Abstract
Fragile X syndrome (FXS) is the leading cause of inherited intellectual disability. Comorbidities of FXS such as autism are increasingly linked to imbalances in excitation and inhibition (E/I) as well as dysfunction in GABAergic transmission in a number of brain regions including the amygdala. However, the link between E/I imbalance and GABAergic transmission deficits in the FXS amygdala is poorly understood. Here we reveal that normal tonic GABAA receptor-mediated neurotransmission in principal neurons (PNs) of the basolateral amygdala (BLA) is comprised of both δ- and α5-subunit-containing GABAA receptors. Furthermore, tonic GABAergic capacity is reduced in these neurons in the Fmr1 knockout (KO) mouse model of FXS (1.5-fold total, 3-fold δ-subunit, and 2-fold α5-subunit mediated) as indicated by application of gabazine (50 μM), 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP, 1 μM), and α5ia (1.5 μM) in whole cell patch-clamp recordings. Moreover, α5-containing tonic GABAA receptors appear to preferentially modulate nonsomatic compartments of BLA PNs. Examination of evoked feedforward synaptic transmission in these cells surprisingly revealed no differences in overall synaptic conductance or E/I balance between wild-type (WT) and Fmr1 KO mice. Instead, we observed altered feedforward kinetics in Fmr1 KO PNs that supports a subtle yet significant decrease in E/I balance at the peak of excitatory conductance. Blockade of α5-subunit-containing GABAA receptors replicated this condition in WT PNs. Therefore, our data suggest that tonic GABAA receptor-mediated neurotransmission can modulate synaptic E/I balance and timing established by feedforward inhibition and thus may represent a therapeutic target to enhance amygdala function in FXS.
Keywords: tonic inhibition, GABA, amygdala, fragile X syndrome
patients with fragile x syndrome (FXS) display a variety of symptoms associated with autism spectrum disorders (ASDs) including mild to severe intellectual disability, social anxiety/withdrawal, increased incidence of epilepsy, attention-deficit hyperactivity disorder (ADHD), and sensory hypersensitivity (Fu et al. 1991; Hagerman et al. 2009; Verkerk et al. 1991). Current evidence implicates a number of developmental defects that likely underlie these symptoms including dysregulated synaptic plasticity (He and Portera-Cailliau 2013), brain region connectivity (Geschwind and Levitt 2007), and excitation/inhibition (E/I) imbalance in critical neuronal circuits such as the cerebral cortex, hippocampus, and amygdala (Gibson et al. 2008; Hays et al. 2011; Olmos-Serrano et al. 2010; Paluszkiewicz et al. 2011b; Zhang and Alger 2010). From the standpoint of neuronal excitation, many of these defects in synaptic plasticity and network imbalance can be attributed to elevated basal and activity-dependent protein translation downstream of enhanced Group I metabotropic glutamate receptor signaling caused by the loss of a critical translational regulator, FMRP (Fragile-X Mental Retardation Protein) (Bear et al. 2004).
However, a growing literature also implicates widespread, brain region-specific disruption in GABAergic inhibitory transmission in FXS, particularly in brain regions such as the sensory cortices and amygdala that are crucially implicated in the most prominent features of the disorder (e.g., sensory hypersensitivity and social withdrawal) (Paluszkiewicz et al. 2011a). For instance, numerous studies identify decreased GABAA receptor (GABAR) subunit expression (D'Hulst et al. 2006; Gantois et al. 2006), altered expression of GABAergic system components such as GAD and GAT-1 (Adusei et al. 2010; El Idrissi et al. 2005), and functional and anatomical disruption of inhibitory synapses (Olmos-Serrano et al. 2010; Vislay et al. 2013).
Accordingly in the basolateral amygdala (BLA), a region essential for sensory integration, assignment of emotional saliency, and regulation of acquired fear (Ehrlich et al. 2009), pervasive GABAergic deficits exist. These deficits include reductions in the number of GABAergic synapses, GABA production and release, and phasic and tonic inhibitory currents in principal excitatory neurons (PNs). Furthermore, this GABAergic dysfunction correlates with a neuronal hyperexcitable phenotype that is pharmacologically rescued by specifically enhancing tonic GABAergic transmission (Olmos-Serrano et al. 2010). Indeed, tonic GABAergic conductance, maintained by low levels of ambient GABA in the extrasynaptic space (Farrant and Nusser 2005), provides a persistent background inhibitory conductance that regulates E/I balance to affect not only intrinsic neuronal excitability (Bonin et al. 2007) but also the integration of synaptic inputs (Mitchell and Silver 2003; Semyanov et al. 2004) and synaptic plasticity (Martin et al. 2010). Yet despite the clear potential of GABAergic inhibitory tone to regulate these common cellular and network dysfunctions in neurodevelopmental disorders including FXS and, in particular, to regulate synaptic plasticity underlying fear processing (acquisition, expression, extinction) (reviewed in Ehrlich et al. 2009), this form of GABAergic transmission remains understudied in the amygdala and FXS.
Here we investigated the state of tonic GABAergic conductance in wild-type (WT) and Fmr1 knockout (KO) mouse BLA to identify specific alterations in FXS that might inform the role of tonic conductance in regulating cellular and synaptic balance in the region. We found that tonic conductance in BLA PNs is comprised of at least δ- and α5-subunit-containing receptors. In addition, total and δ- and α5-mediated tonic capacity are deficient in Fmr1 KO PNs. We also identify a functional preference of α5-containing tonic GABAA receptors for modulation of synaptic events distal from our somatic whole cell patch-clamp recording site. Furthermore, we determined the dynamics of the feedforward circuit comprised of excitatory afferents within the external capsule and local BLA interneurons. Our results reveal that, surprisingly, Fmr1 KO PNs exhibit overall balanced evoked excitatory (Ge) and feedforward inhibitory (Gi) conductance compared with WT. However, slower excitatory response kinetics in Fmr1 KO slices results in a more narrow time window between Ge and Gi peaks in the feedforward response. Furthermore, blockade of α5-containing GABAA receptors in WT slices mimics this condition. Thus we reveal the importance of this selectively activated tonic GABAergic conductance to regulate E/I dynamics in BLA PNs. Therefore, augmenting tonic transmission in Fmr1 KO mice may enhance synaptic integration and network function in this important feedforward inhibitory circuit and thereby improve amygdala-based symptoms of this disorder.
METHODS
Animal use.
Control (FVB.129P2, stock no. 4828) and Fmr1 KO mice (strain name: FVB.129P2-Fmr1tm1Cgr/J; stock no. 4624) on the congenic FVB strain background were obtained from The Jackson Laboratory (Genetics Research, Bar Harbor, ME) and maintained as separate congenic stocks in our own facility. Here, we refer to control animals as wild type (WT) throughout the text and figures. Only male animals were included in the study. Animals were housed and utilized in accordance with protocols approved by the Children's National Medical Center Institutional Animal Care and Use Committee.
Slice preparation for electrophysiology.
Acute slices were prepared from male WT or Fmr1 KO mice, age postnatal day (P)21–P30. Animals were briefly anesthetized with CO2 and decapitated. Brains were removed quickly and placed in cold (4°C) sucrose-based oxygenated (95% O2-5% CO2) cutting solution composed of (in mM) 234 sucrose, 11 glucose, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4·H2O, 10 MgSO4·7H2O, and 0.5 CaCl2·H2O. Coronal slices containing the BLA were obtained with a slicing vibratome (Leica VT1200s) by removing the cerebellum with a perpendicular cut to the rostral-caudal plane and gluing the caudal side down on the vibratome stage submerged in cold cutting solution. Slice thickness was 300 μm for all experiments with the exception of conductance experiments, in which case slices were cut at 400 μm to preserve afferent connections to PNs in the external capsule. The slices were immersed in oxygenated (95% O2-5% CO2) artificial cerebrospinal fluid (ACSF) at 34°C for 30–45 min. ACSF was composed of (in mM) 126 NaCl, 26 NaHCO3, 10 glucose, 2.5 KCl, 1.25 NaH2PO4·H2O, 2 MgCl2·7H2O, and 2 CaCl2·2H2O (pH 7.4, osmolarity maintained at 290–300 mosM).
Electrophysiology.
For all experiments slices were placed in a submerged slice chamber and continuously perfused with ACSF at 2–4 ml/min maintained at 26–28°C with an in-line heater system (Warner Instruments). We used temperatures below physiological (∼37°C), consistent with our previous investigations of the BLA and GABAergic transmission (Olmos-Serrano et al. 2010), to ensure cell and slice health over long recordings. These temperatures were essential in conductance experiments (see Fig. 5) to ensure robust and consistent evoked responses from the external capsule afferents over the length of the experiment. Slices were visualized on a fixed-stage upright microscope (Nikon) equipped with ×10 and ×60 objectives using differential interference contrast (DIC) optics, infrared illumination, and an infrared-sensitive camera (COHU). Whole cell patch-clamp recordings were performed with glass pipettes with resistance of 2.0–4.0 MΩ when filled with intracellular solution. Access resistance of recordings was <25 MΩ and monitored throughout the experiment with brief 5-mV steps every 20 s. Data were discarded if the access resistance changed by >25%. Membrane potentials were adjusted for junction potential (12 mV). Data were acquired with a Multiclamp 700A amplifier and digitized with a Digidata 1322A using pCLAMP 9.2 acquisition software (Molecular Devices). All recordings were made from PNs identified first visually as having a large, pyramidal-like soma with two to seven primary dendrites and then physiologically with prolonged depolarizing and hyperpolarizing current injections (600 ms) (Fig. 1, A and B). PNs typically display broad (∼1.2 ms), accommodating action potentials (APs) in combination with long afterhyperpolarizing potentials (AHPs) (Sah et al. 2003). In experiments utilizing tetrodotoxin (TTX) to record AP-independent synaptic events or using cesium-based intracellular solution, visual identification combined with physiological responses to hyperpolarizing current injections recorded within 30 s of membrane rupture were used exclusively to identify PNs. GABAergic tonic currents and phasic spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded in voltage clamp and isolated by blocking ionotropic glutamatergic transmission with 6,7-dinitroquinoxaline-2,3-dione (DNQX, 20 μM final concentration, AMPA/kainate antagonist; Tocris Bioscience) and dl-2-amino-5-phosphonopentanoic acid (APV, 50 μM final concentration, NMDA receptor antagonist; Tocris Bioscience) in the ACSF. To determine δ-subunit-mediated GABAergic tonic currents and the effect of α5-GABAA receptor blockade on sIPSCs (Figs. 1 and 3), a K-gluconate-based intracellular solution was used (in mM): 70 K-gluconate, 70 KCl, 10 HEPES, 1 EGTA, 2 MgCl2, 4 Mg-ATP, and 0.3 Na-GTP (ECl− = −16 mV). For tonic current capacity experiments and to investigate the role of α5-GABAA receptors on sIPSCs recorded at the soma (Figs. 2 and 4), a cesium chloride-based intracellular solution was used (in mM): 135 CsCl, 10 HEPES, 10 EGTA, 5 QX-314, 2 MgCl2, 4 Mg-ATP, and 0.3 Na-GTP (ECl− = 0 mV). This solution reduces potassium channel currents, allowing better visualization of distal events recorded at the soma. Both intracellular solutions allow visualization of GABAergic currents as downward when the holding potential is near rest (−70 mV to −60 mV).
