Abstract
Rapid escape swims in fish are initiated by the Mauthner cells, giant reticulospinal neurons with unique specializations for swift responses. The Mauthner cells directly activate motoneurons and facilitate predator detection by integrating acoustic, mechanosensory, and visual stimuli. In addition, larval fish show well-coordinated escape responses when exposed to electric field pulses (EFPs). Sensitization of the Mauthner cell by genetic overexpression of the voltage-gated sodium channel SCN5 increased EFP responsiveness, whereas Mauthner ablation with an engineered variant of nitroreductase with increased activity (epNTR) eliminated the response. The reaction time to EFPs is extremely short, with many responses initiated within 2 ms of the EFP. Large neurons, such as Mauthner cells, show heightened sensitivity to extracellular voltage gradients. We therefore tested whether the rapid response to EFPs was due to direct activation of the Mauthner cells, bypassing delays imposed by stimulus detection and transmission by sensory cells. Consistent with this, calcium imaging indicated that EFPs robustly activated the Mauthner cell but only rarely fired other reticulospinal neurons. Further supporting this idea, pharmacological blockade of synaptic transmission in zebrafish did not affect Mauthner cell activity in response to EFPs. Moreover, Mauthner cells transgenically expressing a tetrodotoxin (TTX)-resistant voltage-gated sodium channel retained responses to EFPs despite TTX suppression of action potentials in the rest of the brain. We propose that EFPs directly activate Mauthner cells because of their large size, thereby driving ultrarapid escape responses in fish.
Keywords: escape response, Mauthner cell, electric pulse, nitroreductase, SCN5
teleost fish have among the fastest reaction times for escape responses in the animal kingdom, rapidly accelerating away from the strike path of predators within tens of milliseconds. Response latency is a critical factor in predator evasion, enabling prey to escape the strike zone and freely maneuver while predators are engaged in stereotyped capture movements. Escape responses in fish are initiated by the Mauthner cells, giant reticulospinal neurons with unique specializations for swift responses (Faber and Korn 1978). Underlining their central role in the escape circuit, Mauthner cells are activated by sudden stimuli in diverse sensory modalities. Mauthner cells receive monosynaptic chemical and electrical input from statoacoustic, trigeminal, and lateral line ganglia and visual input relayed via the tectum (Faber and Korn 1978; Kimmel et al. 1990). A single action potential transmitted down the large-diameter Mauthner axon is sufficient to activate a startle response (Nissanov et al. 1990), depolarizing motoneurons through both mono- and disynaptic pathways (Fetcho 1992). The short neuronal pathway from sensory input to motoneuron activation thus guarantees fast reaction times.
Low survival rates among fish larvae necessitate that escape responses emerge very early in development, and indeed by 5 days postfertilization (dpf) zebrafish larvae execute rapid escape swims when threatened by acoustic, tactile, or looming visual cues (reviewed in Fero et al. 2011). Natural predators of zebrafish larvae include adult fish and dragonfly nymphs (Engeszer et al. 2007), which use a hydraulic mechanism to achieve high-velocity labial strikes (Tanaka and Hisada 1980). Accordingly, behavioral response latencies in larvae are short—3 to 10 ms for acoustic and tactile stimuli (Kohashi and Oda 2008; Liu and Fetcho 1999) and ∼400 ms for looming visual stimuli (Facchin et al. 2009). However, the fastest reaction time for escape responses, frequently <2 ms, is seen in response to an electric field pulse (EFP). Many fish species initiate well-coordinated escape swims in response to EFPs (Dunlop et al. 2006; Webb 1976, 1980; Yokogawa et al. 2012). Apart from their use in analyzing escape responses, EFPs are widely used in behavioral assays in fish, especially in paradigms for learning and memory where EFPs act as unconditioned aversive stimuli (Aoki et al. 2013; Bitterman 1964; Portavella et al. 2002; Rawashdeh et al. 2007; Shcherbakov et al. 2005; Valente et al. 2012). In addition, EFPs have been used to assess arousal states (Yokogawa et al. 2007), study nociception (Dunlop et al. 2006), and analyze autonomic and behavioral responses to stressors (Agetsuma et al. 2010; Lee et al. 2010; Mann et al. 2010).
Despite this extensive use in research, the sensory modality through which EFPs trigger behavioral responses in fish has not been identified, although it has been assumed that, as in mammals, EFPs act on cutaneous nociceptors (Dunlop et al. 2006). However, we observed that in zebrafish larvae the first trunk movements in response to an EFP are detectable within 2 ms of the stimulus onset. The extreme short latency of EFP responses suggested that they are mediated by an unusual neuronal mechanism. Using genetic and physiological techniques we investigated the neuronal circuitry by which EFPs elicit ultrarapid escape responses.
MATERIALS AND METHODS
Fish husbandry.
Zebrafish husbandry and tol1 transgenesis have been described previously (Yokogawa et al. 2012). Et(SCP1:Gal4ff)y264 (y264), Et(REx2-cfos:Gal4ff)y269 (y269), and Et(REx2-cfos:Gal4ff)y270 (y270) were isolated in an enhancer trap screen (Bergeron et al. 2012). y264 shows strong expression of Gal4 in the Mauthner cell and has additional stochastic expression in other reticulospinal neurons, the anterior lateral line ganglia, and sparse cells in the spinal cord, hindbrain, cerebellum and forebrain (see Fig. 3B). For Tg(UAS-E1b:BGi-epNTR-TagRFPT-oPre)y268 (UAS:epNTR-RFP), zebrafish codon optimized nfsB was synthesized (Genscript), fused to TagRFPT, and cloned into pT1UMP (Yokogawa et al. 2012). Mutations T41L, N71S, and F124W (Jaberipour et al. 2010; LinWu et al. 2012) were introduced by PCR mutagenesis to generate an “enhanced-potency” nitroreductase (epNTR). Recently, a similar set of mutations was independently tested in zebrafish and also found to improve nitroreductase activity (Mathias et al. 2014). For Tg(UAS-E1b:BGi-SCN5a-v2a-TagRFPT)y266 (UAS:SCN5), human SCN5a (9053152, Thermo Scientific) was fused to v2a-TagRFPT and cloned into pT1UMP. For Tg(UAS-E1b:BGi-GCaMP6s-v2a-mCherry-v2a-lynCFP-oPre)y267 (UAS:GCaMP6s), K78H, T381R, S383T, and R392G mutations (Chen et al. 2013) were introduced into zebrafish optimized GCaMP5g (Genscript). Tg(UAS:nfsB-mCherry) was made with the construct reported by Davison et al. (2007). Other fish larvae were fathead minnows (Pimephales promelas; MBL Aquaculture), Mexican cavefish (Astyanax mexicanus; kind gift of William Jeffery, University of Maryland), and medaka (Oryzias latipes; kind gift of Fumihito Ono, National Institute on Alcohol Abuse and Alcoholism). In vivo experimental protocols were approved by the local animal care and use committee.
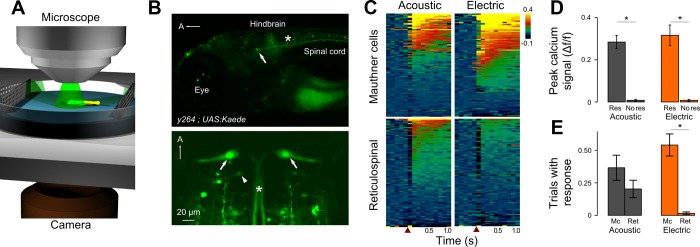
Fig. 3.
Mauthner cells are activated in EFP responses. A: apparatus for imaging reticulospinal neuron calcium responses and tail movements in head-embedded fish. B: lateral (top) and dorsal views (bottom) of Mauthner somas (arrows) and axons (asterisks) in y264;UAS:Kaede larvae. Stochastic expression in other reticulospinal neurons is also seen (arrowhead). A, anterior. C: raster plot of y264;UAS:GCaMP6s calcium-dependent fluorescence changes (Δf/f) in the Mauthner (n = 15) and other reticulospinal neurons (n = 16) evoked by acoustic and EFP stimuli. Stimulus indicated by arrow; 8 trials of each stimulus type per cell. D: Mauthner activity in trials with (Res) and without (No Res) tail movement responses. n = 10 cells. E: fraction of trials with above-threshold calcium change indicating a firing response for Mauthner and other reticulospinal neurons for the trials represented in C. *Paired t-test, P < 0.01.
Immunohistochemistry.
