Abstract
It is widely held that the frontal eye field (FEF) in prefrontal cortex (PFC) modulates processing in visual cortex with attention, although the evidence for a necessary role is equivocal. To help identify critical sources of attentional feedback to area V4, we surgically removed the entire lateral PFC, including the FEF, in one hemisphere and transected the corpus callosum and anterior commisure in two macaques. This deprived V4 of PFC input in one hemisphere while keeping the other hemisphere intact. In the absence of PFC, attentional effects on neuronal responses and synchrony in V4 were significantly reduced and the remaining effects of attention were delayed in time indicating a critical role of PFC. Conversely, distracters captured attention and influenced V4 responses. However, because the effects of attention in V4 were not eliminated by PFC lesions, other sources of top-down attentional control signals to visual cortex must exist outside of PFC.
The prefrontal cortex (PFC) has been implicated in the control of numerous cognitive functions including visual attention. Evidence from lesion and inactivation studies in both monkeys and humans has suggested that executive function and attention is impaired when PFC function is compromised1-4. Recording studies in monkeys5-8 and brain imaging studies in humans (for review see9,10) have also implicated PFC in the control of attention. It has thus been suggested that PFC is part of an attentional control network that exerts top-down control by sending to extrastriate cortex feedback signals, which bias the competition among visual representations in favor of the attended stimuli11.
For spatial attention, the frontal eye field (FEF) in PFC seems to play a central role in enhancing the responses of visual cells in areas such as V4 to behaviorally relevant stimuli. Electrical stimulation or pharmacological manipulation of a visual field location in FEF mimics the effects of attention on V4 neuronal responses12-14. Moreover, activity in the FEF is synchronized with activity in V4 in an attention task and this oscillatory coupling seems to be initiated by FEF neurons7. These findings, however, provide only indirect evidence that FEF acts as a necessary source of attentional signals to area V4.
To examine whether FEF is necessary for the attentional effects on V4 responses one would need to abolish FEF activity while recording in V4 in an attention task. Although it has been shown that local deactivation in FEF leads to impaired target selection at the affected location and affects orientation selectivity during a fixation task14 it has never been tested whether FEF deactivation affects attentional modulation in V4 during an attention task. Moreover, the role of PFC regions outside the low-threshold FEF in providing feedback to V4 is unknown. Thus, it remains an open question whether PFC provides necessary attention-related feedback to V4 and other ventral stream areas.
To test for a critical role of PFC in feedback to V4, we performed a unilateral lesion of the lateral PFC, including FEF and all of the lateral PFC areas with connections to the ventral stream. Thus, no parts of PFC providing critical feedback to V4 for spatial attention could be missed. Moreover, we cut the corpus callosum and anterior commissure to eliminate cross-hemisphere PFC feedback. We recorded neuronal responses in area V4 during an attention task in the lesion-affected and intact hemisphere, with the latter serving as a control for the former. We also measured neuronal synchronization in V4 to assess how the loss of PFC affects different attentional mechanisms.
Results
Following the lesion (Fig. 1a,b) only visual processing in the left hemisphere (right hemifield) could be affected by PFC input (Fig. 1c). We recorded single unit activity and LFPs from area V4 while the monkeys performed an attention task and we compared responses in the control and lesion-affected hemisphere. The monkeys were trained to discriminate the orientation of a grating stimulus among distracter gratings of different colors. The color of a central cue presented at the beginning of the trial identified the target grating (Fig. 1d). Monkeys were required to respond by releasing a bar if the target grating was vertical (Fig. 1d, lower row) and keep holding the bar if it was non vertical (Fig. 1d upper row).
Figure 1.
