Abstract
Recent studies have demonstrated that vision influences the functional remodeling of the mouse retinogeniculate synapse, the connection between retinal ganglion cells and thalamic relay neurons in the dorsal lateral geniculate nucleus (LGN). Initially, each relay neuron receives a large number of weak retinal inputs. Over a 2- to 3-wk developmental window, the majority of these inputs are eliminated, and the remaining inputs are strengthened. This period of refinement is followed by a critical period when visual experience changes the strength and connectivity of the retinogeniculate synapse. Visual deprivation of mice by dark rearing from postnatal day (P)20 results in a dramatic weakening of synaptic strength and recruitment of additional inputs. In the present study we asked whether experience-dependent plasticity at the retinogeniculate synapse represents a homeostatic response to changing visual environment. We found that visual experience starting at P20 following visual deprivation from birth results in weakening of existing retinal inputs onto relay neurons without significant changes in input number, consistent with homeostatic synaptic scaling of retinal inputs. On the other hand, the recruitment of new inputs to the retinogeniculate synapse requires previous visual experience prior to the critical period. Taken together, these findings suggest that diverse forms of homeostatic plasticity drive experience-dependent remodeling at the retinogeniculate synapse.
Keywords: vision, thalamus, synaptic plasticity, synapse development, critical period
experience shapes neuronal circuits during well-defined critical periods in development. In the visual system, cortical circuits are initially established by a combination of molecular cues and spontaneous activity and later refined by visual experience (Espinosa and Stryker 2012; Katz and Shatz 1996). Features of the mature cortical circuit such as ocular dominance (Fagiolini et al. 1994; Gordon and Stryker 1996; Hubel and Wiesel 1970; Wiesel and Hubel 1963), orientation selectivity (Crair et al. 1998; White et al. 2001), and direction selectivity (Daw and Wyatt 1976; Koenig et al. 2006) are shaped by vision during precise developmental time periods. In contrast to the visual cortex, subcortical structures such as the retina, superior colliculus, and thalamus were thought to complete development earlier and be less sensitive to experience. However, recent evidence has challenged this view. For example, in the retina, visual experience affects the segregation of ON-OFF retinal ganglion cells (RGCs) (Landi et al. 2007; Tian and Copenhagen 2003) and has a differential effect on the maturation of rod and cone photoreceptor pathways (Dunn et al. 2013). In the superior colliculus, visual deprivation results in a progressive loss of refinement of receptive fields (Carrasco et al. 2005).
The synapse between RGCs and thalamic relay neurons, the retinogeniculate synapse, transmits visual information from the retina to thalamic relay neurons in the dorsal lateral geniculate nucleus (LGN), which in turn project to the visual cortex. Initially during development, RGC axon inputs from the two eyes overlap in their target regions in the LGN. Gradually, inputs from the ipsilateral and contralateral eyes become segregated into distinct segments. This eye-specific segregation occurs before eye opening and is driven by molecular signals as well as activity-dependent binocular competition in the form of spontaneous retinal waves (Campbell and Shatz 1992; Huberman et al. 2008; Penn et al. 1998; Sretavan and Shatz 1986; Sretavan et al. 1988). After eye-specific segregation completes, changes in retinogeniculate synaptic function continue to occur over a developmental time period spanning eye opening (Chen and Regehr 2000; Jaubert-Miazza et al. 2005; Ziburkus and Guido 2006). Excess retinal afferents to relay neurons prune and existing afferents strengthen. Spontaneous retinal activity, not vision, drives these changes (Hooks and Chen 2006).
Recent studies have uncovered an unexpected role for visual experience in retinogeniculate synapse development. Visual deprivation by dark rearing mice starting at postnatal day (P)20 for at least 1 wk, a paradigm called late dark rearing (late DR), not only weakens existing inputs innervating thalamic relay neurons but also recruits additional retinal inputs, effectively reversing the state of functional connectivity of the retinogeniculate synapse to before eye opening (Hooks and Chen 2006). In contrast, chronic visual deprivation (chronic dark rearing, or chronic DR) of mice from birth results in only modest changes in synapse maturation with notably no significant changes in afferent number or strength. Further work has characterized more precisely the temporal parameters during which visual deprivation triggers changes in connectivity at the retinogeniculate synapse. Visual deprivation earlier in development (from P16), later in development (from P25), or for shorter periods of time (less than 4 days) does not trigger the same robust plasticity as seen in the late DR paradigm (Hooks and Chen 2008). Thus visual experience influences the functional maturation of the retinogeniculate synapse during a precise critical period.
What are the mechanisms that underlie the critical period for experience-dependent change at the retinogeniculate synapse? In the present study we ask whether synapse remodeling in response to late DR represents a homeostatic response to visual experience. Does simply changing the visual environment of mice at P20 induce plasticity at the retinogeniculate synapse? To address this question, we examined the synaptic response to the inverse visual experience of late DR. We found that visual deprivation from birth followed by normal visual experience starting at P20 elicited plasticity in input strength, but not in input number. To recruit additional afferent RGC inputs onto relay neurons, a window of visual experience prior to P20 was necessary. These results suggest that homeostatic mechanisms regulate the total retinal drive onto LGN relay neurons during experience-dependent synaptic remodeling and that vision prior to the critical period influences the expression of this plasticity.
MATERIALS AND METHODS
Animals.
All experimental procedures were performed in compliance with animal protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Children's Hospital Boston. C57BL/6 mice P9–34 were used for all experiments. Control (light-reared, LR) animals were raised in micro-isolator cages in a standard animal facility under 12:12-h light-dark cycles. Dark-reared animals were placed in a light-tight container in which temperature, humidity, and luminance were continually monitored for the time periods indicated. In all cases, dark-reared animals were either transferred back into light-rearing conditions (for experiments involving light rearing after dark rearing) or transferred to the laboratory for slice preparation in an opaque box to minimize light exposure prior to death. All recordings were performed blind to experimental condition.
LGN slice preparation.
In compliance with the IACUC at Children's Hospital Boston, experimental mice were anesthetized with isoflurane and decapitated. The brain was removed and placed in an ice-cold choline cutting solution (in mM: 130 choline chloride, 26 NaHCO3, 25 glucose, 1.25 NaH2PO4, 2.5 KCl, 7 MgCl2, and 0.5 CaCl2). LGN slices (250 μm) preserving the optic tract containing RGC axons were cut using a sapphire blade (Delaware Diamond Knives, Wilmington, DE) on a vibratome (VT1000S; Leica, Deerfield IL) as previously described (Chen and Regehr 2000). Following sectioning, slices were bathed in a chamber filled with the choline cutting solution at 31°C for 20 min, followed by an additional 20 min in an isotonic saline solution (in mM: 125 NaCl, 2.6 NaHCO3 1.25 NaH2PO4, 2.5 KCl, 1 MgCl2, 2 CaCl2, and 25 glucose). Oxygen (95% O2-5% CO2) was supplied continuously to the chamber.