Antibodies were GFP (1:1,000; A-11122, Invitrogen), Kaede (1:1,000; PM012, MBL International), and SCN5a (1:1,000; C144743, LifeSpan BioSciences) and Alexa Fluor 488- and 546-conjugated secondary antibodies (1:400; Invitrogen). Images were captured with a Leica TCS-SP5 II confocal microscope.
Behavioral analysis.
Individual and group testing were performed in a 4.2-cm2 arena above a translucent diffuser illuminated with an LED array. Individual testing of acoustic responses was performed in a grid of 1-cm2 chambers. Responses were recorded with a high-speed camera (DRS Lightning RDT/1; DEL Imaging) at 1,000 frames/s and analyzed with Flote software (Burgess and Granato 2007). Acoustic startle responses, elicited as previously described, occur in two waves: short-latency C-starts (SLC) and long-latency C-starts (Burgess and Granato 2007). Except for Fig. 1C, only data from SLCs were analyzed. As larvae were most responsive to EFPs when oriented toward the anode, except for Fig. 2, A and B, results are for larvae oriented within 22.5° of this direction. Stimuli were generated with a digital-to-analog card (PCI-6221; National Instruments). EFPs were square DC pulses (2-ms duration, 1- to 9-V amplitude except as otherwise noted) or sinusoidal AC pulses (0.5- to 2-ms period, 2-ms duration, 9-V amplitude) across stainless steel wire mesh electrodes 1 or 4.2 cm apart for free-swimming larvae or 2.3 cm apart for head-embedded larvae.
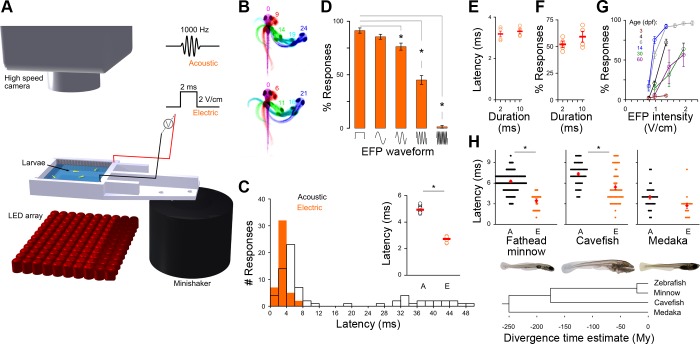
Fig. 1.
Short reaction times for larval fish electric field pulse (EFP) responses. A: apparatus: larvae swim in a chamber attached to a minishaker for acoustic stimuli and flanked by electrodes for EFPs. B: C-start responses to an acoustic (top) or an EFP (bottom) stimulus. Times after stimulation are indicated (ms). C: C-start response latencies, aggregated from 15 larvae tested individually, evoked by EFP (0.8 V/cm, orange) or acoustic (18 m/s2, black) stimuli. Inset: response latencies (n = 14 groups of 30 larvae, circles show mean for each group) and overall average (red bar) for acoustic (A) and EFP (E) responses. *Paired t-test, P < 0.001. D: responsiveness to EFPs (1.2 V/cm amplitude) with square step (left) or sinusoidal waves of 0.5, 1, 2, or 4 kHz (n = 5 groups of 30 fish). *Paired t-test, P < 0.01. E and F: C-start latencies relative to stimulus onset (E) and responsiveness (F) evoked by short (2-ms duration) and long (10-ms duration) EFPs (1.0 V/cm): mean latencies for each group (orange circles) and overall average (red bars) for EFP responses. n = 4 groups of 30 fish. G: responsiveness to EFPs of zebrafish across age [days postfertilization (dpf) indicated on graph]. (3 dpf: n = 4 groups of 35 fish, 4 dpf: n = 5 groups of 30 fish, 6 dpf: n = 14 groups of 30 fish, 14 dpf: n = 5 groups of 8 fish, 30 dpf: n = 4 individual fish, 60 dpf: n = 4 individual fish). H, top: response latency to EFPs (E) and acoustic pulses (A) in fathead minnows (4 dpf; n = 23 EFP, 102 acoustic responses, 24 fish), Mexican cavefish (5 dpf; n = 53 EFP, 53 acoustic responses; 24 fish), and medaka (3 dpf; n = 18 EFP, 36 acoustic responses, 16 fish). Means are shown in red. *Paired t-test P < 0.05. Bottom: chronogram showing the evolutionary relationship of these species (Wang et al. 2012).
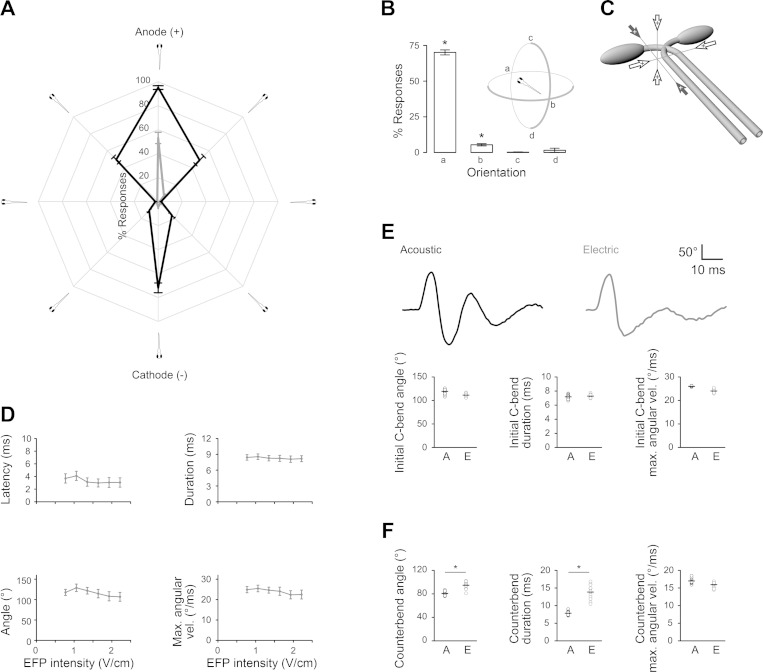
Fig. 2.
Orientation selectivity and kinematics for larval fish EFP responses. A: EFP responsiveness for larval orientations in the field. Azimuth: orientation, range: % of zebrafish responding (1.0 V/cm, gray; 1.3 V/cm, black; n = 14 groups of 30 fish). B: responsiveness for electrodes positioned with the anode above (c) and below (d) the larva. Intensity: 2.6 V/cm. n = 3 groups of 35 fish. *One-sample t-test against 0, P < 0.05. C: anode orientations that trigger (filled arrows) or do not trigger escapes (open arrows) relative to Mauthner cell bodies and axons. D: C-bend response kinematics to EFPs of increasing intensity. n = 6 groups of 30 fish. E, top: graphs show body curvature during acoustic and EFP-triggered C-starts. Bottom: kinematic analysis of initial C-bend of responses triggered by EFPs (E) and acoustic stimuli (A) showing angle, duration, and maximum angular velocity. n = 14 groups of 30 fish. F: kinematic analysis of counterbend of responses triggered by EFPs (E) and acoustic stimuli (A) showing angle, duration, and maximum angular velocity. n = 14 groups of 30 fish. *Paired t-test, P < 0.01.
Calcium imaging.
Calcium fluorescence was monitored with either y264;UAS:GCaMP6s larvae or Mauthner cells retrogradely labeled by injecting Calcium Green-1 dextran (C3713; Invitrogen) into the spinal cord. Larvae were head-embedded in 2% agarose with tail movement unrestricted. Fluorescence was monitored with an Axio Imager compound microscope (Carl Zeiss), and tail movements were recorded with a μEye camera (IDS Imaging). Fluorescence intensities were extracted with custom software. The microscope stage was affixed with a speaker, and a collar with electrodes was positioned inside the dish.
Pharmacology.
dl-2-Amino-5-phosphonopentanoic acid (APV, 100 μM; A5282, Sigma), carbenoxolone (CBX, 1 μM; C4790, Sigma), NBQX (20 μM; N183, Sigma), strychnine (50 μM; S8753, Sigma) and tetrodotoxin (TTX, 1 μM; T-500, Alomone labs) were dissolved in E3 medium. Picrotoxin (100 μM; P1675, Sigma) was dissolved in DMSO. Larvae were embedded in agarose, and drugs were added to the bath solution and injected in the brain ventricle (2–4 nl) with a picospritzer (PV820, World Precision Instruments). Fish were tested before and 10 (TTX) or 30 (all others) min after drug delivery. For behavior testing y264;UAS:SCN5 larvae, RFP expression was visually confirmed at 4 dpf after anesthetization with 0.03% tricaine (MS-222, Sigma). Larvae were allowed to recover 40 h in E3 before testing.
