Abstract
BACKGROUND/OBJECTIVES
In this study, we determined the anti-inflammatory activities and the underlying molecular mechanisms of the methanol extract from Erigeron Canadensis L. (ECM) in LPS-stimulated RAW264.7 macrophage cells.
MATERIALS/METHODS
The potential anti-inflammatory properties of ECM were investigated by using RAW264.7 macrophages. We used western blot assays and real time quantitative polymerase chain reaction to detect protein and mRNA expression, respectively. Luciferase assays were performed to determine the transactivity of transcription factors.
RESULTS
ECM significantly inhibited inducible nitric oxide synthase (iNOS)-derived NO and cyclooxygenase-2 (COX-2) derived PGE2 production in LPS-stimulated RAW264.7 macrophages. These inhibitory effects of ECM were accompanied by decreases in LPS-induced nuclear translocations and transactivities of NFκB. Moreover, phosphorylation of mitogen-activated protein kinase (MAPKs) including extracellular signal-related kinase (ERK1/2), p38, and c-jun N-terminal kinase (JNK) was significantly suppressed by ECM in LPS-stimulated RAW264.7 macrophages. Further studies demonstrated that ECM by itself induced heme oxygenase-1 (HO-1) protein expression at the protein levels in dose-dependent manner. However, zinc protoporphyrin (ZnPP), a selective HO-1 inhibitor, abolished the ECM-induced suppression of NO production.
CONCLUSIONS
These results suggested that ECM-induced HO-1 expression was partly responsible for the resulting anti-inflammatory effects. These findings suggest that ECM exerts anti-inflammatory actions and help to elucidate the mechanisms underlying the potential therapeutic values of Erigeron Canadensis L.
Keywords: Erigeron Canadensis L., anti-inflammation, nitric oxide, heme oxygenase-1, NFκB
INTRODUCTION
Inflammation is a complex reaction against many pathological conditions including tissue injury and microbial invasions. Many stimuli can activate inflammatory leukocytes, such as macrophages, resulting in the induction and synthesis of proinflammatory proteins and enzymes [1]. When the body is stimulated by pathologic injuries, the activated macrophage cells secrete nitric oxide (NO) synthesized from L-arginine by inducible nitric oxide synthase (iNOS) and prostaglandin E2 (PGE2) produced from arachidonic acid metabolites by cyclooxygeanse-2 (COX-2) [2]. The production of these proinflammatory mediators was associated with increased activation of the gene transcriptional regulators, mitogen-activated protein kinases (MAPKs) and nuclear factor κB (NFκB). MAPKs and NFκB play a pivotal role as mediators of cellular responses to extracellular signals [3]. Of the five subunits of the NFκB family (p50, p65 (RelA), c-Rel, p52, and RelB) in mammals, the p50/p65 heterodimers and p50 homodimers are normally sequestered in the cytoplasm by inhibitors of κB (IκB) in normal conditional cells. NFκB activation in response to pro-inflammatory stimuli involves the rapid phosphorylations of IκBs by the IκB kinase (IKK) complex. The NFκB subunits of p50/p65 released from IκBα is translocated into the nucleus where they binds into specific sequences in the cis-acting κB enhancer element of target genes, which leads to the transcription of inflammation-associated genes, such as iNOS and COX-2 [1,4]. Moreover, NFκB is activated by phosphorylation of IκBα via MAPKs families such as p38, extracellular signal regulated kinase (ERK) and c-jun N terminal kinase (JNK). MAPKs react to extracellular stimuli and control various cellular activities including gene expressions, mitosis, differentiations and cell survivals/apoptosis [5]. Once activated by kinases, MAPK is able to phosphorylate the transcription factors such as NFκB, or transcriptionally co-regulate or phosphorylate downstream kinases that lead to the expression of pro-inflammatory mediators of extracellular stimuli [6]. Therefore, MAPKs and NFκB are known as important targets for anti-inflammatory molecules.
Heme oxygeanse-1 (HO-1) is a rate-limiting enzyme in heme catabolism, leading to the formation of carbon monoxide (CO), free Fe2+ irons, and biliverdin [7]. HO-1 has been considered as a cytoprotective enzyme due to the antioxidant activities of biliverdin and its metabolite, bilirubin, as well as the anti-inflammatory ability of CO [8]. Recent studies have shown that a number of therapeutic agents exert anti-inflammatory activities via the upregulation of HO-1 expression [9].
The medicinal plant Erigeron Canadensis (L.) Cronq. is winter annual or biennial in the Comosite family, native to and commonly found around North America [10]. The blossoming parts of this species have been used as a traditional folk medicine to treat a wide range of diseases including edema, hematuria, hepatitis, and cholecystitis. Phytochemical studies of Erigeron canadensis L. (EC) revealed the presence of C10 acetylenes, sesquiterpene hydrocarbons, polyphenolic-polysaccharide, flavonoids, sterols, triterpenes, and tannins which are reported to have remarkable anti-fungal, anti-platelet and anti-inflammatory effects [10,11,12,13]. However, the molecular mechanisms underlying the anti-inflammatory effects of EC are yet to be established. Therefore, this study investigates the anti-inflammatory effect of methanol extract of Erigeron Canadensis L. (ECM) and the underlying molecular mechanism involved in LPS-stimulated RAW264.7 macrophages.
