Abstract
We recently defined a component of motor skill learning as “motor acuity,” quantified as a shift in the speed-accuracy trade-off function for a task. These shifts are primarily driven by reductions in movement variability. To determine the neural correlates of improvement in motor acuity, we devised a motor task compatible with magnetic resonance brain imaging that required subjects to make finely controlled wrist movements under visual guidance. Subjects were imaged on day 1 and day 5 while they performed this task and were trained outside the scanner on intervening days 2, 3, and 4. The potential confound of performance changes between days 1 and 5 was avoided by constraining movement time to a fixed duration. After training, subjects showed a marked increase in success rate and a reduction in trial-by-trial variability for the trained task but not for an untrained control task, without changes in mean trajectory. The decrease in variability for the trained task was associated with increased activation in contralateral primary motor and premotor cortical areas and in ipsilateral cerebellum. A global nonlocalizing multivariate analysis confirmed that learning was associated with increased overall brain activation. We suggest that motor acuity is acquired through increases in the number of neurons recruited in contralateral motor cortical areas and in ipsilateral cerebellum, which could reflect increased signal-to-noise ratio in motor output and improved state estimation for feedback corrections, respectively.
Keywords: fMRI, motor skill, pointing, reaching, speed-accuracy trade-off, wrist, motor cortex, cerebellum
motor skill is a general term that has been used to describe improvement across a wide range of motor learning paradigms. We recently operationally defined a component of motor skill as the training-related change in the speed-accuracy trade-off function for a task (Reis et al. 2009; Shmuelof et al. 2012). We introduced the term “motor acuity” for this aspect of improvement, both to contrast it with motor learning tasks that do not emphasize improved motor execution and to draw parallels with perceptual learning (Censor et al. 2012). Functional imaging has been extensively used to investigate the neural basis of motor learning in humans, but motor acuity has been relatively neglected. The emphasis has instead been on finger sequence tasks, like the serial reaction time task (SRTT) (Grafton et al. 1995; Robertson et al. 2001; Stagg et al. 2011), and on visuomotor adaptation tasks (Diedrichsen et al. 2005; Inoue et al. 1997; Krakauer et al. 2004). In such tasks, subjects modify the selection of movements that are already skilled (such as button pressing and straight reaching movements) and so do not need to improve the acuity of the movements themselves.
A landmark study by Karni and colleagues was an exception to the emphasis on learning of sequence order and adaptation in human imaging studies (Karni et al. 1995). In this study a voxel counting method was used to show that the ability to perform a short finger-opposition sequence faster and more accurately was associated with an increased number of activated voxels in contralateral primary motor cortex (M1) compared with an unlearned sequence, even when the two sequences were matched for rate and component movements (Karni et al. 1995). The control of movement frequency is important because changes in this parameter can lead to activation changes (Jenkins et al. 1997; Orban et al. 2011; Turner et al. 1998). Since the study by Karni and colleagues, however, an association between activation changes in contralateral cortical areas and learning has been elusive. Notably, in a recent meta-analysis of 70 imaging studies of motor learning in humans, the authors found that there was no converging evidence for learning-related activation in contralateral M1, once motor execution was controlled for (Hardwick et al. 2013). This conclusion stands in apparent contradiction with the original result by Karni and colleagues, which was not included in the meta-analysis because a direct statistical comparison between learning and control tasks was not performed. The conclusion of the meta-analysis also contradicts single-unit and structural studies in nonhuman animal models that have consistently shown motor learning-related changes in contralateral motor cortical areas including M1 (Harms et al. 2008; Nudo et al. 1996; Rioult-Pedotti et al. 2000; Xu et al. 2009).
A potential explanation for the discrepancy between nonhuman animal studies that have shown changes in contralateral motor cortical areas and human functional imaging studies, which for the most part have not, is the nature of the motor learning tasks used. We have recently argued that sequence and adaptation tasks predominantly challenge learning processes upstream of skilled motor execution itself (Shmuelof and Krakauer 2011). For example, in the SRTT, the kinematics of the movements themselves are very simple and only the response time is relevant to the task (Nissen and Bullemer 1987). Similarly, for visuomotor rotation, the movements themselves are no more difficult to execute than baseline movements and indeed show no changes in variability (Cunningham 1989; Krakauer et al. 2000). It is notable that the studies included in the meta-analysis reported above were classified as either SRTT variants or sensorimotor tasks. Two other prominent imaging approaches are tracking tasks (Grafton et al. 2008; Miall et al. 2001; Miall and Jenkinson 2005) and bimanual coordination tasks (Kelso 1984) in which subjects learn to make one-dimensional wrist movements at different frequencies (Puttemans et al. 2005) or phases (Debaere et al. 2004) in each hand. Here again, it is either tracking error in cursor space or synchronization between two skilled movements that is changing. In neither case does execution of the movements themselves have to become faster or less variable. The finger sequence task used by Karni, in contrast, requires a change in movement kinematics and in accuracy, and therefore a change in how movements themselves are executed (Karni et al. 1995).
With the goal of studying core aspects of motor skill learning that are not captured by adaptation or sequence tasks, we recently devised a novel visually guided pointing task (“arc pointing task,” APT) in which subjects control a screen cursor through a narrow semicircular channel by rotating their hand about the wrist, using equipment that is compatible with the magnetic resonance (MR) scanner environment (Shmuelof et al. 2012). This task differs from more widely used finger sequencing tasks in that it requires precise visually guided pointing movements that are not overlearned (unlike straight reaching movements), allows for detailed trajectory kinematics to be collected throughout a single movement, and makes it possible to impose specific kinematics for single movements. The APT is, to the best of our knowledge, the first MR-compatible task that allows subjects to make two-dimensional visually guided movement trajectories with the wrist, analogous to arm reaches, that can be characterized kinematically.
In a recent psychophysical study using the APT, we showed that 3 days of practice led to a change in the speed-accuracy trade-off function for the task, driven predominantly by decreased variability around a fairly constant mean trajectory (Shmuelof et al. 2012). In the present study we sought to use functional magnetic resonance imaging (fMRI) to detect a practice-dependent change in brain activation for the APT while controlling for changes in movement execution. The experiment was performed over 5 days: subjects were scanned on day 1, trained on the APT outside the scanner on days 2, 3, and 4, and then rescanned on day 5. We chose to perform a multiday study because in our previous psychophysical study variability was still coming down after 3 days of training (Shmuelof et al. 2012); thus we reasoned that we would increase our chances of detecting the neural correlates of this change by allowing it to be as large as possible. Importantly, performance of the APT on day 1 and day 5 in the scanner was matched for kinematics: subjects performed the task at an enforced slow speed on both days and generated the same mean trajectories. In this way we were able to separate the neural correlates of learning from the neural correlates of the improved motor ability that was achieved through such learning.
