Abstract
Processing of signals within the cerebral cortex requires integration of synaptic inputs and a coordination between excitatory and inhibitory neurotransmission. In addition to the classic form of synaptic inhibition, another important mechanism that can regulate neuronal excitability is tonic inhibition via sustained activation of receptors by ambient levels of inhibitory neurotransmitter, usually GABA. The purpose of this study was to determine whether this occurs in layer II/III pyramidal neurons (PNs) in the prelimbic region of the mouse medial prefrontal cortex (mPFC). We found that these neurons respond to exogenous GABA and to the α4δ-containing GABAA receptor (GABAAR)-selective agonist gaboxadol, consistent with the presence of extrasynaptic GABAAR populations. Spontaneous and miniature synaptic currents were blocked by the GABAAR antagonist gabazine and had fast decay kinetics, consistent with typical synaptic GABAARs. Very few layer II/III neurons showed a baseline current shift in response to gabazine, but almost all showed a current shift (15–25 pA) in response to picrotoxin. In addition to being a noncompetitive antagonist at GABAARs, picrotoxin also blocks homomeric glycine receptors (GlyRs). Application of the GlyR antagonist strychnine caused a modest but consistent shift (∼15 pA) in membrane current, without affecting spontaneous synaptic events, consistent with the tonic activation of GlyRs. Further investigation showed that these neurons respond in a concentration-dependent manner to glycine and taurine. Inhibition of glycine transporter 1 (GlyT1) with sarcosine resulted in an inward current and an increase of the strychnine-sensitive current. Our data demonstrate the existence of functional GlyRs in layer II/III of the mPFC and a role for these receptors in tonic inhibition that can have an important influence on mPFC excitability and signal processing.
Keywords: glycine, GABA, tonic current, prefrontal cortex
the prefrontal cortex (PFC) is a heavily interconnected cortical subregion that plays a prominent role in the organization of behavior. Abnormal PFC activity has been implicated in psychiatric illnesses such as schizophrenia and depression (Elliott et al. 1997; Weinberger et al. 1986; Yoon et al. 2008), and environmental influences like stress and drug addiction (e.g., alcohol, cocaine) can lead to a long-term depression of PFC activity (Abernathy et al. 2010; Goldstein and Volkow 2011). The rodent PFC has a considerably smaller volume relative to brain size than is the case in humans, yet it remains a valuable model to study PFC signaling as it retains many essential features of the primate PFC, including the same cell types and circuitry, while mediating similar behavioral functions like working memory, behavioral flexibility, and attention (Chudasama and Robbins 2006; Seamans et al. 2008; Uylings et al. 2003). A better understanding of PFC connectivity and physiology is essential in providing the context in which prefrontal dysfunction can be understood.
The cortex has a distinct laminar organization that serves to integrate cortical and subcortical signals. In the rodent medial (m)PFC, the conventional view is that the superficial layers (I, II, III) are input layers receiving cortical and mediodorsal thalamic afferents while the deeper layers (V, VI) project to output regions like striatum and thalamus (Krettek and Price 1977; Ongur and Price 2000). Local cortical processing between layers is complex, with a myriad of interneuron subtypes forming distinct connections with dendritic, somatic, and axonal subcompartments of pyramidal neurons (PNs) and other interneurons (DeFelipe et al. 2013; Fino et al. 2013; Rudy et al. 2011). Layer II/III (LII/III) PNs are known to be sparsely activated and receive a dense inhibitory synaptic input from a rich variety of interneurons including parvalbumin-containing fast-spiking (FS), somatostatin-containing, and neurogliaform interneurons, to provide tight control of neuronal excitability (Olah et al. 2009; Petersen and Crochet 2013).
Neuronal activity in the cortex may also be regulated by tonic inhibition. Tonic inhibition occurs when ambient levels of GABA (originating from synaptic “spillover” or via release from glia) cause sustained activation of a population of highly sensitive receptors located in the peri- or extrasynaptic space (Belelli et al. 2009; Farrant and Nusser 2005). This phenomenon has been described in several brain regions, including the cerebellum (Brickley et al. 1996), hippocampus (Nusser and Mody 2002), thalamus (Cope et al. 2005; Jia et al. 2005), and somatosensory cortex (Salin et al. 1995; Yamada et al. 2007). The magnitude of these tonic currents can be increased by inhibiting GABA transporters (Semyanov et al. 2003; Vardya et al. 2008), emphasizing the important role of transporters in regulating tonic currents through the local control of neurotransmitter availability.
GABAA receptors (GABAARs) that are located extrasynaptically have been identified as essential to the generation of tonic inhibition. These receptors are composed primarily of combinations of α4/6-, β2/3-, and δ-subunits (Belelli et al. 2009) or α5-, β2/3-, and γ2-subunits (Belelli et al. 2009). Extrasynaptic receptors have a high affinity for GABA, with a slow desensitization rate, permitting a sustained elevation in chloride conductance even at very low concentrations of GABA (Bai et al. 2001; Brickley et al. 1996; Saxena and Macdonald 1994). In the ventrobasal thalamus, α4β2δ-containing GABAARs mediate tonic current and control the excitability of relay neurons (Jia et al. 2005, 2008). In the superficial layers of the rodent PFC these GABA α4- and δ-subunits appear to be highly expressed (Peng et al. 2002; Pirker et al. 2000), yet GABA-mediated tonic inhibition has not been reported (Hoestgaard-Jensen et al. 2010). Here we test the hypothesis that tonic inhibition occurs in layer II/III of the prelimbic region of the mPFC, and we show that a tonic current mediated by glycine receptors (GlyRs), but not GABAARs, exists in the majority of these neurons.
METHODS
Experimental procedures were performed in male C57BL/6J mice (Jackson Laboratories) under the guidelines set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with the approval of the Institutional Animal Care and Use Committee of Columbia University.
Brain slice preparation.
Mice (25–50 days old) were fully anesthetized with sevoflurane and decapitated into ice-cold (4°C) artificial cerebrospinal fluid (ACSF) containing (in mM) 124 NaCl, 2.5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 2 MgSO4, and 10 glucose. Brains were dissected and sectioned in cold ACSF with a vibrating microtome (Leica VT1000S) into coronal slices (300 μm) that contained the prelimbic region of the mPFC at between −2.8 and −1.78 mm from bregma, according to a mouse brain atlas (Franklin and Paxinos 1997). Slices were then incubated at 32°C in oxygenated (bubbled with 95% O2-5% CO2) ACSF for ∼30–45 min and then moved to room temperature (22–25°C) for at least 45 min before recordings began.
Slice electrophysiology.