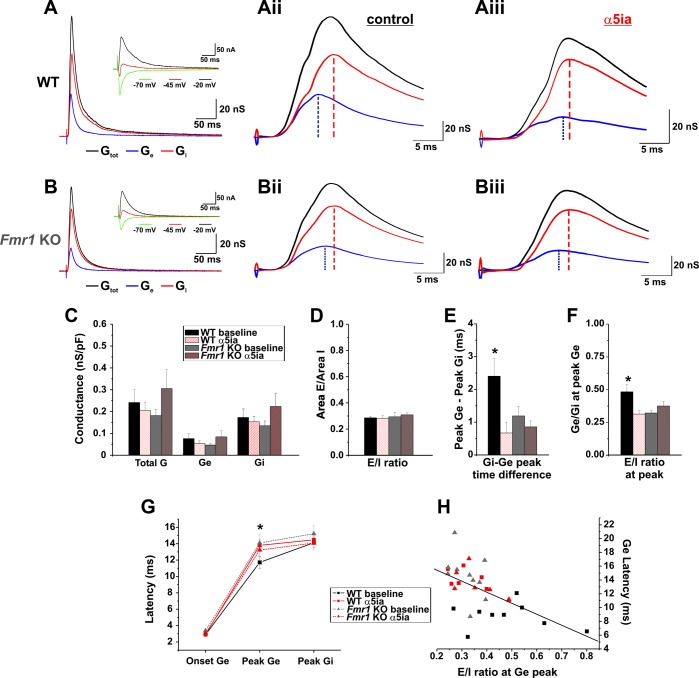
Fig. 5.
The presence of α5-GABAA receptor activity affects evoked response kinetics and synaptic balance. A and B: representative conductance measurements [total conductance (Gtot), black; excitatory conductance (Ge), blue; inhibitory conductance (Gi), red] derived from current/voltage (I/V) curves taken from evoked responses recorded in voltage clamp at 3 different holding potentials (inset: −20 mV, black; −45 mV, red; −70 mV, green) from WT (A) and KO (B) PNs. Aii and Bii: representative examples of baseline conductance kinetics for WT (Aii) and Fmr1 KO (Bii). Aiii and Biii: representative examples of conductance kinetics in the presence of α5-GABAA receptor blockade (α5ia, 1.5 μM) in WT (Aiii) and Fmr1 KO (Biii) cells. C: conductance measurements of WT and Fmr1 KO cells reveal no significant differences in Gtot, Ge, or Gi between genotypes or conditions (baseline or α5ia) [conductance density (nS/pF)]. D: in addition, overall E/I balance is similar among genotypes and conditions [Conductance Area: Ge (nS/ms)/Gi (nS/ms)]. E: conductance kinetics demonstrate a significantly longer duration between Ge and Gi peaks in WT baseline cells compared with WT cells in the presence of α5ia or Fmr1 KO cells (*P < 0.05). F: the E/I conductance ratio at the Ge peak is increased in WT baseline compared with WT cells in the presence of α5ia and Fmr1 KO baseline cells (WT baseline vs. WT α5ia and Fmr1 KO baseline, *P < 0.05; vs. Fmr1 KO α5ia, P = 0.06). G: increased Ge-to-Gi peak times in WT baseline cells associate with changes solely in the Ge latency (center) and not the Ge onset (left) or Gi latency (right) compared with other conditions (WT baseline vs. WT α5ia and Fmr1 KO baseline, *P < 0.05; vs. Fmr1 KO α5ia, P = 0.09). H: summary data from all cells indicate that Ge latency (G) and E/I ratio at peak Ge (F) are negatively correlated (linear regression, r = −0.587, P < 0.0001).
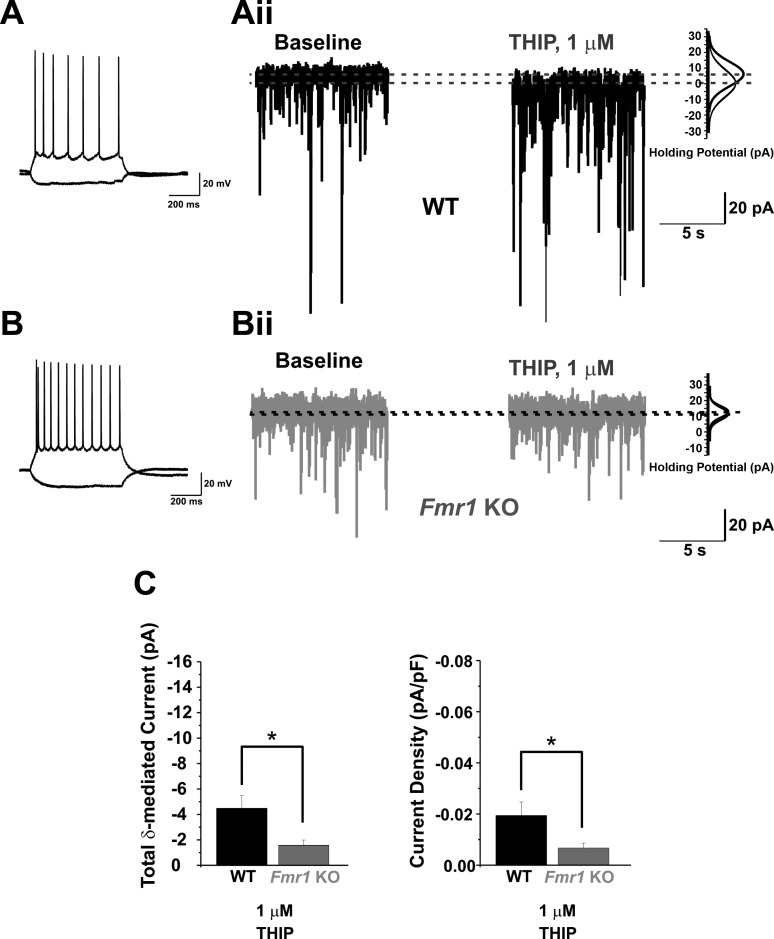
Fig. 1.
Fmr1 knockout (KO) principal neurons (PNs) of the basolateral amygdala (BLA) have reduced δ-subunit-mediated tonic GABAergic currents. A and B: representative current-clamp traces showing typical responses of PNs to depolarizing (+150 pA) and hyperpolarizing (−100 pA) current injections (600 ms). Aii and Bii: representative whole cell voltage-clamp traces recorded from wild-type (WT; A) and Fmr1 KO (B) PNs showing 10-s samples before (baseline) and after (THIP) bath application of 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP, 1 μM) [holding potential (Vhold) = −60 mV]. Gaussian distributions (right) for each sample indicate the differences in mean holding current at each condition. C: averaged group data reveal significantly reduced δ-subunit-mediated current (left) and current density (right) in Fmr1 KO cells vs. WT at 1 μM THIP. *P < 0.05.
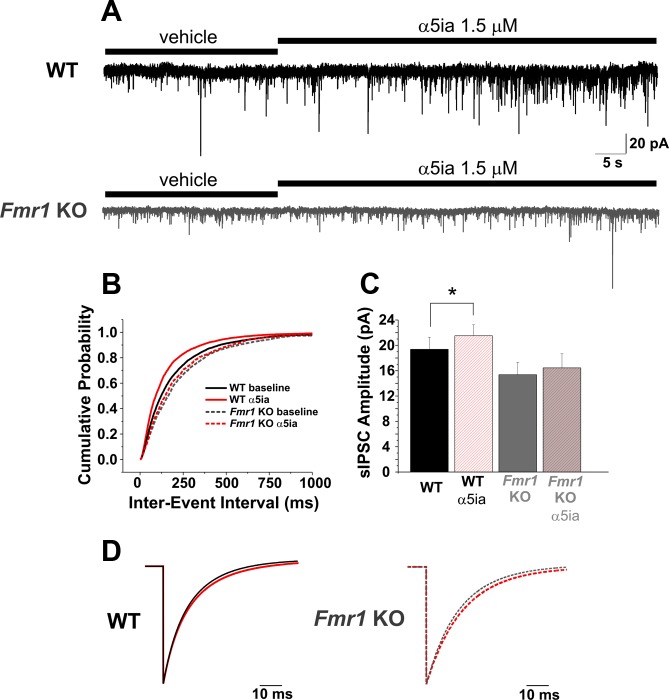
Fig. 3.