It is important to clarify here why we chose a task in which mean kinematics were matched before and after learning in the scanner. Although motor learning leads to improved motor performance, it is not possible to assay neural correlates of learning by comparing brain activation at different performance levels because execution-related changes confound the interpretation. Instead, we recognized that the core result of motor learning is to change motor ability, i.e., the potential or capacity to perform at higher levels. Improved motor ability presumably consists of stable changes in neural circuitry that affect how a given movement is controlled. Hence, these changes should be measurable at any level of execution.
We hypothesized that learning-induced changes in motor acuity will be a result of improved representation of the task in the cortical execution network, achieved through recruitment of additional neurons. This recruitment hypothesis would be consistent with an overall increase in task-related activation, as measured with the blood oxygen level-dependent (BOLD) signal in fMRI.
MATERIALS AND METHODS
Subjects.
Thirteen right-handed subjects (8 women, 5 men; 18–27 yr of age), naive to the task, participated in the study. All subjects gave written informed consent and received token compensation to participate in the study. The study was approved by the Columbia University Institutional Review Board.
MRI acquisition.
Data were acquired on a Philips Intera 3T scanner using a Philips SENSE head coil. The functional scans were acquired with a gradient echo EPI, with voxel size of 3 × 3 × 3 mm (240 × 240 × 120-mm matrix); TR = 2 s, flip angle = 77°, axial slices, TE = 25 ms. Forty slices were acquired in an interleaved sequence at a thickness of 3 mm (no gap). Ninety-six volumes were collected in each experimental run. The first two volumes were discarded to allow magnetization to reach equilibrium. A single T1-weighted anatomical scan was also acquired for each subject (MPRAGE, 1 mm3). The field of view covered the entire cerebrum and most of the cerebellum. The inferior part of the cerebellum was not covered in some of the subjects.
Arc pointing task outside the scanner.
Subjects participated in a protocol consisting of five daily sessions in the lab and two fMRI scans on days 1 and 5. The sessions in the lab were composed of Test sessions (days 1 and 5), in which the performance of subjects in the APT was assessed at 5 movement times (MTs), and Train sessions (days 2, 3, and 4), in which subjects performed the APT at the same MT (see below). The APT required subjects to guide a cursor from one circle to the other through a semicircular channel, presented on a monitor, by moving their left (nondominant) wrist in a clockwise direction, without crossing the borders of the channel. The width of the channel was the same as the targets' diameter (0.7 cm). At the beginning of each trial, one of the two horizontal targets became white (start circle) and the other red (target). A left white target indicated that subjects had to make a movement through the upper semicircular channel to the target, whereas a right white target indicated that they had to move through the lower semicircular channel. After a variable delay, the red circle changed to green, and a tone was played indicating that subjects could start the movement. The cursor was visible throughout the movement. After the trial, the entire trajectory of the cursor appeared on the screen. During Test and Train sessions, subjects were required to make the movements in a predefined MT range, indicated by a computer-generated demonstration of the cursor moving through the channel in the required MT, which was presented at the beginning of each session block. Valid movements (inside the channel and within MT range for the constrained blocks) were followed by a pleasant sound and rewarded with symbolic coins in proportion to the MT. During days 2–4 subjects trained by making movements in a single constrained speed range (Train sessions, 520–780 ms). On days 1 and 5, subjects' overall speed-accuracy trade-off function was sampled by testing their performance at 5 different MTs (Test sessions, 240–420 ms, 400–600 ms, 640–960 ms, 800–1,200 ms, 1,200–1,800 ms), presented in different blocks. Test and Train sessions in the lab lasted ∼1 h. For more detailed information, see Shmuelof et al. (2012).
Arc pointing task inside the scanner.
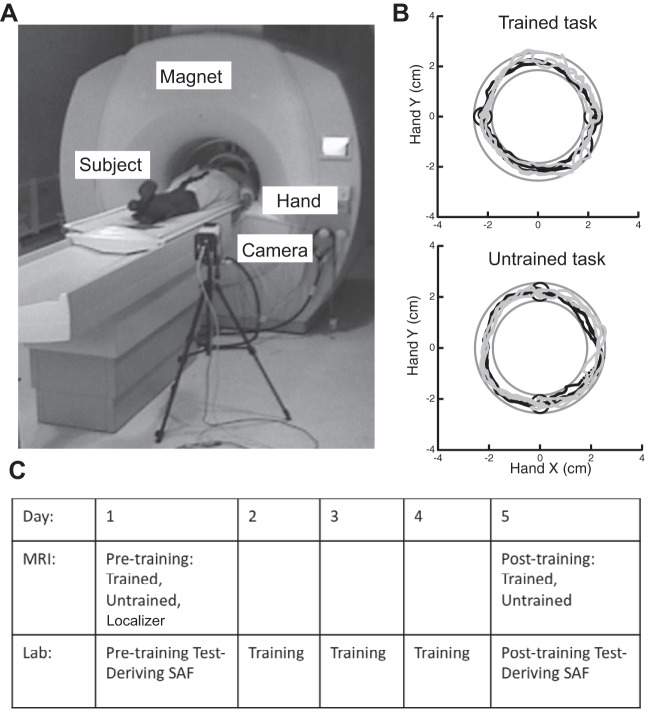
Subjects were scanned before the Test sessions in the lab on days 1 and 5. During the scans, subjects performed movements with their nondominant left wrist while lying in a supine position (Fig. 1A). They viewed, through video goggles (Resonance Technology, Los Angeles, CA), the same display of targets and cursor as in the behavioral sessions. A Qualysis (Gothenburg, Sweden) infrared camera, positioned inside the MRI room (distance >2 m from magnet), recorded the wrist pointing direction as the position of a spherical reflective marker on the index finger's proximal interphalangeal joint (the hand was closed in a fist) at a sampling rate of 100 Hz. Subjects moved the screen cursor horizontally and vertically by pointing with their closed fist (Fig. 1B). Each subject's forearm was placed in a splint to prevent forearm supination, so that the screen x and y positions were mapped, respectively, to wrist flexion-extension and radial-ulnar deviation. A laptop computer (Apple, Cupertino, CA) was used to control the visual display and to collect cursor position data with custom software.
Fig. 1.
A: experimental setup in the MRI scanner. Subjects performed the arc pointing task (APT) task while lying supine and moving their left wrist. The position of the marker was captured by the infrared camera that was positioned in the scanner room. Subjects received feedback through goggles. B: sample hand paths from the Trained (top) and Untrained (bottom) tasks, recorded in the scanner, before (gray, day 1) and after (black, day 5) training. The task was to move the cursor in a clockwise direction from one circle to the other through a circular channel, without crossing the channel's boundaries. Day 1 trajectories show greater trial-to-trial variability than day 5 trajectories for the Trained task but not for the Untrained task. C: experimental protocol. Subjects participated in a 5-day protocol, which was composed of 5 daily sessions in the lab and 2 MRI scans on days 1 and 5. After the MRI sessions a speed-accuracy trade-off function (SAF) for the APT was derived for each subject.
