Abstract
BACKGROUND/OBJECTIVES
High sensitivity C-reactive protein (hsCRP) is a strong independent predictor of future cardiovascular disease (CVD) risk. We evaluated the relationship between hsCRP and dietary intake in apparently healthy young women living in southern Vietnam.
SUBJECTS/METHODS
Serum hsCRP was measured and dietary intake data were obtained using the 1-day 24-hour recall method in women (n = 956; mean age, 25.0 ± 5.7 years) who participated in the International Collaboration Study for the Construction of Asian Cohort of the Korean Genome and Epidemiology Study (KoGES) in 2011.
RESULTS
Women in the high risk group (> 3 mg/L) consumed fewer fruits and vegetables, total plant food, potassium, and folate than those in the low risk group (< 1 mg/L). A multiple regression analysis after adjusting for covariates revealed a significant negative association between hsCRP and fruit and vegetable consumption. A logistic regression analysis showed that the odds ratio (OR) of having a high hsCRP level in women with the highest quartiles of consumption of fruits and vegetables [OR, 0.391; 95% confidence interval (CI), 0.190-0.807], potassium [OR, 0.425; 95% CI, 0.192-0.939] and folate [OR, 0.490; 95% CI, 0.249-0.964] were significantly lower than those in the lowest quartiles.
CONCLUSIONS
These results suggest that, in young Vietnamese women, an increased consumption of fruit and vegetables might be beneficial for serum hsCRP, a risk factor for future CVD events.
Keywords: hsCRP, fruits and vegetables, dietary intake, Vietnamese women
INTRODUCTION
Inflammation is involved in the progression of atherosclerosis and cardiovascular disease (CVD) [1,2]. Several studies have reported that an increased level of hsCRP, a marker for low grade inflammation, is associated with an increased CVD risk among various populations across the world [3,4,5]. CVD mortality is twice as high in a group with hsCRP > 3 mg/L, a level considered high risk by the American Heart Association (AHA) and the Center for Disease Control and Prevention (CDC) [6], compared to those with < 1 mg/L (low risk) [7].
hsCRP is a strong independent predictor for future CVD outcomes. Among apparently healthy non-smoking, normoglycemic, normolipidemic, premenopausal women participating in the Women's Health Study, the risk of future cardiovascular events was four times higher in those with the highest hsCRP than in those with the lowest hsCRP [8]. hsCRP has also been proposed as a more powerful predictor of cardiovascular events than other markers of inflammation or lipids in the plasma such as amyloid A, soluble intercellular adhesion molecule, interleukin-6, homocysteine [9], and even low-density lipoprotein cholesterol, a well-established conventional risk factor for CVD [10].
Several studies have reported a significant association between hsCRP and various dietary factors, suggesting that hsCRP can be modifiable by manipulating the diet. hsCRP level is higher in subjects who consume high quantities of meat, total fat, saturated fat, and cholesterol [11,12,13], and is lower in those who consume high quantities of fruits and vegetables, fish and poultry [14], and dietary fiber [15]. Furthermore, a healthy pattern (high intake of fruits, vegetables, tomatoes, poultry, legumes, tea, fruit juices, and whole grains) is inversely associated, whereas a western pattern (high intake of refined grains, red meat, butter, processed meat, high-fat dairy, sweets and desserts, pizza, potato, eggs, hydrogenated fats, and soft drinks) is positively associated with high hsCRP [16].
Vietnam is undergoing a rapid change in economic development, urbanization, and diet transition with an increasing trend in the number of people with CVD risk factors [17,18]. CVD mortality in Vietnam now accounts for nearly 40% of the mortality due to noncommunicable diseases [19]. The prevalence of some CVD risk factors such as obesity and dyslipidemia has been reported for Vietnamese women [20,21], but not for serum hsCRP levels, which might be a better predictor of future CVD risk. Although many studies have demonstrated an association between hsCRP and dietary intake, very few studies have investigated such a relationship in young women in their 20s and 30s [11]. Given that hsCRP is a powerful predictor of future CVD events, and that its level can be modified by diet, studies on the relationship between hsCRP and diet may help develop core messages for dietary intervention strategies. Therefore, we investigated the relationship between serum hsCRP and diet in healthy Vietnamese young women.
SUBJECTS AND METHODS
Study subjects
This study was part of the International Collaboration Study for the Construction of Asian Cohort of the Korean Genome and Epidemiologic Study (KoGES). This study protocol was approved by the Human Investigation Review Board of Ewha Womans University College of Medicine (ECT 200-18 200-19) and informed consent for participation was obtained from all subjects. The subjects were Vietnamese women living in the city of Can Tho (a suburb of Ho Chi Minh City, Southern Vietnam), Vietnam. Subjects were invited to participate in a comprehensive baseline health screening at a local health center in 2011. Among the 1,005 women (non-pregnant, non-lactating) without history of chronic disease, those with missing information on dietary intake (n = 1), energy consumption < 500 kcal (n = 2) or > 4,000 kcal (n = 7), and those without anthropometric variables and blood profiles (n = 39) were excluded. Therefore, 956 women were finally eligible for this study.
General characteristics
The subjects were individually interviewed by trained technicians using standard protocols. Vietnamese translators helped in the process whenever needed. A questionnaire was developed for this study that included questions about age, education, income, occupation, alcohol consumption, dietary supplement use and exercise. The education status of the Vietnamese women was categorized as ≤middle school, and ≥ high school. Household monthly income was classified as ≤ 3.0, and > 3.0 million Vietnam Dong. Occupation was classified into professional or office worker, student, labor worker, housewife, or unemployed. Alcohol consumption was classified into never, former and current drinkers. Dietary supplement user was defined as a person who currently used any vitamin or mineral supplements. A regular exerciser was defined as a person who performed exercise currently and regularly enough to induce sweating.
Anthropometric parameters, blood pressure, and blood biochemical profiles
Anthropometric variables and blood pressure were obtained by trained nurses or medical personnel. Standing height and body weight were measured using an automatic height/weight measuring instrument (Dong Sahn Jenix Co., Seoul, Korea). Body mass index (BMI) was calculated as kg/m2. Waist and hip circumferences were measured with a tape measure (anthropometric tape; Preston 5193, Seoul, Korea). Body composition was assessed with an INBODY instrument (INBODY 230; Biospace Co., Seoul, Korea). These measurements were taken once. Systolic and diastolic blood pressures were measured using an automatic blood pressure calculator (FT-500R; Jawon Medical, Gyeongsan, Korea) and read by attending medical doctors after a 10-minute rest in the sitting position; the average of two measurements was used.