Electrophysiology.
Whole cell voltage-clamp recordings of thalamic relay neurons from the contralateral monocular region of the dorsal LGN were performed as previously described (Chen and Regehr 2000; Hooks and Chen 2008). Glass electrodes of 1–2 MΩ were filled with a CsF-based internal solution (in mM: 35 CsF, 100 CsCl, 10 EGTA, and 10 HEPES at pH 7.3 and 290 mosM). D600 (0.1 mM methoxyverapamil hydrochloride; Tocris, Ellisville, MO) was added to block voltage-gated calcium channels. Recordings were conducted in isotonic saline solution with 20 μm bicuculline (Tocris, Ellisville, MO), a GABAA receptor antagonist.
Saline-filled glass pipettes were used as stimulating electrodes. They were placed in the optic tract at least 500 μm from the patched cell and to different positions in the optic tract until the location with the largest postsynaptic response was reached. Stimulus intensities ranged from 10 μA to 1 mA. Holding potential alternated between −70 mV (for currents composed of mainly AMPAR-mediated currents) and +40 mV (for both AMPAR- and NMDAR- mediated currents). Because of magnesium block of the NMDAR at negative holding potentials, the peak of the excitatory postsynaptic current (EPSC) evoked at −70 mV was used as a measurement of the AMPAR current. At a holding potential of +40 mV, both AMPAR- and NMDAR-mediated currents contribute to the EPSC; however, the peak current measured after the first 10 ms of the EPSC provided a good estimate of the NMDAR current because AMPAR currents decay with a time constant of 2–3 ms (Chen and Regehr 2000). Cells were not used if access resistance changed more than 20% from baseline during the recording or if leak current was greater than 500 pA. All experiments were performed at room temperature.
Evaluation of synaptic connectivity.
Data acquisition and initial analysis were performed using IgorPro (WaveMetrics, Portland, OR). To assay strengthening and pruning, determination of single-fiber and maximal currents was performed as previously described (Hooks and Chen 2008). We quantified the synaptic connectivity of retinogeniculate afferents to LGN relay cells by using the fiber fraction ratio, as previously published (Hooks and Chen 2006, 2008). The fiber fraction ratio, calculated for each neuron, represents the proportion of total synaptic current contributed by the single fiber and is inversely related to estimated connectivity. A lower fiber fraction ratio represents a smaller contribution of the single-fiber current to the total synaptic response and thus more estimated connected retinal afferents. On the other hand, a higher fiber fraction ratio represents less estimated connected retinal afferents (Hooks and Chen 2006). Moreover, the fiber fraction ratio allowed us to statistically test differences in connectivity between populations of cells from different treatment conditions.
Statistical analysis.
Data analysis and statistics were performed using MATLAB (The MathWorks), Prism (GraphPad Software), and Excel (Microsoft, Redmond, WA). Normality of current amplitude distributions was tested by comparison to a theoretical normal distribution using a Kolmogorov-Smirnov test. The majority of our data did not follow a normal distribution. Unless otherwise noted, the nonparametric two-sided Mann-Whitney U-test was used to compare differences between treatment conditions. Statistical significances in graphs are indicated as *P < 0.05, **P < 0.05, and ***P < 0.001.
RESULTS
Visual experience following early visual deprivation weakens retinal inputs.
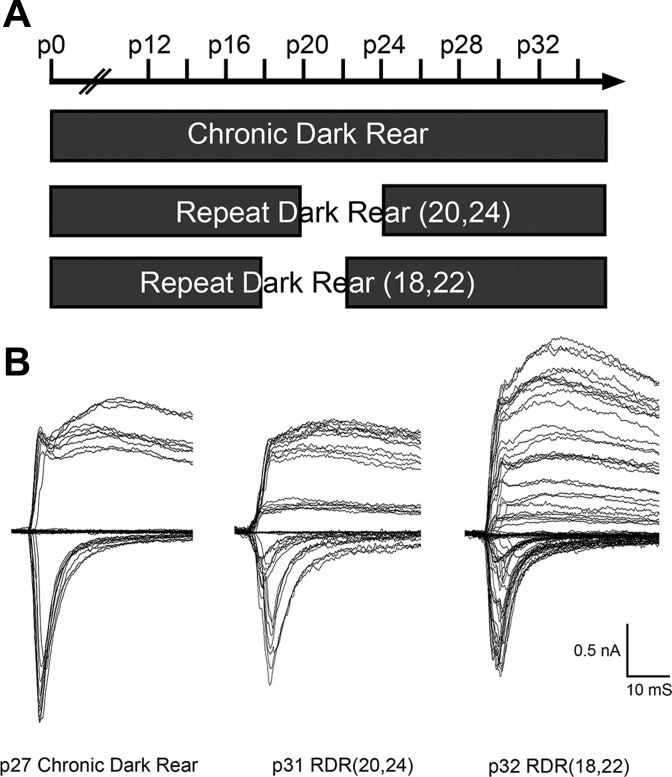
If plasticity observed in the late DR condition were completely attributable to the change in visual environment regardless of the direction of the change (i.e., light to dark vs. dark to light), we hypothesized that exposing a chronically dark-reared mouse to light at P20 would elicit a similar remodeling of synaptic inputs. Thus we dark-reared mice from near birth, P0 or P1, until P20 and then abruptly changed the visual environment by moving these mice to a normal environment of 12:12-h light-dark cycles. We termed this experiment early dark rear, or early DR, because it is the exact inverse of the late DR experiment (Fig. 1A).
Fig. 1.
A: experimental paradigm of early dark rear (early DR) experiment. Filled blocks show time periods in development when mice were dark-reared. In early DR, mice were dark-reared from birth until postnatal day (P)20, at which time they were exposed to normal 12:12-h light-dark cycles for the rest of development. The early DR experiment is the inverse of the late DR experiment, previously published and shown in schematic form (Hooks and Chen 2006). Chronic DR mice were reared in the dark for all of development. Light rear (LR) control mice were exposed to normal 12:12-h light-dark cycles for all of development. All recordings were conducted at mature ages (P27–34). B: representative whole cell patch-clamp recordings from different experimental groups. Left, superimposed traces of evoked excitatory postsynaptic currents (EPSCs) recorded while increasing stimulus intensity from a dorsal lateral geniculate nucleus (LGN) relay neuron of a P28 LR control mouse. Middle, representative LGN relay neuron from a P27 chronic DR mouse. Right, representative LGN relay neuron from a P32 early DR mouse. Stimulus intensity ranged from 10 μA to 1 mA in all experiments. Note the reduction in currents elicited by all levels of stimulation in early DR mice compared with other experimental groups.