Study design inside the scanner.
Subjects performed three experimental runs (Localizer, Trained, and Untrained) in the scanner on day 1 and two (Trained and Untrained) on day 5 (Fig. 1C). To obtain maximum sensitivity to task effects, a block design was used. Horizontal (Trained) and vertical (Untrained, control) versions of the APT (see below) were performed in separate runs before and after training. Six movements were performed in 18-s blocks (repeated 6 times) at a slow speed (1.5 s per movement). Movement blocks were interleaved with 12-s rest periods.
During rest periods, subjects were instructed to relax their wrist and wait for the visual cue indicating the beginning of the next block. During the movement blocks, subjects performed semicircular movements through a channel (0.7 cm wide) between two circular targets (0.7-cm diameter) separated by 4.4 cm. These dimensions refer to the position of the reflective marker as recorded by the motion capture camera. In each trial, subjects moved the cursor from one target to the other in a curved clockwise motion, attempting to keep the cursor within the arc channel (Fig. 1B). The “go” signal for each movement was a visual cue (target color changed from red to green). The instruction to the subjects was to move the cursor between the targets without crossing the boundaries of the channel, and to maintain the required MT.
During movement blocks, subjects received online feedback of cursor position but no further information about their success or failure, or about their movement speed. To control for MT across sessions, subjects had a short training session before the experimental run, in which feedback about MT was given.
Tasks.
Subjects performed three types of movement task. The Trained task consisted of APT movements as described above with the two targets arranged along a horizontal line, in the same configuration as during the behavioral training in the lab. The Untrained task differed in the target arrangement, which was vertical (rotated in 90°) and was never practiced outside of the scanner. In both tasks, movements were always made in a clockwise direction (Fig. 1B). In addition, subjects performed a Localizer task on day 1, which served as a functional localizer to identify brain areas involved in planning and execution of visually guided left wrist reaching movements. Subjects had to guide a cursor between a start target (diameter 0.7 cm) presented at the center of the screen and targets (diameter 0.7 cm) presented 3.5 cm to the left and to the right of the start target by making a sequence of straight out-and-back visually guided movements. As for the APT experiments, this task had a block design: six out-and-back movements were performed in each 18-s block.
Imaging analysis.
Preprocessing and computing activation maps were all performed with Brain Voyager QX 1.10 (Brain Innovation, Maastricht, The Netherlands). Before statistical analysis, head motion correction using trilinear interpolation, high-pass temporal filtering in the frequency domain (3 cycles/total scan time), and spatial smoothing (FWHM = 8 mm) was applied to remove drifts and to improve the signal-to-noise ratio (SNR). The first two functional images of each run were discarded to allow for stabilization of the signal. Functional images were incorporated into the three-dimensional data sets through trilinear interpolation and transformed into Talairach space and Z-normalized. Group analysis was performed with a random-effects multisubject general linear model (GLM). Regressors were defined as a boxcar function peaking during each block, convolved with a two-gamma hemodynamic response function. The task × day interaction analysis was performed with the Brain Voyager QX ANOVA/ANCOVA module.
Voxel-based analysis.
We constrained the voxel-based analysis to the execution network for visually guided wrist movement, using a mask generated from the multisubject contrast map of the functional localizer scan obtained during performance of the Localizer task on day 1 (straight reaching movements > rest, P < 0.05). To correct for multiple comparisons, a cluster threshold of 112 contiguous functional voxels was used for the mask contrast and a cluster threshold of 19 contiguous functional voxels was used for the rest of the contrasts. The thresholds were computed with a Brain Voyager QX Cluster-level Statistical Threshold Estimator plug-in by running 1,000 iterations of a Monte Carlo simulation to estimate the probability of getting a cluster of a given size by chance (taking into account the number of activated voxels and spatial smoothing).
Global ranking analysis.
We designed a nonparametric analysis to capture global changes in activation following training. This analysis was based on individual unmasked and unsmoothed images. For each voxel, the contrast for the day effect comparing the Trained and Untrained tasks was computed based on first-level standard GLM (Friston et al. 1994) images computed in SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5) after slice time correction, high-pass filter (5 cycles per scan), and image normalization to a standard brain using 4th-degree B-spline interpolation. Ranks (integers representing orderings for all contrast estimates in the ROI) were calculated regardless of condition for each subject. The sum of the ranks across conditions is the sum of the integers from 1 to the number of voxels in the image (V) times the number of conditions (C), which is equal to C × V(C × V + 1)/2. This ranking procedure is identical to calculating a Wilcoxon rank sum statistic. The subject-specific proportion of the rank values devoted to each condition was then calculated and subsequently averaged over subjects within conditions. The average proportion in the first condition was retained as a test statistic. A low value of this statistic generally represents lower activation contrast values for this condition relative to the other and vice versa for a high value. A null distribution was obtained by permuting the condition labels within subjects and recalculating the statistics values. The result is a robust nonparametric test of contrast differences using the ensemble of voxels rather than separate interaction tests per voxel.
Behavioral analysis.
Custom routines written within the IGOR software package (WaveMetrics, Lake Oswego, OR) were used to compute error rate, MT, peak speed, and average trajectory. Cursor position data were low-pass filtered (zero-lag, 3rd-order Butterworth filter, cutoff frequency 14 Hz). A trial was considered an error if the cursor's radial position exceeded the channel's boundaries or if the cursor did not reach the target by the end of the trial duration (1.5 s). Error rate is the fraction of error trials out of all trials. Error rate, MT, and peak speed comparisons were performed with paired t-tests for the behavioral data from the scanner and an ANOVA for the behavioral data obtained in the laboratory. For average trajectory and variance calculations, we discarded from each movement the first and last 10° of cursor position, corresponding to the area within the initial and final targets (polar coordinate angle relative to an origin midway between the 2 targets). Trajectories were then interpolated to 200 points, using linear interpolation. Correction for multiple comparisons when comparing the averaged trajectories and the trial-by-trial variability measures was conducted with a random field Gaussian distribution correction for temporal correlation in the data (Shmuelof et al. 2012). This analysis focused on the time-normalized radial position of the cursor, which was the task-relevant control variable, using paired t-tests run repeatedly for every normalized time point (n = 200). To correct for the probability of false positives due to multiple comparisons, we addressed temporal correlations in the data that resulted from temporal smoothing. Corrected thresholds were thus computed based on the estimated number of truly independent samples present within the sampled vector using random field theory (Worsley et al. 1992).
RESULTS
Subjects showed improvement in the trained APT both in and outside the scanner.
