Abstract
The role of ipsilateral primary motor cortex (M1) in hand motor control during complex task performance remains controversial. Bilateral M1 activation is inconsistently observed in functional (f)MRI studies of unilateral hand performance. Two factors limit the interpretation of these data. As the motor tasks differ qualitatively in these studies, it is conceivable that M1 contributions differ with the demand on skillfulness. Second, most studies lack the verification of a strictly unilateral execution of the motor task during the acquisition of imaging data. Here, we use fMRI to determine whether ipsilateral M1 activity depends on the demand for precision in a pointing task where precision varied quantitatively while movement trajectories remained equal. Thirteen healthy participants used an MRI-compatible joystick to point to targets of four different sizes in a block design. A clustered acquisition technique allowed simultaneous fMRI/EMG data collection and confirmed that movements were strictly unilateral. Accuracy of performance increased with target size. Overall, the pointing task revealed activation in contralateral and ipsilateral M1, extending into contralateral somatosensory and parietal areas. Target size-dependent activation differences were found in ipsilateral M1 extending into the temporal/parietal junction, where activation increased with increasing demand on accuracy. The results suggest that ipsilateral M1 is active during the execution of a unilateral motor task and that its activity is modulated by the demand on precision.
Keywords: motor performance, fMRI, motor control, motor cortex
the role of primary motor cortex (M1) in the control of voluntary hand movements is still unclear. In functional (f)MRI studies of unilateral hand motor performance, strictly contralateral M1 (cM1) activation is demonstrated by some investigators (Butefisch et al. 2005; Catalan et al. 1998) while bilateral M1 activation is observed by others (Diedrichsen et al. 2012; Hummel et al. 2003; Lotze et al. 2006; Seidler et al. 2004; Winstein et al. 1997). There are more recent reports of a relationship between the level of precision or complexity of a motor task and this additional ipsilateral M1 (iM1) activation (Hummel et al. 2003; Seidler et al. 2004; Verstynen et al. 2005). These results suggest that iM1 plays a role in the control of hand function and that its functional relevance for motor performance changes as a function of difficulty of a motor task.
However, the interpretation of these neuroimaging data is limited by two factors. First, in most studies of motor demand-related brain activity, qualitatively different movements were tested, and therefore, resultant brain activation patterns are confounded by the tasks not being matched for their kinematics (e.g., force, amplitude, and frequency) and participating muscle groups. Second, most studies lack the verification of a strictly unilateral execution of the motor task during the acquisition of imaging data. Measuring unilateral performance is important as without it the presence of bilateral upper extremity activity with increasing difficulty of the task referred to as “mirror movements” cannot be ruled out and may contribute to the observed bilateral M1 activation. This bilateral upper extremity activity is observed is healthy children (Muller et al. 1997) but may occur in healthy adults when executing complex movements (Mayston et al. 1999) or adults after stroke, when moving the affected hand (Butefisch et al. 2005). In the present study, we addressed these two limiting factors in the neuroimaging of motor demand-dependent activation of motor cortexes using a pointing task allowing for parametric manipulation of task demand to determine whether iM1 activity depends on the demand for precision. We verified strictly unilateral performance of the task with electromyographic recording from bilateral extensor carpi ulnaris (ECU) muscles during imaging of the motor task. We selected a middle-aged population because of the need to characterize motor control in this population in more detail (Talelli et al. 2008a, 2008b; Ward and Frackowiak 2003) as this population is more appropriate for comparison with stroke patients and related studies of motor recovery after stroke.
MATERIALS AND METHODS
Overview of the Experimental Plan
Experiments were carried out to determine the effect of an increasingly demanding parametric motor task on the blood oxygen level-dependent (BOLD) response of iM1 to the executing hand. The study was approved by the Institutional Review Boards (IRB) of West Virginia University, and written, informed consent was obtained from all subjects before entering the study.
Subjects
Thirteen subjects (10 females and 3 males, age 55.4 ± 10.9 yr) fulfilled the following inclusion criteria and were included in this study: age >40 yr, normal MRI of the brain, normal neurological examination, no neurological disorders, no contraindication for transcranial magnetic stimulation (TMS) or MRI, and no intake of central nervous system active drugs. All subjects were strongly right handed according to the Edinburgh handedness inventory (Oldfield 1971).
Motor Task Used During the fMRI Experiment
The motor task was designed as a pointing task that allowed parametric variation of the level of difficulty through change in target size. Decreasing the target size increases the level of difficulty and results in longer movement times when accuracy must be maintained. Similarly, if subjects are forced to complete the task in a short, predefined time, accuracy is expected to decrease for smaller target sizes. This is the speed-accuracy tradeoff described in Fitts' Law (Fitts 1954). The present experiments were performed using Presentation software (www.neurobs.com). Subjects had to manipulate a joystick with the right hand (see Fig. 1 for details). Subjects were instructed to manipulate the joystick with the thumb and middle finger while keeping their index finger on the push button located on its top. The subject's wrist rested on the base of the joystick at a 30–45° angle in reference to the ulnar aspect of the forearm. The manipulation of the joystick in response to the visual stimuli required wrist extension/flexion movements of ±5° in addition to the finger movements. The forearm was resting on a soft pad and foam cushions to ensure that the movements were executed by manipulating the joystick with the hand only. During the training period (see below), involvement of more proximal muscles was monitored and discouraged when observed. Real-time feedback about the joystick position was provided by a cursor moving on a computer screen.
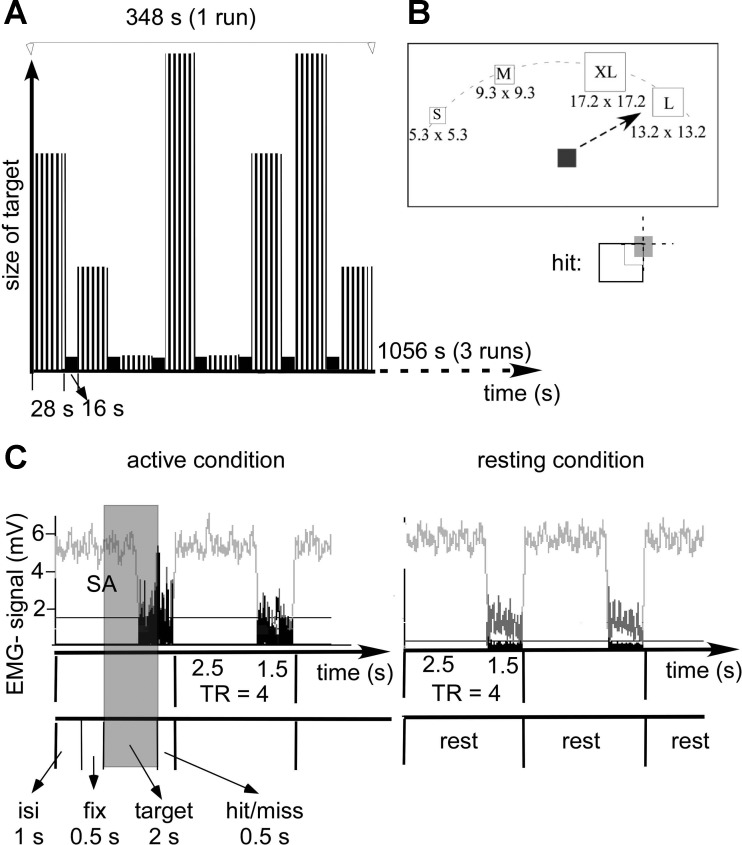
Fig. 1.
Schematic diagram of the experimental design with sparse sampling and EMG recording. A: single run: active condition (grey bar) and resting (black bar) are plotted against time. In the active condition, the 4 different sizes of targets are reflected in the different length of the bars. Targets were presented in blocks of 7 trials (indicated by the black stripes in the active condition, total block duration 28 s) with 16 s of rest between blocks. Two blocks of each target size were presented per run, with block order randomized within each of the 3 runs. Target presentation and data acquisition were time locked by the first pulse [repetition time (TR)] from the scanner. B: target presentation: subjects were asked to move a cursor (5.3 × 5.3 mm) as quickly as possible to the center of targets of different sizes upon their appearance on the screen. Once located in the target (see inset), subjects were asked to push a response button located on its top. A single target was presented every 4 s at any of 4 evenly spaced locations on the upper half field of a PC monitor (30, 60, 300, and 330°). Targets were of varying sizes [5.3 × 5.3 mm (small), 9.3 × 9.3 mm (medium), 13.2 × 13.2 mm (large), 17.2 × 17.2 mm (extra-large)]. Correct movements were defined as completion of the movement in the allocated time of 2 s with the cursor display in the target area (defined as overlap of the target and cursor of an area of ≥25% of the cursor area including the center of the cursor). C: EMG recording of the performing hand: clustered acquisition with scanning in the first 2.5 s of the TR. Note that during these time periods, EMG signals are not visible due to the scanner artifacts (SA). The onset of the TR was time locked to the intertrial time of 1 s to allow EMG sampling void of scanner noise for 50% of the pointing task. The beginning of the shaded area indicates the onset of the motor task starting with the presentation of the target (target 2 s). Note that EMG signal is increased in the extensor carpi ulnaris (ECU) supporting of the performing hand in the active condition but remains small in resting condition. The raw EMG signal is indicated in light grey. The rectified and low-pass filtered EMG signal is indicated in black. For each experimental condition, the integral of EMG signals in the epoch between acquisitions were calculated (see text for more details).