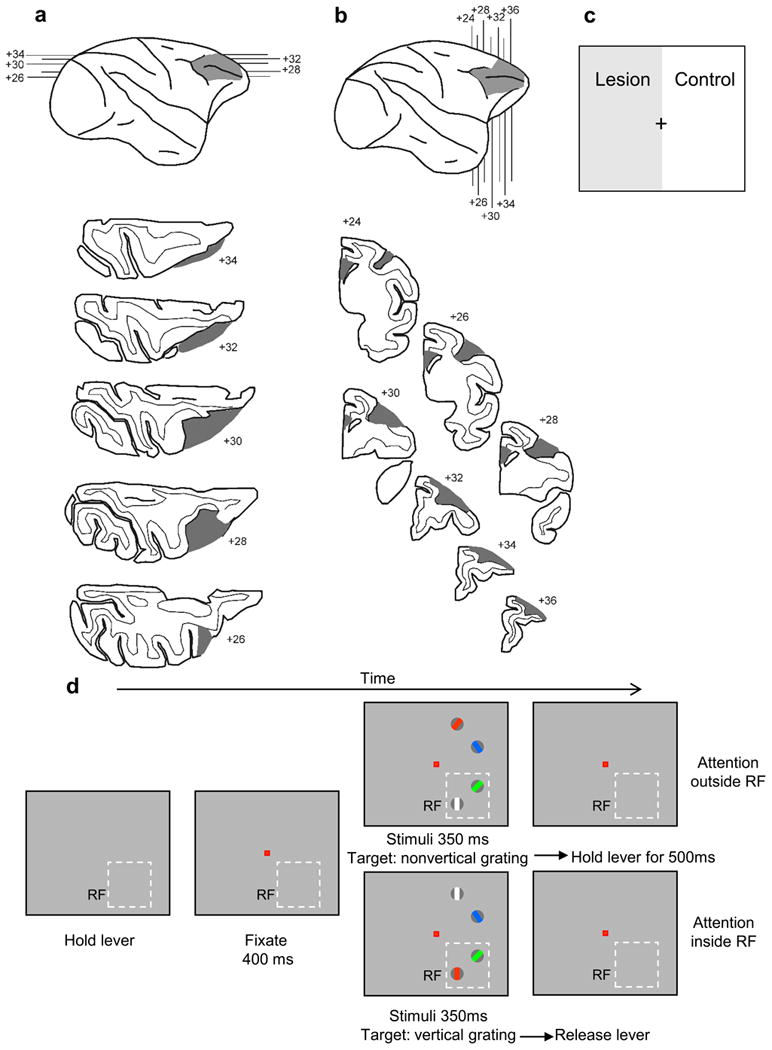
Surgical lesion and task. (a) Reconstruction of the lesion on the lateral surface of the right hemisphere of monkey 1 (top) and horizontal sections through the lesion. Numbers correspond to dorsoventral distance in mm from stereotaxic zero. Lesion is shown in gray shading (b) Reconstruction of the lesion on the lateral surface of the right hemisphere of monkey 2 (top) and coronal sections through the lesion. Numbers correspond to anteroposterior distance in mm from the interaural plane. Cortical lesion and transection of corpus callosum and anterior commissure are shown in gray shading. (c) As a result of the lesion the contralesional visual hemifield (left), shown as gray, is processed without PFC, whereas the ipsilesional visual hemifield serves as an experimental control. (d) Task. The monkey was required to hold a lever to initiate the task. Subsequently a central fixation spot appeared and the monkey was required to fixate it. Successful fixation was followed by presentation of the stimuli array, which consisted of gratings of different color. The monkey was required to discriminate the orientation of the grating that was cued by the color of the fixation spot, while maintaining central fixation, and respond by releasing the lever for a vertical grating (bottom row) within 600 ms or keep holding the lever for a non-vertical grating for 500 ms following the offset of the stimuli (top row). The relative positions of the colored gratings were randomly assigned in each trial. Dashed rectangles indicate the position of a hypothetical receptive field (RF).
To make sure that monkeys would be able to perform the task and complete enough trials for the analysis of the recording data we used orientations that were well above the discrimination thresholds measured previously with a staircase procedure in a similar task1. As a result, the first monkey did not exhibit a significant decrease in performance on the lesion affected side (mean percent correct across sessions: control hemifield 75%, n = 19 sessions, average trials/session = 985; lesion-affected hemifield 78%, n = 7 sessions, average trials/session = 906; unpaired t-test, t24 = 1.08, p = 0.29). The second monkey did show a small but significant decrease in performance on the lesion-affected hemifield (mean percent correct across sessions: control hemifield 78%, n = 74 sessions, average trials/session = 1134; lesion-affected hemifield 71%, n = 43 sessions, average trials/session = 1475; unpaired t-test, t115= 4.09, p < 0.001). Both monkeys had significantly longer reaction times in release trials when stimuli were presented contralateral to the lesion (monkey 1, control hemifield: mean RT = 420 ms, n = 3461 trials, lesion-affected hemifield: mean RT = 433 ms, n = 1800 trials, unpaired t-test, t5259 = −10.2, p<0.001; monkey 2, control hemifield: mean RT = 392 ms, n = 29012 trials, lesion-affected hemifield: mean RT =457 ms, n = 20311 trials, unpaired t-test, t49321 = −97.6, p<0.001).
Effects of Attention on Firing Rates
We recorded neuronal activity from 283 visually responsive neurons (Wilcoxon sign-rank test, p<0.01) in V4 of the intact hemisphere (monkey 1: 119 neurons,; monkey 2: 164 neurons) and 118 visually responsive neurons in the lesion-affected hemisphere (monkey 1: 30 neurons, monkey 2: 88 neurons). Results were qualitatively similar in both monkeys and were thus combined unless stated otherwise.
We first confirmed that neuronal responses to two stimuli inside the RF are determined by the interaction between the two stimuli and that the sensory interaction is modulated by attention as previously described15. Sensory interaction (SI) for two stimuli in the RF (stim1 and stim2) was related to the selectivity (SE) for the two stimuli, in that adding stim1 drove the neuronal response toward the response elicited by stim1 alone (Supplementary Fig. 1). The absolute SE values were not significantly different in the two hemispheres (control:median SE = 0.028, lesion: median SE = −0.04; Wilcoxon ranksum test, p = 0.25) suggesting that the absence of PFC did not affect visual selectivity in V4, at least for the two stimuli presented in the RF in the trials we considered for our analyses. Subsequently, we directed the monkeys' attention to each of the two stimuli in the RF. In agreement with previous studies15, neuronal responses to the pair were driven toward the response to the attended stimulus presented alone in both hemispheres (Supplementary Fig. 1). SI values for pairs of stimuli in the RF changed significantly when attention was directed to stim1, stim2 or outside the RF (one-way repeated measures ANOVA, control: F2,448 = 56.96, lesion: F2,206 = 15.9, p<0.001 in both hemispheres).