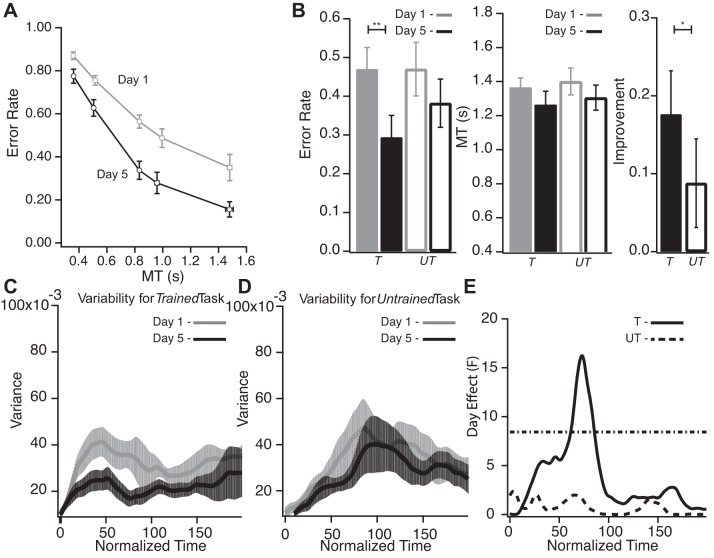
Subjects showed a significant improvement in APT performance across the tested range of movement times MTs when assessed outside the scanner after 3 days of training (comparison of performance on days 1 and 5, P < 0.001; Fig. 2A). Consistent with our previous report (Shmuelof et al. 2012), the improvement generalized to MTs not experienced during training.
Fig. 2.
A: performance in the sessions in the lab before (gray, day 1) and after (black, day 5) training. Error rate (fraction of movements outside the channel) is plotted against average movement time for the 5 imposed movement times (MTs). This plot illustrates the group's SAF and its change after practice. Subjects showed reduction in error rate in all measured speeds, i.e., a shift across the SAF to a higher level of performance. Error bars denote SE. B: performance measures from the Trained (T) and Untrained (UT) tasks (performed in the scanner). *P < 0.05; **P < 0.01. Left: error rate reduction following training for the Trained (filled bars) and Untrained tasks (open bars). Center: subjects did not significantly change MT in either task after training. Right: improvement was greater for the Trained task. Error bars denote SE. C: average trial-by-trial variability from day 1 (gray) and day 5 (black) scanning sessions of the Trained task. Averaged variability is plotted against normalized time. After training, there was a reduction in variability mainly during the first half of the movement. Error bars denote SE. D: average trial-by-trial variability from day 1 (gray) and day 5 (black) scanning sessions for the Untrained task. Averaged variability is plotted against normalized time. Variability for the Untrained task did not change with time. E: comparison of variability measures across days: day effect (F values) as a function of normalized time. Dotted horizontal line represents the threshold (corrected for multiple comparisons) above which F values are statistically significant. Significant changes in variability can be seen for the Trained task (solid line) but not for the Untrained task (dashed line).
During the imaging sessions on days 1 and 5, subjects performed both the Trained (horizontal arc; Fig. 1B) and Untrained (vertical arc) tasks. The Untrained task was introduced to control for a possible order effect: putative learning-related imaging effects for the Trained task on day 5 might instead be a nonspecific effect of performing the same task twice in the scanner regardless of training. If activation changes were merely due to an order effect, comparable activation changes would be seen from day 1 to day 5 for the Untrained task. Subjects showed improvement in accuracy for the Trained APT performed in the scanner from day 1 to day 5 (P = 0.007; Fig. 2B) with no associated change in MT (P = 0.38; Fig. 2B), peak movement speed (P = 0.362), and mean trajectory (P > 0.05 throughout the trajectory; see materials and methods). Crucially, the Trained task showed a decrease in trial-by-trial variability, with a maximal F value of 16.278 (P < 0.001; Fig. 2, C and E). The improvement in performance in the scanner is consistent with the behavioral results obtained outside the scanner (Fig. 2A). The observed reduction in variability is consistent with our previous behavioral work that showed reduction in trial-by-trial variability following training in the APT (Shmuelof et al. 2012). The Untrained task did not show changes in movement speed, MT, mean trajectory, and mean variability across days (P = 0.29, P = 0.31, and P > 0.05, respectively; Fig. 2B).
There was a significant difference in the degree of improvement for the Trained compared with the Untrained task (P = 0.049; Fig. 2B). The small improvement for the Untrained task, although not significant (P = 0.15), likely reflects partial generalization from the horizontal to the vertical task. It should be emphasized that we were looking for neural and behavioral differences between the Trained and Untrained tasks; such differences are not dependent on an absence of changes for the Untrained task.
Skill learning was associated with changes in contralateral motor cortical areas and ipsilateral cerebellum.
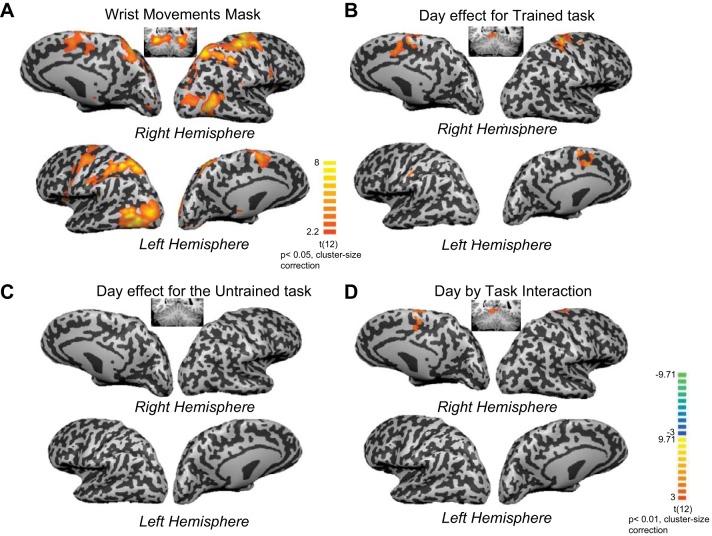
The functional imaging data were analyzed with a GLM (Friston et al. 1994). We were specifically interested in learning-related activation changes in brain areas associated with execution of wrist movements. Therefore the voxelwise analysis was constrained to the execution network for visually guided pointing movements of the left wrist. We used a localizer scan based on straight reaching movements of the left wrist on day 1 to identify the wrist movement network (Fig. 3A, Table 1). Notably, the mask was constructed based on the averaged contrast image from the Localizer scan using a low threshold of P = 0.05 (cluster size correction of 112 functional voxels), resulting in an inclusive mask of the execution network for wrist reaching movements.
Fig. 3.