We assessed experience-dependent synapse remodeling by quantifying two aspects of the retinogeniculate synapse, single-fiber strength and fiber fraction ratio, as previously described (Hooks and Chen 2008). Briefly, while patched onto a relay neuron in the LGN and alternating the holding potential between −70 and +40 mV, we stimulated the optic tract. We first found the single-fiber (SF) response. Stimulation of the optic tract at intensities less than the minimal stimulation response resulted in failure to evoke a synaptic current. With incremental increases in stimulus intensity, an intensity level is eventually reached that reliably evokes small inward synaptic currents at a holding potential of −70 mV through mainly AMPARs and corresponding outward synaptic currents at a holding potential of +40 mV through both AMPARs and NMDARs. We refer to the minimal stimulation response as the single-fiber synaptic input. As the stimulation intensity is gradually increased from the minimal stimulation response, the AMPAR and NMDAR currents increase, consistent with the recruitment of more retinal inputs. The synaptic response to high-intensity stimulation, which theoretically recruits all the afferents preserved in the brain slice, is recorded as the maximal synaptic current for both AMPAR and NMDAR. Figure 1B shows example traces from our experiments. To estimate RGC afferent connectivity to each relay neuron, we compared the relative contribution of the single-fiber current to the maximal evoked synaptic current using the fiber fraction ratio (see materials and methods). We assessed these synaptic properties in early DR mice starting 1 wk after they were removed from the dark, between ages P27 and P34, the same developmental period during which late DR mice were assessed previously (Hooks and Chen 2006).
We found that early DR mice exhibited a significant reduction in single-fiber AMPAR and NMDAR currents compared with similar aged chronic DR mice (SF AMPAR, P < 0.05; SF NMDAR, P < 0.01). Figure 2A, top, shows the distribution of single-fiber AMPAR currents for LR control, chronic DR, and early DR mice. Comparison of the cumulative plots of the three conditions (Fig. 2A, bottom) demonstrates a shift of the early DR distribution to the left of the control and chronic DR distributions. Early DR mice exhibited significantly fewer single fibers that were stronger than 400 pA compared with control mice and chronic DR mice (9/66 in early DR vs. 23/82 in control and 24/78 in chronic DR, Fisher's exact test, P < 0.05). The value 400 pA was chosen for this comparison because it corresponds to the smallest EPSC that can drive action potential firing at the mature retinogeniculate synapse (Liu and Chen 2008). We also found that early DR mice exhibited a significant reduction in maximal AMPAR and NMDAR currents compared with both control and chronic DR mice (early DR vs. LR control and early DR vs. chronic DR: maximal AMPAR, P < 0.05, maximal NMDAR, P < 0.05; Fig. 2B). Notably, the ratio of AMPAR to NMDAR current for early DR mice (A/N ratio; 1.22 ± 0.10) was not significantly different from that for controls (A/N ratio: LR control 1.15 ± 0.09, chronic DR 1.08 ± 0.07, P > 0.05). Taken together, these data suggest that visual experience starting at P20 in visually deprived mice resulted in a significant weakening of retinal input strength.
Fig. 2.
Early visual deprivation decreases input strength at the retinogeniculate synapse. A, top: analysis of single-fiber AMPAR currents for LR control, chronic DR, and early DR mice. Single-fiber AMPAR currents were measured at a holding potential of −70 mV. Histograms are divided into bins of 50 pA. Bottom, cumulative probability plots for the 3 conditions. B: synaptic currents from mice subjected to different visual manipulations measured at −70 mV (AMPAR) and +40 mV (NMDAR, slow component). The peak current amplitude in response to activation of a single retinal afferent was measured as single-fiber current, and maximal current was measured in response to maximal excitation of the optic tract. Average single-fiber (left) and maximal current amplitude (right; note changes in scale) for AMPAR (top) and NMDAR current (bottom) was assessed in LR control, chronic DR, and early DR. Data are means ± SE. All recordings occurred at mature ages (P27–34). LR control, 26 cells from 14 animals; chronic DR, 28 cells from 15 animals; early DR, 25 cells from 12 animals. *P < 0.05; **P < 0.01.
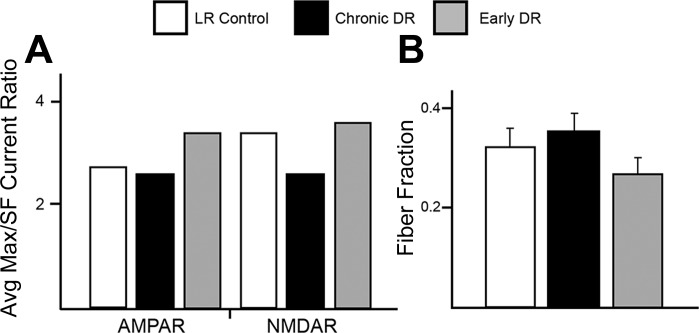
Despite the decrease in input strength observed in early DR mice, the ratios of the average maximal strength to the average single-fiber strength were not notably different between early DR, chronic DR, and LR control mice, suggesting that there was little change in the number of retinal inputs that innervate relay neurons with early DR (Fig. 3A). To make these results statistically testable, we calculated the fiber fraction ratio for populations of neurons in each experimental condition. The population mean of fiber fraction ratio for the early DR condition was not significantly different from that for control or late DR conditions (P > 0.05; Fig. 3B). We conclude that early visual deprivation followed by normal visual experience starting at P20 triggers plasticity in strength of inputs but does not recruit additional inputs to the retinogeniculate synapse.
Fig. 3.
Early visual deprivation does not significantly alter retinal input number. Estimates of number of retinal ganglion cell (RGC) inputs were calculated by 2 different methods. A: fibers remaining for experimental groups, computed as ratio of average maximal current to average single-fiber current (Avg Max/SF), calculated independently for AMPAR and NMDAR currents. B: fiber fraction for chronic and early DR manipulations. No significant changes in connectivity were observed in early DR mice compared with LR control and chronic DR mice. Data are means ± SE; n = 82, 78, and 66 for LR control, chronic DR, and early DR mice, respectively.
Visual experience starting at P20 is not sufficient to trigger input recruitment in response to subsequent visual deprivation.
