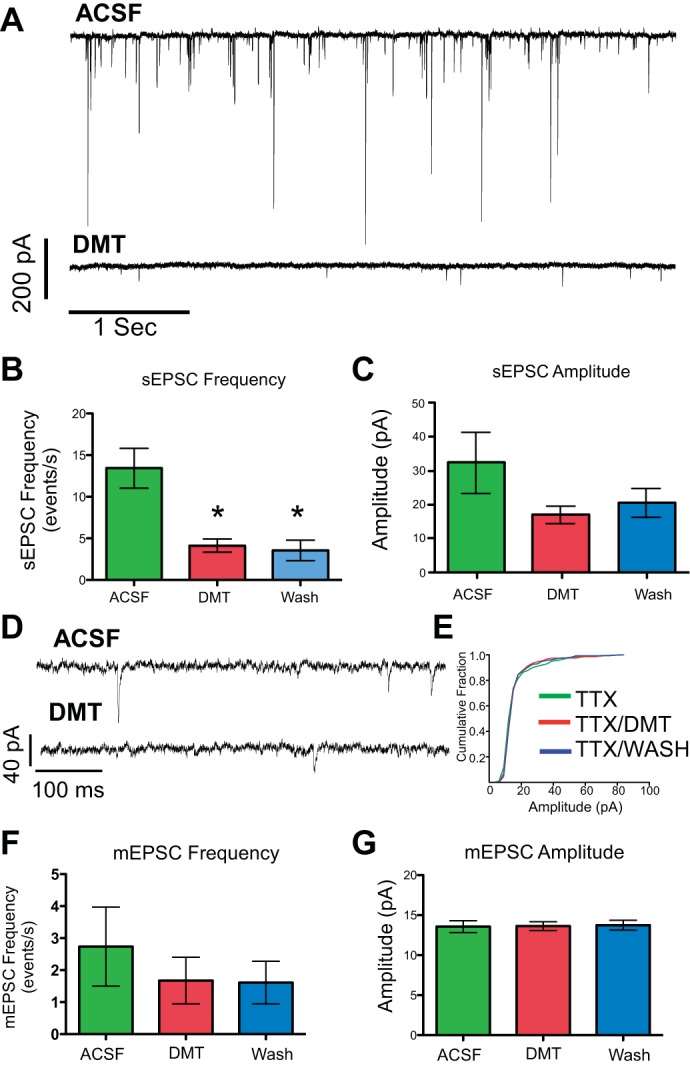
A: representative traces in normal ACSF (top) and after 5 min of DMT (bottom) showing suppression of sEPSCs. B: DMT (100 nM) significantly decreased the frequency of sEPSCs by 72% of control values (*P = 0.0002, n = 11), and there was no washout effect, i.e., the frequency was significantly different (*) compared with baseline (ACSF). C: DMT also reduced the amplitude of sEPSCs; however, the reduction did not reach significance (P = 0.18). D: representative trace of miniature EPSCs (mEPSCs) after TTX (1 μM) pretreatment (top) and in the presence of DMT (bottom). E: cumulative histogram of mEPSCs from the same case showing no change in the slope, indicating that the distribution of mEPSC amplitudes is the same in all treatment groups. F and G: in the presence of TTX, the 26% reduction in mEPSC frequency (F) to DMT was not significant (n = 5) and the mEPSC amplitude (G) remained constant.
While the reductions in sEPSC frequency observed with DMT suggest a presynaptic site of action, the reduction in sEPSC amplitude could be due to either pre- or postsynaptic sites. To further disambiguate the site of action, we recorded miniature EPSCs (mEPSCs) in the presence of the sodium channel blocker TTX (1 μM). With TTX in the medium, DMT did not significantly reduce mEPSC amplitude (Fig. 4G; ACSF: 13.6 ± 0.7 pA, DMT: 13.6 ± 0.55 pA, P ≥ 0.52). In addition, when current amplitudes were plotted with a cumulative histogram, no significant changes in mEPSC amplitude distribution were detected (KS: all P > 0.05). If the site of action was on the postsynaptic cell, then we would expect to observe decreases in the amplitude of mEPSCs when slices are exposed to DMT in the presence of TTX. Unlike sEPSCs, there was no group effect for DMT to reduce the frequency of mEPSCs (Fig. 4F; P > 0.05), although two of five neurons did show significantly reduced frequencies (KS: both P < 0.001). Membrane resistance and holding current did not vary with DMT in either experimental paradigm (TTX or no TTX). These results strongly support the assertion that the site of action for DMT-induced changes in sEPSCs is presynaptic. Hence, the tendency for a reduction in sEPSC amplitudes caused by DMT in ACSF is likely due to the inhibition of spontaneous action potentials in presynaptic neurons and not to postsynaptic activation of the receptor.
α1-Adrenoreceptor
Prolonged application of the α1-adrenoreceptor agonist phenylephrine increased the holding current to levels at which we were unable to maintain adequate space clamp without damaging the postsynaptic neuron even at concentrations as low as 5 μM. This is perhaps not surprising given that prehypoglossal neurons can have very long (>1,000 μm) dendrites with multiple branches (Nasse et al. 2008). Therefore, we were unable to reach an adequate equilibration state that would allow us to characterize the effects of α1-adrenoreceptor agonists on evoked synaptic currents. However, at the beginning of the equilibration period before the holding current became unstable, we observed a dramatic increase in sEPSC frequency coincident with increases in holding current. This led us to hypothesize that presynaptic release of glutamate was acting in conjunction with postsynaptic activation of the α1-adrenoreceptor, leading to a dual excitatory effect. To test this hypothesis, we initially performed calcium imaging studies with ionotropic glutamate receptor antagonists in the medium. Subsequently, we conducted a series of patch-clamp experiments in which we used shorter applications of phenylephrine both with and without TTX to evaluate the pre- and postsynaptic effects.
Calcium imaging.
To screen the interaction of phenylephrine and glutamate across a large population of reticular formation neurons, we used live-cell confocal calcium imaging to record changes in intracellular calcium dynamics in response to short (30 s) exposures of phenylephrine (20 μM) with and without ionotropic glutamate antagonists. A total of 11 pups were used to image 245 cells, from 26 individual slices. Although we attempted to label prehypoglossal neurons with a retrograde marker, this was not very successful and there were few such neurons that took up the calcium indicator.