Blood samples were drawn into EDTA-containing tubes by medical technicians after an 8-hour overnight fast. The samples were centrifuged at 3,500 rpm for 10 minutes at 4℃, and plasma samples were stored at -70℃ until analysis. Fasting blood sugar, total cholesterol, high density lipoprotein (HDL) cholesterol, and triglycerides were measured with an auto analyzer (ADVIA 1550, Bayer Diagnostics, Tarrytown, NY, USA). Low density lipoprotein (LDL) cholesterol was calculated by the following equation described by Friedewald [22]; LDL cholesterol = Total cholesterol-HDL cholesterol-(triglycerides/5). Serum hsCRP was measured with an auto analyzer (ADVIA1800, Siemens Medical Solutions, Malvern, PA USA) using a hsCRP-Latex (II) X2 kit (Seiken Laboratories Ltd., Tokyo, Japan).
Dietary intake
Dietary intake was estimated by a trained dietitian using a 1-day, 24-hour recall method. Guidelines and photos for estimating portion sizes were used in the interview and a Vietnamese/Korean bilingual Vietnamese who is familiar with foods consumed in Vietnam, was consulted before the data entry and analysis. Foods were categorized into cereals/potatoes/sugar products, legumes/nuts and seeds, fruits and vegetables, meat and meat products, eggs and egg products, fishes and shellfishes, and milk and dairy products. Fruits and vegetable group included all fresh and frozen fruits and vegetables, and 100% fruit and vegetable juices. Food and nutrient intake data were analyzed using the Computer Aided Nutritional Analysis program version 3.0 software (CAN-Pro 3.0, Nutritional Assessment Program, 2006, The Korean Nutrition Society, Seoul, Korea) [23]. Vietnamese food composition data based on Vietnamese Food Composition Table in 2007 [24] were entered into the CAN-pro food database program to analyze food and nutrient intake.
Statistical analysis
Data are expressed as means and standard deviations (continuous) or as frequencies and percentages (categorical). Blood profiles and dietary intake data were log transformed to normalize their distributions before analysis. hsCRP levels were divided into three groups: high risk, > 3 mg/L; average, 1-3 mg/L; and low risk, < 1 mg/L by the AHA and CDC. After adjusting for age, BMI, total energy intake, alcohol consumption, dietary supplement use and exercise, a general linear model procedure was performed to examine the differences in the anthropometric variables, blood profiles, and daily food and nutrient intake among the three groups including Tukey's post-hoc comparisons. Binary logistic regression analysis with adjusted models controlling for confounders (age, BMI, total energy intake, alcohol consumption, dietary supplement use and exercise) was also used to identify the odds ratios (ORs) and 95% confidence intervals (CIs) for a high hsCRP level depending on the quartile of food and nutrient intake. Multiple linear regression analyses were used to examine the relationship between hsCRP and dietary intake after adjusting for confounders. All analyses were performed using SAS 9.3 software (SAS Institute., Cary, NC, USA).
RESULTS
General characteristics
The subjects had a mean age of 25.0 ± 5.7 years, and 85.3% of the subjects had a high school education. Nearly 60% of the subjects had a monthly household income of > 3.0 million Vietnamese Dong. The proportion of subjects who were never, former and current drinkers were 80.1%, 8.2%, and 11.6%, respectively. The proportions of subjects who used dietary supplements and exercised regularly were 24.7% and 44.6%, respectively (Table 1).
Table 1.
General characteristics of Vietnamese women (n = 956)
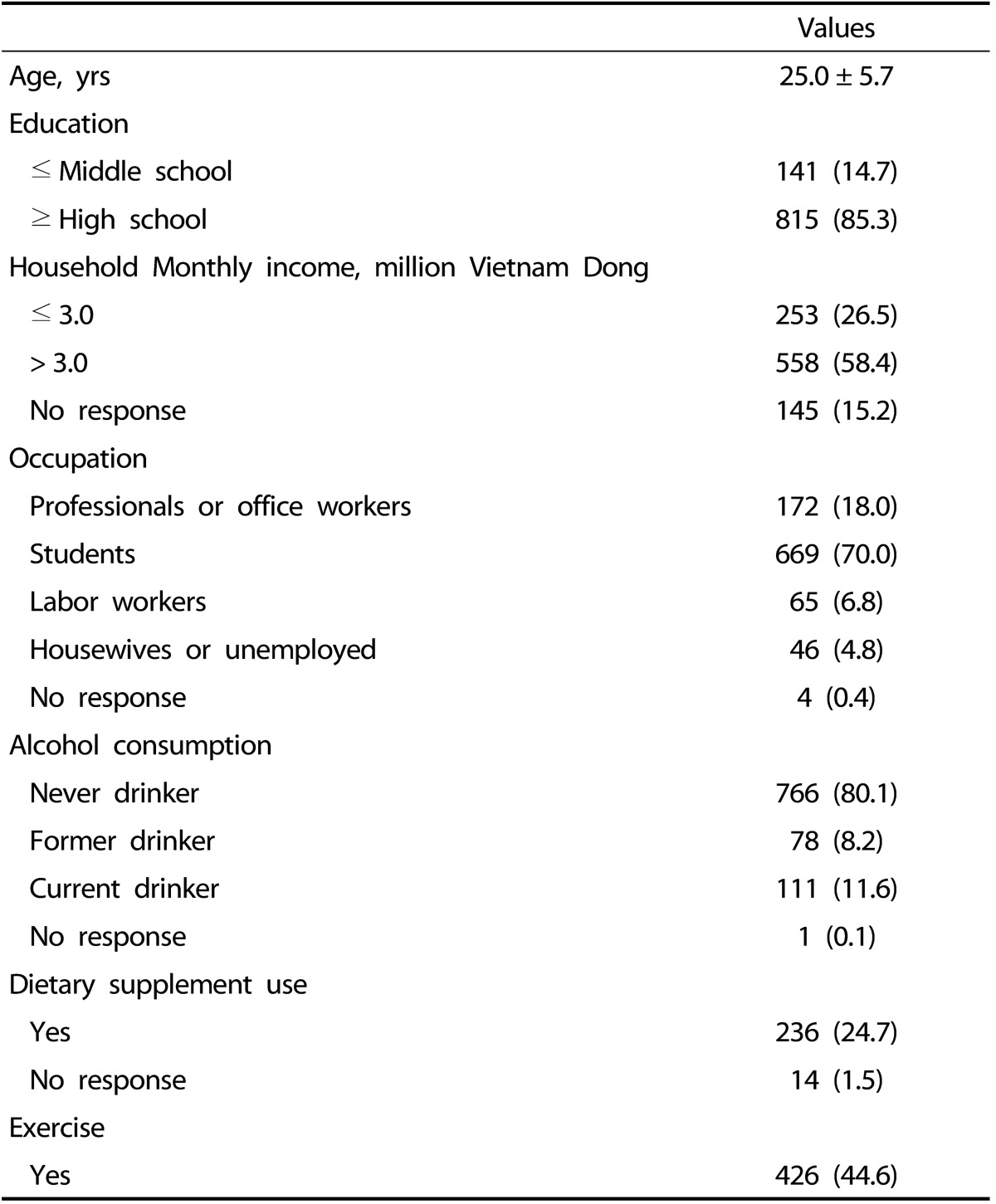
Values are mean ± standard deviation or frequency (%).