A: blood oxygen level-dependent (BOLD) activation increase associated with the wrist localizer task. Voxel-based and ROI analyses were masked by mean activation pattern for straight reaching movements with the left wrist (Localizer scan, wrist movements > baseline). Average activation patterns are shown on inflated brain surfaces. Average activation in the cerebellum is shown on a coronal slice (y = −50). Reaching movement with the wrist was associated with a broad increase in activation in both hemispheres, in visual and motor areas and in the cerebellum. B: contrast map for the Trained task. Subjects were scanned while performing the Trained horizontal arc task before and after training (on days 1 and 5). A contrast analysis between day 1 and day 5 activation patterns within the task mask (subset a) is shown. Increase in activation after training is shown in red-yellow colors; decrease in activation is shown in blue-green colors (color coding is shown at bottom right). Training in the APT was associated with increased activation in the right primary motor, premotor, and supplementary motor cortices. Reduction of activation after training was not detected. C: contrast map for the Untrained vertical arc task. A contrast analysis for the Untrained task within the task mask did not result in any significant change in activation. D: task × training interaction analysis. An interaction analysis (using ANCOVA) between training (day 1 vs. day 5) and task (Trained vs. Untrained) within the task mask resulted in significant activation in premotor dorsal and supplementary motor cortex.
Table 1.
Execution- and learning-related brain activation: summary of activation loci for execution- and learning-related contrasts
| Talairach Coordinates |
|||||
|---|---|---|---|---|---|
| Region | Cluster Size, mm3 | Peak X | Peak Y | Peak Z | t-Value |
| Wrist movement day 1 > baseline (Localizer task, P < 0.05, cluster size correction) | |||||
| lPMC | 10,763 | 33 | −13 | 52 | 6.45 |
| SMA | 10,506 | 4 | −8 | 54 | 7.25 |
| rM1 | 24,365 | 25 | −23 | 48 | 10.65 |
| rAIP | 12,858 | 22 | −56 | 48 | 9.72 |
| lAIP | 13,167 | −20 | −62 | 54 | 11.37 |
| rLOG | 18,251 | 43 | −70 | 7 | 10.43 |
| lLOG | 23,615 | −44 | −71 | 3 | 13.99 |
| rPut | 7,092 | 22 | 4 | 6 | 6.52 |
| lPut | 4,427 | −26 | 1 | 0 | 4.78 |
| rCBL—lobe VI | 14,729 | 31 | −59 | −18 | 10.43 |
| lCBL—lobe VI | 21,287 | −29 | −53 | −18 | 12.82 |
| lCBL—lobe V | 18,534 | −2 | −59 | −12 | 10.62 |
| Trained task day 5 > day 1 (P < 0.01, cluster size correction) | |||||
| rM1 | 2,811 | 19 | −21 | 63 | 3.29 |
| rdPMC | 1,378 | 23 | −8 | 57 | 4.11 |
| SMA | 5,006 | −4 | −14 | 54 | 7.53 |
| rAIP | 596 | −44 | −37 | 47 | 2.06 |
| lCBL—lobe V | 1,340 | 2 | −50 | −9 | 4.70 |
| Task × day interaction (P < 0.01, cluster size correction) | |||||
| rdPMC | 1,619 | 11 | −14 | 66 | 3.71 |
| SMA | 1,065 | −4 | −14 | 54 | 4.57 |
| lCBL—lobe V | 2,104 | −5 | −47 | −12 | 5.02 |
dPMC, dorsal premotor cortex; SMA, supplementary motor cortex; M1, primary motor cortex; AIP, anterior intraparietal cortex; LOG, lateral occipital gyrus; Put, putamen; CBL, cerebellum.
Separate contrast maps were generated for a comparison between task-related activation patterns for days 1 and 5 (P < 0.01, cluster size correction of 19 contiguous functional voxels) within the task mask (Fig. 3A, Table 1) for the Trained (Fig. 3B, Table 1) and Untrained (Fig. 3C) tasks. Training on the horizontal APT was associated with increased activation in contralateral M1, contralateral dorsal premotor cortex (dPMC), and contralateral anterior intraparietal cortex (AIP), supplementary motor cortex (SMA), and ipsilateral cerebellum (Fig. 3B, Table 1). There were no significant reductions in activation following training within the task mask. For the Untrained vertical APT, there were no significant activation increases or decreases (Fig. 3C).
To quantitatively test whether acquisition of skill could be associated with a net global increase in activation across all voxels in the unmasked brain, we designed a nonparametric ranking procedure to compare the distributions of activation for all unthresholded voxels before and after training (see materials and methods). For every subject, day 5 and day 1 activation values for the Trained task from every voxel were ranked together. The proportion of ranks for day 5 activation values was then computed and compared to a null distribution, obtained by permuting condition labels within subjects. The average proportion of ranks across subjects for day 5 observations was 0.52, which was significantly higher than chance (P = 0.03, see materials and methods), indicating a global increase in activation for the Trained task following training. A similar analysis for the Untrained task did not indicate a global change in activation after training (0.5, P = 0.68).
Increases in activation were greater for the Trained task.
While the voxelwise and ROI results showed that the Trained horizontal APT was associated with significant changes in activation and the Untrained vertical APT was not, these results are not sufficient to establish a selective learning effect for the Trained APT compared with the Untrained APT. To reach this conclusion it is necessary to show a significant day × task interaction (Nieuwenhuis et al. 2011). There were significant day × task interactions for the voxelwise analysis in contralateral dPMC, in SMA, and in the ipsilateral cerebellum (Fig. 3D, Table 1).
Given the low sensitivity of the voxel-based analysis, we also used a global multivariate approach to show a day × task interaction for activation across all unthresholded and unmasked voxels. For the global interaction measure we used the same ranking analysis as described above, but this time ranked according to day 5 − day 1 activation values in each voxel for both the Trained and Untrained tasks together. The average proportion of ranks across subjects for the Trained task turned out to be 0.53, indicating that voxels changed more for the Trained than the Untrained task. Permutation analysis showed that the average proportion of ranks was significantly different from the null distribution (P = 0.03). Figure 4 demonstrates the shift of the distribution of the day 5 − day 1 activation pattern for the Trained task compared with the Untrained task for a single subject.
Fig. 4.