Blockade of α5-GABAA receptors increases GABAergic inhibitory efficacy as recorded at the soma. A and B: application of α5ia (1.5 μM) increases the frequency of spontaneous inhibitory postsynaptic currents (sIPSCs) in both WT and Fmr1 KO PNs of the BLA shown here as a decrease in the distribution of the cumulative probability of the interevent interval (B) before and after application of α5ia (Vhold = −60 mV). C: in the presence of α5ia, amplitude also increases in both WT and Fmr1 KO cells. However, both frequency (B) and amplitude (C) changes are reduced in the Fmr1 KO cells vs. WT. D: average event fits from WT and Fmr1 KO sIPSCs [WT: left, baseline (black solid), α5ia (red solid); Fmr1 KO: right, baseline (gray dotted), α5ia (red dotted)] show slight but significant increases in decay constant τ (Table 1). *P < 0.05.
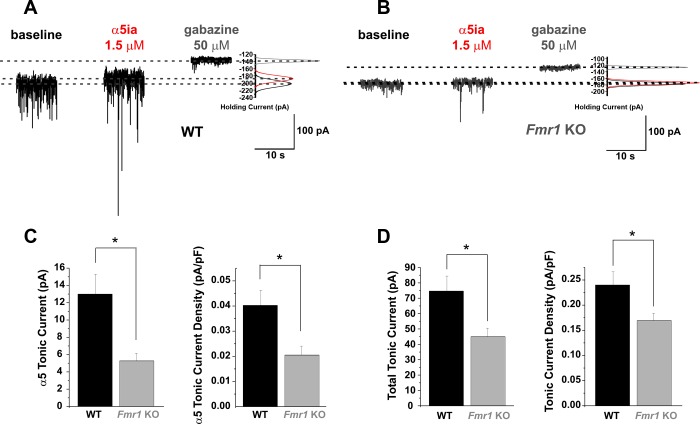
Fig. 2.
Fmr1 KO PNs in the BLA have diminished α5-subunit-specific and total tonic current capacity compared with WT cells. A and B: representative whole cell voltage-clamp traces recorded from WT (A) and Fmr1 KO (B) PNs showing 10-s samples recorded at baseline (black), after application of α5ia (1.5 μM; red), and after application of gabazine (50 μM; gray) (Vhold = −70 mV). Gaussian distributions (right) for the samples indicate the differences in mean holding current at each condition. C: averaged group data reveal significantly reduced α5-subunit-mediated capacity (left) and current density (right) in Fmr1 KO cells vs. WT. Similarly in D, averaged group data reveal significantly reduced total tonic current capacity (left) and current density (right) in Fmr1 KO cells vs. WT. *P < 0.05.
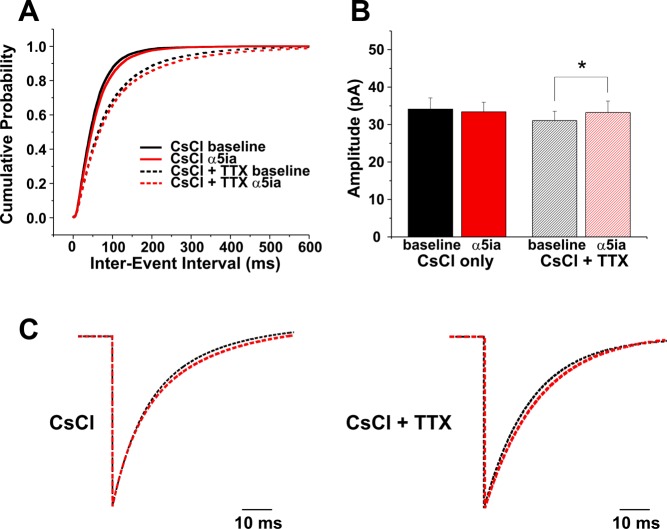
Fig. 4.
Recording IPSCs with CsCl-based pipette solution occludes increases in inhibitory efficacy in response to α5-GABAA receptor blockade. A: application of α5ia (1.5 μM) decreases rather than increases sIPSC (solid lines) and mIPSC (dotted lines) as observed with K-gluconate (K-Gluc) recordings. B: amplitude increases only occur in the presence of TTX in CsCl recordings [miniature IPSCs (mIPSCs), right], whereas no significant changes in amplitude occur in sIPSC recordings after application of α5ia (left). C: in both sIPSC (left) and mIPSC (right) recordings the decay constant τD increases slightly and significantly in response to α5ia application (Table 1). *P < 0.05.
Tonic currents were acquired and analyzed as reported in our previous work (Krook-Magnuson et al. 2008). Briefly, 10-s samples were taken from voltage-clamp recordings [holding potential (Vhold) = −60 mV, K-gluconate-based solution; Vhold = −70 mV, CsCl-based solution] at each experimental condition [baseline (IBSLN), α5ia (Iα5ia, α5-subunit-specific GABAR inverse agonist, 1.5 μM), gabazine (IGBZ, GABAR antagonist SR-95531, 50 μM), or 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP) (ITHIP, 1 μM)]. To minimize bias from phasic events, a Gaussian distribution was fit to the right side of an all-points histogram from each sample from a point 1–3 pA left of the peak (Glykys and Mody 2007). The Gaussian peak determined the mean current for the sample. Total tonic current capacity was calculated from the difference in mean baseline and gabazine currents (IGBZ − IBSLN), and α5-subunit mediated tonic current capacity was calculated from the difference in mean baseline and α5ia currents (Iα5ia − IBSLN). In a separate set of experiments, δ-subunit-specific tonic currents were calculated (in the absence of extracellular GABA supplementation) from the difference in mean baseline and THIP currents (ITHIP − IBSLN). To control for differences in cell size/capacitance, calculated currents were converted to tonic current densities for each cell based on cell capacitance [current density = current (pA)/capacitance (pF)]. Capacitance was determined in voltage clamp with brief 10-mV biphasic voltage steps delivered immediately after whole cell configuration was established.
To evaluate the role of α5-GABAA receptor-mediated tonic conductance in control of IPSC parameters recorded at the soma, AP-dependent IPSCs were recorded with K-gluconate-based internal solution alone (sIPSCs). Subsequent experiments then utilized the CsCl-based internal solution to better visualize distal-originating events at the soma by decreasing potassium conductance (Nicoll et al. 1993) and were recorded without and in the presence of 1 μM TTX in order to block sodium channels [AP-independent miniature IPSCs (mIPSCs)]. IPSCs were analyzed for changes in frequency, amplitude, and kinetics before and after the application of α5ia (1.5 μM). For IPSC measurements drugs were applied locally via gravity-fed Y-tube application, and for evoked conductance experiments drugs were bath applied.
Evoked conductance was derived from the slopes of synaptic current/voltage (I/V) plots utilizing methods similar to those described in Wehr and Zador (2003) and Cruikshank et al. (2007). Excitatory and inhibitory components of total evoked synaptic conductance were then determined based on assumed reversal potentials for excitation and inhibition. First, synaptic currents were evoked in voltage-clamp mode with at least three different holding potentials (typically −20 mV, −45 mV, −70 mV) utilizing a Cs-gluconate-based internal solution composed of (in mM) 130 Cs-gluconate, 4 KCl, 2 NaCl, 10 HEPES, 0.2 EGTA, 0.2 QX-314 (Br−), 4 ATP-Mg, 0.3 GTP-Tris, and 14 phosphocreatine-Tris (pH 7.25, 290 mosM, ECl− = −69 mV) and with 50 μM APV in the bath to remove any nonlinearities in the I/V relationship introduced by activation of synaptic NMDA receptors. Evoked currents were initiated by a 25-μm-diameter concentric bipolar Pt-Ir external stimulating electrode (FHC) placed in the external capsule (mostly cortical inputs) at the level of the central nucleus of the amygdala. Threshold stimulus intensity was determined with single 200-μS pulses at a holding potential of −70 mV (reversal potential of IPSCs) and was considered to be the stimulus amplitude at which there occurred a ∼50% failure rate of monosynaptic evoked excitatory postsynaptic currents (eEPSCs), typically 15–25 μA. Stimulus intensity was then adjusted to 4 times the measured threshold amplitude for the duration of the experiment.
Stimuli were delivered at three or four different holding potentials with a step protocol beginning with the highest holding potential and descending to the lowest in 25-mV increments (i.e., 5 mV, −20 mV, −45 mV, −70 mV). Steps lasted at least 10 s at each holding potential before the stimulus was delivered, and each stimulus was preceded 500 ms by a 5-mV biphasic voltage step to assess input resistance (Rin). The protocol was repeated 6–15 times (typically 10 times), and the responses at each step were averaged (see Fig. 5A). Series resistance was also determined before each group of descending voltage steps with the membrane test feature of pCLAMP 9 using a 20-mV biphasic pulse at 20 Hz. Series resistance was compensated for off-line to wholly account for voltage errors due to series resistance, versus online methods that can leave up to 50% of the voltage error uncompensated. Holding potential was compensated for with the following equation:
where Vcorr(t) is the corrected voltage at time t, Vrec(t) is the recorded voltage at time t, Irec(t) is the recorded current at time t, and Rs is the series resistance measured before each set of voltage steps with the membrane test. Next, recorded current was compensated for Rin and thus any nonsynaptic current affecting somatic voltage change (McNaughton et al. 1981), using the following equation to calculate the evoked synaptic current at each holding potential:
where Isyn(t) is the derived synaptic current at time t, Rin is the input resistance derived with Ohm's law from the 5-mV voltage step preceding the stimulus,
and
After Isyn(t) was derived, an I/V curve for each point in the average response was generated with Isyn(t) values and corresponding Vcorr(t) values [Isyn(t) vs. Vcorr(t)]. The most depolarizing step (5 mV) was removed from analysis because, as has been reported previously, the most depolarizing step introduced nonlinearities into the I/V relationship related to high voltage escape during synaptic responses (Cruikshank et al. 2007). The slope of the I/V relationship at each time point was the total synaptic conductance at each time point, Gsyn(t). The x-intercept of the I/V plot was the synaptic reversal potential at each time point, Esyn(t). The excitatory synaptic reversal potential, Ee, was assumed to be 0 mV, and the inhibitory synaptic reversal potential, Ei, was assumed to be −69 mV based on the calculated reversal potential of Cl−1 ions for the Cs-gluconate-based intracellular solution. With these measures and the following equations (Cruikshank et al. 2007), total conductance [Gsyn(t)] and the excitatory [Ge(t)] and inhibitory [Gi(t)] components of that conductance were calculated and plotted:
therefore,
Statistical analysis.