Comparison of subjects according to hsCRP range
Anthropometric variables, blood pressure, and blood biochemical profiles
The mean BMI of all subjects was 20.0 ± 2.4 kg/m2, and 29.6% and 11.7% of the subjects were underweight (< 18.5 kg/m2) and overweight (≥ 23.0 kg/m2), respectively.
Body weight, BMI, waist and hip circumference, WHR, body fat (%), diastolic blood pressure, white blood cell count, and triglyceride level were higher in the high risk group (hsCRP, > 3 mg/L) than those in the low risk group (hsCRP, < 1 mg/L) (P < 0.0001). In contrast, HDL cholesterol level in the high risk group was significantly lower than that in the low risk group (P = 0.0209) (Table 2).
Table 2.
Anthropometric variables, blood pressure and blood profiles of Vietnamese women according to hsCRP
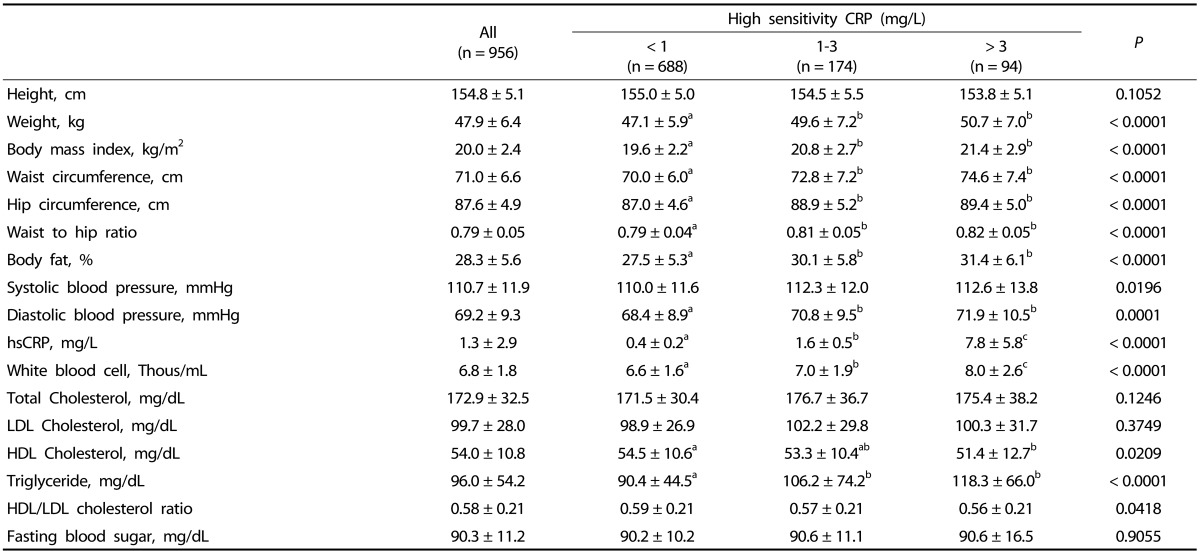
Values are mean ± standard deviation.
Values with different alphabets are significantly different among the three groups by GLM at P < 0.05 as appropriate; including the post hoc comparisons of Tukey.
Daily food and nutrient intake according to the hsCRP range
The high risk group consumed fewer fruits and vegetables (P = 0.0024), less total plant food (P = 0.0404), total food (P = 0.0256), and potassium (P = 0.0281) than those in the low risk group after adjusting for age, BMI, total energy intake, alcohol consumption, dietary supplement use and exercise. The high risk group consumed less amount of folate (P = 0.0284) than the average risk group did (Table 3).
Table 3.