Slices were placed in a submersion chamber and continuously superfused with room-temperature oxygenated ACSF. mPFC neurons were visualized under an upright light microscope (Olympus BX51WI) using infrared and differential interference contrast. PFC cortical layers were identified under a ×4 objective (layer II/III between ∼100 and 300 μm and layer V/VI between 350 and 500 μm from the pial surface), and PNs were identified under a ×40 objective by their characteristic size and shape. Pipettes (open tip resistance 2–5 MΩ for CsCl and 3–6 MΩ for K-gluconate solutions) were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL) by a pipette puller (Sutter Instrument, Novato, CA) and used for electrophysiological recordings. Data were collected with a Multiclamp 700B amplifier (Axon Instruments, Union City, CA) and Clampex 10.2 Software (Molecular Devices, Sunnyvale, CA) in identified PNs after reaching a >1-GΩ seal and after minimization of capacitative currents. Data were collected at 10 kHz and low-pass filtered at 2 kHz. For whole cell recordings under current-clamp conditions, a standard intracellular pipette solution was used (in mM: 130 K+-gluconate, 5 NaCl, 2 MgCl2, 10 HEPES, 0.2 EGTA, 2 ATP-K+, 0.3 GTP-Na+) and data collection was initiated ∼5 min after achieving whole cell configuration. Passive membrane properties [input resistance (IR), membrane capacitance, time constant] were measured from the resting membrane potential (RMP) with 20-pA command increments (6 steps starting at −60 pA, 500 ms), and firing properties (amplitude, frequency, accommodation, I/O relationship) were measured with 40-pA command increments (21 steps starting at −400 pA, 500 ms). To maximize chloride currents, recordings made under voltage-clamp conditions used a high-chloride intracellular solution (in mM: 140 CsCl, 4 NaCl, 1 MgCl2, 10 HEPES, 0.05 EGTA, 2 Mg-ATP, 0.3 GTP-Na+) adjusted to ∼295 mOsm by adding sucrose. To allow equilibration, 7 min of recording occurred prior to data acquisition and only cells demonstrating a stable baseline current were used in the experiment. For phasic events, neurons were voltage clamped at −70 mV, 30-s epochs were collected for analysis of spontaneous postsynaptic currents (sIPSCs), and 45-s epochs were collected for analysis of miniature inhibitory postsynaptic currents (mIPSCs) before and after drug application, allowing time for stabilization of the baseline and drug application. Access resistance was monitored for all cells in voltage clamp via 10-mV hyperpolarizing test pulses, and any cell with access resistance that changed >25% from the start of recording or reached >20 MΩ was omitted from the data analysis.
Biocytin visualization.
To identify neurons, recordings were performed with a modification of the current-clamp procedure and slices were cut at 150-μm thickness to enable better visualization of 0.5% biocytin (Invitrogen, Carlsbad, CA), which was added to the pipette solution. After measurement of passive membrane and firing properties, depolarizing current (+200 pA) was injected at 2 Hz for 15 min. After recording, slices were incubated in ACSF at room temperature for 45 min and then placed in 4% PFA for 1 h. Slices were then processed with the Vectastain ABC kit (Vector Labs, Burlingame, CA) and reacted with the chromogen diaminobenzidine. Filled cells were then examined under a low-power microscope, and images of labeled cells were collected with imaging software (HC Image) on a light microscope (Olympus BX51WI).
Pharmacology.
Drug solutions were applied to the slice by bath perfusion. Stock solutions were made by dissolving drugs in ACSF, adjusting pH to 7.4, and freezing. On the day of recording, drug stock solutions were diluted to yield the appropriate concentration. Glycine, taurine, strychnine, picrotoxin, and sarcosine were purchased from Sigma (St. Louis, MO). Gabazine, gaboxadol {4,5,6,7-tetrahydroisothiazolo-[5,4-c]pyridine-3-ol hydrochloride (THIP)}, tetrodotoxin (TTX), 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline-2,3-dione (NBQX), and d-(−)-2-amino-5-phosphonopentanoic acid (d-AP5) were purchased from Tocris (Bristol, UK).
Data analysis.
Off-line analyses of current-clamp and voltage-clamp data were performed with Clampfit 10.3.02 software. For changes in baseline current, 0.75- to 1.5-s epochs before and after experimental drug applications that contained minimal spontaneous events were selected and all-points histograms were created. These data were fitted with a single Gaussian curve. Responses to concentrations of GABA and glycine were normalized to a common agonist concentration in each cell (1 mM for GABA, 3 mM for glycine), analyzed with a nonlinear regression and fit to the Hill equation I = Imax × [agonist]nH/([agonist]nH + EC50nH), where I is the peak current, Imax is the maximal whole cell current amplitude, [agonist] is the agonist concentration eliciting a half-maximal current response, and nH is the Hill coefficient. Mini Analysis software (Synaptosoft, Decatur, GA) was used to detect synaptic events >18 pA in amplitude 4 times root mean square noise for all cells and 30 pA·ms in area. Amplitude, frequency, rise time (10–90%), and decay time were measured and compared before and after drug application. Graphing and statistical analyses were performed with GraphPad Prism software. Where appropriate, Student's paired t-test and ANOVA were used to test for statistical significance (P > 0.05). Data are expressed as means ± SE.
RESULTS
Characterization of pyramidal neurons in mouse prefrontal cortex.
Many electrophysiological studies in the mouse PFC have focused on PNs in layer V/VI. In the present study, we recorded from a large number of neurons in layer II/III and measured their passive and active membrane properties using pipettes filled with K-gluconate-based intracellular solutions. A subset of layer II/III cells identified by their large size and triangular cell body shape were filled with biocytin (Fig. 1A) and confirmed to be LII/III PNs of the prelimbic region of the mPFC on the basis of morphological and electrophysiological properties. PFC PNs have specific intrinsic and firing properties that distinguish them from GABAergic interneurons, the other principal neuronal subtype within layer II/III (Amatrudo et al. 2012; Kawaguchi 1993).
Fig. 1.
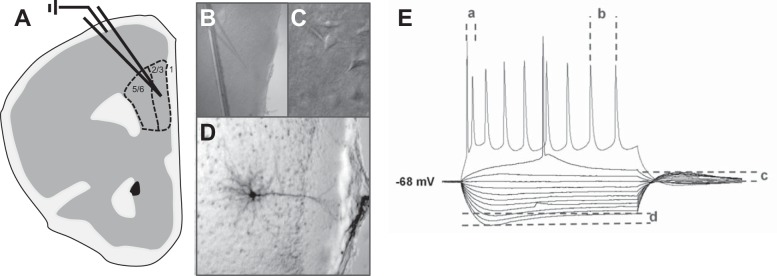
A: schematic illustrating location of whole cell recordings in layer II/III region of prelimbic cortex (dashed line). B and C: pipettes were directed at layer II/III (×4, B), and pyramidal neurons (PNs) were identified by their triangular shape with infrared-differential interference contrast (IR-DIC) optics (×40, C). D: biocytin-filled neuron confirming PN morphology in recorded cell. E: current-clamp recording of firing pattern of filled neuron in D at resting membrane potential (−68 mV) showing single action potential after 80-pA stimulus (black spike) and multiple action potentials after 380-pA stimulus (gray spikes). Accommodation (index measured from a/b) that is characteristic of cortical PNs is present. Additionally, other properties of PNs including sag (d) and an afterdepolarization (c) are present in this neuron. Active and passive membrane properties of layer (L)II/III PNs are summarized in Table 1.