All recordings were analyzed off-line (Clampfit v. 9.2, Molecular Devices; Mini Analysis v. 6.0.7, Synaptosoft; Microsoft Excel; and MATLAB). Expressed values are means ± SE. Statistical analyses utilized the two-tailed Student's t-test or the nonparametric Mann-Whitney U-test where appropriate for measures of tonic currents, sIPSC amplitudes, and properties of synaptic conductance (Origin v. 7.0552, OriginLab). We used the paired Student's t-test to determine significance for within-group comparisons between conditions (i.e., baseline and drug) and ANOVA to compare baseline and drug conditions across the two genotypes (WT and Fmr1 KO). The Kolmogorov-Smirnov (K-S) test was used to compare probability distributions of interevent interval (frequency, sIPSCs, and mIPSCs) for within-group comparisons between conditions (i.e., baseline and drug) and across baseline conditions for the two genotypes (WT and Fmr1 KO) (MATLAB).
RESULTS
Tonic GABAA-mediated capacity in BLA principal neurons is reduced in Fmr1 KO mice.
To determine whether deficits in tonic GABAA receptor expression contribute to the decreased tonic currents previously observed in Fmr1 KO PNs (Olmos-Serrano et al. 2010), we performed whole cell voltage-clamp experiments to measure tonic current capacity in both WT and Fmr1 KO PNs. We specifically targeted those currents mediated by the most common known tonically active GABAA receptor subunits, the δ- and α5-subunit-containing receptors (reviewed in Brickley and Mody 2012). These receptor subunits have been previously shown to be expressed in the BLA via immunohistochemistry (Fritschy and Mohler 1995; Pirker et al. 2000) and therefore likely contribute to the overall tonic conductance. First we tested BLA PNs in WT and Fmr1 KO mice for the presence of δ-subunit-mediated tonic currents, using the δ-subunit-preferring GABAA receptor superagonist THIP (1 μM). Recent studies conducted in δ-subunit KO mice (δ−/−) combined with efficacy studies of THIP on various GABAA receptor subtype combinations have shown that concentrations of THIP specific for efficacy at δ-subunit-containing GABAA receptors likely reside in the low micromolar to submicromolar range (Brown et al. 2002; Stórustovu and Ebert 2006). Therefore we used THIP at 1 μM in our experiments. THIP application at this concentration generated δ-subunit-mediated currents in WT BLA PNs, verifying the presence of δ-subunit-mediated tonic currents in these cells (Fig. 1Aii). These currents were significantly reduced in Fmr1 KO PNs by about threefold (Fig. 1Bii) [WT: 4.47 ± 1.01 pA (n = 11), Fmr1 KO: 1.57 ± 0.42 pA (n = 10); t-test, P = 0.02; Fig. 1C, left]. Moreover, 1 μM THIP-induced current densities were also significantly reduced in Fmr1 KO PNs [WT: 0.019 ± 0.005 pA/pF (n = 11), Fmr1 KO: 0.007 ± 0.002 pA/pF (n = 10); t-test, P = 0.04; Fig. 1C, right].
In parallel experiments we assessed α5-subunit-mediated current capacity and total tonic current capacity (Fig. 2), using the α5-subunit-specific inverse agonist α5ia and the GABAA receptor antagonist gabazine, respectively, to quantify the removal of baseline tonic current. Since our previous studies revealed that extrasynaptic GABA levels differ between WT and Fmr1 KO (Olmos-Serrano et al. 2010), we controlled for decreased GABA availability in the Fmr1 KO BLA by equalizing the extrasynaptic GABA between the two genotypes with 5 μM bath-applied exogenous GABA, which more closely matches in vivo extracellular GABA concentration (Glykys and Mody 2007) and produces larger currents generally than our previous investigations with the lower concentrations of native extrasynaptic GABA in our submerged slices (Olmos-Serrano et al. 2010). Application of α5ia (1.5 μM) revealed the α5-subunit-specific tonic current capacity, and subsequent application of gabazine (50 μM) revealed the total tonic current capacity (Fig. 2, A and B). Exogenous GABA was applied for at least 10 min prior to other drugs to allow synaptic receptors to desensitize to minimize their contribution to measured tonic currents. Additionally, we used a cesium-based intracellular solution (CsCl) in our recording pipettes. Replacing potassium with cesium increases voltage control of the membrane to enhance the strength of more distal synaptic transmission by blocking potassium channels whose conductance otherwise filters events occurring far from the somatic recording site (Stuart and Spruston 1998). Under these conditions application of α5ia revealed that BLA PNs indeed have the capacity for α5-subunit-specific tonic current and that Fmr1 KO PNs have reduced α5-subunit-mediated tonic currents [WT: 13.0 ± 2.3 pA (n = 12 cells), Fmr1 KO: 5.28 ± 0.88 pA (n = 12); t-test, P = 0.007; Fig. 2C] and current density (WT: 0.04 ± 0.020 pA/pF, Fmr1 KO: 0.02 ± 0.011 pA/pF; P = 0.012) compared with WT. Subsequent application of gabazine showed that total tonic current capacity is also reduced, as indicated by reduced gabazine-dependent changes in holding current [WT: 74.61 ± 9.81 pA (n = 12), Fmr1 KO: 44.97 ± 5.56 pA (n = 12); t-test, P = 0.015; Fig. 2D] and current density (WT: 0.24 ± 0.026 pA/pF, Fmr1 KO: 0.17 ± 0.015 pA/pF; t-test, P = 0.028). These data confer a reduced capacity for tonic currents (δ-subunit mediated, Fig. 1; α5-subunit mediated/total capacity, Fig. 2) in Fmr1 KO PNs independent of reduced GABA availability (Olmos-Serrano et al. 2010), since direct activation and saturating GABA still produce smaller currents in these cells compared with WT.
α5-GABAA receptors regulate synaptic efficacy in BLA principal neurons.
In addition to the change in holding current observed in our measurements of tonic capacity by reducing α5-GABAA receptor activity (Fig. 2), we surprisingly observed an apparent increase in the amplitude of GABAA receptor-mediated sIPSCs in both WT and Fmr1 KO PNs [Fig. 2, A and B (α5ia)]. To further explore this observation, we first recorded sIPSCs with our standard potassium-gluconate (K-gluc) intracellular solution in our recording pipettes in the absence of exogenous GABA (Fig. 3). Under these conditions we did not observe any significant changes in holding current after application of the α5-subunit-specific inverse agonist α5ia (Fig. 3A). However, in both WT and Fmr1 KO PNs, reduction of α5-subunit-containing receptor activity caused an overall increase in inhibitory synaptic efficacy as recorded from the soma. Specifically, we observed a decrease in the interevent interval of sIPSCs indicating an increase in sIPSC frequency in both WT and Fmr1 KO PNs. This increase occurred in both WT and Fmr1 KO PNs [Fig. 3, A and B; WT: baseline 203.25 ± 3.65 ms (n = 4,498 events from 13 cells), α5ia 153.54 ± 2.63 ms (n = 5,203 events from 13 cells) (K-S test, P < 0.0001); Fmr1 KO: baseline 244.57 ± 6.86 ms (n = 1,499 events from 7 cells), α5ia 225.7 ± 6.26 ms (n = 1,601 events from 7 cells) (K-S test, P = 0.02)]. Additionally, the amplitude of these sIPSCs also increased in WT on α5ia application but not Fmr1 KO cells [Fig. 3, A and C; WT: baseline 19.36 ± 1.91 pA, α5ia 21.52 ± 1.73 pA (n = 13 cells) (paired t-test, P = 0.021); Fmr1 KO: baseline 15.36 ± 1.95 pA, α5ia 16.44 ± 2.23 pA (n = 7 cells) (paired t-test, P = 0.182)]. Furthermore, the weighted decay time constant (τD) of sIPSCs increased slightly but significantly after α5ia application while the mean 10–90% rise time showed no increase in either WT or Fmr1 KO cells (Fig. 3D; Table 1). In general our results are consistent with our previous published data (Olmos-Serrano et al. 2010; Vislay et al. 2013) indicating that Fmr1 KO BLA PNs display decreased baseline inhibitory synaptic efficacy compared with their WT counterparts (interevent interval, Fig. 3B: WT baseline vs. Fmr1 KO baseline, K-S test, P < 0.0001; amplitude, Fig. 3C: WT vs. Fmr1 KO, ANOVA with Bonferroni correction, P = 0.035). Interestingly, α5ia has the most potent effect on WT cells (frequency and amplitude of sIPSCs) versus Fmr1 KO cells (frequency only), consistent with the conclusion that Fmr1 KO PNs express fewer α5-GABAA receptors. We corroborated the specificity of α5ia effects on sIPSC properties by repeating the experiments in WT slices with another commonly utilized α5-subunit-preferring inverse agonist of the imidazobenzodiazepine class, L-655,708 (100 nM) (Atack et al. 2006; Quirk et al. 1996) This inverse agonist has less specificity and potency at α5- over α1-, α2-, and α3-GABAA receptors compared with α5ia (Atack 2010). Application of this compound in recordings using K-Gluc-based intracellular solution also produced no significant changes in holding current; however, we did observe similar decreases in sIPSC interevent interval determined by a significant leftward shift in the cumulative probability function (similar to Fig. 3B, α5ia) [WT: baseline 193.66 ± 3.89 ms (n = 3,116 events from 6 cells); L-655,708 178.49 ± 3.49 ms (n = 3457 events from 6 cells); K-S test, P < 0.01] as well as a frequency increase by pairwise comparison of mean frequency before and after L-655,708 application (WT: baseline 5.16 ± 1.08 Hz, L-655,708 5.66 ± 1.15 Hz; n = 6 cells, paired t-test, P = 0.03). In addition, amplitude of sIPSCs increased in four of six cells with L-655,708 application (data not shown). These results support a consistent effect of α5-subunit inverse agonists on sIPSC properties in BLA PNs and additionally highlight a more potent action of α5ia versus L-655,708. Therefore, we solely utilized α5ia for the rest of the study.