Following the presentation of a fixation square (0.5 s), subjects were asked to move the cursor as quickly as possible to the center of a target square immediately upon its appearance on the screen. Once the cursor was in the center of the target, subjects were asked to push a response button located on the top of the joystick. Because we were not able to record x, y coordinates continuously, subjects pushed the button to indicate target acquisition. This push button response was used to estimate the subject's movement time (see below). Targets were one of four different sizes (Fig. 1, A and B). A maximum of 2 s was given to perform the task after appearance of the target. The size of the targets and the length of the time interval were determined during pilot testing of the pointing task with the MRI-compatible joystick to achieve an accuracy of >50% (>5/10 trials) at all target sizes. After each trial, subjects were given feedback about their performance. Correct movements were defined as completion of the movement in the allocated time of 2 s with the cursor display in the target area (defined as overlap of the target and cursor of an area of ≥25% of the cursor area including the center of the cursor in Fig. 1B). If the trial was deemed correct, the word “hit” was displayed for 0.5 s (see Fig. 1C). If the subject failed to hit the target area within the allotted time, the word “miss” was displayed. This determination of accuracy was processed in real time by Presentation and was independent of the subject's button press. After the display of this feedback, subjects were asked to move the cursor back into a central position on the display screen. Consecutive target presentations were separated by an intertrial time of 1 s where subjects viewed a grey screen (see Fig. 1B). Targets were presented every 4 s at four evenly spaced locations on the upper half field of a PC monitor (30, 60, 300, and 330°). Blocks of seven trials of a single target size were presented in random order to each participant, with 16 s of rest between each block. Target locations were randomly selected within each block.
All subjects practiced the motor task at least 1 day before the MRI experiment to achieve a stable performance on all targets and >50% accuracy on the smallest target. Subjects practiced the task again on the day of scanning for a total of 20 trials before being placed in the scanner. After this practice, the imaging experiment began. Subjects were positioned on the scanner bed. The joystick was strapped to subjects' upper thighs using Velcro straps. It was positioned in such a way that the subject was able to rest the wrist on the base of the joystick comfortably and to manipulate the joy stick without moving the distal or proximal arm. The arm was supported by pads so the participant did not have to use any muscles to actively support the arm. The nonperforming arm rested on the torso supported by pads. This ensured relaxation of the arm during rest and a standardized manipulation strategy across subjects. A piece of tape was placed across subjects' foreheads and attached to either side of the head coil. Subjects were told that if they felt a tug on the tape, this meant that they were moving their heads, and they should try to stop. The stimuli were projected onto a screen at the foot of the scanner bed. Before the subjects were placed in the scanner bore, the mirror on the head coil was adjusted until subjects could see the screen without having to move their heads or elevate their eyes.
Movement epochs (28 s × 8) alternated with resting epochs (16 s × 7). Trials were blocked by target size (4 s per trial, 7 trials per movement epoch), with the target appearing randomly in one of four possible locations on each trial. Each target size block was presented twice in each run. The order of target size blocks was randomized within each run. The subjects completed three runs, which resulted in the presentation of each target size in six blocks total (see Fig. 1).
We used a separate finger-tapping task to localize M1 hand areas active in controlling contralateral hand movements to determine whether areas modulated by ipsilateral hand performance in the more demanding task (i.e., manipulating the joystick between digits 1 and 3) were distinct. Subjects performed self-paced finger tapping (opposition of the index finger and the thumb) at ∼1-Hz frequency. Movement epochs (20 s × 5 per hand) alternated with resting epochs (20 s × 5). The subject was instructed via visual display projected onto a screen at the foot of the scanner bed. For left-hand finger tapping, a green arrow pointed to the left, and for right-hand finger tapping, a green arrow pointed to the right. Resting was indicated by a red STOP sign.
EMG
The sparse sampling technique with scanning in the first 2.5 s of the repetition time (TR) allowed EMG data collection without scanner artifact during the last 1.5 s of the TR to confirm that movements were strictly unilateral (see below for details, Fig. 1). The onset of the TR was time locked to the intertrial time of 1 s to allow EMG sampling void of scanner noise for 50% of the pointing task (Fig. 1, shaded area). The subject's EMG activity from both ECU muscles was recorded with shielded MRI compatible surface electrodes mounted 5 cm apart in a belly tendon montage (Schwarzer), band-pass filtered (5 Hz to 1 kHz), amplified (MRI compatible amplifier; IED, Hamburg, Germany) digitized, sampled at 1.0-kHz frequency, and stored for offline analysis (LabVIEW; National Instruments) (Butefisch et al. 2005). The ECU muscle was selected because of its activity during the manipulation of the joystick, which required some extension/flexion movements of the hand.
MRI of the Brain
The imaging was performed on the GE Signa 3T in the Center for Advanced Imaging at West Virginia University. We collected high resolution anatomical images with the following parameters: spoiled gradient-recalled echo (SPGR); field of view (FOV) = 240 mm; matrix 256 × 256; slice thickness = 1.5 mm; 124 slices. Three functional runs were collected with the following imaging parameters: spiral in/out; axial plane; TR = 4 s; echo time (TE) = 36 ms; FOV 240 mm; matrix = 128 × 128; slice thickness = 4 mm; 30 slices; 93 volumes. We used a block design with sparse sampling where the volumes were obtained in the first 2.5 s of the TR, so that EMG data could be collected during the last 1.5 s with no interference from the scanner (Fig. 1, A and C).
Data Analysis
Motor performance.
ACCURACY.
Correct movements were defined as completion of the movement in the allocated time of 2 s with the cursor display in the target area (defined as overlap of the target and cursor of an area of ≥25% of the cursor area including the midpoint of the cursor Fig. 1B). The number of correct movements was expressed as the percentage of all movements for the target size.
MOVEMENT TIME.
Movement time was defined as the time interval between the onset of the target display and time of the button push indicating the successful completion of each trial. Only movements where subjects were able to hit the target in the allocated time were considered for the analysis of movement time. Mean movement time was calculated for each subject and target size.
EMG.
EMG data were fed into a custom made analysis program (LabVIEW; National Instruments). Scanner acquisition artifacts (2.5 s) were discarded. The remaining drift was removed by subtracting a linear fitted curve from the data. The EMG signal was rectified, and then the mean for each time epoch (1.5 s) was calculated resulting in a measure of EMG activity for each time interval. The mean of these derivatives was used to express the EMG activity for each condition (right-hand performance vs. rest). For each subject, an increase of mean EMG activity in the nonperforming (left) hand during the right-hand performance condition of >2 SD over the mean EMG activity in the nonperforming hand during the right-hand resting condition was used as a cut off to exclude subjects for the possibility of coactivation of the nonperforming hand during right-hand performance (Butefisch et al. 2005). Separate paired t-tests were used to compare mean EMG activity during right-hand performance and rest for each ECU muscle. For better illustration, the EMG ratio for each ECU muscle was calculated by dividing the mean EMG activity during right-hand performance by the mean EMG activity during rest. A ratio of ∼1 in the ECU muscle of one hand indicates that EMG activity was similar across time.
fMRI data analysis.