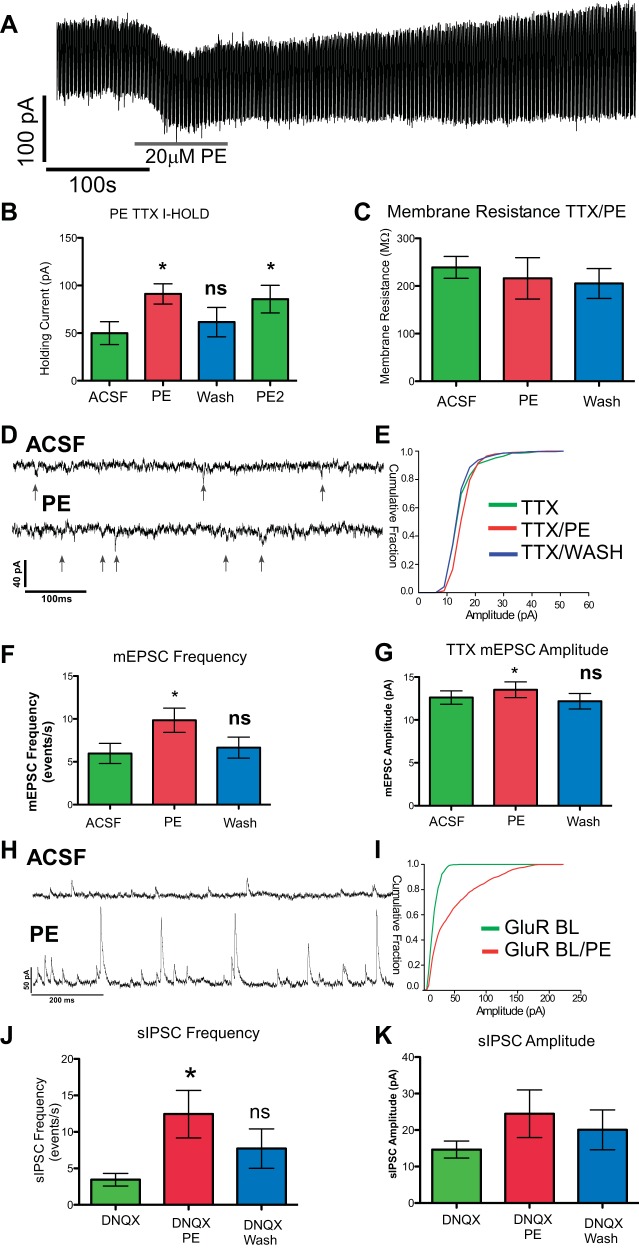
The application of phenylephrine in ACSF resulted in a dramatic increase in fluorescent intensity values (Fig. 5A, ACSF PE1: 20.1 ± 1.3, ΔF/F, n = 193). To assess the contribution of presynaptic glutamate release to this increase in Ca2+, we blocked ionotropic glutamate receptors with DNQX (10 μM) (AMPA) and MK-801 (10 μM) (NMDA) after the first exposure to phenylephrine and observed a significant 34% decrease in fluorescent intensity levels compared with the first phenylephrine exposure (Fig. 5C, PE2 GluR Block: 13.4 ± 1.2, ΔF/F, 126 cells, paired t-test: P ≤ 0.05). The attenuation in the response to phenylephrine in the presence of glutamate antagonists was greater than the nonsignificant 20% attenuation to a second application of phenylephrine alone (Fig. 5C, ACSF PE2; paired t-test: P = 0.17). To verify that glutamate contributes to changes in intracellular calcium and that the decrease in the phenylephrine response was not merely due to receptor desensitization or rundown of the Ca2+ response, DNQX/MK-801 was added to the bath prior to the first exposure of phenylephrine, and again the increase in fluorescent intensity evoked by the α1 agonist was significantly smaller (31%) than that observed in ACSF (Fig. 5C, GluR Block PE1: 14.2 ± 1.2, P ≤ 0.002, 52 cells). Thus phenylephrine applied in the presence of ionotropic glutamatergic blockade still elicits an increase in intracellular calcium, but less so than when phenylephrine is applied alone. The blockade of ionotropic glutamate receptors revealed an additional interesting phenomena in reticular formation neurons. When AMPA and NMDA receptors were blocked prior to the first phenylephrine exposure, we observed significantly more oscillations in fluorescent intensity during drug exposure compared with when phenylephrine was delivered in normal ACSF (Fig. 5D, number of peaks: ACSF 3.5 ± 0.21, PE 7.9 ± 0.72, 2-sample t-test: P ≤ 0.001). These data suggest that glutamate released from presynaptic sources contributes to the excitatory response elicited by α1-adrenoreceptor agonists, and that there are multiple sites of action within the oromotor circuit where phenylephrine can cause changes in neuronal physiology.
Fig. 5.
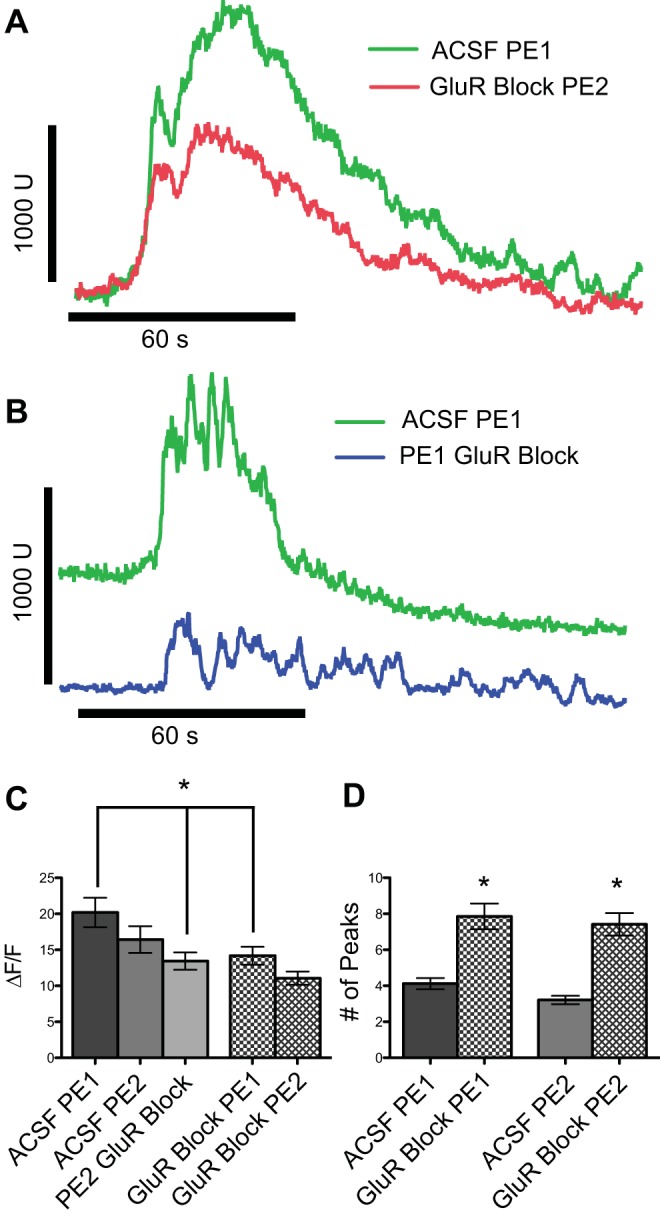
A: representative traces of responses to bath-applied phenylephrine (PE, 20 μM) showing increase in the fluorescent intensity (green trace) that is attenuated after a second application of PE combined with NMDA and AMPA receptor antagonists (DNQX and MK-801: red trace). B: when glutamate receptors were blocked prior to the first exposure of PE (blue trace) there was a reduced response to PE compared with the first exposure of PE in ACSF (green trace). C: mean Ca2+ responses (change in fluorescence intensity, ΔF/F) to PE applied in the presence or absence of ionotropic glutamate receptor blockade. An initial application of PE alone (ACSF PE1) evoked a large response. A subsequent application of PE evoked only a nominally smaller response (ACSF PE2: P = 0.17, n = 67 cells), but when the second application of PE occurred in the presence of glutamate blockade the reduction was larger and significant (PE2 GluR Block: *P ≤ 0.05, n = 126 cells). In a second set of experiments, an initial application of PE in the presence of glutamate blockade elicited a significantly smaller response than the initial PE response observed in the first experiment (GluR Block PE1: *P ≤ 0.002, n = 52) and a second application of PE under the same conditions elicited only a nominally smaller response. D: blocking ionotropic receptors prior to the first phenylephrine application significantly increases the oscillations of intracellular calcium (P ≤ 0.001). U, arbitrary fluorescent intensity units.
Patch clamp.
