Abstract
The non-phase-locked EEG response to painful stimuli has usually been characterized as decreased oscillatory activity (event-related desynchronization, ERD) in the alpha band. Increased activity (event-related synchronization, ERS) in the gamma band has been reported more recently. We have now tested the hypothesis that the non-phase-locked responses to nonpainful electric cutaneous stimuli are different from those to painful cutaneous laser stimuli when the baseline salience of the two stimuli is the same and the salience during the protocol is modulated by count laser and count electric tasks. Both of these stimuli were presented in random order in a single train at intensities that produced the same baseline salience in the same somatic location. The response to the laser stimulus was characterized by five windows (designated windows I–V) in the time-frequency domain: early (200–400 ms) and late (600–1,400 ms) delta/theta ERS, 500–900 ms alpha ERD, 1,200–1,600 ms beta ERS (rebound), and 800–1,200 ms gamma ERS. Similar ERS/ERD windows of activity were found for the electric stimulus. Individual participants very commonly had activity in windows consistent with the overall analysis. Linear regression of ERS/ERD for parietal channels was most commonly found for sensory (pain or unpleasantness)- or attention (salience)-related measures. Overall, the main effect for modality was found in window I-delta/theta and window V-gamma, and the Modality with Task interaction was found in all five windows. All significant interaction terms included Modality as a factor. Therefore, Modality was the most common factor explaining our results, which is consistent with our hypothesis.
Keywords: attention, cortex, event-related synchronization, EEG, human, pain
event-related changes in EEG spectral power can be measured by a decrease in oscillatory activity (event-related desynchronization, ERD) or an increase in oscillatory activity (event-related synchronization, ERS) (Lopes da Silva and Pfurtscheller 1999). These spectral responses (ERS/ERD) are not phase locked to the event but are analyzed by signal averaging in the frequency domain. Different frequency bands exhibit different temporal, spatial, and task-related characteristics, which are consistent with their involvement in different aspects of cerebral processing (Bastiaansen and Brunia 2001; Boiten et al. 1992; Klimesch et al. 1998; Tiihonen et al. 1991). Event-related spectral modulation of scalp EEG has also been applied to studies of the cortical processing of painful stimuli.
In response to painful stimuli, ERD has been found most frequently in the alpha band (Babiloni et al. 2006; Ferracuti et al. 1994; Hu et al. 2013; Mouraux et al. 2003; Ploner et al. 2006). ERS has also been reported at longer latency in the gamma band (Croft et al. 2002) or both the beta (rebound) and gamma bands (Hauck et al. 2007; Mouraux and Iannetti 2008). Some of these ERS/ERD activities have been associated with the perception of pain (Babiloni et al. 2006; Croft et al. 2002) and with endogenous or exogenous attention to the painful stimulus (Hauck et al. 2007; Hu et al. 2013).
It is not clear that any one of these somatic sensory pain-related activities is specific for painful as opposed to nonpainful somatic stimuli, or to the salience of the stimuli (Iannetti et al. 2008; Legrain et al. 2011). We have now tested the hypothesis that the non-phase-locked responses to nonpainful electric cutaneous stimuli are different from those to painful cutaneous laser stimuli when the baseline salience of the two stimuli is the same and the salience during the experimental protocol is modulated by count laser and count electric tasks. In these tasks, participants attended by counting either the painful laser stimuli (count laser) or the nonpainful electrical stimuli (count electric) while both modalities of stimulation were presented in random order in a single train of stimuli, as in previous studies (Bushnell et al. 1985, 1999; Bushnell and Duncan 1989; Longe et al. 2001; Tremblay et al. 1993). The effect of directed attention toward or distraction from a stimulus is to increase or decrease the salience of the stimulus relative to baseline (Downar et al. 2002; Legrain et al. 2011).
The present behavioral paradigm was focused on activities specific to pain. To control for the effect of somatotopic location of the stimulus, both painful laser and nonpainful electrical stimuli were presented in the same somatic location (left dorsal forearm and hand). To control for the effect of expectancy, both stimuli were presented in random order and with random interstimulus intervals. To control for the effect of intrusiveness, both stimuli were presented at intensities that produced the same baseline salience, as determined for each participant in a baseline session prior to the experiment. Therefore, the protocol focused on ERD/ERS interactions specific to painful versus nonpainful stimuli, as modulated by attention.
MATERIALS AND METHODS
Participants and EEG recordings.
Sixteen healthy participants (10 men and 6 women; aged 22–57 yr) were recruited in this study. The protocol for this study was approved by the Institutional Review Board of the Johns Hopkins University School of Medicine; all participants signed an informed consent form prior to the study. The 19-channel EEG signals were recorded with Ag-AgCl electrodes (Grass) placed on the scalp according to the International 10-20 System with a referential montage to a reference of linked earlobes (Fp1, Fp2, Fz, F3, F4, F7, F8, Cz, C3, C4, T3, T4, Pz, P3, P4, T5, T6, O1, O2). Signals were amplified and digitized at the sampling rate of 500 Hz (SynAmps 5083, Neuroscan). The timings for the onset of the laser and electrical stimuli were acquired and digitally embedded in the recordings. The same data have previously been used to analyze causal functional interactions between signals recorded from different electrodes (Markman et al. 2013).
Experimental design.
Painful cutaneous laser and nonpainful electrical pulses were delivered in four blocks of 80 stimuli (40 of each modality) in random order with random interstimulus intervals of 7–8 s. The randomization procedures were carried out by the use of a standard random number generator (java.util.Random, Oracle, Redwood Shores, CA).
In this study, attention was directed as participants were instructed prior to each block to count either the number of laser stimuli (count laser task) or the number of electrical stimuli (count electric task). The protocol consisted of two blocks of “count laser” followed by two blocks of “count electric,” or vice versa. Therefore, there were a total of four blocks (blocks 1, 2, 3, and 4), and the task of the first two blocks (blocks 1 and 2) was randomly assigned to either the count laser task or the count electric task and counterbalanced across participants. The task of counting stimuli was the same whether attention was directed to the laser or the electrical stimulus. The experimental design of directing attention to one of the two stimulus modalities in a single train of stimuli has been used in studies of the attentional modulation of painful stimuli (Bushnell et al. 1985, 1999; Bushnell and Duncan 1989; Tremblay et al. 1993). This protocol was designed to examine activity induced by painful and nonpainful stimuli across a range of salience as manipulated by the experimental task, against a baseline salience that was equal for both stimuli.
Before the first block of stimuli, participants were not told that they would be asked to rate psychophysics of the stimuli at the end of the block. However, at the end of each block, participants were asked to report the number of attended stimuli in that block and to rate the pain intensity, pain unpleasantness, and salience of the laser stimulus. In addition, the participants were asked to rate unpleasantness and salience for the electrical stimulus. All the ratings in this study ranged from 0 to 10. The effect of priority upon these ratings is summarized in results, based upon a detailed presentation of these results in an earlier publication (Markman et al. 2013).