Daily food and nutrient intakes according to hsCRP
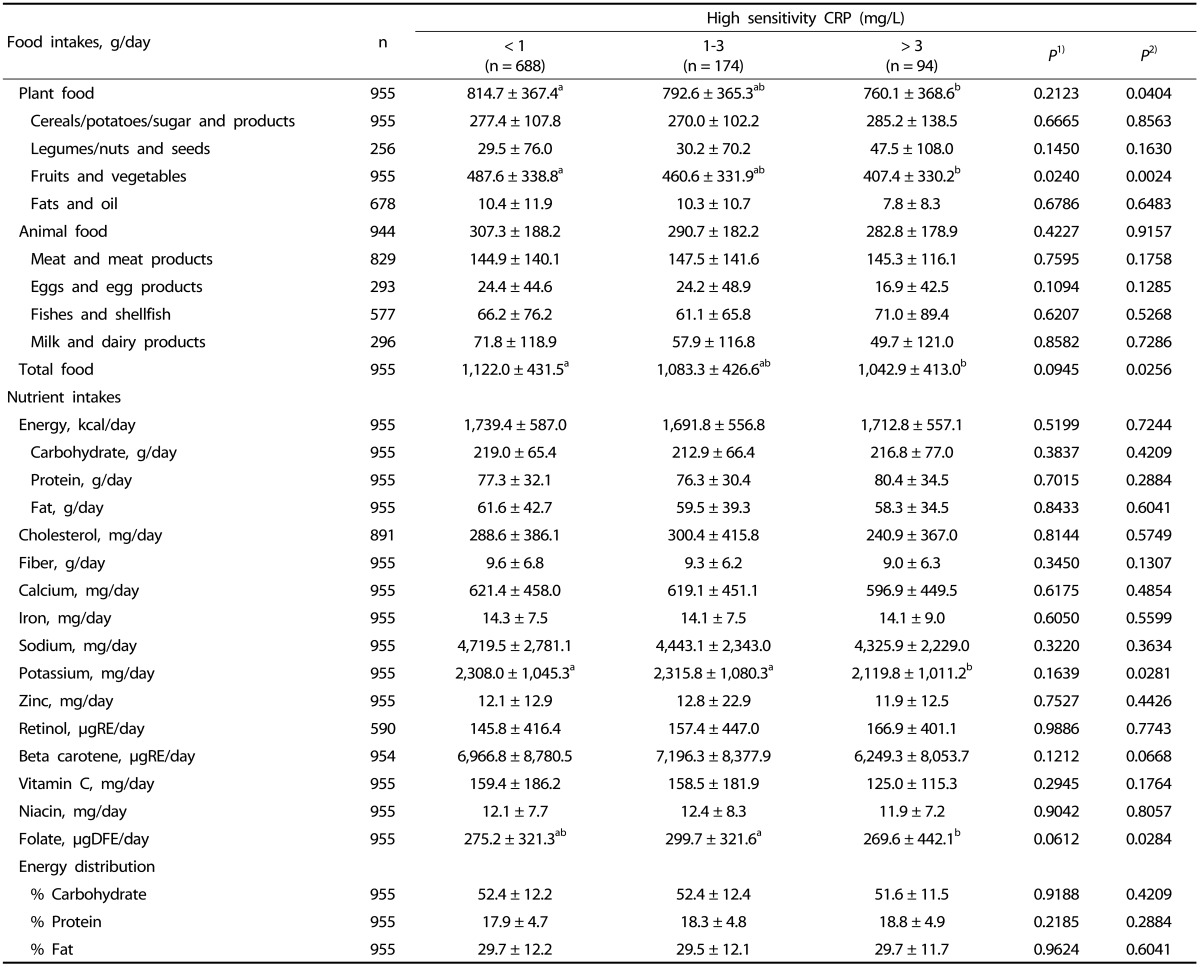
Fruits and Vegetables; Vegetables, mushrooms, seaweeds, fruits
Values are mean ± standard deviation.
Values are log transformed before analysis.
1)From GLM test; Unadjusted for variables
2)From GLM test; adjusted for age, body mass index, total energy intake, alcohol consumption, dietary supplement use, exercise.
Values with different alphabets are significantly different among the three groups by GLM at P < 0.05 as appropriate; including the post hoc comparisons of Tukey.
ORs and 95% CIs of hsCRP (> 3 mg/L) according to the food and nutrient intake quartiles
After adjusting for covariates, a significant negative relationship was observed between hsCRP (> 3 mg/L) and the consumption of fruits and vegetables [OR (95% CI) for the highest quartile compared to the lowest: 0.391 (0.190-0.807), P for trend = 0.0012], potassium [OR (95% CI) for the highest quartile compared to the lowest: 0.425 (0.192-0.939), P for trend = 0.2220] and folate [OR (95% CI) for the highest quartile compared to the lowest: 0.490 (0.249-0.964), P for trend = 0.0696] (Table 4).
Table 4.
Odds ratio and 95% confidence interval of hsCRP (> 3 mg/L) according to quartile of food and nutrient intakes
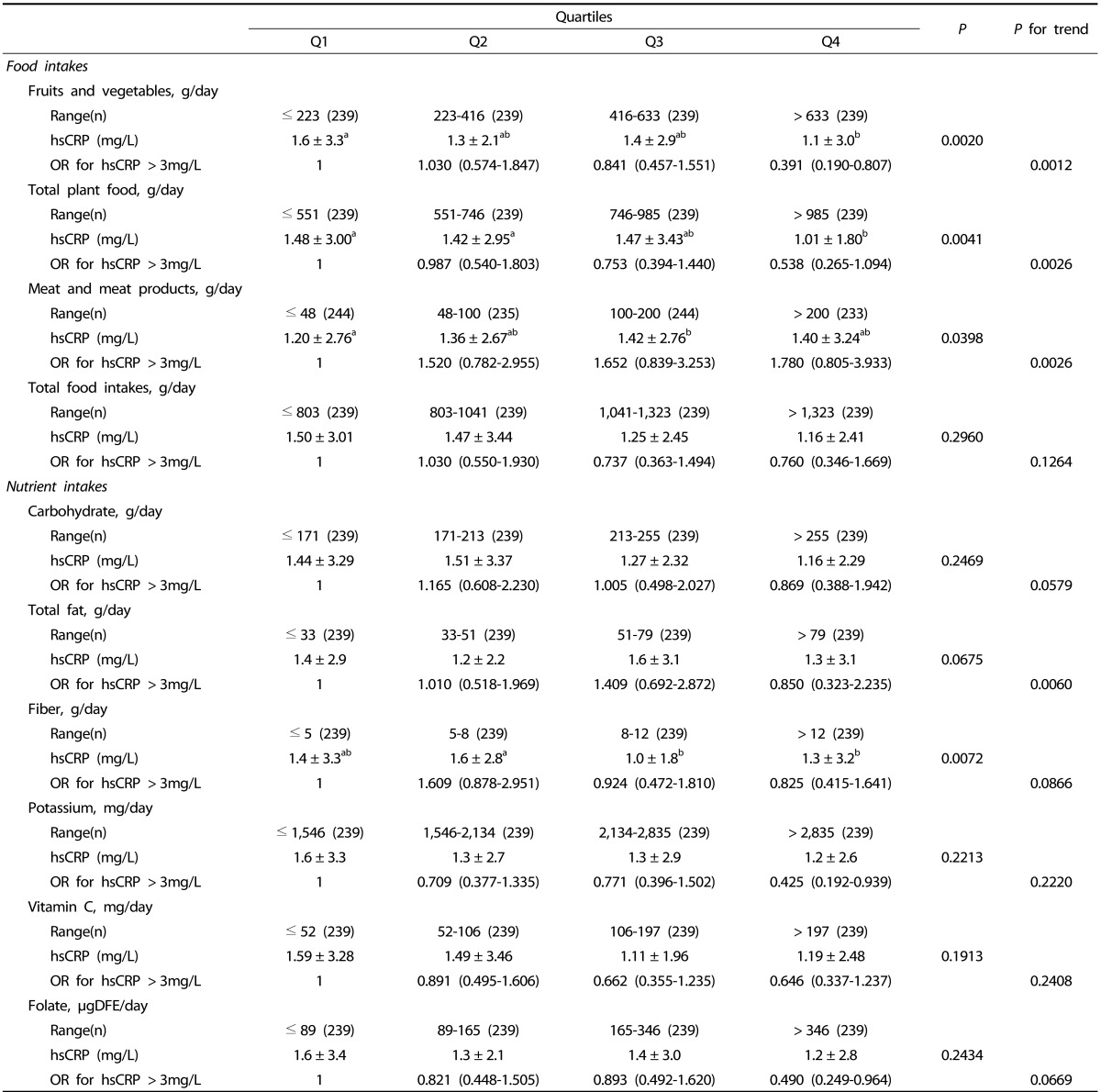
Values are mean ± Standard deviation.