Because the Ca2+ imaging experiments included few prehypoglossal neurons, similar experiments were conducted in patch clamp to further differentiate pre- and postsynaptic effects elicited by phenylephrine and to explore the phenylephrine -glutamate interaction in this defined population of cells. First, we blocked action potential-dependent synaptic activity by including TTX in the medium and limited the time of phenylephrine application. With TTX in the medium, a 90-s application of phenylephrine (20 μM) produced a significant increase in holding current across all cells tested (n = 6; Fig. 6, A and B). The average increase in holding current was 41.2 ± 3.7 pA (P ≤ 0.0001). We also quantified the frequency and amplitude of mEPSCs in response to phenylephrine in the presence of TTX. Compared with ACSF, there was a significant increase in the frequency of mEPSCs with phenylephrine (Fig. 6, D and F; ACSF: 5.9 ± 1.2, PE: 9.9 ± 1.4 mEPSCs/s, P ≤ 0.003), an effect reversed during washout. In addition, there was a small but significant increase in the mean amplitude of mEPSCs (Fig. 6, E and G; ACSF: 12.6 ± 0.78 pA, PE: 13.5 ± 0.92 pA, P ≤ 0.02) and a significant rightward shift in the cumulative histogram in five of six neurons (KS: all P ≤ 0.019). This increase in mEPSC amplitude, along with the increase in holding current, suggests that α1-adrenoreceptors are housed on the postsynaptic neuron. Furthermore, since we also observed an increase in mEPSC frequency, it is likely that α1-adrenoreceptors are also located on presynaptic neurons.
Fig. 6.
A: representative trace showing an increase in holding current in response to PE in the presence of TTX. B: when the cells were held at −60 mV, PE significantly increased the holding current by ∼84% (*P ≤ 0.0001, n = 6). A second exposure of phenylephrine after a 5-min recovery (PE2) reached a similar value (*P ≤ 0.001). C: no statistically significant changes in membrane resistance were observed. D: representative trace of mEPSCs with TTX in the medium. When PE is perfused through the chamber, both the frequency and amplitude of mEPSCs are increased (bottom) compared with TTX alone (top). E: cumulative histogram from the cell in A. PE produced a significant rightward shift, indicating an increase in the frequency of higher-amplitude mEPSCs [Kolmogorov-Smirnov 2-sample test(KS): P ≤ 0.02]. F and G: there was a significant increase in mEPSC frequency (F, *P ≤ 0.003) and amplitude (G, *P ≤ 0.02) in the presence of PE, which returned to baseline after washout. H: representative trace showing an increase in sIPSCs in the presence of glutamate antagonists (DNQX and MK-801) after PE exposure (bottom) compared with control (top). I: cumulative histogram of sIPSC amplitudes from the cell in H (KS: P ≤ 0.0001). J: phenylephrine significantly increased the frequency of sIPSCs by 314% (*P ≤ 0.006, n = 5). K: the mean amplitude of sIPSCs was not significantly increased after PE exposure (P ≤ 0.112), but individual tests for each cell were highly significant (KS: all P ≤ 0.0001).
In a small number of cells (n = 3) we measured the response to phenylephrine in the absence of TTX and observed a significant increase in either or both frequency and amplitude of sEPSCs (KS: all P < 0.02), similar to that observed under TTX. In addition, phenylephrine increased sIPSC frequency in all three neurons (KS: all P < 0.005) and caused a change in amplitude in one (KS: P < 0.0001), an effect not observed with TTX in the medium. This increase in both the frequency and amplitude of spontaneous postsynaptic currents is again consistent with phenylephrine acting at both pre- and postsynaptic sites. However, in contrast to the TTX data, where we observed an increase in holding current in every instance, a short exposure of phenylephrine produced no significant change in holding current without TTX (data not shown) and one cell actually presented with a reduction in holding current of −34.1 pA, similar to what we observed in group 1 cells with norepinephrine application. This suggests that action potential-driven IPSCs can mitigate the depolarizing current induced by postsynaptic α1-adrenoreceptor activation and lead to an overall inhibitory effect in some cells. To provide further evidence that the α1-adrenoreceptor is expressed directly on presynaptic inhibitory neurons, we blocked ionotropic glutamate receptors with DNQX and MK-801 (10 μM) prior to phenylephrine. After ionotropic glutamate receptors were blocked, phenylephrine exposure increased the frequency of sIPSCs by 314% (Fig. 6J; DNQX/MK-801: 3.4 ± 0.87 IPSCs/s, PE DNQX/MK-801: 12.4 ± 3.3 IPSCs/s, P ≤ 0.006), an effect reversed during washout. Although there was no group effect on sIPSC amplitude (Fig. 6K: DNQX/MK-801: 14.7 ± 2.4 pA, PE DNQX/MK-801: 24.5 ± 6.5 pA), individual tests were highly significant in all cells (KS: all P < 0.0001). These data indicate that increases in sIPSCs induced by phenylephrine are not due to a network effect involving glutamate and suggest that the α1-adrenoreceptor is expressed directly on presynaptic inhibitory neurons.