Table 1.
Summary of α5ia-induced changes
| Condition | Genotype | Property | Baseline | α5ia | P Value | n |
|---|---|---|---|---|---|---|
| K-Gluc | ||||||
| Wild type | 10–90% Rise time | 1.23 ± 0.07 ms | 1.27 ± 0.05 ms | 0.23 | 13 | |
| Weighted τ | 15.32 ± 0.89 | 17.62 ± 0.91 | 0.002† | |||
| Fmr1 KO | 10–90% Rise time | 1.42 ± 0.12 ms | 1.60 ± 0.11 ms | 0.11 | 6 | |
| Weighted τ | 15.98 ± 0.78 | 16.94 ± 0.97 | 0.007† | |||
| K-Gluc + TTX | Wild type | 10–90% Rise time | 1.00 ± 0.03 ms | 0.91 ± 0.03 | 0.06 | 6 |
| Weighted τ | 13.04 ± 0.88 | 13.43 ± 0.81 | 0.32 | |||
| CsCl | Wild type | 10–90% Rise time | 1.05 ± 0.12 ms | 1.20 ± 0.13 ms | 0.08 | 8 |
| Weighted τ | 17.43 ± 1.02 | 18.08 ± 0.99 | 0.02* | |||
| CsCl + TTX | Wild type | 10–90% Rise time | 1.38 ± 0.10 ms | 1.37 ± 0.11 ms | 0.53 | 9 |
| Weighted τ | 18.73 ± 0.62 | 20.32 ± 0.69 | 0.01* |
Values are means ± SE. K-Gluc, K-gluconate; KO, knockout.
Boldface values are statistically significantly different between baseline and α5ia conditions.
P < 0.05;
P < 0.01.
Pre- and/or postsynaptic actions of α5-GABAA receptors could underlie the rapid increase in inhibitory synaptic efficacy (frequency and amplitude) following reduction of α5-containing GABAA receptor activity. Since tonic conductance can modulate neuronal gain (Mitchell and Silver 2003), we reasoned that the increase in inhibitory efficacy may have resulted from the removal of an α5-GABAA receptor-mediated conductance that unmasked more distal synaptic events by increasing detectability of our recordings. To test the possibility that blockade of α5-containing receptors uncovers more distally originating synaptic events, we repeated sIPSC recordings in the absence of exogenous GABA, using a CsCl-based internal recording solution in our recording pipettes (Fig. 4) instead of potassium-based (K-Gluc) solution as utilized above (Fig. 3) to reduce potassium currents that filter current changes originating far from the recording site (Stuart and Spruston 1998). By observing a more complete sample of synaptic events including those that originate farther from the recording site under baseline conditions with CsCl recording solution, any effect of α5-GABAA receptors revealed by application of α5ia should be reduced.
As expected, after α5-GABAA receptor blockade the increase in efficacy previously observed with K-Gluc intracellular solution was partially occluded (Fig. 4). Rather than an increase in frequency, we observed a slight decrease in frequency [Fig. 4A; interevent interval: baseline 55.27 ± 0.40 ms (n = 14,293 events from 8 cells), α5ia 61.19 ± 0.46 ms (n = 14,150 events from 8 cells); K-S test, P < 0.0001] and no significant change in sIPSC amplitude (Fig. 4B, left; amplitude: baseline 34.13 ± 2.99 pA, α5ia 33.41 ± 2.56 pA; n = 8 cells, paired t-test, P = 0.29). However, there was still a slight increase in sIPSC τD with α5-GABAA receptor blockade (Fig. 4C, left; Table 1), with no significant change in event 10–90% rise time. Furthermore, unlike cells recorded with K-Gluc intracellular solution (Fig. 3), α5-GABAA receptor blockade was also accompanied by decreases in tonic current in six of eight cells. As expected, these currents were smaller than those observed with CsCl and exogenous saturating GABA (Fig. 2) [CsCl + 5 μM GABA 0.04 ± 0.020 pA/pF (n = 12), CsCl + native GABA 0.02 ± 0.005 pA/pF (n = 6); t-test, P = 0.03]. The presence of α5-subunit-mediated tonic currents in CsCl recordings with only native GABA levels suggests that lack of exogenous GABA in our K-Gluc recordings (Fig. 3) did not contribute to our observation of no α5-GABAA receptor-mediated tonic currents in those recordings. Instead, the results are consistent with an α5-subunit-mediated tonic conductance that regulates neuronal membrane normally electrically out of reach of the K-Gluc intracellular recordings.
It is unlikely that presynaptic mechanisms are involved in the observed increase in inhibitory efficacy following α5-GABAA blockade (Fig. 3), as simply replacing pipette potassium with cesium can prevent frequency increases in response to α5-GABAA receptor blockade (Fig. 4). However, the possibility remains that an increase in the excitability of presynaptic interneurons may be induced by α5-GABAA receptor blockade if these cells are heavily modulated by an α5-specific tonic conductance. Reduction of that conductance might thereby increase the sIPSC frequency on PNs via an increase in presynaptic APs and GABA release. To test for this possibility we recorded mIPSCs with the CsCl-based recording solution in the presence of TTX (1 μM) to block APs. AP-independent mIPSCs showed a slight decrease in frequency rather than an increase similar to sIPSCs recorded with CsCl [Fig. 4A; interevent interval: baseline 96.71 ± 1.31 ms (6,304 events from 9 cells); α5ia 108.80 ± 1.61 ms (6,219 events from 9 cells); K-S test, P = 0.0006]. Under these conditions we also observed a slight increase in amplitude (Fig. 4B; amplitude: baseline 31.05 ± 2.51 pA, α5ia 33.22 ± 3.06 pA; n = 9, paired t-test, P = 0.027), an increase in τD (Fig. 4C; Table 1), and no change in event 10–90% rise time. We subsequently recorded mIPSCs with the K-Gluc-based solution to record primarily more proximal somatic synapses. In these recording conditions we observed no significant change in frequency [interevent interval: baseline 155.36 ± 2.47 ms (n = 4,668 events from 6 cells); α5ia 151.68 ± 2.31 ms (n = 4,809 events from 6 cells); K-S test, P = 0.159] and no significant change in amplitude (amplitude: baseline 21.69 ± 1.22 pA, α5ia 21.10 ± 1.05 pA; n = 6 cells, paired t-test, P = 0.94), τD, or event 10–90% rise time (Table 1) after the application of α5ia to block α5-GABAA receptors. Collectively, these results suggest that α5-GABAA receptors in BLA PNs have a functional preference to modulate postsynaptic events more distal from the soma than those typically recorded with K-Gluc intracellular solution.
α5-GABAA receptors affect evoked response kinetics and synaptic balance in BLA PNs.
BLA PNs receive a variety of direct afferent excitatory inputs from hippocampus and frontal cortex traveling within the external capsule (McDonald and Mascagni 1996; Ottersen 1982) as well as projections from output neurons in the lateral nucleus of the amygdala that relay sensory cortical and thalamic input to the BLA (Krettek and Price 1978). BLA PNs also receive a variety of heterogeneous feedforward and feedback inhibitory inputs from a diverse pool of interneurons that preferentially synapse in the perisomatic, proximal dendritic, or distal dendritic compartments of these cells (Manko et al. 2012; McDonald et al. 2005; Muller et al. 2007). The distinct ability of α5-GABAA receptors to regulate more distal synaptic efficacy in BLA PNs places the receptor subtype in a position to perhaps modulate integration of excitatory and inhibitory inputs locally on dendrites and subsequently affect summation at the soma and axon initial segment. Therefore, we examined whether α5-GABAA receptor-mediated conductance affects the integration of excitatory and inhibitory synaptic transmission in BLA PNs. We investigated this possibility by measuring evoked synaptic conductance in the presence or absence of α5-GABAA receptor activity in both WT and Fmr1 KO amygdala slices (Fig. 5). Synaptic currents were evoked in BLA PNs at three different holding potentials in voltage clamp (−20 mV, −45 mV, −70 mV) by stimulating the external capsule at 4 times the threshold to evoke an inward excitatory current recorded at −70 mV (the reversal potential for Cl−1) (Fig. 5, A and B, insets). This stimulus strength was used because it reliably evoked strong outward inhibitory currents when the cell was held at −20 mV or above. Current thresholds for WT and Fmr1 KO slices were not significantly different from each other [WT: 19.1 ± 1.00 μV (n = 16), Fmr1 KO: 21.7 ± 1.76 μV (n = 18); P = 0.22]. Total synaptic (Gtot), excitatory (Ge), and inhibitory (Gi) conductance were derived from I/V curves taken from average traces at each holding potential (7–15 sweeps/potential) with established methods (see methods) (Fig. 5, A and B, insets). Gtot was large with a fast onset (∼3 ms; Fig. 5G) and slow latency to peak (∼11–14 ms) in both WT and Fmr1 KO slices. Ge and Gi onset occurred within 1–2 ms of each other (Fig. 5, A and B), indicating that stimulated external capsule afferents monosynaptically innervate PNs and GABAergic interneurons of the BLA. The conductance profile corresponds well with analogous feedforward circuits such as those in layer 4 somatosensory cortex (thalamocortical) and hippocampal area CA1 (Cruikshank et al. 2007; Gabernet et al. 2005; Pouille and Scanziani 2001), with the exception of a longer overall latency to peak conductance (11–14 ms vs. 5–7 ms) and longer decay times (100–200 ms vs. 40–60 ms) despite similar stimulus strength (∼40–120 μA, 100–200 μS). Extensive BLA-specific reverberant synaptic connections in PNs (Smith and Paré 1994) reinforced by our strong stimulus together with lower experimental temperatures (26–28°C vs. 32°C) may support this uniquely longer time course by recruiting a relatively larger summation of feedforward excitatory/inhibitory transmission.
