Abstract
The central auditory system has traditionally been divided into lemniscal and nonlemniscal pathways leading from the midbrain through the thalamus to the cortex. This view has served as an organizing principle for studying, modeling, and understanding the encoding of sound within the brain. However, there is evidence that the lemniscal pathway could be further divided into at least two subpathways, each potentially coding for sound in different ways. We investigated whether such an interpretation is supported by the spatial distribution of response features in the central nucleus of the inferior colliculus (ICC), the part of the auditory midbrain assigned to the lemniscal pathway. We recorded responses to pure tone stimuli in the ICC of ketamine-xylazine-anesthetized guinea pigs and used three-dimensional brain reconstruction techniques to map the location of the recording sites. Compared with neurons in caudal-and-medial regions within an isofrequency lamina of the ICC, neurons in rostral-and-lateral regions responded with shorter first-spike latencies with less spiking jitter, shorter durations of spiking responses, a higher proportion of spikes occurring near the onset of the stimulus, lower thresholds, and larger local field potentials with shorter latencies. Further analysis revealed two distinct clusters of response features located in either the caudal-and-medial or the rostral-and-lateral parts of the isofrequency laminae of the ICC. Thus we report substantial differences in coding properties in two regions of the ICC that are consistent with the hypothesis that the lemniscal pathway is made up of at least two distinct subpathways from the midbrain up to the cortex.
Keywords: functional organization, inferior colliculus, lemniscal, medial geniculate, auditory cortex
the ascending auditory pathway has traditionally been separated into two pathways, the lemniscal “core” pathway and the nonlemniscal pathway (Andersen et al. 1980; Ehret and Romand 1997; Rauschecker and Romanski 2011; Rouiller 1997). The lemniscal pathway includes neurons in the central nucleus of the inferior colliculus (ICC), the ventral division of the medial geniculate body (MGV), and core auditory cortex regions (ACC). Neurons within the lemniscal pathway are tonotopically organized and primarily code for auditory information. Within the ICC and MGV, the tonotopicity derives from an arrangement of isofrequency laminae, where each lamina is a two-dimensional sheet formed by the dendritic arbors of neurons that respond optimally to similar “best frequencies” (Cetas et al. 2001; Imig and Morel 1985; Malmierca et al. 1993; Winer and Schreiner 2005). In contrast, neurons within the nonlemniscal pathway include regions outside of ICC, MGV, and ACC, have poor or no tonotopic organization, code for multimodal information, and are thought to modulate lemniscal responses (Andersen et al. 1980; Ehret and Romand 1997; Lee and Sherman 2010; Malmierca and Hackett 2010; Oliver 2005; Winer and Schreiner 2010). This classical view of the central auditory system has served as an organizing principle for studying, modeling, and understanding the encoding of sound within the brain.
The lemniscal system appears to be further divided into subpathways or regions that may be coding for sound information in different ways. At the level of the midbrain, several anatomic studies have revealed spatially distinct regions or functional zones within the ICC that receive inputs from different brain stem nuclei (Brunso-Bechtold et al. 1981; Cant and Benson 2006; Loftus et al. 2004; Oliver et al. 1997; Roth et al. 1978; Shneiderman and Henkel 1987). For example, within lower frequency laminae of the ICC (<5 kHz) in cat, projections from the medial superior olivary nucleus (MSO) were more frequently located in the caudal portion of a lamina, whereas those from the lateral superior olivary nucleus (LSO) dominated in the rostral and somewhat lateral portion (Loftus et al. 2004). For higher frequency laminae, the inputs from the dorsal cochlear nucleus projected throughout the laminae, but inputs from the LSO dominated in more ventrolateral regions. There is further evidence that some of these functional zones within the ICC may be maintained as two distinct sublemniscal pathways through the MGV to the ACC. In the ICC of gerbil, the cochlear nuclei and nuclei of the lateral lemniscus were shown to project throughout the ICC, but the LSO and MSO projected predominantly to the rostral and lateral regions vs. the caudal and medial regions of the ICC (Cant and Benson 2006). These two ICC regions were shown to project to the rostral vs. caudal regions of the MGV, respectively (Cant and Benson 2007). In the cat and rat, neurons in the rostral parts of the MGV have shown to project throughout the auditory cortex, including the primary auditory cortex (A1), whereas those in the caudal MGV project to ACC regions primarily outside of A1 (Morel and Imig 1987; Polley et al. 2007; Rodrigues-Dagaeff et al. 1989; Storace et al. 2010, 2012). Although these studies have been conducted in different species, the available evidence is consistent with the hypothesis that there are two separate lemniscal pathways arising in the ICC that project to rostral vs. caudal parts of the MGV and then project differentially throughout the ACC. We shall refer to this hypothesis as the dual lemniscal pathway hypothesis.
The dual lemniscal projections from the ICC up to the ACC are further supported by electrophysiological studies suggesting that a “caudal” pathway exhibits weaker and less temporally precise activation than a “rostral” pathway. In the guinea pig, electrical stimulation of the caudal-and-medial regions compared with the rostral-and-lateral regions along an isofrequency lamina of the ICC (note that hyphens refer to locations along an ICC lamina) required higher current levels for activating A1 and elicited weaker spiking and local field potential (LFP) magnitudes, longer latencies, greater spiking jitter, and larger discriminable level steps (Lim and Anderson 2007). In the cat, neurons responding to acoustic stimuli in caudal MGV compared with rostral MGV exhibited weaker excitatory activation, less precise time-locking to click trains, longer latencies, greater spiking jitter, and less strict tonotopic organization with wider tuning curves (Rodrigues-Dagaeff et al. 1989). Consistent with these results, the caudal MGV has been shown to project mainly to ACC regions outside of A1 that can exhibit responses with longer latencies, greater spiking jitter, less excitatory activity, and less precise tonotopic organization compared with A1, which receives its inputs primarily from the rostral MGV (Morel and Imig 1987; Polley et al. 2007; Rodrigues-Dagaeff et al. 1989; Schreiner et al. 2011; Storace et al. 2010, 2012).
Although the anatomic and physiological evidence cited above supports a dual lemniscal organization, the hypothesis would be strengthened by evidence for a functional division of acoustic response properties within the ICC that correspond to the anatomic divisions shown within the ICC. A number of previous studies of the ICC have demonstrated regional differences in response properties such as best modulation frequency, threshold, and latency (Hage and Ehret 2003; Hattori and Suga 1997; Langner et al. 2002; Portfors and Wenstrup 2001; Schreiner and Langner 1988; Stiebler 1986). However, these functional maps have varied between different species, properties, and studies, and none have matched the expected anatomic locations described above for the dual lemniscal pathway hypothesis.
In this article we present a systematic mapping study within the ICC to further investigate whether maps of temporal response properties align with the dual lemniscal pathways as previously defined by anatomic projection studies. We recorded acoustic responses to pure tones throughout and up to the borders of the ICC laminae using multisite arrays and detailed histological and computational reconstruction techniques. We then analyzed a wide range of response features based on LFPs and multiunit spiking activity. For multiple response features, we observed obvious and substantial differences between the caudal-and-medial vs. the rostral-and-lateral regions along an ICC lamina, consistent with the expected dual lemniscal organization. These results provide further support for the hypothesis that distinct pathways project from the ICC up to MGV and ACC, and that these pathways code for sound information differently even at the level of the ICC.
MATERIALS AND METHODS
Neural responses were recorded in the ICC of 12 anesthetized guinea pigs with the use of a 4-shank, 32-site electrode array. The array was inserted into multiple locations in each experiment. We used histological and computational reconstruction techniques to ensure that we fully sampled locations throughout and up to the borders of a given isofrequency lamina. We then analyzed a wide range of response features based on LFPs and spikes, and mapped changes in these responses with location along an ICC lamina.
Surgery
Basic surgical procedures and methods for neural recording and acoustic stimulation were similar to those presented in previous work (Lim and Anderson 2006; Straka et al. 2013). Ketamine-xylazine-anesthetized guinea pigs were used according to protocols approved by the University of Minnesota's Institutional Animal Care and Use Committee. Experiments were performed on 12 male and female Hartley guinea pigs (391 ± 57 g; Elm Hill Breeding Labs, Chelmsford, MA). Animals were initially anesthetized with an intramuscular injection of ketamine (40 mg/kg) and xylazine (10 mg/kg) and were given periodic supplements to maintain an areflexive state. Atropine sulfate (0.05 mg/kg) was periodically administered via an intramuscular injection to reduce bronchial secretion. A warm water heating blanket was used to maintain the body temperature at 38 ± 0.5°C, and temperature was monitored with a rectal thermometer. The heart rate and blood oxygen level were also monitored with a pulse oximeter.
After placing the animal into a stereotaxic frame (David Kopf Instruments, Tujunga, CA), we exposed the right side of the cortex from the caudal end of the occipital lobe to the caudal end of the temporal lobe. The dura was removed, and micromanipulators were used to insert a silicon-substrate, 32-site electrode array (NeuroNexus Technologies, Ann Arbor, MI) into the ICC. The array consisted of four 8-mm-long shanks separated by 500 μm. Sites were linearly spaced at 100 μm along the shank (8 sites per shank), and each had an area of about 700 μm2. The array was placed at a 45° angle to the sagittal plane through the visual cortex into the inferior colliculus in order to be aligned along the tonotopic axis of the ICC (Malmierca et al. 1995; Snyder et al. 2004). Array placements were confirmed with acoustically driven responses (Lim and Anderson 2007; Snyder et al. 2004), and the exposed brain was covered with agarose gel.
Recording Setup
All experiments were performed in an acoustically and electrically shielded chamber and controlled by a computer interfaced with TDT System 3 hardware (Tucker-Davis Technology, Alachua, FL) using custom software written in MATLAB (The MathWorks, Natick, MA). Sound was presented via a speaker coupled to the left ear through a hollow ear bar. The speaker-ear bar system was calibrated using a 0.25-in. condenser microphone (ACO Pacific, Belmont, CA) connected to the ear bar via a short plastic tube in place of the ear canal.
All neural signals were passed through analog DC-blocking and anti-aliasing filters from 1.6 Hz to 7.5 kHz. The sampling frequency used for acoustic stimulation was 195 kHz, and that used for neural recording was 24 kHz. The recording ground needle was positioned directly in the brain in the parietal lobe.
Placement of Array
To guide the array placement, various levels of pure tones and broadband noise (50 ms in duration with 5-ms and 0.5-ms rise-fall ramp times, respectively) were presented in the left ear to elicit acoustically driven activity in the contralateral ICC. Poststimulus time histograms (PSTHs) and frequency-response maps (FRMs) were plotted online to confirm the array's position along the tonotopic axis of the ICC. All FRMs and responses to pure tones were later analyzed offline. Details on these analysis methods and example plots for similar types of arrays are presented in previous publications (Lenarz et al. 2006; Lim and Anderson 2006). Briefly, we bandpass filtered the neural signals (300–3,000 Hz) on each site and detected spikes that exceeded a threshold of three standard deviations (SD) above the background activity. To create FRMs, four trials were presented for each pure tone (1–40 kHz, 8 steps/octave) and level (0–70 dB, 10 dB steps) combination in a randomized sequence. The best frequency (BF) was taken as the centroid of frequencies at 10 dB above the visually determined threshold.