With TTX in the medium the mean change in holding current with phenylephrine application was 41.2 pA. When MK-801/DNQX was added to the medium, the mean change in holding current induced by PE (−16.2 pA) was significantly less than the change induced in TTX alone (unpaired t-test, P = 0.037, n = 6 TTX, n = 5 non-TTX). This suggests that network inhibitory currents counteract the inward current in response to phenylephrine.
DISCUSSION
Summary
This study demonstrates that the cNST, the main sensory nucleus receiving signals from the viscera, sends both inhibitory and excitatory connections to preoromotor neurons in the caudal reticular formation. The evoked currents demonstrated properties consistent with both mono- and polysynaptic connectivity, similar to the results obtained by stimulating the (gustatory) rNST (Chen et al. 2012; Nasse et al. 2008). This suggests a common blueprint through which oral and visceral signals impact oromotor neurons associated with brain stem-mediated consummatory responses.
Both pre- and postsynaptic properties of prehypoglossal neurons were modulated by adrenoreceptor agonists. Norepinephrine suppressed cNST-driven EPSCs in prehypoglossal neurons, an effect duplicated with the α2-adrenoreceptor agonist DMT. Changes in the holding current in response to norepinephrine could be accounted for by a combination of both pre- and postsynaptic effects, further delineated by the use of selective adrenoreceptor agonists. The inward current (depolarization) produced by norepinephrine was likely caused by α1-adrenoreceptor activation via postsynaptic mechanisms and through increases in EPSCs via presynaptic (network) pathways. The reduction in holding current (inhibition) seen with norepinephrine was not likely induced by a postsynaptic α2-adrenoreceptor mechanism, but more likely by α1-adrenoreceptor activation of inhibitory presynaptic neurons from an as yet unknown location.
Prehypoglossal neurons, especially those receiving input from the cNST, provide a pathway for the visceral modulation of consummatory oromotor function. These neurons, however, are likely involved in other functions of the oral cavity, e.g., respiratory entrainment of the tongue. Pre- and postsynaptic modulation of these neurons by multiple classes of adrenoreceptors provides a substrate for the multifunctional role these neurons play in homeostatic regulation.
Technical Considerations
The medullary reticular formation becomes heavily myelinated after postnatal day 12 and renders it impossible to visualize individual neurons with infrared-differential interference optics used for slice preparations. As such, P7–P12 pups were used for the experiments in this study. Significant developmental changes do occur over this time period. Nevertheless, neonates in this age range respond in a manner similar to adults with regard to both satiety behavior (Smith et al. 1991) and oromotor pattern generation (Ganchrow et al. 1986; Nasse and Travers 2006). While it is true that neonatal animals can suckle and breathe simultaneously, experimental data from an in vitro brain stem block preparation in young (P0–P3) animals demonstrated that swallowing-like activity briefly inhibits respiratory activity in C3 nerve rootlets (Kogo et al. 2002), i.e., respiration and the consummatory response of suckling interact at the brain stem level. Therefore, while the use of young animals entails limitations, this preparation has the important advantage of allowing identified single-cell physiological experiments in a key brain region that are not possible in adult animals.
α2-Adrenoreceptors
α2-Adrenoreceptor stimulation provided direct evidence for noradrenergic modulation of cNST input onto prehypoglossal neurons by decreasing the amplitude of cNST-evoked EPSCs. Because cNST-evoked synaptic currents were blocked with ionotropic glutamate receptor antagonists, and there were no changes in membrane resistance, holding current, or mEPSC amplitude in response to DMT with TTX pretreatment, it is likely that α2-adrenoreceptors are expressed on the presynaptic terminals or cell bodies of glutamatergic neurons but not on the preoromotor neurons themselves. Consistent with this, α2-adrenoreceptor mRNA expression in the (presynaptic) cNST is denser than in the subjacent reticular formation (Rosin et al. 1993). In contrast to the effects on sEPSCs, α2-adrenoreceptor agonists affected neither the frequency nor the amplitude of sIPSCs, suggesting that α2-adrenoreceptors are not expressed on presynaptic inhibitory neurons projecting to prehypoglossal neurons (at least those within the confines of the slice).
The present results are similar to those of previous studies focused on other components of the medullary circuits of the gastrointestinal and feeding systems, where α2-adrenoreceptor activation was observed to suppress the activity of both cNST and dorsal motor nucleus of the vagus neurons in vitro and in vivo (Martinez-Peña y Valenzuela et al. 2004; Moore and Guyenet 1983). Furthermore, studies in many different brain regions show that α2-adrenoreceptor activation attenuates EPSC amplitude (Bertolino et al. 1997; Hayar and Guyenet 1999; Jiménez-Rivera et al. 2012). The α2-adrenoreceptor is coupled to Gi/o intracellular signaling pathways, and its activation can inhibit both neuronal activity and neurotransmitter release from synaptic terminals. For example, when the G protein subunits are dissociated upon receptor binding, the βγ-subunit activates G protein-activated inwardly rectifying potassium currents (GIRKs) and increases outward potassium conductance, leading to hyperpolarization of the neuronal membrane potential (Hein 2006). In fact, in light of the concordant decrease in holding current and membrane resistance seen in group 1 neurons after norepinephrine application, we predicted that such an α2-adrenoreceptor-mediated effect would be observed after stimulation with DMT in the presence of TTX. This was not the case, however, suggesting that the decrease in holding current observed with norepinephrine had a different origin, e.g., increases of inhibitory (likely GABA or glycine) input to prehypoglossal neurons mediated by α1-adrenoreceptor activation (see below). In addition to membrane potential hyperpolarization, inhibition of transmitter release can also be attributed to α2-adrenoreceptor activation through dissociation of the βγ-subunit. In this case the βγ-subunit binds directly to N- and P/Q-type calcium channels in a voltage-dependent manner and reduces channel conductance. This results in lower intraterminal calcium concentration and can inhibit neurotransmitter release (Bean 1989; Hille 1994; Tedford and Zamponi 2006; Zamponi and Snutch 1998). Indeed, this may be the mechanism for the reduction in evoked-response amplitude and increase in failure rate we observed with DMT application in patch-clamp experiments.