All fMRI data analysis was performed using AFNI (Cox 1996). Each participant's data were motion corrected and coregistered with the individual's anatomical scan, which was normalized to Talairach space. This transform was applied to the coregistered functional data. Data were smoothed with an 8-mm full width at half-maximum kernel before regression analysis. All four target size conditions were modeled as 28-s block functions, with head movement vectors included as regressors of no interest. Baseline was modeled implicitly. The low accuracy in the small target performance introduced an uncertainty about the movement trajectories in this condition (see results for motor performance below and Fig. 2 for details). We therefore had to exclude these trials from the group analysis. Individual beta parameters for the three primary conditions of interest (medium, large, and extra-large target size) were submitted to further group level statistical analysis. All results were corrected for multiple comparisons using AFNI's Monte Carlo simulation technique. Unless otherwise noted, activation was thresholded at a family-wise error-corrected P < 0.05, resulting from an uncorrected P < 0.005 with a 2,360-mm3 minimum cluster size. Data from the finger-tapping task were analyzed separately using the same procedure.
Fig. 2.
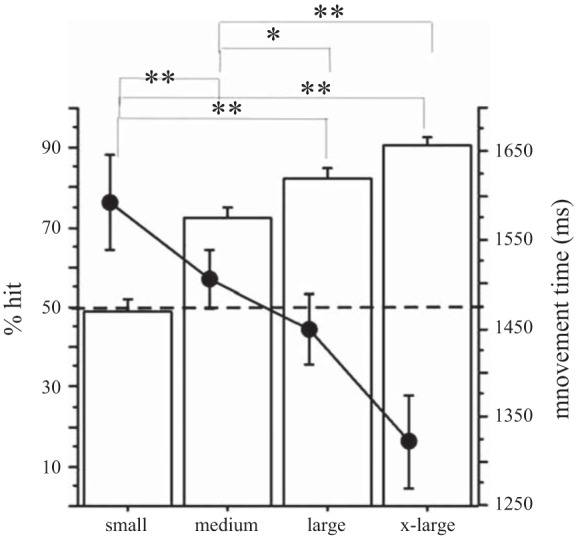
Accuracy and movement time of task performance as a function of target size: as indicated by the increasing percentage of target hits (bars), the subject's accuracy improved as targets got bigger (left axis). At the same time, movement time (●) decreased as targets became larger (right axis). Level of accuracy differed significantly between each target size pair except for the comparison between large and extra-large targets. Note that the performance level dropped below 50% hits (dotted line) for the smallest target. Error bars reflect SE. *P = 0.001, **P < 0.0001 (comparisons are significant at an alpha-corrected P < 0.05 if the corresponding P < 0.005).
Individual anatomical regions of interest (ROIs) consisting of the anterior and posterior portions of the precentral gyrus were hand drawn for each hemisphere on each subject's anatomical scan in native space. The posterior aspect of the gyrus precentralis was included in its entire extent and is commonly the site of primary motor cortex while premotor area corresponds to the anterior portion of precentral gyrus (Duvernoy 1999; Zilles and Rehkaemper 1998). This was done without consulting the functional activation maps for the whole brain analysis.
RESULTS
Subjects
Of the 13 participants studied in the present experiments, the data of 3 participants were excluded because of task-related head movements of >4 mm between consecutive volumes during the scanning. Only the data for the remaining 10 subjects (8 females, age: 53.1 ± 11.5 yr) were considered in the analysis of motor performance, EMG data, and fMRI data.
Motor Performance
Accuracy.
There was a significant effect of target size on accuracy (Figs. 2 and 3) as indicated by a repeated-measures ANOVA with percent hits as the dependent variable and target size (4 levels) and number of runs (3 runs) as independent variables [target size: F(3,9) = 82.98, P < 0.0001; number of runs: F(2,9) = 6.26, P < 0.01]. The interaction between run and target size was not significant. As expected, post hoc comparison with t-tests of the performance in the pointing task revealed that the accuracy (expressed as %hits/target size) was significantly greater as target sizes increased (see Fig. 2 and legend for P values). For the smallest target size, the accuracy was 50% and participants reported significant difficulty in performing the task for the small targets in the scanner despite adequate performance with this target size during training.
Fig. 3.
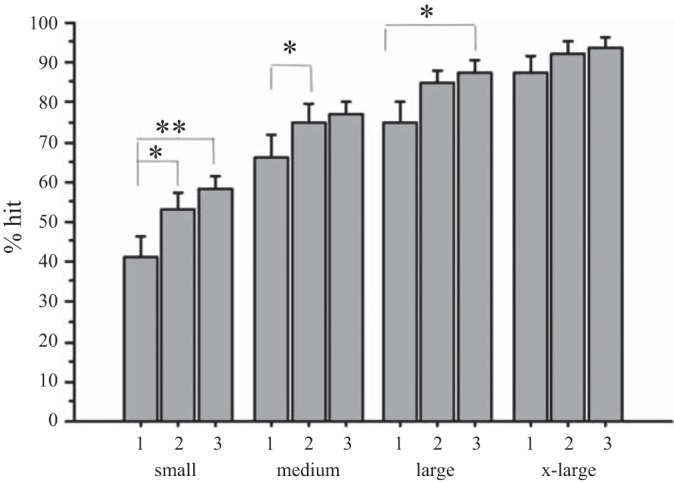
Accuracy of performance as a function of target size and number of runs (3 runs): as indicated by the increasing percentage of target hits, subjects' pointing accuracy improved over time for the small, medium, and large targets, indicating that subjects may have learned during the scanning process. The numbers 1, 2, and 3 indicate the run number. Error bars reflect standard error. *P < 0.05, **P < 0.005.
In addition, there was an increase in accuracy across the three runs for the small, medium, and large targets, with t-tests showing statistically significant increases in accuracy between the first and second run or third run (see Fig. 3 and legend for P values). In contrast, accuracy for the extra-large target was constant across time. These results indicate that subjects may have learned during performance on the small, medium, and large targets while performance remained stable for the extra-large target size.
Movement time.
Analysis of the movement times revealed that in some trials, subjects did not push the response button located on the top of the joystick in time to indicate the time of successful completion of the trial. Because of the missing data, we summarized the movement time in a plot to demonstrate the overall tendency of movement time to increase as target sizes decreased but did not perform statistical analysis (Fig. 2).
EMG Data of Motor Task
All subjects showed strictly unilateral performance and were included in further analysis. Each subject's mean EMG activity in left nonperforming hand during right-hand performance was smaller than the mean +2 SD of the left nonperforming hand EMG activity during right-hand rest (see materials and methods for details). This resulted in a group mean EMG ratio of 1.067 ± 0.03 (left-hand mean EMG activity during right-hand performance/left-hand mean EMG activity during rest; Fig. 4) Statistical testing revealed no significant difference in the magnitude of nonperforming left-hand EMG activity between periods of right-hand rest, and right-hand activity [paired t-test: NS, t(9) = 1.31, P = 0.22]. For the performing right hand the EMG activity was significantly greater in periods of performance compared with rest [paired t-test: t(9) = 3.63, P < 0.01]resulting in an EMG ratio of 1.6 ± 0.23 (Fig. 4).
Fig. 4.
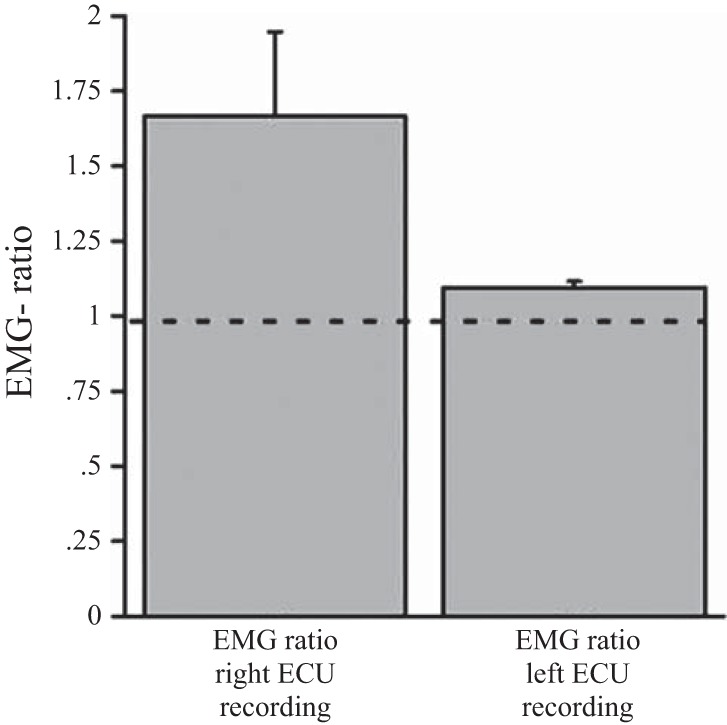
EMG activity during performance of the motor task: the EMG activity for the ECU muscle was expressed as a ratio where the mean EMG activity during performance was divided by the mean EMG activity during rest. The EMG ratio of the left (nonperforming) ECU muscle was 1.067 ± 0.03, indicating that EMG activity in that muscle was similar in rest and active task blocks, thereby confirming unilateral performance with the right hand. In contrast, the EMG ratio of the right (performing) ECU muscle was 1.61 ± 0.23.