MATERIAL AND METHODS
Chemicals
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS) and PSN antibiotic solution (penicillin-streptomycin-neo-mycin) were obtained from Gibco BRL (Gaithersburg, MD, USA). LPS (Escherichia coli, serotype O111:B4), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO) and Griess reagent were purchased from Sigma (St. Louis, MO, USA). Zinc protoporphyrin (ZnPP) was obtained from Porphyrin Products (Logan, UT, USA). Antibodies were from the following sources: iNOS, COX-2, HO-1, NFκB p65, NFκB p50, IκB-α, p-IκB-α, lammin B and Anti-rabbit, anti-goat and anti-mouse IgG conjugated to horseradish-peroxidase (HRP) (Santa Cruz, CA, USA), and β-actin (Sigma). Anti-ERK1/2 (where ERK indicates extracellular regulated kinase) and antiphospho-ERK1/2 (p-ERK), anti-JNK1/2 (where JNK indicates c-Jun N-terminal kinase) and antiphospho-JNK1/2 (p-JNK1/2), anti-p38 (where where p38 kinase) obtained from Cell Signaling Technology (Izasa, Madrid, Spain). Lipofectamine 2000 was obtained from Invitrogen (Carlsbad, CA, USA) and Dual-Glo Luciferase assay kit was obtained from Promega (Madison, CA, USA). All other chemicals were of the highest pure grade available on the market.
Sample preparation
The lyophilized aboveground part of EC was obtained from Rural Development Administration in Korea. Approximately 5g of The EC was extracted with 300 mL methanol by shaking for 24 h at room temperature and filtered through Whatman No. 2 filter paper, and the extracts were evaporated under vacuum. A yield of 1.53 g (30.6%) was obtained. The dried residues were redissolved in DMSO and subsequently passed through a 0.22 µm sterile filter. The sample was stored at -20℃ until analysis. ECM was diluted in the medium so that the final concentration of DMSO was less than 0.1% v/v.
Cell culture
The murine macrophage cell line RAW264.7 cells were obtained from the Korean Cell Line Bank (KCLB). RAW264.7 macrophages were grown in DMEM and supplemented with 10% FBS, 2 mM glutamine, 100 unit/ml penicillin, 50 µg/ml streptomycin at 37℃ in a humidified atmosphere of 5% CO2.
Cell viability assay
We evaluated the protective effects of ECM by using MTT colorimetric assay [14]. RAW264.7 macrophages were seeded in 96-well plate at a density of 1.5 × 105 cells/well. After 6 h, the culture medium was replaced with FBS-free medium containing various concentration of ECM (25, 50, 100, and 200 µg/ml). After 24 h, 20 µL of MTT (1 mg/ml) was added to each well and incubated for 4 h at 37℃ (5% CO2). After incubation, the supernatant was removed and the blue crystal formazan crystals produced in viable cells were solubilized with DMSO. We quantified relative cell viability by measuring the absorbance at 550 nm using a microplate reader (BioTek, Inc., Winooski, VT, USA). All experiments were performed in a triplicate manner.
Measurement of NO and PGE2
RAW 264.7 macrophages were seeded in 96-well plate at a density of 1.5 × 105 cells/well for 6 h, followed by treatment with or without LPS (1 µg/ml) and different concentrations of ECM (25, 50, 100, and 200 µg/ml) for a further 24 h. In some experiments, ZnPP was added to the plates together with ECM. The nitrite accumulation in the supernatant was determined by Griess reaction. Each 100 µL of culture supernatant was mixed with an equal volume of Griess reagent and incubated for 10 minutes at 37℃ (5% CO2). The absorbance of the mixture at 550 nm was determined by using a microplate reader, and nitrite concentration was determined with the dilution of sodium nitrite as a standard. The amount of PGE2 in the culture supernatant was estimated using PGE2 ELISA kit (Minneapolis, MN, USA) according to the manufacturer's guidelines.
Westernblot analysis
Expression levels of protein were examined by Westernblot analysis. Whole cell extracts were obtained after treatment by harvesting cells, washing them with ice-cold phosphate buffered saline (PBS), and lysing in a Pro-Prep™ sample buffer (iNtRON Biotech, Seongnam, Korea). To obtain nuclear and cytoplasmic lysates, the cell pellets were lysed using NE-PER® nuclear and cytoplasmic extraction reagents (Pierce Biotechnology Inc., Rockford, IL, USA) according to the manufacturer's guidelines. The protein concentration of each sample was quantified by use of the BCA protein assay reagent. Equal amounts of proteins were denatured and separated on SDS-polyacrylamide gels, and then transferred to nitrocellulose membranes. Membranes were blocked with 5% nonfat milk in Tris-buffered saline and Tween 20 (TBST) for 1 h and incubated overnight with antibodies at 4℃ against iNOS (1:1000), COX-2 (1:1000), HO-1 (1:1000), β-actin (1:2000), IκBα (1:500), phospho-IκBα (1:500), p65 (1:500), p50 (1:500), ERK1/2 (1:1000), phospho-ERK1/2 (1:1000), JNK1/2 (1:500), phospho-JNK1/2 (1:500), p38 (1:500), phospho-p38 (1:500) and lammin B (1:1000). After washing with TBST buffer, a 1:1000 dilution of horseradish peroxidase-labeled IgG was added at room temperature for 60 minutes. Protein bands were visualized on X-ray film activated by chemiluminescence using the super signal west pico chemiluminescent substrate (Thermo scientific, MA, USA).