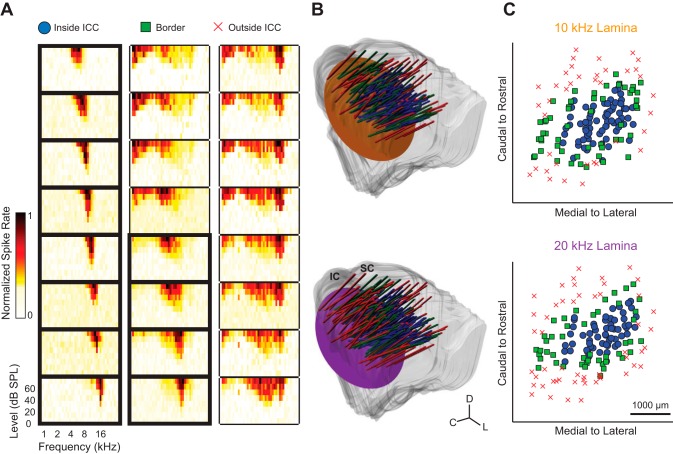
As shown in Fig. 1A, FRMs for each site were found to determine the frequency tuning properties for each neural population. To record from both the 10- and 20-kHz BF-matched neurons for each placement, the depth of the array was adjusted for each stimulus. For each depth, FRMs were acquired and placements were classified as inside, on the border, or outside the ICC. Placement in the ICC was defined by observing FRMs that exhibited an orderly shift from low to high BFs for superficial to deeper sites, respectively, along a shank (Lim and Anderson 2006; Snyder et al. 2004). Shanks were labeled “border” if the FRMs showed broad tuning with orderly shifts or if a neighboring shank (which was simultaneously recorded) was outside the ICC. In addition, shanks were designated as a border position if the sites along the shank showed tonotopic shifts for only four to six consecutive sites, one being the BF-matched site to the stimulus. Shanks were determined to be outside the ICC if the tonotopic shift of increasing BFs with deeper sites was not present.
Fig. 1.
Histological reconstructions and physiological responses were used to determine recording locations and delineate borders in the central nucleus of the inferior colliculus (ICC). A: each placement was determined to be inside the ICC, on the border, or outside the ICC based on frequency-response maps (FRMs) recorded on a single-array shank, which had 8 sites spanning different frequency layers. Placements were considered to be inside the ICC when shallow to deep sites along the shank systematically showed low to high best frequencies (BFs), respectively. Placements were considered to be on the border of the ICC if there was only a partial BF shift across the shank, as shown, or if a neighboring shank within the same array placement was outside the ICC. Placements that did not show any systematic BF shift were considered to be outside the ICC. The FRMs enclosed by bold lines were sites considered to be inside the ICC, and of these sites, only those with a BF of 10 or 20 kHz were further analyzed. B: the midbrains and array placements were reconstructed in 3 dimensions and normalized onto a single brain (see materials and methods, Histology and midbrain reconstructions, for details). For each midbrain reconstruction, individual slices were traced and then aligned. The midbrain surface was then reconstructed by creating a mesh over all slices, where the deviations on the surface reflect differences across slices due to minor misalignments. The array placements (cylinders) were determined by calculating the average trajectory of placement locations observed across relevant slices and are extended above the inferior colliculus (IC) for visualization. The 10- and 20-kHz isofrequency laminae (orange and purple planes, respectively) were approximated by planes at depths corresponding to neurons with those respective BFs. C, caudal; D, dorsal; L, lateral; SC, superior colliculus. C: the location of each recording site was determined across the isofrequency laminae. Symbols are defined in A.
Acoustic Stimuli
Two durations of pure tones at 10 or 20 kHz were each presented 20 times at varying levels in 2-dB increments. In all 12 animals, we presented short tones, which were 5 periods of a stimulus (i.e., 0.5 or 1 ms for 10 and 20 kHz, respectively) with a 2.5-period cos2 up and down ramps. In four of these animals, we also presented 50-ms-long tones, with 5-ms cos2 up and down ramps. The longer stimulus was chosen so that we could compare the results of our short tones with those of long tones presented in literature. Both the 10- and 20-kHz stimuli were randomly presented across varying level and stimulus durations, at a rate of 2/s. The stimuli were presented at levels of up to 80 dB SPL, although this upper limit could be lower for different placements in which we already observed strong or saturating activity at lower levels. For each shank in the ICC, we analyzed the site that responded with a BF closest to the frequency of the stimulus presented. For the 10-kHz stimuli, the average BF was 10 ± 1 kHz (mean ± SD) for short tones (n = 119 sites) and long tones (n = 54). For the 20-kHz, the average BF was 19 ± 1 kHz for short tones (n = 99) and long tones (n = 52). An ICC isofrequency lamina has a bandwidth of ∼0.28 octave in cat (Schreiner and Langner 1997) and 0.29 octave in rat (Malmierca et al. 2008). For all of the stimuli, our bandwidths of 0.15 octave SD for 10 kHz and 0.11 octave SD for 20 kHz were much smaller than the expected bandwidths of each isofrequency lamina (i.e., 0.28 or 0.29 octave) and support the conclusion that the BF-matched sites were within a given 10- or 20-kHz lamina.
Histology and Midbrain Reconstructions
Before placement, the array was dipped into a red dye (DiI: 1,1-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; Sigma-Aldrich, St. Louis, MO) to enable later identification of the array locations across the ICC during histological analysis. Detailed description of the histological procedure, midbrain reconstruction, normalization, and approximation of frequency lamina is provided in a previous publication (Markovitz et al. 2012). Briefly, the midbrain was fixed by a diffusion bath in 3.7% paraformaldehyde and cut into sagittal sections at 60 μm using a sliding microtome (Leica, Buffalo Grove, IL). Images of each slice were taken using a Leica MZ FLIII fluorescence stereomicroscope (Buffalo Grove, IL), Leica DFC412 C Peltier-cooled charge-coupled device camera, and Image-Pro software (MediaCybernetics, Bethesda, MD). A single-reflection white light image was taken using a variable-intensity fiber-optic light source (Fiber-Lit-PL800; Dolan-Jenner Industries, Boxborough, MA) to determine the outline of each slice. Fluorescence images were later superimposed on the white light images for visualization of the reference and array shank points stained with DiI. The brain was then reconstructed in three dimensions using Rhinoceros (Seattle, WA), and the positions of the arrays were estimated by creating best-fit lines through the points on individual slices.
The reconstructions for each brain were then normalized to one standard using the curvature of the IC and a reference needle point placed at the intersection of the superior colliculus, thalamus, and lateral extension from the IC. These anatomic features have been shown to be reliable and consistent for normalizing brains across animals in previous studies from our laboratory (Markovitz et al. 2012, 2013). The laminae were approximated by creating a plane orthogonal to the average insertion angle of all best-fit lines of the array placements. To determine the depth of each lamina, we calculated the distance from the surface of the IC, where neurons do not respond to broadband noise, to locations where neurons respond with specific BFs from previously published data (Markovitz et al. 2012). This distance was multiplied by a scaling factor to account for tissue changes due to the histological process. Although the true frequency laminae are curved throughout the ICC (Malmierca et al. 1995, 2008), the approximation of the lamina as a flat plane was necessary for the analysis of how responses vary across each lamina. Thus the locations along a lamina are a projection of points onto this plane.
The reconstructions of each array placement within the normalized brain, as well as the 10- and 20-kHz laminae, can be seen in Fig. 1B. For the shanks that were inside or on the border of the ICC (Fig. 1C), sites with similar BFs to the stimulus were analyzed for the different LFP and spiking properties. Although large vasculature on the surface of the visual cortex impeded our ability to insert the arrays more medially than shown in Fig. 1C, the FRMs indicated that our most medial sites were located at the border of the ICC.
Data Analysis
Local field potential activity.
LFPs were obtained by averaging (across 20 trials) the neural signals recorded on one site. LFP threshold was determined by finding a response that was 3 SD above the background activity, which was taken from the 40-ms window preceding the acoustic stimulus of pre-averaged data. The time of the averaged LFP peak (i.e., for the minimum point of the negative peak) was calculated at the lowest level that elicited an LFP peak at threshold. The magnitude of the negative averaged LFP peak, which was subtracted from the baseline, was determined at a level 4 dB above the threshold for each site. We found these criteria to be the minimum stimulation levels that were not strongly influenced by noise. The most caudal-and-medial locations of the ICC usually did not exhibit activity that surpassed these criteria even up to our maximum level of 80 dB SPL. Despite this limitation, similar trends in response properties were still observed across ICC locations.
Spiking activity.
Spikes were detected offline using the same online method described above. The window length of the PSTH was then visually assessed by estimating the start and end times. A signal detection theory (SDT) paradigm was used to calculate d′, which describes the separation between the driven and spontaneous activity distributions in the units of the standard deviation of the spontaneous activity distribution obtained for the 40-ms window preceding the acoustic stimulus (Britten et al. 1992; Green and Swets 1966). All spiking parameters, including PSTH duration, were calculated at the lowest level that elicited a spiking response of d′ = 1 and d′ = 3, which we refer to as threshold and suprathreshold, respectively, throughout this article.
During our analysis, it was evident that PSTHs had different shapes along with different durations, especially for long tone stimuli. To quantify these shapes, we created a parameter called “halftime.” The halftime is the time required for half of the total spikes within the PSTH to occur. Therefore, a short halftime is indicative that the majority of the spikes occur at the beginning of the PSTH (i.e., with a predominant onset shape), whereas a long halftime means that the majority of spikes occur later in the PSTH (i.e., with a predominant sustained shape).
The first spike latency (FSL) and FSL jitter were calculated by computing the mean and SD, respectively, of the first spike latencies across all 20 trials. The minimum latency for a given trial was at least 4 ms after the onset of the acoustic tone, which was the shortest latency previously reported for the ICC (Schreiner and Langner 1988; Syka et al. 2000). If the first spike for a given trial did not occur by the “lock-out” time, which was the end of the PSTH window, it was counted as a “miss” and that trial was not included in the analysis.
Steepest gradient axis.
Response properties were fit to the ICC locations using two-dimensional linear multiple regression analysis, in which a site's location across the lamina was a predictor of the response property. The model determined the slope parameter, which we call the steepest gradient axis (i.e., the vector of greatest increase) for each response parameter.
Clustering analysis.
To determine the location and number of clusters across an isofrequency lamina, we performed k-means clustering analysis for all spiking parameters for both thresholds (d′ = 1 and d′ = 3) in response to a stimulus. Only sites with spiking that met both threshold criteria were analyzed (i.e., the few sites that did not show a spiking response of d′ = 3 were excluded). We did not include LFP parameters because of their limited spatial resolution and nonexistent responses in some caudal-and-medial regions that would confound the clustering analysis (see results for further details). For the cluster analysis, the spiking parameters were first normalized by taking the natural log of the values. After this transformation, the variance for each parameter was similar (i.e., the largest variance for a parameter was no more than 3 times the smallest variance for a parameter included in the clustering), which is necessary to ensure similar weights across parameters (Everitt et al. 2001; SAS Institute 1999). Using only the normalized spiking parameters, we performed the k-means clustering algorithm in R (R Core Team 2013) and compared results when fitting with up to five clusters. To determine the optimum number of clusters without overfitting, we calculated the Bayesian information criterion (BIC) for each clustering fit:
where SSE is the total within-cluster sum of square error, k is the number of parameters estimated (i.e., the number of clusters multiplied by the 8 spiking parameters), and n is the number of sites. Thus the BIC calculates the fit error and includes a penalty for increasing the number of clusters, and the optimal number of clusters is indicated by the lowest BIC value.
Thresholds.
Within a single placement of the four-shank array, the thresholds of BF-matched sites on the most rostral shank to the most caudal shank in the ICC were compared. Comparisons were only made for placements with at least two shanks inside the ICC. At least one of the sites had to reach threshold activity; if the other site did not reach threshold, threshold was estimated by adding 2 dB to the highest level presented for that site. The highest stimulus level used for each placement was varied within experiments to accommodate the rising thresholds that occurred with each placement, and this allowed us to ensure that activation of most ICC neurons was sufficient for analysis. This estimated threshold did not affect our findings because it was a more conservative estimate of threshold for our analysis (i.e., the threshold for such a site would be equal to or even higher than what we estimated). Spiking thresholds were determined at threshold and suprathreshold levels, which were the lowest levels that elicited a spiking response of d′ = 1 and d′ = 3, respectively. LFP thresholds were determined at the level that elicited a response 3 SD above the background activity. The difference between the caudal shank threshold and rostral shank threshold was determined for each placement, and this difference was determined to be positive or negative. Significance across all placements was determined using the sign test, which uses the binomial test to assess the null hypothesis that there are equal numbers of positive and negative differences.