To examine the effect of attention on firing rates in the two hemispheres in more detail we compared responses with attention directed inside the RF to those when attention was directed outside the RF. For the former (attend in condition) we limited analysis to trials in which attention was directed to the preferred color (stim1 or stim 2) because of the dependence of attentional modulation on neuronal selectivity. In the control (intact) hemisphere 71% of the neurons showed a significant enhancement in their firing rates (5% showed a significant decrease) with attention (average response in a window 150-300 ms after stimuli onset; Wilcoxon rank-sum test, p < 0.05). At the population level (Fig. 2a), activity was enhanced with attention by 25% (150-300 ms poststimulus, Wilcoxon sign-rank test, p < 0.001).
Figure 2.
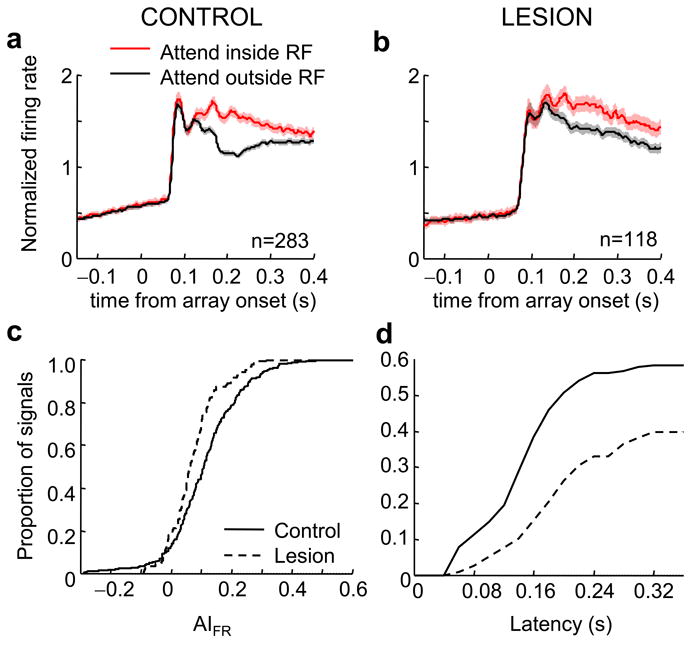
Effect of attention on firing rate responses in V4 of the control and lesion-affected hemisphere. (a) Normalized population average firing rates from the control hemisphere. (b) Normalized population average firing rates from the lesion-affected hemisphere. Responses in a,b are aligned on the presentation of the stimuli array. Red lines correspond to responses in the condition in which the target stimulus appeared inside the RF of the recorded neuron and the black line corresponds to the responses when the target stimulus appeared outside the RF. Shaded areas represent mean ± standard error of the mean. (c) Cumulative distributions of attentional indices computed from firing rates for the control (solid line) and lesion (dashed line) hemisphere. (d) Cumulative distributions of attentional latencies in firing rates for the control (solid line) and lesion (dashed line) hemisphere.
Compared to the control hemisphere, attentional effects were smaller in the lesion-affected hemisphere. Specifically, 55% of the neurons showed a significant enhancement in their firing rates (2% showed a significant decrease) with attention (average response 150-300 ms after stimuli onset; Wilcoxon rank-sum test, p < 0.05). At the population level (Fig. 2b) activity was significantly albeit modestly affected by attention showing a 15% increase for firing rates (average response 150-300 ms, poststimulus, Wilcoxon sign-rank test, p < 0.001). A distribution of effects in the control and lesion-affected hemisphere is shown in Supplementary Fig. 2. Attention indices for firing rate modulation (AIFR) were significantly lower in the lesion-affected side (Fig. 2c, control hemisphere: median AIFR = 0.11, lesion-affected hemisphere: median AIFR = 0.06, Wilcoxon rank-sum test p < 0.001). Thus, in the absence of PFC, attentional modulation of firing rates in V4 is decreased, strongly supporting the notion that PFC is a source of attentional modulation in V4.