Painful laser and nonpainful electrical stimulation.
The experiment was conducted in a silent, dimly lit room with the room temperature between 22°C and 24°C. Participants sat in a chair and rested their arms on a table in front of them. Insert earphones (ER1-14A Eartips, Etymotic Research, Elk Grove Village, IL) delivered a constant white noise (60–80 dB) throughout the experiment (Click-Tone control module, Astro-Med, Grass Instrument Division).
Participants were asked to keep their eyes closed and sit quietly throughout the experiment while trains of painful laser and nonpainful electrical stimuli were delivered. Both painful and nonpainful stimuli were applied within the territory of the left superficial radial nerve. The nonpainful electrical stimuli were constant-current square-wave pulses of 1-ms duration and were delivered by a Grass S12 Isolated Biphasic Stimulator through skin electrodes (0.5-cm diameter, 1-cm interelectrode distance) on the dorsum of the left wrist. The laser stimuli were generated and delivered with a thulium YAG laser (Neurotest, Wavelight, Starnberg, Germany) with a laser beam that had a wavelength of 2 μm, a diameter of 6 mm, and a duration of 1 ms. The laser stimuli were applied on the dorsum of the left hand. The stimuli locations were slightly different each time to avoid fatigue or sensitization.
At the end of each block, numerical rating scales were used to rate the participant's psychophysical metrics regarding both painful and nonpainful stimuli. For the nonpainful electrical stimulus, unpleasantness was rated on a numerical rating scale that was anchored by 0 for the absence of unpleasantness and 10 for the greatest imaginable unpleasantness. For the painful laser stimulus, pain intensity and unpleasantness were rated separately on numerical rating scales. The pain intensity scale was anchored by 0 for the absence of pain and 10 for the maximum imaginable pain. For both modalities, salience was described as “the ability of the stimulus to capture attention” and was rated on a numerical rating scale for which 0 was the absence of salience and 10 was the most salient stimulus imaginable.
For each participant, a series of laser pulses was delivered at eight energy levels in an increasing order ranging from 400 to 900 mJ, i.e., 400, 480, 560, 640, 720, 800, 850, and 900 mJ. Prior to the experiment task, the participant was asked to rate the pain intensity for the given energy levels. The laser energy for the experimental task was selected to be the level rated as a pain of 4–6 by the participant. In addition, at each given energy level the participant was asked to rate unpleasantness and salience. The average energy level corresponding to a 4–6 pain intensity rating was 730 ± 170 mJ.
In a separate session prior to the experiment task, all participants were familiarized with electrical stimuli by a series of electric pulses at intensity levels from 5.5 to 18 mA, all of which were nonpainful. Participants were asked to rate the unpleasantness and salience for each intensity level. For each participant, the final intensity level of the electrical stimulus was selected so that the electrical stimulus was nonpainful and produced a salience rating that was equal to the salience of the selected laser energy level (average current 12.8 ± 5.2 mA). For each participant, both levels of electric and laser stimulation were fixed and applied during all experiments so that at baseline painful and nonpainful stimuli were considered equally salient.
Event-related spectral power (ERS/ERD) analysis.
In this study, the event-related spectral perturbation was used to estimate the event-related non-phase-locked responses induced by the laser and electric stimulations (Delorme and Makeig 2004). This technique measures significant event-related changes in the power spectrum across different frequency bands in the poststimulus interval. To detect power changes across different frequencies, each poststimulus spectral estimate was divided by the mean baseline power spectrum, and this ratio was the ERS/ERD.
Prior to the ERS/ERD analysis, the event-related potentials (ERPs) were estimated by averaging signals across trials and channels for each participant and task; these ERPs were subtracted from the signals (Fig. 1, top). In the statistical analysis of ERS/ERD data, we established the levels for significance (upper and lower boundaries) after the FFT application. We used a bootstrap procedure that was performed over a 0.2-s time period immediately before the stimulus onset. The significant level was set to α = 0.05. The final results from this analysis were presented as a ratio between the baseline and poststimulus estimates. It was called ERS if this ratio was >1 and ERD if <1. The following provides a more detailed account of the statistical analysis of the power spectrum.
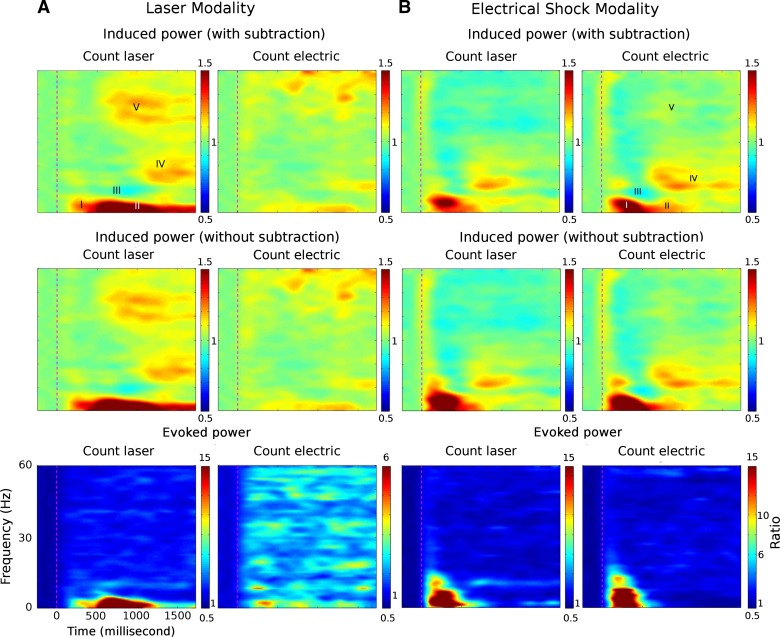
Fig. 1.
Time-frequency plots of event-related synchronization (ERS)/event-related desynchronization (ERD) in the count laser and count electric tasks as averaged across all participants and channels for the response to the painful laser stimulus (A) and the nonpainful electrical stimulus (B), as labeled. Top and middle: these plots with and without subtraction of the laser-evoked potential (A) and electrical cutaneous-evoked potential (B). Bottom: the phase-locked time-frequency plot evoked by the laser (A) and the electrical cutaneous stimulus (B). In all rows, hot and cold colors indicate increases and decreases in power relative to the background level. For example, 1.5 and 0.5 in the color bar scale denote 1.5 and 0.5 times the baseline power, and therefore the ERS and ERD; 1 denotes the baseline power. The color bar for the response evoked by the laser stimulus in the count electric plot (A) is different from the other plots in order to show activity in this category more clearly.