Luciferase assay
RAW264.7 macrophages were transfected using the Lipofectamine 2000 and pNFκB-Luc reporter plasmid, as instructed by the manufacturer's instruction. The RAW264.7 macrophages were seeded in black 96-well plate at a density of 1.5 × 105 cells/well. After the start of transfection for 24 h, cells were pretreated with ECM (25, 50, 100, and 200 µg/ml) for 1 h and treated with LPS (1 µg/ml). Following 24 h of stimulation, cells were lysed and the luciferase activity was determined using the Promega luciferase assay system (Madison, CA, USA) and luminometer (Panomics, Fremont, CA, USA).
Statistical analysis
All experiments were repeated at least three times. Results are reported as mean ± standard error (SE). Statistical comparisons were determined by one-way analysis of variance (ANOVA) with SAS version 8.1 (SAS Institute, Cary, NC, USA), followed by a Duncan's multiple comparison test. A P-value of 0.05 was considered significant.
RESULTS
Effects of ECM on the LPS-induced NO and PGE2 production and cell viability
The potential anti-inflammatory properties of ECM were investigated by using RAW264.7 macrophages, which can produce NO and PGE2 upon stimulation with LPS. RAW264.7 cells were stimulated with LPS (1 µg/ml) and different concentrations of ECM (25, 50, 100 and 200 µg/ml). LPS treatment significantly increased the concentrations of NO and PGE2. ECM significantly suppressed the LPS-induced NO and PGE2 production at 100 and 200 µg/ml of ECM in RAW264.7 cells (Fig. 1A and B). ECM did not influence the cytotoxicity of RAW264.7 cells at the employed concentrations (25, 50, 100 and 200 µg/ml). Thus, the inhibitory effects were deemed as to be not attributed to cytotoxic effects (Fig. 1A)
Fig. 1.
Inhibitory effects of ECM on nitrite and PGE2 production in LPS-stimulated RAW264.7 macrophages. Cells were co-incubated with indicated concentrations of ECM and LPS for 24 h. The treated culture media were used to measure the amounts of nitrite production (A) and PGE2 production (B). Cytotoxic effect of ECM was measured by MTT assay (A). Each value is expressed as a mean ± standard error (n = 3). #P < 0.05 compared to control, *P < 0.05 compared to LPS.
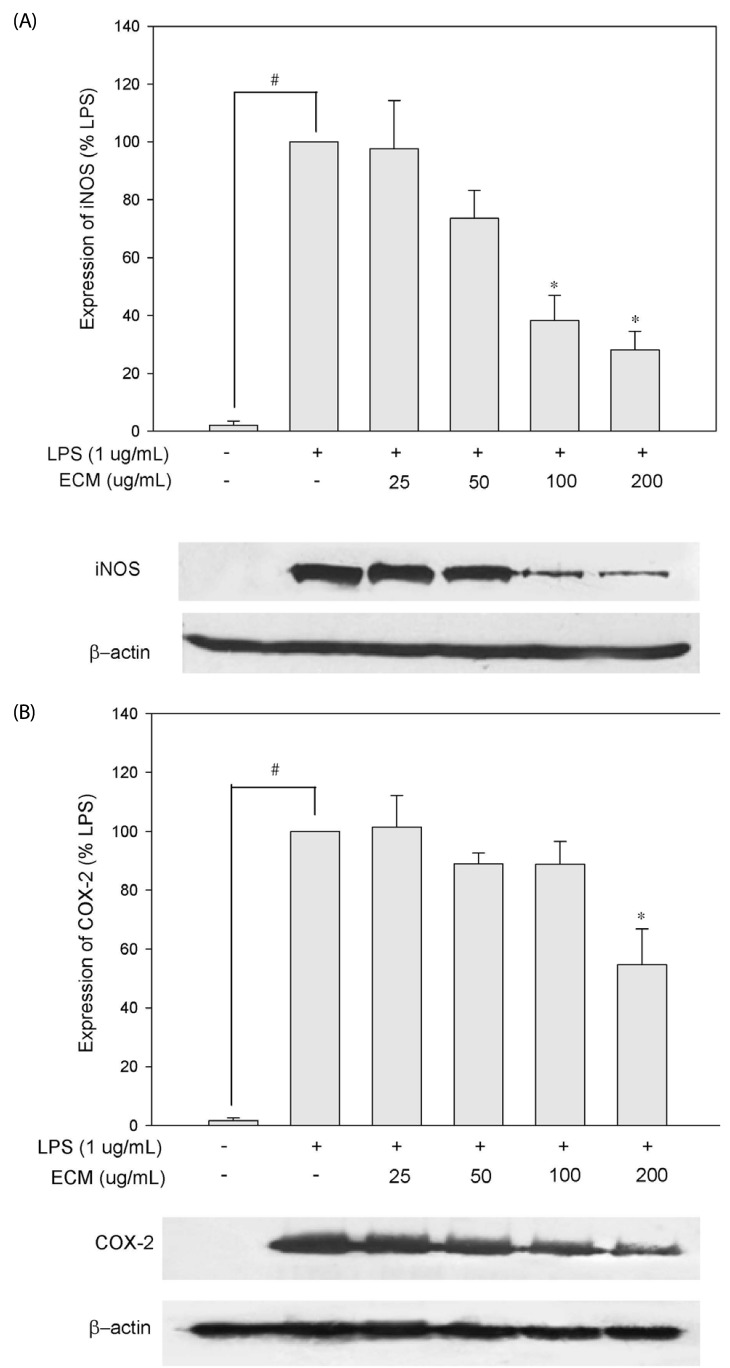
Effects of ECM on the LPS-induced iNOS and COX-2 protein expression
