Abstract
BACKGROUND/OBJECTIVES
The number of diabetic patients has recently shown a rapid increase, and delayed wound healing is a major clinical complication in diabetes. In this study, the wound healing effect of Hominis placenta (HP) treatment was investigated in normal and streptozotocin-induced diabetic mice.
MATERIALS/METHODS
Four full thickness wounds were created using a 4 mm biopsy punch on the dorsum. HP was injected subcutaneously at the middle region of the upper and lower wounds. Wounds were digitally photographed and wound size was measured every other day until the 14th day. Wound closure rate was analyzed using CANVAS 7SE software. Wound tissues were collected on days 2, 6, and 14 after wounding for H/E, immunohistochemistry for FGF2, and Masson's trichrome staining for collagen study.
RESULTS
Significantly faster wound closure rates were observed in the HP treated group than in normal and diabetes control mice on days 6 and 8. Treatment with HP resulted in reduced localization of inflammatory cells in wounded skin at day 6 in normal mice and at day 14 in diabetic mice (P < 0.01). Expression of fibroblast growth factor (FGF) 2 showed a significant increase in the HP treated group on day 14 in both normal (P < 0.01) and diabetic mice (P < 0.05). In addition, HP treated groups showed a thicker collagen layer than no treatment groups, which was remarkable on the last day, day 14, in both normal and diabetic mice.
CONCLUSIONS
Taken together, HP treatment has a beneficial effect on acceleration of cutaneous wound healing via regulation of the entire wound healing process, including inflammation, proliferation, and remodeling.
Keywords: Diabetes, wound, Hominis placenta, inflammation, fibroblast growth factor 2
INTRODUCTION
Diabetes, a complex metabolic disorder, is becoming a major health concern [1]. Among diabetic complications, impaired wound healing, which often results in infection, chronic ulceration, and possible amputation of extremities can give rise to major clinical problems [2,3]. Despite a large number of studies for development of effective treatments for wound repair in diabetes, a new treatment for wound healing remains to be determined [2].
The physiology of wound healing is a complex and sequential process, which can be broadly categorized according to three interrelated phases; inflammation, proliferation, and regeneration [4,5,6,7]. Diabetes-induced impairment of wound healing is characterized by inhibition of the inflammatory response, angiogenesis, fibroplasia, and defects in collagen deposition. In normal inflammation phase, necrotic tissues are removed in order to create a platform for growth of healthy tissue. Therefore, the initial inflammatory response after injury is important for rapid wound healing. In a pathological condition, such as that of diabetics, slow removal of necrotic tissue delays the onset of healing and results in chronic inflammation [8,9]. Angiogenesis, the formation of new vessels from preexisting ones, is essential to successful wound healing. Newly formed blood vessels play pivotal roles in formation of provisional granulation tissue and supply of nutrition and oxygen to growing new tissues [10]. Cooperative expression of angiogenic growth factors, including vascular endothelial growth factor (VEGF), transforming growth factor beta (TGF-β), fibroblast growth factor (FGF), and angiopoietins is important in wound angiogenesis [10]. FGF-2, called bFGF, is a strong mitogenic factor of endothelial cells [11] and promotes endothelial cell proliferation and differentiation [12]. During this last phase, synthesis of structural proteins, such as collagen, is important for tissue regeneration and remodeling. Increasing collagen deposition improves tensile strength of skin wounds, and has an important role in interference with scar formation.
Hominis placenta (HP) is a dried placenta isolated from healthy pregnant women after delivery [13,14]. HP is a rich source of various bioactive substances, including peptides, nucleic acids, fatty acids, amino acids, enzymes, minerals, and trace elements [14,15]. It is known to have various pharmacological effects, such as anti-oxidation, anti-inflammation, and regeneration, especially acceleration of wound healing [16,17,18]. Therefore, we supposed that HP extract is effective in all phases of the physiology of wound healing.
In this study, the wound healing effect of Hominis placenta (HP) treatment was investigated in normal and streptozotocin-induced diabetic mice.
MATERIALS AND METHODS
Animals
Male C57BL/6 mice, 6-7 weeks of age, weighing 20-25 g (Samtaco, Seoul, Korea), were used in all experiments. They were maintained on a 12 h/12 h light/dark schedule with free access to food and water. All experiments were approved by the Kyung Hee University Animal Care Committee for animal welfare [KHUASP(SE)-11-016] and were performed according to the guidelines of the NIH and the Korean Academy of Medical Sciences.