Pointing task-related brain activity.
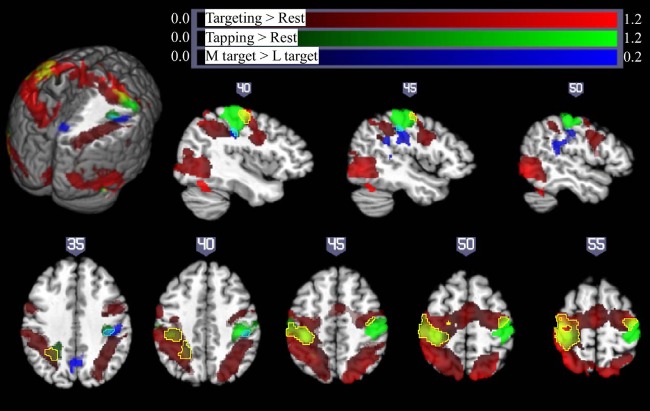
As illustrated in Figure 5, strictly unilateral performance of the pointing task (collapsed across extra-large, large, and medium sized targets) resulted in extensive activation of bilateral sensorimotor cortex in the precentral and postcentral gyri/sulci, bilateral posterior parietal cortex, bilateral medial frontal gyrus and supplementary motor area (SMA), bilateral middle occipital gyrus, and ipsilateral cerebellum (Fig. 5, red; for details, see Table 1). This contrasts with activation arising from the qualitatively different right- and left-handed finger-tapping task (Fig. 5, green, with overlap between finger-tapping and pointing task performance shown in yellow), which resulted in activation restricted to contralateral sensorimotor areas and the corresponding ipsilateral cerebellum. Left M1 is robustly activated by both the targeting task and the finger-tapping task performed by the right hand. However, Fig. 5 shows that ipsilateral activation in the pointing task is largely anterior to the activation arising from the left-handed finger-tapping task.
Fig. 5.
Pointing and finger-tapping task-related brain activation: activity related to the pointing task (collapsed across XL, L, and M targets) is indicated in red. Activation related to right- and left-handed finger tapping is indicated in green, with overlap between finger-tapping and pointing task performance shown in yellow. Note that while there was extensive bilateral activation for the pointing task, M1 activation in the finger-tapping tasks was only seen contralateral to the performing hand, so that the left hemisphere is due solely to right-handed finger tapping (with left hemisphere yellow areas show overlap between right-handed finger-tapping and right-handed pointing task performance) and the right hemisphere activity is due solely to left-handed finger tapping (yellow colors in the right hemisphere show overlap between activity due to the right-handed targeting task and left-handed finger-tapping task, outlined with a yellow border for ease of visualization). Significant activation related to increasing motor demand (M targets > L targets) is indicated in blue (overlap between this region and left-handed finger tapping shown in cyan, outlined for clarity). All activations are shown overlaid on the Colin27 template in standard space, thresholded at a corrected P < 0.05 (uncorrected threshold P < 0.005 and cluster size > 2,360 mm3) Increased color intensity corresponds to higher estimates of percent signal change. Cuts in the 3-dimensional rendering are shown at x = 0, y = −15, and z = 35. The right hemisphere is depicted at top. The right (R) and left (L) side of the brain are indicated at bottom. Numerical labels above each slice show slice coordinates in the x dimension (sagittal sections) or z dimension (axial sections).
Table 1.
Overall task effects on the BOLD response
| Contrast/Anatomical Location | Coordinates of Center of Mass | Volume, mm3 |
|---|---|---|
| Right-hand targeting | ||
| L postcentral gyrus, | −34.3, −36.0, | 14,363 |
| L precentral gyrus, bilateral superior parietal lobule | 48.7 | |
| R cerebellum | 18.8, −49.8, −19.4 | 6,447 |
| L middle occipital gyrus | −47.8, −65.6, −6.0 | 2,294 |
| R precentral gyrus, R postcentral gyrus | 27.8, −13.0, 51.2 | 1,887 |
| Bilateral medial frontal gyrus, SMA | 0.8, −6.7, 54.2 | 1,308 |
| R middle occipital gyrus | 8.2, −58.7, −4.2 | 959 |
| Right-hand finger tapping | ||
| R cerebellum | 14.6, −54.5, −20.1 | 17,279 |
| L superior temporal | −40.9, −59.7, | 16,641 |
| Gyrus, middle temporal gyrus, inferior parietal lobule | 11.3 | |
| L precentral and postcentral gyri (areas 1, 2, 3a, 3b, 4a, 4p, 6) | −36.5, −26.3, 55.3 | 14,433 |
| L putamen, insula | −30.1, −8.7, 6.9 | 2,562 |
| Left-hand finger tapping | ||
| R precentral and postcentral gyri (areas 1, 2, 3a, 3b, 4a, 4p, 6) | 40.6, −24.0, 52.1 | 11,507 |
| L cerebellum | −10.3, −53.8, −15.0 | 7,401 |
Talaraich coordinates for the center of mass were derived from a random-effects group analysis. BOLD, blood oxygen level dependent. All contrasts were thresholded at a corrected threshold of P < 0.05, using an uncorrected P < 0.005 with a minimum cluster size of 2,360 mm3. For the joystick-targeting task, this resulted in extensive activation in bilateral frontal, parietal, and temporal cortex. To better clarify the anatomical regions activated by the pointing task, the pointing data reported in this table were rethresholded at a corrected P < 0.05 using an uncorrected P < 0.0001 with a cluster size of >333 mm3. Brodmann areas, where listed, are from the Juelich atlas (Eickhoff et al. 2005).
Effect of increasing motor demand on primary motor cortex activity.
In keeping with our stated aim, the effects of parametric task difficulty increases on both contralateral and iM1 activity were investigated through targeted ROI analyses. This is especially important since the ipsilateral activation seen during the pointing task was largely anterior to the M1 region defined by contralateral finger tapping and we wish to determine whether iM1 and not just premotor areas are modulated by the difficulty of the targeting task. The selection of appropriate ROIs is not always straightforward (Friston et al. 2006; Saxe et al. 2006). Functional localizers are often used to define ROIs, but with the increasing evidence for M1 heterogeneity (Kalaska 2009; Rathelot and Strick 2006), we did not expect the areas involved in executing contralateral finger movements to be the same areas modulated by ipsilateral task demand. The present results confirmed that, indeed, the left-handed finger-tapping task used to localize M1 hand area was largely separate from the ipsilateral activation seen during the pointing task. Further, the right-handed finger tapping did not result in any iM1 activity (Fig. 5 and Table. 1). Therefore, the areas delineated by the finger-tapping task were not used as functional ROIs in further analysis.