Since overall inhibitory synaptic efficacy is decreased in Fmr1 KO PNs (Olmos-Serrano et al. 2010), we expected that total evoked synaptic E/I balance in Fmr1 KO slices might be increased. However, surprisingly, we instead detected no significant differences in peak levels of Gtot, Ge, or Gi between WT and Fmr1 KOs (Fig. 5C, Table 2; reported as conductance density to control for differences in cell size/capacitance, nS/pF; WT: n = 9; Fmr1 KO: n = 11). In addition, overall E/I balance as measured by the ratio of Ge to Gi over time (Fig. 5, A, B, and D) was also unchanged between WT and Fmr1 KOs {Area E/Area I (integral) = [Ge(nS)/time(ms)]/[Gi(nS)/time(ms)]}.
Table 2.
Total conductance and excitatory/inhibitory components are not significantly different between wild-type and Fmr1 KO slices and α5ia does not affect conductance in either genotype
| Property | Genotype | Condition | Conductance Density, nS/pF | P Value vs. Condition | P Value vs. Genotype |
|---|---|---|---|---|---|
| Gtot | Wild type | Baseline | 0.24 ± 0.06 | 0.64 | |
| α5ia | 0.21 ± 0.04 | ||||
| Fmr1 KO | Baseline | 0.18 ± 0.03 | 0.15 | 0.36 | |
| α5ia | 0.31 ± 0.09 | 0.60 | |||
| Ge | Wild type | Baseline | 0.08 ± 0.02 | 0.47 | |
| α5ia | 0.05 ± 0.01 | ||||
| Fmr1 KO | Baseline | 0.05 ± 0.01 | 0.15 | 0.22 | |
| α5ia | 0.08 ± 0.03 | 0.35 | |||
| Gi | Wild type | Baseline | 0.17 ± 0.04 | 0.70 | |
| α5ia | 0.15 ± 0.03 | ||||
| Fmr1 KO | Baseline | 0.14 ± 0.02 | 0.13 | 0.39 | |
| α5ia | 0.22 ± 0.06 | 0.31 | |||
| Ge/Gi | Wild type | Baseline | 0.29 ± 0.01 | 0.83 | |
| α5ia | 0.28 ± 0.02 | ||||
| Fmr1 KO | Baseline | 0.29 ± 0.04 | 0.75 | 0.84 | |
| α5ia | 0.31 ± 0.02 | 0.31 |
Values are means ± SE.
Gtot, total conductance; Ge, excitatory conductance; Gi, inhibitory conductance.
Interestingly, instead of total evoked conductance and overall synaptic E/I balance differences we observed a striking difference between genotypes in the kinetics of the evoked responses. Evoked conductance in Fmr1 KO PNs showed a significant decrease in the time between peak Ge and peak Gi compared with WT PNs (Fig. 5, Aii, Bii, and E; Gi peak time–Ge peak time: WT baseline 2.41 ± 0.54 ms, Fmr1 KO baseline 1.19 ± 0.28 ms; ANOVA, P = 0.023). The decreased Ge peak-to-Gi peak time was associated with significantly decreased E/I conductance ratio at the Ge peak in Fmr1 KO PNs compared with WT (Fig. 5F; WT baseline 0.48 ± 0.05, Fmr1 KO baseline 0.32 ± 0.02; ANOVA, P = 0.005) consistent with a decreased window for synaptic integration in these Fmr1 KO cells (Isaacson and Scanziani 2011; Pouille and Scanziani 2001). We then investigated the underlying cause of this alteration in synaptic timing by determining the conductance onset and peak latencies of each component conductance (Ge and Gi). We determined that the difference in Ge and Gi response kinetics correlated with a significantly decreased Ge peak latency in WT PNs compared with Fmr1 KO PNs (Fig. 5G; WT baseline 11.72 ± 0.73 ms, Fmr1 KO baseline 14.09 ± 1.06 ms; ANOVA, P = 0.048) but without a difference in the latency of Gi (Fig. 5G; Gi latency: WT baseline 14.22 ± 0.58 ms, Fmr1 KO baseline 15.22 ± 0.99 ms; ANOVA, P = 0.38). Therefore, these data suggest that differences in kinetics in WT and Fmr1 KO slices depend solely on the faster latency of Ge (faster rise time) in WT PNs and not on Gi latency. The increased Ge kinetics result in an increased window for cell responsiveness in WT PNs in which there is less overlap of excitation and inhibition compared with Fmr1 KO PNs, resulting in an increase in the E/I ratio at the Ge peak for WT neurons (Fig. 5F).
To determine whether this difference in Ge kinetics and altered E/I timing could be related to the reduction and/or loss of α5-GABAA receptors, we next examined evoked synaptic conductance in a separate set of cells in the absence of α5-GABAA receptor activity. If α5-GABAA receptors heavily regulate synaptic efficacy and integration (Figs. 3 and 4), these receptors might underlie the kinetic differences, providing a tonic conductance to maintain local membrane resistance and ensure faster evoked synaptic kinetics, thereby controlling the spread of synaptic activity. In recordings from both WT and Fmr1 KO slices we bath-applied α5ia (1.5 μM) to the bath solution after threshold stimulation levels were established and then assessed evoked synaptic conductance as described above. The threshold stimulus did not significantly change for either group after application of α5ia [WT before: 21.1 ± 1.76 μV, WT after α5ia: 22.2 ± 1.31 (n = 7; paired t-test, P = 0.66); Fmr1 KO before: 18.1 ± 2.85 μV, Fmr1 KO after α5ia: 18.7 ± 2.97 μV (n = 7; paired t-test, P = 0.89); WT vs. Fmr1 KO, ANOVA, P = 0.39]. Measurements of peak conductance density (Gtot, Ge, and Gi) did not differ significantly from WT baseline or Fmr1 KO baseline groups for either genotype in the presence of α5ia (Fig. 5C; Table 2), so α5-GABAA receptor blockade does not affect overall conductance or components of that conductance as recorded at the soma (Ge and Gi).
In WT slices, blockade of α5-GABAA receptors significantly reduced the Ge peak-to-Gi peak time compared with the WT baseline group [Fig. 5, Aii, Aiii, and E; WT α5ia: 0.67 ± 0.33 ms (n = 7 cells) vs. WT baseline, t-test, P = 0.012]. Notably, peak-to-peak times mimicked those of both the Fmr1 KO baseline and Fmr1 KO α5ia conditions [WT α5ia vs. Fmr1 KO baseline, t-test, P = 0.26; vs. Fmr1 KO α5ia: 0.86 ± 0.18 ms (n = 7 cells), t-test, P = 0.63]. Concurrently, blockade of α5-GABAA receptors in WT cells also resulted in significant reduction of the E/I conductance ratio at the Ge peak compared with the WT baseline group. Again, these values were similar to those recorded from Fmr1 KO slices in either the baseline or α5ia condition (Fig. 5F; WT α5ia: 0.31 ± 0.03 vs. WT baseline, t-test, P = 0.016, vs. Fmr1 KO baseline, t-test, P = 0.79; Fmr1 KO α5ia: 0.37 ± 0.03, t-test, P = 0.19). In addition, this reduction correlated with an increased latency to the Ge peak similar to both Fmr1 KO groups (Fig. 5G; WT α5ia: 13.8 ± 0.68 ms, vs. WT baseline, t-test, P = 0.03, vs. Fmr1 KO baseline, t-test, P = 0.84; Fmr1 KO α5ia: 13.2 ± 0.82 ms, t-test, P = 0.60) but without a change in the latency to the Gi peak (WT α5ia: 14.5 ± 0.54 ms, vs. WT baseline, t-test, P = 0.67, vs. Fmr1 KO baseline, t-test, P = 0.58; Fmr1 KO α5ia: 14.2 ± 0.79 ms, t-test, P = 0.69). Consistent with a lack of α5-GABAA receptor function in Fmr1 KO slices, blockade with α5ia did not result in any significant measureable changes compared with the Fmr1 KO baseline groups in control ACSF (Fig. 5, E–G; Ge-to-Gi peak time, t-test, P = 0.19; E/I conductance ratio at Ge peak, t-test, P = 0.08; latency to Ge peak, t-test, P = 0.29; latency to Gi peak, t-test, P = 0.43).
These data show that in WT slices α5-containing GABAA receptors are involved in controlling evoked synaptic conductance balance in BLA PNs by primarily modulating Ge latencies. Summary data from all PNs reveals that Ge latency negatively correlates with E/I balance at the Ge peak (Fig. 5H) such that longer Ge latencies in the absence of α5-GABAA receptor activity generally coincide with lower E/I ratios. In general, the Fmr1 KO PN population displays a tendency toward narrower Ge-to-Gi temporal windows associated with decreased α5-GABAA receptor expression that may imply decreased capacity for accurate input integration and plasticity in a circuit that is crucial for regulating fear and anxiety.
DISCUSSION
Tonic GABAergic inhibition is a critical regulator of cellular and network E/I balance (Farrant and Nusser 2005); however, in neurodevelopmental disorders such as FXS that are characterized by a network imbalance the role of this powerful conductance is poorly understood, particularly in key brain regions such as the amygdala. Here we demonstrated that tonic GABAergic current capacity in BLA PNs of Fmr1 KO mice is reduced independently of decreased GABA availability, a prominent characteristic in the Fmr1 KO amygdala (Olmos-Serrano et al. 2010). Furthermore, this tonic conductance consists of at least δ- and α5-GABAA receptor components, both of which are reduced in Fmr1 KO cells. Interestingly, we show that α5-GABAA receptors functionally modulate cellular compartments distal from the soma. In addition, although overall E/I synaptic conductance balance appears to be maintained in Fmr1 KO slices in response to external capsule stimulation, the window of peak excitation to peak inhibition generated by feedforward inhibition is significantly narrowed compared with WT. Finally, deactivation of an α5-GABAA receptor-mediated conductance in WT slices tightens E/I timing to Fmr1 KO levels, indicating that this tonic conductance can at least partially regulate synaptic E/I dynamics in BLA PNs.