Next, we tested whether in the absence of PFC, attentional selection latencies in V4 would be shifted later. We were able to estimate the latencies of attentional effects for 165 neurons in the control hemisphere and 47 neurons in the lesion-affected hemisphere. In the control hemisphere attentional latencies were significantly earlier relative to those measured in the lesion-affected hemisphere (Fig. 2d, control: median latency = 161 ms, lesion: median latency = 199 ms, Wilcoxon rank-sum test p < 0.001). To rule out the possibility that earlier latencies in the control hemisphere might be due to the larger attentional effect on the control side we repeated the analysis, including only neurons with attentional effects of similar size (20-40% increase with attention). In this subset of signals too (52 and 18 neurons in the control and lesion-affected hemisphere, respectively) the distribution of latencies in the control hemisphere was shifted earlier compared to that in the lesion-affected hemisphere (control median latency: 178 ms, lesion median latency: 214 ms; Wilcoxon rank-sum test, p < 0.02). The delayed onset of attentional effects in the lesion-affected hemisphere confirms that the absence of PFC deprives V4 of an important source of attentional modulation.
Effects of Attention on Neuronal Synchronization
Previous studies have shown that in addition to modulating firing rates, attention enhances neuronal synchronization within V4 as well as within other areas of the attentional network7,16-21. Moreover, we have shown that oscillatory coupling between FEF and V4 in the gamma frequency range is enhanced with attention and that this coupling is initiated by the FEF visual neurons7,19. We therefore asked how the absence of PFC input affects neuronal synchronization in V4, using both LFP power analysis and spike-field coherence (SFC) estimates.
We recorded 252 LFP signals from V4 of the control hemisphere and 152 LFPs from the lesion-affected hemisphere. At the population level, we found a significant attentional enhancement of LFP power in gamma frequencies between 50 and 90 Hz in both hemispheres (Fig. 3a-d, average power 200-300 ms post stimuli onset, paired t-test, control: t251 = 12.11, lesion: t127 = 11.44, p <0.001 in both hemispheres). Gamma power was enhanced by 10% in the intact hemisphere and by 7% in the lesion-affected hemisphere (for a distribution of effects see Supplementary Fig. 3). Attentional indices (AIpowergamma) were significantly lower in the lesion-affected hemisphere (Fig. 3e; median AIpowergamma control: 0.05, lesion: 0.03; Wilcoxon rank-sum test p <0.05). The decrease in attentional modulation indicates that the PFC input to V4 is responsible at least in part for the increase in gamma power with attention.
Figure 3.
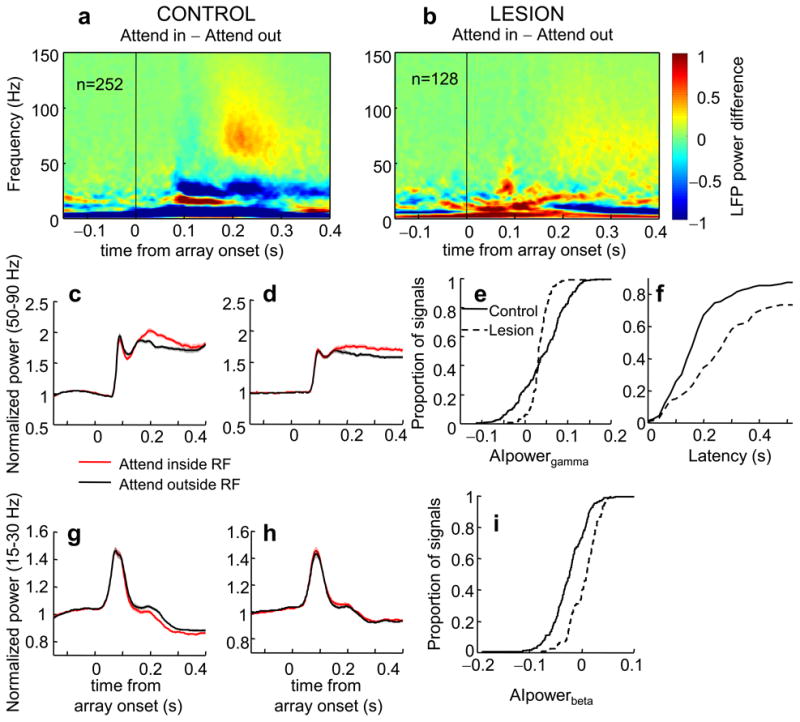
Effect of attention on V4 LFP power in the control and lesion-affected hemisphere. (a) Population average of attentional effects (attention inside RF-attention outside RF) on LFP power in the control hemisphere as a function of trial time. (b) Population average of attentional effects (attention inside RF-attention outside RF) on LFP power in the lesion-affected hemisphere as a function of trial time. (c,d) Normalized LFP gamma power averaged between 50-90 Hz in the control (c) and lesion (d) hemisphere. (e) Cumulative distributions of attentional indices in gamma power for the control (solid line) and lesion (dashed line) hemisphere. (f) Cumulative distributions of attentional latencies in gamma power in the control (solid line) and lesion (dashed line) hemisphere. (g,h) Normalized LFP beta power averaged between 15-30 Hz in the control (g) and lesion (h) hemisphere. (i) Cumulative distributions of attentional indices in beta power for the control (solid line) and lesion (dashed line) hemisphere. Conventions as in Fig. 2.