α1-Adrenoreceptors
Prolonged stimulation with the α1-adrenoreceptor agonist phenylephrine caused a large increase in holding current prior to reaching an equilibration point that precluded determining whether this agonist modulated cNST-evoked currents in prehypoglossal neurons. Nevertheless, we did observe postsynaptic changes in the presence of TTX with short (90 s) applications of the drug. Furthermore, we also observed presynaptic modulation of spontaneous and miniature postsynaptic currents, evidenced by increases in the frequency of these currents.
In the presence of TTX, phenylephrine consistently produced an inward current similar to what was observed in a subset of neurons stimulated with norepinephrine (in the absence of TTX). Although this indicates depolarization in response to α1-adrenoreceptor activation, we did not see a simultaneous increase in membrane resistance as would be expected if this inward current was mediated by inhibition of two-pore potassium channels via the PLC/diacylglycerol (DAG) intracellular signaling pathway (Talley et al. 2000). A lack of change in the membrane resistance might be explained by the simultaneous activation and inactivation of two different channels after α1-adrenoreceptor stimulation. Yamanaka et al. (2006) demonstrated that α1-adrenoreceptor activation induced an inward current in hypothalamic orexin neurons through the opening of a nonselective cation channel, potentially a member of the canonical transient receptor potential (TRPC) family, which should decrease membrane resistance. On the other hand, the same PLC/DAG signaling pathway that opens TRPC channels also inhibits GIRK and two-pore potassium channels (reviewed in Albert 2011), which would increase the membrane resistance. As such, it is possible that the inward current seen with phenylephrine is the result of the simultaneous activation of TRPC channels and the inhibition of GIRK channels with no net change in membrane resistance.
In addition to the increased holding current observed with phenylephrine in TTX, we also observed a significant increase in both the frequency and amplitude of sEPSCs and sIPSCs in ACSF. This suggests that α1-adrenoreceptors are expressed directly on prehypoglossal neurons and on the cell bodies or terminals of neurons that synapse onto them. Since our patch-clamp studies demonstrated that electrical stimulation of the cNST produced excitatory glutamatergic currents in prehypoglossal neurons, it is presumed that increased cNST activity in response to phenylephrine would also increase glutamatergic excitation in prehypoglossal neurons. Indeed, Hermann et al. (2005) demonstrated that phenylephrine causes oscillations in intracellular calcium and increased activity in cNST neurons that respond to gastric stretch. In our studies, the α1-adrenoreceptor agonist phenylephrine produced a robust increase in intracellular calcium signal that was attenuated by blocking ionotropic glutamate receptors. Although we could not identify these reticular formation neurons as prehypoglossal, they were located in the same region as the identified prehypoglossal cells and thus suggest that presynaptic glutamate release contributes to the increased intracellular calcium signal produced by α1-adrenoreceptor activation.
Very few inhibitory currents were observed when slices were pretreated with TTX in any paradigm tested in this study. However, both norepinephrine and phenylephrine significantly increased the frequency and amplitude of spontaneous inhibitory currents when TTX was not included. This may indicate that spontaneous inhibitory currents require action potentials in spontaneously active inhibitory neurons. This result is consistent with respiratory and cardiovascular circuits, where presynaptic GABA and glycine currents can influence autonomic output (Boychuk et al. 2011; O'Brien et al. 2004; O'Brien and Berger 1999). Inhibitory currents recorded from cardiac vagal neurons located in the nucleus ambiguus were potentiated in a fashion similar to our prehypoglossal neurons when exposed to norepinephrine or phenylephrine (Boychuk et al. 2011), that is, the increase in spontaneous inhibitory currents was dependent on action potentials since the potentiation was not observed in the presence of TTX.
β-Adrenoreceptors are also expressed in low to moderate levels in the region where we recorded (Wanaka et al. 1989). Therefore, it is possible that some of the effects observed with norepinephrine and attributed to α1-adrenoreceptors could also be mediated by the β-adrenoreceptor, which we did not test. For example, β-adrenoreceptor activation can inhibit K+ channels, leading to postsynaptic depolarization (Madison and Nicoll 1986a, 1986b), and thus could also lead to an increase in the frequency of EPSCs and IPSCs via presynaptic network activity (Bateman et al. 2012).
Functional Considerations
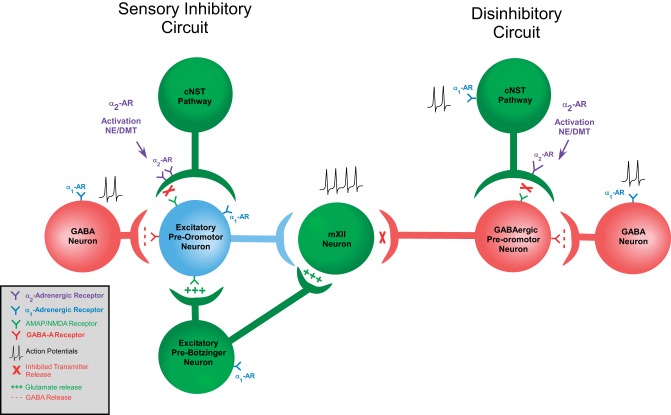
The main findings of this study indicate that norepinephrine activates multiple adrenoreceptor subtypes both pre- and postsynaptic to prehypoglossal neurons and exerts a host of physiological changes in reticular neurons. These complex effects are consistent with the complex and multifunctional nature of this region of the reticular formation as well as the known involvement of norepinephrine with a wide variety of functions. We propose that the excitatory and inhibitory effects of norepinephrine on these reticular neurons may represent two arms of autonomic regulation (Fig. 7). Suppression of excitatory currents from the cNST through α2-adrenoreceptor mechanisms may support a role for inhibiting sensory stimuli from propagating through the system during the satiety phase of ingestion, or in times of high sympathetic activation. Here we posit that an excitatory viscero-oromotor pathway from the cNST to (excitatory) prehypoglossal neurons is suppressed by norepinephrine acting presynaptically via α2-adrenoreceptors. For instance, suppression of afferent signals from the oral cavity that activate oromotor and swallowing central pattern generators could be suppressed after a meal. Indeed, it has been shown that both locally infused norepinephrine and leptin, which are released during satiety, can inhibit reflexive swallowing induced by stimulation of the superior laryngeal nerve (Félix et al. 2006; Kessler and Jean 1986b). Furthermore, the swallow central pattern generator is located in the cNST and acts to both recruit oropharyngeal muscles to perform a swallow and inhibit respiration (reviewed in Jean 2001; Lang 2009). In addition, the suppression of excitatory currents in prehypoglossal neurons elicited from the (taste) rNST are also attenuated by norepinephrine (Chen et al. 2012), suggesting that taste and visceral NST afferents modulate prehypoglossal neurons through similar pathways. The origin of the norepinephrine could potentially be A2 neurons located in the cNST that show enhanced activity during satiety (Appleyard et al. 2007; Rinaman 2003; Rinaman et al. 1998; Wellman 2005).