In total, identified LII/III PNs (n = 30) had a RMP of −67.9 ± 1 mV, IR = 156 ± 9 MΩ, membrane time constant (τm) = 28 ± 2 ms, membrane capacitance (Cm) = 176 ± 7 pF, rheobase = 111 ± 10 pA, and firing threshold = −38 ± 1 mV (Table 1), all of which are consistent with previous reports for PNs in this area (Amatrudo et al. 2012; Kawaguchi 1993). When prolonged depolarizing current injections were made at RMP, the LII/III neurons fired in a pattern characteristic of PNs, including pronounced accommodation of firing rate. Accommodation index (AI) = 0.5 ± 0.02 was expressed as the ratio of the interspike frequency for the last two spikes versus the first two spikes (Fig. 1E, a and b) during a 500-ms, 240-pA command. A pronounced “sag” in the electrotonic potential (+3.0 ± 0.5 mV) was observed in response to larger hyperpolarizing currents. This was expressed as the voltage difference between the peak and the steady state during a 500-ms, −400-pA current (Fig. 1Ed). Afterdepolarization was defined as the peak voltage difference between RMP and the overshoot following −400-pA current (Fig. 1Ec). Morphological analysis of LII/III neurons successfully filled with biocytin and retrieved after processing the slices (n = 8) revealed extensive apical dendrites that extended into layer I and cell bodies located in layer II/III of the prelimbic region of the PFC (Franklin and Paxinos 2008). We conclude that we have been able to identify neurons in layers II/III with the morphological and electrical signatures typical of PNs (accommodation, voltage sag).
Table 1.
Passive and active membrane properties of layer II/III and V/VI PNs located in the prelimbic region of the PFC
| Layer II/III | Layer V/VI | P value | |
|---|---|---|---|
| RMP, mV | −67.9 ± 1 | −69.5 ± 1 | 0.21 |
| Input resistance, MΩ | 156 ± 9 | 102 ± 9 | <0.001 |
| Membrane capacitance, pF | 176 ± 7 | 170 ± 13 | 0.73 |
| τm, ms | 28 ± 2 | 16 ± 1 | <0.001 |
| Rheobase, pA | 111 ± 10 | 155 ± 14 | <0.05 |
| Sag, mV | 2.7 ± 0.4 | 4.0 ± 0.5 | <0.05 |
| ADP, mV | 3.2 ± 0.4 | 3.5 ± 0.4 | 0.61 |
Values are means ± SE; n = 30 for each group.
PN, pyramidal neuron; PFC, prefrontal cortex; RMP, resting membrane potential; τm, membrane time constant; ADP, afterdepolarization. P values, unpaired t-test; significant values are in boldface.
PNs were then recorded from layers V/VI to compare membrane and firing properties between layers (Table 1). These neurons showed a slightly hyperpolarized RMP of −69.5 ± 1. Student's t-test revealed several significant differences in PNs between layers, where layer V/VII PNs had higher IR (102 ± 9 MΩ, P < 0.001), τm (16 ± 1 ms, P < 0.001), and rheobase (155 ± 14 pA, P < 0.05) while Cm was similar (170 ± 13 pF). Additionally, sag was significantly greater in layer V/VI (sag = 4.0 ± 0.5, P < 0.05). It should be noted that when recorded at room temperature (Amatrudo et al. 2012; Kawaguchi 1993), cortical PNs have higher IR and lower rheobase than at more physiological temperatures without effects on RMP and Ih (Day et al. 2005; Thuault et al. 2013). For a detailed description of the effects of recording temperature on cortical PNs, see Hedrick and Waters (2012).
Synaptic events.
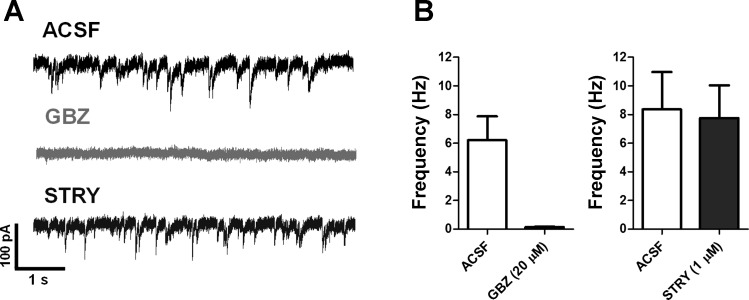
First, we recorded spontaneous synaptic events in layer II/III of the mPFC, using a CsCl-based intracellular solution. We found that gabazine (20 μM, n = 6) blocked 98% of all spontaneous transient events (Fig. 2A) recorded under voltage clamp at −70 mV (Fig. 2B), which indicated that the majority of synaptic events were sIPSCs mediated by GABA acting at GABAARs. The GlyR antagonist strychnine (1 μM) did not cause any significant changes in frequency (Fig. 2) or amplitude of the sIPSCs. Next, we isolated mIPSCs by blocking sodium channels with TTX and AMPA and NMDA receptor-mediated currents with NBQX (10 μM) and d-AP5 (50 μM). The properties of these mIPSCs are summarized in Table 2 and indicate that essentially all of these events are fast, with rise times ∼2 ms and decay time constant lasting ∼4–5 ms. After application of strychnine we found that amplitude and frequency of mIPSCs were unchanged, although we noted a small change in decay time of mIPSCs (Table 2; P < 0.05, t-test).
Fig. 2.
A: representative recordings of spontaneous synaptic events under voltage-clamp conditions using high-internal chloride solution under control (ACSF), 20 μM gabazine (GBZ, light gray), or 1 μM strychnine (STRY, dark gray) demonstrating near-complete block of events by gabazine and no effect of strychnine. B: frequency (Hz) of synaptic events is plotted before (ACSF) and after application of 20 μM gabazine or 1 μM strychnine. Properties of synaptic inhibitory currents are summarized in Table 2.
Table 2.
Characteristics of synaptic events (sIPSCs and mIPSCs) summarized for neurons before and after application of 1 μM strychnine
| Events (cells) | Freq, Hz | Amp, pA | Rise, ms | Decay, ms | |
|---|---|---|---|---|---|
| sIPSC | |||||
| ACSF | 1,239 (6) | 6.8 ± 2 | 39.4 ± 2 | 2.6 ± 0.1 | 8.6 ± 0.6 |
| STRY | 1,175 (6) | 6.7 ± 2 | 38.8 ± 3 | 2.7 ± 0.1 | 7.8 ± 0.6 |
| mIPSC | |||||
| ACSF | 578 (5) | 2.4 ± 0.8 | 33 ± 4 | 2.2 ± 0.2 | 5.3 ± 0.4 |
| STRY | 439 (5) | 1.8 ± 0.7 | 29 ± 3 | 2.2 ± 0.2 | 4.3 ± 0.3 |
Values are mean ± SE characteristics of synaptic events [spontaneous (sIPSC) and miniature (mIPSC) inhibitory postsynaptic currents] summarized for neurons before (ACSF) and after application of 1 μM strychnine (STRY). Significant changes in mIPSC decay time (P < 0.05) are in boldface.