Ablations.
y264;UAS:epNTR-RFP, y269;UAS:epNTR-RFP, and y269;UAS:nfsB-mCherry larvae and RFP-negative siblings (controls) were treated with 10 mM metronidazole in E3 from 3 to 5 dpf. To detect cell death during genetic ablation, larvae were immersed for 1 h in 8 μM PhiPhiLux G1D2 (OncoImmunin) and washed three times before live imaging as previously described (Yokogawa et al. 2012). Laser ablations were performed in Gt(T2KSAG)j1229a (J1229) larvae, which express GFP in the Mauthner cell (Burgess et al. 2009). Mauthner cells, or RoV3 neurons for controls, were ablated with a Leica TCS-SP5 II 2-photon confocal microscope with a ×20 0.95 NA objective and an 800-nm laser. Immunolabeling against GFP confirmed successful ablation of 70% of targeted cells. Only data from confirmed ablations were analyzed.
Statistical analysis.
Analyses were performed with Gnumeric (http://projects.gnome.org/gnumeric/). Data in figures and text are means and SE.
RESULTS
EFPs induce well-coordinated swimming movements in larval zebrafish that strongly resemble C-start escape responses triggered by acoustic stimuli (Fig. 1, A and B, Supplemental Movie S1).1 Larvae show two types of responses to acoustic stimuli distinguished by latency and kinematics (Burgess and Granato 2007). In contrast, the vast majority of EFP responses were initiated within 10 ms of the stimulus (Fig. 1C). Since both AC and DC EFPs have been used in behavioral assays in fish, we tested the behavioral responsiveness to both stimulus types. Responsiveness to EFPs was greater for square (DC) field pulses compared with sinusoidal (AC) pulses of the same duration and amplitude (Fig. 1D). Using long-duration field pulses (10 ms), we observed that most EFP responses were evoked by the onset of the positive field potential, not the pulse offset (Fig. 1E), and increasing the duration of the square pulse did not increase responsiveness (Fig. 1F). Surprisingly, larvae showed a faster reaction time to EFPs than to acoustic stimuli (Fig. 1C, inset; electric 2.7 ± 0.06 ms, acoustic 5.1 ± 0.07 ms, P < 0.001) and we frequently noted EFP responses beginning within 2 ms of the stimulus onset, indicating a very short path for sensory-motor transmission. Ultrafast EFP responses are not a unique specialization of early-stage larval teleosts, because we observed the EFP response in 4–14 dpf larvae, 1-mo-old juveniles, and 2-mo-old adults (Fig. 1G). Moreover, these responses are not a unique specialization of zebrafish, because we recorded similarly rapid responses in larvae of three other phylogenetically well-separated lineages of teleost species (Fig. 1H; Supplemental Movies S2–S4). In each species, the EFP response was a well-coordinated swimming movement, initiated with a C-bend to one side, followed by a counterbend and swim. Similar to larval zebrafish, medaka, fathead minnows, and cavefish larvae all had shorter EFP response latencies than acoustic response latencies (although the difference was not statistically significant for medaka). The remarkably short reaction time of larvae to EFPs led us to analyze the neural circuitry responsible for this behavior.
Fish that sense weak electric fields show orientation selectivity of responsiveness (Yager and Hopkins 1993). Likewise, larvae showed an orientation selectivity of responsiveness in the electric field, with maximal responsiveness to fields oriented along the rostro-caudal axis of the larva and almost no reaction to laterally or vertically oriented fields (Fig. 2, A–C). Larvae were more responsive when oriented toward the anode than toward the cathode (Fig. 2, A and B). At voltage intensities up to 2 V/cm, the initial C-bend of the escape response was highly stereotyped and little affected by voltage intensity, indicating that the EFP response is an all-or-nothing event (Fig. 2D). At intensities of 6–9 V/cm, larvae responded with C-starts; however, we observed greater variability in the duration and angular velocity of the initial bend (data not shown). SLC responses to acoustic stimuli are also all-or-nothing events, initiated by the Mauthner cells (Burgess and Granato 2007). Direct comparison of EFP responses to SLC responses in fish alternately exposed to EFP and acoustic stimuli (Fig. 1A) revealed that the initial C-bends of both responses were similar in the angle of the turn, bend duration, and maximal angular velocity, although the angle of the EFP C-bend was slightly smaller than the acoustic C-bend (EFP: 111 ± 3.5°, acoustic: 119 ± 5°; paired t-test, P = 0.07) (Fig. 2E). Likewise, for medaka, fathead minnows, and cavefish, the initial C-bend angle of EFP responses was slightly lower than the acoustic response, although again the differences were not significant (data not shown). In contrast, kinematic measures for the counterbends were significantly different between responses to EFP and acoustic stimuli (Fig. 2F). As escape responses triggered by reticulospinal neurons other than the Mauthner cell have different kinematic profiles (Burgess and Granato 2007; Liu and Fetcho 1999), the close similarity of the initial C-bend to Mauthner-mediated acoustic responses suggested that the Mauthner cells are involved in the EFP response.
We next monitored Mauthner cell activity during EFP responses. For this we used the genetically encoded calcium indicator GCaMP6s to report Mauthner activity in head-embedded fish while monitoring tail movements (Fig. 3A). We generated a UAS:GCaMP6s transgenic line and directed GCaMP expression to the Mauthner cell using enhancer trap line y264, which shows robust expression of Gal4 in the Mauthner cells and sparse stochastic expression in other reticulospinal neurons (Fig. 3B). GCaMP fluorescence was monitored in response to EFPs and acoustic stimuli presented on alternating trials. Each stimulus was calibrated to produce a behavioral (tail movement) response on 75% of trials. EFPs triggered large increases in calcium-dependent fluorescence in Mauthner cells similar to changes induced by acoustic stimuli (Fig. 3, C and D). Only small changes in fluorescence (Δf/f: 0.023 ± 0.004, n = 9 cells; paired t-test, P < 0.001), possibly reflecting subthreshold EPSPs, were seen in acoustic trials with no behavioral response, confirming that large signals report Mauthner cell firing (Fig. 3D). For the 15 Mauthner cells tested with 8 trials each, we detected superthreshold changes in fluorescence indicating neuronal activity in 44 acoustic trials and 65 EFP trials (Fig. 3E). All superthreshold changes in fluorescence were accompanied by a behavioral response for both types of stimulus. This demonstrates that EFPs activate the Mauthner cell at a level similar to acoustic stimuli and that Mauthner cell firing strongly correlates with behavioral responses to EFPs.
Do EFPs broadly activate neurons or selectively activate the Mauthner cell? To address this, we imaged calcium responses in additional reticulospinal neurons. As expected (Gahtan et al. 2002), other reticulospinal neurons also responded during acoustic trials; for the 16 reticulospinal neurons, we observed 26 responses to acoustic stimuli (from 8 cells). However, across all trials, only two responses to EFPs (1 each from 2 neurons) were observed (Fig. 3, C and E). As EFP responses in Mauthner cells had a larger calcium signal than acoustic responses, it is unlikely that we failed to detect EFP responses in other reticulospinal neurons. These results show that the Mauthner cell is more sensitive to EFPs than other reticulospinal neurons.