RESULTS
Case Example of Responses
As evidenced by both LFPs and PSTHs, evoked responses to pure tones varied systematically across an isofrequency lamina of the ICC. Figure 2 shows a case example, where an electrode array was placed several times within a single animal and neural activity in response to short and long 10-kHz tones at 70 dB SPL was recorded on sites within the 10-kHz lamina. Only sites that were within the ICC are shown and were analyzed.
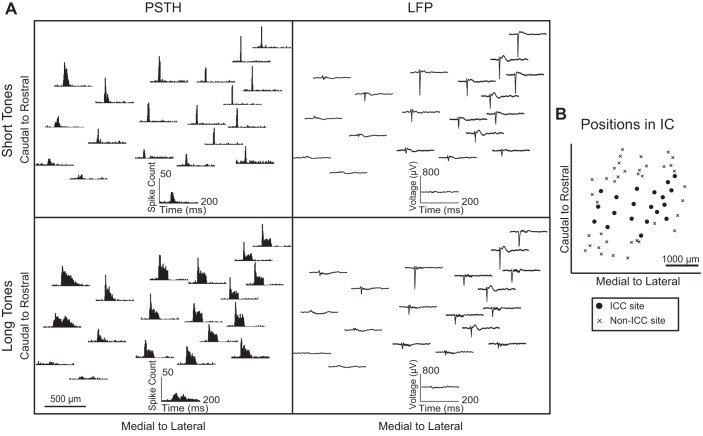
Fig. 2.
Typical responses across an isofrequency lamina. A: the poststimulus time histogram (PSTH) and local field potential (LFP) responses to 10-kHz short and long tone stimuli at 70 dB SPL were determined for each site (insets) and plotted across an ICC lamina (each panel) based on locations from histological reconstructions. All sites shown were within the ICC and were recorded in a single animal. The PSTHs of sites in the rostral-and-lateral locations had pronounced onset spiking that grew wider in caudal-and-medial regions in response to short tones. In response to long tones, most sites responded with sustained spiking, but the onset was more pronounced in rostral-and-lateral regions, whereas the sustained activity was more pronounced in the caudal-and-medial regions. In addition, sites in rostral-and-lateral regions responded with larger LFPs to both stimuli, whereas caudal-and-medial regions had weak LFPs. B: the positions of sites with responses shown in A are plotted with the non-ICC sites from Fig. 1C to show the span of sites across the lamina.
In response to the short tone stimuli, PSTHs in the rostral-and-lateral areas had a sharp onset response that grew wider in units located caudally and medially. In response to the long tone stimuli, PSTHs throughout a lamina showed sustained activity. However, in the rostral-and-lateral area, the amount of spiking in the onset portion is much larger than the sustained portion. In contrast, caudal-and-medial locations exhibit more complex and/or longer lasting responses. For both short and long tone stimuli, LFP peaks were large in the rostral-and-lateral area and grew weaker in more caudal-and-medial locations. In the most caudal-and-medial area, LFPs were small and never exceeded threshold above background activity regardless of the stimulus level. Despite the weak LFPs, these caudal-and-medial locations still exhibited strong spiking responses, and thus the inputs must not have sufficient temporal and spatial synchrony to elicit larger LFPs.
Response Parameter Maps Across the ICC Lamina
The consistent trends observed in response types encouraged us to further investigate how specific properties of the responses varied across the ICC lamina. In this analysis, neural responses at a similar activation level were analyzed for all ICC locations. For spiking, this was accomplished by using SDT to determine levels that elicited threshold and suprathreshold responses at d′ levels of 1 and 3, respectively. Several spiking parameters were analyzed, including PSTH duration, halftime, FSL, and the FSL jitter, and as shown in Fig. 3. Since we observed different PSTH shapes, we also analyzed halftime, which is the amount of time for half of the total spikes to occur. Halftime is indicative of the PSTH shape, since longer halftimes occur with stronger sustained spiking, whereas shorter halftimes occur with stronger onset spiking.
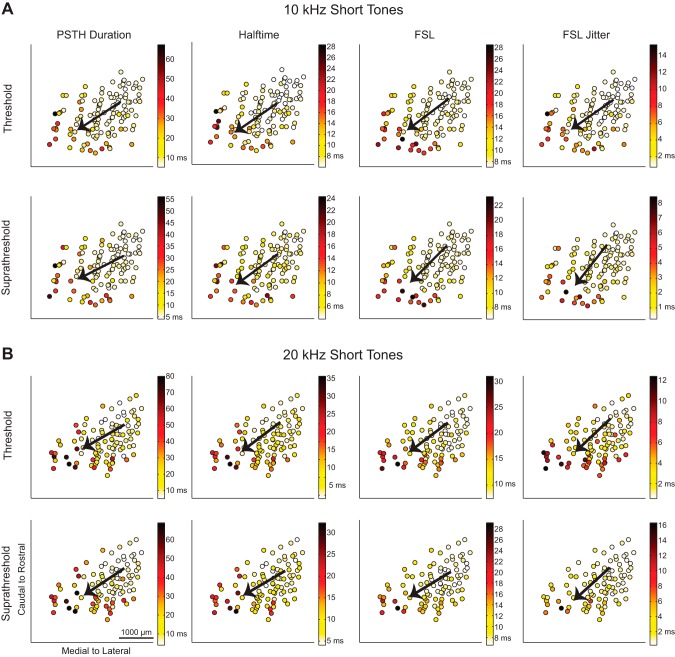
Fig. 3.
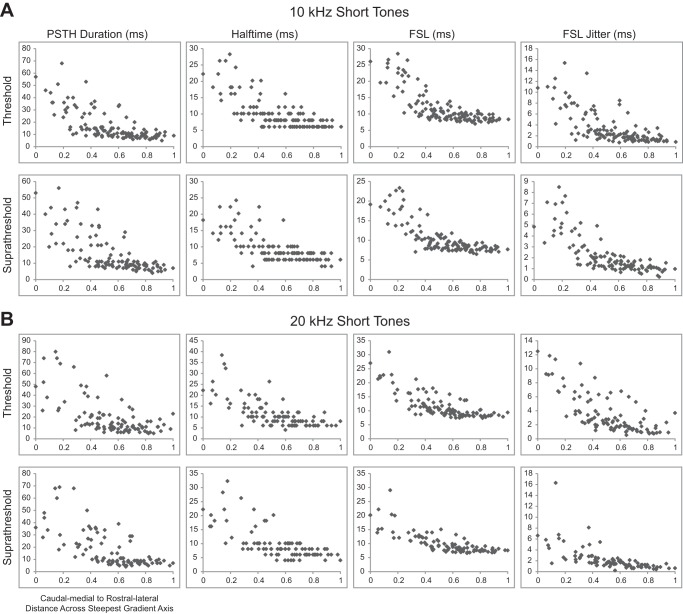
Spiking responses to short tones vary from caudomedial to rostrolateral across the lamina. The maps of spiking in response to 10-kHz (A) and 20-kHz short tones (B) show response parameters recorded at each location. Multiple regression was performed to determine the steepest gradient axis (arrows). The spiking properties of PSTH duration, halftime, first spike latency (FSL), and FSL jitter were determined for each site at threshold and suprathreshold levels. See Table 1 for details on the regression statistics and Fig. 4 for spiking response maps for the long tone stimuli.
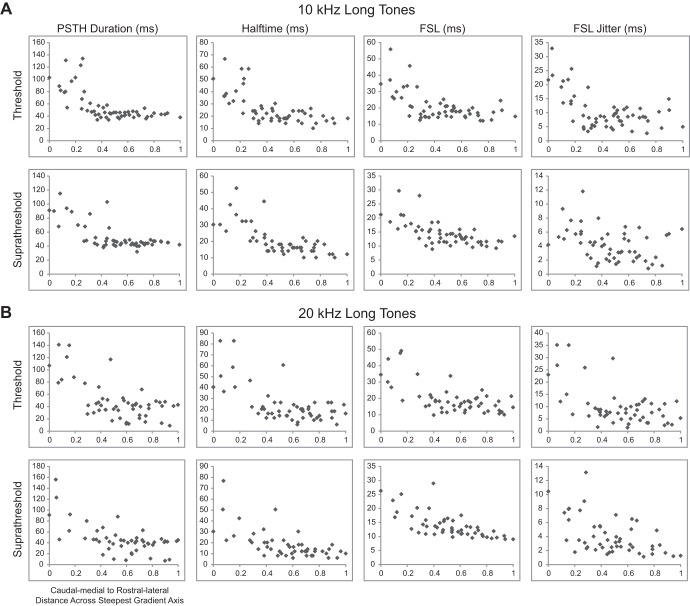
In response to both 10- and 20-kHz tones, the values decreased from large PSTH durations, halftimes, FSLs, and FSL jitters caudally and medially to smaller values rostrally and laterally for both threshold and suprathreshold levels. This trend was also similar for 10- and 20-kHz long tone responses, as shown in Fig. 4.
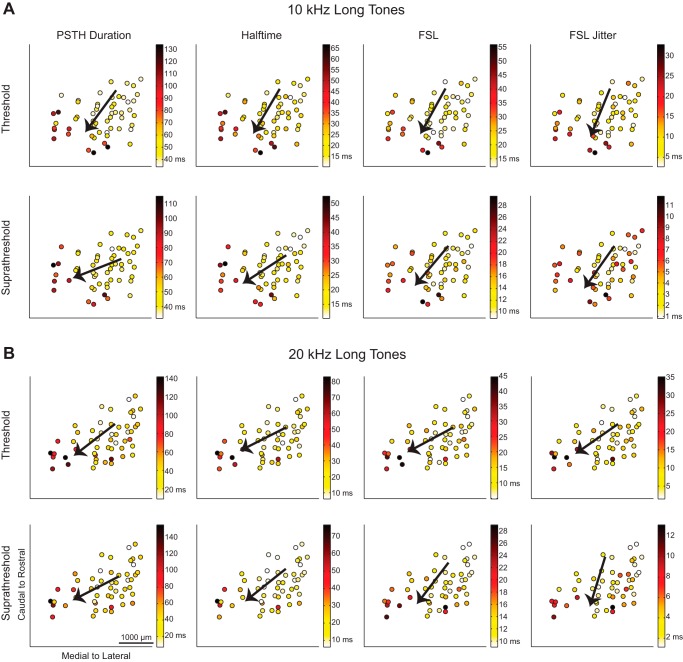
Fig. 4.
Spiking responses to long tones vary from caudomedial to rostrolateral across the lamina. The maps of spiking in response to 10-kHz (A) and 20-kHz long tones (B) show response parameters recorded at each location. Similar to short tone responses in Fig. 3, the responses varied from caudomedial to rostrolateral regions across the lamina.
In addition to spiking parameters, we also analyzed how LFP parameters varied across the ICC lamina. The LFP threshold was defined as three times the standard deviation of the spontaneous activity. LFP peak time was determined at the lowest stimulus level that surpassed this threshold, and the magnitude of the LFP peak was found at 4 dB above this threshold. In choosing these criteria, we attempted to find the lowest elicited threshold that would provide consistent results while minimizing noise effects. However, the responses in the most caudal area still did not exceed spontaneous activity. These caudal locations were set to 0 μV for the analysis of LFP peak magnitudes but were not included for the analysis of LFP peak time. The maps of LFP parameters in response to 10- and 20-kHz short and long tones are shown in Fig. 5, where caudal-and-medial regions have smaller or subthreshold LFP peaks that occur later, whereas rostral-and-lateral regions have larger LFP peaks that occur earlier.
Fig. 5.