The results of the early DR experiment, when interpreted in the context of our previous studies of late dark rearing, suggest that weakening of synaptic strength can be elicited by a change of the visual environment at P20 regardless of sign, that is, from light to dark or from dark to light. However, an increase in the number of retinal inputs that innervate a given relay neuron only occurred in response to late DR. These findings are consistent with our proposed hypothesis that previous visual experience (i.e., a period of normal 12:12-h light-dark cycles before P20) is necessary to trigger changes in retinal afferent connectivity in response to later changes in visual environment (Hooks and Chen 2008).
To explicitly test this hypothesis, we designed an experiment that would expose chronically dark-reared mice at P20 to a brief window of normal 12:12-h light-dark cycles before these animals were visually deprived for a second time. We visually deprived mice starting from near birth (P0 or P1) until P20, as in the early DR condition, brought them into normal 12:12-h light-dark cycles for 4 days until P24, and then visually deprived them again for the rest of development. Four days was chosen empirically as the length of exposure to visual experience because our previous experiments had shown that changes in synaptic strength and connectivity could occur with 4 days of visual experience (exposure to light after late DR; Hooks and Chen 2008). We labeled this experimental group repeat dark rear (20,24), or RDR(20,24) (Fig. 4A). Figure 4B shows example traces from these experiments.
Fig. 4.
A: experimental paradigm and example traces of repeat dark rear (RDR) experiments. Filled blocks show time periods in development when mice were dark-reared. All recordings occurred at mature ages (P27–34) at least 7 days after the last manipulation of visual experience. B: representative electrophysiological recordings for different experimental groups in A. Left, superimposed traces of evoked EPSCs recorded while increasing stimulus intensity from an LGN relay neuron of a P27 chronic DR mouse. Middle, representative relay neuron from a P31 RDR(20,24) mouse. Right, representative relay neuron from a P32 RDR(18,22) mouse. Stimulus intensity ranged from ∼10 μA to 1 mA in all experiments. Note the dramatic increase in distinct steps of elicited current for RDR(18,22) mice with a relative preservation of maximal current elicited compared with the other experimental groups.
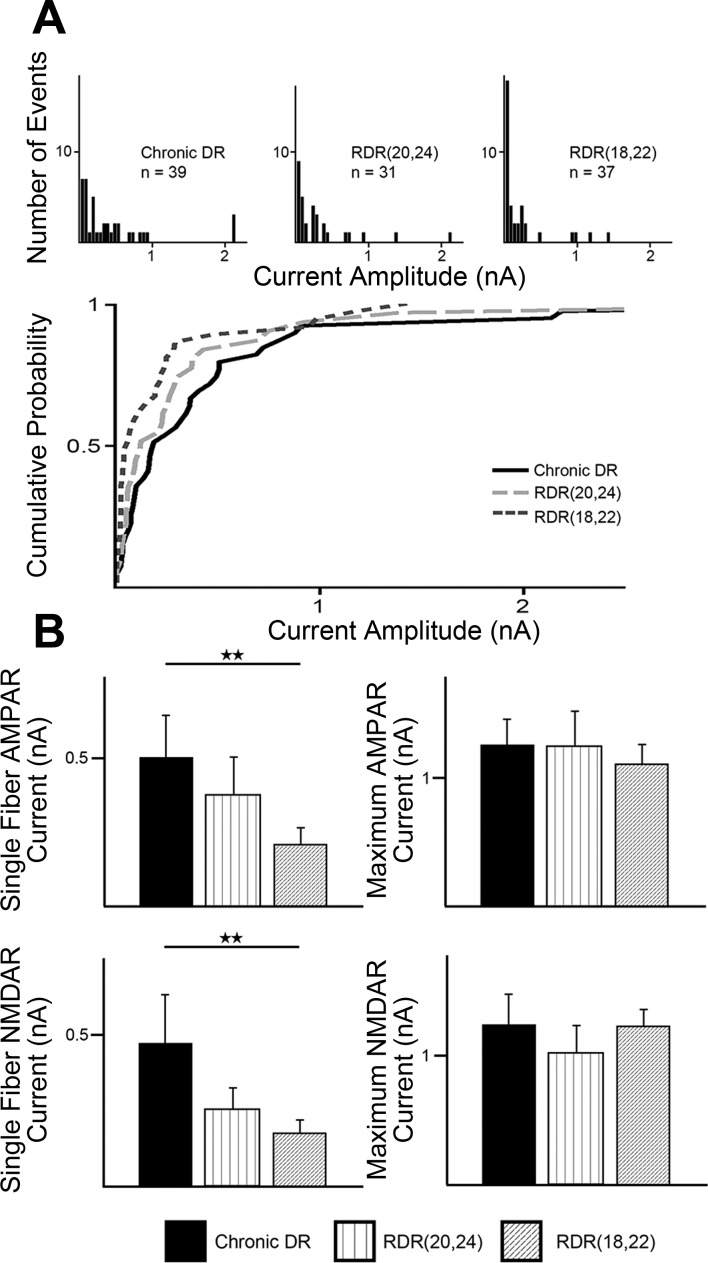
We assessed synaptic properties in RDR(20,24) mice starting 1 wk after their later period of visual deprivation, thus between P31 and P34. No significant differences were observed in single-fiber or maximal currents between RDR(20,24) (SF AMPA 371.94 ± 132.84 pA, maximum AMPA 1,237.20 ± 298.55 pA) and chronic DR mice (SF AMPA 500.90 ± 147.24 pA, maximum AMPA 1,260.20 ± 207.60 pA; P > 0.05; Fig. 5) or LR control mice (SF AMPA 451.32 ± 102.96 pA, maximum AMPA 1,212.10 ± 167.72 pA; P > 0.05). There were also no significant differences in single-fiber or maximal strength when RDR(20,24) mice were compared with early DR mice (SF AMPA 237.93 ± 76.19 pA, maximum AMPA 818.06 ± 167.60 pA; P > 0.05). These results show that RDR(20,24) mice did not exhibit significant changes in retinal input strength compared with chronic DR or early DR mice.
Fig. 5.