The EEG recordings were rereferenced to an averaged reference and filtered 0.1 to 250 Hz using a Hanning window finite impulse response filter. The event-related EEG epochs were extracted from every trial with a fixed interval of 0.5 s before and 2 s after at the onset of the stimulations. All EEG epochs were visually inspected by two independent individuals for artifact rejections. The time-frequency analysis was performed partly with newtimf.m in the EEGLAB, an open source toolbox, running in the 64-bit MATLAB [R2012a (7.14.0.739)] environment (Delorme and Makeig 2004). Briefly, using the windowed FFT approach, the analysis estimated the significant event-related changes in the amplitude of the power spectrum across different times/frequencies. A sliding window with a length of 512 ms was used for the FFT, and this window was advanced 5 ms throughout the whole epoch. The final time-frequency matrix for each epoch (∼80 epochs per subject per modality) was set to have 410 linear-spaced frequencies from 0.2 Hz to 100 Hz and 400 time stamps ranging from −244.0 to 1,744.0 ms, and thus the resolutions for time and frequency were 0.24 Hz and 4.97 ms. To establish the levels of the significances (upper and lower boundaries) after the windowed FFT application for each frequency step (from 0.2 to 100 Hz, step size = 0.24 Hz), a bootstrap procedure was performed by randomly selecting the spectral estimates in a shorter time period across epochs (i.e., 0.2 s immediately before the stimulus onset) (Delorme and Makeig 2004). The final results from this analysis were presented as a ratio between the baseline and poststimulus spectral estimates. It is called ERS if this ratio was >1 and ERD if <1.
RESULTS
Psychophysical ratings.
The psychophysics of the stimuli and the behavioral paradigm have been presented in a preceding paper (Markman et al. 2013) and so are described briefly here. Across all participants, the painful laser evoked sharp or pinprick pain sensations and the electrical stimulus produced a nonpainful tingling sensation. The pain intensity was significantly greater (5.4 ± 2 vs. 1.9 ± 3; P < 0.01, paired t-test) during the count laser task than during the count electric task. Error rates in this paradigm (total presented stimuli minus reported stimuli) were low both for counting the total number of painful laser stimuli in the count laser task and for counting the nonpainful electric stimuli in the count electric task (0.12 ± 0.09 vs. 0.10 ± 0.12; P > 0.05).
Stimulus intensities were adjusted so that both stimuli produced the same baseline salience (prior to the task blocks); the average salience level for both the laser and the electrical stimulus was 5.3 ± 1.9. The salience level for the laser stimulus during the count laser task (6.2 ± 2.6) was not significantly different from that for the electric stimulus during the count electric task (4.8 ± 2.0; P > 0.05, t-test). Similarly, the salience level for the laser stimulus under the count electric task was not significantly different from that for the electric stimulus under the count laser task (2.0 ± 1.4 vs. 2.0 ± 1.7; P > 0.05). This was consistent with prior demonstrations that nonpainful somatic stimuli can be as salient as painful somatic stimuli (Legrain et al. 2011; Mouraux and Iannetti 2009). Salience, pain (laser only), and unpleasantness ratings were always higher for the stimulus that was counted in each task. Error rates were low, which indicated correct performance of the tasks overall.
To examine the effect of priority upon the salience ratings, we compared the first versus the second block in the first pair of blocks and the first versus the second block in the second pair of blocks. The first pair of blocks examined the laser stimulus under the count laser task, and the second pair of blocks examined the electric stimulus under the count electric task (see materials and methods). The order of the tasks between the first two blocks versus the second two blocks was randomized and counterbalanced across subjects. Overall, the results demonstrate that none of the differences in the ratings between the blocks of the same tasks and stimuli was significant (Table 2 in Markman et al. 2013).
Table 2.
Consistency of a significant change in ERS/ERD in a window across individual subjects vs. the window overall
| Window | F3 | F4 | C3 | C4 | Cz | P3 | P4 | Pz |
|---|---|---|---|---|---|---|---|---|
| Count laser | ||||||||
| I | 0.81 | 1 | 0.88 | 0.94 | 1 | 0.94 | 0.94 | 1 |
| II | 0.94 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| III | 0.88 | 1 | 0.88 | 0.88 | 0.75 | 0.81 | 0.88 | 0.75 |
| IV | 1 | 1 | 0.94 | 1 | 1 | 1 | 0.94 | 1 |
| V | 0.94 | 0.94 | 1 | 1 | 1 | 1 | 0.94 | 1 |
| Count electric | ||||||||
| I | 0.88 | 0.81 | 0.94 | 0.94 | 1 | 0.81 | 1 | 0.94 |
| II | 0.88 | 0.81 | 1 | 0.94 | 0.94 | 0.94 | 0.88 | 1 |
| III | 0.94 | 0.88 | 0.75 | 0.81 | 0.56 | 0.69 | 0.63 | 0.56 |
| IV | 0.88 | 0.88 | 0.88 | 0.94 | 1 | 0.75 | 0.94 | 1 |
| V | 1 | 1 | 1 | 1 | 0.94 | 0.94 | 0.94 | 1 |
If all subjects had significance in the same window as the overall analysis, then the consistency had a value of 1; if no subject had a significant window, then the consistency had a value of 0.
ERS/ERD spectral windows: laser vs. electrical stimuli.
The basis of all our analyses was time-frequency plots that were constructed for painful laser stimulation and nonpainful electrical cutaneous stimulation (Fig. 1). The selection of windowing parameters (Table 1) was arbitrary and was based upon the overall results (Fig. 1) without accounting for differences in results between subjects, tasks, or modalities. Therefore, the consistency between data overall versus individual subjects and factors was unlikely to be a product of windowing of the overall results. The color bar for the plot of the laser stimulus and count electric task was different from the other plots in order to optimally show activity in this category. Evidence of induced activity in this category (Fig. 2, top right) occurred in windows I and II-delta/theta and channels Cz, Pz, C4, and P3.
Table 1.
Time and frequency dimensions for windows for ERS/ERD
| Window I-delta/thetaERS | Window II-delta/thetaERS | Window III-alphaERD | Window IV-betaERS | Window V-gammaERS | |
|---|---|---|---|---|---|
| Laser time, ms | 200–400 | 600–1,400 | 500–900 | 1,200–1,600 | 800–1,200 |
| Laser frequency, Hz | 0–8 | 0–8 | 8–10 | 15–25 | 40–50 |
| Electric time, ms | 150–500 | 700–900 | 400–550 | 700–1,600 | 800–1,200 |
| Electric frequency, Hz | 0–8 | 0–8 | 8–10 | 10–20 | 40–50 |
ERS, event-related synchronization; ERD, event-related desynchronization.