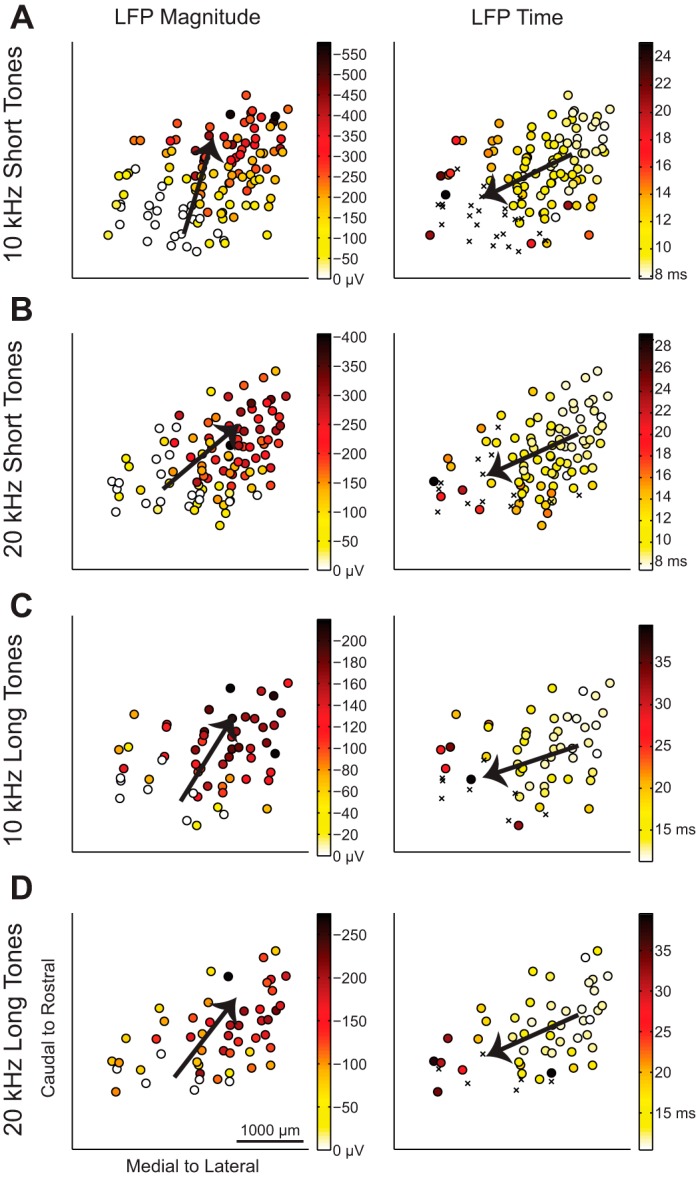
LFP response properties vary from caudomedial to rostrolateral across the lamina. The maps of LFP properties in response to 10-kHz short tones (A), 20-kHz short tones (B), 10-kHz long tones (C), and 20-kHz long tones (D) show response parameters recorded at each location. Multiple regression was performed to determine the steepest gradient axis (arrows). The LFP peak time was determined at threshold, and the LFP magnitude was determined at 4 dB above threshold. If the site did not exceed threshold, the LFP magnitude was labeled as 0 μV and included in the data analysis; those locations were not included in the LFP peak time analysis and are indicated (×) in the LFP time plots. See Table 1 for details on the regression statistics.
To quantify the gradient directionality of these different response maps, a line was fit to the data using linear, two-dimensional multiple regression analysis to find the steepest gradient axis, or the direction in which the response increased the most. The steepest gradient axes (the directional arrows) for each parameter are overlaid on the location data in Fig. 3 for the short tone spiking parameters, in Fig. 4 for the long tone spiking parameters, and in Fig. 5 for short and long tone LFP responses.
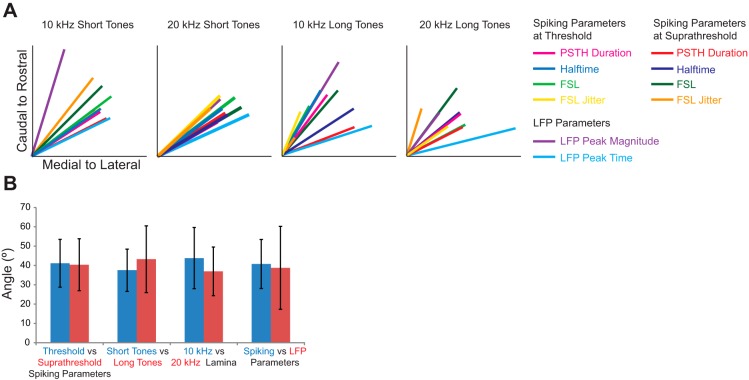
Directions for all parameters, summarized by the steepest gradient axes lines in Fig. 6A, show that all of the different response properties varied in a caudomedial to rostrolateral direction. The regression fit for each parameter was found to be significant, and details on the angle value and statistics are presented in Table 1. Across the 10- and 20-kHz laminae, the spiking and LFP response gradients varied from 24° to 74° rostrolaterally in response to short tones and from 14° to 73° rostrolaterally in response to long tones, where 90° would be aligned along the caudal-to-rostral axis and 0° would be aligned along the medial-to-lateral axis.
Fig. 6.
Response parameters vary in the caudomedial to rostrolateral direction irrespective of threshold, stimulus, or parameter type. A: multiple regression was performed to determine the steepest gradient axis (colored lines), which highlights the directionality for each parameter across the ICC lamina. The directions of the line vectors are represented by the arrows in Fig. 3 for the short tone spiking parameters, in Fig. 4 for the long tone spiking parameters, and in Fig. 5 for short and long tone LFP responses. The length of each line vector indicates the strength of the regression, where the longest line corresponds to an R2 value of 0.64 (see Table 1 for details on each regression). Responses to all spiking and LFP parameters varied caudomedially to rostrolaterally for the 10- and 20-kHz short and long tone responses. B: the angles of the responses, averaged across relevant response parameters (i.e., all other parameters and stimuli conditions except those directly being compared), were similar when compared between different conditions. Average angles across all spiking parameters, BF laminae, and stimuli length were comparable between threshold and suprathreshold conditions. Average angles across all LFP and spiking parameters were similar when compared between tone lengths or BF laminae. Finally, the average angles were similar between spiking and LFP parameters. Data are means ± SD.
Table 1.
Multiple regression statistics summary
| Spiking Parameters |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| At threshold |
At suprathreshold |
LFP Parameters |
||||||||
| PSTH duration | Halftime | FSL | FSL jitter | Width | Halftime | FSL | FSL jitter | Peak magnitude | Peak time | |
| 10-kHz short tones | ||||||||||
| θ | 33.3 | 34.6 | 37.4 | 35.0 | 27.1 | 36.1 | 45.4 | 51.5 | 73.5 | 25.8 |
| n | 119 | 119 | 119 | 119 | 113 | 113 | 113 | 113 | 119 | 95 |
| R2 | 0.47 | 0.48 | 0.57 | 0.48 | 0.48 | 0.45 | 0.57 | 0.57 | 0.64 | 0.50 |
| P | 7.E-17 | 3.E-17 | 4.E-22 | 4.E-17 | 2.E-16 | 5.E-15 | 4.E-21 | 1.E-20 | 3.E-26 | 2.E-14 |
| 10-kHz long tones | ||||||||||
| θ | 53.3 | 59.4 | 61.3 | 67.3 | 21.1 | 33.0 | 49.3 | 54.6 | 58.8 | 18.0 |
| n | 55 | 55 | 55 | 55 | 53 | 53 | 53 | 53 | 55 | 47 |
| R2 | 0.43 | 0.43 | 0.32 | 0.27 | 0.44 | 0.49 | 0.39 | 0.12 | 0.62 | 0.54 |
| P | 5.E-07 | 5.E-07 | 4.E-05 | 3.E-04 | 5.E-07 | 4.E-08 | 4.E-06 | 4.E-02 | 1.E-11 | 3.E-08 |
| 20-kHz short tones | ||||||||||
| θ | 30.0 | 36.5 | 37.3 | 44.2 | 32.6 | 30.6 | 30.4 | 42.2 | 42.5 | 24.6 |
| n | 99 | 99 | 99 | 99 | 93 | 93 | 93 | 93 | 99 | 85 |
| R2 | 0.40 | 0.46 | 0.56 | 0.50 | 0.46 | 0.47 | 0.56 | 0.41 | 0.49 | 0.58 |
| P | 3.E-11 | 1.E-13 | 9.E-18 | 4.E-15 | 1.E-12 | 3.E-13 | 1.E-16 | 5.E-11 | 1.E-14 | 3.E-16 |
| 20-kHz long tones | ||||||||||
| θ | 37.8 | 27.8 | 28.0 | 35.3 | 27.0 | 39.3 | 53.4 | 72.6 | 52.8 | 14.1 |
| n | 52 | 52 | 52 | 52 | 50 | 50 | 50 | 50 | 52 | 46 |
| R2 | 0.39 | 0.38 | 0.38 | 0.29 | 0.36 | 0.39 | 0.48 | 0.28 | 0.31 | 0.64 |
| P | 7.E-06 | 8.E-06 | 9.E-06 | 2.E-04 | 3.E-05 | 9.E-06 | 3.E-07 | 4.E-04 | 1.E-04 | 4.E-10 |
Multiple regression was performed to determine the directionality of how each response parameter varied across the isofrequency lamina. The angle θ (in degrees) across the isofrequency lamina is the inverse tangent of the slope of the regression, where caudal to rostral would be 90° and medial to lateral would be 0°. Each regression was performed on the number of sites (n), with the descriptive statistics of a coefficient of determination (R2) and probability (P). PSTH, poststimulus time histogram; FSL, first spike latency; LFP, local field potential.
Next, we investigated whether the variation in angles across the ICC lamina depended on different properties, laminae, or stimulus duration. We compared the mean angles between these specific variables by averaging across all other properties and stimulus conditions, as summarized in Fig. 4B. The mean angle was 41 ± 12° (mean ± SD, n = 16 parameters) for threshold and 40 ± 13° (n = 16) for suprathreshold spiking parameters, when averaged across the four parameters each for the 10- and 20-kHz short and long tone stimuli. The similarity between the two values suggests that this directionality is maintained across stimulation levels for spiking responses. The mean angle across all properties was 37.5 ± 11° (n = 20) for the short tones and 43 ± 17° (n = 20) for long tones, when averaged across the four spiking parameters at each of the two threshold levels and LFP parameters for both frequency laminae. Therefore, response properties were similar between short and long tone stimuli, although long tones resulted in greater scatter of angles, potentially because of the fewer number of sites sampled. In addition, the average angle for all properties and stimuli was 44 ± 16° (n = 20) for the 10-kHz lamina and 39 ± 22° (n = 20) for the 20-kHz lamina, which indicates that the two lamina showed similar trends across properties. Across all stimuli, the average angle was 41 ± 13° (n = 32) for spiking parameters and 39 ± 22° (n = 8) for LFP parameters. The similarity of trends for both LFP and PSTH response characteristics further supports the main trends in response differences between rostral-and-lateral vs. caudal-and-medial areas of the ICC. There were no significant differences between all four of these group comparisons (P > 0.05, using the Mann-Whitney test). Therefore, we conclude that the directionality of property differences from caudomedial to rostrolateral is consistent across the parameters examined in this study, including short and long tone stimuli, the 10-kHz and 20-kHz laminae, and threshold and suprathreshold spiking activity, as well as spiking and LFP activity.
Two Spatially Distinct Areas Along an ICC Lamina
Although the steepest gradient axis was an important tool in determining the directionality of response gradients, we needed to further investigate how the responses vary along these gradients. On the basis of previous anatomic and physiological studies, it has been proposed that at least two spatially segregated lemniscal pathways arise from the ICC (i.e., caudal-and-medial vs. rostral-and-lateral areas) up to the forebrain. Thus it might be expected that response parameters would exhibit at least two clusters of response values between the caudal-and-medial vs. rostral-and-lateral areas of the ICC, rather than a smooth gradient of values across that dimension. To address this question, we projected each recording location onto the steepest gradient axis by determining the shortest distance between the two. We then plotted the response properties for each location as a function of distance from the vertex (i.e., the most caudomedial point on the steepest gradient axis), as shown in Fig. 7 in response to short tones.
Fig. 7.