Tonic GABAergic neurotransmission in BLA principal neurons.
Tonic GABAergic conductance has been observed in a number of brain regions including the cerebral cortex, hippocampus, striatum, thalamus, and cerebellum and is commonly mediated to varying degrees by both δ- and α5-GABAA receptors in a region-specific manner (Farrant and Nusser 2005). Our results show that BLA PNs utilize at least these two receptor subtypes to maintain tonic GABAergic conductance. Furthermore, the magnitude of both of these component currents is relatively small, consistent with the diffuse levels of protein expression of these subunits in the region (Fritschy and Mohler 1995). The data here, combined with recent data implicating strong tonic modulation mediated by the atypical α3-subunit-containing GABAA receptor in BLA PNs (Marowsky et al. 2012), underscore the diversity of GABAA receptor subtypes that can tonically control membrane excitability in these cells.
Recordings performed with two intracellular solutions that enable relatively strong (CsCl) and relatively weak (K-Gluc) voltage control demonstrate a functional preference of α5-GABAA receptors to affect synaptic conductance at membrane locations distal from our somatic recording site in BLA PNs. This preference implies that these receptors likely lie in proximal dendrites rather than the soma. However, these functional data only indirectly identify this subcellular location preference for α5-GABAA receptors. Ultrastructural studies comparing α5-subunit expression surrounding somatic and dendritic synapses will be required to confirm these observations. Nevertheless, the data are consistent with an established role of these receptors in analogous principal cells that express α5-GABAA receptors in other brain regions. For instance, functional and ultrastructural studies of neocortical layer 5 and hippocampal pyramidal neurons reveal a preference of these receptors to specifically modulate dendritic subcellular compartments (Ali and Thomson 2008; Christie and De Blas 2002).
α5-GABAA receptor transmission affects synaptic balance in BLA PNs.
Integration windows (Lloyd 1946), the time frame within which excitatory potentials can effectively summate to generate an AP, are influenced by the relative timing and strength of synaptic excitatory and inhibitory input and have been well established to exist in feedforward circuits especially in sensory cortices (Isaacson and Scanziani 2011). Most importantly, they determine the window of responsiveness to coincident activity in the neuron and determine the tuning of the neuron to incoming stimuli [the population of inputs to which the neuron can respond (König et al. 1996)]. The more narrow the response window (Ge to Gi), the more synchronized afferent inputs must be to generate an AP response in the neuron (Gabernet et al. 2005; Pouille and Scanziani 2001). In contrast, the broader the integration window, the less sensitive the neuron is to input timing and therefore the broader the range of inputs to which the neuron can respond and the higher the coding capacity (Poo and Isaacson 2009). In the present study we revealed that in WT BLA PNs reduction of a distally located α5-GABAA receptor-mediated tonic conductance restricted the feedforward temporal window established by a mixed evoked excitatory/inhibitory conductance. Therefore, tonic α5-GABAA receptor-mediated conductance could at least partially modulate integration of postsynaptic excitatory and inhibitory potentials at the soma in these cells. In this manner, tonic conductance might support the proper feedforward integration of various concurrent afferent inputs to BLA PN dendrites from thalamus, lateral amygdala, prefrontal cortex, and hippocampus, for example, for proper processing of conditioned fear and extinction via regulation of activity-dependent synaptic plasticity and coincidence detection (Ehrlich et al. 2009). However, future direct investigations of the integration window are necessary to determine whether changes in this α5-GABAA receptor-mediated conductance indeed affect the spike probability of BLA PNs in response to coincident inputs.
Furthermore, evidence shows that even small changes in inhibition can significantly alter E/I balance and network function (Isaacson and Scanziani 2011). Although it is well established that GABAergic inhibition generally supports a more narrow integration window and increased neuron selectivity (Wehr and Zador 2003), studies to elucidate the role of GABAergic inhibition in these processes generally block all GABAA receptors (phasic and tonic) with an antagonist such as bicuculline, which limits the interpretation of experimental results. In contrast, using blockade specifically of α5-GABAA receptors, we show that a tonic, perhaps dendritically based, conductance can function to support a broader E/I temporal window independent of the feedforward inhibitory phasic synaptic conductance (Gi), by primarily affecting Ge kinetics. Since tonic conductance modulates gain by altering membrane resistance (Mitchell and Silver 2003), the loss of α5-GABAA receptor activity could increase membrane resistance locally, thereby increasing the membrane time constant and slowing the response rise time and decay in these cells. This situation could support the increased summation of synaptic events from multiple recurrent excitatory synaptic connections (Smith and Paré 1994) to produce longer Ge latencies. Increases in τD in the presence of α5ia in our IPSC recordings further support this phenomenon.
Implications of reduced tonic GABAergic inhibition for FXS.
Our data showing compromised tonic GABA conductance in Fmr1 KO slices add to the growing evidence of E/I imbalances in FXS (Paluszkiewicz et al. 2011b) and related ASDs in general (Markram and Markram 2010; Zhang et al. 2008). As a powerful regulator of cellular excitability, the significant reduction of this conductance likely directly underlies the hyperexcitability of Fmr1 KO BLA PNs (Olmos-Serrano et al. 2010), and although it has only specifically been examined in a limited number of circuits in FXS (Curia et al. 2009), decreased tonic GABAergic conductance may in fact represent a common feature of the disorder across the FXS brain given the broad reduction of GABAergic components, including the tonically active δ-subunit (reviewed in Paluszkiewicz et al. 2011a).
Our observations of significantly tightened E/I temporal windows in Fmr1 KO BLA PNs in response to afferent input represent a novel physiological phenotype that could also result from impaired tonic GABAergic inhibition, given that reduction of α5-subunit-mediated tonic conductance in WT slices reproduces the Fmr1 KO phenotype. However, we cannot discount other cellular and network changes that may occur in the Fmr1 KO BLA that could also account for the narrow temporal window, including maturity, number, and location of excitatory and inhibitory synapses on Fmr1 KO PNs. For instance, hyperconnectivity of neocortical layer 5 pyramidal cells has been observed in these mice (Testa-Silva et al. 2012). A similar condition in the BLA would support increased summation of recurrent excitatory synaptic conductance in our experiments independent of decreased α5-GABAA receptor-mediated conductance. Alternatively, intrinsic alterations in membrane properties related to ion channelopathies could alter summation of inputs at the soma and/or integration of orthodromic and anterograde dendritic E/I currents (Brager and Johnston 2014) and modify the observed temporal window. These factors combined with our strong evoked stimulus could have similarly influenced the surprisingly unchanged total synaptic conductance and overall E/I balance we observed in Fmr1 KO slices compared with WT by reinforcing and/or amplifying recurrent connections. Also of note, although our recordings were performed at temperatures near those used in analogous examinations of feedforward circuits in acute brain slices (Cruikshank et al. 2007; Gabernet et al. 2005), they were below physiological temperature (∼37°C). Given the variable temperature sensitivity of excitatory and inhibitory synapses (Huntsman and Huguenard 2000; Postlethwaite et al. 2007), the observed differences in integration windows between genotypes could be more or less pronounced in vivo or at a higher temperature. Despite this potential caveat, our results show a clear difference in regulation of E/I inputs in Fmr1 KO PNs and a role of α5-GABAA receptors in that regulation.
Even if decreased α5-subunit expression is not the principal cause of the restricted E/I windows in Fmr1 KO PNs, augmentation of deficient tonic GABAergic conductance could still support more efficient BLA network function by increasing coding capacity (Isaacson and Scanziani 2011) and improving cellular hyperexcitability (Olmos-Serrano et al. 2010). In fact, these cellular and network phenotypes may go hand in hand in FXS since narrow integration windows could be partially overcome with increased neuron excitability to support coincidence detection within a restricted time window. Consequently, hyperexcitability could develop as a homeostatic response to narrow integration windows in FXS subsequent to abnormal synaptic development (He and Portera-Cailliau 2013) or vice versa. Given the pervasiveness of cellular hyperexcitability and network hypersynchrony in FXS networks, particularly sensory cortices (Gibson et al. 2008; Gonçalves et al. 2013), and its association with narrowed E/I temporal windows here, altered E/I synaptic integration windows may represent a hallmark of FXS networks generally. If so, sensory integration dysfunction could be explained by cellular/network hyperexcitability during developmental critical periods in FXS (Gonçalves et al. 2013) combined with decreased coding capacity to affect experience-dependent plasticity within sensory cortices as well as the proper assimilation of those networks with subcortical structures like the BLA. Enhancement of tonic GABAergic inhibition in FXS, then, may support both a normalization of coding capacity and network excitability to enhance brain function in patients with this disorder.
GRANTS
This work was supported by grants from National Institute of Neurological Disorders and Stroke (M. M. Huntsman; Grant NS-053719), the Epilepsy Foundation (B. S. Martin), Autism Speaks (M. M. Huntsman and J. G. Corbin), and the FRAXA Foundation (M. M. Huntsman and J. G. Corbin).
DISCLOSURES
M. M. Huntsman and J. G. Corbin are paid consultants of SAGE Therapeutics, Inc. (Cambridge, MA).
AUTHOR CONTRIBUTIONS
Author contributions: B.S.M., J.G.C., and M.M.H. conception and design of research; B.S.M. performed experiments; B.S.M. and M.M.H. analyzed data; B.S.M. and M.M.H. interpreted results of experiments; B.S.M. prepared figures; B.S.M. and M.M.H. drafted manuscript; B.S.M., J.G.C., and M.M.H. edited and revised manuscript; B.S.M., J.G.C., and M.M.H. approved final version of manuscript.