Diabetic mouse model and experimental groups
Diabetic mice were established by intraperitoneal injection of 50 mg/kg STZ (Sigma-Aldrich, Gyunggi-do, Korea) dissolved in 0.01 M sodium citrate buffer after 8 hour starvation for five consecutive days. Normal mice received equal volumes of sodium citrate buffer. Blood glucose levels in diabetic mice were measured under fasted conditions by tail vein sampling on the first day, the last day, and three days after the last day (eighth day) of STZ injection. Mice with blood glucose levels higher than 200 mg/ml on the eighth day were included in the diabetic experiment. Normal mice underwent equal procedures, except STZ injection.
Mice were divided into four groups: 1) wounded and untreated normal mice (N/W, n = 17), 2) wounded and HP treated normal mice (N/W+P, n = 20), 3) wounded and untreated diabetic mice (DB/W, n = 17), and 4) wounded and HP treated diabetic mice (DB/W+P, n = 15).
Full thickness wound preparation and pharmacopuncture treatment
Before wound preparation, hair on the dorsal skin of mice was shaved. After mice were slightly anesthetized with ethyl ether, four full-thickness wounds were made using a disposable sterile 4 mm biopsy punch (Kai industries, Seki city, Japan). A 10 ml ampule extracted from human placenta (Korean Pharmacopuncture Institute, Seoul, Korea) was used for HP injection. Mice received subcutaneous injection of 0.02 ml of HP at the middle region of the upper and lower wound every other day for 14 days. An equal volume of saline (0.02 ml) was injected into the control mice as a control for HP injection.
Measurement of wound closure rate
For measurement of wound size, four wounds were measured for each mouse. The wound areas were measured every other day from the day of wounding (d0) until 14 days after wounding (d14). Wounds were digitally photographed and wound closure was quantified using CANVAS 7SE software (Deneba System Inc., Miami, Florida, USA). The rate of wound closure was expressed as the ratio of wound area compared to the area of d0.
Sample harvesting
On the day of wounding (d0) and days 2, 6, and 14 after wounding, mice were sacrificed and the skins were harvested each time. Then, tissue samples around wounds were harvested and immediately placed in 3% formalin solution for fixation. A few days later, the samples were embedded in paraffin and cut into 4 µm slices and placed on microscope slides, and then used for histological staining.
Histological analysis
Before the histological procedure, the slides were incubated in xylene and then hydrated in 100% ethanol and 95% ethanol for removal of paraffin. Slides were stained with Harris hematoxylin and eosin for inflammatory cells, and Masson Trichrome for collagen fiber.
For immunohistochemical staining of FGF2, the slides were incubated in 3% hydrogen peroxide for 10 minutes, washed in wash buffer (TBS-T, Tris Buffered Saline containing 0.1% Tween-20), and incubated in blocking solution (TBS-T with 5% normal goat serum) for 1 hour. Then, the rabbit polyclonal FGF2 antibody (Santa Cruz, CA, USA) was diluted in blocking solution and added to each section. These solutions were incubated overnight at 4℃. The sections were then rinsed with wash buffer and incubated with biotinylated anti-rabbit IgG (Vector Laboratories Inc., Burlingame, CA, USA) for 1 hour. Slides were incubated with ABC reagent (Vector Laboratories Inc., Burlingame, CA, USA) and incubated in 0.02% diaminobenzidine and 0.003% hydrogen peroxide in 1M Tris-buffered saline (pH 7.5). As soon as the sections were developed, the slides were immersed in distilled water. The sections were counterstained with hematoxylin. After staining, slides were dehydrated in 95% and 100% ethanol, incubated in xylene, and mounted. Pictures of the skin layers were taken using a bright-field microscope (BX51; Olympus, Tokyo, Japan).
Statistical analysis
All data were expressed as the mean ± standard deviation (SD). Statistical analysis was performed using a student t-test and two way ANOVA followed by Bonferroni post-test analysis using Prism ver.5 (GraphPad Software, Inc., California, USA). Differences were considered statistically significant at P < 0.05.