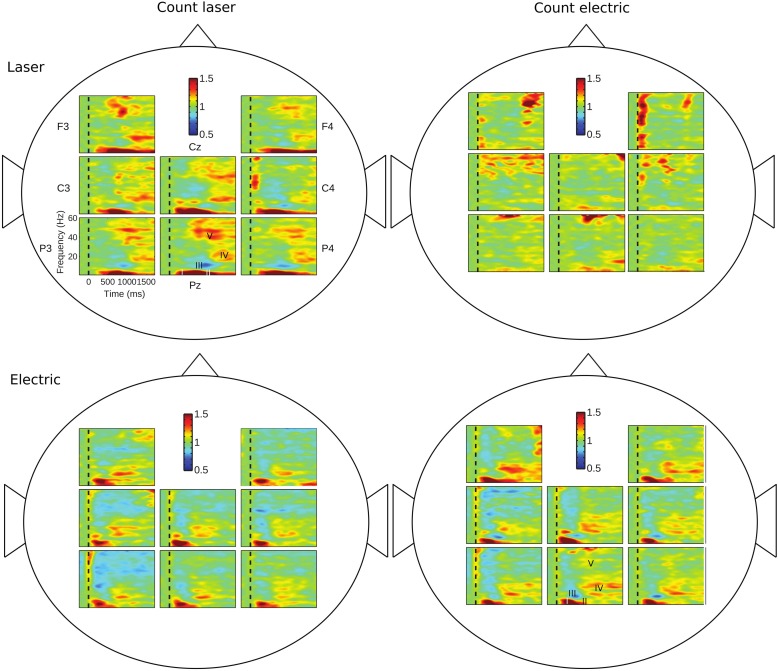
Fig. 2.
Time-frequency plots of ERS/ERD in all 4 Task by Modality combinations as averaged across all participants and within channels, as labeled in top left panel (Attention Task, Laser Modality). Conventions for axes and color scales are as described in the legend for Fig. 1 and as shown in top left panel of this figure.
The present ERS/ERD analysis was based upon results that were calculated with subtraction of the ERP, i.e., of Fig. 1, top. This analysis revealed patterns of ERS/ERD with five clearly distinct windows of induced activity, as shown in Fig. 1. Windows I and II, both delta/theta, were the only windows showing significant phase-locked activity (Fig. 1, bottom), which indicated that activity in these windows reflected both evoked and induced activity. Other windows showed only non-phase-locked (induced) activity. Nevertheless, the time-frequency plots did not show differences between induced power with and without subtraction of the evoked activity (Fig. 1, top and middle), which suggested that the ERP did not influence the non-phase-locked time-frequency plot. The results of induced power with and without subtraction of ERPs and evoked power were critical for the interpretation of ERS/ERD activity.
Time-frequency plots for both modalities of stimulation showed low-frequency (delta/theta band) early windows (Table 1) that were bimodal with earlier and later windows (Fig. 1A, top; windows I and II-delta/theta). This was more evident in the count electric task for the electrical stimulus (Fig. 1B) and in the count laser task for the laser stimulus (Fig. 1A). Both the laser and the electric modalities showed an alpha ERD (window III-alpha, Table 1) followed by a beta rebound ERS (window IV-beta), which was more pronounced in the count electric task for the electrical stimulus and in the count laser task for the laser stimulus. The latencies of these induced low- and high-frequency non-phase-locked responses were longer than those in previous studies (Gross et al. 2007; Mouraux et al. 2003; Zhang et al. 2012). Some of those studies employed passive tasks, and none employed the two-stimulus simultaneous presentation task of the present study, which was cognitively demanding and may have led to longer latencies of activation.
These measures of ERS/ERD are all expressed as ratios of pre- to poststimulus power [see Event-related spectral power (ERS/ERD) analysis]. To give an indication of the raw power by task modality category, we present the following median (95th–5th percentiles) values across all windows and channels: count laser: laser stimulus 17 (31–9) μV2/Hz, electric stimulus 29 (48–17); and count electric: laser stimulus 18 (30–11), electric stimulus 32 (57–18). These are consistent with the results for wakeful ongoing scalp EEG recording in healthy individuals (Niedermeyer and da Silva 2005).
ERS/ERD: consistency between participants of ERS/ERD across EEG channels.
We next examined the consistency of the results by tabulating the total number of subjects for which an ERS/ERD window was consistent with the same window and channel as in the overall analysis (Fig. 1). This was expressed as the ratio of the number of subjects with consistent ERS/ERD channels by window and channel (see Table 2) divided by the total number of subjects (n = 16).
As an example of this ratio, for the count laser task, window V-gamma, and channel F3, 15 of 16 subjects had the same significant ERS/ERD as the overall analysis for a ratio of 15/16 or 0.94. This is termed as 1 miss since one channel did not have the same significant ERS/ERD as the overall analysis. For the count laser task (Table 2), each window within channel had ratios of 1–0.68. For the count electric task, the ratios were between 1 and 0.5. This analysis was an indicator of the generalizability of the present results, which will be a basis for comparisons with other studies.
These results demonstrate a congruent pattern of ERS/ERD between individual participants versus the overall analysis. Table 2 shows that most participants had no misses for the majority of the window channel pairs. This might suggest that those windows for which ERS/ERD in individual participants were different from the overall analysis occurred equally among all participants. Therefore, we examined the results by looking for misses by channel and window combinations within individuals.
The windows that were different from the overall analysis were mostly in window III-alpha. These window III-alpha misses were found in participants 1, 5, 9, 11, 12, and 15, who were missing activity in 1, 3, 5, 3, 3, and 5 channels, respectively. The windows III-alpha with missing ERS/event-related causality (ERC) results (Table 2) were largely found in channels C3, C4, Cz, P3, P4, and Pz. The frequent absence of activity in window III-alpha may be reflected by the variability between channels in average time-frequency plots, such as count laser task—laser stimulus (Fig. 2, top left). However, in the case of window I-delta/theta, only one channel lacked ERS, i.e., participant 3. Such misses were infrequent for the other windows, as shown in Table 2. Overall, the number of misses for window III-alpha were significantly greater than those for window I-delta/theta (6/16 vs. 1/16; P = 0.041, Fisher) and any other window (6/15 vs. 0/16; P = 0.009).
The consistency of activations in windows across subjects suggests that the results of the overall analysis will be generalizable. In the case of window III-alpha, five subjects had misses at multiple channels, which suggested that a population of subjects was characterized by common misses in this window. However, subjects with multiple channels missing window III-alpha showed no differences versus other subjects in ratings of task-related pain and unpleasantness or error rates or laser power settings.
Laser ERS/ERD: effect of stimulus, task, and channel within window.
We next undertook an analysis of variance (ANOVA) model of ERS/ERD with Channel, Task, and Modality factors for each window. The F3, F4, C3, C4, Cz, P3, P4, and Pz channels for each subject were selected for the analysis of the ERS and ERD (Hu et al. 2013; Mouraux et al. 2003; Ohara et al. 2004a). For each time-frequency window, the ERS/ERD results were modeled by the three factors in a within-subject ANOVA (2 Tasks × 2 Modalities × 8 Channels). The Wilcoxon signed-rank test was used for the post hoc analysis, and the significance level was set at P < 0.05 with Bonferroni correction for multiple comparisons.