LII/III PN pharmacology: responses to GABA and THIP.
To further study the presence and sensitivity of GABAARs on PNs, exogenous GABA (1 μM–3 mM) was applied to PNs under voltage-clamp conditions after stabilization of baseline recording current. Responses to GABA were then normalized to the response to 1 mM GABA (n = 7). A paired t-test revealed a significant inward current following application of 10 μM GABA (−40 ± 13 pA, P < 0.05) and above (Fig. 3A). A concentration-response curve was generated (Fig. 3B) and fitted to the data with the Hill equation, yielding a GABA EC50 of 353 μM and Hill slope = 1.43.
Fig. 3.
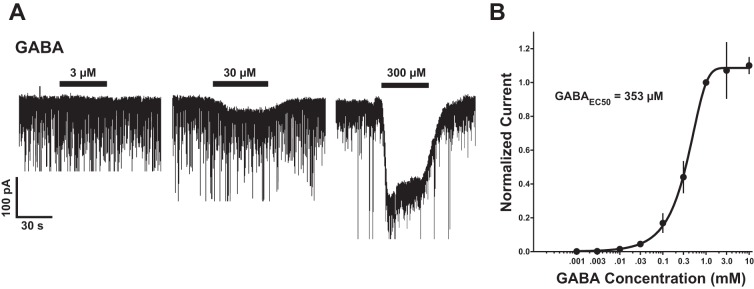
A: representative traces recorded under voltage-clamp conditions using high-chloride internal solution demonstrating prefrontal cortex (PFC) LII/III PN response to 3, 30, and 300 μM GABA. Significant inward current was observed at concentrations ≥ 10 μM, −40 ± 13 pA, P < 0.05. B: concentration-response curve of GABA as normalized to 1 mM GABA. EC50 = 353 μM; Hill slope = 1.43.
We also investigated whether δ-containing GABAARs were present with the selective agonist THIP. We observed a modest but significant response (−14 ± 4 pA, P < 0.05, paired t-test) to a low concentration of THIP (0.3 μM, n = 5) that selectively activates δ-containing GABAARs. Application of 1.0 μM THIP (n = 9) elicited a larger response (−56 ± 7 pA, P < 0.005, paired-t-test) (Fig. 4A). Responses to THIP were fully blocked by the GABAAR antagonist gabazine (20 μM) (Fig. 4). These results suggest that δ-containing GABAARs are likely present on LII/III PNs and that they can be fully inhibited by gabazine.
Fig. 4.
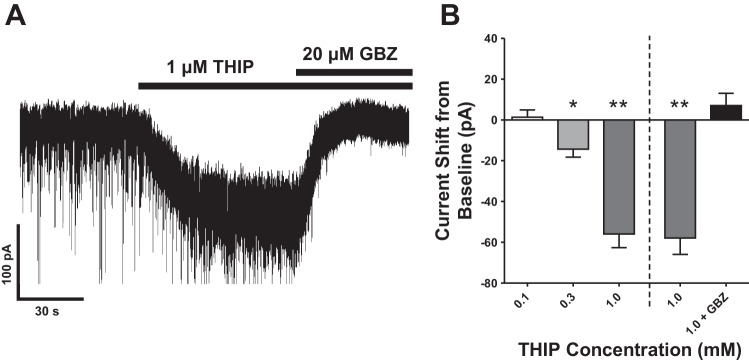
A: representative trace demonstrating large inward current after application of the GABA agonist THIP (1 μM) that was completely blocked by the GABA antagonist gabazine (20 μM). B: results including response to 0.3 and 1 μM THIP (*P < 0.05, **P < 0.005).
Picrotoxin- and strychnine-sensitive tonic inhibitory current.
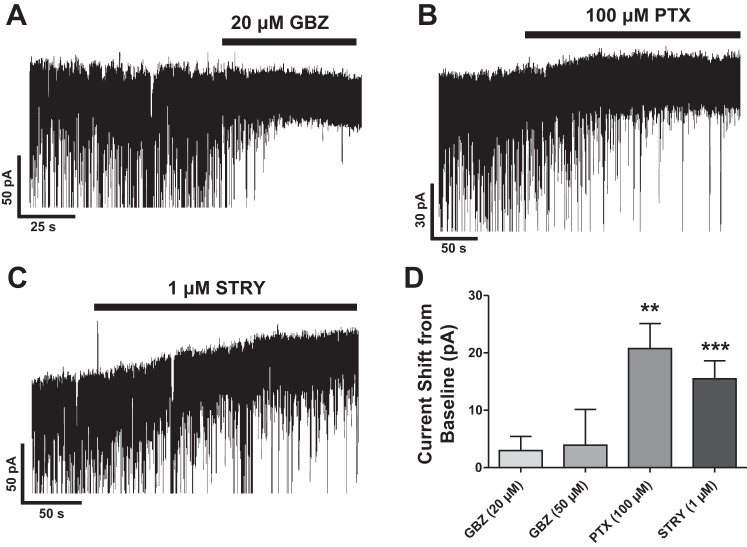
The presence of a tonic inhibitory current is common in forebrain regions and can be routinely demonstrated by applying a GABAA receptor (GABAAR) antagonist and measuring an outward (positive) shift in baseline current under conditions where internal chloride is high and tonic currents are inwardly directed at −70 mV (e.g., Jia et al. 2009). To investigate the presence of a GABAergic tonic current in mPFC LII/III PNs, we applied gabazine (20 μM) at a concentration that has previously been shown to block GABA-mediated tonic inhibition in thalamic relay neurons (Jia et al. 2009). We did not observe a significant reproducible change in baseline current (+3.0 ± 3 pA, n = 13, only 4 of 13 neurons with a shift > 6 pA), despite a near-total block of synaptic events by gabazine (Fig. 5A), indicating the absence of a GABA-mediated tonic current in these neurons. Some labs report that higher concentrations of gabazine are needed to demonstrate a tonic current (Bai et al. 2001). Increasing the concentration of gabazine (50 μM) also did not alter the results in our hands (+3.9 ± 6 pA, n = 7, 3 of 7 neurons with a shift > 6 pA). In our next series of experiments, we applied picrotoxin (PTX, 100 μM), a compound that blocks the chloride channel of open GABAARs. After application of PTX, we routinely observed a large positive shift in baseline current (+21 ± 4 pA, P < 0.005, n = 9); eight of nine neurons had a shift > 6 pA (Fig. 5, B and D). In addition to GABAARs, PTX is known to inhibit other ligand-gated chloride channels, including homomeric GlyRs (Pribilla et al. 1992; Yoon et al. 2008). We subsequently found that application of strychnine (1 μM), a selective GlyR antagonist, caused a reproducible (9 of 12 neurons > 6 pA) and significant shift (15 ± 3 pA, P < 0.005, n = 12) in baseline holding current (Fig. 5, C and D). From these results, we conclude that LII/III neurons have a strychnine- and PTX-sensitive tonic current.
