Abstract
Hundreds of mutations in the SCN1A sodium channel gene confer a wide spectrum of epileptic disorders, requiring efficient model systems to study cellular mechanisms and identify potential therapeutic targets. We recently demonstrated that Drosophila knock-in flies carrying the K1270T SCN1A mutation known to cause a form of genetic epilepsy with febrile seizures plus (GEFS+) exhibit a heat-induced increase in sodium current activity and seizure phenotype. To determine whether different SCN1A mutations cause distinct phenotypes in Drosophila as they do in humans, this study focuses on a knock-in line carrying a mutation that causes a more severe seizure disorder termed Dravet syndrome (DS). Introduction of the DS SCN1A mutation (S1231R) into the Drosophila sodium channel gene para results in flies that exhibit spontaneous and heat-induced seizures with distinct characteristics and lower onset temperature than the GEFS+ flies. Electrophysiological studies of GABAergic interneurons in the brains of adult DS flies reveal, for the first time in an in vivo model system, that a missense DS mutation causes a constitutive and conditional reduction in sodium current activity and repetitive firing. In addition, feeding with the serotonin precursor 5-HTP suppresses heat-induced seizures in DS but not GEFS+ flies. The distinct alterations of sodium currents in DS and GEFS+ GABAergic interneurons demonstrate that both loss- and gain-of-function alterations in sodium currents are capable of causing reduced repetitive firing and seizure phenotypes. The mutation-specific effects of 5-HTP on heat-induced seizures suggest the serotonin pathway as a potential therapeutic target for DS.
Keywords: SCN1A epilepsy, Drosophila, sodium channel, Dravet syndrome, GEFS+
the majority of mutations causing epilepsy occur in ion channel or neurotransmitter receptor genes (Noebels 2003). In SCN1A, the NaV1.1 sodium channel gene, over 600 mutations result in a spectrum of seizure disorders ranging from genetic epilepsy with febrile seizure (GEFS+) to more severe conditions such as Dravet syndrome (DS) (Catterall et al. 2010; Escayg and Goldin 2010; George 2005). GEFS+ is characterized by frequent febrile seizures that persist beyond 6 yr of age (Scheffer and Berkovic 1997; Scheffer et al. 2009). In DS, febrile seizures apparent in the first year of life often progress to prolonged or continuous seizures along with a variety of comorbidities including psychomotor delay, ataxia, and cognitive impairment (Dravet 2011; Oguni et al. 2001). The large number of distinct GEFS+ and DS-causing SCN1A mutations has made determination of the allele-specific cellular mechanisms difficult, hampering development of effective therapeutics.
The effects of epilepsy-causing SCN1A mutations on sodium channel function were initially explored using heterologous expression systems. However, the observation that channel properties varied with expression system limited the conclusions drawn from these studies (George 2005; Lossin et al. 2003; Mantegazza et al. 2010; Spampanato et al. 2003). To circumvent these problems, recent studies have made use of two mouse knock-in models of SCN1A epilepsy: R1407X DS and R1648H GEFS+ (Martin et al. 2010; Ogiwara et al. 2007). Although located in different regions of the channel, both mutations result in constitutively reduced sodium currents, suggesting loss-of-function mutations that inhibit repetitive firing in GABAergic neurons may be a common mechanism underlying seizures caused by SCN1A mutations.
We have been developing Drosophila as a low-cost, complementary model system to explore the cellular mechanism contributing to seizures associated with SCN1A mutations. In a recent study, we demonstrated that knock-in of the GEFS+ mutation K1270T into the fly sodium channel gene para confers a temperature-sensitive seizure phenotype (Sun et al. 2012). Electrophysiology performed in the adult brain revealed this specific GEFS+ mutation causes a conditional gain in sodium current function associated with a shift in the voltage-dependent deactivation threshold of the persistent sodium current at high temperatures. In GABAergic interneurons, this leads to prolonged depolarization that also results in reduced repetitive firing (Sun et al. 2012). These data suggest that both gain- and loss-of-function SCN1A mutations can result in epileptic activity, and understanding the cellular mechanism of distinct mutations will be important for treating these disorders.
To explore further the use of Drosophila knock-ins as models of human epilepsy, we asked if different SCN1A mutations cause distinct behavioral phenotypes in flies as they do in humans. If so, are the underlying cellular mechanisms also distinct? Finally, can knock-in flies be used to identify mutation-specific therapies? To address these questions, we created a knock-in fly line carrying an SCN1A mutation (S1231R) that causes DS (Fujiwara et al. 2003). The DS flies exhibit spontaneous as well as heat-induced seizures with increased heat sensitivity compared to GEFS+ flies, consistent with the difference in disease severity in humans. The underlying changes in sodium currents are distinct in the two mutants: DS is characterized by both a constitutive and conditional loss of sodium current function as opposed to the GEFS+ mutation, which is primarily a conditional gain in sodium current function. Both, however, result in reduced repetitive firing in GABAergic neurons. We also present evidence implicating the serotonin pathway as a novel therapeutic target for DS.
MATERIALS AND METHODS
Fly lines.
The GEFS+ knock-in line, in which the K1270T mutation was introduced into the Drosophila para sodium channel gene, was established using ends-out homologous recombination as previously described (Sun et al. 2012). The DS S1231R knock-in line was made using identical procedures with the same targeting vector, which was made possible by the close proximity of the specific DS and GEFS+ mutations selected. The control knock-in line was made using the vector carrying the wild-type substitutions S1231S and K1270K.
All three knock-in lines were back-crossed to w1118, a white-eyed genetic background, for five generations. Following this, each was crossed to a line with UAS-GFP, w+ on the second chromosome. Flies homozygous for the recombinant alleles on the X chromosome and the UAS-GFP, w+ on the second chromosome were selected to establish the three strains used in the experiments reported. These are referred to as GEFS+, DS, and control. Heterozygotes were generated by crossing these lines to each other. All flies were grown at room temperature (22–24°C) under a 12:12-h light-dark cycle.
High-volume heat-induced seizure assay.
In our previous study, seizure assays in knock-in flies were conducted by heating 1 fly/vial in a water bath with continuous monitoring of seizure activity for 120 s (Sun et al. 2012). With this method, the seizure activity could be modeled by the equation t = 120 p, where t is total time spent seizing in seconds and p is the average probability of seizing, calculated by evaluating seizure probability at 1-s intervals (1 Hz). To establish a more efficient assay, we evaluated seizing behavior from 2-min videos of vials containing multiple flies and found that it was possible to accurately assess seizing activity in up to 5 flies/vial.
In addition, we calculated the minimal sampling rate that would accurately model the total seizing time t determined using a 1-Hz sampling rate. From our database containing entries from 3,520 flies in 70 independent experiments with a sampling rate of 1 Hz, data were binned in groups of 5 and the average probability of seizing at various sampling rates was calculated. This indicated 0.05 Hz as the minimal sampling rate where the average probability of seizing p maintained the linear relationship with the total seizing time t with R2 > 0.99. The absolute difference between the true total seizing time and the calculated total seizing time is 1.5 ± 0.94 s (mean ± SD).
To validate this strategy empirically, we repeated heat-induced seizure experiments in 2 independent experiments with 990 flies. Total seizing time from continuously monitoring seizing activity was not significantly different from that obtained by monitoring 5 flies/vial with a 0.05-Hz sampling rate. On the basis of this analysis, heat-induced seizure assays were carried out by heating 5 flies/vial in a water bath under continuous video recording, and the probability of seizing among the 5 flies (i.e., 1/5 = 20%, 4/5 = 80%) was determined at 20-s (0.05 Hz) intervals during video review. The total seizing time t was calculated using the equation t = 110.7p − 1.5. All behavior experiments were conducted blind with respect to genotype and/or drug treatment.
Whole cell recordings from local neurons in isolated whole brain.
All brains were obtained from adult DS or control flies (2–5 days old). The entire brain was removed from the head and prepared for recording as previously described (Gu and O'Dowd 2006, 2007). Pipettes were visually targeted to a small subset of local neurons (LNs) in an anatomically distinct region of the dorsal lateral aspect of the antenna lobe. Whole cell sodium currents, depolarization-evoked action potentials, and spontaneous burst of firing were recorded with standard whole cell pipettes of 10–11 MΩ. All voltages reported refer to pipette potentials at the soma.