In window I-delta/theta, Modality and Channel were the main effects (P = 0.02 for Modality and P < 0.00001 for Channel) and there were significant interactions of Task with Modality (F1,15 = 5.92, P < 0.03) and of Task with Channel with Modality (F7,105 = 2.34, P = 0.03). Post hoc testing revealed that the Cz channel had significantly larger ERS responses in the count laser than the count electric task for the laser modality (Table 3), which was reflected in Fig. 2 [top left (dense red) vs. top right (yellow)]. The interaction terms were reflected in F3, F4, C3, C4, Cz, and Pz, which were found to have significantly larger ERS for the electric stimulus in the count laser task than laser ERS in the count electric task (Table 3; Wilcoxon signed-rank test, P < 0.0063). This was reflected in Fig. 2 [bottom left (dense red) vs. top right (light colors)] in the window I-delta/theta ERS levels for all channels except for P3 and P4 (Wilcoxon signed-rank test, P < 0.0063).
Table 3.
Channels with significantly higher ERS/ERD by Window, Task, and Modality as determined in post hoc tests of ANOVA described in text
| Modality |
||
|---|---|---|
| Task | Laser | Electric |
| Window I-delta/theta | ||
| Count laser | Cz* | |
| Count electric | F3*†, F4*†, C3*†, C4*†, Cz*†, Pz*† | |
| Window II-delta/theta | ||
| Count laser | F4*, Cz* | |
| Count electric | ||
| Window III-alpha | ||
| Count laser | C3* | |
| Count electric | ||
| Window V-gamma | ||
| Count laser | ||
| Count electric | C3† | |
Indicates greater ERS/ERD activity for the task with the * vs. the other task, while both tasks are under the same modality. For example, in window I-delta/theta under the laser modality and attention task the * by Cz indicates that the Cz has greater ERS for the attention than the distraction task, while the laser modality remains the same.
Indicates greater ERS/ERD for the modality with the † vs. the other modality, while the task remains the same. For example, under the laser modality and count electric task the † beside the C3 in window V-gamma indicates that the C3 has greater ERS for the laser than electric modality, while the count electric task remains the same.
Indicates greater ERS/ERD for the modality and task of the channel with the *† vs. both the other modality and task, i.e., interaction term. For example, in window I-delta/theta and channel F3 the electric stimulus and the count electric task had larger ERS than the laser stimulus and the attention task.
For window II-delta/theta, Task and Channel were the main effects (P = 0.026 and P < 0.0003, respectively) and there was significant interaction of Task with Modality (F1,15 = 10.80, P = 0.005). Post hoc testing revealed that F4 and Cz had significantly larger ERS activity in the count laser than the count electric task for laser modality, as summarized in Table 3 (Wilcoxon signed-rank test, P < 0.0063). This was reflected in the laser modality for the count laser task (Fig. 2, top left, dense red) compared with the count electric (Fig. 2, top right, light colors).
In window III-alpha, Channel was a main effect (P = 0.003) and there were significant interactions of Task with Modality (F1,15 = 19.52, P = 0.005) and of Task with Modality with Channel (F7,105 = 2.538, P = 0.02). Post hoc testing revealed that C3 had significantly larger ERD responses in the count laser than the count electric task for the laser modality (Table 3). This was reflected in the laser modality for the count laser (Fig. 2, top left, intense blue) versus count electric task (Fig. 2, top right, yellow green), which may be related to the small ERD variability for this pair. The high mean ERD values for Cz, Pz, and P4 were influenced by the variability of a population of subjects having multiple misses in window III, particularly in channels C3, C4, Cz, P3, P4, and Pz (see ERS/ERD: consistency between participants of ERS/ERD across EEG channels; Table 2).
In window IV-beta no main effect was found, but a significant interaction of Task with Modality was found in this window (F1,15 = 8.934, P = 0.01). However, the post hoc test showed no significant result after correction for multiple comparisons. This was reflected in the yellow to red window IV-beta activity, which was found in all four modality and task displays in Fig. 2.
In window V-gamma, Modality was a main effect (P = 0.04) and there was a significant interaction of Task with Modality (F1,15 = 16.64, P = 0.001). Post hoc testing revealed that C3 had greater laser ERS in the count electric task (Fig. 2, top right, yellow and red patchwork) than electric ERS in the count laser task (Fig. 2, bottom left, solid blue) (Wilcoxon signed-rank test, P = 0.0011).
Overall, the interaction of Modality with Task was the only effect that was found in all windows in this statistical analysis, and that was reflected by the contrasts in Fig. 2. Modality was a main effect in window I-delta/theta and window V-gamma, and all interactions included Modality as a factor. These results indicate that Modality was an important component in the model of 2 Tasks × 2 Modalities × 8 Channels.
Regression of laser pain psychophysics vs. ERD/ERS by channel.
The overall changes for psychophysical ratings (poststimulus minus baseline prestimulus) and task error rates for the laser stimulus were correlated with the ERS or ERD as appropriate to the window (e.g., window III-alpha ERD) and the tasks by linear regression analysis (P < 0.05). All significant results showed linear regression with a positive slope, as indicated in Table 4 by the + sign following the channel at which significant regression was found.
Table 4.
Channels with significant linear regression results of ERD/ERS vs. psychophysical measures in the laser modality
| Window | EEG Change | Task | Psychophysics | Channel |
|---|---|---|---|---|
| I-delta/theta | ERS | Count electric | Task error score | P3+, P4+ |
| II-delta/theta | ERS | Count laser | Task error score | C3+, P3+ |
| Count electric | Pain rating | P3+, P4+, Pz+ | ||
| Unpleasantness | Pz+ | |||
| III-alpha | ERD | Count laser | Task error score | F3+, Pz+ |
| IV-beta | ERS | Count laser | Task error score | F3+ |
| Count electric | Unpleasantness | C4+ | ||
| Salience | Pz+ | |||
| V-gamma | ERS | Count laser | Salience | P4+ |
| Count electric | Pain rating | Pz+ |
For parietal channels across several windows (P3, P4, Pz), sensory (pain or unpleasantness) or attention (salience) ratings most commonly had significant linear regression on ERS/ERD. Specifically, the P3 and P4 electrodes had significant regression more commonly than all other electrodes (11/15 vs 4/15; P = 0.027, Fisher). A relationship between electrodes with ERS/ERD activation was not more common at parietal channels for sensory and attention measures versus task error rates (7/15 vs. 4/15; P = 1). These results suggest that parietal structures may have a disproportionate role in ratings of sensory and attention.
DISCUSSION
Overall, the main effect for Modality was found for window I-delta/theta and window V-gamma, and the Modality with Task interaction was found for all five windows. Task main effects were found for window II-delta/theta, and Task with Modality interactions were found for all windows, as above. Channel was a main effect for windows I-delta/theta, II-delta/theta, and III-alpha, and there were no interactions of Channel with Modality or Task. However, Channel with Task with Modality interactions were found for windows I-delta/theta and III-alpha. There was no significant interaction term that did not include Modality as a factor. Therefore, the Modality-dependent main effects and the interactions of Modality with Task and of Task with Modality with Channel together were the most common effects explaining these results. These results are consistent with our hypothesis that the non-phase-locked responses to the nonpainful electrical cutaneous stimulus modality are different from those to the painful laser modality.