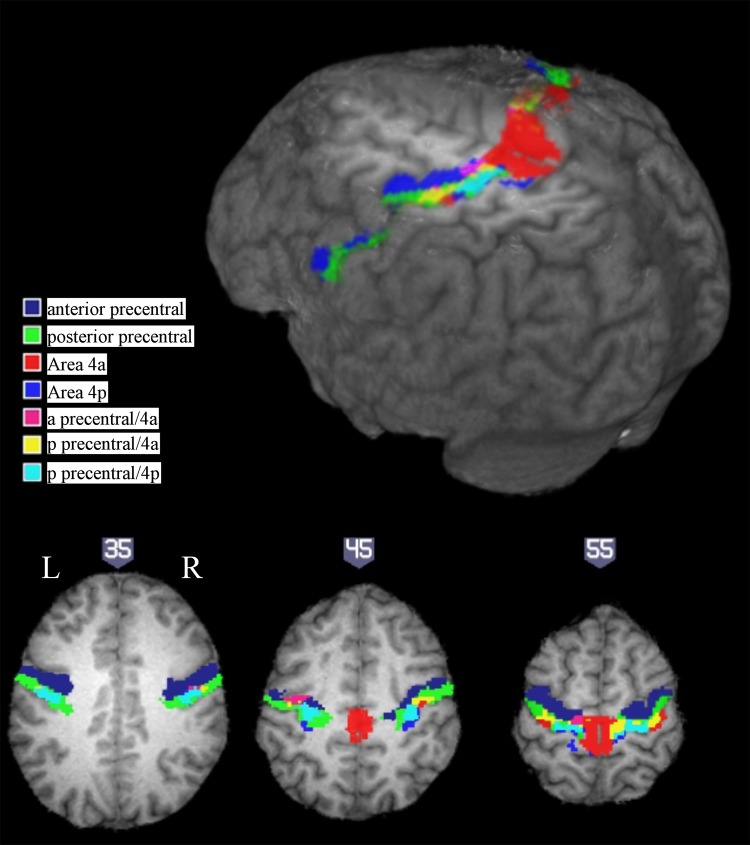
Another approach is to select anatomical ROIs based on a probabilistic atlas of cytoarchitectonic organization across a group of 10 individuals. Here, anatomical ROIs consisting of the primary motor cortex areas 4a and 4p were defined using the Juelich atlas (Eickhoff et al. 2005). Areas 4a and 4p are two parallel bands within area 4 (rostral band: 4a; caudal band: 4p) running mediolaterally from the midline to the Sylvian fissure. For each participant, mean signal change in each ROI was calculated for the three target sizes of interest. A 2 × 2 × 3 repeated-measures ANOVA with hemisphere (ipsilateral/contralateral), region (4a/4p), and target size (M, L, and XL) as factors showed a significant main effect of hemisphere [F(1,9) = 51.9, P < 0.001]. There was also a significant interaction between hemisphere and target size [F(2,18) = 4.42, P < 0.05]. No other effects or interactions reached significance. Individual analysis of each ROI revealed a significant effect of target size in ipsilateral areas 4p [F(2,18) = 4.72, P < 0.05] and 4a [F(2,18) = 3.78, P < 0.05] but no significant effect of target size in either contralateral ROI (both P > 0.1; Fig. 6, top). This indicates that activity in iM1 is modulated by the demand for precision while cM1 is unmodulated or modulated to a lesser extent.
Fig. 6.
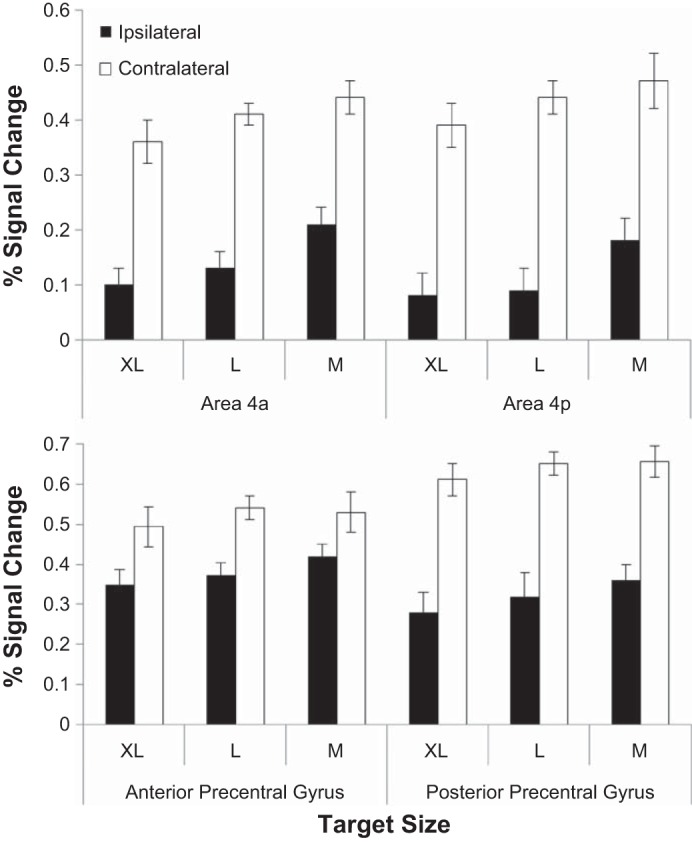
Pointing task-related brain activity in the atlas-based regions of interest (ROIs; areas 4a and 4p) and the individualized ROIs (anterior and posterior aspect of precentral gyrus). Top: group data, means ± SE activation in areas 4a and 4p: In addition to a significant effect of hemisphere, there is a significant hemisphere × target size interaction carried by significant effects of target size in both ipsilateral ROIs (4a and 4p) but no effect of target size in either contralateral ROIs. Bottom: group data, means ± SE activation in individual anatomical anterior and posterior aspect of precentral gyrus ROIs showing a significant effect of hemisphere (activation in all three target conditions is larger in contralateral precentral gyrus than ipsilateral precentral gyrus) and significant hemisphere × target size and hemisphere × ROI position (anterior/posterior aspect of precentral gyrus) interactions. There is a trend toward increasing activation with decreasing target size in ipsilateral precentral gyrus ROIs but not in the contralateral ROIs.
While normalization and the use of probabilistic atlases reduce some of the problems of combining data from different subjects, individual differences in anatomy and the cortical localization of function can contribute variability even to spatially normalized data. This can be seen in the illustration in Fig. 7, which shows the overlap between the atlas-based ROIs and the anatomically defined ROIs for a representative subject. While smoothing of functional data is often used to reduce the impact of individual variability in anatomy in normalized group data, it additionally has the effect of blurring together activations from nearby cortical regions. We therefore attempted to confirm the results of our atlas-based ROI analysis by applying each subject's hand-drawn anterior and posterior precentral gyrus ROI masks described above to the unsmoothed data in native space (Fig. 6, bottom) to ensure that the effects seen in the posterior aspect of precentral gyrus (corresponding to primary motor cortex) were not driven by blurred-in activation from anterior portion of precentral gyrus (corresponding to premotor area). A 2 × 2 × 3 repeated-measures ANOVA with hemisphere (ipsilateral/contralateral), ROI position (anterior/posterior), and target size (medium, large, extra-large) as factors of interest showed a significant main effect of hemisphere [F(1,9) = 41.5, P < 0.001]. Significant interactions between hemisphere and target size [F(2,18) = 6.02, P < 0.01] and hemisphere and position [F(1,9) = 8.38, P < 0.05] warranted further exploration. Although the effect of target size did not reach significance in any of the four ROIs, the data pattern was consistent with the results of the probabilistic atlas based analysis, with no effect of target size in contralateral precentral gyrus (F < 1) but with a slight trend toward increasing activation with increased demand in both anterior and posterior aspects of the ipsilateral precentral gyrus [F(2,18) = 2.3, P = 0.13].
Fig. 7.
Cytoarchitectonic organization based area 4a and 4p and anatomically based hand-drawn anterior and posterior precentral gyrus ROI locations from a single representative subject (S2), overlaid on the participant's T1 image. Rendering cut shown at z = 50. Anatomical ROIs shown in blue (anterior precentral gyrus) and green (posterior precentral gyrus). Cytoarchitectonic organization based ROIs shown in red/violet/yellow (4a) and cyan/blue (4p) where secondary colors correspond to overlap with anterior precentral (4a: violet) or posterior precentral gyrus (4a: yellow; 4p: cyan). For illustrative purposes, the participant's structural image and anatomical ROI tracings have been normalized to template space. Note that the actual anatomical ROI analysis reported in Fig. 6 uses unsmoothed data in each subject's native space.
Exploring the effects of motor learning during on quantitatively different motor task-related brain activity.
Improved accuracy over blocks for the medium and large target sizes suggested that participants may have learned while performing the pointing task in the scanner. While the experiment was not designed to study motor learning, we wanted to explore the question of motor learning-related effects on motor demand-related differences in M1 activity. We contrasted brain activity between two target sizes with comparable improvement in accuracy over time (medium and large targets), suggesting the presence of motor learning in both tasks, as well as between two target sizes with different amount of improvement in accuracy over time (medium and extra-large targets), suggesting the presence of motor learning in one condition but not the other. The effect of motor demand should be isolated by comparing the brain activity related to pointing to medium and large targets, as learning is present in both conditions (Fig. 3). As indicated in Table 2 and Fig. 5 (blue), the contrast between pointing to medium and large targets reveals a significant difference in activation spanning ipsilateral precentral and postcentral gyri. This activation is located below what is typically the hand area and extends back into the temporal/parietal junction. This area, which is more active for targets requiring higher precision (M > L), is largely distinct from areas activated by the pointing task overall (Fig. 5, red) and from activity related to left-handed finger movement in a finger-tapping task (Fig. 5, green, and Table 1). In addition, there is a significant increase in activation for medium relative to large targets in the precuneus and posterior cingulate cortex. No areas showed the reverse pattern, with more reliable activation for less demanding targets (L > M).
Table 2.