Moreover, similar to our firing rate results attentional latencies in LFP gamma power in the lesion-affected hemisphere were shifted significantly later compared to latencies in the intact hemisphere (Fig. 3f, control median: 169 ms, lesion median: 242 ms; Wilcoxon rank-sum test p < 0.001). This difference in latencies did not depend on the magnitude of the attentional effect in the two hemispheres. When we considered only signals with effects of similar magnitude (61 signals in the control and 71 signals in the lesion-affected hemisphere with gamma enhancements with attention 5-15%), latencies in the control hemisphere were significantly earlier (control median: 185 ms, lesion median: 239 ms, Wilcoxon rank-sum test p < 0.05).
LFP power was also modulated in beta frequencies. We found a statistically significant decrease in power with attention in frequencies 15-30 Hz in the control hemisphere but the difference did not reach significance in the lesion-affected hemisphere (Fig. 3a,b,g,h; average power 200-300ms post stimulus, paired t-test: control: t251 = −9.40, p < 0.001, 95% CI [−0.875,−0.572], lesion: t127 = 1.78, p = 0.08, 95% CI [−0.011, 0.26]) suggesting a larger effect of attention on beta power modulation in the control hemisphere. At the population level LFP beta power was decreased by 5% in the control hemisphere and was enhanced by 0.9% in the lesion-affected hemisphere (for a distribution of attentional effects see Supplementary Fig. 3). Attentional indices for beta power were significantly lower in the control hemisphere (Fig. 3i; median AIpowerbeta control: −0.02, lesion: 0.009; Wilcoxon rank-sum test p < 0.001). LFP power in alpha frequencies (9-14Hz) was also significantly decreased with attention albeit in both hemispheres (Fig. 3a,b, p < 0.001 in both cases) and the magnitude of attentional modulation was not significantly different in the two hemispheres (median AIpoweralpha control: −0.008, lesion: −0.01; Wilcoxon rank-sum test, p = 0.07). At the population level LFP alpha power was decreased by 3% in both hemispheres.
We next considered spike-LFP coherence analysis (SFC), which provides a direct measure of phase locking between spike trains and LFPs in the frequency domain. Our dataset consisted of 339 spike-LFP pairs in the intact hemisphere (167 pairs from monkey 1 and 172 pairs from monkey 2) and 265 pairs in the lesion-affected hemisphere (42 from monkey 1 and 223 from monkey 2). SFC coherence was significantly enhanced with attention between 50 and 90 Hz in both hemispheres (intact: Fig. 4a, paired t-test, t338 = 8.95, p < 0.001; lesion-affected hemisphere: Fig. 4b, t264 = 3.64, paired t-test p < 0.01; for a distribution of effects on gamma coherence see Supplementary Fig. 4). However, the attentional modulation of coherence between 50 and 90 Hz was significantly lower in the lesion-affected hemisphere compared to the intact hemisphere (Fig. 4c; Wilcoxon rank-sum test, p < 0.01, median AIcohgamma, control: 0.09, lesion: 0.03). This result confirms and extends our LFP gamma power findings providing direct evidence for a causal role of PFC input in the enhancement of gamma synchronization in V4.
Figure 4.
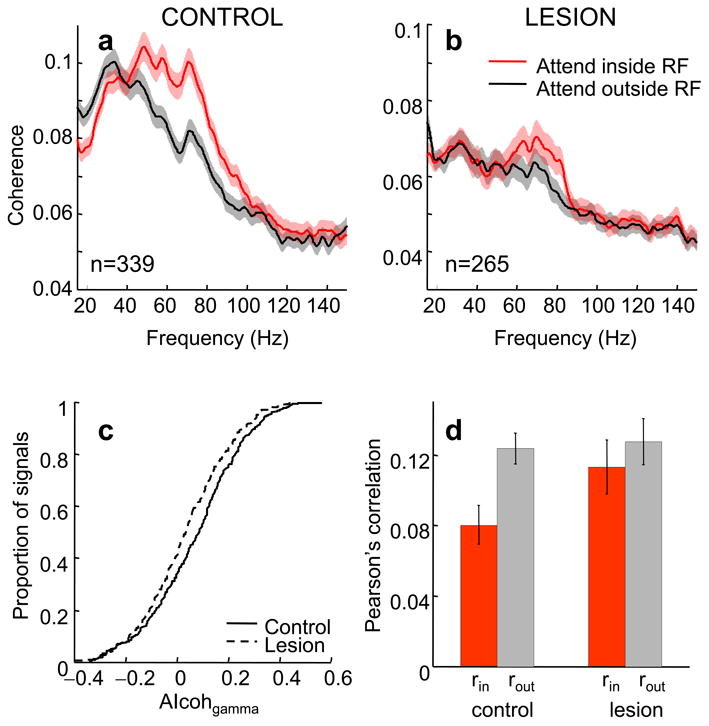
Effect of attention on synchronization and noise correlation in V4 in the control and lesion-affected hemisphere. Spike-field coherence in V4 of the control (a) and lesion (b) hemisphere.(c) Cumulative distribution of attentional indices for gamma coherence (averaged between 50 and 90Hz) in the control (solid line) and lesion (dashed line) hemisphere.(d) Mean Pearson's correlation (noise correlation, r) between spike counts of pairs of single neurons in V4 in the control and lesion-affected hemisphere in the two attention conditions (red bars: attention inside RF, grey bars: attention outside RF). Other conventions as in Fig. 2.