Many of the latency differences between windows for the laser and electrical modalities may reflect the longer laser receptor utilization time and transmission delay of the Adelta and C fibers versus the Abeta fibers, which are activated by the electrical cutaneous stimulus (Kakigi et al. 1991; Kakigi and Shibasaki 1991; Vallbo et al. 1979). These later onset latencies are evident for the laser versus the electrical modality in Fig. 1, window I-delta/theta, as well as the alpha ERD and the beta ERS rebound (Fig. 1, windows III-alpha and IV-beta). Later offset latencies are found at windows II-delta/theta, III-alpha, and IV-beta of the laser versus the electrical stimulus, and may also reflect processes that occur after transduction and transmission of the afferent signal.
The time-frequency plots of ERD/ERS activity are similar to previous reports that phasic painful stimuli produce non-phase-locked alpha ERD alone (Babiloni et al. 2006; Hu et al. 2013; Mouraux et al. 2003; Ploner et al. 2006), gamma alone (Croft et al. 2002), or alpha ERD followed by beta ERS rebound (Hauck et al. 2007; Iannetti et al. 2008). These findings are consistent with another study of changes in non-phase-locked responses to phasic pain stimuli (Hauck et al. 2007). The same pattern of alpha ERD followed by beta ERS rebound is reported with somatic sensory stimuli such as electrical stimulation of the median nerve (Bauer et al. 2006; Salmelin and Hari 1994), with different motor tasks (Neuper et al. 2006; Neuper and Pfurtscheller 1996), and with electrical cutaneous stimuli, as in the present case.
Psychophysical protocols can exert powerful influences upon evoked ERS/ERD activity. Several studies have examined the effect of directed attention in an oddball protocol upon magnetoencephalographic (MEG) sources activated by a painful intracutaneous electrical stimulus. When count laser was directed toward one stimulus, then a MEG alpha ERD (present window III-alpha) was recorded (Hu et al. 2013; see also Mouraux et al. 2003), which is consistent with the effect of count laser on window III-alpha ERD (Fig. 1, top). Early gamma ERS (present window V-gamma) was greater with directed attention to the high-intensity target (oddball) stimulus (Hauck et al. 2007). These results suggest that attention to a phasic painful stimulus induces alpha ERD and gamma ERS, which is consistent with the present results.
ERD/ERS vs. pain perception.
The present results show evidence of correlation between ERD/ERS activity in all five windows and pain perception. This is consistent with a study that examined responses to three laser stimuli in a short train. That study reported that pain intensity of the first stimulus was correlated with ERD and ERS (Iannetti et al. 2008), although alpha ERD was not correlated with pain intensity. Direct correlations with pain perception were also reported in the case of “prefrontal” gamma power (Croft et al. 2002) and in the case of three alpha EEG subbands that showed ERD with the anticipation of pain (Babiloni et al. 2006).
The early gamma ERS found at channel C4 during the count electric and laser tasks for the laser modality (Fig. 2) might be consistent with that reported in primary somatosensory cortex (SI) of a prior study (Babiloni et al. 2006; Zhang et al. 2012), based on visual comparison. In the present results higher pain ratings were found for the count laser task versus the count electric task, and the C4 early gamma was correspondingly larger (Fig. 2). The early gamma ERS found at the F4 channel for the laser modality and the count electric task might reflect the cognitive aspect for the prefrontal cortical regulation of pain (Babiloni et al. 2006; Coghill et al. 1999; Villemure and Bushnell 2002), consistent with reference (Croft et al. 2002).
The identity of structures in the brain generating the present scalp EEG results must be interpreted cautiously. Nevertheless, these results seem to be consistent with the correlation between stimulus intensity and pain rating in a study of alpha ERD carried out through subdural electrodes (Ohara et al. 2004a). In that report, alpha ERD was maximal and more widespread over SI and parasylvian cortex during directed attention to the painful laser stimulus (counting stimuli) versus distraction (reading for comprehension) (Ohara et al. 2004a). The relationship between pain perception and neural recordings has also been explored through analysis of the intracranial recordings of human thalamic neuronal firing and cortical local field potentials (Lee et al. 1999; Lenz et al. 2010; Ohara et al. 2004b). The relationship of ERD/ERS activity to pain perception and attention is strong evidence of the relationship between this activity and pain sensations.
Methodological considerations.
To study the changes for the non-phase-locked activities precisely, it is important to determine that there are minimal phase-locked components in the overall power spectrum. This is accomplished by subtracting the ERP from every epoch to eliminate phase-locked components and to retain only non-phase-locked signals (Fig. 1) (Bastiaansen and Hagoort 2003; Tallon-Baudry and Bertrand 1999). The difference between the ERS/ERD with subtraction and ERS/ERD without subtraction has been both predicted and observed to be very small relative to the ongoing EEG (Makeig 1993) (see also Fig. 1). However, the non-phase-locked activities cannot be precisely discriminated when both (phase and nonphase) activities are present within the same window. Furthermore, individual channels do not always reflect the overall analysis, which is described above (Table 2) and which may reflect the non-phase-locked activity described by Zhang et al. (2012). Therefore, early-latency ERS found in this study might originate to some degree from both phase-locked and non-phase-locked activity in windows I and II-delta/theta (Bastiaansen and Hagoort 2003; Kalcher and Pfurtscheller 1995). From a practical point of view, the ERP subtraction procedure has been widely used for both EEG and MEG signals to investigate the induced non-phase-locked activities in humans (David et al. 2006; Hauck et al. 2007; Tallon-Baudry and Bertrand 1999).
Salience is a property of painful and nonpainful somatic sensory stimuli, and of stimuli of special sense, e.g., vision (Downar et al. 2002, 2003; Mouraux and Iannetti 2009). The response to the painful cutaneous laser may reflect the salience of the stimulus, defined as “the ability of the stimulus to capture attention” (Mouraux and Iannetti 2009; see also Downar et al. 2002, 2003; Legrain et al. 2005; Lorenz and Garcia-Larrea 2003; Zaslansky et al. 1995). We controlled for the effect of baseline salience of the two stimuli within each participant by matching the salience of the electric stimulus to that of the painful laser stimulus through adjustments in the intensity of the stimuli. Salience of a stimulus was altered by task so that salience was not equal under different tasks. In addition, both stimuli were applied in the same somatotopic location. This method was designed so that the presence or absence of pain was the main difference between the two stimuli. The present results are an example of “the usefulness of laser-related activity… to explore the effect of a given experimental factor on the transmission and processing of nociceptive input” (Mouraux and Iannetti 2009).
It could be suggested that the consistency of these results is a function of the windows selected for this analysis (Table 1). Time-frequency plots were constructed for painful laser stimuli and nonpainful electrical cutaneous stimuli (Fig. 1). The selection of windowing parameters (Table 1) was descriptive and was based upon the overall results (Fig. 1) without accounting for differences in results between subjects, Tasks, or Modalities. Therefore, the consistency between data overall versus individual subjects and factors is unlikely to be a product of windowing of the overall results.