The expression of the pro-inflammatory enzymes, such as iNOS and COX-2, plays critical roles in immune-activated macrophages and at inflammatory sites by producing COX-2-derived PGE2 and iNOS-derived NO [15]. To understand whether the inhibitory effects of ECM on NO and PGE2 productions are related to the regulation of the expression in iNOS and COX-2, respectively, we performed the Western blot analysis using cell extracts from RAW264.7 macrophages exposed to LPS in the absence or presence of ECM. The iNOS and COX-2 signals were barely detected in normal macrophages but were remarkably increased upon exposures to the LPS alone (Fig. 2A and B). ECM treatment significantly inhibited this LPS-stimulated iNOS expression at 100 and 200 µg/ml, while COX-2 protein expressions were partially inhibited by 200 µg/ml of ECM in RAW264.7 cells.
Fig. 2.
Inhibitory effects of ECM on iNOS (A) and COX-2 (B) expression in LPS-stimulated RAW264.7 macrophages. Cells were co-incubated with indicated concentrations of ECM and LPS for 24 h. Each value is expressed as a mean ± standard error (n = 3). #P < 0.05 compared to control, *P < 0.05 compared to LPS.
Effects of ECM on the LPS-induced NFκB translocation and activation
The transcription factor of NFκB is implicated in the regulation of many genes that code for mediators of pro-inflammatory responses, e.g. iNOS and COX-2 [16]. Thus, we considered that the NFκB signaling pathway might be involved in the ECM-mediated down-regulations of iNOS and COX-2. Accordingly, we also examined the effects of ECM at concentrations of 25-200 µg/ml on the NFκB signaling pathway. As shown in Fig. 3A, the level of nuclear p65 and p50 protein was increased in LPS-activated RAW264.7 macrophages for 1 h. However, this was gradually inhibited by increasing concentrations of ECM. To investigate effects of ECM on LPS-stimulated NFκB promoter activity, we also performed transfections of RAW264.7 cells with a pNFκB-luc plasmid generated by inserting NFκB-binding sites into pLuc-promoter vector. Exposure of cells to LPS increased the luciferase activity in the cells transfected with the pNFκ B-luciferase reporter construct (Fig. 3B). In the presence of ECM from 25 to 200 µg/ml, the luciferase activity of NFκB was suppressed in a dose-dependent manner (P < 0.05). These results demonstrated that ECM inhibits the LPS-induced nuclear translocation and transactivity of NFκB, and ultimately suppresses the production of COX-2-derived PGE2 and iNOS-derived NO.
Fig. 3.
Inhibitory effects of ECM on NFκB translation (A) and promoter activity (B) in LPS-stimulated RAW264.7 macrophages. Cells were co-incubated with indicated concentrations of ECM and LPS for 1 h. Each value is expressed as a mean ± standard error (n = 3). #P < 0.05 compared to control, *P < 0.05 compared to LPS.