Isolated sodium currents were recorded using a pipette solution containing (in mM) 102 d-gluconic acid, 102 CsOH, 0.085 CaCl2, 1.7 MgCl2, 17 NaCl, 0.94 EGTA, 8.5 HEPES, and 4.5 ATP. The pH was adjusted to 7.2 and osmolarity to 235 mosM. The external solution contained (in mM) 120 NaCl, 1.8 CoCl2, 0.8 MgCl2, 3 KCl, 5 glucose, 10 HEPES, 2.5 tetraethylammonium (TEA), and 1.0 4-aminopyridine (4-AP); it also contained the synaptic receptor blockers d-turbocurarine (curarine; 20 μM) and picrotoxin (PTX; 10 μM). The pH was adjusted to 7.2 and osmolarity to 250 mosM. Data shown were corrected for the 5-mV liquid junction potential generated in these solutions. Depolarization-evoked action potentials were recorded using the same solutions as those used for sodium currents except that cesium gluconate in the internal solution was replaced by potassium gluconate, CoCl2 in the external solution was replaced by CaCl2, and there was no TEA or 4-AP. For examination of the evoked firing properties, the membrane potential was held at −75 mV by injection of hyperpolarizing holding current. Spontaneous burst firing was recorded using the same internal and external solution used for depolarization-evoked action potentials, with the omission of PTX and curarine.
The chamber was continuously perfused at 1 ml/min with external solution, and the temperature in the chamber was controlled and monitored using a CL-100 Bipolar Temperature Controller (Harvard Apparatus). Although there were small variations in the heating profile between individual neurons during the 2-min heating period, there was no difference between the genotypes in the maximal temperatures examined (control 35.2 ± 0.7°C, DS 35.2 ± 0.9°C, means ± SD).
Data were acquired with a patch-clamp L/M-EPC7 amplifier (List Medical), a Digidata 1322A digital-to-analog converter (Molecular Devices), a Dell computer (Dimension 8200), and pClamp9 software (Molecular Devices).
Three-day drug administration.
Two-day-old female DS and GEFS+ flies were transferred into vials with standard cornmeal food that contained various concentrations of drugs. Flies were transferred once to fresh vials after 24–48 h on drug-containing food, and seizure assays were done at 72–74 h. Experiments were conducted blind with respect to drug concentrations.
For drug-containing food preparation, the drugs were dissolved in 1 ml of vehicle, which was added to 19 ml of liquefied cornmeal-based fly food (approximately ≤60°C), and the mixture was vortexed for 5 s. Deionized water was used as vehicle for 5-hydroxytryptophan (5-HTP; Sigma), histamine bisphosphate (histamine; Sigma), and cimetidine (Sigma), and 1 N HCl was the vehicle for 4-chloro-dl-phenylalanine (PCPA; Sigma), 3,4-dihydroxy-l-phenylalanine (l-DOPA; Sigma), and 3-iodo-l-tyrosine (3-IY; Sigma). Approximately 1.5–2 ml of food were poured into 25-mm plastic vials to cover the bottom of each vial. After the food solidified, the vials were stored at 4°C for up to 3 days.
Statistics
Comparisons between GEFS+, DS, and control flies were done with two-tailed independent Student's t-test (2 genotypes) or one-way ANOVA followed by the Bonferroni post hoc test (more than 2 genotypes). Comparisons between permissive and elevated temperature within the same genotype were done with the paired t-test. Differences between mean values were considered significant at P ≤ 0.05.
RESULTS
Increased sensitivity to heat-induced seizure in DS vs. GEFS+ knock-ins.
At room temperature, both DS and GEFS+ knock-in flies exhibit abnormal behaviors that appear to mimic partial seizures, such as repetitive grooming that often results in broken wings in aged flies. There are also aberrant behaviors that are distinct to each mutant line. Some DS flies exhibit rapid wing flapping while walking that can cause the flies to lose balance and bounce around in the vials, whereas GEFS+ flies exhibit repetitive jerks of one or more legs that can result in the flies flipping onto their back (see Supplemental Movies S1 and S2). (Supplemental material for this article is available online at the Journal of Neurophysiology website.) However, at the normal rearing temperature (22–24°C), the spontaneous seizure-like events in both mutant lines occur at low frequency and are variable in nature and duration, limiting their utility for quantitative analysis of seizure behavior.
We next examined the effect of the DS mutation on sensitivity to heat-induced seizures. Since the para gene is located on the X chromosome, we assessed activity in hemizygous male (DS/Y) and homozygous female (DS/DS) mutants separately. When 2-day-old DS knock-in flies in plastic vials were immersed in a 40°C water bath, they exhibited a rapid decline in coordinated walking movements, followed by falling onto their backs or sides. This was classified as a seizure on the basis of continuous movement of the legs and abdomen in the fallen flies. Within 20 s of immersion in the water bath, 100% of both DS/Y and DS/DS were seizing and continued to do so throughout the 2-min heating period (Fig. 1A). When the vials were removed from the water bath, the flies rapidly resumed walking/climbing behavior. No temperature-sensitive seizure phenotype was apparent in the control strain under the same conditions (Fig. 1A).
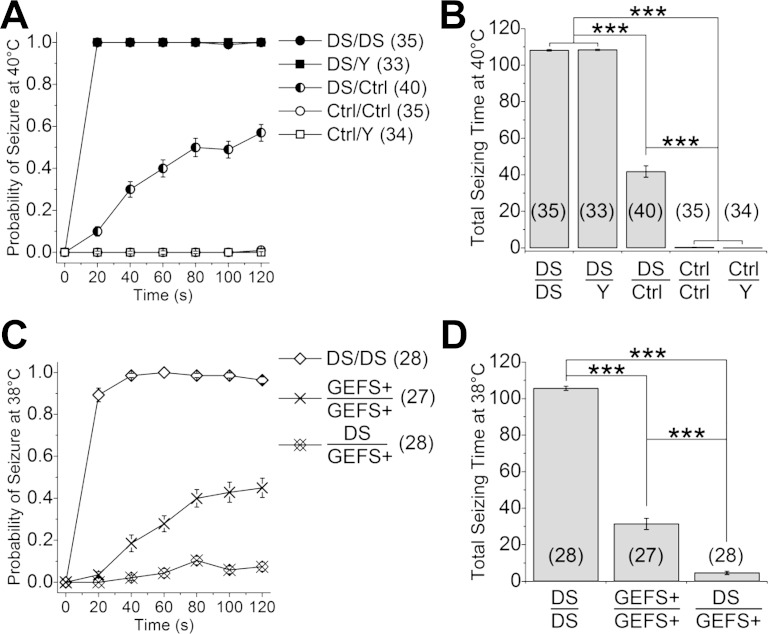
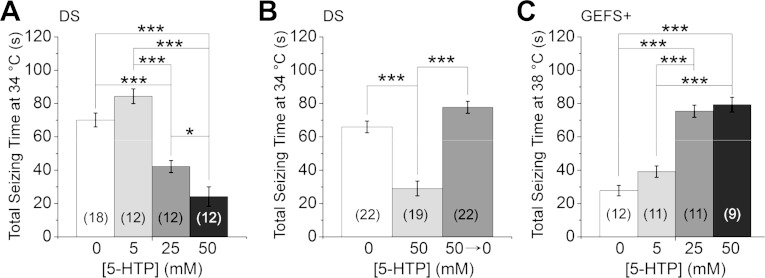
Fig. 1.
Dravet syndrome (DS) flies are more sensitive to heat-induced seizure than GEFS+ flies. A: DS (S1231R) knock-ins display semidominant seizure phenotype when exposed to 40°C. Note that the probability of seizure of DS/Y and Ctrl/Y flies is the same as that of DS/DS and Ctrl/Ctrl flies, respectively, and overlap on the graph. B: total time spent seizing during 120 s of heating is significantly different between the mutants (DS/DS and DS/Y), heterozygotes (DS/Ctrl), and controls (Ctrl/Ctrl and Ctrl/Y). C: DS flies display higher probability of seizure than GEFS+ (K1270T) flies at 38°C. The reduced probability of seizure in DS/GEFS+ flies suggests the mechanisms underlying seizure conferred by the 2 mutations are different. D: total seizing time at 38°C is significantly shorter in DS/GEFS+ than in DS/DS and GEFS+/GEFS+ flies, respectively. ***P < 0.001, ANOVA, Bonferroni post hoc test. Symbols and error bars represent means ± SE from the number of groups of 5 flies indicated (n).