Values are log transformed before analysis.
Adjusted for age, body mass index, total energy intake, alcohol consumption, dietary supplement use, exercise.
Values with different alphabets are significantly different among the three groups by GLM at P < 0.05 as appropriate; including the post hoc comparisons of Tukey.
Multiple regression analysis of hsCRP and dietary intake
Serum hsCRP was positively correlated with the consumption of eggs and egg products, meat and meat products, and fat (P < 0.05). However, hsCRP was negatively correlated with fruit and vegetable, total plant food, total food, carbohydrate, fiber, potassium, vitamin C, and folate consumption P < 0.05) (data not shown). A multiple regression analysis after adjusting for covariates revealed a significant negative association between hsCRP and consumption of fruits and vegetables (β = -0.2565, P = 0.0029) (Table 5).
Table 5.
Coefficients from multiple regression analysis between hsCRP and dietary intakes in Vietnamese women
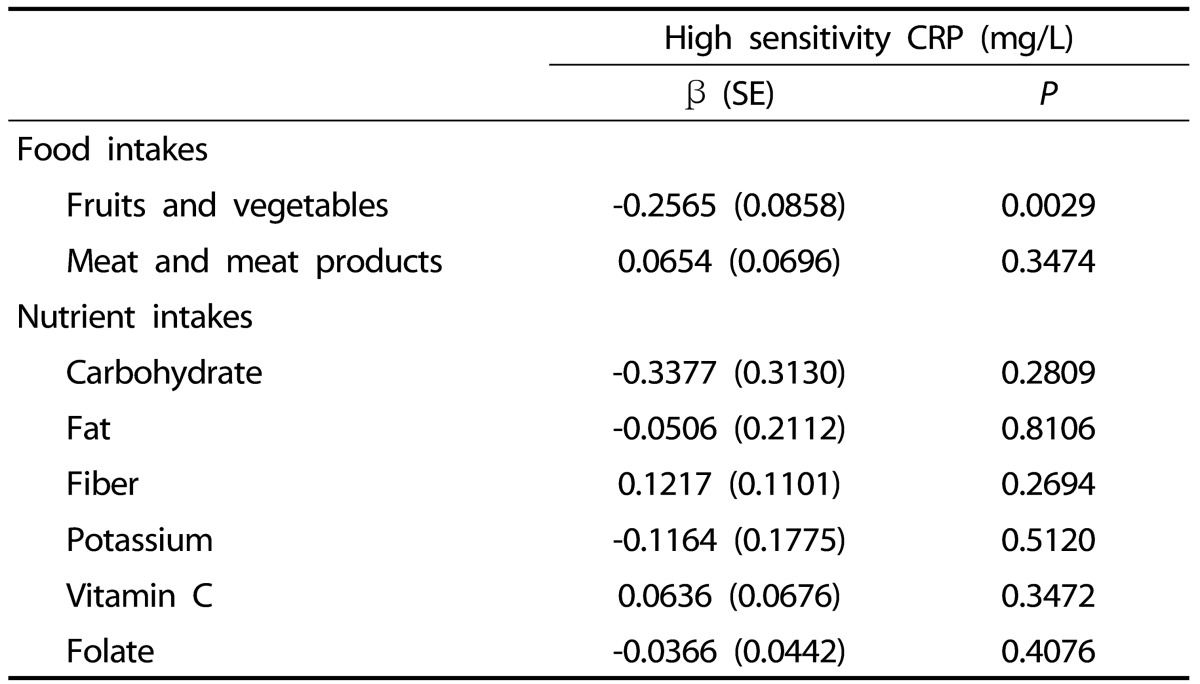
Values are log transformed before analysis.
Adjusted for age, body mass index, total energy intake, alcohol consumption, dietary supplement use, exercise.
DISCUSSION
In this study of apparently healthy Vietnamese women, the high risk group (serum hsCRP > 3 mg/L) consumed fewer fruits and vegetables and less potassium and folate than those in the low and average risk groups. We found an inverse association between serum hsCRP and fruit and vegetable, total plant food, total food, potassium, and folate consumption after adjusting for age, BMI, total energy intake, alcohol consumption, dietary supplement use and exercise. A multiple regression analysis after adjusting for covariates revealed a significant negative association between hsCRP and fruit and vegetable consumption, confirming the results of other investigators [25,26].