Effects of target difficulty and motor learning on the BOLD response
| Contrast Anatomical Location | Coordinates of Center of Mass | Volume, mm3 |
|---|---|---|
| M > L | ||
| R precentral and postcentral gyrus (areas 2, 3a, 3b, 4p, OP1, OP2) | 46.7, −29.3, 26.7 | 3,328 |
| Bilateral precuneus, posterior cingulate | 0.3. −58.6, 30.6 | 2,562 |
| M > XL | ||
| Bilateral anterior cingulate and medial frontal gyrus | 5.1, 34.4, 26.9 | 6,324 |
| Precuneus, posterior cingulate, L superior temporal gyrus | −1.0, −54.9, 29.4 | 6,174 |
| L insula, L superior temporal gyrus, L parahippocampal gyrus | −35.3, −33.8, −3.2 | 5,552 |
| R insula, R superior temporal gyrus, R parahippocampal gyrus | 41.0, −20.0, −3.2 | 5,322 |
Note: The contrast M > L compares the effect of increasing the demand on precision on task-related brain activity. The contrast M > XL compares the effect of increasing demand on precision and presence of motor learning. Talaraich coordinates for the center of mass of clusters with increased activity during pointing to the medium-sized target (M) when compared with pointing to the large and extra-large targets (L and XL). Coordinates were derived from a random-effects group analysis (n = 10). All contrasts were thresholded at a corrected threshold of P = 0.05 (uncorrected P < 0.005, with cluster size of 2,360 mm3).
The comparison of brain activity related to pointing to the medium and extra-large targets included a comparison of learning and nonlearning, since extra-large targets had stable accuracy rates over time (Fig. 3) and medium targets did not. As indicated in Fig. 8, this contrast (M > XL) shows differential activation in bilateral medial frontal gyri, bilateral precuneus, the anterior and posterior cingulate cortex, as well as in bilateral temporal regions including the parahippocampal and superior temporal gyri and extending into the insula (Table 2).
Fig. 8.
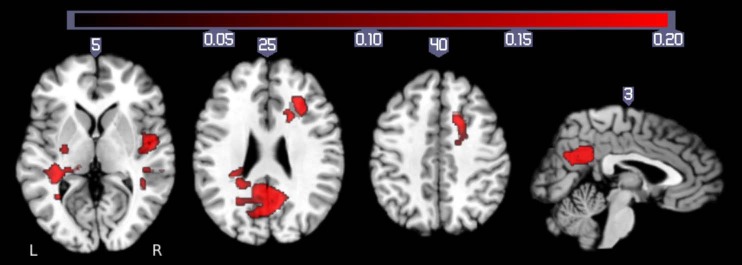
Brain areas that show significantly more activation during pointing to medium-sized targets than during pointing to extra-large targets (M > XL). Behaviorally, the contrast between these conditions shows both difficulty and learning effects since XL targets had stable accuracy rates over time and medium targets did not (see Fig. 3). Activation is thresholded at a corrected P < 0.05 (uncorrected threshold P < 0.005 and cluster size > 2,360 mm3).
DISCUSSION
The purpose of the study was to determine whether iM1 is active during the performance of a hand motor task when executed strictly unilaterally and whether the extent of iM1 activity depends on the demand for precision in a hand motor task. The research was prompted by results of imaging studies demonstrating increased activity of ipsilateral motor areas when healthy subjects executed more difficult tasks (Hummel et al. 2003; Seidler et al. 2004; Talelli et al. 2008a; Verstynen et al. 2005; Winstein et al. 1997) or patients moving the affected hand after stroke (Butefisch et al. 2005; Schaechter and Perdue 2008; Ward et al. 2003). These results raise several questions. First, as most studies lack EMG recording from the nonperforming hand during the execution of the motor task, a strictly unilateral performance of the task was not confirmed and the possibility that some of the brain activity is indeed related to bilateral task performance cannot be ruled out (Diedrichsen et al. 2012; Seidler et al. 2004; Talelli et al. 2008a; Verstynen et al. 2005; Verstynen and Ivry 2011). Second, results from several investigators (Hummel et al. 2003; Seidler et al. 2004; Talelli et al. 2008a; Verstynen et al. 2005; Verstynen and Ivry 2011; Winstein et al. 1997) suggest motor performance relies more on bilateral motor areas as the task becomes more demanding. However, in most studies qualitatively different movements were tested, and, therefore resultant brain activation patterns are confounded by the tasks not being matched for their kinematics (e.g., force, amplitude, and frequency) and participating muscle groups.
In the present study we aimed to answer the questions of whether iM1 activation is seen in middle-aged subjects with strictly unilateral hand performance and whether the extent or strength of the iM1 activity depends on the demand on precision. We chose a task that allowed a parametric manipulation of task demand in the form of an increased need for precision to maintain accuracy, while holding important kinematic features of the motor task constant. As indicated by the increases in the movement time and decreases in accuracy as a function of target size (Fig. 2), the employed task indeed had different demands on precision. To match for effort, we asked subjects to give their best effort in pointing to the targets of different sizes by moving the cursor as quickly as possible. This resulted in slight differences in the movement time as a function of target size. The change in movement time is a trade-off to match for effort.
EMG activity from both hands was recorded to determine the presence of coactivation during task performance. Although the quantification of the EMG changes as a function of condition was limited to the short time intervals between the scanner artifacts, the 1.5 s allowed capturing 50% of the performance and should therefore represent the task-related activity in EMG for the different conditions. Moreover, increased EMG activity was clearly noted in the performing hand, indicating that task-related increases were indeed captured. The similarity of EMG activity in the nonperforming left hand during time periods of activity and rest of the performing right hand resulted in a EMG ratio close to 1 (Fig. 4) and confirmed a similar level of EMG activity throughout the experiment. As a result, the observed right-hand performance-related changes in the iM1 BOLD response cannot be explained by changes in left-hand activity. The main finding of the present study is, therefore, that iM1 is active with strictly unilateral execution of the pointing task as confirmed by the absence of increased EMG activity in the nonperforming left hand (Fig. 4). Further, iM1 is modulated in a motor demand-dependent manner as indicated by the results of the atlas based and anatomical ROI analyses where an increased BOLD signal is seen as the target size becomes smaller.
The interpretation of the current fMRI results is limited by the fact that changes in inhibitory and excitatory activity cannot be distinguished. Increased BOLD in iM1 with increasing demand on the motor task could result from increases of inhibitory or excitatory activity or any combination of these. Evidence from electrophysiological experiments using TMS demonstrated that inhibiting iM1 by means of low-frequency repetitive (r)TMS improves performance of the hand ipsilateral to the stimulated M1 (Buetefisch et al. 2011; Dafotakis et al. 2008; Kobayashi et al. 2003), supporting the notion that there may be a need for suppression of task performance-related iM1 excitatory activity. Further, in a study by Talelli et al. (2008a), right-hand grip-related iM1 activity was explored as a function of inhibitory effect from the left M1(contralateral M1) on the right M1 (interhemispheric inhibition). It was demonstrated that peak forces for a hand grip were positively correlated with increases in the iM1 BOLD response when interhemispheric inhibition between motor cortexes was weak. This positive correlation changed to a negative correlation when interhemispheric inhibition was strong. This would indicate that activity in iM1 is controlled to some extent by the inhibitory effect of the contralateral M1.
Whether iM1 exerts its effects directly through ipsilateral cortico-spinal or cortico-reticulo-spinal (Ziemann et al. 1999) connections or indirectly through its cortico-cortical connections or a combination of both is not known. As indicated in Fig. 5, the iM1 area active for overall pointing task performance as well as the iM1 area that is more active for the conditions with a higher demand on accuracy is largely distinct from the M1 area active during contralateral hand movements. This is consistent with results from previous TMS (Ziemann et al. 1999) and fMRI (Verstynen et al. 2005; Verstynen and Ivry 2011) studies where ipsilateral hand-related activity (Verstynen et al. 2005; Verstynen and Ivry 2011) or evoked responses in ipsilateral hand muscles (Ziemann et al. 1999) were distinct and anterior compared with the contralateral hand movement-related activity or corresponding hot spot. In fact, Verstynen and Ivry (2011) suggest that this may not be a primary motor region but an area of PMd that is concerned with coupling activity between left and right M1. As the distinction between premotor areas and primary motor cortex is based on the cytoarchitectonic structure, the employed technique cannot distinguish between these areas on a single subject level. In the present study, the probabilistic atlas of cytoarchitectonic organization across a group of 10 individuals was used to determine the location of these areas (Eickhoff et al. 2005). Based on that analysis, the majority of ipsilateral activity is within the premotor area but some activity was localized in iM1. The involvement of iM1 is further confirmed in the analysis based on each individual's anterior and posterior precentral gyrus anatomical mask and the corresponding unsmoothed functional data in native space.