Since DS is an autosomal dominant disorder in human, we tested the temperature-sensitive behavior of flies heterozygous for the DS mutation (DS/control). The time to seizure onset in the DS/control flies was delayed, and the maximal probability of seizing was lower than in the homozygous mutants (Fig. 1A). Furthermore, there was marked variability in the response of the heterozygotes, with 11% showing no seizures. The majority (89%) exhibited seizure activity, but the seizure events could start and stop more than once during the 2-min period. A significant difference in total seizing time was apparent between the three genotypes with homozygotes > heterozygotes > control (Fig. 1B). These data indicate that, similar to its presentation in human, the S1231R DS mutation in Drosophila is semidominant with regard to heat-induced seizure behavior.
Although our data indicate the DS mutation results in semidominant inheritance of a heat-induced seizure phenotype in flies, as we previously reported for the GEFS+ mutation (Sun et al. 2012), the seizures in the two knock-in lines, like their human counterparts, are clearly distinct. This includes hypoactivity in DS flies at room temperature and before onset of heat-induce seizures, whereas GEFS+ flies have a level of activity similar to that of control flies at room temperature but are hyperactive before onset of seizures (Supplemental Movie S3). Most distinctively, after being removed from heated water bath, all of the DS flies (n = 35) resume normal locomotion without experiencing the period of postseizure nonresponsiveness (9–28 min) characteristic of the GEFS+ flies (Sun et al. 2012).
To directly compare the seizure behavior caused by the DS and GEFS+ mutations, the temperature sensitivity of the seizures was examined. Even at the lower temperature of 38°C, homozygous DS flies still exhibited rapid seizure onset and a high maximal seizure probability. In contrast, seizure onset was markedly slower and the total seizing time significantly reduced in GEFS+ flies (Fig. 1, C and D). In addition, at 38°C, flies heterozygous for the two mutations (DS/GEFS+) showed a reduced rather than an intermediate seizure probability compared with either the DS/DS or GEFS+/GEFS+ flies (Fig. 1, C and D). These data indicate that different cellular mechanisms, rather than a common mechanism with varying severity, underlie the temperature-sensitive seizure phenotypes in the DS and GEFS+ mutants. This raises the interesting possibility that the two mutations co-suppress each other's seizure phenotype via offsetting cellular mechanisms.
DS mutation S1231R causes both constitutive and heat-induced reductions in sodium currents.
To determine if the cellular mechanisms contributing to temperature-sensitive seizures in DS and GEFS+ knock-in flies are distinct, we examined sodium currents in the GABAergic LNs in brains isolated from adult male flies (Gu and O'Dowd 2006, 2007). The LNs examined were in the dorsal lateral aspect of the antennal lobe, the same population previously studied in the GEFS+ mutants (Sun et al. 2012).
Isolated sodium currents in both Control and DS LNs exhibited two distinct components: a transient current (INaT) and a persistent current (INaP) (Fig. 2A). The LN sodium currents are not well space-clamped in either the control or DS mutant LNs, consistent with most channels being located in neuronal processes that are electrotonically distant from the cell body. Whereas this precludes obtaining an accurate biophysical description of the current, threshold measurements can be used to identify differences associated with genotype and/or response to elevated temperature.
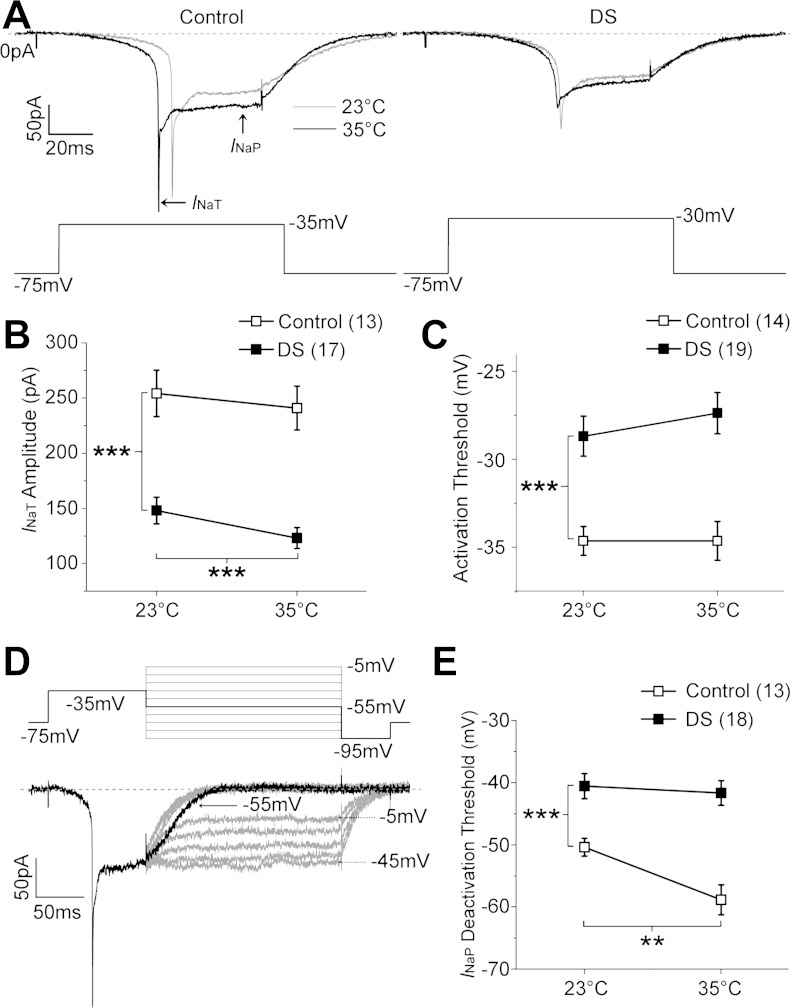
Fig. 2.
DS local neurons (LNs) exhibit both constitutive and conditional reduction in sodium current activity. A: sodium currents elicited by depolarizing voltage steps in control and DS LNs at 23 and 35°C. B: transient current (INaT) amplitude in DS LNs is significantly reduced compared with that in control LNs at 23°C (independent t-test) and is further reduced at 35°C (paired t-test). C: sodium current activation threshold is significantly more depolarized in DS compared with control LNs. D: deactivation threshold is defined as the least negative voltage step that results in the decline of persistent current (INaP) toward baseline, as illustrated in a control LN. E: deactivation threshold is significantly more depolarized in DS compared with control LNs at 23°C (independent t-test). Deactivation threshold is more depolarized in control LNs at 35°C compared with 23°C (paired t-test), but it is insensitive to heating in DS LNs (P = 0.54, paired t-test). **P < 0.01; ***P < 0.001. Symbols and error bars represent means ± SE from the number of LNs indicated (n).
The peak INaT amplitude, although likely to be an underestimate due to the similarly poor space clamp in both genotypes, is significantly reduced in DS compared with control LNs at 23°C (Fig. 2B). The change in current amplitude is unlikely to be due to difference in cell size, since there is no difference in the mean cell capacitance between DS (24.0 ± 1.3 pF) and control LNs (25.2 ± 1.2 pF). The reduced amplitude in the mutant could be due to changes in the number, localization, and/or function of the channels. However, in DS LNs there is also a significant further reduction (P < 0.001, paired t-test) in current amplitude as the temperature is raised to 35°C that is not seen in control LNs (Fig. 2, A and B). This indicates that the DS S1231R mutation directly results in reduced channel function at elevated temperatures. Although the threshold voltage for activating sodium currents is also significantly more depolarized in DS compared with control LNs, the activation threshold does not change at elevated temperature in either control or DS (Fig. 2C).
We previously reported an increased hyperpolarizing shift in INaP deactivation threshold in GEFS+ LNs at elevated temperature (Sun et al. 2012). To determine if this property is also changed in DS LNs, we used the same three-step protocol: a prepulse to inactivate INaT, followed by a family of test pulses from −5 to −95 mV (Fig. 2D). The threshold voltage for INaP deactivation is defined as the least negative test pulse that results in current returning to baseline. In the control LN illustrated in Fig. 2D, INaP persists during the test pulses from −5 to −45 mV and returns to baseline when the test pulses are between −55 to −95 mV. Therefore, the deactivation threshold for INaP in this LN is −55 mV. The average INaP deactivation threshold is significantly less hyperpolarized in DS LNs compared with control LNs at 23°C (Fig. 2E). Whereas increasing the temperature to 35°C has no effect on the deactivation of INaP in DS LNs, the difference in INaP deactivation threshold between control and DS LNs is magnified due to the significant shift toward a hyperpolarized voltage in control LNs (Fig. 2E). These data indicate that the DS mutation causes a less hyperpolarized deactivation voltage of the INaP than the control at room temperature and impairs temperature-sensitive hyperpolarizing shift of the INaP deactivation voltage at elevated temperature.