The timing of visual experience is critical for rewiring the retinogeniculate synapse. A, top: analysis of single-fiber AMPAR currents for chronic DR, RDR(20,24), and RDR(18,22) mice. Single-fiber AMPAR currents were measured at a holding potential of −70 mV. Histograms are divided into bins of 50 pA. Bottom, cumulative probability plots for the same experimental groups. B: synaptic currents after manipulations of visual experience measured at −70 mV (AMPAR) and +40 mV (NMDAR, slow component). Average single-fiber (left) and maximal current amplitude (right; note changes in scale) for AMPAR (top) and NMDAR current (bottom) assessed in chronic DR, RDR(20,24), and RDR(18,22) mice. All recordings occurred at mature ages (P27–34) at least 7 days after final manipulation of visual experience. Data are means ± SE. Chronic DR, 28 cells from 15 animals (data also shown in Fig. 2); RDR(20,24), 20 cells from 11 animals; RDR(18,22), 24 cells from 11 animals. *P < 0.05; **P < 0.01.
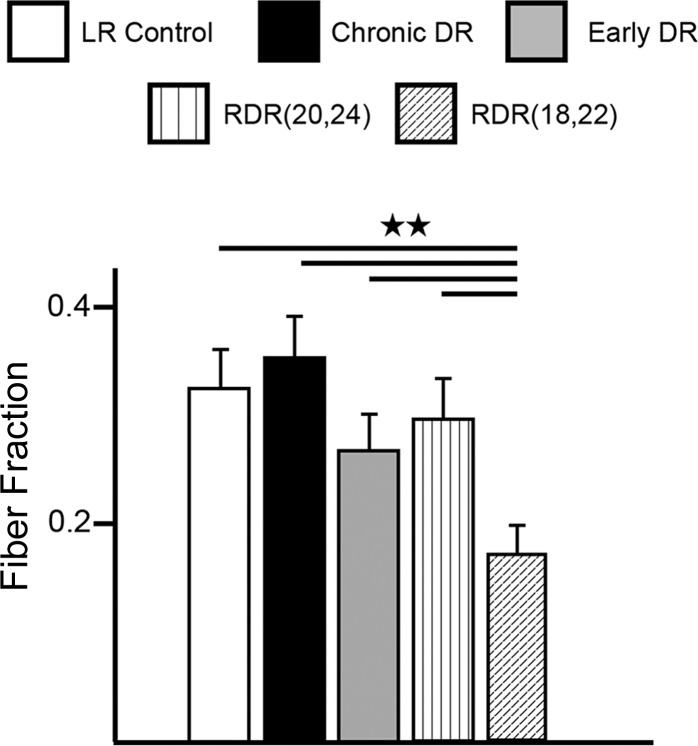
Estimating the number of connected retinal afferents in RDR(20,24) mice using the fiber fraction ratio revealed no significant differences between the fiber fraction of RDR(20,24), early DR, chronic DR, and LR control mice (Fig. 6). Thus a 4-day window of visual experience starting after P20 is not sufficient to trigger the same plasticity as observed in the late DR condition.
Fig. 6.
Visual experience in the precritical period is necessary for triggering recruitment of new inputs to the retinogeniculate synapse. Fiber fraction ratio is shown for all experimental groups. Each single-fiber current divided by the maximal current for the same relay neuron estimates the fraction of the cell's total current contributed by the single-fiber input. The population average of all fiber fraction ratios for a given experimental condition gives an estimate of retinogeniculate connectivity: a decrease in fiber fraction ratio signifies a relative increase in the number of connected afferents. Note that the RDR(18,22) group exhibits a significantly decreased fiber fraction ratio compared with each and all of the other experimental groups tested. Data are means ± SE; n = 82, 78, 66, 62, and 74 for LR control, chronic DR, early DR, RDR(20,24) and RDR(18,22) mice, respectively. Data for LR control, chronic DR, and early DR are also represented in Fig. 3. **P < 0.01 for all comparisons.
Visual experience starting at P18 triggers experience-dependent recruitment of afferent inputs.
The results from our RDR(20,24) experiment reveal that providing a brief window of visual experience after P20 is not sufficient to trigger the recruitment of additional inputs as seen in our late DR condition. A major difference between the late DR condition and the RDR(20,24) condition is that the visual experience in late DR mice occurs between eye opening (P12–14) and P20, whereas visual experience in RDR(20,24) occurs between P20 and P24. Thus we next tested whether shifting the period of visual experience earlier by a few days was sufficient to trigger the recruitment of additional inputs.
Our previous studies had shown that dark rearing mice from P15 onward did not induce significant recruitment of additional fibers (Hooks and Chen 2008). This experimental group received visual experience for ∼3 days, between eye opening at P12 and dark rear at P15. Because of the lack of plasticity observed in these mice, we focused on the later period between P15 and P20. We created an additional experimental group in which mice were visually deprived from near birth (P0 or P1) until P18, brought into normal 12:12-h light-dark cycles for 4 days until P22, and then dark-reared again for the rest of development. We labeled this group repeat dark rear (18,22), or RDR(18,22). The 4-day window of visual experience for this experimental group occurred between P18 and P22, representing a shift of 2 days toward birth compared with the RDR(20,24) group. Furthermore, RDR(18,22) mice received 2 days of vision before P20 and 2 days of vision after P20. The rationale behind the RDR(18,22) experiment was to hold the length of the window of visual experience constant compared with the RDR(20,24) experiment while systematically shifting into the period just before the critical period (Fig. 4).
We assessed RDR(18,22) mice starting at P29, 1 wk after their later period of visual deprivation. RDR(18,22) mice showed decreased single-fiber AMPAR- and NMDAR-mediated currents compared with chronic DR mice (P < 0.01; Fig. 5, A and B). The decrease in single-fiber strength is also shown clearly by a left shift in the cumulative probability plot of single-fiber AMPAR currents in RDR(18,22) mice compared with chronic DR and RDR(20,24) mice (Fig. 5A, bottom). The decrease in single-fiber strength in RDR(18,22) mice was reminiscent of both the early DR and late DR mice. However, unlike early DR mice, RDR(18,22) mice did not show a decrease in maximal currents compared with chronic DR and control mice (P > 0.05; Fig. 5B). Notably, the ratio of AMPAR to NMDAR current for RDR(18,22) mice (A/N ratio 0.87 ± 0.07) was significantly lower than that for LR control and early DR mice (P < 0.05), but not chronic DR mice (P > 0.05).
RDR(18,22) mice showed a significant decrease in the fiber fraction ratio compared with all experimental groups including LR control, chronic DR, early DR, and RDR(20,24) mice [P < 0.01, RDR(18,22) vs. all other groups pairwise; Fig. 6]. In the RDR(18,22) condition, each fiber represented only 17.4 ± 2.6% of the total synaptic current to a given LGN relay neuron, whereas in all other groups each fiber accounted for ∼30% of the total synaptic current (Fig. 6). This represents an approximate doubling in the number of retinal afferents connected to each relay neuron. The increase in connectivity in RDR(18,22) is reminiscent of the late DR condition, suggesting a process of plasticity whereby new afferents are actively recruited. Therefore, shifting the 4-day window of visual experience earlier by 2 days (from P20 to P18) is sufficient to trigger plasticity of both strength and number of inputs at the retinogeniculate synapse. Figure 7 summarizes the changes in connectivity and strength in all experimental groups tested and discussed in this study.