RESULTS
Wound closure rate
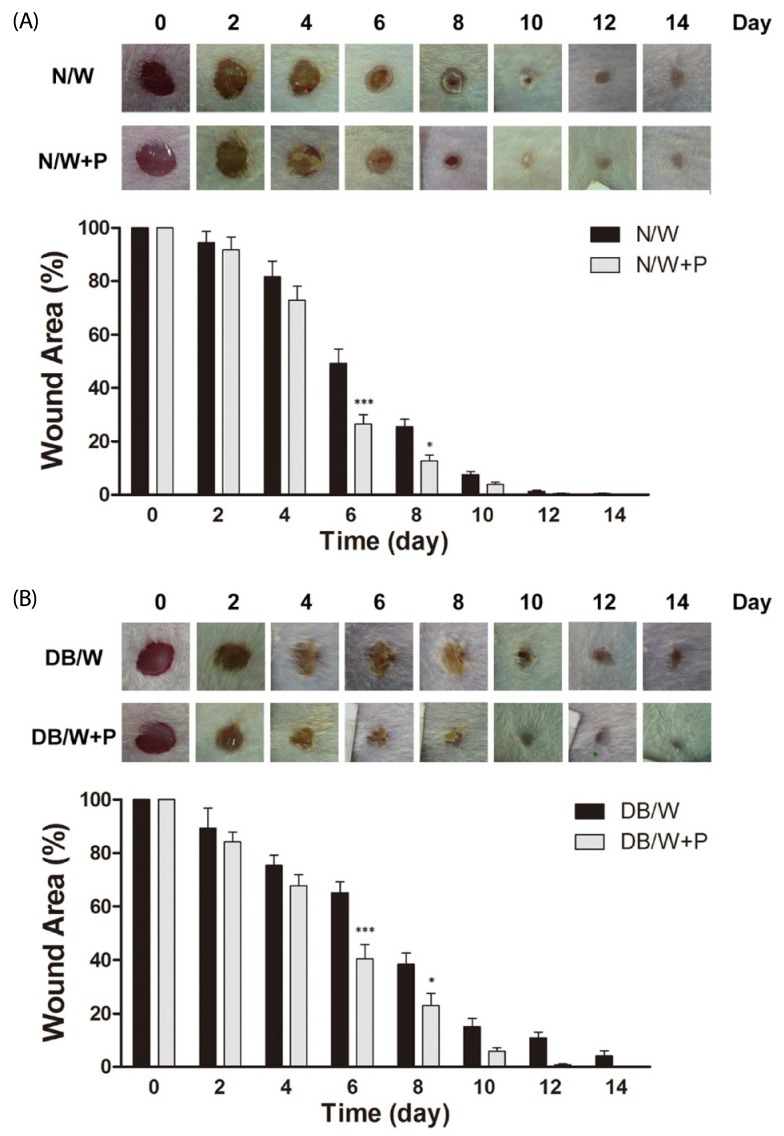
Delayed wound healing process was observed in diabetic mice compared to normal mice from day 6 (P < 0.05 on day 6, P < 0.01 on day 8, P < 0.05 on day 10, P < 0.001 on day 12, P < 0.05 on day 14). Treatment with HP resulted in a more rapid reduction in wound areas in both normal and diabetic mice, and significant changes were observed on days 6 and 8 in both normal (P < 0.001 on day 6 and P < 0.05 on day 8) (Fig. 1A) and diabetic mice (P < 0.001 on day 6 and P < 0.05 on day 8) (Fig. 1B). In addition, wounds remained until the 14th day in normal and diabetic control mice, whereas mice in HP treated groups recovered from their wounds.
Fig. 1.
Effects of Hominis placenta treatment on wound closure in normal and diabetic mice. Values with different superscripts are significantly different by 2-way ANOVA followed by least significant difference test (*P < 0.05, ***P < 0.001). * means statistical difference vs. group W. Values are expressed as mean ± SD. (A) N/W: untreated group after wounding, N/W+P: Hominis placenta treated group after wounding. (B) DB/W: untreated diabetes group after wounding, DB/W+P: Hominis placenta treated diabetes group after wounding.
Localization of Inflammatory cells in wounded area
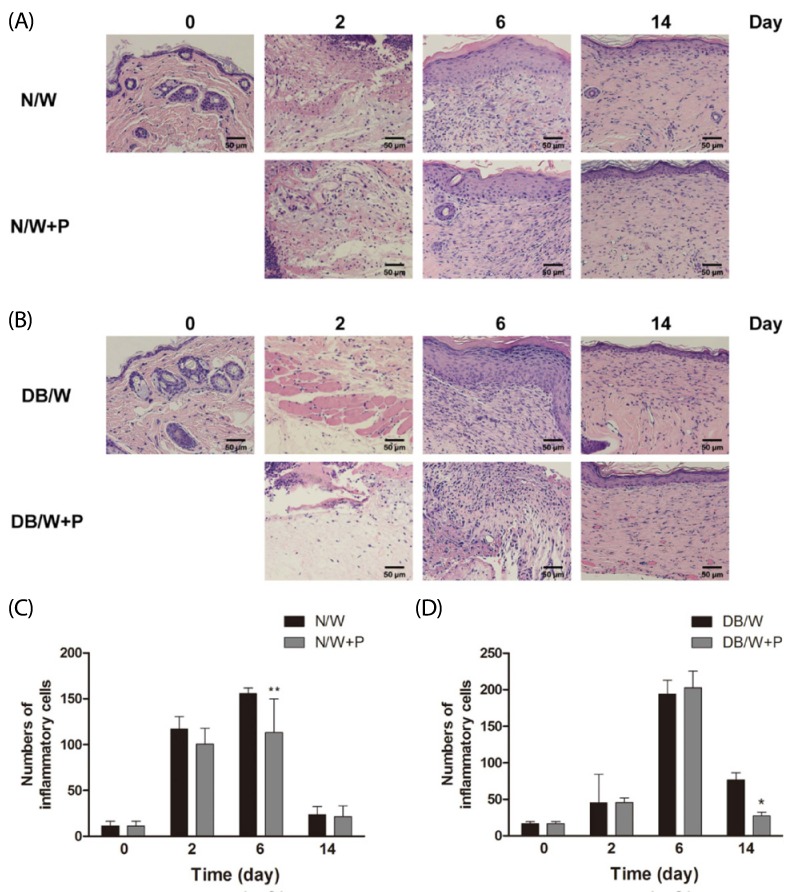
HE staining was performed for analysis of localization of inflammatory cells. The numbers of neutrophils and macrophages were counted after staining. In normal mice, the number of inflammatory cells were increased until day 6, and then showed a rapid decrease.