DS LNs exhibit reduced evoked firing frequency.
To investigate how the DS-induced changes in sodium currents affect excitability in LNs, we first examined firing properties in response to depolarizing current injections. To reduce the spontaneous activity in LNs, all cells were held at −75 mV and synaptic blockers PTX and curarine were added to the recording solution.
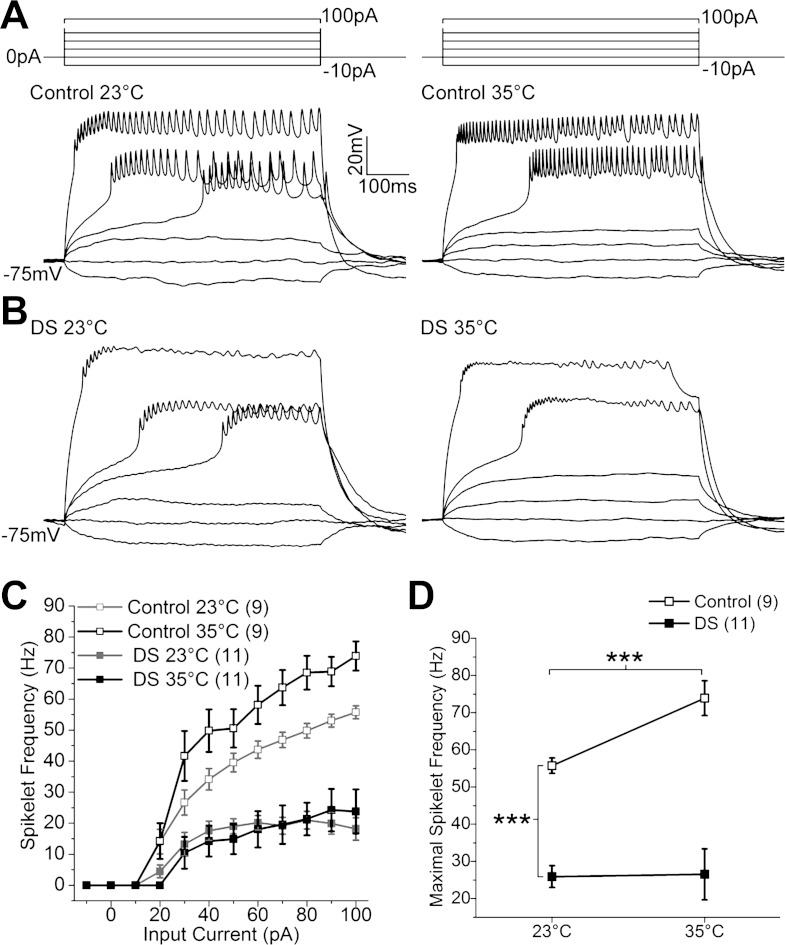
In both control and DS LNs, suprathreshold current injections evoked depolarizations capped by a train of small-amplitude spikelets characteristic of sodium-dependent action potentials (Fig. 3, A and B). Spikelet frequency was quantified by counting the number of spikelets that are 2 mV or greater in amplitude. In control LNs, the spikelet frequency increased with increasing current injection at both 23 and 35°C (Fig. 3, A and C). In contrast, the spikelet frequency remained unchanged in DS LNs at higher input currents (Fig. 3, B and C). In addition, the maximal firing frequency in DS LNs is significantly lower compared with that in control LNs at 23°C (Fig. 3D). When the temperature was raised to 35°C, trains of spikelets were maintained throughout the current step in control LNs. In contrast, spikelets in DS LNs often terminated before the end of the current step. The significant increase in maximal firing frequency characteristic of control LNs when temperature is elevated from 23 to 35°C is absent in DS LNs (Fig. 3D).
Fig. 3.
Evoked firing frequency is constitutively decreased in DS LNs. A and B: trains of spikelets recorded from control (A) and DS LNs (B) at 23 and 35°C evoked by the illustrated stimulus protocols. C: average spikelet frequency of control and DS LNs plotted as a function of injected current at 23 and 35°C. D: maximal spikelet frequency is significantly reduced in DS compared with control LNs at 23°C (independent t-test). Maximal spikelet frequency in control LNs increases at 35°C (paired t-test). There is no change in maximal spikelet frequency in DS LNs when temperature is raised to 35°C (P = 0.91, paired t-test). ***P < 0.001. Symbols and error bars represent means ± SE from the number of LNs indicated (n).
Together, these data indicate that the constitutive reduction in sodium current amplitude and smaller voltage range over which DS sodium currents are active contribute to the reduced firing frequency in DS LNs at permissive temperature. Further reduction of the current amplitude at higher temperature and absence of a temperature-dependent hyperpolarizing shift in INaP deactivation threshold may be responsible for the inability of spikelet frequency to increase in DS LNs at the higher temperature.
DS LNs show decreased spontaneous burst frequency.
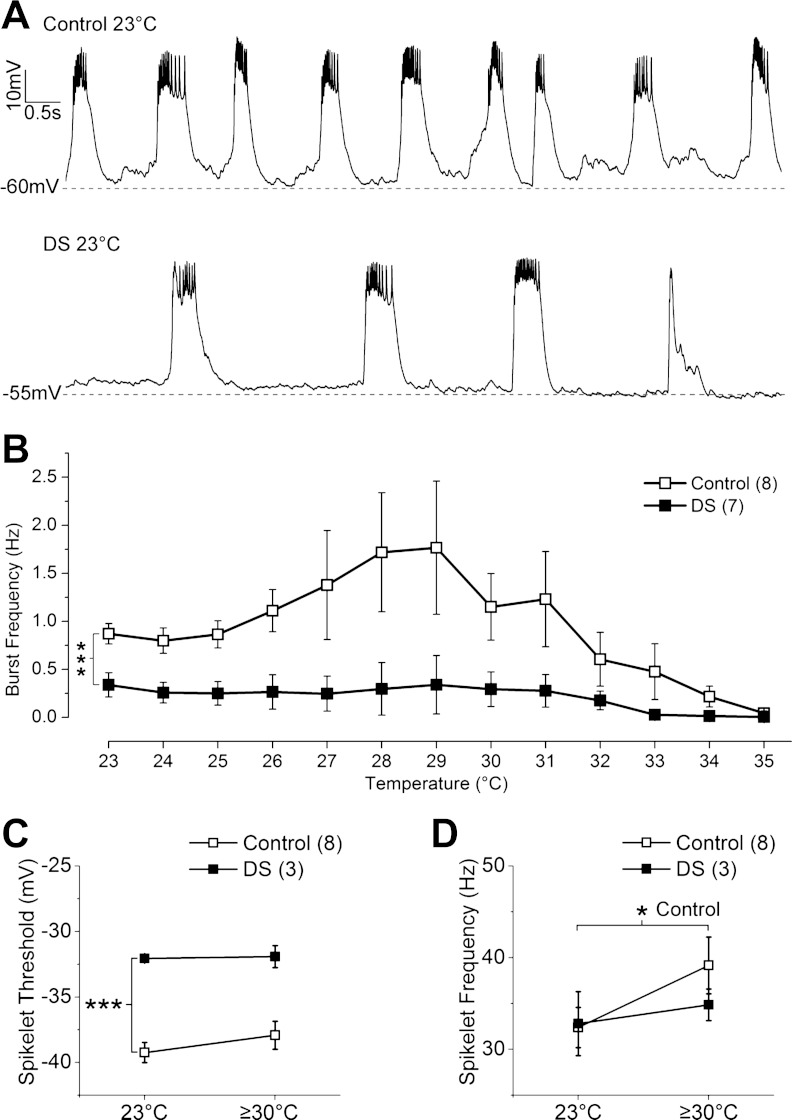
To examine how the DS mutation affects neuronal circuit activity, spontaneous activity in LNs was monitored in the absence of synaptic current blockers and without application of hyperpolarizing holding current. Spontaneous activity was recorded continuously for 15 min: 5 min at 23°C, 2 min during which time temperature was increased to 35°C, 3 min as temperature was returned to 23°C, and 5 min at 23°C.