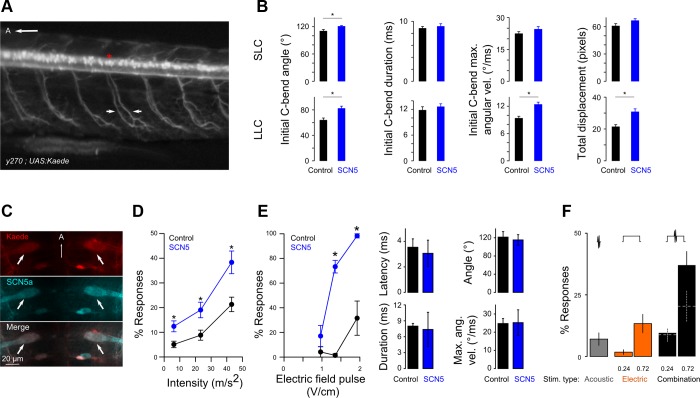
To test the role of the Mauthner cell in EFP responses, we overexpressed the α-subunit of a voltage-gated sodium channel to increase Mauthner cell membrane excitability. In contrast to methods such as channelrhodopsin stimulation that require exogenous stimuli and acutely drive neuronal activity irrespective of behavioral context, this strategy allows us to assess the contribution of a given neuron to a behavioral response under normal assay conditions. For these experiments, we used the voltage-gated sodium channel α-subunit from human cardiomyocyte (SCN5a), which activates and shows 50% conductance at ∼15 mV more negative potentials than α-subunits from brain or skeletal muscle and is slower to inactivate (Deschênes et al. 2001; Mantegazza et al. 2001). We generated a UAS:SCN5 transgenic line and, as proof of principle for SCN5a exogenous expression increasing neuronal excitability, drove expression in motoneurons using Gal4 enhancer trap line Et(REx2-cfos:Gal4ff)y270 (Fig. 4A). In zebrafish larvae, stimuli that trigger large-angle escape turns recruit a larger fraction of motoneurons into the active pool, and these neurons fire more actively (Bhatt et al. 2007). Consistent with increased motoneuron excitability, y270;UAS:SCN5 larvae showed larger angle C-bends during escape responses. The larger angle of long-latency C-starts was due to an increase in the maximal angular velocity of the bend, as would be expected if a larger fraction of motoneurons were recruited (Fig. 4B). SLC responses are normally initiated with extremely high angular velocity, and thus the relatively small increase in bend angle after SCN5 expression may be due to a ceiling effect. We next drove expression of SCN5a in the Mauthner cell using the UAS:SCN5 transgenic line and enhancer trap line y264. To confirm SCN5 expression we used immunohistochemistry and saw expression in the Mauthner cell in y264;UAS:SCN5 larvae (Fig. 4C). y264;UAS:SCN5 larvae showed increased responsiveness to an acoustic stimulus, confirming that overexpression sensitized the Mauthner cell (Fig. 4D). The latency and kinematics of the acoustic short- and long-latency responses were similar between larvae expressing SCN5 in their Mauthner cells and clutchmates without the transgene (data not shown). y264;UAS:SCN5 larvae also showed greater responsiveness to an EFP but no change in response kinematics (Fig. 4E). This experiment correlates increased excitability of the Mauthner cell with enhanced responsiveness to the EFP implying that the Mauthner cell initiates this behavior. As an independent line of evidence, we examined the behavior of larvae presented with simultaneous subthreshold EFP and acoustic stimuli. Because acoustic stimuli are known to depolarize the Mauthner cell, we reasoned that if the EFP acts through a non-Mauthner response circuit simultaneous presentation with the acoustic stimulus would have an additive effect on responsiveness, whereas if both stimuli activate the Mauthner cell the combined presentation would have a supra-additive effect on responsiveness. We observed supra-additive responsiveness to simultaneous subthreshold acoustic and EFP stimuli, as expected if both stimuli stochastically trigger the Mauthner cell to fire (Fig. 4F).
Fig. 4.
Mauthner cells participate in EFP responses. A: lateral view of motoneuron axons (arrows) and cell bodies (asterisk) in y270;UAS:Kaede larvae. A, anterior. B: bend angle, duration, and maximum angular velocity of the initial C-bend and total displacement for short-latency (SLC, top) and long-latency (LLC, bottom) responses for y270;UAS:SCN5 larvae expressing exogenous SCN5 (blue) or nonexpressing siblings (black) triggered by acoustic stimuli (rms: 29 m/s2). n = 40 control fish and 44 SCN5 fish. *t-Test, P < 0.01. C: single confocal slice in y264;UAS:SCN5;UAS:Kaede larva. Top: immunohistochemistry for Kaede (red). Middle: immunohistochemistry for SCN5a (cyan). Bottom: merge of channels. Arrows indicate Mauthner cell bodies. A, anterior. D and E: acoustic (D) and EFP (E) responsiveness for y264;UAS:SCN5 larvae expressing exogenous SCN5 (blue) or nonexpressing siblings (black). n = 4 groups of 8 fish. *t-Test P < 0.01. Kinematics of EFP responses are not changed by SCN5 expression. F: responsiveness to weak acoustic (gray; 2-ms pulse, 6.2 m/s2) and EFP (orange; 10-ms pulse, intensity in V/cm indicated) pulses presented separately or together (black). White dashes in combination trials indicate sum of acoustic and EFP responses tested separately. n = 5 groups of 30 fish.
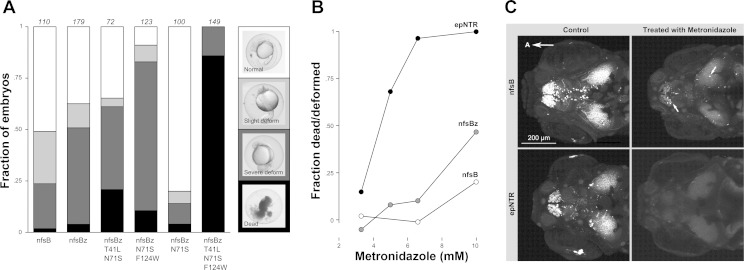
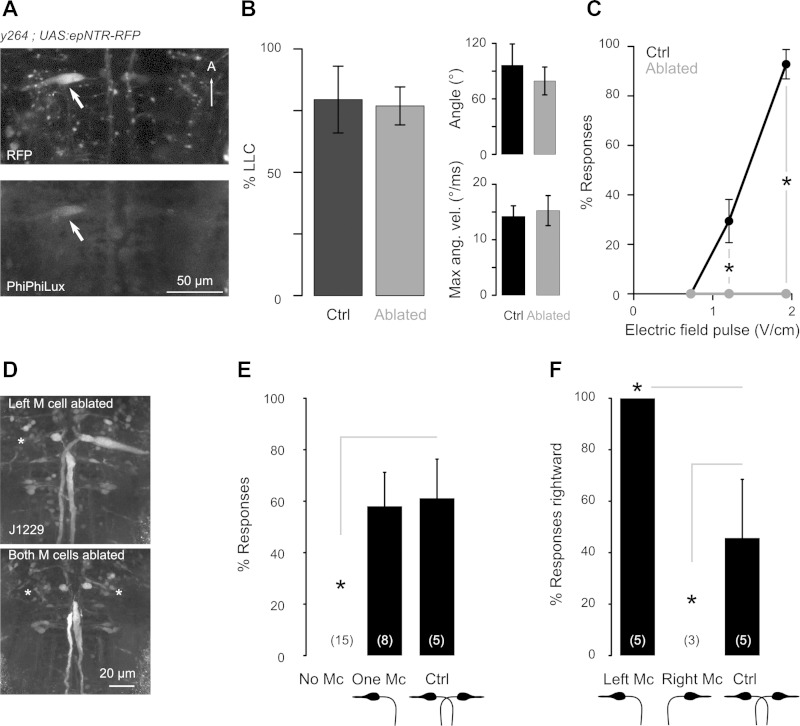
To determine whether the Mauthner cells are required for EFP responses, we used the nitroreductase nfsB, which metabolizes metronidazole into a cell-impermeant cytotoxin, allowing cell-specific ablation (Pisharath et al. 2007). To improve ablation efficacy, we used a modified nfsB with increased activity through codon optimization and introduction of three amino acid changes, T41L, N71S, and F124W (Jaberipour et al. 2010; LinWu et al. 2012) (enhanced-potency nitroreductase, “epNTR”). Improved ablation efficiency by epNTR was initially shown by injection of mRNA into embryos (Fig. 5, A and B). After generating a stable UAS:epNTR-RFP line, we confirmed this by comparing the extent of cell loss after ablation to an unmodified UAS:nfsB transgenic line (Fig. 5C). We drove expression of epNTR in the Mauthner cells using y264;UAS:epNTR-RFP larvae. After 12 h of metronidazole treatment, we detected apoptosis in RFP-expressing Mauthner cells of y264;UAS:epNTR-RFP larvae with PhiPhiLux G1D2, a live fluorescent reporter of caspase-3-like activity (Fig. 6A) (Packard and Komoriya 2008; Yokogawa et al. 2012). After ablation of both Mauthner cells in y264;UAS:epNTR-RFP larvae, the Mauthner cell-independent long-latency response to acoustic stimuli was executed with normal response rates and kinematics (Fig. 6B) but the EFP response was completely lost (Fig. 6C). To confirm that the loss of the EFP response was due to ablation of the Mauthner cell and not due to ablation of other NTR-expressing cells in y264;UAS:epNTR-RFP larvae, we performed single-cell ablations of the Mauthner cells. Using J1229 larvae that express GFP in the Mauthner cells and other reticulospinal neurons (Burgess et al. 2009), we targeted and laser ablated Mauthner cells, or RoV3 neurons in controls. Similar to genetic ablations, EFP responses were lost after bilateral laser ablation of the Mauthner cells (Fig. 6, D and E), while after unilateral ablation all responses were initiated in a direction contralateral to the remaining Mauthner cell (Fig. 6, D and F). Together, these experiments show that the Mauthner cell is required for EFP responses.