Beta coherence (15-30 Hz) was significantly decreased with attention in the intact hemisphere (Fig. 4a, paired t-test t338 = −4.16, p <0.001, 95% CI [−0.012,−0.004]) although this effect was mainly driven by the first monkey's data (monkey1: paired t-test, p<0.001; monkey 2: p = 0.12). In the lesion-affected hemisphere the effect of attention on beta coherence did not reach significance (Fig. 4b, paired t-test, t264 = −0.27, p = 0.79, 95% CI [−0.004, 0.003]). These results indicate that the effect of attention on beta coherence modulation was larger on the control side. Indeed, a direct comparison of beta modulation in the two hemispheres showed that the modulation was significantly lower in the lesion compared to the intact hemisphere (median AIcohbeta intact: −0.060, lesion: 0.016; Wilcoxon rank-sum test p < 0.05) with attention indices in the lesion-affected hemisphere not significantly different from zero (sign-rank test, p = 0.59).For a distribution of attentional effects on beta coherence in the two hemispheres see Supplementary Fig. 4.
Attention has also been shown to reduce noise correlation22,23 which corresponds to the correlation of spike counts over trials between pairs of neurons. Given that such correlated “noise” would reduce the fidelity of neuronal signals it has been suggested that a reduction in the shared variability could increase the signal-to-noise ratio and thus improve neural coding24. We computed the Pearson's correlation coefficient of spike counts (within a window 150-300 ms poststimulus) over trials between pairs of neurons in V4 (226 neuronal pairs in the control hemisphere and 96 pairs in the lesion-affected hemisphere) in the two attention conditions. In accordance with previous studies we found that attention significantly reduced noise correlation (r) in the control hemisphere (Fig. 4d, Wilcoxon sign rank test, p < 0.001, 95% CI [−0.06,−0.02]). However, the change in noise correlation with attention did not reach significance in the lesion-affected hemisphere (p = 0.27, 95% CI [−0.04, 0.015]). Thus, the effect of attention on noise correlation was larger on the control side. Indeed, a direct comparison showed that correlation change (rattended−runattended) was significantly larger in the intact hemisphere compared to the lesion-affected hemisphere (Wilcoxon rank-sum test p < 0.05; control median difference: −0.04, lesion median difference: −0.007).
Attentional Modulation in Correct and Error Trials
We next addressed the relative magnitude of attentional modulation in trials with correct and incorrect responses. Incorrect responses included incorrect release on hold trials and incorrect hold on release trials but not fixation breaks. The population results are shown in Figs 5 and 6 (see also Supplementary Fig. 5). When data from both monkeys were pooled together the effects of attention on firing rates, alpha and gamma power, and gamma SFC were reduced or eliminated in both hemispheres on error trials compared with correct trials. Thus, all of these measures were sensitive to behavioral performance. By contrast, modulation of beta SFC, beta power and noise correlation did not depend on the monkeys' performance but only on the presence of PFC, in that all measures were reduced in the lesion-affected hemisphere, regardless of performance. A comparison of attentional effects on correct and error trials in both the intact and the lesion-affected hemispheres is provided in Table 1 for each monkey separately.
Figure 5.
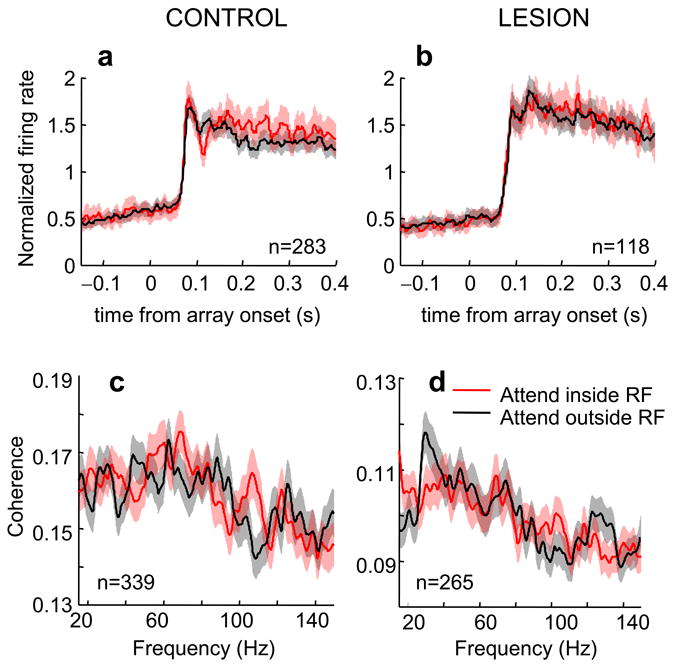
Effect of attention on V4 firing rates responses and spike-field coherence in error trials. (a,b) Normalized population average firing rates in incorrect trials. Activity averaged over V4 neurons recorded in the control (a) and lesion (b) hemisphere. (c,d) Spike field coherence in V4 of the control (c) and lesion-affected hemisphere (d) in incorrect trials. Conventions as in Fig. 2.