Previous studies on the relationship between hsCRP and dietary intake have reported that hsCRP levels are inversely associated with fruit and vegetable intake [25], and that the risk of high hsCRP (> 3 mg/L) increases with low consumption of fruits and vegetables [26]. Among nutrients, hsCRP is inversely associated with dietary intake of vitamin C [27], folate [28], and dietary fiber [15], for all of which nutrients fruits and vegetables are great sources. Most of these studies were conducted in middle-aged or elderly women in western populations (England, Portugal, Sweden, and Iran). Only one study is available in young Indian women in their 20s and 30s, which was similar to our study subjects, and that study reported a decreased hsCRP level in the group that consumed many fruits and vegetables compared to that of low consumption. This suggests that the beneficial role of fruits and vegetables against hsCRP may be universal across many population groups including eastern countries and also several age groups including young adults [11] despite differences in baseline hsCRP level and fruit and vegetable consumption. A healthy or prudent diet pattern with high fruit and vegetable consumption is inversely associated with hsCRP in diverse ethnic populations including those participated in the Nurses' Health Study [14] and Japanese [29]).
Increased hsCRP levels due to inflammation lead to a risk for endothelial dysfunction [30] and cause progression of atherosclerosis and cardiovascular disease [31]. Fruits and vegetables or nutrients such as vitamin C, folate, and potassium, which are found in high concentrations in those food groups, may directly and indirectly influence serum hsCRP levels, inflammation, and endothelial function. The mechanisms associated with dietary factors that influence hsCRP levels and inflammation are still unclear. However, high consumption of fruits and vegetables, which are good sources of antioxidant vitamins and folate, has beneficial effects on endothelial function [32,33]. Antioxidant vitamins contribute to endothelial function by reducing LDL oxidation [34], and folate by reducing plasma homocysteine concentration which can increase platelet aggregation, thrombosis [35] and decrease nitric-oxide (NO) production [36]. Potassium, another nutrient found in high concentrations in fruits and vegetables, improves vascular function by softening endothelial cells and stimulating NO release [37,38].
The mean hsCRP of the subjects was 1.3 ± 2.9 mg/L, and 9.8% of the subjects were in the high risk hsCRP group, which was similar to the level in young Indian (mean hsCRP levels, 1.3 ± 2.5 mg/L, 12.8% of subjects belonged to the high risk group) [11], but higher than that of other Asian young women [39,40] and Turkish women [41] as well as young women in European countries (mean hsCRP levels, 0.67-0.89 mg/L) [42,43]. In our subjects, hsCRP was highly correlated with many of the metabolic disease parameters such as BMI, WHR, body fat, blood pressure, white blood cell count, triglycerides, and HDL cholesterol, confirming the results of other studies, mostly in middle-aged populations [44,45]. We found such a relationship between hsCRP and metabolic disease parameters in Vietnamese young women as well.
Due to limited data, it is difficult to compare our results to those of other studies conducted among Vietnamese young women of same age, geographical or socioeconomic groups. The BMI of our subjects (mean 20.0 ± 2.4) was higher to that reported in women (mean of BMI 19.5 ± 1.9) of Thai Nguyen's 9 districts, rural region (n = 4,983; mean age, 26.2 ± 4.6 years) [46] and lower than that of women of reproductive age (mean of BMI 21.1 ± 0.1) of urban region (n = 730; mean age, 32.7 ± 0.4 years) [47]. The energy intake in our subjects was about 1,728 kcal/day (data not shown), which was lower than that reported in young women living in the rural area (2,196 kcal/day) [46] and those in the urban area (1,899 kcal/day) [48]. The differences in energy intake may have been partly due to differences in food intake survey methods, a 24-hour recall method in our study, semiquantitative food frequency method in rural areas [46], and 24-hour recall combined with controlled food weighing method in urban areas [48].
Vietnam is undergoing a rapid change in economic development, urbanization, and diet transition with an increasing trend in the number of people with CVD risk factors [17,18]. The CVD mortality in Vietnam now accounts for nearly 40% of mortality due to noncommunicable diseases [19]. A high CVD risk is particularly noticeable in urban areas of Vietnam, similar to this study area. The prevalence of diabetes and having a cluster of (≥ 2/4) metabolic CVD risk factors (hypertension, dyslipidaemia, diabetes, and obesity) in women in urban areas is approximately 1.4 and 2.6 times higher, respectively than that in rural areas [20,49]. The prevalence of some CVD risk factors in Vietnamese women such as obesity, dyslipidemia, diabetes has been reported [20,21], but not for serum hsCRP levels, which might be a better predictor of future CVD risk.
Although many studies have demonstrated an association between hsCRP level and dietary intake, very few studies have investigated such a relationship in young women in their 20s and 30s. If intervention is implemented early enough, the impact of diet and lifestyle interventions can be maximized. Given that hsCRP is a powerful predictor for future CVD, and that hsCRP level might be modifiable by dietary factors, studies on the relationship between hsCRP level and diet are important for developing diet intervention strategies.
This study has several limitations. First, a 1-day 24-hour recall may be insufficient to assess typical daily intake due to potentially large intra-individual variability in food and nutrient intake. However, our dietary interviewers were well-trained, which minimized potential errors when assessing dietary intake. Second, nearly 70% of our subjects were college students; therefore, our data may not be extrapolated to most women in Vietnam. Third, we cannot infer causality because of the cross-sectional nature of the study. Therefore, a prospective study should be conducted to explore the association between hsCRP and dietary intake in Vietnamese women. Nevertheless, our study had certain strengths. This is the first study on the relationship between hsCRP and diet in young Vietnamese adult women. This study was the International Collaboration Study for the Construction of Asian Cohort of the Korean Genome and Epidemiology Study (KoGES) performed on a large scale, and information on the identified and potential confounding factors was included in the analysis.
In conclusion, we found that hsCRP level was closely related to dietary intake in healthy Vietnamese women. Fruit and vegetable, potassium, and folate intake were inversely associated with hsCRP level. Therefore, to prevent the occurrence of future CVD by reducing hsCRP levels, it is necessary to increase consumption of fruits, vegetables, and all plant foods.
APPROVAL FROM INSTITUTIONAL ETHICS COMMITTEE
This study protocol was approved by the Human Investigation Review Board of Ewha Womans University College of Medicine (ECT 200-18 200-19).
Footnotes
This research was supported by a fund (2011-E71003-00) by Research of Korea Centers for Disease Control and Prevention and by the Brain Korea 21 Plus.