Fig. 7.
Schematic representation of hypothesized integrative circuits that inhibit orosensory pathways and allow respiratory input to entrain the hypoglossal motor nucleus. On left, glutamatergic sensory input from the NST is inhibited by presynaptic activation of α2-adrenoreceptors and prevents information from propagating through the circuit by inhibiting glutamate release onto glutamatergic preoromotor neurons. Additionally, GABAergic interneurons are activated through an α1-adrenoreceptor mechanism to further inhibit preoromotor neurons that govern ingestive oromotor activity. Respiratory-related neurons from other medullary regions, such as the pre-Bötzinger complex, could still entrain either the preoromotor neuron or the hypoglossal motor neurons themselves to maintain airway patency. On right, a disinhibitory circuit that withdraws tonic inhibition from the hypoglossal neuron is diagrammed. Here activation of α2-adrenoreceptors inhibits excitatory currents from the NST that project to a GABAergic preoromotor neuron. However, this GABAergic preoromotor neuron is inhibited through α1-adrenoreceptor-mediated activation of a GABAergic interneuron, leading to the disinhibition of the hypoglossal neuron. This would then allow direct projections from the pre-Bötzinger complex to entrain the hypoglossal motor neuron directly and entrain it to inspiratory activity.
While it may be advantageous to suppress chemosensory inputs from the tongue during satiety to inhibit consummatory behavior, respiratory-related signals need access to the tongue regardless of metabolic state. Here, we posit a role for α1-adrenoreceptors influencing GABAergic prehypoglossal neurons via a second GABAergic interneuron (Fig. 7). GABAergic neurons are well represented in the reticular formation lateral to the hypoglossal nucleus in the region we recorded (Li et al. 1997; Travers et al. 2005), and electrical stimulation of this area can elicit inhibitory currents in hypoglossal motor neurons (O'Brien et al. 2004; O'Brien and Berger 1999). While the infusion of GABA receptor agonists into the hypoglossal nucleus reduces inspiratory phase-related hypoglossal nerve activity (Okabe et al. 1994), the infusion of GABAA receptor agonists into the reticular area subjacent to the cNST in the region from which we recorded results in increased activity of the hypoglossal motor nucleus. This suggests a mechanism of disinhibition for potentiating hypoglossal inspiratory-phase activity (Chamberlin et al. 2007). Thus, simultaneous with norepinephrine suppressing a viscero-oromotor circuit related to metabolic state via α2-adrenoreceptors on excitatory projections to prehypoglossal neurons, norepinephrine could also be disinhibiting lingual motor neurons via α1-adrenoreceptor excitation of GABAergic interneurons projecting to GABAergic prehypoglossal neurons.
While we did not test the phenotypes of neurons recorded in this study, it is possible that a proportion of the cells are GABAergic and the increased inhibitory drive we observed is evidence for an α1-adrenoreceptor-mediated disinhibition of hypoglossal activity. Future studies could test this hypothesis with a transgenic mouse line currently available that expresses a variant of yellow fluorescent protein (Venus) in inhibitory neurons (Wang et al. 2009). While the exact details of mechanisms and the overall neurophysiological circuit remain to be determined, this study provides evidence for noradrenergic modulation of a multifunctional substrate that could support both respiratory and oral consummatory behavior.
GRANTS
This work was supported by National Institutes of Health Grants DC-00416, DC-00417, and DE 014320.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.S.N. and J.B.T. conception and design of research; J.S.N. performed experiments; J.S.N. and J.B.T. analyzed data; J.S.N. and J.B.T. interpreted results of experiments; J.S.N. prepared figures; J.S.N. drafted manuscript; J.S.N. and J.B.T. edited and revised manuscript; J.S.N. and J.B.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Alexandra Toole for excellent technical assistance and Dr. Susan Travers for invaluable insights and critical reading of the manuscript. Calcium imaging experiments were performed with the instruments and services at the Campus Microscopy and Imaging Facility at The Ohio State University.
REFERENCES
- Albert AP. Gating mechanisms of canonical transient receptor potential channel proteins: role of phosphoinositols and diacylglycerol. Adv Exp Med Biol 704: 391–411, 2011 [DOI] [PubMed] [Google Scholar]
- Appleyard SM, Marks D, Kobayashi K, Okano H, Low MJ, Andresen MC. Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. J Neurosci 27: 13292–13302, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Boychuk CR, Philbin KE, Mendelowitz D. Beta adrenergic receptor modulation of neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Neuroscience 210: 58–66, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature 340: 153–156, 1989 [DOI] [PubMed] [Google Scholar]
- Bertolino M, Vicini S, Gillis R, Travagli A. Presynaptic alpha2-adrenoceptors inhibit excitatory synaptic transmission in rat brain stem. Am J Physiol Gastrointest Liver Physiol 272: G654–G661, 1997 [DOI] [PubMed] [Google Scholar]