Figure 6.
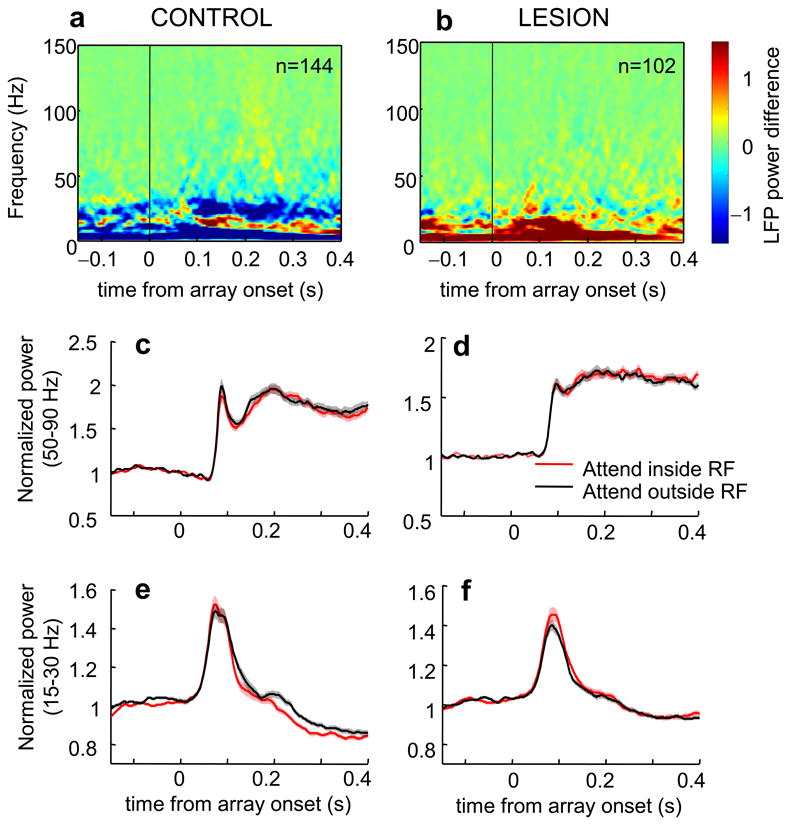
Effect of attention on V4 LFP power in error trials. (a,b) Population average of attentional effects (attention inside RF-attention outside RF) on LFP power of V4 in the control (a) and lesion (b) hemisphere for incorrect trials as a function of trial time. (c,d) Normalized LFP gamma power averaged between 50-90 Hz in the control (c) and lesion (d) hemisphere. (e,f) Normalized LFP beta power averaged between 15-30 Hz in the control (e) and lesion (f) hemisphere. Conventions as in Fig. 2.
Table 1. Comparison of attentional modulation in correct and error trials in the two hemispheres of the two monkeys.
| Control Hemisphere | Lesion Hemisphere | Main Effect | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correct trials Mean AI | Error trials Mean AI | Diff | Correct trials Mean AI | Error trials Mean AI | Diff | Hemisphere | Trial type | |||||||||
| M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | |
| Firing Rate | 0.14 | 0.10 | 0.06 | 0.04 | +++ | +++ | 0.11 | 0.06 | 0.03 | 0.01 | +++ | +++ | + | + | +++ | +++ |
| Gamma Coherence (50-90Hz) | 0.12 | 0.03 | 0.02 | -0.01 | +++ | + | 0.08 | 0.02 | -0.006 | -0.001 | +++ | + | + | + | +++ | ++ |
| Beta coherence (15-30 Hz) | -0.06 | -0.04 | 0.02 | -0.01 | - | - | -0.05 | 0.02 | -0.07 | 0.03 | - | - | + | ++ | + | - |
| Gamma LFP Power (50-90Hz) | 0.09 | 0.03 | 0.01 | -0.007 | +++ | +++ | 0.04 | 0.02 | -0.003 | 0.005 | +++ | +++ | +++ | + | +++ | +++ |
| Beta LFP power (15-30 Hz) | -0.04 | -0.02 | -0.04 | -0.03 | - | - | -0.01 | 0.008 | -0.001 | 0.002 | - | - | +++ | +++ | - | - |
| Alpha LFP power (9-14 Hz) | -0.02 | -0.007 | 0.01 | 0.008 | +++ | + | -0.04 | -0.01 | 0.005 | 0 | +++ | + | - | - | +++ | ++ |
| Noise correlation (rin-rout) | -0.05 | -0.03 | -0.05 | -0.006 | - | - | -0.03 | 0.006 | 0.009 | 0.05 | - | - | + | + | - | - |
Mean attentional indices calculated for six different measures (firing rate, gamma and beta spike-field coherence, gamma, beta and alpha LFP power) as AttendIn-AttendOut/AttendIn+AttendOut in correct and error trials. For noise correlation, the attentional effect was quantified as rin−rout. Data from monkey 1 (M1) and monkey 2 (M2) are presented separately. Values in bold indicate mean indices values significantly different from zero (t-test, p<0.05). Statistical comparisons were carried out using a two way ANOVA to assess a significant main effect of hemispheres and/or trial type (two rightmost columns) followed by a Tukey Kramer post-hoc test to evaluate differences between correct and error trials (Diff).