At 23°, both DS and control LNs exhibit regular burst firing characterized by slow-wave depolarizations capped by a variable number of spikelets (Fig. 4A). The most striking change is a significant reduction in the burst frequency in DS compared with control LNs (Fig. 4, A and B). As the temperature of the recording solution increases toward 35°C, the burst frequency in control LNs initially increases and then decreases before ceasing completely (Fig. 4B). Normal burst firing resumes as the temperature returns to 23°C (data not shown). In contrast, DS LNs do not exhibit the increase in burst frequency at 27–31°C, so the difference between the control and DS LNs is magnified at these temperatures (Fig. 4B).
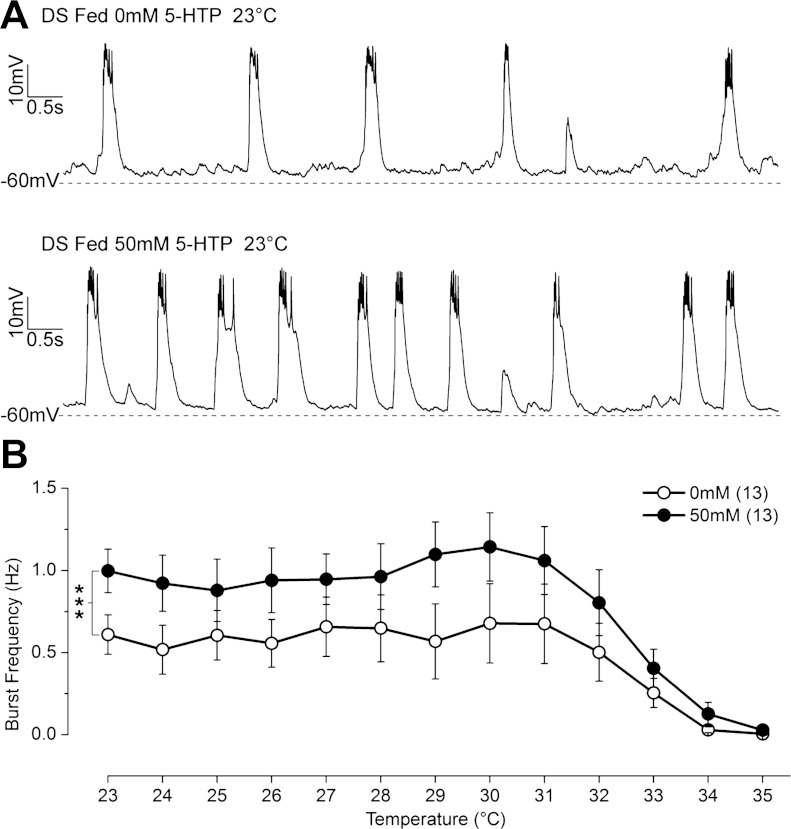
Fig. 4.
In DS flies, spontaneous burst firing frequency is constitutively reduced and does not show normal heat-induced increase. A: spontaneous burst firing recorded in control and DS LNs at 23°C. B: spontaneous burst frequency in DS LNs is significantly lower at 23°C and responds differently to temperature elevation than control LNs (***P < 0.001, 2-way ANOVA). Spontaneous burst frequency in DS LNs does not increase at lower temperatures of 27-31°C as seen in the control LNs. C: spikelet threshold is more depolarized in DS than in control LNs at 23°C (***P < 0.001, independent t-test) and does not change in either control (P = 0.15) or DS (P = 0.88, paired t-test) when temperature is elevated. D: spikelet frequency in DS LNs does not increase (P = 0.37) as it does in control LNs (*P < 0.05, paired t-test) when the temperature is raised to 35°C. Symbols and error bars represent means ± SE from the number of LNs indicated (n).
The mechanism underlying cessation of burst firing in control LNs at high temperature is not well understood. However, since the LNs can fire repetitively at 35°C when given depolarizing current injection (Fig. 3A), we suspect that reduced synaptic excitation at elevated temperature contributes to cessation of burst firing in this excised brain preparation. The comparison of burst firing between DS and control LNs under similar conditions shows that the DS LNs lose the ability to increase burst firing frequency during the initial heating, strongly implicating an increased susceptibility to loss of synaptic input in DS LNs. These data suggest that increasing burst firing frequency in GABAergic neurons in the control flies is likely to play a role in preventing heat-induced seizures, and failure to do so, as in the DS mutant, may contribute to heat-induced seizures.
As predicted from the threshold sodium current measurements, the spikelet threshold is significantly more depolarized in DS compared with control LNs at 23°C, and there is no change in either control or DS LNs at elevated temperature (Fig. 4C).
The spikelet frequency (number of spikelets/burst duration) is not significantly different in control vs. DS LNs at 23°C (Fig. 4D). However, the spikelet frequency in control LNs is increased above 30°C compared with the frequency at 23°C (Fig. 4D). The measurement of spikelet frequency at elevated temperature is complicated in DS LNs, since 4 of the 7 DS LNs had fewer than 3 bursts above 30°C, preventing reliable measurement of spikelet frequency in those LNs. In the remaining 3 DS LNs, however, spikelet frequency above 30°C is not significantly different from that at 23°C (Fig. 4D). The lack of increase in spikelet frequency with increasing temperature is consistent with the findings in evoked spikelet frequency.
There are no significant differences in resting membrane potential, burst amplitude, or burst duration between control and DS LNs at permissive or high temperatures (data not shown).
These data indicate that changes in sodium channels caused by the DS mutation not only reduce intrinsic excitability in the LNs but also result in a reduction of spontaneous burst firing frequency at room temperature that is more pronounced at high temperature. No evidence of prolonged depolarizations characteristic of GEFS+ mutant LNs at high temperatures (Sun et al. 2012) was observed, supporting the conclusion that distinct cellular mechanisms mediate heat-induced seizures in DS and GEFS+ mutants.
Feeding flies 5-HTP suppresses heat-induced seizures in DS but not GEFS+ knock-ins.
Seizures in Dravet syndrome patients are often resistant to treatment with pharmaceutical agents (Chiron and Dulac 2011). An important question therefore is, can the knock-in flies be used to identify modifiers that suppress the seizures? To this end we established an efficient heat-induced seizure assay to test and analyze the total seizing time during heating for 120–150 flies per hour following extended drug treatment (see materials and methods).
Both DS and GEFS+ knock-in lines were generated in a white eye (w−) mutant background. Before the studies reported in this and our previous paper were begun, the second chromosome was replaced with one containing the mini-white gene (w+), restoring the normal red eye color. However, comparison of heat-induced seizures in DS and GEFS+ flies revealed unexpected differences in temperature sensitivity in the red vs. white eye backgrounds (data not shown). Previous studies showing mutation of the white gene, an ABC transporter, reduces expression of monoamines in the adult brain (Borycz et al. 2008) led us to explore the role of monoamine signaling on seizure behavior.
Initial studies were carried out in homozygous and heterozygous female DS flies to determine if there is a gene dosage-dependent response to drug treatments. However, the large variability in sensitivity to heat-induced seizures in the heterozygous females made it more difficult to quantify the effects of drug treatments. Therefore, only homozygous females were used to test the effects of drugs in mutant flies. To modulate monoamine levels, 2-day-old adult knock-in female flies were transferred to vials with food containing different concentrations of the test drug for 3 days prior to behavioral testing. To allow for detection of positive or negative effects of drug treatments, seizure assays were carried out at a temperature where untreated female knock-in flies showed an intermediate level of seizures. This feeding paradigm with 5-HTP, the immediate precursor to serotonin, results in a 15- to 20-fold increase in serotonin levels in adult fly head (Dierick and Greenspan 2007).
DS flies fed with 5-HTP exhibited a significant dose-dependent reduction in total seizing time (Fig. 5A). To determine if suppression of heat-induced seizures was reversible, three groups of DS flies were processed in parallel: group 1 was fed 0 mM, and group 2 was fed 50 mM 5-HTP continuously for 6 days, and group 3 was fed 50 mM 5-HTP for 3 days followed by 0 mM 5-HTP for another 3 days. Total seizing time was evaluated in the 3 groups in the 8-day-old adults. Consistent with the previous result, total seizing time was significantly reduced in DS flies fed continuously with 5-HTP compared with the nontreated DS flies. However, seizure time of flies that were returned to normal food following 5-HTP exposure was indistinguishable from that of the nontreated DS flies (Fig. 5B). These data suggest that increasing serotonin levels in DS flies reversibly suppresses sensitivity to heat-induced seizures. Manipulation of dopaminergic or histaminergic signaling, using dopamine synthesis modulators l-DOPA and 3-IY (Bainton et al. 2000; Neckameyer 1996) or histamine bisphosphate and the histamine receptor blocker cimetidine (Hong et al. 2006), failed to suppress seizure activity in the DS mutants (data not shown).