Fig. 5.
Improved ablation efficiency with epNTR. A: optimization of nitroreductase for ablation using embryo injections. Embryos were injected with mRNA (50 pg) and treated with 10 mM metronidazole (Met) overnight. At 24 hpf, the dead, severely deformed, slightly deformed, and normal embryos were counted (total n for each injection is indicated above the columns). nfsBz is codon optimized for zebrafish with a custom algorithm. Variants with the amino acid changes were also tested. The triple mutant is significantly better than the next best double mutant (χ2 = 11.7, P < 0.001). B: Met dose response curve for nfsB, nfsBz, and epNTR (nfsBz with T41L, N71S, and F124W). For each data point, the fraction of dead or deformed embryos is normalized to a matched population of injected embryos not treated with Met. C: confocal image stacks in y269;UAS:nfsB-mCherry (top) or y269;UAS:epNTR-RFP (bottom) larvae comparing RFP expression in control larvae (left) to larvae after ablation (right). Arrows indicate cells remaining after ablation with the original nsfB. A, Anterior.
Fig. 6.
EFP responses are Mauthner cell dependent. A: selective apoptosis in neurons expressing epNTR during metronidazole treatment. In a y264;UAS:epNTR-RFP larva with stochastic epNTR-RFP expression in the left Mauthner cell (arrow, top), PhiPhiLux G1D2 fluorescence is observed only in the same cell (arrow, bottom). A, anterior. B: acoustic LLC responsiveness and kinematics in y264;UAS:epNTR-RFP ablated larvae (gray) and nonablated sibling controls (black) (n = 4 plates of 5 fish for each group). C: EFP responsiveness in y264;UAS:epNTR-RFP ablated larvae (gray, n = 15 fish) and nonablated sibling controls (black, n = 17 fish). *t-Test, P < 0.01. D: immunolabeling of GFP in J1229 larvae after laser ablation of the left (top) or both (bottom) Mauthner cells. E and F: EFP responsiveness (E) and response direction (F; % of responses directed rightward) after unilateral or bilateral Mauthner cell laser ablations. Remaining Mauthner cell(s) are depicted. n is indicated in parentheses. *t-Test, P < 0.01.
The extreme short latency of EFP responses prompted us to ask how stimulus detection and transmission to the Mauthner cell could be accomplished so quickly. We detected responses within 2 ms of the onset of the EFP, remarkable given that the latency from Mauthner spike to trunk muscle contraction is ∼2 ms (Eaton et al. 1977a). We hypothesized that EFPs directly activate the Mauthner cell, bypassing sensory structures. To evaluate this, we silenced neuronal inputs to Mauthner cells by injecting larvae with a cocktail of antagonists (APV, NBQX, picrotoxin, strychnine, CBX; see materials and methods) designed to block glutamatergic, GABAergic, and glycinergic receptors and gap junctions (Fig. 7A). Injection of blockers drastically suppressed tail movements but was not lethal to larvae, which recovered and resumed spontaneous swimming after 3 h. For each larva, calcium responses to EFP and acoustic stimuli were recorded before and 30 min after drug injection, allowing us to examine the effect of synaptic block on individual Mauthner cells. Calcium imaging confirmed that after drug injection Mauthner cell responses to acoustic stimuli were completely lost (Fig. 7, B and C). In contrast, Mauthner cells in the same larvae retained responsiveness to EFPs, indicating that EFPs do not require synaptic transmission to drive Mauthner cell activity. These experiments are thus consistent with the notion that EFPs directly act on the Mauthner cell. However, although the cocktail of antagonists was designed to block the types of neurotransmission known to act on the Mauthner cell (Faber and Korn 1978) and did in fact effectively block the acoustic response, we cannot exclude the possibility that an uncharacterized neurotransmitter type relays EFP signaling to the Mauthner cell.
Fig. 7.
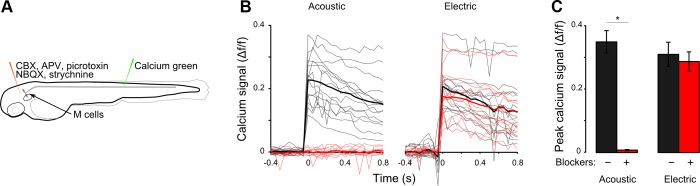
Neurotransmission is not required for EFPs to activate Mauthner cells. A: protocol for Calcium Green imaging in Mauthner cells before and after injection of synaptic blockers into the brain ventricle. B: before pharmacological blockade of synaptic transmission, acoustic and EFP stimuli (black) evoke increases in fluorescence intensity in Mauthner cells. After blockade, the calcium response to the acoustic stimulus is eliminated but the EFP response remains (red). Mean of responses in bold. C: quantification of B showing mean of peak of calcium signals. n = 5 cells from 5 fish. *Paired t-test, P < 0.01.
To address the possibility that other inputs to the Mauthner cell drive the EFP response, we isolated the Mauthner cell from other brain activity by taking advantage of the TTX resistance of SCN5a (Satin et al. 1992). We hypothesized that after action potentials were blocked throughout the brain by TTX injection into y264;UAS:SCN5 larvae, the expression of TTX-resistant SCN5a in the Mauthner cell should enable it to generate action potentials in response to an EFP. As with the cocktail of antagonists, TTX injection paralyzed larvae but was not lethal (Fig. 8A). For each larva, calcium responses to EFP and acoustic stimuli were recorded before and 10 min after TTX injection, allowing us to examine the effect on individual Mauthner cells. Calcium imaging in control larvae confirmed that TTX injection abolished Mauthner cell responses to acoustic and EFP stimuli (Fig. 8, B–D). In SCN5-expressing larvae, Mauthner cell responses to acoustic stimuli were also lost as expected because of TTX block of action potentials in the VIIIth nerve (Fig. 8C). In contrast, EFPs induced responses in SCN5-expressing Mauthner cells of TTX-injected fish (Fig. 8D), indicating that neuronal activity elsewhere in the brain is not required for EFPs to activate the Mauthner cell and thus strongly suggesting that EFPs directly activate the Mauthner cell.
Fig. 8.
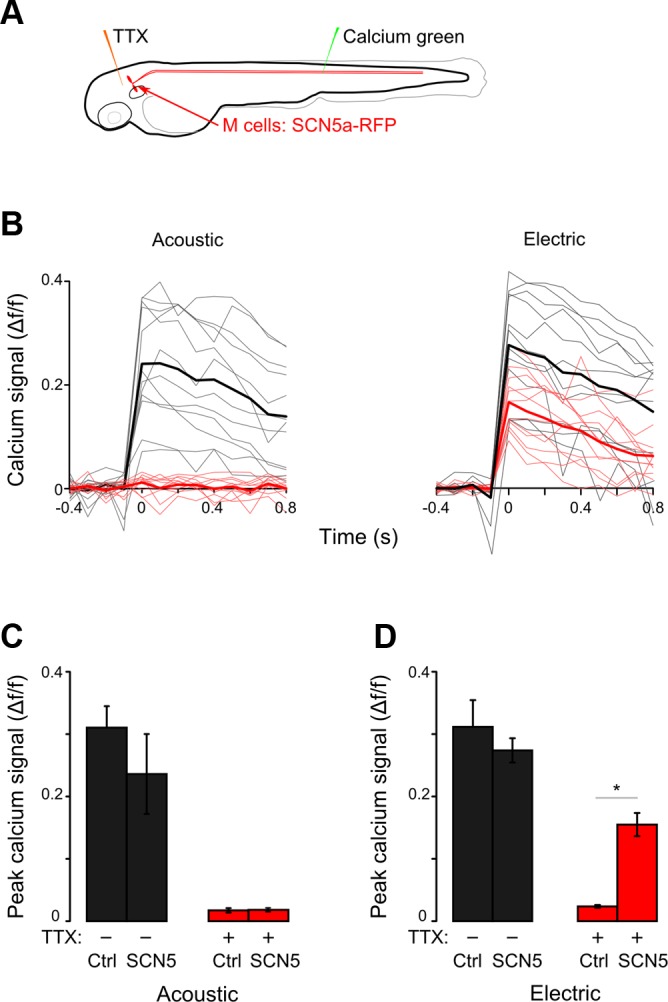
EFPs directly activate Mauthner cells. A: protocol for Calcium Green imaging in Mauthner cells before and 10 min after injection of TTX into the brain ventricle. B: Mauthner cell calcium responses in y264;UAS:SCN5 larvae. Calcium responses to EFPs are seen both before (black) and after (red) TTX injection. Acoustic responses are seen before (black) but not after (red) TTX injection. Mean of responses in bold. C and D: mean of peak calcium signals in SCN5-expressing larvae and nonexpressing siblings (Ctrl) before and after TTX injection for acoustic (C) and EFP (D) stimuli. Both groups, n = 4 cells from 4 fish. *Paired t-test, P < 0.01.