Fig. 7.
Role of visual experience in wiring and rewiring the retinogeniculate synapse. Summary schematic shows different experimental paradigms and how they each affect retinal afferent maturation and pruning. In early stages of development (P8–16), regardless of visual experience, LGN relay neurons undergo pruning of initially weak and redundant inputs. At older ages (P20+), late visual deprivation, but not chronic dark rearing, results in the weakening of existing inputs and the recruitment of additional afferents. Early visual deprivation (early DR experiment) results in the weakening of retinal afferents but no recruitment of additional inputs. Repeat dark rear experiments [RDR(20,24) and RDR(18,22)] reveal that visual experience before P20 is critical for triggering the later recruitment of additional inputs in response to visual deprivation. Thus the timing of early visual experience is critical for the expression of plasticity at the retinogeniculate synapse.
DISCUSSION
Homeostatic plasticity at the retinogeniculate synapse.
Two major forms of activity-dependent plasticity have been associated with synaptic development in the visual system. Hebbian plasticity is thought to underlie the strengthening of some synapses at the expense of others during periods of refinement (Katz and Shatz 1996; Shatz 1990). Consistent with this idea, recent studies have shown that synaptic connections between neurons with correlated firing patterns become stronger, whereas those with uncorrelated activity become weaker and are eventually eliminated (Butts et al. 2007; Ziburkus et al. 2009). At the retinogeniculate synapse, increased correlated firing between RGCs and relay neurons, for example, during retinal waves, are thought to strengthen connections between active neurons.
Homeostatic plasticity has also been shown to play an important role in shaping connectivity in the developing visual system (Turrigiano and Nelson 2004; Whitt et al. 2014). This form of plasticity maintains global activity of a neuron, input, or neural circuit near a set firing range to ensure a wide and dynamic range of neural responses (Turrigiano et al. 1998). In the visual cortex, monocular deprivation by intraocular injections of TTX or by lid-suture around the time of eye opening as well as dark rearing increases the miniature EPSC (mEPSC) amplitude of layer 2/3 pyramidal neurons (Desai et al. 2002; Goel et al. 2006). In the superior colliculus, disruption of correlated retinal waves during development activates homeostatic mechanisms to conserve the total retinocollicular input (Chandrasekaran et al. 2005, 2007). In the visual thalamus, homeostatic plasticity in corticothalamic inputs to visual thalamus occurs in response to monocular deprivation (Krahe and Guido 2011).
At the retinogeniculate synapse, our previous studies dark rearing mice from P20 (late DR) resulted in weakened RGC input strength and recruitment of additional afferent inputs. To ask whether this synaptic response to a change in visual experience at P20 represented a Hebbian or homeostatic response, we performed the early DR experiment, in which mice were dark-reared from birth until P20 and then subsequently reared in normal visual conditions for 1 wk. One might predict that if retinogeniculate synapse plasticity were Hebbian, a sudden increase in visually driven information at P20 would strengthen synaptic connections, resulting in an increased total current. On the other hand, a homeostatic response to incoming visual experience would result in a decrease in total current.
We found that visual deprivation of mice until P20, followed by 1 wk of normal visual experience, resulted in a global reduction in retinal input strength onto thalamic relay neurons with no changes in input number compared with control animals. The average strength of individual RGC inputs decreased to nearly one-half of control values, and there was a similar decrease in maximal currents elicited. These results are consistent with a homeostatic “scaling down” of retinal input strength onto LGN relay neurons in response to a change in visual environment at P20.
Although synaptic strength decreases in our early DR experiment, no change was seen in A/N ratio compared with control animals. AMPAR- and NMDAR-mediated currents are scaled proportionally in synaptic scaling (Watt et al. 2000). Maintaining a constant ratio of AMPAR-to-NMDAR current in homeostatic plasticity may serve the function of normalizing activity without fundamentally altering the transmission of information across the synapse. Taken together, our data show that homeostatic plasticity occurs during the time period when the retinogeniculate synapse is sensitive to visual experience.
Distinct forms of homeostatic plasticity.
One interesting finding in comparing the results of our early DR experiment with those of the late DR experiment (Hooks and Chen 2006) was that changes from light to dark vs. dark to light at P20 did not produce symmetrically opposite effects. Dark rearing mice at P20 caused a significant weakening of retinal input strength and recruitment of additional inputs. That the net synaptic response (maximal currents) in late DR increased is consistent with a homeostatic compensation for visual deprivation whereby the input “scales up” the number of afferent inputs and total synaptic charge transfer from retina. On the other hand, early DR elicited a homeostatic response to increased visual experience at P20 by “scaling down” the strength of retinal inputs.
Thus, at the retinogeniculate synapse, different forms of homeostatic plasticity regulate the distinct responses to early DR and late DR. This observation is consistent with the idea that there are diverse means of achieving homeostatic plasticity. For example, in cultured cortical pyramidal neurons, homeostatic plasticity scales the entire distribution of mEPSC amplitudes at individual neurons up or down proportionally in response to changes in chronic activity (Turrigiano et al. 1998; Turrigiano and Nelson 2004), whereas in cultures of hippocampal synapses, homeostatic plasticity alters the number of neurotransmitter release sites (Murthy et al. 2001). Manipulations of sensory experience in vivo have also been shown to result in diverse expressions of homeostatic plasticity that are age dependent. Changes in afferent inputs, probability of release, and postsynaptic membrane excitability have all been described as different forms of homeostatic plasticity (Desai et al. 1999; Goel et al. 2011; Petrus et al. 2011; Turrigiano 2011; Whitt et al. 2014).
Visual experience and the precritical period.
Our previous studies comparing late DR with chronic DR suggest that prior visual experience is necessary to elicit changes in afferent pruning of the retinogeniculate synapse (Hooks and Chen 2008). To explore the role of visual experience further, we provided a short 4-day period of vision to chronically deprived mice from birth at two different time points (P18–22 and P20–24). If vision is needed for the expression of plasticity, then “pulsing” vision in different developmental windows, before repeat visual deprivation, should lead to distinct synaptic responses. Indeed, we found that transient light exposure for the first time at P20–24 did not elicit significant changes in input strength or number of afferent inputs. However, the same manipulation at P18–22 elicited both a weakening of synaptic inputs similar to early DR as well as a recruitment of more RGC inputs as in late DR. These experiments show that not only is prior visual experience needed to trigger the recruitment of new inputs in response to visual deprivation, but also that the timing of this initial sensory stimulation, specifically that it occurs before P20, is crucial.