Fig. 5.
Systemic upregulation of serotonin has differential effects on temperature-sensitive seizures in DS and GEFS+ flies. A: total seizing time decreased in a dose-dependent manner in DS flies fed with the serotonin precursor 5-hydroxytryptophan (5-HTP) for 3 days. B: the effect of 5-HTP treatment is reversible. Total seizing time in flies fed 5-HTP for 6 days (50 mM) was significantly lower than flies fed vehicle (0 mM) or flies fed 5-HTP for 3 days followed by 3 days of vehicle only (50→0 mM). C: in contrast, 5-HTP treatment increases total seizing time in GEFS+ flies in a dose-dependent manner. *P < 0.05; ***P < 0.001, ANOVA with Bonferroni post hoc tests. Symbols and error bars represent means ± SE from the number of groups of 5 flies indicated (n).
To test whether the effect of 5-HTP on seizure phenotype is mutation specific, GEFS+ flies were also fed 5-HTP. Interestingly, the heat-induced seizure was exacerbated in a dose-dependent manner (Fig. 5C). The divergent effects of increasing serotonin levels are consistent with two distinct mechanisms of seizure generation in the DS vs. the GEFS+ knock-in.
5-HTP partially rescues reduced LN burst firing frequency in DS knock-ins.
To begin exploring the cellular mechanism underlying suppression of heat-induced seizures in DS knock-ins, we examined the spontaneous activity in GABAergic LNs in DS flies fed 5-HTP for 3 days. For consistency with the behavioral studies using 5-HTP, these experiments were conducted in female flies. There were no differences in physiological properties of LNs in 5-day-old females not fed 5-HTP and the 2-day-old males in the previous studies.
The spontaneous burst frequency in DS flies fed 5-HTP is significantly increased compared with untreated DS flies at 23°C (Fig. 6, A and B). As the temperature rises toward 35°C, the burst frequency in both 5-HTP treated and untreated DS LNs is stable up to 32°C, at which point the frequency in both groups begins to decline before complete cessation at 35°C. However, the burst firing frequency of LNs in 5-HTP treated DS flies is significantly increased compared with that in untreated DS flies for most of the temperature range (Fig. 6B).
Fig. 6.
5-HTP partially normalizes spontaneous burst firing frequency in DS LNs. A: spontaneous bursts of firing recorded at 23°C in LNs from 5-day-old DS flies fed with 0 and 50 mM 5-HTP for 3 days. B: spontaneous burst frequency at 23°C is significantly increased in LNs in DS flies fed with 50 mM 5-HTP and are overall more resistant to temperature elevation than the LNs in untreated DS flies (***P < 0.001, 2-way ANONA). Symbols and error bars represent means ± SE from the number of LNs indicated (n).
There was no change in LN spikelet frequency, spikelet threshold, or burst duration in DS flies fed 5-HTP (data not shown), indicating that the treatment had no effect on intrinsic properties of the LNs. In addition, there were no significant changes in sodium current properties following 5-HTP treatment (data not shown).
These data suggest that 5-HTP does not directly reverse the effects of the DS mutation on sodium channels but suppresses the seizure phenotype by modulating circuit activity.
DISCUSSION
Different SCN1A mutations cause distinct seizure phenotypes in flies consistent with their effects in humans.
In humans, the S1231R mutation in SCN1A causes Dravet syndrome, whereas the K1270T mutation, less than 40 amino acids away, is associated with GEFS+. Although both of these are disorders in which individuals exhibit febrile and afebrile seizures, DS is a more severe disorder than GEFS+ (Abou-Khalil et al. 2001; Dravet 2011; Fujiwara et al. 2003; Scheffer and Berkovic 1997). Remarkably, inserting these mutations into the Drosophila sodium channel also results in mutant lines with distinct seizure phenotypes. At normal rearing temperature (22–24°C), both GEFS+ and DS flies exhibit a range of abnormal behaviors that appear to mimic partial seizures. Whereas the low frequency of these spontaneous events makes quantitative comparison between the lines difficult, the conditional nature of febrile seizures makes them well suited for such measures.
Our data demonstrate that the S1231R and K1270T mutations individually confer a semidominant heat-induced seizure behavior in Drosophila, with each mutant exhibiting unique characteristics. The increase in sensitivity to heat-induced seizures in DS relative to GEFS+ flies is consistent with the increased overall severity of DS compared with GEFS+ in humans.
The DS and GEFS+ flies also exhibit distinct postseizure behaviors: DS flies return to normal activity without experiencing the extended period of nonresponsiveness following heat-induced seizure characteristics of the GEFS+ flies. The period of nonresponsiveness in GEFS+ flies could be a result of altered state of consciousness or paralysis; nevertheless, it may resemble the postictal states in humans (Fisher and Schachter 2000; Widdess-Walsh and Devinsky 2010). The differences in heat sensitivity and postseizure behavior, along with the other distinct characteristics of the seizures, demonstrate that the GEFS+ and DS mutations result in two different seizure phenotypes in flies as well as in humans.
In addition to febrile and afebrile seizures, most DS patients also exhibit comorbidities including developmental delays, ataxia, and cognitive deficits (Dravet 2011). Interestingly, the DS flies exhibit altered locomotion at room temperature, characterized by uncoordinated leg movements and abnormal wing extension during walking that are absent in control and GEFS+ flies (data not shown). The reduced coordination during locomotion suggests additional neuronal abnormalities in the DS mutants. Future studies will be important in exploring how these mutations affect locomotion and other behaviors including learning and memory. Longevity assays may also be useful in exploring if/how these mutations affect life span.
Distinct alterations in sodium currents in DS and GEFS+ knock-in flies.
Studies in a mouse SCN1A knock-out and a SCN1A DS nonsense mutation knock-in (R1407X) report sodium currents with reduced magnitude and reduced excitability in inhibitory neurons at behaviorally permissive temperatures (Ogiwara et al. 2007; Yu et al. 2006). Since more than half of the SCN1A mutations found in DS patients result in truncations of Nav1.1 channels, constitutive loss of function of Nav1.1 channel is likely to be a common mechanism of DS (Catterall 2012). Our study demonstrates for the first time that a missense mutation that causes DS results in a constitutive reduction as well as a novel temperature-dependent decrease in sodium current magnitude in an in vivo model system (Fig. 7A, arrowhead). In addition, in DS LNs the more depolarized activation threshold for the sodium current along with less repolarization required to deactivate the INaP results in sodium currents that are active over a smaller voltage range than in control LNs (Fig. 7B). Therefore, we have classified the S1231R DS as a loss-of-function mutation that has both a constitutive and heat-sensitive component.
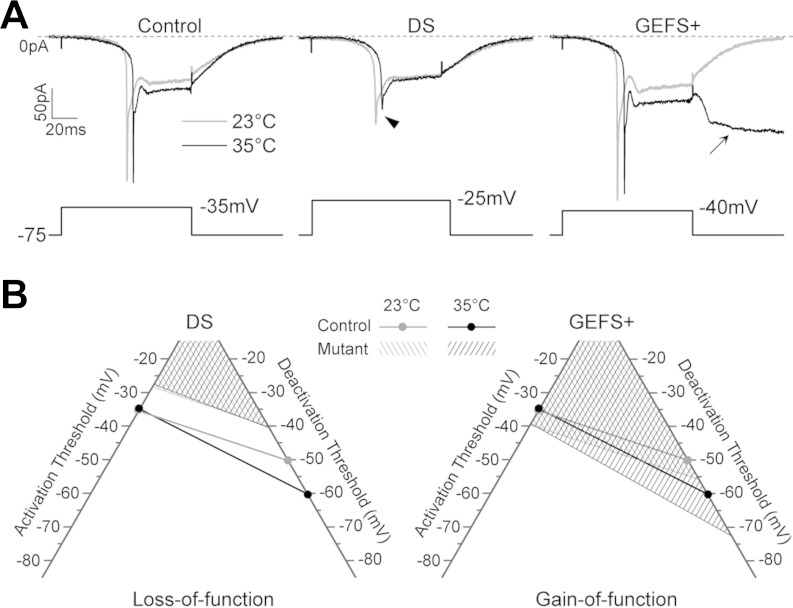
Fig. 7.