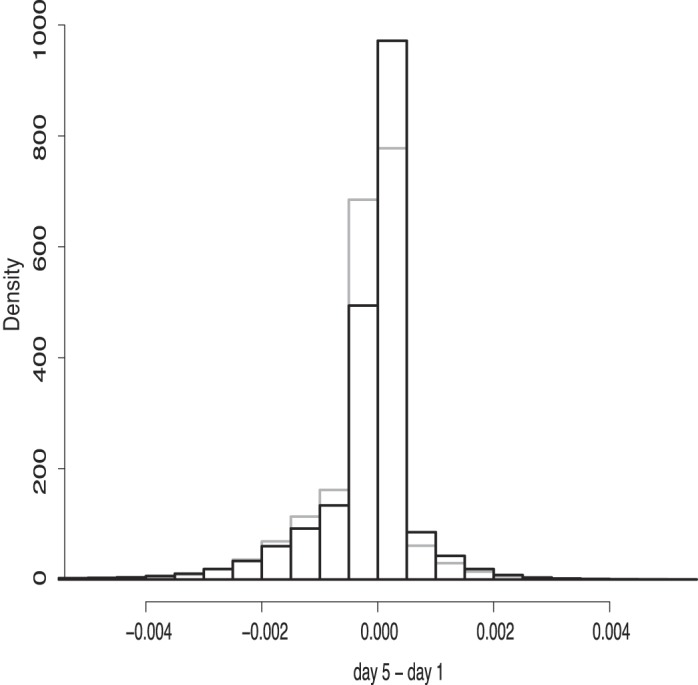
Sample of the global interaction analysis from a single subject: distribution of the training effect (day 5 − day 1) of all voxels for the Trained (black) and Untrained (gray) tasks. The Trained distribution is shifted to the right of the Untrained distribution, indicating fewer negative values and more positive values relative to the Untrained.
In summary, when kinematics were successfully constrained on day 1 and day 5 (same MT and average trajectory) for both the Trained horizontal APT and the Untrained vertical APT, there were significant learning-related increases in activation for the Trained task in contralateral motor cortical areas and in the ipsilateral cerebellum. Corroborating these results, a global test of activation across all voxels showed that there was greater activation overall for the Trained task compared with the Untrained task.
DISCUSSION
We sought to dissociate brain activation related to motor learning from brain activation related to motor execution. We were specifically interested in the neural correlates of decreased movement variability (improved motor acuity) when controlling visually guided cursor trajectories with the wrist. We found learning-related increases in activation in contralateral motor cortical areas and in the ipsilateral cerebellum when the task was performed with matched kinematics on the pre- and posttraining days.
We have recently suggested that it is motor acuity that requires learning-related changes in contralateral primary and premotor cortical areas (Krakauer and Mazzoni 2011; Shmuelof and Krakauer 2011). Studies of motor learning in rodents have consistently shown changes in contralateral M1 after practice on visually guided pellet prehension tasks (Greenough et al. 1985; Kargo and Nitz 2004; Kleim et al. 2002; Xu et al. 2009). These changes, which take days to weeks to develop, include expansion in motor maps, long-term potentiation, and synaptogenesis. In one study, rats were trained on a pellet prehension task over 12 days. Over the first 6 days, pellet retrieval success rates were associated with changes in the action selected and changes in the ratio of muscle activation for a particular EMG pattern. Reduction in the variability of the muscle recruitment pattern only occurred over days 7–12, and it was only this reduction in variability that correlated with improvements in SNR in M1 cells (Kargo and Nitz 2004). This result is entirely consistent with our results: the APT was designed to emphasize variability reduction over action selection. This result also provides a potential explanation for why so many human imaging studies have not shown learning-related changes in contralateral motor cortical areas after controlling for execution. The kind of learning seen in the first 6 days in the rat study is probably what is being emphasized in most human studies, namely, adaptation and action selection rather than motor acuity.
In previous work we have shown that training on the APT at slow speeds leads to improvements at untrained fast speeds (Shmuelof et al. 2012). We suggested that this generalization supports a representation of skilled movements that can be scaled across a range of difficulty (speed) levels. This idea is supported by our present result that learning-related activation was detectable even when performing at a slow speed. One possibility is that specific arrangements of controllers in M1 can be learned and associated with task-specific synergies. A fairly simple scalar input control onto these cortical representations could then allow these synergies to scale across speeds (d'Avella et al. 2008; Overduin et al. 2012). The degree of task specificity of these synergies is yet to be determined. The lack of a significant task × day interaction in M1 may support a partial overlap between learned synergies for the two tasks performed with the same effector. Thus we would conjecture that the nonsignificant interaction effect for the trained versus untrained task in M1 is due to generalization rather than a lack of learning-related changes in this region.
We found that training in the APT was associated with increases in activation in motor cortical areas and the cerebellum without any significant decrease in activation (Fig. 2B). In contrast, previous studies of motor learning that have focused on average activity changes, as we did here, have shown both increases and decreases in activation in several brain areas (Kelly and Garavan 2005; Petersen et al. 1998; Steele and Penhune 2010; Wu et al. 2004). Increased accuracy and precision in motor performance with training are presumably driven by increased SNR in brain representations. There is evidence from the perceptual learning literature that there are at least two cortical mechanisms for increasing SNR (Reed et al. 2011; Yang et al. 2009). Training-related improvements in frequency discrimination in rats are first associated with auditory cortex map expansion and then with map renormalization (Reed et al. 2011). The expanded representation may improve encoding through summation over more units, while selective stabilization of specific dendritic spines during the renormalization phase may be associated with improved encoding through selection of the most informative units, i.e., through reduction in the noise correlations between task-related units (Bejjanki et al. 2011). Increased accuracy and precision in motor performance with training, as for perceptual learning, are presumably also driven by increased SNR in brain representations. It may be that the reported bidirectionality of brain activation responses in motor learning tasks (Dayan and Cohen 2011; Hardwick et al. 2013) reflects the fact that the SNR can be improved by either increases in the number of neurons recruited for a task or selection of a subset of neurons specifically tuned to the task. The former would lead to increases in average activation and the latter to decreases. The relative balance of these competing mechanisms for a learned representation may be dependent on a variety of factors that include the task itself and the time spent practicing the task. Thus we propose that in our task motor acuity was associated with an increase in the number of neurons recruited. It is possible that with more prolonged training we would have seen activation return to day 1 levels (Puttemans et al. 2005; Reed et al. 2011; Xu et al. 2009). It should be noted that although statistical maps cannot distinguish increased extent (more voxels) from increased intensity (increased activation of same number of voxels), the latter would decrease, not increase, SNR.
A new approach to the study of learning is to use multivoxel pattern analysis (Cox and Savoy 2003; Kamitani and Tong 2005). With this approach it has been shown that improvement in perceptual orientation discrimination was associated with increased orientation discrimination in the BOLD signal taken from visual areas, without any changes in average activation in the same areas (Jehee et al. 2012). In the motor domain, it has recently been shown that those areas that showed the largest learning-related increases in classification accuracy of four separate trained finger sequences were in areas that showed no changes in average activation (Wiestler and Diedrichsen 2013). Areas that did show a change in average activation for the direct contrast between trained versus untrained sequences showed decreases in activation (bilateral dPMC and along the intraparietal sulcus) and no increases. How to reconcile these results with our and other studies (in multiple species) that suggest a predominant role for contralateral motor cortical areas for skill? In the study by Wiestler and Diedrichsen, subjects executed sequences faster by overlapping presses of each individual finger (Wiestler and Diedrichsen 2013). There was no measure of precision of either the individual finger presses or the two-finger transitions. Thus it could be argued that subjects were learning to choose the specific finger transitions needed for each sequence through a better representation of each sequence, i.e., faster selection of the required transitions. The ability to quickly execute any particular transition may, however, already have been at ceiling before learning even began. The decrease in mean activation in this case could be a result of the reduction in the cognitive effort required to select the right sequence of finger presses. Indeed, such automatization effects in sequence learning have been shown to be associated with reduction in activation in cortical motor areas (dPMC, SMA, and parietal regions) (Puttemans et al. 2005; Wu et al. 2004). Thus sequence tasks may for the most part emphasize action selection over action execution. In our task, in contrast, it is clear to subjects from the outset what action is needed, down to the submovements, and it is the variability of this single action that needs to be reduced with training. An increase in neural bandwidth may only be needed when speed and accuracy of a particular action increase and not when the only difference is whether the actions are released in parallel rather than serially.