Possible generators for ERD/ERS non-phase-locked activity.
In the present results, maxima during the count laser task were found in central electrodes for windows I and II-delta/theta and III-alpha and during the count electric task for window V-gamma (Table 3). Consistent with this finding are a number of studies in which alpha ERD was found at C4 and Cz (Mouraux et al. 2003), over sensorimotor channels (Ploner et al. 2006), or over contralateral central channels (Hu et al. 2013). These results seem to be consistent with MEG studies that generate a three-dimensional estimate of the generator of activations. In the case of MEG recordings, delta ERD was maximal over sensorimotor channels, alpha ERD over sensorimotor structures (Hauck et al. 2007), and beta ERD over primary motor cortex (Raij et al. 2004). Functional imaging studies also reflect the possible generators of the non-phase-locked ERS/ERD activity reported here.
The anatomical relationship of non-phase-locked EEG to functional imaging techniques is complex. PET and fMRI studies have demonstrated modulation of blood flow or BOLD in SI and secondary somatosensory cortex (SII), anterior cingulate cortex (ACC), orbitofrontal cortex, and insula by attention to painful stimuli (Bantick et al. 2002; Brooks et al. 2002; Davis et al. 1997; Frankenstein et al. 2001; Hofbauer et al. 2001; Petrovic et al. 2000). These findings may be consistent with the significant ERS at central and midline channels during the count laser task (Table 3). Distraction produced decreased activations in SI, SII, and insula (Brooks et al. 2002; Petrovic et al. 2000), which may be consistent with decreased ERS/ERD over central channels.
ERD/ERS and ERC.
In a prior study the majority of individual participants had ERC in the same channel as classified by task and direction of functional interaction as in the overall analysis (Markman et al. 2013). Similarly, the majority of individual participants had ERS/ERD activity in the same channels as in the overall analysis. These results point to substantial consistency across participants for causality and activation. Channels with ERC were significantly more common during the count laser task, and channels with ERD/ERS were more common during the count laser than during the count electric task. These findings raise the possibility that ERC is related to ERD/ERS as a function of Task.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-38493 to F. A. Lenz and by the Hopkins Neurosurgery Pain Research Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.-H.C., C.-C.L., and J.H.K. analyzed data; J.-H.C., C.-C.L., J.H.K., and F.A.L. interpreted results of experiments; J.-H.C. and J.H.K. prepared figures; J.-H.C., C.-C.L., J.H.K., T.M.M., and F.A.L. edited and revised manuscript; J.-H.C., C.-C.L., and F.A.L. approved final version of manuscript; C.-C.L., T.M.M., and F.A.L. conception and design of research; C.-C.L. and T.M.M. performed experiments; F.A.L. drafted manuscript.
REFERENCES
- Babiloni C, Brancucci A, Del Percio C, Capotosto P, Arendt-Nielsen L, Chen AC, Rossini PM. Anticipatory electroencephalography alpha rhythm predicts subjective perception of pain intensity. J Pain 7: 709–717, 2006 [DOI] [PubMed] [Google Scholar]
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain 125: 310–319, 2002 [DOI] [PubMed] [Google Scholar]
- Bastiaansen M, Hagoort P. Event-induced theta responses as a window on the dynamics of memory. Cortex 39: 967–992, 2003 [DOI] [PubMed] [Google Scholar]
- Bastiaansen MC, Brunia CH. Anticipatory attention: an event-related desynchronization approach. Int J Psychophysiol 43: 91–107, 2001 [DOI] [PubMed] [Google Scholar]
- Bauer M, Oostenveld R, Peeters M, Fries P. Tactile spatial attention enhances gamma-band activity in somatosensory cortex and reduces low-frequency activity in parieto-occipital areas. J Neurosci 26: 490–501, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiten F, Sergeant J, Geuze R. Event-related desynchronization: the effects of energetic and computational demands. Electroencephalogr Clin Neurophysiol 82: 302–309, 1992 [DOI] [PubMed] [Google Scholar]
- Brooks JC, Nurmikko TJ, Bimson WE, Singh KD, Roberts N. fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage 15: 293–301, 2002 [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH. Sensory and affective aspects of pain perception: is medial thalamus restricted to emotional issues? Exp Brain Res 78: 415–418, 1989 [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH, Dubner R, Jones RL, Maixner W. Attentional influences on noxious and innocuous cutaneous heat detection in humans and monkeys. J Neurosci 5: 1103–1110, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proc Natl Acad Sci USA 96: 7705–7709, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol 82: 1934–1943, 1999 [DOI] [PubMed] [Google Scholar]
- Croft RJ, Williams JD, Haenschel C, Gruzelier JH. Pain perception, hypnosis and 40 Hz oscillations. Int J Psychophysiol 46: 101–108, 2002 [DOI] [PubMed] [Google Scholar]
- David O, Kilner JM, Friston KJ. Mechanisms of evoked and induced responses in MEG/EEG. Neuroimage 31: 1580–1591, 2006 [DOI] [PubMed] [Google Scholar]
- Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ. Functional MRI of pain- and attention-related activations in the human cingulate cortex. J Neurophysiol 77: 3370–3380, 1997 [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21, 2004 [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J Neurophysiol 87: 615–620, 2002 [DOI] [PubMed] [Google Scholar]
- Downar J, Mikulis DJ, Davis KD. Neural correlates of the prolonged salience of painful stimulation. Neuroimage 20: 1540–1551, 2003 [DOI] [PubMed] [Google Scholar]
- Ferracuti S, Seri S, Mattia D, Cruccu G. Quantitative EEG modifications during the Cold Water Pressor Test: hemispheric and hand differences. Int J Psychophysiol 17: 261–268, 1994 [DOI] [PubMed] [Google Scholar]
- Frankenstein UN, Richter W, McIntyre MC, Remy F. Distraction modulates anterior cingulate gyrus activations during the cold pressor test. Neuroimage 14: 827–836, 2001 [DOI] [PubMed] [Google Scholar]
- Gross J, Schnitzler A, Timmermann L, Ploner M. Gamma oscillations in human primary somatosensory cortex reflect pain perception. PLoS Biol 5: e133, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck M, Lorenz J, Engel AK. Attention to painful stimulation enhances gamma-band activity and synchronization in human sensorimotor cortex. J Neurosci 27: 9270–9277, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer RK, Rainville P, Duncan GH, Bushnell MC. Cortical representation of the sensory dimension of pain. J Neurophysiol 86: 402–411, 2001 [DOI] [PubMed] [Google Scholar]