DS S1231R is a loss-of-function mutation and GEFS+ K1270T is a gain-of function mutation. A: representative sodium currents recorded in a control, DS, and GEFS+ LN at 23 and 35°C. Note the reduced sodium current magnitude in the DS LN (arrowhead) and the nondeactivating INaP at −75 mV in the GEFS+ LN (arrow). B: the voltage range over which sodium currents are active, denoted by the area above the activation and deactivation threshold, is smaller in DS than in control LNs at 23°C and does not increase at 35°C as in control LNs. In contrast, the GEFS+ mutation causes a more significantly increased sodium current conductive range than the control, particularly at 35°C, at which the deactivation threshold of INaP is below −70 mV.
The changes associated with the DS knock-ins are clearly distinct from the alterations we previously reported in the GEFS+ knock-ins that were made using the same targeting vector (Sun et al. 2012). First, the amplitude of INaT that is reduced in DS LNs at room temperature and further decreased at elevated temperature is not affected by the GEFS+ mutation (Fig. 7A). Second, in GEFS+ GABAergic LNs the sodium currents are active over a larger voltage range than in control LNs, especially at elevated temperature, due to the less depolarized activation threshold for the sodium current and the greater repolarization required to deactivate the INaP (Fig. 7B). The K1270T GEFS+ is therefore classified as a primarily conditional gain-of-function mutation.
These data demonstrate that both loss-of-function and gain-of-function mutations in a single sodium channel gene can result in a heat-induced seizure phenotype. Furthermore, the temperature-dependent changes in sodium current function seen in both the DS and GEFS+ knock-in flies suggest that future studies in mammalian models should evaluate sodium current function at high as well as permissive temperatures (Martin et al. 2010; Ogiwara et al. 2007; Yu et al. 2006).
How could the amino acid changes in the DS and GEFS+ missense mutants affect the voltage range over which sodium currents are active? The voltage-gated sodium channel in Drosophila, as in mammals, is composed of four homologous domains (DI-DIV), with each domain consisting of six transmembrane segments (S1–S6) (Loughney et al. 1989). The four domains create a central ion-conducting pore surrounded by four voltage sensors composed of the S1–S4 segments (Yu and Catterall 2004). A recent modeling study indicates the outward movement of S4 during channel activation involves interactions between the positively charged arginines in S4 and the negatively charged residues in S1–S3 (Yarov-Yarovoy et al. 2012). Based on this gating model, substitution of amino acid residues with different charge or size in S1–S3 may interfere with S4 movement, resulting in alterations in sodium channel gating properties. In DS, the replacement of a small polar amino acid serine (S1231) by a large positively charged arginine in DIIIS1 could contribute to the reduced voltage range over which the channels are active. The opposite may occur in GEFS+ where a large positively charged lysine (K1270) in DIIIS2 is replaced by a small polar amino acid threonine. This can be tested by creating knock-in mutants where different amino acids substitutions are made at these same locations.
Both loss-of-function and gain-of-function mutations can result in reduced repetitive firing.
In DS knock-in flies, the decrease in sodium current and more limited voltage range over which the current is active are consistent with the reduced firing frequency in LNs at permissive temperatures. Furthermore, the novel temperature-sensitive reduction in current amplitude and lack of normal increase in conductance range could account for the absence of the typical increase in spikelet frequency seen in control LNs as the temperature is raised. This is a distinct mechanism, underlying reduced repetitive firing, from that of the GEFS+ mutation, in which the increased hyperpolarizing shift in INaP deactivation voltage results in sustained membrane depolarization, preventing repetitive firing (Sun et al. 2012). Decreased repetitive firing in GABAergic neurons would reduce inhibition, increasing the probability of seizure activity.
The different but complementary changes in sodium current caused by the DS and GEFS+ mutations are also consistent with the behavioral data demonstrating that the GEFS+/DS flies exhibit lower sensitivity to heat-induced seizures than either DS/DS or GEFS+/GEFS+ flies. The hyperpolarizing shift in voltage required to activate the 50% of the channels encoded by the sodium channel gene with the GEFS+ mutation could increase the probability of reaching the more depolarized membrane potential required to activate the other half of the channels encoded by sodium channel genes with the DS mutation. Once activated, however, the smaller-magnitude currents associated with the DS mutation could reduce the overall magnitude of the membrane depolarization, thereby facilitating the deactivation of the current mediated by the GEFS+ mutant channels. This predicts that the repetitive firing frequency of LNs in GEFS+/DS flies will be higher than that of LNs in either GEFS+/GEFS+ or DS/DS flies.
Cellular localization of mutation-induced changes in sodium current.
In Drosophila, para is the only gene encoding voltage-gated sodium channels, and both the DS and GEFS+ mutations examined in this study are within an obligate exon. This leads to the prediction that the mutation-associated changes in sodium currents also occur in other neuronal subtypes. Therefore, generation of seizures in the mutants is likely to result from changes in many different neurons that affect network activity, rather than from an isolated change in the firing of one population of GABAergic neurons in the antennal lobes. To examine the issue of cell specificity, we have begun to look at excitatory motor neurons in the adult thoracic ganglion. As predicted, preliminary data indicate that motor neurons (MNs) in the adult thoracic ganglion in DS flies have reduced sodium currents. However, unlike the LNs, repetitive firing of these excitatory MNs is not reduced (data not shown). We are currently exploring whether differential expression of other ion channels giving rise to the unique firing properties of MNs might account for this result. To more fully address the issue of cell specificity, it will be important to examine the effects of DS and GEFS+ mutations in other identified neuronal populations.
Serotonin differentially regulates seizure sensitivity in GEFS+ and DS mutants.
By using a simple behavior assay, our study shows that it is possible to rapidly test the effects of pharmacological modifiers on temperature-sensitive seizures in the mutant flies. Feeding flies the serotonin precursor 5-HTP for 3 days had differential effects on the GEFS+ and DS flies. Whereas GEFS+ flies showed an increase in seizure probability at high temperature, this treatment suppressed heat-induced seizures in the DS knock-ins. The concentration of 5-HTP used in feeding paradigm was chosen because it was previously shown to increase serotonin levels in adult fly heads by 15- to 20-fold (Dierick and Greenspan 2007).
Analysis of the underlying currents in DS LNs demonstrates that increasing serotonin does not restore mutant sodium channel function. However, spontaneous burst frequency was partially rescued, indicating that the mechanism of seizure suppression is likely to be a compensatory change in other intrinsic membrane properties and/or network activity. The increase in burst frequency is also consistent with serotonin exacerbating heat-induced seizures in GEFS+ flies, since the major abnormality in GEFS+ LNs is prolonged membrane depolarizations preventing repetitive firing at high temperature. It seems likely that increasing burst frequency would increase the frequency of prolonged membrane depolarizations.
The effect of 5-HTP in DS flies is consistent with two clinical studies reporting that increasing serotonin levels, using the serotonin reuptake inhibitors fluoxetine and citalopram, reduced seizure frequency in all participating patients (17 and 11, respectively) with refractory epilepsies (Albano et al. 2006; Favale et al. 1995, 2003). On the basis of our studies in flies, it is possible that the clinical efficacy of these drugs in humans is also a compensatory change in other channel types or network connections. Identification of new drugs that target such pathways in flies may be useful in developing therapies to treat patients with refractory epilepsies.
Recent advances in GAL4-UAS binary system have made it possible to manipulate the activity of small sets of neurons, providing a tool for identifying specific sets of serotonergic neurons that are involved in modulating temperature-sensitive seizures in the DS flies (Alekseyenko et al. 2013; Venken et al. 2011). Identification of those neurons and their signaling pathway should increase of our knowledge of seizure regulation as well as provide potential therapeutic targets for treating these epileptic disorders.