It is important to avoid the error of reverse inference when speculating about the meaning of the activations observed in an imaging study (Poldrack 2006). Our main prediction was that contralateral motor cortical areas would show a learning effect if the task emphasized the requirement for motor acuity. That said, we were agnostic as to whether we would see a learning effect in the ipsilateral motor cerebellum or not; we observed increased activation in lobule V of the anterior lobe, which has been shown to be involved in visuomotor rotation learning (Donchin et al. 2012). The cerebellum is a critical structure for adaptation, returning behavior to baseline levels in the setting of external perturbations and maintaining a calibrated forward model of an ever-changing plant (Barash et al. 1999; Tseng et al. 2007). What is not clear is the degree to which the cerebellum is involved in improving motor acuity. We have recently shown that feedback-based corrections improve from day 1 to day 5 in the APT (Shmuelof et al. 2012). Such improved feedback responses could occur through improved state estimation by the cerebellum. In this framework, the decrease in variability seen with learning could be due to more precise feedback corrections enabled by the cerebellum and increased SNR via increased neuronal recruitment in motor cortical areas. The learning-related activation we observed in the cerebellum was medial to the previously reported hand area in superior cerebellar cortex (lobules V and VI) (Grodd et al. 2001; Kuper et al. 2012; Rijntjes et al. 1999). Indeed, we saw cerebellar activation in this hand area in our Localizer task (Fig. 3A). Changes in activation associated with learning a new internal model also occur outside the cerebellar hand area (Imamizu et al. 2000). The results of this previous study and our present study suggest that both acquisition of a new forward model and improvement of state estimates in an existing forward model may depend on the same cerebellar representation.
An alternative explanation for our results could be that the activation differences are driven by differences in observed errors before and after training. Indeed, both motor cortical areas and the cerebellum have been shown to have error-related activation (Diedrichsen et al. 2005; Imamizu et al. 2000; Schlerf et al. 2012). Critically, however, in these cases, activation increased as errors increased and, in the case of the cerebellum, occurred in the hand area. Here we show that activation increased with training as errors decreased and this activation was medial to the previously reported error-related cerebellar hand area activations.
Conclusions.
We show that improvements in motor acuity over days are associated with learning-related increases in activation in areas within the baseline execution network: contralateral motor cortical areas and the ipsilateral cerebellum. A global nonlocalizing analysis confirmed that learning was associated with net increases in activation. Thus the observed decreases in movement variability could be accounted for by a learning-related increase in the number of neurons recruited for the task. We conclude that when humans improve in the performance of a task that in many ways can be considered an analog for visually guided reaching, learning-related changes occur within the execution network in a manner analogous to that seen in rodent and nonhuman primate models (Nudo et al. 1996; Xu et al. 2009).
GRANTS
This research was supported by National Institute of Neurological Disorders and Stroke Grant R01 NS-052804 (J. W. Krakauer, P. Mazzoni), Machiah Foundation/Jewish Community Federation, Feinberg Graduate School and EU Grant FP7 TANGO (L. Shmuelof), and the Parkinson's Disease Foundation (P. Mazzoni).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.S., P.M., and J.W.K. conception and design of research; L.S. performed experiments; L.S., J.Y., and B.C. analyzed data; L.S., P.M., and J.W.K. interpreted results of experiments; L.S. prepared figures; L.S., P.M., and J.W.K. drafted manuscript; L.S., J.Y., B.C., and J.W.K. edited and revised manuscript; L.S., P.M., and J.W.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Eric Zarahn for assistance with experimental design and insightful discussion; Terry Sanger for discussions about variability; Juan Camilo Cortes and R. J. Delnicki for technical assistance; and Robert S. Sainburg for sharing experiment-control software.
REFERENCES
- Barash S, Melikyan A, Sivakov A, Zhang M, Glickstein M, Thier P. Saccadic dysmetria and adaptation after lesions of the cerebellar cortex. J Neurosci 19: 10931–10939, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejjanki VR, Beck JM, Lu ZL, Pouget A. Perceptual learning as improved probabilistic inference in early sensory areas. Nat Neurosci 14: 642–648, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censor N, Sagi D, Cohen LG. Common mechanisms of human perceptual and motor learning. Nat Rev Neurosci 13: 658–664, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DD, Savoy RL. Functional magnetic resonance imaging (fMRI) “brain reading”: detecting and classifying distributed patterns of fMRI activity in human visual cortex. Neuroimage 19: 261–270, 2003 [DOI] [PubMed] [Google Scholar]
- Cunningham HA. Aiming error under transformed spatial mappings suggests a structure for visual-motor maps. J Exp Psychol Hum Percept Perform 15: 493–506, 1989 [DOI] [PubMed] [Google Scholar]
- d'Avella A, Fernandez L, Portone A, Lacquaniti F. Modulation of phasic and tonic muscle synergies with reaching direction and speed. J Neurophysiol 100: 1433–1454, 2008 [DOI] [PubMed] [Google Scholar]
- Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron 72: 443–454, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Changes in brain activation during the acquisition of a new bimanual coordination task. Neuropsychologia 42: 855–867, 2004 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R. Neural correlates of reach errors. J Neurosci 25: 9919–9931, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin O, Rabe K, Diedrichsen J, Lally N, Schoch B, Gizewski ER, Timmann D. Cerebellar regions involved in adaptation to force field and visuomotor perturbation. J Neurophysiol 107: 134–147, 2012 [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189–210, 1994 [Google Scholar]
- Grafton ST, Hazeltine E, Ivry R. Functional mapping of sequence learning in normal humans. J Cogn Neurosci 7: 497–510, 1995 [DOI] [PubMed] [Google Scholar]
- Grafton ST, Schmitt P, Van Horn J, Diedrichsen J. Neural substrates of visuomotor learning based on improved feedback control and prediction. Neuroimage 39: 1383–1395, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Larson JR, Withers GS. Effects of unilateral and bilateral training in a reaching task on dendritic branching of neurons in the rat motor-sensory forelimb cortex. Behav Neural Biol 44: 301–314, 1985 [DOI] [PubMed] [Google Scholar]