Treatment with HP resulted in a significant reduction in the number of inflammatory cells on day 6 in normal mice (P < 0.01) (Fig. 2A, C). In diabetic mice, the number of inflammatory cells showed a sharp increase on day 6, and then decreased. Treatment with HP also resulted in a significant reduction in the number of inflammatory cells on day 14 in diabetic mice (P < 0.05) (Fig. 2B, D).
Fig. 2.
Changes of inflammatory cells in normal and diabetic mice. Values with different superscripts are significantly different by 2-way ANOVA followed by least significant difference test (*P < 0.05, **P < 0.01). * means statistical difference vs. group W. Values are expressed as mean ± SD. (A, C) N/W: untreated group after wounding, N/W+P: Hominis placenta treated group after wounding. (B, D) DB/W: untreated diabetes group after wounding, DB/W+P: Hominis placenta treated diabetes group after wounding.
FGF2 expression
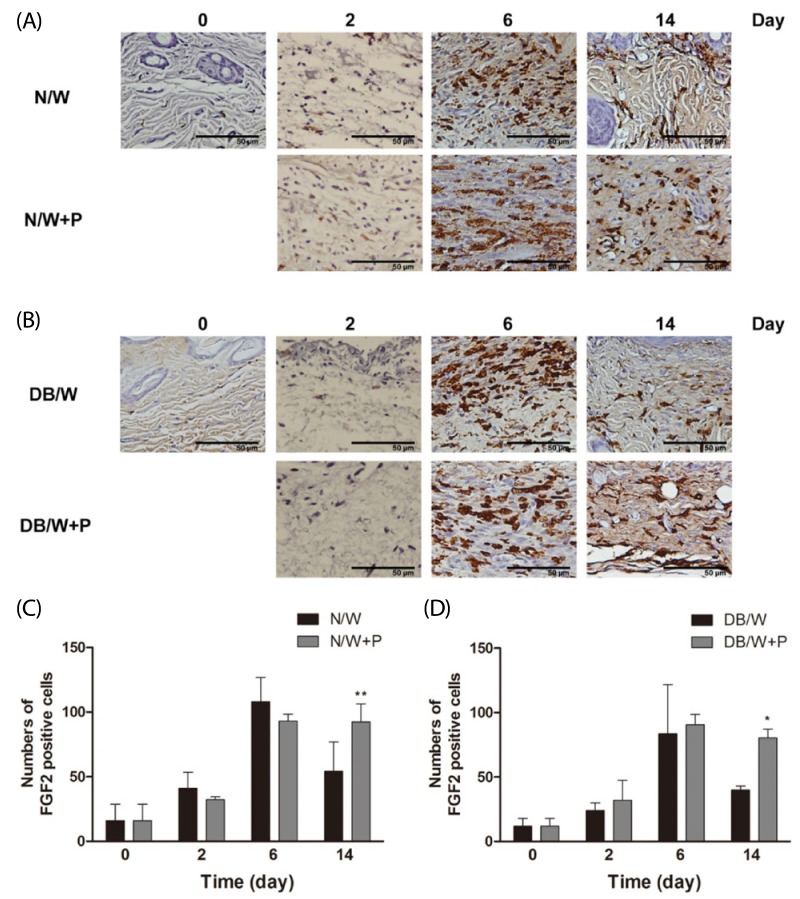
Wound tissues were stained immunohistologically for FGF2, and the expression level of FGF2 at the granulation tissue was analyzed. The number of FGF2 positive cells increased until day 6 in normal and diabetic control mice, and then decreased. However, HP treated mice exhibited higher numbers of FGF2 positive cells in both normal and diabetic mice on day 14 (P < 0.01 in normal mice and P < 0.05 in diabetic mice) (Fig. 3).