References
- 1.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 2.Corrado E, Rizzo M, Coppola G, Fattouch K, Novo G, Marturana I, Ferrara F, Novo S. An update on the role of markers of inflammation in atherosclerosis. J Atheroscler Thromb. 2010;17:1–11. doi: 10.5551/jat.2600. [DOI] [PubMed] [Google Scholar]
- 3.Hamer M, Chida Y, Stamatakis E. Association of very highly elevated C-reactive protein concentration with cardiovascular events and all-cause mortality. Clin Chem. 2010;56:132–135. doi: 10.1373/clinchem.2009.130740. [DOI] [PubMed] [Google Scholar]
- 4.Jiang S, Bao Y, Hou X, Fang Q, Wang C, Pan J, Zuo Y, Zhong W, Xiang K, Jia W. Serum C-reactive protein and risk of cardiovascular events in middle-aged and older chinese population. Am J Cardiol. 2009;103:1727–1731. doi: 10.1016/j.amjcard.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 5.Arima H, Kubo M, Yonemoto K, Doi Y, Ninomiya T, Tanizaki Y, Hata J, Matsumura K, Iida M, Kiyohara Y. High-sensitivity C-reactive protein and coronary heart disease in a general population of Japanese: the Hisayama study. Arterioscler Thromb Vasc Biol. 2008;28:1385–1391. doi: 10.1161/ATVBAHA.107.157164. [DOI] [PubMed] [Google Scholar]
- 6.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F Centers for Disease Control and Prevention; American Heart Association. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 7.Koenig W, Khuseyinova N, Baumert J, Meisinger C. Prospective study of high-sensitivity C-reactive protein as a determinant of mortality: results from the MONICA/KORA Augsburg Cohort Study, 1984-1998. Clin Chem. 2008;54:335–342. doi: 10.1373/clinchem.2007.100271. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 11.Arya S, Isharwal S, Misra A, Pandey RM, Rastogi K, Vikram NK, Dhingra V, Chatterjee A, Sharma R, Luthra K. C-reactive protein and dietary nutrients in urban Asian Indian adolescents and young adults. Nutrition. 2006;22:865–871. doi: 10.1016/j.nut.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Azadbakht L, Esmaillzadeh A. Red meat intake is associated with metabolic syndrome and the plasma C-reactive protein concentration in women. J Nutr. 2009;139:335–339. doi: 10.3945/jn.108.096297. [DOI] [PubMed] [Google Scholar]
- 13.Montonen J, Boeing H, Fritsche A, Schleicher E, Joost HG, Schulze MB, Steffen A, Pischon T. Consumption of red meat and whole-grain bread in relation to biomarkers of obesity, inflammation, glucose metabolism and oxidative stress. Eur J Nutr. 2013;52:337–345. doi: 10.1007/s00394-012-0340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, Hu FB. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2004;80:1029–1035. doi: 10.1093/ajcn/80.4.1029. [DOI] [PubMed] [Google Scholar]
- 15.Ajani UA, Ford ES, Mokdad AH. Dietary fiber and C-reactive protein: findings from National Health and Nutrition Examination Survey data. J Nutr. 2004;134:1181–1185. doi: 10.1093/jn/134.5.1181. [DOI] [PubMed] [Google Scholar]
- 16.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr. 2007;137:992–998. doi: 10.1093/jn/137.4.992. [DOI] [PubMed] [Google Scholar]
- 17.Khoi HH. Actual Nutrition Problems of Vietnam and Japan; Proceedings of Joint Symposium Organized by the Japanese National Institute of Health and Nutrition, and the Vietnamese National Institute of Nutrition; September 1/1997; Hanoi. Hanoi: Medical Publisher; 1998. [Google Scholar]
- 18.Hanh TT, Komatsu T, Hung NT, Chuyen VN, Yoshimura Y, Tien PG, Yamamoto S. Nutritional status of middle-aged Vietnamese in Ho Chi Minh city. J Am Coll Nutr. 2001;20:616–622. doi: 10.1080/07315724.2001.10719066. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization (CH) Noncommunicable Diseases Country Profiles 2011. Geneva: World Health Organization; 2011. [Google Scholar]
- 20.Nguyen QN, Pham ST, Do LD, Nguyen VL, Wall S, Weinehall L, Bonita R, Byass P. Cardiovascular disease risk factor patterns and their implications for intervention strategies in Vietnam. Int J Hypertens. 2012;2012:560397. doi: 10.1155/2012/560397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Son le NT, Kunii D, Hung NT, Sakai T, Yamamoto S. The metabolic syndrome: prevalence and risk factors in the urban population of Ho Chi Minh City. Diabetes Res Clin Pract. 2005;67:243–250. doi: 10.1016/j.diabres.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 23.The Korean Nutrition Society. Nutritional assessment program 'CAN-Pro 3.0' [CD-ROM] Seoul: The Korean Nutrition Society; 2006. [Google Scholar]
- 24.Ministry of Health, National Institute of Nutrition (VN) Vietnamese Food Consumption Table. Honoi: Medical Publishing Housing; 2007. [Google Scholar]
- 25.Oliveira A, Rodríguez-Artalejo F, Lopes C. The association of fruits, vegetables, antioxidant vitamins and fibre intake with high-sensitivity C-reactive protein: sex and body mass index interactions. Eur J Clin Nutr. 2009;63:1345–1352. doi: 10.1038/ejcn.2009.61. [DOI] [PubMed] [Google Scholar]
- 26.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am J Clin Nutr. 2006;84:1489–1497. doi: 10.1093/ajcn/84.6.1489. [DOI] [PubMed] [Google Scholar]
- 27.Chun OK, Chung SJ, Claycombe KJ, Song WO. Serum C-reactive protein concentrations are inversely associated with dietary flavonoid intake in U.S. adults. J Nutr. 2008;138:753–760. doi: 10.1093/jn/138.4.753. [DOI] [PubMed] [Google Scholar]
- 28.Holt EM, Steffen LM, Moran A, Basu S, Steinberger J, Ross JA, Hong CP, Sinaiko AR. Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J Am Diet Assoc. 2009;109:414–421. doi: 10.1016/j.jada.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nanri A, Yoshida D, Yamaji T, Mizoue T, Takayanagi R, Kono S. Dietary patterns and C-reactive protein in Japanese men and women. Am J Clin Nutr. 2008;87:1488–1496. doi: 10.1093/ajcn/87.5.1488. [DOI] [PubMed] [Google Scholar]