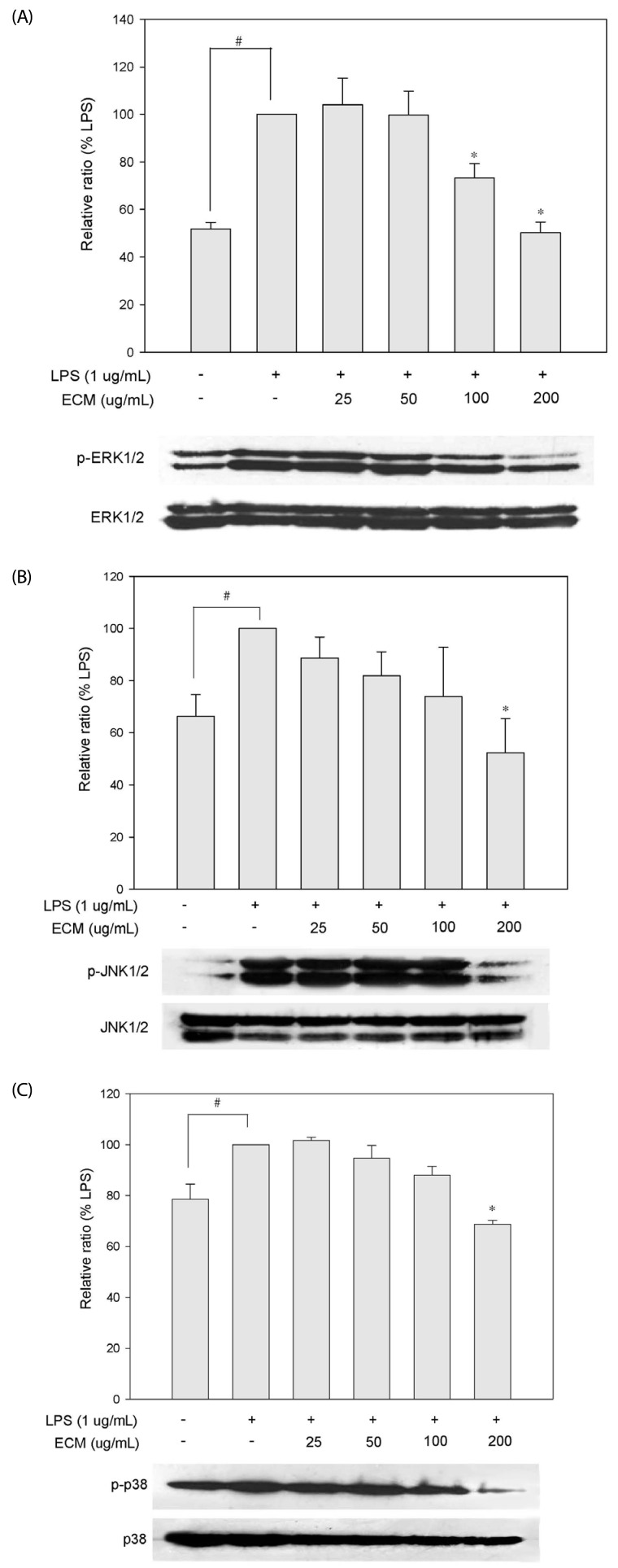
Effects of ECM on the LPS-induced phosphorylation of MAPKs
MAPKs are important mediators involved in the interacellular network of proteins that transduce extracellular cues to intracellular responses [17]. It is well known that the phosphorylation and activation status of kinases in the MAPK system has crucial impacts on the secretion of inflammatory factors and transcriptional activation of NFκB in LPS-induced macrophages [18]. To further investigate whether the inhibition NFκB activation and inflammatory mediators by ECM is modulated through the MAPK pathway, we evaluated the effects of ECM for the LPS-induced phosphorylation of ERK1/2, JNK, and p38. As shown in Fig. 4, ECM significantly suppressed the expressions of phosphorylated forms on ERK1/2, JNK and p38 at 200 µg/ml. Non-phosphorylated forms were not altered by treatments. These results suggest that ECM blocks ERK1/2, JNK, and p38 phosphorylation in the MAPK pathways to suppress the inflammatory responses of LPS-induced RAW264.7 cells.
Fig. 4.
Inhibitory effects of ECM on phosphorylation of MAPKs in LPS-stimulated RAW264.7 macrophages. Cells were co-incubated with indicated concentrations of ECM and LPS for 30 minutes and whole-cell lysates were analyzed by Westernblot analysis using various antibodies against activated MAPKs such as (A) ERK, (B) JNK, and (C) p38. Each value is expressed as a mean ± standard error (n = 3). #P < 0.05 compared to control, *P < 0.05 compared to LPS.
Involvement of HO-1 in the inhibitory effect of the ECM on LPS-induced NO production
HO-1 acts as a major protective factor because of its antioxidant, anti-apoptotic and anti-inflammatory properties. In light of the cytoprotective role of HO-1, the specific pharmacological activation of HO-1 gene expression may represent a novel target for therapeutic intervention. Several studies have reported that the induction of HO-1 enables anti-inflammatory function in macrophages [7,19]. Therefore, we examined whether the ECM induces HO-1 expression in RAW264.7 macrophages. The RAW264.7 cells were treated with the increasing concentrations of ECM for 24 h and the expression level of HO-1 was determined by the Westernblot analysis (Fig. 5A). HO-1 expression reached its maximum at the highest ECM concentration (200 µg/ml). We also assessed HO-1 expression levels in RAW264.7 macrophages incubated with ECM in the presence of LPS. Compared with the control, LPS induced a moderate increase of HO-1 expression, and this response was augmented by ECM (Fig. 5B). Based on observations that the treatment of the macrophages with ECM was able to inhibit the production of LPS-stimulated pro-inflammatory mediators and enzymes to induce the HO-1 expression, we tested that the inhibitory effects of NO production by treatment with ECM was mediated through HO-1 expressions. RAW264.7 macrophages were treated with ECM in the presence of ZnPP, a specific HO-1 competitive inhibitor, followed by LPS for 24 h. The ZnPP at 20 µM reversed the inhibition of LPS-stimulated NO productions caused by prolonged ECM treatment (Fig. 6). These results demonstrated that inhibitory effects of ECM on LPS-induced NO productions were coincidentally correlated with ECM-enhanced HO-1 expressions. Although the exact mechanisms involved in the anti-inflammatory effects of HO-1 have not yet been fully elucidated, the enzymatic by-products of HO-1 have been evaluated as possible factors [15,20].