DISCUSSION
These experiments demonstrate that sudden electrical signals in the water trigger a Mauthner-dependent escape response. The remarkably short reaction time suggested that EFPs might directly activate the Mauthner cell. Supporting this, Mauthner cell activity during EFP responses was not abolished when the Mauthner cells were synaptically or electrically isolated from other neurons. Thus these results support a mechanism in which EFPs directly activate the Mauthner cell, bypassing sensory apparatus to trigger escape responses.
Escape responses activated by EFP stimuli were not identical to those triggered by acoustic stimuli. C-start responses triggered by antidromically stimulating the Mauthner cell axon with electrodes chronically implanted in the spinal cord have a 12% smaller initial C-bend angle and a greatly reduced counterbend angle compared with acoustically evoked C-starts (Nissanov et al. 1990). This was interpreted to suggest that additional reticulospinal neurons participate in the escape network activated by acoustic stimuli. Our experiments largely support this model, as we also observed a small decrease in initial C-bend angle and a strong change in the duration and angle of the counterbend for EFP responses compared with acoustically evoked C-starts. However, an alternative explanation for the kinematic differences in the initial C-bend arises from how the Mauthner cell is activated by each stimulus. Action potentials in the Mauthner axon triggered by antidromic stimuli are smaller in amplitude than those elicited by orthodromic stimuli (Furshpan and Furukawa 1962), potentially leading to reduced synaptic drive to motoneurons. If correct, this could explain the reduced angular velocity and magnitude of the initial C-bend of the escape response evoked by electrical stimuli.
How do external electrodes selectively trigger escape responses? Action potentials generated by the Mauthner cell in larvae can be detected by external bath electrodes, demonstrating that the integument offers relatively low electrical resistance (Featherstone 1991; Prugh et al. 1982). We used EFPs of a magnitude comparable to those in previous studies with zebrafish (for example, Pradel et al. 1999; Valente et al. 2012). At these levels, calcium imaging revealed selective activation of the Mauthner cell and a specific short-latency behavioral response. Larger EFPs might activate other reticulospinal neurons, producing distinct behavioral responses, or broadly activate neurons in the brain, producing poorly coordinated movements. Two special characteristics of the Mauthner cell likely cause larvae to perform an escape response to the EFPs used here. First, unlike most other neurons, a single action potential in one Mauthner cell is sufficient to drive a coordinated C-start response (Nissanov et al. 1990), leading to the notion that the Mauthner cell is a “command-like” neuron (Eaton et al. 2001). Second, the large size of the Mauthner axons increases susceptibility to depolarization by electric fields. Large neuronal structures show greater sensitivity to electric stimulation because membrane resistance decreases as surface area increases (in proportion to length squared) while cytoplasmic resistance decreases more because of the larger increase in volume (in proportion to length cubed). Indeed, stimulating electrodes in the spinal cord selectively activate the Mauthner cells among other reticulospinal neurons (Eaton and Farley 1975; Nissanov et al. 1990), and we rarely observed responses in non-Mauthner reticulospinal neurons using an EFP that activated Mauthner cells on 50% of trials. The EFP likely stimulates the Mauthner axons rather than soma, as larvae showed maximal responsiveness when the axons were longitudinally aligned with the field. This is the orientation most susceptible to a voltage gradient between stimulating electrodes (Ranck 1975).
Our findings raise the question as to the adaptive value of the large size of the Mauthner cells. It has been proposed that the large diameter of Mauthner axons [4–5 μm at 6 dpf (Kimmel 1972)] facilitates rapid propagation of action potentials for 1) short response latencies (Bennett 1984), 2) synchronous muscle activation during vigorous escape movements (Eaton et al. 1977b), and 3) suppressing slower competing motor commands enabling reliable escape behaviors (Eaton et al. 1995). However, there are arguments against each of these ideas. The increase in conduction velocity provided by the larger diameter of the Mauthner axon makes only a small contribution to response latency because of the short distance to motoneurons in the spinal cord (DiDomenico et al. 1988; Eaton et al. 1995). To estimate this contribution in 6 dpf larvae we used the coefficient of conduction velocity to axon diameter (1.26 m/s per μm of diameter) determined for Mauthner cells (Funch et al. 1981). The difference in diameter between the Mauthner axon and other reticulospinal axons (4.7 and 1.1 μm) should increase conduction velocity by 4.5 m/s, allowing signals to reach the distal end of the spinal cord ∼0.6 ms more quickly. Contrary to the second notion that the large diameter of Mauthner axons is necessary for synchronous muscle activation during vigorous escape movements, escape responses triggered by reticulospinal neurons with smaller axon diameters are similar in angle to or larger than those triggered by the Mauthner cells (Liu and Fetcho 1999). The reliability hypothesis as yet lacks empirical support. When both Mauthner cells fire near-simultaneously, the first to fire activates interneurons that suppress contralateral motoneurons enabling normal escape behavior (Satou et al. 2009). However, non-Mauthner escape responses are initiated 3–30 ms after Mauthner-mediated escapes (Burgess and Granato 2007; Kohashi and Oda 2008; Liu and Fetcho 1999) and could therefore be suppressed even if the Mauthner axon was similar in size to other reticulospinal neurons.
An intriguing hypothesis is that the large size of the Mauthner cell enhances its susceptibility to ephaptic coupling. We observed that subthreshold field potentials (72 mV/mm) sensitize the Mauthner cell to concurrent auditory stimuli. The Mauthner axon's sensitivity to electric fields may make it susceptible to ephaptic coupling from neighboring axons and trunk muscles. Large-magnitude extracellular potentials (∼20 mV) at the axon hillock are known to inhibit action potential generation in Mauthner cells (Furshpan and Furukawa 1962). In mammals, even smaller-magnitude endogenous positive extracellular potentials (∼0.2 mV) inhibit Purkinje cell firing (Blot and Barbour 2014) and application of negative extracellular potentials (−0.2 mV) near the axon initial segment enhances Purkinje cell firing (Blot and Barbour 2014). Whether ephaptic coupling enhances transmission along the Mauthner axon has yet to be determined. A potential source of ephaptic signaling is the extracellular muscle potential generated by the synchronous contraction of tail muscles during turns and swimming. Single fast-twitch muscle fibers can repetitively depolarize >100 mV during fictive swimming (Horstick et al. 2013). Large biphasic muscle potentials (∼0.8-mV amplitude) evoked during escape responses of larvae have been recorded with bath electrodes (Issa et al. 2011). Recordings from chronically implanted electrodes (Eaton et al. 1981) showed large extracellular potentials adjacent to the Mauthner cell during escape responses. These are likely to be generated by action potentials in trunk muscle (Issa et al. 2011). Muscle potentials generated during swimming bouts may sensitize the Mauthner cell to its other neuronal inputs, possibly contributing to the heighted startle responsiveness exhibited by moving, compared with stationary, larvae (Burgess and Granato 2007). The possible ephaptic coupling of these muscle potentials to the Mauthner axon is an intriguing avenue for future research.