While there is evidence for ipsilateral corticospinal projections in humans, their potential role in controlling the ipsilateral upper extremity is less well understood. In intact humans, stimulation of M1 using TMS elicits motor-evoked potentials in ipsilateral hand muscles but these are difficult to obtain and require high stimulation intensity and preinnervations of the target muscle (Ziemann et al. 1999). In nonhuman primates, recording of iM1 neurons during upper limb movements demonstrated that cells in iM1 were modulated by the task but that the timing of this activity was best correlated with weak muscle activity in the contralateral nonmoving arm (Soteropoulos et al. 2011). Taken together, these results suggest that the control of the hand movements via ipsilateral corticospinal connections is weak. Alternatively, task-related effects in iM1 could be mediated by cortico-reticulo-spinal connections. In contrast to cortico-spinal connection, cortico-reticulo-spinal projections are bilateral and are thought to be involved in the execution of selective finger movements (Soteropoulos et al. 2012). The involvement of this pathway is supported by TMS-derived evidence of longer latencies of motor-evoked potentials elicited in the ipsilateral hand muscles (Ziemann et al. 1999).
One could also argue that this M1 area may be concerned with the integration of afferent input from other motor areas. Recent evidence of bilateral M1 projections from posterior parietal (Koch et al. 2008, 2009) and dorsal premotor areas, likely conveying some task-related information such as visuospatial and motor planning information, supports a more indirect effect and the notion that M1 functions at a higher level in motor control by integrating afferent information and then generating a descending motor command that defines the spatiotemporal form of the movement (Kalaska 2009). A higher level role for M1 in motor control is also supported by the results of a recent rTMS study where low-frequency rTMS applied to left M1 improved performance in both hands for the task with the highest demand on precision, while performance remained unchanged for the tasks with lower demands (Buetefisch et al. 2011). However, as left M1 was stimulated and there is evidence for left hemispheric dominance in motor programming (Terao et al. 2005), the comparison with the results of the present study are limited.
In the present study, activity in cM1 did show a tendency to increase as a function of target size but this was not statistically significant (Fig. 6). Our findings are in contrast to the results by Seidler et al. (2004), who demonstrated decreasing cM1 activity as targets became smaller. However, direct comparison of the results is difficult as their subjects were younger (mean 25.1 yr compared with 55.4 yr), a factor that influences motor task-related BOLD activation (Talelli et al. 2008a). Further, subjects did not practice the task before scanning, did not receive any feedback about performance, and were given more time to complete the task. In addition, some of the identified activation areas would likely not survive more stringent multiple comparisons correction done in the present study. Furthermore, by only evaluating the linear size effect in areas where the main effect (task > rest) is significant, they might be masking out additional areas of activation.
The comparison of our findings with results of other imaging studies is limited by the fact that in these studies qualitatively different tasks were compared. While some studies report increased cM1 activity in a power grip vs. precision grip (Ehrsson et al. 2000; Kuhtz-Buschbeck et al. 2008), others report no change (Takasawa et al. 2003). In a static precision grip task, Kuhtz-Buschbeck et al. (2001) found stronger BOLD activity of the contralateral M1 when the forces were lower but were controlled more accurately. Results from single cell recordings in monkeys indicate that power grip and discrete finger movements in a precision grip are encoded differently in M1 (Kalaska 2009). Specifically, recordings of CM neurons in primate cM1 indicate more activity in CM cells when monkeys made more controlled movements than during more forceful movements in a precision grip task (Hepp-Reymond et al. 1999; Maier et al. 1993) or wrist flexion/extension or pinch task using the thumb and index finger (Cisek et al. 2003).
In the present study, subjects' performance improved during the acquisition of the imaging data indicating that subjects may have learned during the scanning. This would introduce a confounding factor of “motor learning” especially in the more demanding conditions (Fig. 3). In previous studies, performance was not analyzed for evidence of motor learning during the scanning (Butefisch et al. 2005; Schaechter and Perdue 2008; Seidler et al. 2004). Therefore, it is not clear to what extent the imaging findings may reflect activity related to motor learning in addition to activity related to task demand. Although the present study was not designed to study motor learning, the concern about motor learning as a confounding variable was explored by comparing task demand differences in conditions with equivalent motor learning (medium and large targets) and task demand differences in conditions where motor learning differed (medium and extra-large targets). When motor learning was controlled for, we found that activity in ipsilateral brain areas was modulated by the demand on accuracy of a motor task. Specifically, the ipsilateral sensorimotor cortex as well as a midline precuneus and posterior cingulate gyrus region are more active when pointing to the medium size target compared with the large target (Table 2 and Fig. 5). However, when contrasting the performance of a demanding motor task showing evidence of motor learning (pointing to medium-sized targets) and the performance of a less demanding motor task without evidence of learning (pointing to XL targets), additional activity was seen in bilateral superior temporal gyrus, insula and parahippocampal gyrus, the anterior cingulate cortex, and bilateral medial frontal gyrus (Fig. 8 and Table 2).
Previous reports of demand-related BOLD responses have shown more extensive activation than is seen in our comparison of conditions with different demand for precision but equivalent motor learning (Seidler et al. 2004; Winstein et al. 1997). Specifically, Winstein et al. (1997) examined a continuous repetitive aiming task of increasing difficulty by varying the target size in a positron emission tomography study. They demonstrated an association between increasing task difficulty and greater activation in bilateral dorsal premotor cortex, SMA, caudate nucleus, and visual and parietal cortical areas. Seidler et al. (2004) studied discrete movements in a similar aiming task and demonstrated an association between increasing task difficulty and ipsilateral motor cortex, insular cortex, cingulate cortex, and cerebellum. It is conceivable that some of the described activation in the prior work is due to the presence of motor learning as cingulate cortex, parietal areas, premotor cortex, SMA, dorsolateral prefrontal cortex, and cerebellum are involved in motor learning (Halsband and Lange 2006) and could contribute to the increased activity seen when subjects executed the more demanding task. However, as this study was not designed to test the effect of motor learning on brain activation, further analysis of this important question would require a different experimental setup. Other limitations of the study are the relatively small sample size and the predominance of female participants as well as a design limitation that reduced the range of level of difficulty due to the exclusion of the smallest target and prevented post hoc sorting of trials to examine effects of accuracy or comparing of successful trials across conditions. While we cannot completely exclude the possibility of some spillover effect of premotor-related activity into M1, we feel that the interpretation of our results is supported by the data and is consistent with data from monkey neurophysiology experiments and several other human functional neuroimaging studies (Diedrichsen et al. 2012; Hummel et al. 2003; Lotze et al. 2006; Seidler et al. 2004; Verstynen et al. 2005; Winstein et al. 1997).
In summary, the results of the present study provide evidence that iM1 is active during the execution of a strictly unilateral skilled motor task. By using a pointing task that was designed for parametric manipulation of task demand, we demonstrated that task-related differences in iM1 were related to the demand of the motor task his is consistent with the notion of M1 functioning at a higher level in motor control by integrating afferent input from nonprimary motor areas.
GRANTS
This work was supported by a Research Development Grant of West Virginia Univeristy and partially supported by National Institutes of Health Grants R56-NS-070879, R21-HD-067906, and R01-NS-060830.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.M.B. and M.P. conception and design of research; C.M.B., L.S., B.H., and M.P. performed experiments; C.M.B., K.P.R., L.S., and B.H. analyzed data; C.M.B. and K.P.R. interpreted results of experiments; C.M.B. prepared figures; C.M.B. and K.P.R. drafted manuscript; C.M.B. and K.P.R. edited and revised manuscript; C.M.B., K.P.R., L.S., B.H., and M.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank our subjects for participation in the study, Drs. Aina Puce and Susanne Lemieux for help with the experimental setup in the MRI environment, Dr. Cornelia Stoeckel for help with the data analysis, Dr. Sebastian Buetefisch for technical support, and Dr. Xiaping Hu for critical discussion of the MRI data analysis.
Present address of L. Shuster: Dept. Communication Sciences and Disorders, Grand Valley State University, Grand Rapids, MI.
Present address of M. Parsons: Neurologic Institute, Cleveland Clinic, Cleveland, OH.