- 30.Fichtlscherer S, Rosenberger G, Walter DH, Breuer S, Dimmeler S, Zeiher AM. Elevated C-reactive protein levels and impaired endothelial vasoreactivity in patients with coronary artery disease. Circulation. 2000;102:1000–1006. doi: 10.1161/01.cir.102.9.1000. [DOI] [PubMed] [Google Scholar]
- 31.Huang PH, Chen JW, Lu TM, Yu-An Ding P, Lin SJ. Combined use of endothelial function assessed by brachial ultrasound and high-sensitive C-reactive protein in predicting cardiovascular events. Clin Cardiol. 2007;30:135–140. doi: 10.1002/clc.20058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuevas AM, Germain AM. Diet and endothelial function. Biol Res. 2004;37:225–230. doi: 10.4067/s0716-97602004000200008. [DOI] [PubMed] [Google Scholar]
- 33.Brown AA, Hu FB. Dietary modulation of endothelial function: implications for cardiovascular disease. Am J Clin Nutr. 2001;73:673–686. doi: 10.1093/ajcn/73.4.673. [DOI] [PubMed] [Google Scholar]
- 34.Rodríguez JA, Grau A, Eguinoa E, Nespereira B, Pérez-Ilzarbe M, Arias R, Belzunce MS, Páramo JA, Martínez-Caro D. Dietary supplementation with vitamins C and E prevents downregulation of endothelial NOS expression in hypercholesterolemia in vivo and in vitro. Atherosclerosis. 2002;165:33–40. doi: 10.1016/s0021-9150(02)00188-0. [DOI] [PubMed] [Google Scholar]
- 35.Haynes WG. Vascular effects of homocysteine: therapeutic implications. Heart Fail. 1999;15:153–163. [Google Scholar]
- 36.Stroes ES, van Faassen EE, Yo M, Martasek P, Boer P, Govers R, Rabelink TJ. Folic acid reverts dysfunction of endothelial nitric oxide synthase. Circ Res. 2000;86:1129–1134. doi: 10.1161/01.res.86.11.1129. [DOI] [PubMed] [Google Scholar]
- 37.Oberleithner H, Callies C, Kusche-Vihrog K, Schillers H, Shahin V, Riethmüller C, Macgregor GA, de Wardener HE. Potassium softens vascular endothelium and increases nitric oxide release. Proc Natl Acad Sci U S A. 2009;106:2829–2834. doi: 10.1073/pnas.0813069106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Büssemaker E, Hillebrand U, Hausberg M, Pavenstädt H, Oberleithner H. Pathogenesis of hypertension: interactions among sodium, potassium, and aldosterone. Am J Kidney Dis. 2010;55:1111–1120. doi: 10.1053/j.ajkd.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 39.Kim M, Kim H, Sohn C. Relationship between vitamin K status, bone mineral density, and hs-CRP in young Korean women. Nutr Res Pract. 2010;4:507–514. doi: 10.4162/nrp.2010.4.6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka M, Yoshida T, Bin W, Fukuo K, Kazumi T. FTO, abdominal adiposity, fasting hyperglycemia associated with elevated HbA1c in Japanese middle-aged women. J Atheroscler Thromb. 2012;19:633–642. doi: 10.5551/jat.11940. [DOI] [PubMed] [Google Scholar]
- 41.Tarkun I, Arslan BC, Cantürk Z, Türemen E, Sahin T, Duman C. Endothelial dysfunction in young women with polycystic ovary syndrome: relationship with insulin resistance and low-grade chronic inflammation. J Clin Endocrinol Metab. 2004;89:5592–5596. doi: 10.1210/jc.2004-0751. [DOI] [PubMed] [Google Scholar]
- 42.Puder JJ, Varga S, Kraenzlin M, De Geyter C, Keller U, Müller B. Central fat excess in polycystic ovary syndrome: relation to low-grade inflammation and insulin resistance. J Clin Endocrinol Metab. 2005;90:6014–6021. doi: 10.1210/jc.2005-1002. [DOI] [PubMed] [Google Scholar]
- 43.Lawson EA, Miller KK, Mathur VA, Misra M, Meenaghan E, Herzog DB, Klibanski A. Hormonal and nutritional effects on cardiovascular risk markers in young women. J Clin Endocrinol Metab. 2007;92:3089–3094. doi: 10.1210/jc.2007-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saito M, Ishimitsu T, Minami J, Ono H, Ohrui M, Matsuoka H. Relations of plasma high-sensitivity C-reactive protein to traditional cardiovascular risk factors. Atherosclerosis. 2003;167:73–79. doi: 10.1016/s0021-9150(02)00380-5. [DOI] [PubMed] [Google Scholar]
- 45.Fröhlich M, Imhof A, Berg G, Hutchinson WL, Pepys MB, Boeing H, Muche R, Brenner H, Koenig W. Association between C-reactive protein and features of the metabolic syndrome: a population-based study. Diabetes Care. 2000;23:1835–1839. doi: 10.2337/diacare.23.12.1835. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen PH, Strizich G, Lowe A, Nguyen H, Pham H, Truong TV, Nguyen S, Martorell R, Ramakrishnan U. Food consumption patterns and associated factors among Vietnamese women of reproductive age. Nutr J. 2013;12:126. doi: 10.1186/1475-2891-12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laillou A, Pham TV, Tran NT, Le HT, Wieringa F, Rohner F, Fortin S, Le MB, Tran do T, Moench-Pfanner R, Berger J. Micronutrient deficits are still public health issues among women and young children in Vietnam. PLoS One. 2012;7:e34906. doi: 10.1371/journal.pone.0034906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laillou A, Berger J, Le BM, Pham VT, Le TH, Nguyen CK, Panagides D, Rohner F, Wieringa F, Moench-Pfanner R. Improvement of the Vietnamese diet for women of reproductive age by micronutrient fortification of staples foods and condiments. PLoS One. 2012;7:e50538. doi: 10.1371/journal.pone.0050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duc Son LN, Kusama K, Hung NT, Loan TT, Chuyen NV, Kunii D, Sakai T, Yamamoto S. Prevalence and risk factors for diabetes in Ho Chi Minh City, Vietnam. Diabet Med. 2004;21:371–376. doi: 10.1111/j.1464-5491.2004.01159.x. [DOI] [PubMed] [Google Scholar]