REFERENCES
- Adusei DC, Pacey LK, Chen D, Hampson DR. Early developmental alterations in GABAergic protein expression in fragile X knockout mice. Neuropharmacology 59: 167–171, 2010 [DOI] [PubMed] [Google Scholar]
- Ali AB, Thomson AM. Synaptic alpha5 subunit-containing GABAA receptors mediate IPSPs elicited by dendrite-preferring cells in rat neocortex. Cereb Cortex 18: 1260–1271, 2008 [DOI] [PubMed] [Google Scholar]
- Atack JR. Preclinical and clinical pharmacology of the GABAA receptor alpha5 subtype-selective inverse agonist alpha5IA. Pharmacol Ther 125: 11–26, 2010 [DOI] [PubMed] [Google Scholar]
- Atack JR, Pike A, Clarke A, Cook SM, Sohal B, McKernan RM, Dawson GR. Rat pharmacokinetics and pharmacodynamics of a sustained release formulation of the GABAA alpha5-selective compound L-655,708. Drug Metab Dispos 34: 887–893, 2006 [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci 27: 370–377, 2004 [DOI] [PubMed] [Google Scholar]
- Bonin RP, Martin LJ, MacDonald JF, Orser BA. Alpha5GABAA receptors regulate the intrinsic excitability of mouse hippocampal pyramidal neurons. J Neurophysiol 98: 2244–2254, 2007 [DOI] [PubMed] [Google Scholar]
- Brager DH, Johnston D. Channelopathies and dendritic dysfunction in fragile X syndrome. Brain Res Bull 103: 11–17, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Mody I. Extrasynaptic GABAA receptors: their function in the CNS and implications for disease. Neuron 73: 23–34, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha4beta3delta GABAA receptors. Br J Pharmacol 136: 965–974, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, De Blas AL. Alpha5 subunit-containing GABAA receptors form clusters at GABAergic synapses in hippocampal cultures. Neuroreport 13: 2355–2358, 2002 [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci 10: 462–468, 2007 [DOI] [PubMed] [Google Scholar]
- Curia G, Papouin T, Séguéla P, Avoli M. Downregulation of tonic GABAergic inhibition in a mouse model of fragile X syndrome. Cereb Cortex 19: 1515–1520, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hulst C, de Geest N, Reeve SP, van Dam D, de Deyn PP, Hassan BA, Kooy RF. Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res 1121: 238–245, 2006 [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron 62: 757–771, 2009 [DOI] [PubMed] [Google Scholar]
- El Idrissi A, Ding XH, Scalia J, Trenkner E, Brown WT, Dobkin C. Decreased GABAA receptor expression in the seizure-prone fragile X mouse. Neurosci Lett 377: 141–146, 2005 [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci 6: 215–229, 2005 [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol 359: 154–194, 1995 [DOI] [PubMed] [Google Scholar]
- Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJ, Holden JJ, Fenwick RG, Warren ST. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell 67: 1047–1058, 1991 [DOI] [PubMed] [Google Scholar]
- Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron 48: 315–327, 2005 [DOI] [PubMed] [Google Scholar]
- Gantois I, Vandesompele J, Speleman F, Reyniers E, D'Hooge R, Severijnen LA, Willemsen R, Tassone F, Kooy RF. Expression profiling suggests underexpression of the GABAA receptor subunit delta in the fragile X knockout mouse model. Neurobiol Dis 21: 346–357, 2006 [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol 17: 103–111, 2007 [DOI] [PubMed] [Google Scholar]
- Gibson JR, Bartley AF, Hays SA, Huber KM. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J Neurophysiol 100: 2615–2626, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mody I. The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J Physiol 582: 1163–1178, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves JT, Anstey JE, Golshani P, Portera-Cailliau C. Circuit level defects in the developing neocortex of Fragile X mice. Nat Neurosci 16: 903–909, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, Kronk R, Delahunty C, Hessl D, Visootsak J, Picker J, Gane L, Tranfaglia M. Advances in the treatment of fragile X syndrome. Pediatrics 123: 378–390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays SA, Huber KM, Gibson JR. Altered neocortical rhythmic activity states in Fmr1 KO mice are due to enhanced mGluR5 signaling and involve changes in excitatory circuitry. J Neurosci 31: 14223–14234, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CX, Portera-Cailliau C. The trouble with spines in fragile X syndrome: density, maturity and plasticity. Neuroscience 251: 120–128, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntsman MM, Huguenard JR. Nucleus-specific differences in GABAA-receptor-mediated inhibition are enhanced during thalamic development. J Neurophysiol 83: 350–358, 2000 [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron 72: 231–243, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- König P, Engel AK, Singer W. Integrator or coincidence detector? The role of the cortical neuron revisited. Trends Neurosci 19: 130–137, 1996 [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol 178: 255–280, 1978 [DOI] [PubMed] [Google Scholar]
- Krook-Magnuson EI, Li P, Paluszkiewicz SM, Huntsman MM. Tonically active inhibition selectively controls feedforward circuits in mouse barrel cortex. J Neurophysiol 100: 932–944, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DP. Facilitation and inhibition of spinal motoneurons. J Neurophysiol 9: 421–438, 1946 [DOI] [PubMed] [Google Scholar]
- Manko M, Bienvenu TC, Dalezios Y, Capogna M. Neurogliaform cells of amygdala: a source of slow phasic inhibition in the basolateral complex. J Physiol 590: 5611–5627, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram K, Markram H. The intense world theory—a unifying theory of the neurobiology of autism. Front Hum Neurosci 4: 224, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marowsky A, Rudolph U, Fritschy JM, Arand M. Tonic inhibition in principal cells of the amygdala: a central role for alpha3 subunit-containing GABAA receptors. J Neurosci 32: 8611–8619, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Zurek AA, MacDonald JF, Roder JC, Jackson MF, Orser BA. Alpha5GABAA receptor activity sets the threshold for long-term potentiation and constrains hippocampus-dependent memory. J Neurosci 30: 5269–5282, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Cortico-cortical and cortico-amygdaloid projections of the rat occipital cortex: a Phaseolus vulgaris leucoagglutinin study. Neuroscience 71: 37–54, 1996 [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Mania I, Rainnie DG. Evidence for a perisomatic innervation of parvalbumin-containing interneurons by individual pyramidal cells in the basolateral amygdala. Brain Res 1035: 32–40, 2005 [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, Andersen P. Synaptic efficacy and EPSP summation in granule cells of rat fascia dentata studied in vitro. J Neurophysiol 46: 952–966, 1981 [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron 38: 433–445, 2003 [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Postsynaptic targets of somatostatin-containing interneurons in the rat basolateral amygdala. J Comp Neurol 500: 513–529, 2007 [DOI] [PubMed] [Google Scholar]
- Nicoll A, Larkman A, Blakemore C. Modulation of EPSP shape and efficacy by intrinsic membrane conductances in rat neocortical pyramidal neurons in vitro. J Physiol 468: 693–710, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos-Serrano JL, Paluszkiewicz SM, Martin BS, Kaufmann WE, Corbin JG, Huntsman MM. Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome. J Neurosci 30: 9929–9938, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen OP. Connections of the amygdala of the rat. IV. Corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. J Comp Neurol 205: 30–48, 1982 [DOI] [PubMed] [Google Scholar]
- Paluszkiewicz SM, Martin BS, Huntsman MM. Fragile X syndrome: the GABAergic system and circuit dysfunction. Dev Neurosci 33: 349–364, 2011a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluszkiewicz SM, Olmos-Serrano JL, Corbin JG, Huntsman MM. Impaired inhibitory control of cortical synchronization in fragile X syndrome. J Neurophysiol 106: 2264–2272, 2011b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101: 815–850, 2000 [DOI] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. Odor representations in olfactory cortex: “sparse” coding, global inhibition, and oscillations. Neuron 62: 850–861, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite M, Hennig MH, Steinert JR, Graham BP, Forsythe ID. Acceleration of AMPA receptor kinetics underlies temperature-dependent changes in synaptic strength at the rat calyx of Held. J Physiol 579: 69–84, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 293: 1159–1163, 2001 [DOI] [PubMed] [Google Scholar]
- Quirk K, Blurton P, Fletcher S, Leeson P, Tang F, Mellilo D, Ragan CI, McKernan RM. [3H]L-655,708, a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the alpha5 subunit. Neuropharmacology 35: 1331–1335, 1996 [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ESL, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev 83: 803–834, 2003 [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci 27: 262–269, 2004 [DOI] [PubMed] [Google Scholar]
- Smith Y, Paré D. Intra-amygdaloid projections of the lateral nucleus in the cat: PHA-L anterograde labeling combined with postembedding GABA and glutamate immunocytochemistry. J Comp Neurol 342: 232–248, 1994 [DOI] [PubMed] [Google Scholar]
- Stórustovu SÍ, Ebert B. Pharmacological characterization of agonists at delta-containing GABAA receptors: functional selectivity for extrasynaptic receptors is dependent on the absence of gamma2. J Pharmacol Exp Ther 316: 1351–1359, 2006 [DOI] [PubMed] [Google Scholar]
- Stuart G, Spruston N. Determinants of voltage attenuation in neocortical pyramidal neuron dendrites. J Neurosci 18: 3501–3510, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa-Silva G, Loebel A, Giugliano M, de Kock CP, Mansvelder HD, Meredith RM. Hyperconnectivity and slow synapses during early development of medial prefrontal cortex in a mouse model for mental retardation and autism. Cereb Cortex 22: 1333–1342, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65: 905–914, 1991 [DOI] [PubMed] [Google Scholar]
- Vislay RL, Martin BS, Olmos-Serrano JL, Kratovac S, Nelson DL, Corbin JG, Huntsman MM. Homeostatic responses fail to correct defective amygdala inhibitory circuit maturation in fragile X syndrome. J Neurosci 33: 7548–7558, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426: 442–446, 2003 [DOI] [PubMed] [Google Scholar]
- Zhang L, Alger BE. Enhanced endocannabinoid signaling elevates neuronal excitability in fragile X syndrome. J Neurosci 30: 5724–5729, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, He J, Jugloff DG, Eubanks JH. The MeCP2-null mouse hippocampus displays altered basal inhibitory rhythms and is prone to hyperexcitability. Hippocampus 18: 294–309, 2008 [DOI] [PubMed] [Google Scholar]