Is the large size of the Mauthner cell a feature that enables fish to rapidly escape from naturally occurring electric fields? Because we found EFP responses in all four species of fish larvae tested here, a natural electric field source that triggers EFP responses should be common. Strongly electric fish that stun prey with electric organ discharges of tens or hundreds of volts are of restricted geographical distribution (Bennett 1971). Weakly electric fish that use electric pulses for communication and navigation are numerous, but these produce discharges of <1 V/cm (Scheich and Bullock 1974). As most other bioelectric sources underwater are at least two orders of magnitude weaker than the field strength required to elicit the EFP response (Patullo and Macmillan 2010), EFP responses may therefore not be triggered frequently under natural conditions. However, the stereotyped nature of escape responses is exploited by some predators to control the behavior of prey fish (Catania 2009). For example, during nocturnal predation, the catfish Malapterurus electricus deploys “prevolleys” of electric organ discharges thought to startle immobile prey into revealing themselves (Belbenoit et al. 1979). An intriguing possibility is that prevolleys take advantage of the hardwired EFP response described here to directly evoke Mauthner-dependent responses. Thus in some circumstances the large size of the Mauthner cells may carry an ethological cost.
In mammals, transcutaneous electric stimuli primarily activate C and Aδ peripheral sensory fibers (Koga et al. 2005). EFPs of magnitudes similar to those used here are commonly used in fish research and have been assumed to elicit reflex responses through a similar mechanism (Dunlop et al. 2006). Our findings strongly suggest that, at least for larval fish, such EFPs act directly on central neurons that trigger escape responses. Signaling to the Mauthner cell via peripheral sensory or other neurons is not required for an EFP response. Experiments using EFPs as sensory stimuli should therefore be interpreted with this in mind, as electric stimuli may not act as acute aversive stimuli.
GRANTS
This work was supported by the Intramural Research Program of the National Institute of Child Health and Human Development.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.M.T., S.A.B., E.J.H., V.A., T.P.-H., G.H., and H.A.B. conception and design of research; K.M.T., S.A.B., E.J.H., D.C.J., V.A., and H.A.B. performed experiments; K.M.T., S.A.B., E.J.H., D.C.J., V.A., T.P.-H., and H.A.B. analyzed data; K.M.T., S.A.B., E.J.H., V.A., T.P.-H., G.H., and H.A.B. interpreted results of experiments; K.M.T., D.C.J., and H.A.B. prepared figures; K.M.T. and H.A.B. drafted manuscript; K.M.T., S.A.B., E.J.H., G.H., and H.A.B. edited and revised manuscript; K.M.T., S.A.B., E.J.H., D.C.J., V.A., T.P.-H., G.H., and H.A.B. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jennifer Strykowski for zebrafish support.
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- Agetsuma M, Aizawa H, Aoki T, Nakayama R, Takahoko M, Goto M, Sassa T, Amo R, Shiraki T, Kawakami K, Hosoya T, Higashijima Si, Okamoto H. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat Neurosci 13: 1354–1356, 2010. [DOI] [PubMed] [Google Scholar]
- Aoki T, Kinoshita M, Aoki R, Agetsuma M, Aizawa H, Yamazaki M, Takahoko M, Amo R, Arata A, Higashijima S, Tsuboi T, Okamoto H. Imaging of neural ensemble for the retrieval of a learned behavioral program. Neuron 78: 881–894, 2013. [DOI] [PubMed] [Google Scholar]
- Belbenoit P, Moller P, Serrier J, Push S. Ethological observations on the electric organ discharge behaviour of the electric catfish, Malapterurus electricus (Pisces). Behav Ecol Sociobiol 4: 321–330, 1979. [Google Scholar]
- Bennett MV. Electric organs. In: Fish Physiology, edited by Hoar WS, Randall DJ. New York: Academic, 1971, p. 347–491. [Google Scholar]
- Bennett MV. Escapism: some startling revelations. In: Neural Mechanisms of Startle Behavior, edited by Eaton RC. New York: Plenum, 1984, p. 353–363. [Google Scholar]
- Bergeron SA, Hannan MC, Codore H, Fero K, Li G, Moak ZB, Yokogawa T, Burgess HA. Brain selective transgene expression in zebrafish using an NRSE derived motif. Front Neural Circuits 6: 110, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt DH, McLean DL, Hale ME, Fetcho JR. Grading movement strength by changes in firing intensity versus recruitment of spinal interneurons. Neuron 53: 91–102, 2007. [DOI] [PubMed] [Google Scholar]
- Bitterman ME. Classical conditioning in the goldfish as a function of the CS-UCS interval. J Comp Physiol Psychol 58: 359–366, 1964. [DOI] [PubMed] [Google Scholar]
- Blot A, Barbour B. Ultra-rapid axon-axon ephaptic inhibition of cerebellar Purkinje cells by the pinceau. Nat Neurosci 17: 289–295, 2014. [DOI] [PubMed] [Google Scholar]
- Burgess HA, Granato M. Sensorimotor gating in larval zebrafish. J Neurosci 27: 4984–4994, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HA, Johnson SL, Granato M. Unidirectional startle responses and disrupted left-right co-ordination of motor behaviors in robo3 mutant zebrafish. Genes Brain Behav 8: 500–511, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania KC. Tentacled snakes turn C-starts to their advantage and predict future prey behavior. Proc Natl Acad Sci USA 106: 11183–11187, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499: 295–300, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison JM, Akitake CM, Goll MG, Rhee JM, Gosse N, Baier H, Halpern ME, Leach SD, Parsons MJ. Transactivation from Gal4-VP16 transgenic insertions for tissue-specific cell labeling and ablation in zebrafish. Dev Biol 304: 811–824, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschênes I, Trottier E, Chahine M. Implication of the C-terminal region of the α-subunit of voltage-gated sodium channels in fast inactivation. J Membr Biol 183: 103–114, 2001. [DOI] [PubMed] [Google Scholar]
- DiDomenico R, Nissanov J, Eaton RC. Lateralization and adaptation of a continuously variable behavior following lesions of a reticulospinal command neuron. Brain Res 473: 15–28, 1988. [DOI] [PubMed] [Google Scholar]
- Dunlop R, Millsopp S, Laming P. Avoidance learning in goldfish (Carassius auratus) and trout (Oncorhynchus mykiss) and implications for pain perception. Appl Anim Behav Sci 97: 255–271, 2006. [Google Scholar]
- Eaton R, Farley R. Mauthner neuron field potential in newly hatched larvae of the zebra fish. J Neurophysiol 38: 502–512, 1975. [DOI] [PubMed] [Google Scholar]
- Eaton R, Farley R, Kimmel C, Schabtach E. Functional development in the Mauthner cell system of embryos and larvae of the zebra fish. J Neurobiol 8: 151–172, 1977a. [DOI] [PubMed] [Google Scholar]
- Eaton R, Lavender W, Wieland C. Identification of Mauthner-initiated response patterns in goldfish: evidence from simultaneous cinematography and electrophysiology. J Comp Physiol A 144: 521–531, 1981. [Google Scholar]
- Eaton RC, Bombardieri RA, Meyer G. The Mauthner-initiated startle response in teleost fish. J Exp Biol 66: 65–81, 1977b. [DOI] [PubMed] [Google Scholar]
- Eaton RC, Hofve JC, Fetcho JR. Beating the competition: the reliability hypothesis for Mauthner axon size. Brain Behav Evol 45: 183–194, 1995. [DOI] [PubMed] [Google Scholar]
- Eaton RC, Lee RK, Foreman MB. The Mauthner cell and other identified neurons of the brainstem escape network of fish. Prog Neurobiol 63: 467–485, 2001. [DOI] [PubMed] [Google Scholar]
- Engeszer RE, Patterson LB, Rao AA, Parichy DM. Zebrafish in the wild: a review of natural history and new notes from the field. Zebrafish 4: 21–40, 2007. [DOI] [PubMed] [Google Scholar]
- Faber DS, Korn H (Editors) Electrophysiology of the Mauthner cell: basic properties, synaptic mechanisms and associated networks. In: Neurobiology of the Mauthner Cell. New York: Raven, 1978. [Google Scholar]
- Facchin L, Burgess HA, Siddiqi M, Granato M, Halpern ME. Determining the function of zebrafish epithalamic asymmetry. Philos Trans R Soc Lond B Biol Sci 364: 1021–1032, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone D. Noninvasive detection of electrical events during the startle response in larval Medaka. J Exp Biol 158: 583, 1991. [Google Scholar]