Fig. 5.
Inductions by ECM of HO-1 protein expression in RAW264.7 cells. Cells were treated (A) without or (B) with LPS and indicated concentrations of ECM for 24 h. The Westernblot is a representative of three independent experiments. Each value is expressed as a mean ± standard error (n = 3). #P < 0.05 compared to control, *P < 0.05 compared to LPS.
Fig. 6.
HO-1 mediates the suppression of LPS-induced NO production in RAW 264.7 cells. Cells were pre-incubated with 200 µg/ml ECM for 30 min before exposure 30 µM ZnPP and LPS (1 µg/ml) for 24 h. The treated culture media were used to measure the amount of nitrite production. Each value is expressed as a mean ± standard error (n = 3). #P < 0.05 compared to LPS, *P < 0.05 compared to LPS + ECM.
DISCUSSION
During the inflammatory process, sizeable quantities of the pro-inflammatory mediators of NO and PGE2 were generated by the inducible isoforms of iNOS and COX-2, respectively. Therefore, the inhibition of these mediators with pharmacological modulators may be an effective therapeutic strategy for the prevention and treatment of a variety of inflammatory reactions and infectious diseases [7,21]. The result of this study demonstrated that treatment with ECM at concentrations of 100 and 200 µg/ml significantly suppressed COX-2 and iNOS expressions in an LPS-activated macrophage culture system, leading to the inhibition of COX-2-derived PGE2 and iNOS-derived NO production. In LPS-stimulated macrophages, the inflammatory mediators such as iNOS-derived NO and COX-2-derived PGE2 are mediated by the regulations of NFκB signaling pathway. To determine whether the NFκB activation, a key signaling pathway leading to iNOS and COX-2 gene expression, is the upstream inhibitory target for ECM, we performed reporter gene analysis by using the NFκB binding sequence and westernblot analysis in nucleus. The results demonstrated that the molecular mechanisms by which ECM inhibits the expression of these inflammatory mediators appeared to involve inhibitions of NFκB activation via the blocking of LPS-stimulated promoter activities of NFκB and the translocations of the NFκB p65/p50 protein in this study.
MAPKs are a group of serine/threonine protein kinases that are activated in response to diverse extracellular stimuli that mediate signal transduction from the cell surface to the nucleus [22]. MAPKs have been implicated in the signaling pathway relevant to LPS-induced inflammations. Previous studies have provided evidence for the role of ERK1/2, JNK, and p38 in the transcriptional activation of pro-inflammatory cytokines and their mediators. The exact signaling pathways that include the three types of MAPKs are still unclear. However, there are cross talks and signal convergences between them. It has been previously reported that ERK1/2 modulates LPS-induced NO production through the NFκB pathway in RAW264.7 macrophage [23]. Moreover, the JNK phosphorylation leads to activation of NFκB transcriptional factor and it suggest that the JNK pathway directly affects expression of the inflammatory proteins, iNOS and COX-2 in the cell nucleus [15,24], while the p38 kinase modulates COX-2 expressions, but not the iNOS expression, in LPS-induced RAW264.7 cells [25]. In this study, we evaluated the effects of ECM on the activation of three MAPKs, and determined that ECM significantly inhibits the phosphorylation of MAPKs. These data suggested that ECM might decrease NO and PGE2 production via inhibitions of the ERK/21, JNK and p38 activations as well as blocking the NFκB activation.
It has been previously reported that inflammatory stimuli induce the expressions of HO-1 thereby suggesting that HO-1 expression is an adaptive cellular response to inflammation. Moreover, data from various studies have suggested that the increased HO-1 expressions play a vital role in the inhibitory modulation of iNOS expression and subsequently, NO production in LPS-activated macrophages [8]. Therefore, HO-1 may be a target for the treatment of inflammatory disorders. Recently, accumulating evidence indicates that induction of HO-1 expression is promoted by activation of the nuclear transcription factor E2-related factor 2 (Nrf2) in RAW 264.7 cells stimulated with LPS [26,27,28], Upon the oxidative stress or inflammatory response, the nuclear translocation of Nrf2 initiated antioxidative and/or cytoprotective cascades. The Nrf2 regulates, basal and inducible expression of numerous detoxifying and antioxidant genes, including HO-1 [29]. Our data showed that HO-1 was induced by treatment with ECM alone and that this effect may be related to the ECM-induced activation of Nrf2, a key transcription factor in HO-1 expression. The present study further examined whether the ECM induces not only the HO-1 expression but also inhibits LPS-induced NO by increasing expression of HO-1 in macrophages. Our results indicated that ECM up-regulates the HO-1 expression and the inhibition of HO-1 activity by the HO-1 inhibitor, ZnPP, partially reversed the inhibitory effects of ECM for NO production in LPS-stimulated macrophages. Although the exact mechanisms involved in anti-inflammatory effects of HO-1 have not been fully elucidated, one or more enzymatic by-products of HO-1 have been evaluated as possible factors capable of inhibiting macrophage mediated inflammations [30]. CO can directly bind the heme-iron contained in heme enzymes like the NADPH oxidase and iNOS, which results in an inhibition of the electron transfer reaction [31].