In response to short tones, differences of spiking properties from the caudomedial to rostrolateral areas suggest that the 2 areas are distinct clusters. The values of spiking parameters are plotted along the steepest gradient axis from the caudomedial (0) to the rostrolateral (1) endpoints along an ICC lamina in response to 10-kHz (A) or 20-kHz kHz short tones (B). The spiking parameters are small and similar in rostrolateral regions and increase rapidly in amount and scatter toward more caudomedial regions. Trends in spiking responses were similar across different stimulus levels. See Fig. 8 for spiking responses to the long tone stimuli.
For the spiking parameters in Fig. 7, a specific pattern of increasing values and heteroscedasticity can be seen along the rostrolateral-to-caudomedial dimension. This is most clearly observed for the FSL parameter, where latencies are short in the rostrolateral region and become longer in the caudomedial region. At a distance of about 0.5 up to 1 (i.e., along the abscissa and in the rostrolateral region), there is no or minimal change in values. However, for smaller distances below about 0.5 (i.e., in the caudomedial region), the values increase rapidly with greater heteroscedasticity. This basic pattern can be observed for all the other spiking parameters and for long tone stimuli (see Fig. 8) and suggests that there may be distinct clusters along an isofrequency lamina.
Fig. 8.
In response to long tones, differences of spiking differences of spiking properties from the caudomedial to rostrolateral areas suggest that the 2 areas are distinct clusters. The values of spiking parameters are plotted along the steepest gradient axis from the caudomedial (0) to the rostrolateral (1) endpoints along an ICC lamina in response to 10-kHz (A) and 20-kHz long tones (B). Similar to short tone stimuli in Fig. 7, the spiking parameters are small and similar in rostrolateral regions and increase rapidly in amount and scatter toward more caudomedial regions.
In addition, Fig. 9 shows that this pattern of increasing heteroscedasticity toward caudomedial regions can be partially observed for LFP peak time but not for LFP peak magnitude. Although the trends observed with LFP data are generally consistent with the spiking data, the differences are likely cause by the limited spatial sampling and resolution for LFPs. Specifically, some LFP responses never exceeded spontaneous activity in caudomedial areas, which limited analysis of LFP but not spiking features. In addition, an LFP corresponds to activity recorded across a large population of neurons surrounding the recording site that can span a distance of hundreds of microns, whereas our multiunit spiking activity corresponds to a spatial span of tens of microns (Eggermont and Smith 1995; Humphrey and Schmidt 1991; Leung 1990; Mitzdorf 1985). Thus the limited spatial resolution of the LFP response likely smeared location effects along the caudomedial-to-rostrolateral distance. Together, these data suggest that there are at least two spatially distinct clusters across an isofrequency lamina. To quantitatively confirm the existence of clusters, we performed k-means clustering analysis on all eight spiking parameters (i.e., 4 parameters at 2 thresholds) but did not include LFP parameters because of their limited spatial resolution and nonexistent responses in some of the caudal-and-medial regions. To normalize the Euclidian distances for the parameters, we first calculated the natural log and ensured that the standard deviations of values for each parameter were similar. We then performed the k-means clustering for these normalized spiking parameters for each tone stimulus.
Fig. 9.
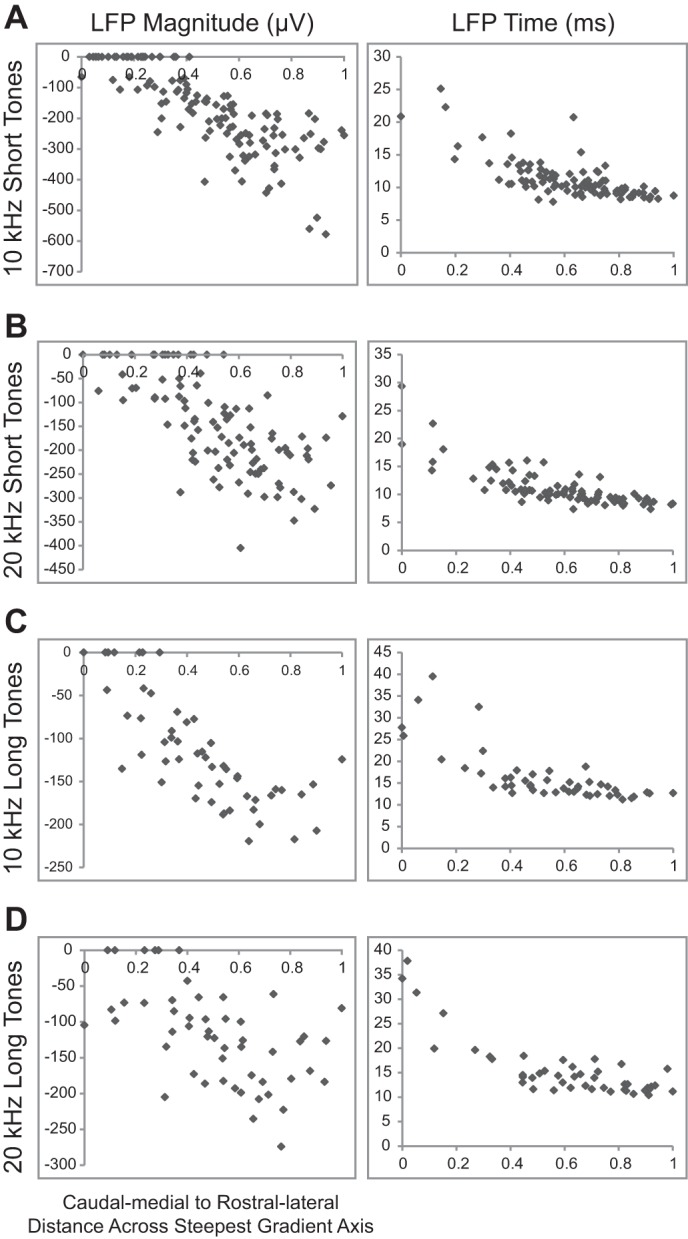
Differences of LFP properties from the caudomedial to rostrolateral areas. The values of LFP parameters are plotted along the steepest gradient axis from the caudomedial (0) to the rostrolateral (1) endpoints along an ICC lamina in response to 10-kHz short tones (A), 20-kHz short tones (B), 10-kHz long tones (C), and 20-kHz long tones (D). The LFP times are small and similar in rostrolateral regions and increase rapidly in amount and scatter toward more caudomedial regions. LFP magnitudes are large in rostrolateral regions while rarely exceeding threshold in caudomedial regions.
Comparing the calculated BIC for up to five clusters, we found that the BIC was optimal for two clusters for the 10- and 20-kHz short and long tones stimuli. During the clustering analysis, it became evident that classification of points was unreliable for the long tone stimuli due to the small number of points and insufficient coverage across the ICC laminae, especially within the caudal-and-medial region. Therefore, we only continued the clustering analysis for short tone stimuli. The location for each classified point can be seen in Fig. 10, where it is evident that sites assigned to the two clusters are located in different regions across the isofrequency laminae. Sites assigned to cluster 1, which had shorter PSTH durations, halftimes, latencies, and jitter at both thresholds, were mainly observed in the rostral-and-lateral regions compared with the sites assigned to cluster 2, which were mainly observed in the caudal-and-medial regions. Therefore, we conclude that there are at least two clusters of responses to acoustic stimuli that exist within the caudal-and-medial vs. rostral-and-lateral regions along an isofrequency lamina.
Fig. 10.
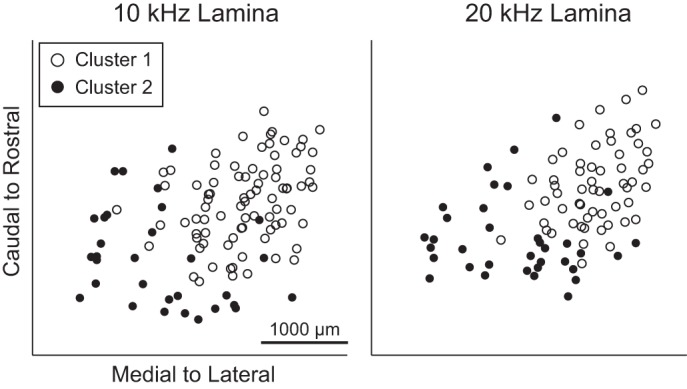
Clustering analysis reveals 2 distinct clusters in the caudal-and-medial vs. rostral-and-lateral areas. For the 10- and 20-kHz short tone stimuli, a k-means clustering algorithm assigned each point into 1 of 2 clusters based on the spiking parameters (after normalization, see text for details). The locations of sites assigned to cluster 1 are found in the rostral-and-lateral regions, whereas the site locations for cluster 2 are typically found in the caudal-and-medial regions across the ICC isofrequency lamina.
Threshold Analysis
The previous results were all compared at levels that elicited a similar extent of activity for each site. We also wanted to analyze whether thresholds varied across locations. Unfortunately, each subsequent array insertion slightly increased the threshold of response regardless of location, possibly due to minor tissue damage, swelling over time, or adaptive effects. This limitation restricted us from analyzing location maps for threshold across all our array shank locations and animals. Since we used a four shank array in which the shanks were separated by 500 μm and aligned along the caudal to rostral direction of the ICC, we instead compared the thresholds of BF-matched sites on the most rostral shank vs. those on the most caudal shank for each placement. These rostral and caudal sites were distributed throughout the rostral and caudal ICC areas over all of the animals, as shown in Fig. 11. As described previously, the spiking threshold and suprathreshold values were determined using SDT at stimulation levels that elicited spiking activity at d′ values of 1 and 3, respectively. For each placement, we determined the differences between the caudal shank and rostral shank threshold. We used the sign test to test the null hypothesis that, across all placements, the number of occurrences of positive differences was equal to that of the number of negative differences.
Fig. 11.
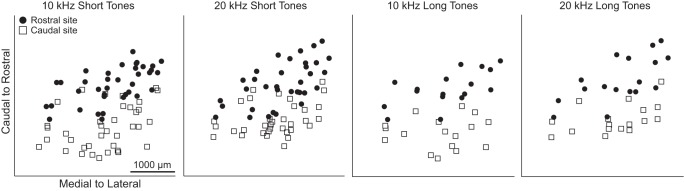
Distribution of the locations of most rostral and most caudal shank for each ICC array placement. The most rostral shank (●) and the most caudal shank (◻) in the ICC for each array placement are shown for the different stimuli. Since placements of rostral and caudal points were distributed throughout the rostral and caudal half of the ICC, respectively, this suggests that comparing the thresholds of the rostral and caudal shank for each placement (i.e., BF-matched sites) is an appropriate way to compare threshold differences between the rostral vs. caudal regions along an ICC lamina.
We found that thresholds were significantly lower for rostral compared with caudal shanks for spiking in response to 10-kHz short tone at spiking threshold (P = 5 × 10−4, n = 37 comparisons) and suprathreshold levels (P = 4 × 10−3, n = 37). Similar trends were observed for the other stimuli, although these trends did not reach significance. In addition, LFP thresholds were lower for rostral compared with caudal shanks for the 10-kHz short tones (P = 9 × 10−10, n = 36), 10-kHz long tones (P = 6 × 10−5, n = 17), and 20-kHz short tones (P = 7 × 10−3, n = 31). A similar trend was observed for the 20-kHz long tones, but it did not reach significance. Perhaps not all of these conditions reached significance because of the orientation of the shanks along the caudal-to-rostral dimension instead of the caudomedial-to-rostrolateral dimension. Nevertheless, all of the conditions still exhibited similar trends of lower thresholds in more rostral areas, which is consistent with what is expected for the dual lemniscal organization. In particular, the rostral (and lateral) ICC projects up to the rostral MGV, which in turn projects to A1, and both of these regions have shown greater sensitivity and/or stronger neural activity compared with the caudal MGV and regions outside of A1 that receive projections from the caudal-and-medial ICC.