Fig. 3.
Changes of FGF2 expression in normal and diabetic mice. Values with different superscripts are significantly different by 2-way ANOVA followed by least significant difference test (*P < 0.05, **P < 0.01). * means statistical difference vs. group W. Values are expressed as mean ± SD. (A, C) N/W: untreated group after wounding, N/W+P: Hominis placenta treated group after wounding. (B, D) DB/W: untreated diabetes group after wounding, DB/W+P: Hominis placenta treated diabetes group after wounding.
HP treatment induced collagen changes
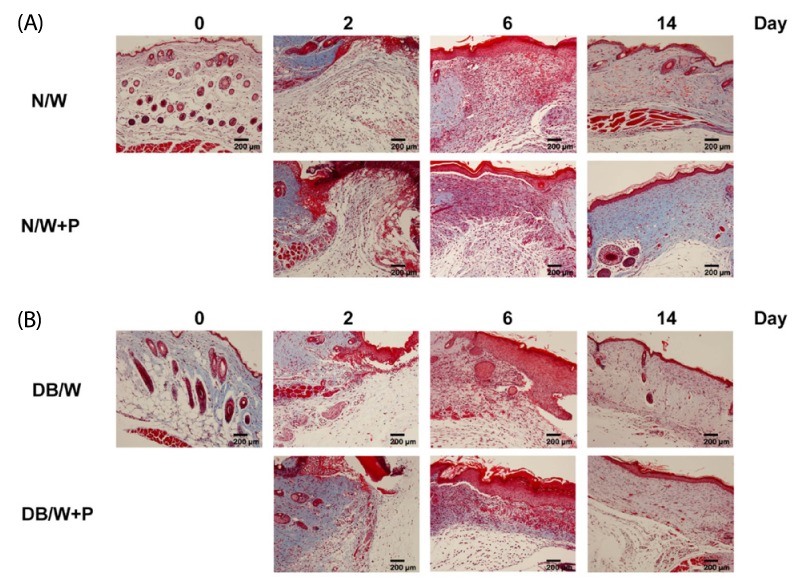
Masson's trichrome staining was performed for analysis of collagen deposition at the granulation tissue and dermis. The blue color indicated the amount of collagen fibers, the red color indicated keratin and muscle fibers, the light red color indicated cytoplasm, and the black color indicated cell nuclei. We found that the collagen layer of diabetic mice was thinner than that of normal mice throughout the entire experiment. HP treatment induced a thicker collagen layer than control in both the normal and diabetic groups, and the collagen layer was markedly increased on day 14 (Fig. 4).
Fig. 4.
Changes of collagen at the granulation tissue and dermis in normal and diabetic mice. (A) N/W: untreated group after wounding, N/W+P: Hominis placenta treated group after wounding. (B) DB/W: untreated diabetes group after wounding, DB/W+P: Hominis placenta treated diabetes group after wounding. Red: keratin and muscle fibers, blue: collagen and bone, light red: cytoplasm, black: cell nuclei.
Blood glucose levels and body weight
To verify physiological conditions of diabetic mice, we measured body weight (g) and blood glucose level (mg/ml) under non-fasted conditions on day 14. The average body weight and blood glucose level of each group is shown in Table 1. No significant difference was observed between groups.
Table 1.
Body weight and blood glucose level of diabetic mice
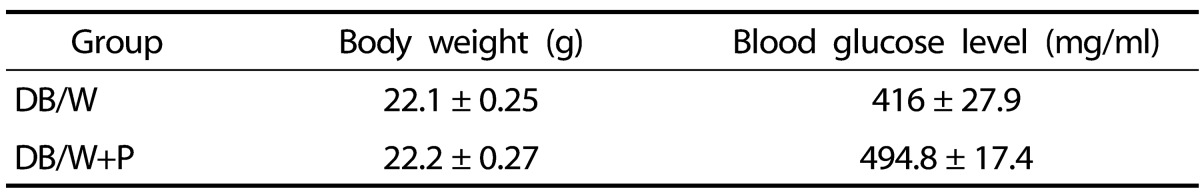
Body weight and blood glucose level of diabetic mice on day 14. No significant difference was observed between groups. DB/W: untreated diabetes group after wounding, DB/W+P: Hominis placenta treated diabetes group after wounding.