In conclusion, our studies demonstrated that ECM inhibits iNOS-derived NO and COX-2-derived PGE2 production in LPS-induced macrophages via inactivation of NFκB and MAPKs signaling pathway. These inhibitory effects, at least in parts, could be mediated via inductions of HO-1 by ECM. Therefore, the present study demonstrates that ECM is a promising therapeutic agent to combat inflammatory diseases.
Footnotes
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the ministry of Education, Science and Technology (2012R1A1A4A0105273).
References
- 1.Joh EH, Gu W, Kim DH. Echinocystic acid ameliorates lung inflammation in mice and alveolar macrophages by inhibiting the binding of LPS to TLR4 in NF-κB and MAPK pathways. Biochem Pharmacol. 2012;84:331–340. doi: 10.1016/j.bcp.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 2.Lee JA, Lee MY, Shin IS, Seo CS, Ha H, Shin HK. Anti-inflammatory effects of Amomum compactum on RAW 264.7 cells via induction of heme oxygenase-1. Arch Pharm Res. 2012;35:739–746. doi: 10.1007/s12272-012-0419-x. [DOI] [PubMed] [Google Scholar]
- 3.Kim SH, Shin TY. Anti-inflammatory effect of leaves of Eriobotrya japonica correlating with attenuation of p38 MAPK, ERK, and NF-kappaB activation in mast cells. Toxicol In Vitro. 2009;23:1215–1219. doi: 10.1016/j.tiv.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 4.Ku KT, Huang YL, Huang YJ, Chiou WF. Miyabenol A inhibits LPS-induced NO production via IKK/IkappaB inactivation in RAW 264.7 macrophages: possible involvement of the p38 and PI3K pathways. J Agric Food Chem. 2008;56:8911–8918. doi: 10.1021/jf8019369. [DOI] [PubMed] [Google Scholar]
- 5.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 6.Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol Cell Biol. 1990;10:1498–1506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee MY, Lee JA, Seo CS, Ha H, Lee H, Son JK, Shin HK. Anti-inflammatory activity of Angelica dahurica ethanolic extract on RAW264.7 cells via upregulation of heme oxygenase-1. Food Chem Toxicol. 2011;49:1047–1055. doi: 10.1016/j.fct.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Chen TY, Sun HL, Yao HT, Lii CK, Chen HW, Chen PY, Li CC, Liu KL. Suppressive effects of Indigofera suffruticosa Mill extracts on lipopolysaccharide-induced inflammatory responses in murine RAW 264.7 macrophages. Food Chem Toxicol. 2013;55:257–264. doi: 10.1016/j.fct.2012.12.056. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y, Sung J, Sung M, Choi Y, Jeong HS, Lee J. Involvement of heme oxygenase-1 in the anti-inflammatory activity of Chrysanthemum boreale Makino extracts on the expression of inducible nitric oxide synthase in RAW264.7 macrophages. J Ethnopharmacol. 2010;131:550–554. doi: 10.1016/j.jep.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 10.Pawlaczyk I, Czerchawski L, Kuliczkowski W, Karolko B, Pilecki W, Witkiewicz W, Gancarz R. Anticoagulant and anti-platelet activity of polyphenolic-polysaccharide preparation isolated from the medicinal plant Erigeron canadensis L. Thromb Res. 2011;127:328–340. doi: 10.1016/j.thromres.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 11.Mukhtar N, Iqbal K, Malik A. Novel sphingolipids from Conyza canadensis. Chem Pharm Bull (Tokyo) 2002;50:1558–1560. doi: 10.1248/cpb.50.1558. [DOI] [PubMed] [Google Scholar]
- 12.Veres K, Csupor-Löffler B, Lázár A, Hohmann J. Antifungal activity and composition of essential oils of Conyza canadensis herbs and roots. ScientificWorldJournal. 2012;2012:489646. doi: 10.1100/2012/489646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie WD, Gao X, Jia ZJ. A new C-10 acetylene and a new triterpenoid from Conyza canadensis. Arch Pharm Res. 2007;30:547–551. doi: 10.1007/BF02977646. [DOI] [PubMed] [Google Scholar]
- 14.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 15.Jung CH, Kim JH, Hong MH, Seog HM, Oh SH, Lee PJ, Kim GJ, Kim HM, Um JY, Ko SG. Phenolic-rich fraction from Rhus verniciflua Stokes (RVS) suppress inflammatory response via NF-kappaB and JNK pathway in lipopolysaccharide-induced RAW 264.7 macrophages. J Ethnopharmacol. 2007;110:490–497. doi: 10.1016/j.jep.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Chiu FL, Lin JK. Tomatidine inhibits iNOS and COX-2 through suppression of NF-kappaB and JNK pathways in LPS-stimulated mouse macrophages. FEBS Lett. 2008;582:2407–2412. doi: 10.1016/j.febslet.2008.05.049. [DOI] [PubMed] [Google Scholar]