Fig. 5.
A and B: representative recordings under voltage-clamp conditions showing that application of 20 μM gabazine (GBZ) did not detect a tonic GABA current; however, application of 100 μM picrotoxin (PTX) did, causing a significant positive shift from baseline current. C: a similar positive shift was revealed after application of 1 μM strychnine. Results are summarized in D (**P < 0.005, ***P < 0.001).
Glycine and taurine.
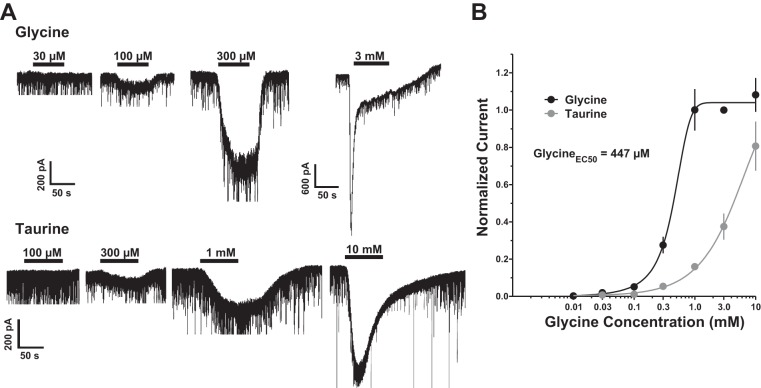
The presence of a tonic strychnine-sensitive current in PFC LII/III neurons was surprising, as there have been few reports on the existence of functional GlyRs in the cortex using slice electrophysiology. In our next experiment, we set out to characterize LII/III PN sensitivity to endogenous ligands of GlyRs, beginning with glycine itself (0.01–10 mM). Bath-applied glycine elicited significant responses at a concentration of 100 μM (P < 0.05, n = 7) and EC50 of 447 μM, Hill slope = 2.56, and very large responses (>1.5 nA) at the highest concentrations tested (3 and 10 mM) (Fig. 6). Application of taurine (0.1–10 mM) also induced measurable current at 100 μM (P < 0.05, n = 7) but was less potent than glycine at several concentrations (100 μM–10 mM). All responses to glycine and taurine were normalized to the response to 3 mM glycine, established at the end of each recording after a washout period (>5 min) (Fig. 6B).
Fig. 6.
A, top: representative recordings under voltage-clamp conditions showing the response to exogenously applied glycine (30 μM–3 mM). Bottom: taurine application (100 μM–10 mM) causes a similar inward current at higher concentrations. B: glycine and taurine applications are shown on concentration-response curve normalized to 3 mM glycine for each neuron, where glycine EC50 = 447 μM and Hill slope = 2.56.
We then elicited responses (112 ± 24 pA) to an ∼EC20 concentration of glycine (200 μM) recorded in the absence and presence of strychnine (1 μM) to determine whether these glycine-activated currents are mediated by strychnine-sensitive GlyRs. Strychnine applications blocked the response to glycine (Fig. 7A) and generated a positive shift in the baseline current (+22 ± 9 pA) (Fig. 7B). Glycine is known to be an essential coagonist at the NMDA receptor (NMDAR). To examine the contribution of NMDARs, we next measured the response to 200 μM glycine before and after inhibition of NMDARs with d-AP5. We did not observe any significant difference of the 200 μM glycine response in the presence of d-AP5 (Fig. 7C).
Fig. 7.
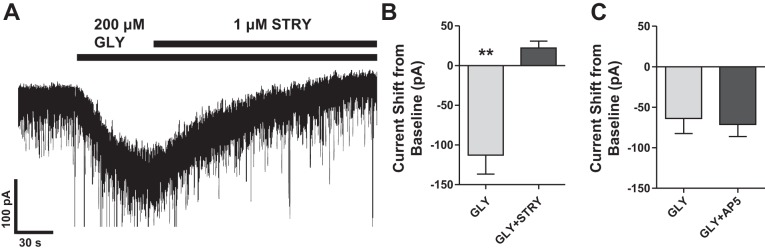
A: representative recording showing that the shift in current by glycine (200 μM) is completely blocked by coapplication of strychnine (1 μM) (summarized in B; **P < 0.01). C: graph demonstrating that the NMDA antagonist d-(−)-2-amino-5-phosphonopentanoic acid (AP5) does not affect the shift in current by glycine (200 μM).
We next sought to examine the possible role of glycine transporters (GlyTs) in regulating the availability of glycine in the slice. We found that application of sarcosine, a potent inhibitor of GlyT1, caused a significant inward current (−63.5 ± 13 pA) at 500 μM (P < 0.001, t-test), which could be largely blocked (∼75%) by strychnine (1 μM) (Fig. 8). In addition, GlyT1 inhibition increased the magnitude of the strychnine-sensitive current by ∼30 pA, from ∼15 pA to 45 pA (Fig. 8B).
Fig. 8.
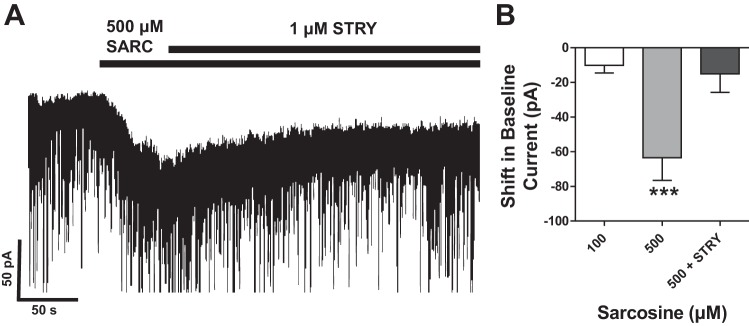
A: representative recording showing that sarcosine (SARC; 500 μM) causes a negative shift in baseline current that can be primarily blocked by coapplication of strychnine (1 μM). B: graph showing concentration-dependent action of sarcosine on holding current and blockade by strychnine (***P < 0.001).
DISCUSSION
Characterization of pyramidal neurons in mouse prefrontal cortex.
Our investigation of passive and active membrane properties in layers II/III and layers V/VI of mouse PFC identified several layer-specific characteristics. PNs in layers II/II had higher IR, longer τm, and decreased rheobase, providing evidence that they are more excitable than PNs located in layer V/VI. Additionally, we found that both layers exhibit a measurable sag and afterdepolarization in response to hyperpolarizing stimuli consistent with the presence of Ih. Layer V/VI PNs showed a larger sag on average, consistent with findings that HCN1 channels predominate in layer V/VI PNs of PFC, where they are important for establishing the RMP and for the generation of persistent firing (Thuault et al. 2013).