- Fero K, Yokogawa T, Burgess HA. The behavioral repertoire of larval zebrafish. In: Zebrafish Models in Neurobehavioral Research, edited by Kalueff A, Cachat J. New York: Springer, 2011, p. 249–291. [Google Scholar]
- Fetcho JR. Excitation of motoneurons by the Mauthner axon in goldfish: complexities in a “simple” reticulospinal pathway. J Neurophysiol 67: 1574–1586, 1992. [DOI] [PubMed] [Google Scholar]
- Funch PG, Kinsman SL, Faber DS, Koenig E, Zottoli SJ. Mauthner axon diameter and impulse conduction velocity decrease with growth of goldfish. Neurosci Lett 27: 159–164, 1981. [DOI] [PubMed] [Google Scholar]
- Furshpan EJ, Furukawa T. Intracellular and extracellular responses of the several regions of the Mauthner cell of the goldfish. J Neurophysiol 25: 732–771, 1962. [DOI] [PubMed] [Google Scholar]
- Gahtan E, Sankrithi N, Campos JB, O'Malley DM. Evidence for a widespread brain stem escape network in larval zebrafish. J Neurophysiol 87: 608–614, 2002. [DOI] [PubMed] [Google Scholar]
- Horstick EJ, Linsley JW, Dowling JJ, Hauser MA, McDonald KK, Ashley-Koch A, Saint-Amant L, Satish A, Cui WW, Zhou W, Sprague SM, Stamm DS, Powell CM, Speer MC, Franzini-Armstrong C, Hirata H, Kuwada JY. Stac3 is a component of the excitation-contraction coupling machinery and mutated in Native American myopathy. Nat Commun 4: 1952, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa FA, O'Brien G, Kettunen P, Sagasti A, Glanzman DL, Papazian DM. Neural circuit activity in freely behaving zebrafish (Danio rerio). J Exp Biol 214: 1028–1038, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaberipour M, Vass SO, Guise CP, Grove JI, Knox RJ, Hu L, Hyde EI, Searle PF. Testing double mutants of the enzyme nitroreductase for enhanced cell sensitisation to prodrugs: effects of combining beneficial single mutations. Biochem Pharmacol 79: 102–111, 2010. [DOI] [PubMed] [Google Scholar]
- Kimmel CB. Mauthner axons in living fish larvae. Dev Biol 27: 272–275, 1972. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Hatta K, Metcalfe WK. Early axonal contacts during development of an identified dendrite in the brain of the zebrafish. Neuron 4: 535–545, 1990. [DOI] [PubMed] [Google Scholar]
- Koga K, Furue H, Rashid MH, Takaki A, Katafuchi T, Yoshimura M. Selective activation of primary afferent fibers evaluated by sine-wave electrical stimulation. Mol Pain 1: 13, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohashi T, Oda Y. Initiation of Mauthner- or non-Mauthner-mediated fast escape evoked by different modes of sensory input. J Neurosci 28: 10641–10653, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Mathuru AS, Teh C, Kibat C, Korzh V, Penney TB, Jesuthasan S. The habenula prevents helpless behavior in larval zebrafish. Curr Biol 20: 2211–2216, 2010. [DOI] [PubMed] [Google Scholar]
- LinWu SW, Wu CA, Peng FC, Wang AH. Structure-based development of bacterial nitroreductase against nitrobenzodiazepine-induced hypnosis. Biochem Pharmacol 83: 1690–1699, 2012. [DOI] [PubMed] [Google Scholar]
- Liu KS, Fetcho JR. Laser ablations reveal functional relationships of segmental hindbrain neurons in zebrafish. Neuron 23: 325–335, 1999. [DOI] [PubMed] [Google Scholar]
- Mann KD, Hoyt C, Feldman S, Blunt L, Raymond A, Page-McCaw PS. Cardiac response to startle stimuli in larval zebrafish: sympathetic and parasympathetic components. Am J Physiol Regul Integr Comp Physiol 298: R1288–R1297, 2010. [DOI] [PubMed] [Google Scholar]
- Mantegazza M, Yu FH, Catterall WA, Scheuer T. Role of the C-terminal domain in inactivation of brain and cardiac sodium channels. Proc Natl Acad Sci USA 98: 15348–15353, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias JR, Zhang Z, Saxena MT, Mumm JS. Enhanced cell-specific ablation in zebrafish using a triple mutant of Escherichia coli nitroreductase. Zebrafish 11: 85–97, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissanov J, Eaton RC, DiDomenico R. The motor output of the Mauthner cell, a reticulospinal command neuron. Brain Res 517: 88–98, 1990. [DOI] [PubMed] [Google Scholar]
- Packard BZ, Komoriya A. Intracellular protease activation in apoptosis and cell-mediated cytotoxicity characterized by cell-permeable fluorogenic protease substrates. Cell Res 18: 238–247, 2008. [DOI] [PubMed] [Google Scholar]
- Patullo BW, Macmillan DL. Making sense of electrical sense in crayfish. J Exp Biol 213: 651–657, 2010. [DOI] [PubMed] [Google Scholar]
- Pisharath H, Rhee JM, Swanson MA, Leach SD, Parsons MJ. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech Dev 124: 218–229, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portavella M, Vargas JP, Torres B, Salas C. The effects of telencephalic pallial lesions on spatial, temporal, and emotional learning in goldfish. Brain Res Bull 57: 397–399, 2002. [DOI] [PubMed] [Google Scholar]
- Pradel G, Schachner M, Schmidt R. Inhibition of memory consolidation by antibodies against cell adhesion molecules after active avoidance conditioning in zebrafish. J Neurobiol 39: 197–206, 1999. [PubMed] [Google Scholar]
- Prugh JI, Kimmel CB, Metcalfe WK. Noninvasive recording of the Mauthner neurone action potential in larval zebrafish. J Exp Biol 101: 83–92, 1982. [DOI] [PubMed] [Google Scholar]
- Ranck JB., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res 98: 417–440, 1975. [DOI] [PubMed] [Google Scholar]
- Rawashdeh O, de Borsetti NH, Roman G, Cahill GM. Melatonin suppresses nighttime memory formation in zebrafish. Science 318: 1144, 2007. [DOI] [PubMed] [Google Scholar]
- Satin J, Kyle JW, Chen M, Bell P, Cribbs LL, Fozzard HA, Rogart RB. A mutant of TTX-resistant cardiac sodium channels with TTX-sensitive properties. Science 256: 1202–1205, 1992. [DOI] [PubMed] [Google Scholar]
- Satou C, Kimura Y, Kohashi T, Horikawa K, Takeda H, Oda Y, Higashijima S. Functional role of a specialized class of spinal commissural inhibitory neurons during fast escapes in zebrafish. J Neurosci 29: 6780–6793, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheich H, Bullock TH. The detection of electric fields from electric organs. In: Electroreceptors and Other Specialized Receptors in Lower Vertebrates, edited by Fessard A. Berlin: Springer, 1974, p. 201–256. [Google Scholar]
- Shcherbakov D, Winklhofer M, Petersen N, Steidle J, Hilbig R, Blum M. Magnetosensation in zebrafish. Curr Biol 15: 161–162, 2005. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Hisada M. The hydraulic mechanism of the predatory strike in dragonfly larvae. J Exp Biol 88: 1–20, 1980. [Google Scholar]
- Valente A, Huang KH, Portugues R, Engert F. Ontogeny of classical and operant learning behaviors in zebrafish. Learn Mem 19: 170–177, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Gan X, Li J, Mayden R, He S. Cyprinid phylogeny based on Bayesian and maximum likelihood analyses of partitioned data: implications for Cyprinidae systematics. Sci China Life Sci 55: 761–773, 2012. [DOI] [PubMed] [Google Scholar]
- Webb PW. The effect of size on the fast-start performance of rainbow trout Salmo gairdneri, and a consideration of piscivorous predator-prey interactions. J Exp Biol 65: 157–177, 1976. [DOI] [PubMed] [Google Scholar]
- Webb PW. Does schooling reduce fast-start response latencies in teleosts? Comp Biochem Physiol A Physiol 65: 231–234, 1980. [Google Scholar]
- Yager DD, Hopkins CD. Directional characteristics of tuberous electroreceptors in the weakly electric fish, Hypopomus (Gymnotiformes). J Comp Physiol A 173: 401–414, 1993. [DOI] [PubMed] [Google Scholar]
- Yokogawa T, Hannan MC, Burgess HA. The dorsal raphe modulates sensory responsiveness during arousal in zebrafish. J Neurosci 32: 15205–15215, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokogawa T, Marin W, Faraco J, Pezeron G, Appelbaum L, Zhang J, Rosa F, Mourrain P, Mignot E. Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants. PLoS Biol 5: 2379–2397, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.