DISCUSSION
The main objective of this study was to investigate the therapeutic effect of HP treatment on cutaneous wound healing in normal and diabetic mice. Our findings indicated that HP treatment remarkably promoted the wound closure rate in normal and diabetic mice and increased FGF2 expression and collagen synthesis. In addition, HP treatment resulted in significantly reduced delayed localization of inflammatory cells.
HP consists of various bioactive molecules, such as hepatocyte growth factor (HGF), nerve growth factor (NGF), interferon, interleukins, colony-stimulating factor (CSF), globulin, albumin, and prostaglandin. HP is usually administrated orally or by intradermal injection. In East Asian medicine, a developed injection technique for injection of refined herbal extract into a specific point, such as an acupuncture point is commonly used in order to produce a synergistic effect of acupuncture and herbs [19]. HP has been used for improvement of physiological function in various diseases [13,14,16,18] and various pharmacological effects of HP have been reported, such as reducing pain, regulation of inflammatory cytokines, promoting regeneration after injury, and anti-oxidative effect [14,20,21,22]. HP is also effective in wound repair [16,18,23], however, therapeutic effect of HP in diabetes and the underlying mechanism of HP treatment has not been clearly defined. This study demonstrated the effect of HP on cutaneous wound healing in diabetes for the first time.
Impaired wound healing, a major clinical complication of diabetes [3,24], is caused by peripheral neuropathy, ischemic arterial disease, infection, repeated mechanical stress, and autonomic deficit [25,26]. Biologically, delayed wound healing in diabetes is a very complicated process involved in infiltration of inflammatory cells, production of growth factors, formation of blood vessels, proliferation of new tissues, and composition of extracellular matrix [23,27,28].
In inflammation phase, wounded tissue secretes growth factors and cytokines for initiation of the wound healing process. Neutrophils phagocytize foreign bodies and bacteria and gradually replace monocytes with macrophages. Macrophage phagocytize microorganisms, fibrin, neutrophils, and fragments of the extracellular matrix (ECM) and secrete biomolecules for effective wound repair [29]. These released molecules proceed to the next phase of the wound in order to provide the basis for formation of the extracellular matrix [26]. In our result, inflammatory cells of normal mice were increased from day 2 and maximized on day 6, and then decreased on day 14. In normal condition, it is a physiological wound healing process. However, in diabetic mice, inflammatory cells did not increase on day 2 and showed a rapid increase on day 6. In addition, inflammation was prolonged until day 14. These results reflect the delayed wound repair in diabetes. The HP treatment group showed a significant decrease in the number of inflammatory cells on day 6 in normal and day 14 in diabetic mice. The number of inflammatory cells of the HP treated group in diabetes on day 14 was the same as that of normal condition. Therefore, HP treatment accelerates the inflammatory process and transition to the next phase of the wound healing process.
The proliferation phase is characterized by tissue granulation, production of ECM, and formation of new blood vessels (angiogenesis). The angiogenic process is a major part of the proliferation phase and involves variable growth factors such as platelet-derived growth factor (PDGF), FGF2, TGF-β, VEGF, NGF, EGF, and angiotensin [26,30,31,32,33,34]. These growth factors stimulate proliferation of fibroblasts, angiogenesis, and collagen synthesis for repair of wounded tissue [30]. FGF2, one of the most important growth factors associated with angiogenesis, accelerates angiogenesis by synergic effect with VEGF and TGF-β [35]. FGF2 is mainly produced by keratinocytes and stimulates fibroblast migration, formation of new blood vessels, and production of ECM [30]. In our result, FGF2 expression was increased until day 6 and then decreased in the wounded group of normal and diabetic mice. However, HP treatment prolonged the expression of FGF2, meaning that HP treatment has an effect in increasing fibroblast production resulting in formation of new tissues.
The regeneration phase, involving extensive tissue remodeling, was replaced with proteoglycan and collagen molecules, which organized into thicker bundles, resulting in stronger tissue [26]. Collagen fiber synthesis is one of the most important events in wound healing. In diabetes, collagen fiber synthesis was impaired and it was accompanied by an increased apoptosis of fibroblasts [36,37]. In our study, the wounded lesions of the HP treated group showed increased collagen synthesis compared with the control group in normal and diabetic mice, and collagen synthesis showed a noticeable increase on the last day, day 14. Therefore, HP treatment is effective in increasing collagen synthesis, suggesting that HP treatment accelerates regeneration of tissues and might also be helpful for other diseases involving delayed wound lesion.