REFERENCES
- Buetefisch CM, Hines B, Shuster L, Pergami P, Mathes A. Motor demand-dependent improvement in accuracy following low-frequency transcranial magnetic stimulation of left motor cortex. J Neurophysiol 106: 1614–1621, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butefisch CM, Kleiser R, Korber B, Muller K, Wittsack HJ, Homberg V, Seitz RJ. Recruitment of contralesional motor cortex in stroke patients with recovery of hand function. Neurology 64: 1067–1069, 2005 [DOI] [PubMed] [Google Scholar]
- Catalan MJ, Honda M, Weeks RA, Cohen LG, Hallett M. The functional neuroanatomy of simple and complex sequential finger movements: a PET study. Brain 121: 253–264, 1998 [DOI] [PubMed] [Google Scholar]
- Cisek P, Crammond DJ, Kalaska JF. Neural activity in primary motor and dorsal premotor cortex in reaching tasks with the contralateral vs. ipsilateral arm. J Neurophysiol 89: 922–942, 2003 [DOI] [PubMed] [Google Scholar]
- Cox R. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173, 1996 [DOI] [PubMed] [Google Scholar]
- Dafotakis M, Grefkes C, Wang L, Fink GR, Nowak DA. The effects of 1 Hz rTMS over the hand area of M1 on movement kinematics of the ipsilateral hand. J Neural Transm 115: 1269–1274, 2008 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Wiestler T, Krakauer JW. Two distinct ipsilateral cortical representations for individuated finger movements. Cereb Cortex 23: 1362–1377, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Brain. Surface, Blood Supply, and Three-Dimensional Section Anatomy. Wien, NY: Springer, 1999 [Google Scholar]
- Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H. Cortical activity in precision- versus power-grip tasks: an fMRI study. J Neurophysiol 83: 528–536, 2000 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25: 1325–1335, 2005 [DOI] [PubMed] [Google Scholar]
- Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol 47: 381–391, 1954 [PubMed] [Google Scholar]
- Friston KJ, Rotshtein P, Geng JJ, Sterzer P, Henson RN. A critique of functional localisers. Neuroimage 30: 1077–1087, 2006 [DOI] [PubMed] [Google Scholar]
- Halsband U, Lange RK. Motor learning in man: a review of functional and clinical studies. J Physiol (Paris) 99: 414–424, 2006 [DOI] [PubMed] [Google Scholar]
- Hepp-Reymond M, Kirkpatrick-Tanner M, Gabernet L, Qi HX, Weber B. Context-dependent force coding in motor and premotor cortical areas. Exp Brain Res 128: 123–133, 1999 [DOI] [PubMed] [Google Scholar]
- Hummel F, Kirsammer R, Gerloff C. Ipsilateral cortical activation during finger sequences of increasing complexity: representation of movement difficulty or memory load? Clin Neurophysiol 114: 605–613, 2003 [DOI] [PubMed] [Google Scholar]
- Kalaska JF. From intention to action: motor cortex and the control of reaching movements. Adv Exp Med Biol 629: 139–178, 2009 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Hutchinson S, Schlaug G, Pascual-Leone A. Ipsilateral motor cortex activation on functional magnetic resonance imaging during unilateral hand movements is related to interhemispheric interactions. Neuroimage 20: 2259–2270, 2003 [DOI] [PubMed] [Google Scholar]
- Koch G, Fernandez Del Olmo M, Cheeran B, Schippling S, Caltagirone C, Driver J, Rothwell JC. Functional interplay between posterior parietal and ipsilateral motor cortex revealed by twin-coil transcranial magnetic stimulation during reach planning toward contralateral space. J Neurosci 28: 5944–5953, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Ruge D, Cheeran B, Fernandez Del Olmo M, Pecchioli C, Marconi B, Versace V, Lo Gerfo E, Torriero S, Oliveri M, Caltagirone C, Rothwell JC. TMS activation of interhemispheric pathways between the posterior parietal cortex and the contralateral motor cortex. J Physiol 587: 4281–4292, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck JP, Ehrsson HH, Forssberg H. Human brain activity in the control of fine static precision grip forces: an fMRI study. Eur J Neurosci 14: 382–390, 2001 [DOI] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck JP, Gilster R, Wolff S, Ulmer S, Siebner H, Jansen O. Brain activity is similar during precision and power gripping with light force: an fMRI study. Neuroimage 40: 1469–1481, 2008 [DOI] [PubMed] [Google Scholar]
- Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J Neurosci 26: 6096–6102, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier MA, Bennett KM, Hepp-Reymond MC, Lemon RN. Contribution of the monkey corticomotoneuronal system to the control of force in precision grip. J Neurophysiol 69: 772–785, 1993 [DOI] [PubMed] [Google Scholar]
- Mayston MJ, Harrison LM, Stephens JA. A neurophysiological study of mirror movements in adults and children. Ann Neurol 45: 583–594, 1999 [DOI] [PubMed] [Google Scholar]
- Muller K, Kass-Iliyya F, Reitz M. Ontogeny of ipsilateral corticospinal projections: a developmental study with transcranial magnetic stimulation. Ann Neurol 42: 705–711, 1997 [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971 [DOI] [PubMed] [Google Scholar]
- Rathelot JA, Strick PL. Muscle representation in the macaque motor cortex: an anatomical perspective. Proc Natl Acad Sci USA 103: 8257–8262, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Brett M, Kanwisher N. Divide and conquer: a defense of functional localizers. Neuroimage 30: 1088–1096; discussion 1097–1089, 2006 [DOI] [PubMed] [Google Scholar]
- Schaechter JD, Perdue KL. Enhanced cortical activation in the contralesional hemisphere of chronic stroke patients in response to motor skill challenge. Cereb Cortex 18: 638–647, 2008 [DOI] [PubMed] [Google Scholar]
- Seidler RD, Noll DC, Thiers G. Feedforward and feedback processes in motor control. Neuroimage 22: 1775–1783, 2004 [DOI] [PubMed] [Google Scholar]
- Soteropoulos DS, Edgley SA, Baker SN. Lack of evidence for direct corticospinal contributions to control of the ipsilateral forelimb in monkey. J Neurosci 31: 11208–11219, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soteropoulos DS, Williams ER, Baker SN. Cells in the monkey ponto-medullary reticular formation modulate their activity with slow finger movements. J Physiol 590: 4011–4027, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasawa M, Oku N, Osaki Y, Kinoshita H, Imaizumi M, Yoshikawa T, Kimura Y, Kajimoto K, Sasagaki M, Kitagawa K, Hori M, Hatazawa J. Cerebral and cerebellar activation in power and precision grip movements: an H2 15O positron emission tomography study. J Cereb Blood Flow Metab 23: 1378–1382, 2003 [DOI] [PubMed] [Google Scholar]
- Talelli P, Ewas A, Waddingham W, Rothwell JC, Ward NS. Neural correlates of age-related changes in cortical neurophysiology. Neuroimage 40: 1772–1781, 2008a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talelli P, Waddingham W, Ewas A, Rothwell JC, Ward NS. The effect of age on task-related modulation of interhemispheric balance. Exp Brain Res 186: 59–66, 2008b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao Y, Furubayashi T, Okabe S, Arai N, Mochizuki H, Kobayashi S, Yumoto M, Nishikawa M, Iwata NK, Ugawa Y. Interhemispheric transmission of visuomotor information for motor implementation. Cereb Cortex 15: 1025–1036, 2005 [DOI] [PubMed] [Google Scholar]
- Verstynen T, Diedrichsen J, Albert N, Aparicio P, Ivry RB. Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity. J Neurophysiol 93: 1209–1222, 2005 [DOI] [PubMed] [Google Scholar]
- Verstynen T, Ivry RB. Network dynamics mediating ipsilateral motor cortex activity during unimanual actions. J Cogn Neurosci 23: 2468–2480, 2011 [DOI] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain 126: 2476–2496, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RS. Age-related changes in the neural correlates of motor performance. Brain 126: 873–888, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstein CJ, Grafton ST, Pohl PS. Motor task difficulty and brain activity: investigation of goal-directed reciprocal aiming using positron emission tomography. J Neurophysiol 77: 1581–1594, 1997 [DOI] [PubMed] [Google Scholar]
- Ziemann U, Ishii K, Borgheresi A, Yaseen Z, Battaglia F, Hallett M, Cincotta M, Wassermann EM. Dissociation of the pathways mediating ipsilateral and contralateral motor-evoked potentials in human hand and arm muscles. J Physiol 518: 895–906, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Rehkaemper G. Funktionelle Neuroanatomie. Wien, NY: Springer, 1998 [Google Scholar]