- Grodd W, Hulsmann E, Lotze M, Wildgruber D, Erb M. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp 13: 55–73, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RM, Rottschy C, Miall RC, Eickhoff SB. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage 67: 283–297, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms KJ, Rioult-Pedotti MS, Carter DR, Dunaevsky A. Transient spine expansion and learning-induced plasticity in layer 1 primary motor cortex. J Neurosci 28: 5686–5690, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, Yoshioka T, Kawato M. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature 403: 192–195, 2000 [DOI] [PubMed] [Google Scholar]
- Inoue K, Kawashima R, Satoh K, Kinomura S, Goto R, Sugiura M, Ito M, Fukuda H. Activity in the parietal area during visuomotor learning with optical rotation. Neuroreport 8: 3979–3983, 1997 [DOI] [PubMed] [Google Scholar]
- Jehee JF, Ling S, Swisher JD, van Bergen RS, Tong F. Perceptual learning selectively refines orientation representations in early visual cortex. J Neurosci 32: 16747–16753, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins IH, Passingham RE, Brooks DJ. The effect of movement frequency on cerebral activation: a positron emission tomography study. J Neurol Sci 151: 195–205, 1997 [DOI] [PubMed] [Google Scholar]
- Kamitani Y, Tong F. Decoding the visual and subjective contents of the human brain. Nat Neurosci 8: 679–685, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargo WJ, Nitz DA. Improvements in the signal-to-noise ratio of motor cortex cells distinguish early versus late phases of motor skill learning. J Neurosci 24: 5560–5569, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377: 155–158, 1995 [DOI] [PubMed] [Google Scholar]
- Kelly AM, Garavan H. Human functional neuroimaging of brain changes associated with practice. Cereb Cortex 15: 1089–1102, 2005 [DOI] [PubMed] [Google Scholar]
- Kelso JA. Phase transitions and critical behavior in human bimanual coordination. Am J Physiol Regul Integr Comp Physiol 246: R1000–R1004, 1984 [DOI] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Cooper NR, Hogg TM, Reidel CN, Remple MS, Nudo RJ. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem 77: 63–77, 2002 [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Ghilardi MF, Mentis M, Barnes A, Veytsman M, Eidelberg D, Ghez C. Differential cortical and subcortical activations in learning rotations and gains for reaching: a PET study. J Neurophysiol 91: 924–933, 2004 [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Mazzoni P. Human sensorimotor learning: adaptation, skill, and beyond. Curr Opin Neurobiol 21: 636–644, 2011 [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Pine ZM, Ghilardi MF, Ghez C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci 20: 8916–8924, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuper M, Thurling M, Stefanescu R, Maderwald S, Roths J, Elles HG, Ladd ME, Diedrichsen J, Timmann D. Evidence for a motor somatotopy in the cerebellar dentate nucleus—an FMRI study in humans. Hum Brain Mapp 33: 2741–2749, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall RC, Jenkinson EW. Functional imaging of changes in cerebellar activity related to learning during a novel eye-hand tracking task. Exp Brain Res 166: 170–183, 2005 [DOI] [PubMed] [Google Scholar]
- Miall RC, Reckess GZ, Imamizu H. The cerebellum coordinates eye and hand tracking movements. Nat Neurosci 4: 638–644, 2001 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Forstmann BU, Wagenmakers EJ. Erroneous analyses of interactions in neuroscience: a problem of significance. Nat Neurosci 14: 1105–1107, 2011 [DOI] [PubMed] [Google Scholar]
- Nissen M, Bullemer P. Attentional requirements of learning: evidence from performance measures. Cogn Psychol 19: 1–32, 1987 [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci 16: 785–807, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban P, Peigneux P, Lungu O, Debas K, Barakat M, Bellec P, Benali H, Maquet P, Doyon J. Functional neuroanatomy associated with the expression of distinct movement kinematics in motor sequence learning. Neuroscience 179: 94–103, 2011 [DOI] [PubMed] [Google Scholar]
- Overduin SA, d'Avella A, Carmena JM, Bizzi E. Microstimulation activates a handful of muscle synergies. Neuron 76: 1071–1077, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, van Mier H, Fiez JA, Raichle ME. The effects of practice on the functional anatomy of task performance. Proc Natl Acad Sci USA 95: 853–860, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends Cogn Sci 10: 59–63, 2006 [DOI] [PubMed] [Google Scholar]
- Puttemans V, Wenderoth N, Swinnen SP. Changes in brain activation during the acquisition of a multifrequency bimanual coordination task: from the cognitive stage to advanced levels of automaticity. J Neurosci 25: 4270–4278, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed A, Riley J, Carraway R, Carrasco A, Perez C, Jakkamsetti V, Kilgard MP. Cortical map plasticity improves learning but is not necessary for improved performance. Neuron 70: 121–131, 2011 [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci USA 106: 1590–1595, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijntjes M, Buechel C, Kiebel S, Weiller C. Multiple somatotopic representations in the human cerebellum. Neuroreport 10: 3653–3658, 1999 [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science 290: 533–536, 2000 [DOI] [PubMed] [Google Scholar]
- Robertson EM, Tormos JM, Maeda F, Pascual-Leone A. The role of the dorsolateral prefrontal cortex during sequence learning is specific for spatial information. Cereb Cortex 11: 628–635, 2001 [DOI] [PubMed] [Google Scholar]
- Schlerf J, Ivry RB, Diedrichsen J. Encoding of sensory prediction errors in the human cerebellum. J Neurosci 32: 4913–4922, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuelof L, Krakauer JW. Are we ready for a natural history of motor learning? Neuron 72: 469–476, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuelof L, Krakauer JW, Mazzoni P. How is a motor skill learned? Change and invariance at the levels of task success and trajectory control. J Neurophysiol 108: 578–594, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Johansen-Berg H. The role of GABA in human motor learning. Curr Biol 21: 480–484, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele CJ, Penhune VB. Specific increases within global decreases: a functional magnetic resonance imaging investigation of five days of motor sequence learning. J Neurosci 30: 8332–8341, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98: 54–62, 2007 [DOI] [PubMed] [Google Scholar]
- Turner RS, Grafton ST, Votaw JR, Delong MR, Hoffman JM. Motor subcircuits mediating the control of movement velocity: a PET study. J Neurophysiol 80: 2162–2176, 1998 [DOI] [PubMed] [Google Scholar]
- Wiestler T, Diedrichsen J. Skill learning strengthens cortical representations of motor sequences. eLife 2: e00801, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 12: 900–918, 1992 [DOI] [PubMed] [Google Scholar]
- Wu T, Kansaku K, Hallett M. How self-initiated memorized movements become automatic: a functional MRI study. J Neurophysiol 91: 1690–1698, 2004 [DOI] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462: 915–919, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature 462: 920–924, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]