In our study, HP treatment showed effectiveness in acceleration of the wound healing process, however, several limitations remain. HP treatment had no effect on the blood glucose level, suggesting that intradermal HP treatment to the local area around the wound works only locally, not systemically. It could differ by the location of HP treatment; therefore, conduct of further studies will be needed in order to determine the difference between local effect and systemic effect of HP treatment.
In summary, our data indicate that HP represents a promising treatment for repair of normal and diabetic wounds. HP improved the entire wound healing process through anti-inflammation, FGF2 activation, and collagen deposition. Further investigations are required in order to elucidate the exact molecular mechanism of HP treatment during the process of wound healing in healthy and diabetic subjects.
Footnotes
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 2005-0049404 and 2010-0008834) and a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (No. HI13C0540).
References
- 1.Morain WD, Colen LB. Wound healing in diabetes mellitus. Clin Plast Surg. 1990;17:493–501. [PubMed] [Google Scholar]
- 2.Hehenberger K, Heilborn JD, Brismar K, Hansson A. Inhibited proliferation of fibroblasts derived from chronic diabetic wounds and normal dermal fibroblasts treated with high glucose is associated with increased formation of l-lactate. Wound Repair Regen. 1998;6:135–141. doi: 10.1046/j.1524-475x.1998.60207.x. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin PJ, Immonen JA, Zagon IS. Topical naltrexone accelerates full-thickness wound closure in type 1 diabetic rats by stimulating angiogenesis. Exp Biol Med (Maywood) 2013;238:733–743. doi: 10.1177/1535370213492688. [DOI] [PubMed] [Google Scholar]
- 4.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 5.Witte MB, Barbul A. Role of nitric oxide in wound repair. Am J Surg. 2002;183:406–412. doi: 10.1016/s0002-9610(02)00815-2. [DOI] [PubMed] [Google Scholar]
- 6.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 7.Steed DL, Donohoe D, Webster MW, Lindsley L Diabetic Ulcer Study Group. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. J Am Coll Surg. 1996;183:61–64. [PubMed] [Google Scholar]
- 8.Firat ET, Dag A, Gunay A, Kaya B, Karadede MI, Kanay BE, Ketani A, Evliyaoglu O, Uysal E. The effects of low-level laser therapy on palatal mucoperiosteal wound healing and oxidative stress status in experimental diabetic rats. Photomed Laser Surg. 2013;31:315–321. doi: 10.1089/pho.2012.3406. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed N. Advanced glycation endproducts--role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003;60:107–114. doi: 10.1002/jemt.10249. [DOI] [PubMed] [Google Scholar]
- 11.Schweigerer L, Neufeld G, Friedman J, Abraham JA, Fiddes J, Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987;325:257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- 12.Montesano R, Vassalli JD, Baird A, Guillemin R, Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci U S A. 1986;83:7297–7301. doi: 10.1073/pnas.83.19.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldfarb G, Doan Ba T, Duran A. Human placenta for chronic leg ulcers. Lancet. 1980;2:40. doi: 10.1016/s0140-6736(80)92921-9. [DOI] [PubMed] [Google Scholar]
- 14.Jang SY, Park JW, Bu Y, Kang JO, Kim J. Protective effects of hominis placenta hydrolysates on radiation enteropathy in mice. Nat Prod Res. 2011;25:1988–1992. doi: 10.1080/14786419.2010.513035. [DOI] [PubMed] [Google Scholar]
- 15.Park SY, Phark S, Lee M, Lim JY, Sul D. Anti-oxidative and anti-inflammatory activities of placental extracts in benzo[a]pyrene-exposed rats. Placenta. 2010;31:873–879. doi: 10.1016/j.placenta.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 16.De D, Chakraborty PD, Bhattacharyya D. Regulation of trypsin activity by peptide fraction of an aqueous extract of human placenta used as wound healer. J Cell Physiol. 2011;226:2033–2040. doi: 10.1002/jcp.22535. [DOI] [PubMed] [Google Scholar]
- 17.De D, Datta Chakraborty P, Mitra J, Sharma K, Mandal S, Das A, Chakrabarti S, Bhattacharyya D. Ubiquitin-like protein from human placental extract exhibits collagenase activity. PLoS One. 2013;8:e59585. doi: 10.1371/journal.pone.0059585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong JW, Lee WJ, Hahn SB, Kim BJ, Lew DH. The effect of human placenta extract in a wound healing model. Ann Plast Surg. 2010;65:96–100. doi: 10.1097/SAP.0b013e3181b0bb67. [DOI] [PubMed] [Google Scholar]