- Boxwell AJ, Yanagawa Y, Travers SP, Travers JB. The mu-opioid receptor agonist DAMGO presynaptically suppresses solitary tract-evoked input to neurons in the rostral solitary nucleus. J Neurophysiol 109: 2815–2826, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boychuk CR, Bateman RJ, Philbin KE, Mendelowitz D. Alpha1-adrenergic receptors facilitate inhibitory neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Neuroscience 193: 154–161, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin NL, Eikermann M, Fassbender P, White DP, Malhotra A. Genioglossus premotoneurons and the negative pressure reflex in rats. J Physiol 579: 515–526, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Travers SP, Travers JB. Activation of NPY receptors suppresses excitatory synaptic transmission in a taste-feeding network in the lower brain stem. Am J Physiol Regul Integr Comp Physiol 302: R1401–R1410, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu TH, Chen MJ, Yang YR, Yang JJ, Tang FI. Action of dexmedetomidine on rat locus coeruleus neurones: intracellular recording in vitro. Eur J Pharmacol 285: 261–268, 1995 [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Sawchenko PE. A circumscribed projection from the nucleus of the solitary tract to the nucleus ambiguus in the rat: anatomical evidence for somatostatin-28-immunoreactive interneurons subserving reflex control of esophageal motility. J Neurosci 9: 1668–1682, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham ET, Sawchenko PE. Dorsal medullary pathways subserving oromotor reflexes in the rat: implications for the central neural control of swallowing. J Comp Neurol 417: 448–466, 2000 [PubMed] [Google Scholar]
- Doyle MW, Andresen MC. Reliability of monosynaptic sensory transmission in brain stem neurons in vitro. J Neurophysiol 85: 2213–2223, 2001 [DOI] [PubMed] [Google Scholar]
- Félix B, Jean A, Roman C. Leptin inhibits swallowing in rats. Am J Physiol Regul Integr Comp Physiol 291: R657–R663, 2006 [DOI] [PubMed] [Google Scholar]
- Fukuda A, Minami T, Nabekura J, Oomura Y. The effects of noradrenaline on neurones in the rat dorsal motor nucleus of the vagus, in vitro. J Physiol 393: 213–231, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganchrow JR, Steiner JE, Canetto S. Behavioral displays to gustatory stimuli in newborn rat pups. Dev Psychobiol 19: 163–174, 1986 [DOI] [PubMed] [Google Scholar]
- Grill HJ. Leptin and the systems neuroscience of meal size control. Front Neuroendocrinol 31: 61–78, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Smith GP. Cholecystokinin decreases sucrose intake in chronic decerebrate rats. Am J Physiol Regul Integr Comp Physiol 254: R853–R856, 1988 [DOI] [PubMed] [Google Scholar]
- Hayar A, Guyenet PG. Alpha2-adrenoceptor-mediated presynaptic inhibition in bulbospinal neurons of rostral ventrolateral medulla. Am J Physiol Heart Circ Physiol 277: H1069–H1080, 1999 [DOI] [PubMed] [Google Scholar]
- Hein L. Adrenoceptors and signal transduction in neurons. Cell Tissue Res 326: 541–551, 2006 [DOI] [PubMed] [Google Scholar]
- Hermann GE, Nasse JS, Rogers RC. Alpha-1 adrenergic input to solitary nucleus neurones: calcium oscillations, excitation and gastric reflex control. J Physiol 562: 553–568, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikasa Y, Akiba T, Iino Y, Matsukura M, Takase K, Ogasawara S. Central alpha-adrenoceptor subtypes involved in the emetic pathway in cats. Eur J Pharmacol 229: 241–251, 1992 [DOI] [PubMed] [Google Scholar]
- Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci 17: 531–536, 1994 [DOI] [PubMed] [Google Scholar]
- Inyushin MU, Arencibia-Albite F, Vázquez-Torres R, Vélez-Hernández ME, Jiménez-Rivera CA. Alpha-2 noradrenergic receptor activation inhibits the hyperpolarization-activated cation current (Ih) in neurons of the ventral tegmental area. Neuroscience 167: 287–297, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev 81: 929–969, 2001 [DOI] [PubMed] [Google Scholar]
- Jiménez-Rivera CA, Figueroa J, Vázquez-Torres R, Vélez-Hernandez ME, Schwarz D, Velásquez-Martinez MC, Arencibia-Albite F. Presynaptic inhibition of glutamate transmission by α2 receptors in the VTA. Eur J Neurosci 35: 1406–1415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler JP, Jean A. Inhibitory influence of monoamines and brainstem monoaminergic regions on the medullary swallowing reflex. Neurosci Lett 65: 41–46, 1986a [DOI] [PubMed] [Google Scholar]
- Kessler JP, Jean A. Effect of catecholamines on the swallowing reflex after pressure microinjections into the lateral solitary complex of the medulla oblongata. Brain Res 386: 69–77, 1986b [DOI] [PubMed] [Google Scholar]
- Kogo M, Yamanishi T, Koizumi H, Matsuya T. Swallowing-like activity elicited in vitro in neonatal rat organ attached brainstem block preparation. Brain Res 955: 24–33, 2002 [DOI] [PubMed] [Google Scholar]
- Lang IM. Brain stem control of the phases of swallowing. Dysphagia 24: 333–348, 2009 [DOI] [PubMed] [Google Scholar]
- Li YQ, Takada M, Kaneko T, Mizuno N. Distribution of GABAergic and glycinergic premotor neurons projecting to the facial and hypoglossal nuclei in the rat. J Comp Neurol 378: 283–294, 1997 [DOI] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Actions of noradrenaline recorded intracellularly in rat hippocampal CA1 pyramidal neurones, in vitro. J Physiol 372: 221–244, 1986a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Cyclic adenosine 3′,5′-monophosphate mediates beta-receptor actions of noradrenaline in rat hippocampal pyramidal cells. J Physiol 372: 245–259, 1986b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Peña y Valenzuela I, Rogers RC, Hermann GE, Travagli RA. Norepinephrine effects on identified neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol 286: G333–G339, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AJ. Oral and pharyngeal reflexes in the mammalian nervous system: their diverse range in complexity and the pivotal role of the tongue. Crit Rev Oral Biol Med 13: 409–425, 2002 [DOI] [PubMed] [Google Scholar]
- Minneman KP, Theroux TL, Hollinger S, Han C, Esbenshade TA. Selectivity of agonists for cloned alpha 1-adrenergic receptor subtypes. Mol Pharmacol 46: 929–936, 1994 [PubMed] [Google Scholar]
- Moore SD, Guyenet PG. Alpha-receptor mediated inhibition of A2 noradrenergic neurons. Brain Res 276: 188–191, 1983 [DOI] [PubMed] [Google Scholar]
- Nasse J, Terman D, Venugopal S, Hermann G, Rogers R, Travers JB. Local circuit input to the medullary reticular formation from the rostral nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 295: R1391–R1408, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasse J, Travers JB. Solitary nucleus-reticular formation projections in a neonatal slice preparation. Chem Senses 31: A1–A144, 2006 [Google Scholar]