- 17.Cho SY, Park SJ, Kwon MJ, Jeong TS, Bok SH, Choi WY, Jeong WI, Ryu SY, Do SH, Lee CS, Song JC, Jeong KS. Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-kappaB pathway in lipopolysaccharide-stimulated macrophage. Mol Cell Biochem. 2003;243:153–160. doi: 10.1023/a:1021624520740. [DOI] [PubMed] [Google Scholar]
- 18.Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression. The role of TATA-binding protein (TBP) J Biol Chem. 1999;274:30858–30863. doi: 10.1074/jbc.274.43.30858. [DOI] [PubMed] [Google Scholar]
- 19.Ryter SW, Otterbein LE, Morse D, Choi AM. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem. 2002;234-235:249–263. doi: 10.1023/A:1015957026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim AN, Jeon WK, Lee JJ, Kim BC. Up-regulation of heme oxygenase-1 expression through CaMKII-ERK1/2-Nrf2 signaling mediates the anti-inflammatory effect of bisdemethoxycurcumin in LPS-stimulated macrophages. Free Radic Biol Med. 2010;49:323–331. doi: 10.1016/j.freeradbiomed.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Xia Z, Triffitt JT. A review on macrophage responses to biomaterials. Biomed Mater. 2006;1:R1–R9. doi: 10.1088/1748-6041/1/1/R01. [DOI] [PubMed] [Google Scholar]
- 22.Li B, Lee DS, Choi HG, Kim KS, Kang DG, Lee HS, Jeong GS, Kim YC. Sauchinone suppresses pro-inflammatory mediators by inducing heme oxygenase-1 in RAW264.7 macrophages. Biol Pharm Bull. 2011;34:1566–1571. doi: 10.1248/bpb.34.1566. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Jiang W, Zhang Z, Qian M, Du B. Nitidine chloride inhibits LPS-induced inflammatory cytokines production via MAPK and NF-kappaB pathway in RAW 264.7 cells. J Ethnopharmacol. 2012;144:145–150. doi: 10.1016/j.jep.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 24.Cheon MS, Yoon T, Lee do Y, Choi G, Moon BC, Lee AY, Choo BK, Kim HK. Chrysanthemum indicum Linné extract inhibits the inflammatory response by suppressing NF-kappaB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophages. J Ethnopharmacol. 2009;122:473–477. doi: 10.1016/j.jep.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 25.Paul A, Cuenda A, Bryant CE, Murray J, Chilvers ER, Cohen P, Gould GW, Plevin R. Involvement of mitogen-activated protein kinase homologues in the regulation of lipopolysaccharide-mediated induction of cyclo-oxygenase-2 but not nitric oxide synthase in RAW 264.7 macrophages. Cell Signal. 1999;11:491–497. doi: 10.1016/s0898-6568(99)00018-2. [DOI] [PubMed] [Google Scholar]
- 26.Choi HG, Lee DS, Li B, Choi YH, Lee SH, Kim YC. Santamarin, a sesquiterpene lactone isolated from Saussurea lappa, represses LPS-induced inflammatory responses via expression of heme oxygenase-1 in murine macrophage cells. Int Immunopharmacol. 2012;13:271–279. doi: 10.1016/j.intimp.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Bang SY, Kim JH, Kim HY, Lee YJ, Park SY, Lee SJ, Kim Y. Achyranthes japonica exhibits anti-inflammatory effect via NF-κB suppression and HO-1 induction in macrophages. J Ethnopharmacol. 2012;144:109–117. doi: 10.1016/j.jep.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 28.Jin GH, Park SY, Kim E, Ryu EY, Kim YH, Park G, Lee SJ. Anti-inflammatory activity of Bambusae Caulis in Taeniam through heme oxygenase-1 expression via Nrf-2 and p38 MAPK signaling in macrophages. Environ Toxicol Pharmacol. 2012;34:315–323. doi: 10.1016/j.etap.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Can'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 30.Hwangbo C, Lee HS, Park J, Choe J, Lee JH. The anti-inflammatory effect of tussilagone, from Tussilago farfara, is mediated by the induction of heme oxygenase-1 in murine macrophages. Int Immunopharmacol. 2009;9:1578–1584. doi: 10.1016/j.intimp.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Srisook K, Palachot M, Mongkol N, Srisook E, Sarapusit S. Anti-inflammatory effect of ethyl acetate extract from Cissus quadrangularis Linn may be involved with induction of heme oxygenase-1 and suppression of NF-kappaB activation. J Ethnopharmacol. 2011;133:1008–1014. doi: 10.1016/j.jep.2010.11.029. [DOI] [PubMed] [Google Scholar]