- 19.Shen FY, Lee MS, Jung SK. Effectiveness of pharmacopuncture for asthma: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2011;2011 doi: 10.1155/2011/678176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeom MJ, Lee HC, Kim GH, Shim I, Lee HJ, Hahm DH. Therapeutic effects of Hominis placenta injection into an acupuncture point on the inflammatory responses in subchondral bone region of adjuvant-induced polyarthritic rat. Biol Pharm Bull. 2003;26:1472–1477. doi: 10.1248/bpb.26.1472. [DOI] [PubMed] [Google Scholar]
- 21.Seo TB, Han IS, Yoon JH, Seol IC, Kim YS, Jo HK, An JJ, Hong KE, Seo YB, Kim DH, Park SK, Yang DC, Namgung U. Growth-promoting activity of Hominis Placenta extract on regenerating sciatic nerve. Acta Pharmacol Sin. 2006;27:50–58. doi: 10.1111/j.1745-7254.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee KW, Ji HM, Kim DW, Choi SM, Kim S, Yang EJ. Effects of Hominis placenta on LPS-induced cell toxicity in BV2 microglial cells. J Ethnopharmacol. 2013;147:286–292. doi: 10.1016/j.jep.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 23.Peplow PV, Baxter GD. Gene expression and release of growth factors during delayed wound healing: a review of studies in diabetic animals and possible combined laser phototherapy and growth factor treatment to enhance healing. Photomed Laser Surg. 2012;30:617–636. doi: 10.1089/pho.2012.3312. [DOI] [PubMed] [Google Scholar]
- 24.Marrotte EJ, Chen DD, Hakim JS, Chen AF. Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice. J Clin Invest. 2010;120:4207–4219. doi: 10.1172/JCI36858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 26.Tsourdi E, Barthel A, Rietzsch H, Reichel A, Bornstein SR. Current aspects in the pathophysiology and treatment of chronic wounds in diabetes mellitus. Biomed Res Int. 2013;2013:385641. doi: 10.1155/2013/385641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D'Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol. 2007;170:1178–1191. doi: 10.2353/ajpath.2007.060018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibran NS, Jang YC, Isik FF, Greenhalgh DG, Muffley LA, Underwood RA, Usui ML, Larsen J, Smith DG, Bunnett N, Ansel JC, Olerud JE. Diminished neuropeptide levels contribute to the impaired cutaneous healing response associated with diabetes mellitus. J Surg Res. 2002;108:122–128. doi: 10.1006/jsre.2002.6525. [DOI] [PubMed] [Google Scholar]
- 29.Peppa M, Stavroulakis P, Raptis SA. Advanced glycoxidation products and impaired diabetic wound healing. Wound Repair Regen. 2009;17:461–472. doi: 10.1111/j.1524-475X.2009.00518.x. [DOI] [PubMed] [Google Scholar]
- 30.Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg. 2005;31:674–686. doi: 10.1111/j.1524-4725.2005.31612. [DOI] [PubMed] [Google Scholar]
- 31.Roberts AB. Transforming growth factor-beta: activity and efficacy in animal models of wound healing. Wound Repair Regen. 1995;3:408–418. doi: 10.1046/j.1524-475X.1995.30405.x. [DOI] [PubMed] [Google Scholar]
- 32.Greenhalgh DG, Sprugel KH, Murray MJ, Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990;136:1235–1246. [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuda H, Koyama H, Sato H, Sawada J, Itakura A, Tanaka A, Matsumoto M, Konno K, Ushio H, Matsuda K. Role of nerve growth factor in cutaneous wound healing: accelerating effects in normal and healing-impaired diabetic mice. J Exp Med. 1998;187:297–306. doi: 10.1084/jem.187.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida S, Matsumoto K, Tomioka D, Bessho K, Itami S, Yoshikawa K, Nakamura T. Recombinant hepatocyte growth factor accelerates cutaneous wound healing in a diabetic mouse model. Growth Factors. 2004;22:111–119. doi: 10.1080/08977190410001701005. [DOI] [PubMed] [Google Scholar]
- 35.Eppley BL, Doucet M, Connolly D, Feder J. Enhancement of angiogenesis by bFGF in mandibular bone graft healing in the rabbit. J Oral Maxillofac Surg. 1988;46:391–398. doi: 10.1016/0278-2391(88)90223-6. [DOI] [PubMed] [Google Scholar]
- 36.Greenhalgh DG. Wound healing and diabetes mellitus. Clin Plast Surg. 2003;30:37–45. doi: 10.1016/s0094-1298(02)00066-4. [DOI] [PubMed] [Google Scholar]
- 37.Spanheimer RG, Umpierrez GE, Stumpf V. Decreased collagen production in diabetic rats. Diabetes. 1988;37:371–376. doi: 10.2337/diab.37.4.371. [DOI] [PubMed] [Google Scholar]