The timeframe between eye opening and susceptibility to visual deprivation at P20 at the retinogeniculate synapse is a period that we refer to as the precritical period (Hooks and Chen 2007, 2008). Whereas it was once thought that circuits in the visual system formed independently of visual experience during the precritical period, more recent evidence from the visual cortex shows that visual experience shapes connectivity during this time at multiple levels, from gene expression patterns (Majdan and Shatz 2006) to postsynaptic receptor subunits (Lu and Constantine-Paton 2004) and retinotopic maps (Smith and Trachtenberg 2007).
How does visual experience that occurs in the precritical period prime the visual circuit to allow for subsequent plasticity at the retinogeniculate synapse? One hypothesis is that vision before P20 activates the expression of a group of genes specific for that developmental period. A possible candidate is Mecp2, a gene encoding the transcriptional regulator, which has been shown to be a critical regulator of homeostatic plasticity in hippocampus as well as primary visual cortex (Blackman et al. 2012; Qiu et al. 2012). We recently showed that Mecp2 also plays an important role in experience-dependent plasticity at the retinogeniculate synapse (Noutel et al. 2011). Perhaps activity-dependent phosphorylation of Mecp2 at a number of sites changes over development, and the patterns of phosphorylation correspond to the expression of distinct groups of genes that impart sensitivity to vision and the subsequent expression of plasticity (Ebert and Greenberg 2013; Lyst et al. 2013; Zhong et al. 2012).
Another candidate for vision-dependent synaptic plasticity is the phosphorylation of the transmembrane AMPAR regulatory protein (TARP), stargazin. We have recently shown that phosphorylation of stargazin in the LGN is regulated by visual experience and that this TARP plays a prominent role during the experience-dependent phase of retinogeniculate remodeling as well as in homeostatic plasticity in cultured neurons (Louros et al. 2014). Interestingly, stargazin has multiple phosphorylation sites, raising the question of whether there is a phosphorylation “code” that regulates which synapses AMPAR are trafficked to (Tomita et al. 2005).
Yet another hypothesis is that vision during the precritical period is necessary for the coordinated maturation of different regions of the visual system, which in turn interact with development at the retinogeniculate synapse. Certainly visual experience in the precritical period is necessary for the maturation of many features of the visual system, including orientation selectivity, direction selectivity, and inhibitory circuits (Fagiolini et al. 1994; Morales et al. 2002). Moreover, our recent study demonstrated that chronic dark rearing disrupts the coordinated development of visual system circuits (Kang et al. 2013). Therefore, visual deprivation may lead to disruption of the proper interaction between different circuits in the visual system, which in turn may lead to abnormal experience-dependent plasticity at the retinogeniculate synapse.
In summary, our studies show that homeostatic plasticity drives experience-dependent remodeling during the thalamic critical period. In response to increased visually evoked activity following visual deprivation, retinal input drive “scaled down” by weakening of synaptic strength. Conversely, compensatory synaptic response to visual deprivation involved “scaling up” the number of afferent inputs and required vision during the precritical period. These bidirectional homeostatic mechanisms allow sensory circuits to adapt to the surrounding environment.
GRANTS
This work was supported by National Institutes of Health Grants R01 EY013613 (to C. Chen and E. Kang) and P01 HD18655 (to C. Chen) and a Howard Hughes Medical Institute Medical Research Fellowship and Nancy Lurie Marks Foundation Scholarship (to D. J. Lin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.J.L. and C.C. conception and design of research; D.J.L. and E.K. performed experiments; D.J.L. analyzed data; D.J.L. and C.C. interpreted results of experiments; D.J.L. prepared figures; D.J.L. drafted manuscript; D.J.L. and C.C. edited and revised manuscript; D.J.L. and C.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Michela Fagiolini, Andrew Thompson, Jonathan Garst Orozco, Jessica Hauser, Kevin Park, Liza Litvina, Yi Zhang, Evi Hock, Joseph Leffler, Jose Morales, and Kate Hong for helpful comments on the manuscript.
Present address for D. J. Lin: Department of Neurology, Massachusetts General Hospital, Boston, MA 02114.
REFERENCES
- Blackman MP, Djukic B, Nelson SB, Turrigiano GG. A critical and cell-autonomous role for MeCP2 in synaptic scaling up. J Neurosci 32: 13529–13536, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts DA, Kanold PO, Shatz CJ. A burst-based “Hebbian” learning rule at retinogeniculate synapses links retinal waves to activity-dependent refinement. PLoS Biol 5: e61, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G, Shatz CJ. Synapses formed by identified retinogeniculate axons during the segregation of eye input. J Neurosci 12: 1847–1858, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco MM, Razak KA, Pallas SL. Visual experience is necessary for maintenance but not development of receptive fields in superior colliculus. J Neurophysiol 94: 1962–1970, 2005 [DOI] [PubMed] [Google Scholar]
- Chandrasekaran AR, Plas DT, Gonzalez E, Crair MC. Evidence for an instructive role of retinal activity in retinotopic map refinement in the superior colliculus of the mouse. J Neurosci 25: 6929–6938, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran AR, Shah RD, Crair MC. Developmental homeostasis of mouse retinocollicular synapses. J Neurosci 27: 1746–1755, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Regehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron 28: 955–966, 2000 [DOI] [PubMed] [Google Scholar]
- Crair MC, Gillespie DC, Stryker MP. The role of visual experience in the development of columns in cat visual cortex. Science 279: 566–570, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw NW, Wyatt HJ. Kittens reared in a unidirectional environment: evidence for a critical period. J Physiol 257: 155–170, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci 5: 783–789, 2002 [DOI] [PubMed] [Google Scholar]
- Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci 2: 515–520, 1999 [DOI] [PubMed] [Google Scholar]
- Dunn FA, Santina Della L, Parker ED, Wong RO. Sensory experience shapes the development of the visual system's first synapse. Neuron 80: 1159–1166, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert DH, Greenberg ME. Activity-dependent neuronal signalling and autism spectrum disorder. Nature 493: 327–337, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron 75: 230–249, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res 34: 709–720, 1994 [DOI] [PubMed] [Google Scholar]