- Hu L, Peng W, Valentini E, Zhang Z, Hu Y. Functional features of nociceptive-induced suppression of alpha band electroencephalographic oscillations. J Pain 14: 89–99, 2013 [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Hughes NP, Lee MC, Mouraux A. Determinants of laser-evoked EEG responses: pain perception or stimulus saliency? J Neurophysiol 100: 815–828, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakigi R, Endo C, Neshige R, Kuroka Y, Shibasaki H. Estimation of conduction velocity of A-delta fibers in humans. Muscle Nerve 14: 1193–1196, 1991 [DOI] [PubMed] [Google Scholar]
- Kakigi R, Shibasaki H. Estimation of conduction velocity of the spino-thalamic tract in man. EEG Clin Neurophysiol 80: 39–45, 1991 [DOI] [PubMed] [Google Scholar]
- Kalcher J, Pfurtscheller G. Discrimination between phase-locked and non-phase-locked event-related EEG activity. Electroencephalogr Clin Neurophysiol 94: 381–384, 1995 [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Russegger H, Pachinger T, Schwaiger J. Induced alpha band power changes in the human EEG and attention. Neurosci Lett 244: 73–76, 1998 [DOI] [PubMed] [Google Scholar]
- Lee J, Dougherty PM, Antezana D, Lenz FA. Responses of neurons in the region of human thalamic principal somatic sensory nucleus to mechanical and thermal stimuli graded into the painful range. J Comp Neurol 410: 541–555, 1999 [DOI] [PubMed] [Google Scholar]
- Legrain V, Bruyer R, Guerit JM, Plaghki L. Involuntary orientation of attention to unattended deviant nociceptive stimuli is modulated by concomitant visual task difficulty. Evidence from laser evoked potentials. Clin Neurophysiol 116: 2165–2174, 2005 [DOI] [PubMed] [Google Scholar]
- Legrain V, Iannetti GD, Plaghki L, Mouraux A. The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol 93: 111–124, 2011 [DOI] [PubMed] [Google Scholar]
- Lenz FA, Casey KL, Jones EG, Willis WD., Jr The Human Pain System: Experimental and Clinical Perspectives. New York: Cambridge Univ. Press, 2010 [Google Scholar]
- Longe SE, Wise R, Bantick S, Lloyd D, Johansen-Berg H, McGlone F, Tracey I. Counter-stimulatory effects on pain perception and processing are significantly altered by attention: an fMRI study. Neuroreport 12: 2021–2025, 2001 [DOI] [PubMed] [Google Scholar]
- Lopes da Silva FH, Pfurtscheller G. Basic concepts on EEG synchronization and desynchronization. In: Handbook of Electroencephalography and Clinical Neurophysiology, edited by Pfurtscheller G, Lopes da Silva FH. New York: Elsevier Science, 1999, p. 3–11 [Google Scholar]
- Lorenz J, Garcia-Larrea L. Contribution of attentional and cognitive factors to laser evoked brain potentials. Neurophysiol Clin 33: 293–301, 2003 [DOI] [PubMed] [Google Scholar]
- Makeig S. Auditory event-related dynamics of the EEG spectrum and effects of exposure to tones. Electroencephalogr Clin Neurophysiol 86: 283–293, 1993 [DOI] [PubMed] [Google Scholar]
- Markman T, Liu CC, Chien JH, Crone NE, Zhang J, Lenz FA. EEG analysis reveals widespread directed functional interactions related to a painful cutaneous laser stimulus. J Neurophysiol 110: 2440–2449, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouraux A, Guerit JM, Plaghki L. Non-phase locked electroencephalogram (EEG) responses to CO2 laser skin stimulations may reflect central interactions between Adelta- and C-fibre afferent volleys. Clin Neurophysiol 114: 710–722, 2003 [DOI] [PubMed] [Google Scholar]
- Mouraux A, Iannetti GD. Across-trial averaging of event-related EEG responses and beyond. Magn Reson Imaging 26: 1041–1054, 2008 [DOI] [PubMed] [Google Scholar]
- Mouraux A, Iannetti GD. Nociceptive laser-evoked brain potentials do not reflect nociceptive-specific neural activity. J Neurophysiol 101: 3258–3269, 2009 [DOI] [PubMed] [Google Scholar]
- Neuper C, Pfurtscheller G. Post-movement synchronization of beta rhythms in the EEG over the cortical foot area in man. Neurosci Lett 216: 17–20, 1996 [DOI] [PubMed] [Google Scholar]
- Neuper C, Wortz M, Pfurtscheller G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog Brain Res 159: 211–222, 2006 [DOI] [PubMed] [Google Scholar]
- Niedermeyer E, Lopes da Silva F. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Philadelphia, PA: Lippincott Williams & Wilkins, 2005 [Google Scholar]
- Ohara S, Crone NE, Weiss N, Lenz FA. Attention to a painful cutaneous laser stimulus modulates electrocorticographic event-related desynchronization in humans. Clin Neurophysiol 115: 1641–1652, 2004a [DOI] [PubMed] [Google Scholar]
- Ohara S, Crone NE, Weiss N, Treede RD, Lenz FA. Amplitudes of laser evoked potential recorded from primary somatosensory, parasylvian and medial frontal cortex are graded with stimulus intensity. Pain 110: 318–328, 2004b [DOI] [PubMed] [Google Scholar]
- Petrovic P, Petersson KM, Ghatan PH, Stone-Elander S, Ingvar M. Pain-related cerebral activation is altered by a distracting cognitive task. Pain 85: 19–30, 2000 [DOI] [PubMed] [Google Scholar]
- Ploner M, Gross J, Timmermann L, Pollok B, Schnitzler A. Oscillatory activity reflects the excitability of the human somatosensory system. Neuroimage 32: 1231–1236, 2006 [DOI] [PubMed] [Google Scholar]
- Raij TT, Forss N, Stancak A, Hari R. Modulation of motor-cortex oscillatory activity by painful Adelta- and C-fiber stimuli. Neuroimage 23: 569–573, 2004 [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hari R. Spatiotemporal characteristics of sensorimotor neuromagnetic rhythms related to thumb movement. Neuroscience 60: 537–550, 1994 [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci 3: 151–162, 1999 [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Hari R, Kajola M, Karhu J, Ahlfors S, Tissari S. Magnetoencephalographic 10-Hz rhythm from the human auditory cortex. Neurosci Lett 129: 303–305, 1991 [DOI] [PubMed] [Google Scholar]
- Tremblay N, Bushnell MC, Duncan GH. Thalamic VPM nucleus in the behaving monkey. II. Response to air-puff stimulation during discrimination and attention tasks. J Neurophysiol 69: 753–763, 1993 [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979 [DOI] [PubMed] [Google Scholar]
- Villemure C, Bushnell MC. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain 95: 195–199, 2002 [DOI] [PubMed] [Google Scholar]
- Zaslansky R, Sprecher E, Tenke CE, Hemli JA, Yarnitsky D. The P300 in pain evoked potentials. Pain 66: 39–49, 1995 [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Hu L, Hung YS, Mouraux A, Iannetti GD. Gamma-band oscillations in the primary somatosensory cortex—a direct and obligatory correlate of subjective pain intensity. J Neurosci 32: 7429–7438, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]