Synaptic GABAAR signaling.
Spontaneous synaptic GABA-mediated transmission was observed in LII/III PNs. Under conditions of high internal chloride, we observed frequent (∼7 Hz) spontaneous gabazine-sensitive currents averaging ∼40 pA in amplitude. Measurements of mIPSCs show rise times of ∼2 ms and decay times of ∼5 ms. The relatively rapid timescale of these events indicates that they were likely mediated by α1β2/3γ2-GABAARs, as in cerebellar Purkinje neurons and elsewhere in the brain (Vicini et al. 2001). Regional expression analysis supports the presence of α1β2/3γ2-GABAARs in layer LII/III of the frontal cortex (Fritschy and Mohler 1995; Pirker et al. 2000), and sIPSCs recorded in cortical LII/III PNs of α1 knockout mice (Bosman et al. 2002) are significantly slower in time course.
Lack of tonic GABAAR current in LII/III PNs.
Tonic inhibition is widespread but by no means ubiquitous in the brain. For tonic inhibition to occur, several requirements must be satisfied. First, there needs to be sufficient expression of extrasynaptic receptor subtypes with high ligand affinity and slow desensitization rates in order to sustain chloride conductance. Second, an inhibitory neurotransmitter needs to be present at sufficiently high levels to activate these receptors. Expression analyses have demonstrated the presence of GABAAR subunits α4 and δ in the outer layers of the PFC (Peng et al. 2002; Pirker et al. 2000). GABAA α4βδ receptors have been shown to have a higher sensitivity to GABA (activation at 100 nM) compared with receptor subtypes associated with synaptic receptors (e.g., α1β2γ2s GABAARs, activation at 3 μM) (Jia et al. 2005).
Determining neurotransmitter concentrations in brain slices is obviously difficult, but we can infer that ambient GABA concentrations in slices of the mouse PFC must be very low, since although THIP (a selective, highly efficacious agonist at α4,δ-containing GABAARs) can elicit a significant current at 0.3 and 1.0 μM, there is little to no gabazine-sensitive tonic current, perhaps <100 nM. The lack of a tonic GABA current here is consistent with previous reports in LII/III PFC neurons in rodents (Drasbek and Jensen 2006; Hoestgaard-Jensen et al. 2010). Variations in brain levels of GABA are presumed to be a result of regional differences in the subtypes and expression levels of the GABA transporters. Tonic inhibition revealed by gabazine has been identified in cortical LII/III PNs, but following extensive blockade of GABA transporters with NO-711 (Vardya et al. 2008). In reports investigating layer V PFC neurons GABA-mediated tonic inhibition was absent (Weitlauf and Woodward 2008), but it has been demonstrated in the somatosensory cortex (Salin et al. 1995), suggesting that in anterior cortical subregions such as PFC ambient GABA may be more tightly regulated.
Presence of tonic glycine currents in LII/III PNs.
An alternative source of cortical inhibition can arise from the presence of glycine, the other major inhibitory neurotransmitter in the central nervous system. Like GABAARs, GlyRs are pentameric ligand-gated ion channels that are permeable to chloride (Kirsch 2006). In the spinal cord, synaptic GlyRs are formed as a heteropentamer of α1- and β-subunits; the presence of a β-subunit confers synaptic localization through its binding to gephyrin, an anchoring protein that is enriched at synaptic sites (Kirsch 2006). In structures of the postnatal forebrain, there is very low expression of GlyRα1 mRNA compared with the spinal cord, but the expression of GlyRα2 and GlyRα3 has been reported in several brain regions, including the PFC (Jonsson et al. 2009; Malosio et al. 1991).
In the hippocampus functional, strychnine-sensitive GlyRs have been identified and are believed to be homomeric assemblies of α-subunits because of their sensitivity to PTX (Chattipakorn and McMahon 2002, 2003; Zhang et al. 2008b) as well as cyclothiazide, a proposed α2-GlyR antagonist (Zhang et al. 2008b). In hippocampal slices, a tonic GlyR current was identified in the CA1 region after strychnine application (Zhang et al. 2008a). These data support the idea that α homomeric GlyRs mediate tonic inhibition, analogous to the role that δ-containing GABAARs play in tonic GABA inhibition.
In layer II/III of the PFC, using PTX (100 μM), we found that we could elicit a positive shift in baseline current. In dissociated PFC neurons, PTX has been shown to block glycine currents (Lu and Ye 2011). We subsequently demonstrated the presence of tonic GlyRs by applying strychnine, which elicited a significant shift (∼15 pA) in baseline current in the absence of an effect on the frequency of GABA-mediated sIPSCs or isolated mIPSCs. Applications of exogenous glycine further showed that a large population of GlyRs is present in PFC LII/III PNs and can generate large inward currents in response to exogenous glycine, which could be fully blocked by strychnine. Previous reports have shown slightly higher sensitivity of exogenous glycine on hippocampal PNs from 3- to 4-wk-old rats (EC50 = 270 μM) and on PFC PNs from periadolescent rats (EC50 = 117 μM) and that these currents could be blocked by strychnine and PTX (Chattipakorn and McMahon 2002; Lu and Ye 2011). The measurements made in PFC PNs were from freshly dissociated neurons, and differences in glycine sensitivity are likely due to GlyTs actively taking up glycine. Importantly, application of sarcosine (500 μM), a GlyT1 antagonist, elicited a 64-pA inward current that could be blocked by strychnine. GlyT1s are primarily located on astrocytes in the cortex (Kunz et al. 2012), and we conclude that astrocytic GlyT1 plays an important role in regulating the magnitude of tonic GlyR inhibition in the mouse PFC.
A recent report in the mouse lateral orbital frontal cortex (LOFC) is consistent with our findings and has implicated extrasynaptic GlyRs as targets of ethanol modulation (Badanich et al. 2013). Specifically, these authors found that high concentrations of ethanol (66 mM) shift the holding current of deep-layer PNs of the LOFC and that this could be blocked by PTX and strychnine (1 μM) but not by the GABAAR antagonist bicuculline. In addition, ethanol, a known positive allosteric modulator of GlyRs, decreased neuronal excitability, an effect that was blocked by the application of strychnine but not bicuculline. They conclude that strychnine-sensitive GlyRs modulate ethanol's effects on deep-layer LOFC neurons. Unlike our findings in LII/III PFC, they did not detect the presence of a strychnine-sensitive tonic current in LOFC. This may be due to a reduced availability of ambient glycine or other GlyR ligands in this subregion.
Endogenous ligand responsible for tonic GlyR activation.