GRANTS
This work was supported by a University of California Irvine/Stanley Behrens Public Impact Fellowship and a California Institute for Regenerative Medicine Postdoctoral Fellowship to R. J. Schutte; an America Epilepsy Society and Sunovion Pharmaceuticals Postdoctoral Fellowship to S. S. Schutte; National Institute of Neurological Disorders and Stroke (NINDS) Grant NS083009 and a Howard Hughes Medical Institute Professor Program Grant to D. K. O'Dowd; and NINDS Grant NS074686 and an Ellison Medical Foundation Senior Scholar Award to R. Reenan.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.J.S., S.S.S., R.R., and D.K.O. conception and design of research; R.J.S., S.S.S., J.A., E.V.B., J.G., C.S., and Y.A.S. performed experiments; R.J.S., S.S.S., J.A., and E.V.B. analyzed data; R.J.S., S.S.S., M.A.S., and D.K.O. interpreted results of experiments; R.J.S. and S.S.S. prepared figures; R.J.S. and S.S.S. drafted manuscript; R.J.S., S.S.S., M.A.S., R.R., and D.K.O. edited and revised manuscript; R.J.S., S.S.S., R.R., and D.K.O. approved final version of manuscript.
Supplementary Material
REFERENCES
- Abou-Khalil B, Ge Q, Desai R, Ryther R, Bazyk A, Bailey R, Haines JL, Sutcliffe JS, George AL. Partial and generalized epilepsy with febrile seizures plus and a novel SCN1A mutation. Neurology 57: 2265–2272, 2001. [DOI] [PubMed] [Google Scholar]
- Albano C, Cupello A, Mainardi P, Scarrone S, Favale E. Successful treatment of epilepsy with serotonin reuptake inhibitors: proposed mechanism. Neurochem Res 31: 509–514, 2006. [DOI] [PubMed] [Google Scholar]
- Alekseyenko OV, Chan YB, Li R, Kravitz EA. Single dopaminergic neurons that modulate aggression in Drosophila. Proc Natl Acad Sci USA 110: 6151–6156, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LT, Singh CM, Moore MS, Neckameyer WS, Heberlein U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr Biol 10: 187–194, 2000. [DOI] [PubMed] [Google Scholar]
- Borycz J, Borycz JA, Kubow A, Lloyd V, Meinertzhagen IA. Drosophila ABC transporter mutants white, brown and scarlet have altered contents and distribution of biogenic amines in the brain. J Exp Biol 211: 3454–3466, 2008. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Sodium channel mutations and epilepsy. In: Jasper's Basic Mechanisms of the Epilepsies (4th ed.), edited by Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-and E, scueta AV. Bethesda, MD: National Center for Biotechnology Information, 2012. [PubMed] [Google Scholar]
- Catterall WA, Kalume F, Oakley JC. NaV1.1 channels and epilepsy. J Physiol 588: 1849–1859, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiron C, Dulac O. The pharmacologic treatment of Dravet syndrome. Epilepsia 52, Suppl 2: 72–75, 2011. [DOI] [PubMed] [Google Scholar]
- Dierick HA, Greenspan RJ. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat Genet 39: 678–682, 2007. [DOI] [PubMed] [Google Scholar]
- Dravet C. The core Dravet syndrome phenotype. Epilepsia 52, Suppl 2: 3–9, 2011. [DOI] [PubMed] [Google Scholar]
- Escayg A, Goldin AL. Sodium channel SCN1A and epilepsy: mutations and mechanisms. Epilepsia 51: 1650–1658, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favale E, Audenino D, Cocito L, Albano C. The anticonvulsant effect of citalopram as an indirect evidence of serotonergic impairment in human epileptogenesis. Seizure 12: 316–318, 2003. [DOI] [PubMed] [Google Scholar]
- Favale E, Rubino V, Mainardi P, Lunardi G, Albano C. Anticonvulsant effect of fluoxetine in humans. Neurology 45: 1926–1927, 1995. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Schachter SC. The postictal state: a neglected entity in the management of epilepsy. Epilepsy Behav 1: 52–59, 2000. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Sugawara T, Mazaki-Miyazaki E, Takahashi Y, Fukushima K, Watanabe M, Hara K, Morikawa T, Yagi K, Yamakawa K, Inoue Y. Mutations of sodium channel alpha subunit type 1 (SCN1A) in intractable childhood epilepsies with frequent generalized tonic-clonic seizures. Brain 126: 531–546, 2003. [DOI] [PubMed] [Google Scholar]
- George AL., Jr Inherited disorders of voltage-gated sodium channels. J Clin Invest 115: 1990–1999, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, O'Dowd DK. Cholinergic synaptic transmission in adult Drosophila Kenyon cells in situ. J Neurosci 26: 265–272, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, O'Dowd DK. Whole cell recordings from brain of adult Drosophila. J Vis Exp 248: 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong ST, Bang S, Paik D, Kang J, Hwang S, Jeon K, Chun B, Hyun S, Lee Y, Kim J. Histamine and its receptors modulate temperature-preference behaviors in Drosophila. J Neurosci 26: 7245–7256, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossin C, Rhodes TH, Desai RR, Vanoye CG, Wang D, Carniciu S, Devinsky O, George ALJ. Epilepsy-associated dysfunction in the voltage-gated neuronal sodium channel SCN1A. J Neurosci 23: 11289–11295, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughney K, Kreber R, Ganetzky B. Molecular analysis of the para locus, a sodium channel gene in Drosophila. Cell 58: 1143–1154, 1989. [DOI] [PubMed] [Google Scholar]
- Mantegazza M, Rusconi R, Scalmani P, Avanzini G, Franceschetti S. Epileptogenic ion channel mutations: from bedside to bench and, hopefully, back again. Epilepsy Res 92: 1–29, 2010. [DOI] [PubMed] [Google Scholar]
- Martin MS, Dutt K, Papale LA, Dube CM, Dutton SB, de Haan G, Shankar A, Tufik S, Meisler MH, Baram TZ, Goldin AL, Escayg A. Altered function of the SCN1A voltage-gated sodium channel leads to γ-aminobutyric acid-ergic (GABAergic) interneuron abnormalities. J Biol Chem 285: 9823–9834, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer WS. Multiple roles for dopamine in Drosophila development. Dev Biol 176: 209–219, 1996. [DOI] [PubMed] [Google Scholar]
- Noebels JL. Exploring new gene discoveries in idiopathic generalized epilepsy. Epilepsia 44, Suppl 2: 16–21, 2003. [DOI] [PubMed] [Google Scholar]
- Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, Takeuchi T, Itohara S, Yanagawa Y, Obata K, Furuichi T, Hensch TK, Yamakawa K. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci 27: 5903–5914, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguni H, Hayashi K, Awaya Y, Fukuyama Y, Osawa M. Severe myoclonic epilepsy in infants–a review based on the Tokyo Women's Medical University series of 84 cases. Brain Dev 23: 736–748, 2001. [DOI] [PubMed] [Google Scholar]
- Scheffer IE, Berkovic SF. Generalized epilepsy with febrile seizures plus. A genetic disorder with heterogeneous clinical phenotypes. Brain 120: 479–490, 1997. [DOI] [PubMed] [Google Scholar]
- Scheffer IE, Zhang YH, Jansen FE, Dibbens L. Dravet syndrome or genetic (generalized) epilepsy with febrile seizures plus? Brain Dev 31: 394–400, 2009. [DOI] [PubMed] [Google Scholar]
- Spampanato J, Escayg A, Meisler MH, Goldin AL. Generalized epilepsy with febrile seizures plus type 2 mutation W1204R alters voltage-dependent gating of Nav1.1 sodium channels. Neuroscience 116: 37–48, 2003. [DOI] [PubMed] [Google Scholar]
- Sun L, Gilligan J, Staber C, Schutte RJ, Nguyen V, O'Dowd DK, Reenan R. A knock-in model of human epilepsy in Drosophila reveals a novel cellular mechanism associated with heat-induced seizure. J Neurosci 32: 14145–14155, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken KJ, Simpson JH, Bellen HJ. Genetic manipulation of genes and cells in the nervous system of the fruit fly. Neuron 72: 202–230, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdess-Walsh P, Devinsky O. Historical perspectives and definitions of the postictal state. Epilepsy Behav 19: 96–99, 2010. [DOI] [PubMed] [Google Scholar]
- Yarov-Yarovoy V, DeCaen PG, Westenbroek RE, Pan CY, Scheuer T, Baker D, Catterall WA. Structural basis for gating charge movement in the voltage sensor of a sodium channel. Proc Natl Acad Sci USA 109: E93–E102, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Catterall WA. The VGL-chanome: a protein superfamily specialized for electrical signaling and ionic homeostasis. Sci STKE 2004: re15, 2004. [DOI] [PubMed] [Google Scholar]
- Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, Spain WJ, McKnight GS, Scheuer T, Catterall WA. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci 9: 1142–1149, 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.