- O'Brien JA, Berger AJ. Cotransmission of GABA and glycine to brain stem motoneurons. J Neurophysiol 82: 1638–1641, 1999 [DOI] [PubMed] [Google Scholar]
- O'Brien JA, Sebe JY, Berger AJ. GABAB modulation of GABAA and glycine receptor-mediated synaptic currents in hypoglossal motoneurons. Respir Physiol Neurobiol 141: 35–45, 2004 [DOI] [PubMed] [Google Scholar]
- Okabe S, Woch G, Kubin L. Role of GABAB receptors in the control of hypoglossal motoneurons in vivo. Neuroreport 5: 2573–2576, 1994 [DOI] [PubMed] [Google Scholar]
- Ono T, Ishiwata Y, Inaba N, Kuroda T, Nakamura Y. Hypoglossal premotor neurons with rhythmical inspiratory-related activity in the cat: localization and projection to the phrenic nucleus. Exp Brain Res 98: 1–12, 1994 [DOI] [PubMed] [Google Scholar]
- Ono T, Ishiwata Y, Kuroda T, Nakamura Y. Swallowing-related perihypoglossal neurons projecting to hypoglossal motoneurons in the cat. J Dent Res 77: 351–360, 1998 [DOI] [PubMed] [Google Scholar]
- Rinaman L. Hindbrain noradrenergic lesions attenuate anorexia and alter central cFos expression in rats after gastric viscerosensory stimulation. J Neurosci 23: 10084–10092, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol 300: R222–R235, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, Baker EA, Hoffman GE, Stricker EM, Verbalis JG. Medullary c-Fos activation in rats after ingestion of a satiating meal. Am J Physiol Regul Integr Comp Physiol 275: R262–R268, 1998 [DOI] [PubMed] [Google Scholar]
- Rogers RC, Travagli RA, Hermann GE. Noradrenergic neurons in the rat solitary nucleus participate in the esophageal-gastric relaxation reflex. Am J Physiol Regul Integr Comp Physiol 285: R479–R489, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Zeng D, Stornetta RL, Norton FR, Riley T, Okusa MD, Guyenet PG, Lynch KR. Immunohistochemical localization of alpha 2A-adrenergic receptors in catecholaminergic and other brainstem neurons in the rat. Neuroscience 56: 139–155, 1993 [DOI] [PubMed] [Google Scholar]
- Saito Y, Ezure K, Tanaka I, Osawa M. Activity of neurons in ventrolateral respiratory groups during swallowing in decerebrate rats. Brain Dev 25: 338–345, 2003 [DOI] [PubMed] [Google Scholar]
- Scheinin H, Virtanen R, MacDonald E, Lammintausta R, Scheinin M. Medetomidine—a novel alpha 2-adrenoceptor agonist: a review of its pharmacodynamic effects. Prog Neuropsychopharmacol Biol Psychiatry 13: 635–651, 1989 [DOI] [PubMed] [Google Scholar]
- Schneider JE, Wise JD, Benton NA, Brozek JM, Keen-Rhinehart E. When do we eat? Ingestive behavior, survival, and reproductive success. Horm Behav 64: 702–728, 2013 [DOI] [PubMed] [Google Scholar]
- Seeley RJ, Grill HJ, Kaplan JM. Neurological dissociation of gastrointestinal and metabolic contributions to meal size control. Behav Neurosci 108: 347–352, 1994 [PubMed] [Google Scholar]
- Shirasaka T, Kannan H, Takasaki M. Activation of a G protein-coupled inwardly rectifying K+ current and suppression of Ih contribute to dexmedetomidine-induced inhibition of rat hypothalamic paraventricular nucleus neurons. Anesthesiology 107: 605–615, 2007 [DOI] [PubMed] [Google Scholar]
- Smith GP, Jerome C, Cushin BJ, Eterno R, Simansky KJ. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science 213: 1036–1037, 1981 [DOI] [PubMed] [Google Scholar]
- Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol Regul Integr Comp Physiol 249: R638–R641, 1985 [DOI] [PubMed] [Google Scholar]
- Smith GP, Tyrka A, Gibbs J. Type-A CCK receptors mediate the inhibition of food intake and activity by CCK-8 in 9- to 12-day-old rat pups. Pharmacol Biochem Behav 38: 207–210, 1991 [DOI] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron 25: 399–410, 2000 [DOI] [PubMed] [Google Scholar]
- Tedford HW, Zamponi GW. Direct G protein modulation of Cav2 calcium channels. Pharmacol Rev 58: 837–862, 2006 [DOI] [PubMed] [Google Scholar]
- Travers JB, DiNardo LA, Karimnamazi H. Medullary reticular formation activity during ingestion and rejection in the awake rat. Exp Brain Res 130: 78–92, 2000 [DOI] [PubMed] [Google Scholar]
- Travers JB, Yoo JE, Chandran R, Herman K, Travers SP. Neurotransmitter phenotypes of intermediate zone reticular formation projections to the motor trigeminal and hypoglossal nuclei in the rat. J Comp Neurol 488: 28–47, 2005 [DOI] [PubMed] [Google Scholar]
- Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specificity and potency of medetomidine as an alpha 2-adrenoceptor agonist. Eur J Pharmacol 150: 9–14, 1988 [DOI] [PubMed] [Google Scholar]
- Wanaka A, Kiyama H, Murakami T, Matsumoto M, Kamada T, Malbon CC, Tohyama M. Immunocytochemical localization of beta-adrenergic receptors in the rat brain. Brain Res 485: 125–140, 1989 [DOI] [PubMed] [Google Scholar]
- Wang Y, Kakizaki T, Sakagami H, Saito K, Ebihara S, Kato M, Hirabayashi M, Saito Y, Furuya N, Yanagawa Y. Fluorescent labeling of both GABAergic and glycinergic neurons in vesicular GABA transporter (VGAT)-venus transgenic mouse. Neuroscience 164: 1031–1043, 2009 [DOI] [PubMed] [Google Scholar]
- Wellman PJ. Modulation of eating by central catecholamine systems. Curr Drug Targets 6: 191–199, 2005 [DOI] [PubMed] [Google Scholar]
- Willing AE, Berthoud HR. Gastric distension-induced c-fos expression in catecholaminergic neurons of rat dorsal vagal complex. Am J Physiol Regul Integr Comp Physiol 272: R59–R67, 1997 [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Muraki Y, Ichiki K, Tsujino N, Kilduff TS, Goto K, Sakurai T. Orexin neurons are directly and indirectly regulated by catecholamines in a complex manner. J Neurophysiol 96: 284–298, 2006 [DOI] [PubMed] [Google Scholar]
- Yamanishi T, Takao K, Koizumi H, Ishihama K, Nohara K, Komaki M, Enomoto A, Yokota Y, Kogo M. Alpha2-adrenoceptors coordinate swallowing and respiration. J Dent Res 89: 258–263, 2010 [DOI] [PubMed] [Google Scholar]
- Zamponi GW, Snutch TP. Decay of prepulse facilitation of N type calcium channels during G protein inhibition is consistent with binding of a single Gbeta subunit. Proc Natl Acad Sci USA 95: 4035–4039, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]