- Goel A, Jiang B, Xu LW, Song L, Kirkwood A, Lee HK. Cross-modal regulation of synaptic AMPA receptors in primary sensory cortices by visual experience. Nat Neurosci 9: 1001–1003, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Xu LW, Snyder KP, Song L, Goenaga-Vazquez Y, Megill A, Takamiya K, Huganir RL, Lee HK. Phosphorylation of AMPA receptors is required for sensory deprivation-induced homeostatic synaptic plasticity. PLoS One 6: e18264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci 16: 3274–3286, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron 52: 281–291, 2006 [DOI] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Critical periods in the visual system: changing views for a model of experience-dependent plasticity. Neuron 56: 312–326, 2007 [DOI] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Vision triggers an experience-dependent sensitive period at the retinogeniculate synapse. J Neurosci 28: 4807–4817, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol 206: 419–436, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci 31: 479–509, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaubert-Miazza L, Green E, Lo FS, Bui K, Mills J, Guido W. Structural and functional composition of the developing retinogeniculate pathway in the mouse. Vis Neurosci 22: 661–676, 2005 [DOI] [PubMed] [Google Scholar]
- Kang E, Durand S, LeBlanc JJ, Hensch TK, Chen C, Fagiolini M. Visual acuity development and plasticity in the absence of sensory experience. J Neurosci 33: 17789–17796, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science 274: 1133–1138, 1996 [DOI] [PubMed] [Google Scholar]
- Koenig MA, Kaplan PW, Thakor NV. Clinical neurophysiologic monitoring and brain injury from cardiac arrest. Neurol Clin 24: 89–106, 2006 [DOI] [PubMed] [Google Scholar]
- Krahe TE, Guido W. Homeostatic plasticity in the visual thalamus by monocular deprivation. J Neurosci 31: 6842–6849, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi S, Cenni MC, Maffei L, Berardi N. Environmental enrichment effects on development of retinal ganglion cell dendritic stratification require retinal BDNF. PLoS One 2: e346, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chen C. Different roles for AMPA and NMDA receptors in transmission at the immature retinogeniculate synapse. J Neurophysiol 99: 629–643, 2008 [DOI] [PubMed] [Google Scholar]
- Louros SR, Hooks BM, Litvina L, Carvalho AL, Chen C. A role for stargazin in experience-dependent plasticity. Cell Rep 7: 1614–1625, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Constantine-Paton M. Eye opening rapidly induces synaptic potentiation and refinement. Neuron 43: 237–249, 2004 [DOI] [PubMed] [Google Scholar]
- Lyst MJ, Ekiert R, Ebert DH, Merusi C, Nowak J, Selfridge J, Guy J, Kastan NR, Robinson ND, de Lima Alves F, Rappsilber J, Greenberg ME, Bird A. Rett syndrome mutations abolish the interaction of MeCP2 with the NCoR/SMRT co-repressor. Nat Neurosci 16: 898–902, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdan M, Shatz CJ. Effects of visual experience on activity-dependent gene regulation in cortex. Nat Neurosci 9: 650–659, 2006 [DOI] [PubMed] [Google Scholar]
- Morales B, Choi SY, Kirkwood A. Dark rearing alters the development of GABAergic transmission in visual cortex. J Neurosci 22: 8084–8090, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Schikorski T, Stevens CF, Zhu Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron 32: 673–682, 2001 [DOI] [PubMed] [Google Scholar]
- Noutel J, Hong YK, Leu B, Kang E, Chen C. Experience-dependent retinogeniculate synapse remodeling is abnormal in MeCP2-deficient mice. Neuron 70: 35–42, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn AA, Riquelme PA, Feller MB, Shatz CJ. Competition in retinogeniculate patterning driven by spontaneous activity. Science 279: 2108–2112, 1998 [DOI] [PubMed] [Google Scholar]
- Petrus E, Anguh TT, Pho H, Lee A, Gammon N, Lee HK. Developmental switch in the polarity of experience-dependent synaptic changes in layer 6 of mouse visual cortex. J Neurophysiol 106: 2499–2505, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z, Sylwestrak EL, Lieberman DN, Zhang Y, Liu XY, Ghosh A. The Rett syndrome protein MeCP2 regulates synaptic scaling. J Neurosci 32: 989–994, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ. Impulse activity and the patterning of connections during CNS development. Neuron 5: 745–756, 1990 [DOI] [PubMed] [Google Scholar]
- Smith SL, Trachtenberg JT. Experience-dependent binocular competition in the visual cortex begins at eye opening. Nat Neurosci 10: 370–375, 2007 [DOI] [PubMed] [Google Scholar]
- Sretavan DW, Shatz CJ, Stryker MP. Modification of retinal ganglion cell axon morphology by prenatal infusion of tetrodotoxin. Nature 336: 468–471, 1988 [DOI] [PubMed] [Google Scholar]
- Sretavan DW, Shatz CJ. Prenatal development of cat retinogeniculate axon arbors in the absence of binocular interactions. J Neurosci 6: 990–1003, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian N, Copenhagen DR. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron 39: 85–96, 2003 [DOI] [PubMed] [Google Scholar]
- Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron 45: 269–277, 2005 [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu Rev Neurosci 34: 89–103, 2011 [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391: 892–896, 1998 [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci 5: 97–107, 2004 [DOI] [PubMed] [Google Scholar]
- Watt AJ, van Rossum MC, MacLeod KM, Nelson SB, Turrigiano GG. Activity coregulates quantal AMPA and NMDA currents at neocortical synapses. Neuron 26: 659–670, 2000 [DOI] [PubMed] [Google Scholar]
- White LE, Coppola DM, Fitzpatrick D. The contribution of sensory experience to the maturation of orientation selectivity in ferret visual cortex. Nature 411: 1049–1052, 2001 [DOI] [PubMed] [Google Scholar]
- Whitt JL, Petrus E, Lee HK. Experience-dependent homeostatic synaptic plasticity in neocortex. Neuropharmacology 78: 45–54, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Effects of visual deprivation on morphology and physiology of cells in the cat's lateral geniculate body. J Neurophysiol 26: 978–993, 1963 [DOI] [PubMed] [Google Scholar]
- Zhong X, Li H, Chang Q. MeCP2 phosphorylation is required for modulating synaptic scaling through mGluR5. J Neurosci 32: 12841–12847, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziburkus J, Dilger EK, Lo FS, Guido W. LTD and LTP at the developing retinogeniculate synapse. J Neurophysiol 102: 3082–3090, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziburkus J, Guido W. Loss of binocular responses and reduced retinal convergence during the period of retinogeniculate axon segregation. J Neurophysiol 96: 2775–2784, 2006 [DOI] [PubMed] [Google Scholar]