Our evidence supports the expression of functional, extrasynaptic GlyRs, but the source of the endogenous ligand for GlyRs in the PFC remains unclear. In vivo microdialysis has shown that 10 μM of extracellular glycine exists in the extracellular space of the hippocampus (Horio et al. 2011). Additionally, glutamatergic synapses (where concentrations of glycine have been estimated to reach 1 mM) may be a second source of glycine due to synaptic spillover (Vandenberg and Aubrey 2001), although it is believed that GlyTs limit glycine spillover 100- to 1,000-fold (Harsing and Matyus 2013). In addition to glycine, the GlyR partial agonists taurine and β-alanine are known to be abundant in the brain (Dahchour et al. 2000; Murakami and Furuse 2010), and we show here that exogenous taurine can cause an inward current, supporting its role as a GlyR agonist on PFC PNs. In early postnatal cortical PNs, taurine has been shown to mediate tonic nonsynaptic GlyR activation (Flint et al. 1998). In hippocampal PNs, the inhibition of taurine and β-alanine transporters by guanidinoethanesulfonic acid can generate a GABA-independent, strychnine-sensitive current of 15 pA (Mori et al. 2002). Our finding that sarcosine, which selectively inhibits GlyT1, increased the measurable strychnine-sensitive tonic current by ∼30 pA indicates that glycine is a major mediator of the strychnine-sensitive current; however, it seems reasonable to propose that sufficient levels of multiple endogenous GlyR ligands can arise to tonically activate GlyRs in the forebrain.
Physiological and functional significance.
In the PFC, LII/III PNs are uniquely positioned to integrate several inputs from cortical and subcortical structures. We have shown that inhibition of these neurons is more diverse than previously realized, including a novel role for tonic GlyR activation. Tonic inhibitory conductances of this scale have previously been shown to inhibit neuronal activity (Jia et al. 2008). This novel mechanism may contribute to the sparse action potentials observed in LII/III neurons around resting potential and permit increased fidelity of neuronal output, a proposed tuning mechanism involved in attention and working memory in the PFC (Rao et al. 2000). As GlyR activation increases, we would expect increased fidelity at lower levels of activation and subsequent silencing of PFC PNs at high concentrations of GlyR ligands or positive GlyR modulation.
Summary.
We investigated excitability and inhibitory currents in neurons of layer II/III of the prelimbic region of the mouse PFC and found distinct roles for GABA and glycine. Our results indicate that GABAARs mediate primarily synaptic inhibitory currents and strychnine-sensitive GlyRs mediate primarily tonic currents on LII/II PNs. These neurons exhibit large currents after the application of glycine and taurine, suggesting the existence of functional GlyRs. These findings reveal a novel form of inhibition in the PFC that may regulate neuronal excitability and contribute to the balance of excitation and inhibition that is critical to maintaining proper cortical function.
GRANTS
This work was supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA) Grant R01 AA-19801 to N. L. Harrison. M. C. Salling was supported by Postdoctoral Training Grant F32 AA-022028 from NIAAA.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.C.S. and N.H. conception and design of research; M.C.S. performed experiments; M.C.S. analyzed data; M.C.S. and N.H. interpreted results of experiments; M.C.S. prepared figures; M.C.S. drafted manuscript; M.C.S. and N.H. edited and revised manuscript; M.C.S. and N.H. approved final version of manuscript.
REFERENCES
- Abernathy K, Chandler LJ, Woodward JJ. Alcohol and the prefrontal cortex. Int Rev Neurobiol 91: 289–320, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatrudo JM, Weaver CM, Crimins JL, Hof PR, Rosene DL, Luebke JI. Influence of highly distinctive structural properties on the excitability of pyramidal neurons in monkey visual and prefrontal cortices. J Neurosci 32: 13644–13660, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Mulholland PJ, Beckley JT, Trantham-Davidson H, Woodward JJ. Ethanol reduces neuronal excitability of lateral orbitofrontal cortex neurons via a glycine receptor dependent mechanism. Neuropsychopharmacology 38: 1176–1188, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acidA receptors in hippocampal neurons. Mol Pharmacol 59: 814–824, 2001 [DOI] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci 29: 12757–12763, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman LW, Rosahl TW, Brussaard AB. Neonatal development of the rat visual cortex: synaptic function of GABAA receptor alpha subunits. J Physiol 545: 169–181, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol 497: 753–759, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattipakorn SC, McMahon LL. Pharmacological characterization of glycine-gated chloride currents recorded in rat hippocampal slices. J Neurophysiol 87: 1515–1525, 2002 [DOI] [PubMed] [Google Scholar]
- Chattipakorn SC, McMahon LL. Strychnine-sensitive glycine receptors depress hyperexcitability in rat dentate gyrus. J Neurophysiol 89: 1339–1342, 2003 [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol 73: 19–38, 2006 [DOI] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J Neurosci 25: 11553–11563, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahchour A, Hoffman A, Deitrich R, de Witte P. Effects of ethanol on extracellular amino acid levels in high-and low-alcohol sensitive rats: a microdialysis study. Alcohol 35: 548–553, 2000 [DOI] [PubMed] [Google Scholar]
- Day M, Carr DB, Ulrich S, Ilijic E, Tkatch T, Surmeier DJ. Dendritic excitability of mouse frontal cortex pyramidal neurons is shaped by the interaction among HCN, Kir2, and Kleak channels. J Neurosci 25: 8776–8787, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Lopez-Cruz PL, Benavides-Piccione R, Bielza C, Larranaga P, Anderson S, Burkhalter A, Cauli B, Fairen A, Feldmeyer D, Fishell G, Fitzpatrick D, Freund TF, Gonzalez-Burgos G, Hestrin S, Hill S, Hof PR, Huang J, Jones EG, Kawaguchi Y, Kisvarday Z, Kubota Y, Lewis DA, Marin O, Markram H, McBain CJ, Meyer HS, Monyer H, Nelson SB, Rockland K, Rossier J, Rubenstein JL, Rudy B, Scanziani M, Shepherd GM, Sherwood CC, Staiger JF, Tamas G, Thomson A, Wang Y, Yuste R, Ascoli GA. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci 14: 202–216, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasbek KR, Jensen K. THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cereb Cortex 16: 1134–1141, 2006 [DOI] [PubMed] [Google Scholar]
- Elliott R, Baker SC, Rogers RD, O'Leary DA, Paykel ES, Frith CD, Dolan RJ, Sahakian BJ. Prefrontal dysfunction in depressed patients performing a complex planning task: a study using positron emission tomography. Psychol Med 27: 931–942, 1997 [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci 6: 215–229, 2005 [DOI] [PubMed] [Google Scholar]
- Fino E, Packer AM, Yuste R. The logic of inhibitory connectivity in the neocortex. Neuroscientist 19: 228–237, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint AC, Liu X, Kriegstein AR. Nonsynaptic glycine receptor activation during early neocortical development. Neuron 20: 43–53, 1998 [DOI] [PubMed] [Google Scholar]