DISCUSSION
Our results indicate a clear segregation of response features within the isofrequency laminae of the ICC. Compared with caudal-and-medial portions of the laminae, rostral-and-lateral portions responded to pure tones with shorter PSTH durations, a higher proportion of spikes occurring earlier in the PSTH, shorter first-spike latencies with less jitter, and larger LFP peaks with shorter latencies. Multiple regression analysis demonstrated a significant caudomedial to rostrolateral directionality of response properties for both short and long stimuli. Further analysis indicated the presence of two clusters of feature values, one at caudal-and-medial regions and the other at rostral-and-lateral regions of an isofrequency lamina. In addition, acoustic thresholds were generally lower in rostral vs. caudal locations. These results are consistent with the regions expected in the dual lemniscal pathway hypothesis, which proposes that there are at least two subprojection pathways emerging from the ICC that then project up to the MGV, and from there to the ACC.
Methodological Considerations
Identification of ICC sites.
One of the first considerations in evaluating responses in the IC was to distinguish between lemniscal and nonlemniscal areas. We defined the ICC physiologically; a site is determined to be within the ICC if the shank in the multielectrode array showed a clear tonotopic organization of low to high BFs from shallow to deep sites. However, the borders for subnuclei of the inferior colliculus have been difficult to delineate because they can vary between physiological and anatomic techniques as well as across different anatomic markers (Oliver 2005). Since one of the primary physiological differences between the lemniscal and nonlemniscal pathways has been the presence of tonotopy, we have used this as the defining characteristic for identifying sites located within the ICC, as shown in Fig. 1. Using this definition, we were able to present differences in response properties for recording sites that were located exclusively within the lemniscal region of the midbrain.
Approximation of an isofrequency lamina.
To analyze how responses vary with location within the ICC, we approximated the is frequency laminae as flat planes. Our approximated laminae have some important differences compared with the true anatomic laminae such as those reconstructed by Malmierca et al. (1995) in the guinea pig. When the ICC points collected on our 20-kHz lamina are compared with their 21-kHz laminar plexus, the two laminae extend similarly in the medial-to-lateral direction, although their plexus appears to extend slightly more rostrally and ventrally. In addition, the true laminae are curved and have an external “wing” that extends laterally and dorsally, whereas our approximated laminae are flat. Although we acknowledge these discrepancies, we do not believe that they affect our findings because the locations of the shanks, which were placed approximately orthogonal to the laminae, would still remain in similar locations relative to each other along an ICC lamina. These discrepancies could affect the absolute distances between the location of sites along an ICC lamina and the exact shape of the two subregions identified in our study, but they would not change our finding that two subregions with different coding properties exist within an ICC lamina.
Systematic mapping of only two laminae.
We targeted only two isofrequency lamina of the ICC (i.e., 10- and 20-kHz laminae) to fully map numerous locations across and up to the border of each of those laminae. Since the typical hearing range of the guinea pig spans frequencies from ∼50 Hz up to 50 kHz (Heffner et al. 1971), we cannot claim that the two subregions with distinct response properties for the two isofrequency laminae observed in our study occur throughout the ICC. In fact, there have been a few anatomic studies showing some differences in the anatomic projections to our identified functional subregions for different frequency laminae in cat (Loftus et al. 2004; Oliver 1987; Oliver et al. 1997). The cochlear nucleus was found to generally project throughout the ICC with LSO terminating in more rostral and/or lateral portions along the laminae of the ICC. For lower frequency laminae (<5 kHz) the LSO projected more dominantly in rostral regions, whereas for higher frequency laminae the LSO projected more dominantly in lateral and ventral regions. In addition, the MSO projected mainly to caudal regions of only low frequency ICC laminae. On the other hand, there are anatomic and physiological studies demonstrating a segregated organization of the ICC that is consistent with our identified functional caudal-and-medial vs. rostral-and-lateral subregions and that occurred across different frequency laminae. In gerbil, this segregated organization was anatomically demonstrated through most of the ICC spanning low-, middle-, and high-frequency regions (Cant and Benson 2006, 2007). Generally consistent with the studies in cat cited above, the cochlear nucleus projected throughout the ICC and the LSO projected to the rostral and lateral portions of the ICC, although no differences across frequency laminae were reported for LSO projections as in cat. This study in gerbil found that the MSO also projected to rostral and lateral portions of the ICC across different frequency regions, which is in contrast to the MSO projections to the caudal portions of the ICC for only low-frequency regions in cat. In guinea pig, this segregated organization was also physiologically demonstrated through differences in activation properties from the ICC up to A1 for various frequency laminae spanning 9–23 kHz (Lim and Anderson 2007). Therefore, although there are some differences in the types and spatial pattern of brainstem inputs into the ICC across frequency laminae and species, the rostral-and-lateral vs. caudal-and-medial division along an ICC lamina appears to be consistent across frequency regions for at least some brain stem inputs and/or functional properties for different species.
Identification of spiking response clusters within ICC.
Although response properties clearly differ across an ICC isofrequency lamina, the question arises as to whether the response maps should be described as a continuum or as two distinct clusters. To address this question, we performed cluster analysis and found that response values were optimally assigned into one of two groups located in either the caudal-and-medial or the rostral-and-lateral regions. As shown in Fig. 10, there is a clear separation of the two clusters with only a few overlapping points mostly near the middle of the two clusters. Because of these overlapping points, we cannot completely rule out the possibility that a gradient exists across the isofrequency laminae, at least for some of the response properties. However, we would expect to see a few points that are not perfectly aligned within each cluster, since we analyzed a large number of different spiking response properties (8 total for each of the 4 stimuli) in this cluster analysis, and there are some spatial errors expected in our three-dimensional site reconstructions. We also believe that the stark difference between the mean and heteroscedasticity in a variety of spiking response values between the two regions (e.g., for FSL in Fig. 7A) supports the existence of two distinct clusters, at least for those response properties. Furthermore, a previous anatomic study in gerbil identified two subregions in ICC consistent with our two clusters that projected to and remained largely separate in the MGV (Cant and Benson 2006, 2007), suggesting that segregated regions do exist within the ICC.
Effects of anesthesia.
One limitation of the present study was the use of an anesthetized preparation to provide sufficient time to position the electrode array into numerous locations throughout the ICC in each experiment. We selected ketamine-xylazine as the anesthetic because previous studies have shown minimal or no alterations in ICC activity compared with the awake state for properties including FSL, FSL jitter, trial-by-trial reliability, temporal synchronization capabilities, firing patterns in response to pure tones, and spontaneous activity (Astl et al. 1996; Suta et al. 2003; Ter-Mikaelian et al. 2007; Torterolo et al. 2002). In one study that used barbiturate-ketamine anesthetic in gerbil (Ter-Mikaelian et al. 2007), some neurons exhibited greater firing rate adaptation over a longer period of time compared with the awake condition. However, this adaptation occurred on a time scale (>500 ms) longer than our analysis period (<160 ms) and thus is unlikely to have affected our results. There is the possibility that the ketamine-xylazine anesthesia may have altered some specific properties we analyzed in this study that were not investigated in those previous studies listed above, such as PSTH duration, halftime, and LFP magnitude. However, we observed a similar and consistent map within an ICC lamina regardless of the response property. Furthermore, different types of anesthetics (i.e., ketamine, pentobarbital, or nitrous oxide) were used in the previous studies cited in the Introduction that demonstrated distinct and consistent response properties between the dual lemniscal pathways through the MGV and ACC, which also matched what we observed in the ICC (see A Dual Lemniscal Organization for further details on this functional organization).
Comparison of Response Maps to Previous Studies
Although many of the response properties we analyzed had not previously been mapped across an ICC lamina, we could at least compare our threshold and latency maps to those of previous studies. We observed shorter latencies in rostral-and-lateral vs. caudal-and-medial regions along an ICC lamina, which is different from previous studies that showed shorter latencies in more lateral vs. medial locations along the ICC lamina in chinchilla and cat (Langner et al. 2002; Schreiner and Langner 1988) and ventral-and-lateral vs. dorsal-and-medial locations in the medial division of the ICC in bat (Hattori and Suga 1997; Portfors and Wenstrup 2001). In addition, we found that rostral regions typically had lower thresholds than caudal regions, whereas previous studies in mouse found a concentric map with lower thresholds in more central regions (Hage and Ehret 2003; Stiebler 1986). The inconsistencies between our results and previous studies could be associated with the use of different animal species or ICC border definitions. Inconsistencies could also be due to differences in histological and site reconstruction techniques.
Although we found an organization of temporal features within an ICC lamina, we did not find a consistent organization for tuning curve width (i.e., Q10 and Q30 values) or tonotopicity (unpublished observations). With regard to frequency tuning properties, it was shown in anesthetized mouse that neurons with narrower tuning are located in more central regions of an ICC lamina that then broaden in more outward locations (Hage and Ehret 2003; Stiebler 1986). However, a more recent study in awake mouse performed detailed histological reconstructions of all the recording site locations and found no spatial trend along an ICC lamina for tuning curve width (Portfors et al. 2011). As suggested by Portfors el al. (2011), the differences in results could be associated with their use of histological reconstructions for more accurately combining response locations across animals that was not performed in the previous studies.
Although we focused on responses to simple pure tones in this study, maps of other features such as binaurality, FM sweep, and localization have also been found across isofrequency regions within the ICC. Whereas units sensitive to interaural time delays are located in the rostral-and-lateral areas of ICC, monaural units are predominantly located in the caudal ICC (Roth et al. 1978; Semple and Aitkin 1979; Wenstrup et al. 1985). In addition, faster following responses (i.e., best modulation frequencies) tend to be observed in more lateral vs. medial regions along an ICC lamina in chinchilla, cat, and guinea fowl (Langner 1992; Langner et al. 2002; Schreiner and Langner 1988), although the topographic map for best modulation frequencies has also been shown to be complex (e.g., concentric and varying across frequency regions) and level dependent (Krishna and Semple 2000; Schreiner and Langner 1988). Harmonics of frequency modulation components also vary from caudal to rostral-and-lateral regions of the ICC in bat (O'Neill et al. 1989). All of these different feature maps further highlight the complexity and diversity in the coding of sounds within the ICC that require further investigation in terms of how they relate to and interact with the dual lemniscal organization proposed in this study.
Mechanisms Underlying Response Maps
The differences in response features between the rostral-and-lateral and the caudal-and-medial areas of the ICC may be due to different sources of input from the brain stem that create spatially distinct zones (Brunso-Bechtold et al. 1981; Cant and Benson 2007, 2006; Loftus et al. 2004; Oliver et al. 1997; Roth et al. 1978; Semple and Aitkin 1979; Shneiderman and Henkel 1987). These inputs have previously been hypothesized to contribute to different responses. For example, Semple and Aitkin (1979) showed that the type of binaural response for neurons is likely correlated to the source of inputs. Monaural units located in the caudal, ventral, and lateral regions of the ICC appear to receive their primary input from the contralateral cochlear nucleus, one of the dominant sources of monaural excitatory input to the ICC (Semple and Aitkin 1979). The location of binaural units varied depending on type, although each was generally segregated from the other types of monaural and binaural units. Delay-sensitive units were typically found in rostral, dorsal, and lateral regions, contralateral-excitatory-and-ipsilateral-inhibitory units were located in rostral regions, and binaurally excitatory units were most common in medial regions of the ICC. These binaural units likely receive their input from a specific combination of contralateral and ipsilateral inputs from brain stem nuclei. However, it is not yet clear how our temporal response properties correlate with the complex combinations of contralateral and ipsilateral projections from the cochlear nucleus, superior olivary complex, and lateral lemniscus (Irvine 2011; Winer and Schreiner 2005).
In addition to brain stem inputs, local midbrain projections may target and interact with neurons differentially across an ICC lamina that could result in subregions with different functional properties. A recent study that differentiated between monosynaptic inputs from local polysynaptic inputs within the inferior colliculus found that monosynaptic inputs have short latencies, whereas local polysynaptic inputs result in variable onset times and evoke long-lasting excitation (Sivaramakrishnan et al. 2013). Together with our results showing that caudal-and-medial regions respond with longer, more diverse latencies and longer PSTH durations than rostral-and-lateral regions, this suggests that local polysynaptic midbrain circuits may be targeting and activating the caudal-and-medial regions to a larger extent.