- Franklin KB, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1997 [Google Scholar]
- Franklin KB, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 2008 [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol 359: 154–194, 1995 [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12: 652–669, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsing LG, Jr, Matyus P. Mechanisms of glycine release, which build up synaptic and extrasynaptic glycine levels: the role of synaptic and non-synaptic glycine transporters. Brain Res Bull 93: 110–119, 2013 [DOI] [PubMed] [Google Scholar]
- Hedrick T, Waters J. Effect of temperature on spiking patterns of neocortical layer 2/3 and layer 6 pyramidal neurons. Front Neural Circuits 6: 28, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoestgaard-Jensen K, Dalby NO, Wolinsky TD, Murphey C, Jones KA, Rottlander M, Frederiksen K, Watson WP, Jensen K, Ebert B. Pharmacological characterization of a novel positive modulator at alpha4beta3delta-containing extrasynaptic GABAA receptors. Neuropharmacology 58: 702–711, 2010 [DOI] [PubMed] [Google Scholar]
- Horio M, Kohno M, Fujita Y, Ishima T, Inoue R, Mori H, Hashimoto K. Levels of d-serine in the brain and peripheral organs of serine racemase (Srr) knock-out mice. Neurochem Int 59: 853–859, 2011 [DOI] [PubMed] [Google Scholar]
- Jia F, Chandra D, Homanics GE, Harrison NL. Ethanol modulates synaptic and extrasynaptic GABAA receptors in the thalamus. J Pharmacol Exp Ther 326: 475–482, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Goldstein PA, Harrison NL. The modulation of synaptic GABAA receptors in the thalamus by eszopiclone and zolpidem. J Pharmacol Exp Ther 328: 1000–1006, 2009 [DOI] [PubMed] [Google Scholar]
- Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol 94: 4491–4501, 2005 [DOI] [PubMed] [Google Scholar]
- Jonsson S, Kerekes N, Hyytia P, Ericson M, Soderpalm B. Glycine receptor expression in the forebrain of male AA/ANA rats. Brain Res 1305: S27–S36, 2009 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Groupings of nonpyramidal and pyramidal cells with specific physiological and morphological characteristics in rat frontal cortex. J Neurophysiol 69: 416–431, 1993 [DOI] [PubMed] [Google Scholar]
- Kirsch J. Glycinergic transmission. Cell Tissue Res 326: 535–540, 2006 [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol 171: 157–191, 1977 [DOI] [PubMed] [Google Scholar]
- Kunz PA, Burette AC, Weinberg RJ, Philpot BD. Glycine receptors support excitatory neurotransmitter release in developing mouse visual cortex. J Physiol 590: 5749–5764, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Ye JH. Glycine-activated chloride currents of neurons freshly isolated from the prefrontal cortex of young rats. Brain Res 1393: 17–22, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malosio ML, Marqueze-Pouey B, Kuhse J, Betz H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J 10: 2401–2409, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Gahwiler BH, Gerber U. Beta-alanine and taurine as endogenous agonists at glycine receptors in rat hippocampus in vitro. J Physiol 539: 191–200, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Furuse M. The impact of taurine- and beta-alanine-supplemented diets on behavioral and neurochemical parameters in mice: antidepressant versus anxiolytic-like effects. Amino Acids 39: 427–434, 2010 [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol 87: 2624–2628, 2002 [DOI] [PubMed] [Google Scholar]
- Olah S, Fule M, Komlosi G, Varga C, Baldi R, Barzo P, Tamas G. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature 461: 1278–1281, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 10: 206–219, 2000 [DOI] [PubMed] [Google Scholar]
- Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR. GABAA receptor changes in delta subunit-deficient mice: altered expression of alpha4 and gamma2 subunits in the forebrain. J Comp Neurol 446: 179–197, 2002 [DOI] [PubMed] [Google Scholar]
- Petersen CC, Crochet S. Synaptic computation and sensory processing in neocortical layer 2/3. Neuron 78: 28–48, 2013 [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101: 815–850, 2000 [DOI] [PubMed] [Google Scholar]
- Pribilla I, Takagi T, Langosch D, Bormann J, Betz H. The atypical M2 segment of the beta subunit confers picrotoxinin resistance to inhibitory glycine receptor channels. EMBO J 11: 4305–4311, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABAA blockade of prefrontal cortical neurons engaged by working memory. J Neurosci 20: 485–494, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol 71: 45–61, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin P, Tseng GF, Hoffman S, Parada I, Prince DA. Axonal sprouting in layer V pyramidal neurons of chronically injured cerebral cortex. J Neurosci 15: 8234–8245, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: role of the delta subunit. J Neurosci 14: 7077–7086, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Lapish CC, Durstewitz D. Comparing the prefrontal cortex of rats and primates: insights from electrophysiology. Neurotox Res 14: 249–262, 2008 [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci 6: 484–490, 2003 [DOI] [PubMed] [Google Scholar]
- Thuault SJ, Malleret G, Constantinople CM, Nicholls R, Chen I, Zhu J, Panteleyev A, Vronskaya S, Nolan MF, Bruno R, Siegelbaum SA, Kandel ER. Prefrontal cortex HCN1 channels enable intrinsic persistent neural firing and executive memory function. J Neurosci 33: 13583–13599, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res 146: 3–17, 2003 [DOI] [PubMed] [Google Scholar]
- Vandenberg RJ, Aubrey KR. Glycine transport inhibitors as potential antipsychotic drugs. Expert Opin Ther Targets 5: 507–518, 2001 [DOI] [PubMed] [Google Scholar]
- Vardya I, Drasbek KR, Dosa Z, Jensen K. Cell type-specific GABAA receptor-mediated tonic inhibition in mouse neocortex. J Neurophysiol 100: 526–532, 2008 [DOI] [PubMed] [Google Scholar]
- Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE. GABAA receptor alpha1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci 21: 3009–3016, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry 43: 114–124, 1986 [DOI] [PubMed] [Google Scholar]
- Weitlauf C, Woodward JJ. Ethanol selectively attenuates NMDAR-mediated synaptic transmission in the prefrontal cortex. Alcohol Clin Exp Res 32: 690–698, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J, Furukawa T, Ueno S, Yamamoto S, Fukuda A. Molecular basis for the GABAA receptor-mediated tonic inhibition in rat somatosensory cortex. Cereb Cortex 17: 1782–1787, 2007 [DOI] [PubMed] [Google Scholar]
- Yoon JH, Minzenberg MJ, Ursu S, Ryan Walter BS, Wendelken C, Ragland JD, Carter CS. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. Am J Psychiatry 165: 1006–1014, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LH, Gong N, Fei D, Xu L, Xu TL. Glycine uptake regulates hippocampal network activity via glycine receptor-mediated tonic inhibition. Neuropsychopharmacology 33: 701–711, 2008a [DOI] [PubMed] [Google Scholar]
- Zhang XB, Sun GC, Liu LY, Yu F, Xu TL. Alpha2 subunit specificity of cyclothiazide inhibition on glycine receptors. Mol Pharmacol 73: 1195–1202, 2008b [DOI] [PubMed] [Google Scholar]