Our LFP and spiking data support the existence of at least two functional zones across the ICC laminae and suggest that these regions have different inputs and outputs. Since LFPs generally correspond to spatially diffuse, synchronous inputs (Eggermont and Smith 1995), our observation further suggesting that LFP responses vary between rostral-and-lateral vs. caudal-and-medial regions is consistent with the studies cited above that found different inputs along the ICC laminae. In addition, the location trends for LFP responses were similar to those for spiking features, which correspond to neural outputs. The differences in spiking responses between regions may be attributed to different types of neurons existing within an ICC lamina that vary in morphology and biophysical properties (Faye-Lund and Osen 1985; Herrera et al. 1988; Malmierca et al. 1993; Oliver and Morest 1984; Oliver et al. 1994; Paloff et al. 1992). Because we used multiunit recordings, the responses presented in this study are from a small population of neurons rather than individual neurons. Therefore, our results may be attributed to different types of neurons with varying intrinsic and synaptic properties and/or a different mixture of these neurons between the caudal-and-medial vs. the rostral-and-lateral regions of the ICC. Within the ICC, specific physiological response characteristics have been correlated with different cell types. For example, cells can be classified by distinct firing patterns such as onset, sustained, or rebound responses, each of which are generated by unique synaptic properties of K+ channels (Sivaramakrishnan and Oliver 2001). Another study found that onset responses are only present in stellate and not in disc-shaped cells in the ICC (Wallace et al. 2012). Combining these studies with our results, the pronounced onset spiking responses we observed in the rostral-and-lateral regions of the ICC may be due to a greater density of neurons that have unique K+ currents and/or morphologically appear to be stellate. However, we are unaware of any studies that directly correlated synaptic or morphological properties with location across an ICC lamina.
A Dual Lemniscal Organization
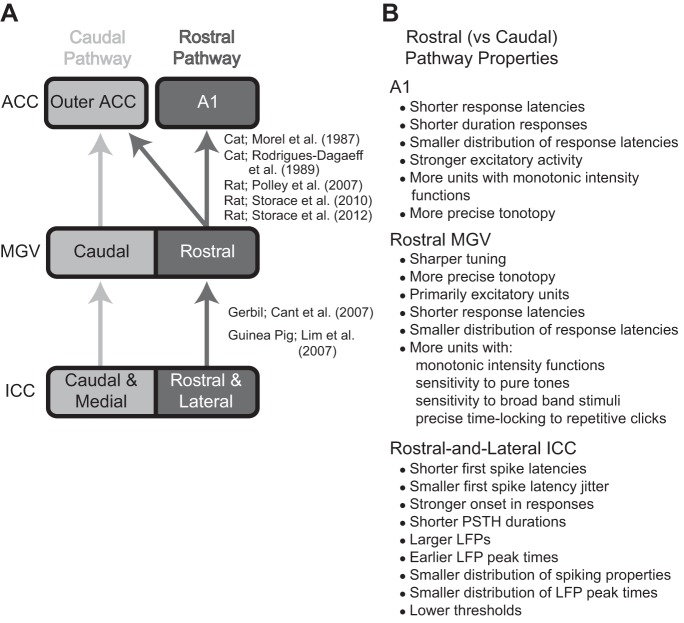
The concept of a dual lemniscal organization in the auditory system was first proposed in the 1980s (Morel and Imig 1987; Rodrigues-Dagaeff et al. 1989), specifically for projections from MGV up to ACC in a cat model. The dual lemniscal pathway hypothesis was further expanded in 2006 to 2007 to include pathways from the brain stem up through the ICC, MGV, and ACC across several species, including gerbil, rat, and guinea pig (Cant and Benson 2006, 2007; Lim and Anderson 2007; Polley et al. 2007). Together, these studies revealed two segregated anatomic pathways through the ICC (caudal-and-medial vs. rostral-and-lateral regions), MGV (caudal vs. rostral regions), and ACC (A1 vs. core regions outside of A1). They also demonstrated different coding properties between these pathways within the MGV and ACC, and for electrical activation of ICC up to ACC. More recently, a series of anatomic and functional studies in rat further support this dual lemniscal organization from the MGV up to ACC and also reveal differences in gene expression for type 1 vesicular glutamate transporter that may contribute to the distinct coding properties between pathways (Storace et al. 2010, 2011, 2012). Figure 12A provides a simplified schematic summarizing the dual lemniscal pathways through the ICC, MGV, and ACC. The differences in coding properties between the rostral and caudal MGV, demonstrated by Rodrigues-Dagaeff (1989) in cat and listed in Fig. 12B, suggest that the rostral pathway is designed for stronger excitatory activation and more temporally and spectrally precise transmission of information up to higher centers. Many of these differences in coding properties between the dual pathways have also been shown in ACC and are listed in Fig. 12B (Phillips et al. 1995; Polley et al. 2007; Schreiner et al. 2011; Storace et al. 2012; Wallace et al. 2000).
Fig. 12.
Schematic of anatomic projections and summary of physiological differences that indicate the lemniscal pathway is segregated into 2 subprojection pathways. A: the rostral and caudal ascending pathways show spatially segregated anatomic projections from the ICC up to the core auditory cortex regions (ACC). Overlapping projections between the 2 pathways are not shown. A1, primary auditory cortex; MGV, ventral division of the medial geniculate body. B: in contrast to the caudal pathway, the rostral pathway also shows different responses to acoustic stimuli in A1 (Phillips et al. 1995; Polley et al. 2007; Storace et al. 2012; Wallace et al. 2000), rostral MGV (Rodrigues-Dagaeff et al. 1989), and the rostral-lateral ICC (as shown in this study). [Adapted from Lim et al. (2008) with permission.]
The goal of our study was to determine whether at least some basic temporal or spectral features exhibited a spatial organization within ICC and whether this organization was consistent with that of previous anatomic studies listed in Fig. 12A to further support the dual lemniscal pathway hypothesis. We found an organization of temporal features for LFP and spiking activity that aligns with the dual lemniscal pathways depicted in Fig. 12. Thus we confirmed the existence of these distinct regions along the ICC laminae that code for sound in different ways. Although we found an organization of temporal features, we did not find a consistent organization for spectral properties across the ICC laminae, perhaps because a dual lemniscal organization for spectral coding properties may not occur until the MGV up to ACC (Polley et al. 2007; Rodrigues-Dagaeff et al. 1989; Storace et al. 2011). Other studies further demonstrate the complex and heterogeneous organization of different sound features across the ICC and up to ACC, some of which are organized into topographic maps that may or may not align with the anatomic segregation for the dual lemniscal organization (see Comparison of Response Maps to Previous Studies). These heterogeneous maps likely arise from the complex organization of brain stem inputs and local circuits within the ICC as well as different types of neurons with unique biophysical properties (see Mechanisms Underlying Response Maps). Although there is some complexity and heterogeneity, a consistent organization of at least two spatially segregated pathways through the ICC, MGV, and ACC with different coding properties has been observed across studies spanning multiple species.
This dual lemniscal pathway organization may serve a critical role in how the brain generally processes sound within the ascending auditory system, but this will require further investigation. The organization of temporal response properties shown here in the ICC, which is consistent with that in the MGV and ACC, suggests that the rostral pathway may be designed for stronger activation and more precise transmission of sound information to higher centers compared with the caudal pathway. In addition to the divergent anatomy shown in ascending pathways, there is also evidence that descending projections differentially affect regions along the isofrequency laminae of the ICC. A recent study demonstrated that electrical stimulation of A1 elicits descending excitatory activation in the caudal-and-medial regions within an ICC lamina but with little or no activation within the rostral-and-lateral regions (Markovitz et al. 2013). Combined with the robust and precise activation properties of the rostral pathway that projects up to A1, these findings suggest that the rostral pathway may serve as the main ascending auditory pathway, whereas the caudal pathway, at least within the midbrain, may serve a more modulatory role for sound processing.
GRANTS
This work was supported by startup funds from the University of Minnesota and National Institute of Deafness and Other Communications Disorders Grant R03 DC011589.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.M.S. and H.H.L. conception and design of research; M.M.S. performed experiments; M.M.S. and S.S. analyzed data; M.M.S. interpreted results of experiments; M.M.S. and S.S. prepared figures; M.M.S. drafted manuscript; M.M.S. and H.H.L. edited and revised manuscript; M.M.S., S.S., and H.H.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Patrick Lee and Melissa McMahon for contributions in the histological reconstructions, and the University of Minnesota Statistics Consulting department for assistance with the clustering analysis.
REFERENCES
- Andersen RA, Roth GL, Aitkin LM, Merzenich MM. The efferent projections of the central nucleus and the pericentral nucleus of the inferior colliculus in the cat. J Comp Neurol 194: 649–662, 1980 [DOI] [PubMed] [Google Scholar]
- Astl J, Popelar J, Kvasnak E, Syka J. Comparison of response properties of neurons in the inferior colliculus of guinea pigs under different anesthetics. Audiology 35: 335–345, 1996 [DOI] [PubMed] [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci 12: 4745–4765, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunso-Bechtold JK, Thompson GC, Masterton RB. HRP study of the organization of auditory afferents ascending to central nucleus of inferior colliculus in cat. J Comp Neurol 197: 705–722, 1981 [DOI] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Multiple topographically organized projections connect the central nucleus of the inferior colliculus to the ventral division of the medial geniculate nucleus in the gerbil, Meriones unguiculatus. J Comp Neurol 503: 432–453, 2007 [DOI] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Organization of the inferior colliculus of the gerbil (Meriones unguiculatus): differences in distribution of projections from the cochlear nuclei and the superior olivary complex. J Comp Neurol 495: 511–528, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetas JS, Price RO, Velenovsky DS, Sinex DG, McMullen NT. Frequency organization and cellular lamination in the medial geniculate body of the rabbit. Hear Res 155: 113–123, 2001 [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Smith GM. Synchrony between single-unit activity and local field potentials in relation to periodicity coding in primary auditory cortex. J Neurophysiol 73: 227–245, 1995 [DOI] [PubMed] [Google Scholar]
- Ehret G, Romand R. The Central Auditory System. New York: Oxford University Press, 1997 [Google Scholar]
- Everitt BS, Landau S, Leese M. Cluster Analysis. London: Arnold, 2001 [Google Scholar]
- Faye-Lund H, Osen KK. Anatomy of the inferior colliculus in rat. Anat Embryol (Berl) 171: 1–20, 1985 [DOI] [PubMed] [Google Scholar]
- Green D, Swets J. Signal Detection Theory and Psychophysics. New York: Wiley, 1966 [Google Scholar]
- Hage SR, Ehret G. Mapping responses to frequency sweeps and tones in the inferior colliculus of house mice. Eur J Neurosci 18: 2301–2312, 2003 [DOI] [PubMed] [Google Scholar]
- Hattori T, Suga N. The inferior colliculus of the mustached bat has the frequency-vs-latency coordinates. J Comp Physiol A 180: 271–284, 1997 [DOI] [PubMed] [Google Scholar]
- Heffner R, Heffner H, Masterton B. Behavioral measurements of absolute and frequency-difference thresholds in guinea pig. J Acoust Soc Am 49: 1888–1895, 1971 [DOI] [PubMed] [Google Scholar]
- Herrera M, Correa J, Sanchez del Campo F, Ruiz A. Stellate cells and their axonal patterns in the central nucleus of the inferior colliculus of the cat (Felis domesticus). J Hirnforsch 29: 393–402, 1988 [PubMed] [Google Scholar]
- Humphrey D, Schmidt E. Extracellular single-unit recording methods. In: Neurophysiological Techniques, edited by Boulton A, Baker G, Vanderwolf C. Clifton, NJ: Humana, 1991, p. 1–64 [Google Scholar]
- Imig TJ, Morel A. Tonotopic organization in ventral nucleus of medial geniculate body in the cat. J Neurophysiol 53: 309–340, 1985 [DOI] [PubMed] [Google Scholar]
- Irvine DR. The Auditory Brainstem: A Review of the Structure and Function of Auditory Brainstem Processing Mechanisms. London, Springer, 2011 [Google Scholar]
- Krishna BS, Semple MN. Auditory temporal processing: responses to sinusoidally amplitude-modulated tones in the inferior colliculus. J Neurophysiol 84: 255–273, 2000 [DOI] [PubMed] [Google Scholar]
- Langner G. Periodicity coding in the auditory system. Hear Res 60: 115–142, 1992 [DOI] [PubMed] [Google Scholar]
- Langner G, Albert M, Briede T. Temporal and spatial coding of periodicity information in the inferior colliculus of awake chinchilla (Chinchilla laniger). Hear Res 168: 110–130, 2002 [DOI] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Drivers and modulators in the central auditory pathways. Front Neurosci 4: 79, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenarz M, Lim HH, Patrick JF, Anderson DJ, Lenarz T. Electrophysiological validation of a human prototype auditory midbrain implant in a guinea pig model. J Assoc Res Otolaryngol 7: 383–398, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LW. Field potentials in the central nervous system. In: Neurophysiological Techniques, edited by Boulton A, Baker G, Vanderwolf C. Clifton, NJ: Humana, p. 277–312 [Google Scholar]
- Lim HH, Anderson DJ. Auditory cortical responses to electrical stimulation of the inferior colliculus: implications for an auditory midbrain implant. J Neurophysiol 96: 975–988, 2006 [DOI] [PubMed] [Google Scholar]
- Lim HH, Anderson DJ. Spatially distinct functional output regions within the central nucleus of the inferior colliculus: Implications for an auditory midbrain implant. J Neurosci 27: 8733–8743, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HH, Lenarz T, Anderson DJ, Lenarz M. The auditory midbrain implant: effects of electrode location. Hear Res 242: 74–85, 2008 [DOI] [PubMed] [Google Scholar]
- Loftus WC, Bishop DC, Saint Marie RL, Oliver DL. Organization of binaural excitatory and inhibitory inputs to the inferior colliculus from the superior olive. J Comp Neurol 472: 330–344, 2004 [DOI] [PubMed] [Google Scholar]
- Malmierca MS, Blackstad TW, Osen KK, Karagulle T, Molowny RL. The central nucleus of the inferior colliculus in rat: a Golgi and computer reconstruction study of neuronal and laminar structure. J Comp Neurol 333: 1–27, 1993 [DOI] [PubMed] [Google Scholar]
- Malmierca MS, Hackett TA. Structural organization of the ascending auditory pathway. In: The Oxford Handbook of Auditory Science: The Auditory Brain, edited by Palmer AR, Rees A. New York: Oxford University Press, 2010, p. 9–41 [Google Scholar]
- Malmierca MS, Izquierdo MA, Cristaudo S, Hernandez O, Perez-Gonzalez D, Covey E, Oliver DL. A discontinuous tonotopic organization in the inferior colliculus of the rat. J Neurosci 28: 4767–4776, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca MS, Rees A, Le Beau FE, Bjaalie JG. Laminar organization of frequency-defined local axons within and between the inferior colliculi of the guinea pig. J Comp Neurol 357: 124–144, 1995 [DOI] [PubMed] [Google Scholar]
- Markovitz CD, Tang TT, Edge DP, Lim HH. Three-dimensional brain reconstruction of in vivo electrode tracks for neuroscience and neural prosthetic applications. Front Neural Circuits 6: 39, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovitz CD, Tang TT, Lim HH. Tonotopic and localized pathways from primary auditory cortex to the central nucleus of the inferior colliculus. Front Neural Circuits 7: 77, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev 65: 37–100, 1985 [DOI] [PubMed] [Google Scholar]
- Morel A, Imig TJ. Thalamic projections to fields A, AI, P, and VP in the cat auditory cortex. J Comp Neurol 265: 119–144, 1987 [DOI] [PubMed] [Google Scholar]
- O'Neill WE, Frisina RD, Gooler DM. Functional organization of mustached bat inferior colliculus: I. Representation of FM frequency bands important for target ranging revealed by 14C-2-deoxyglucose autoradiography and single unit mapping. J Comp Neurol 284: 60–84, 1989 [DOI] [PubMed] [Google Scholar]
- Oliver DL. Neuronal organization in the inferior colliculus. In: The Inferior Colliculus, edited by Winer JA, Schreiner CE. New York: Springer Science and Business Media, 2005, p. 69–114 [Google Scholar]
- Oliver DL. Projections to the inferior colliculus from the anteroventral cochlear nucleus in the cat: possible substrates for binaural interaction. J Comp Neurol 264: 24–46, 1987 [DOI] [PubMed] [Google Scholar]
- Oliver DL, Beckius GE, Bishop DC, Kuwada S. Simultaneous anterograde labeling of axonal layers from lateral superior olive and dorsal cochlear nucleus in the inferior colliculus of cat. J Comp Neurol 382: 215–229, 1997 [DOI] [PubMed] [Google Scholar]
- Oliver DL, Morest DK. The central nucleus of the inferior colliculus in the cat. J Comp Neurol 222: 237–264, 1984 [DOI] [PubMed] [Google Scholar]
- Oliver DL, Winer JA, Beckius GE, Saint Marie RL. Morphology of GABAergic neurons in the inferior colliculus of the cat. J Comp Neurol 340: 27–42, 1994 [DOI] [PubMed] [Google Scholar]
- Paloff AM, Usunoff KG, Hinova-Palova DV. Ultrastructure of Golgi-impregnated and gold-toned neurons in the central nucleus of the inferior colliculus in the cat. J Hirnforsch 33: 361–407, 1992 [PubMed] [Google Scholar]
- Phillips DP, Semple MN, Kitzes LM. Factors shaping the tone level sensitivity of single neurons in posterior field of cat auditory cortex. J Neurophysiol 73: 674–686, 1995 [DOI] [PubMed] [Google Scholar]
- Polley DB, Read HL, Storace DA, Merzenich MM. Multiparametric auditory receptive field organization across five cortical fields in the albio rat. J Neurophysiol 97: 3621–3638, 2007 [DOI] [PubMed] [Google Scholar]
- Portfors CV, Mayko ZM, Jonson K, Cha GF, Roberts PD. Spatial organization of receptive fields in the auditory midbrain of awake mouse. Neuroscience 193: 429–439, 2011 [DOI] [PubMed] [Google Scholar]
- Portfors CV, Wenstrup JJ. Topographical distribution of delay-tuned responses in the mustached bat inferior colliculus. Hear Res 151: 95–105, 2001 [DOI] [PubMed] [Google Scholar]
- Rauschecker J, Romanski L. Auditory cortical organization: evidence for functional streams. In: The Auditory Cortex, edited by Winer JA, Schreiner CE. New York: Springer, 2011, p. 99–116 [Google Scholar]
- Rodrigues-Dagaeff C, Simm G, De Ribaupierre Y, Villa A, De Ribaupierre F, Rouiller EM. Functional organization of the ventral division of the medial geniculate body of the cat: evidence for a rostro-caudal gradient of response properties and cortical projections. Hear Res 39: 103–125, 1989 [DOI] [PubMed] [Google Scholar]
- Roth GL, Aitkin LM, Andersen RA, Merzenich MM. Some features of the spatial organization of the central nucleus of the inferior colliculus of the cat. J Comp Neurol 182: 661–680, 1978 [DOI] [PubMed] [Google Scholar]
- Rouiller EM. Functional organization of the auditory pathways. In: The Central Auditory System, edited by Ehret G, Romand R. New York: Oxford University Press, 1997 [Google Scholar]
- SAS Institute. SAS/STAT User's Guide, Version 8. Cary, NC: SAS Institute, 1999 [Google Scholar]
- Schreiner C, Froemke R, Atencio C. Spectral processing in auditory cortex. In: The Auditory Cortex, edited by Winer JA, Schreiner CE. New York: Springer, 2011, p. 275–308 [Google Scholar]
- Schreiner CE, Langner G. Laminar fine structure of frequency organization in auditory midbrain. Nature 388: 383–386, 1997 [DOI] [PubMed] [Google Scholar]
- Schreiner CE, Langner G. Periodicity coding in the inferior colliculus of the cat. II. Topographical organization. J Neurophysiol 60: 1823–1840, 1988 [DOI] [PubMed] [Google Scholar]
- Semple MN, Aitkin LM. Representation of sound frequency and laterality by units in central nucleus of cat inferior colliculus. J Neurophysiol 42: 1626–1639, 1979 [DOI] [PubMed] [Google Scholar]
- Shneiderman A, Henkel CK. Banding of lateral superior olivary nucleus afferents in the inferior colliculus: a possible substrate for sensory integration. J Comp Neurol 266: 519–534, 1987 [DOI] [PubMed] [Google Scholar]
- Sivaramakrishnan S, Oliver DL. Distinct K currents result in physiologically distinct cell types in the inferior colliculus of the rat. J Neurosci 21: 2861–2877, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaramakrishnan S, Sanchez JT, Grimsley CA. High concentrations of divalent cations isolate monosynaptic inputs from local circuits in the auditory midbrain. Front Neural Circuits 7: 175, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder RL, Bierer JA, Middlebrooks JC. Topographic spread of inferior colliculus activation in response to acoustic and intracochlear electric stimulation. J Assoc Res Otolaryngol 5: 305–322, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiebler I. Tone-threshold mapping in the inferior colliculus of the house mouse. Neurosci Lett 65: 336–340, 1986 [DOI] [PubMed] [Google Scholar]
- Storace DA, Higgins NC, Chikar JA, Oliver DL, Read HL. Gene expression identifies distinct ascending glutamatergic pathways to frequency-organized auditory cortex in the rat brain. J Neurosci 32: 15759–15768, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storace DA, Higgins NC, Read HL. Thalamic label patterns suggest primary and ventral auditory fields are distinct core regions. J Comp Neurol 518: 1630–1646, 2010 [DOI] [PubMed] [Google Scholar]
- Storace DA, Higgins NC, Read HL. Thalamocortical pathway specialization for sound frequency resolution. J Comp Neurol 519: 177–193, 2011 [DOI] [PubMed] [Google Scholar]
- Straka MM, Schendel D, Lim HH. Neural integration and enhancement from the inferior colliculus up to different layers of auditory cortex. J Neurophysiol 110: 1009–1020, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suta D, Kvasnak E, Popelar J, Syka J. Representation of species-specific vocalizations in the inferior colliculus of the guinea pig. J Neurophysiol 90: 3794–3808, 2003 [DOI] [PubMed] [Google Scholar]
- Syka J, Popelar J, Kvasnak E, Astl J. Response properties of neurons in the central nucleus and external and dorsal cortices of the inferior colliculus in guinea pig. Exp Brain Res 133: 254–266, 2000 [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2013 [Google Scholar]
- Ter-Mikaelian M, Sanes DH, Semple MN. Transformation of temporal properties between auditory midbrain and cortex in the awake Mongolian gerbil. J Neurosci 27: 6091–6102, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torterolo P, Falconi A, Morales-Cobas G, Velluti RA. Inferior colliculus unitary activity in wakefulness, sleep and under barbiturates. Brain Res 935: 9–15, 2002 [DOI] [PubMed] [Google Scholar]
- Wallace MN, Rutkowski RG, Palmer AR. Identification and localisation of auditory areas in guinea pig cortex. Exp Brain Res 132: 445–456, 2000 [DOI] [PubMed] [Google Scholar]
- Wallace MN, Shackleton TM, Palmer AR. Morphological and physiological characteristics of laminar cells in the central nucleus of the inferior colliculus. Front Neural Circuits 6: 55, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenstrup JJ, Ross LS, Pollak GD. A functional organization of binaural responses in the inferior colliculus. Hear Res 17: 191–195, 1985 [DOI] [PubMed] [Google Scholar]
- Winer J, Schreiner C. The central auditory system: a functional analysis. In: The Inferior Colliculus, edited by Winer J, Schreiner C. New York: Springer, 2005, p. 1–68 [Google Scholar]
- Winer JA, Schreiner C, editors. The Auditory Cortex. New York: Springer, 2010 [Google Scholar]