p<0.05,
p<0.01,
p<0.001,
no statistically significant difference
We hypothesized that if PFC is an important source of attentional selection signals, the absence of PFC should specifically increase interference from distracters. To this end, we considered trials with a single vertical stimulus (target or distracter) in the array in which the monkey released the bar. These comprised: a) trials in which the monkey released correctly to a vertical target inside the RF (vertical in, correct release, INcr), b) trials in which the monkey released correctly to a vertical target outside the RF (vertical out, correct release, OUTcr), c) trials in which attention should have been directed inside the RF and the monkey should have held the bar but the monkey released incorrectly and there was a vertical distracter outside the RF (vertical out, incorrect release, OUTincr) and d) trials in which attention should have been directed outside the RF and the monkey should have held the bar but the monkey released incorrectly and there was a vertical distracter inside the RF (vertical in, incorrect release, INincr). By comparing incorrect releases in the presence of a vertical distracter to correct releases to a vertical target we wanted to test whether there was any neural evidence that the vertical stimulus captured the monkeys' attention and thus that the absence of PFC results in increased distractibility.
We found a significant main effect of hemisphere and trial type on firing rates and LFP gamma power (Fig. 7; two-way ANOVA, firing rates, main effect of hemisphere: F1,974 = 7.18, p < 0.01; main effect of trial type: F3,974 = 69,1, p < 0.001; LFP gamma power, main effect of hemisphere: F3,1193 = 42.4, p < 0.001; main effect of trial type: F1,1193 = 16.3, p < 0.001). We employed post-hoc tests to examine how responses in the different types of trials compared to each other. Had the monkeys' attention been reliably directed toward the vertical grating in the array we should have seen no significant difference between INcr and INincr as well as between OUTcr and OUTincr. The data on the intact hemisphere did not support this view. There were no significant differences in firing rates between INincr and OUTincr,(Fig. 7a,b, Tukey-Kramer test p = 0.40) with all other pairwise comparisons showing significant differences. Moreover, there were no significant differences in LFP gamma power between INcr, and OUTincr as well as between INincr and OUTincr (Fig. 7c,d, Tukey-Kramer test p > 0.14 in both comparisons) with all other pairwise comparisons showing significant differences. These results indicate that incorrect release responses cannot be attributed reliably to shifts of attention toward the vertical distracter on the intact side.
Figure 7.
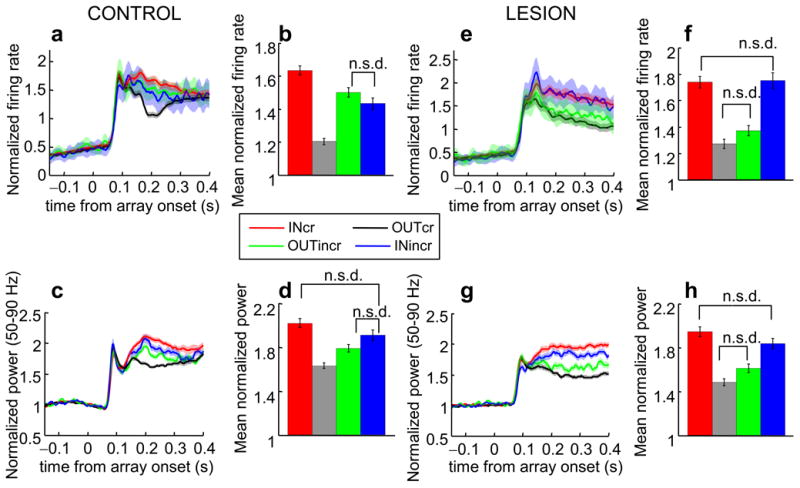
Effect of vertical distracters on neuronal responses and LFP gamma power. (a,e) Normalized population average firing rates of V4 neurons in the control (a) and lesion (e) hemisphere. In all plots red depicts data from trials in which the monkey correctly released to a vertical grating inside the RF (INcr), black/gray depicts data from trials in which the monkey released correctly to a vertical target outside the RF (OUTcr), blue corresponds to data from trials in which there was a vertical distracter inside the RF and the monkey was cued to a non vertical stimulus outside the RF but released incorrectly instead of holding (INincr), green corresponds to data from trials in which there was a vertical distracter outside the RF and the monkey was cued to a non vertical stimulus inside the RF but released incorrectly instead of holding (OUTincr) (b,f) Bar graphs indicating mean normalized firing rates across the population averaged 150-300 ms following array onset in the four trial types in the control (b) and lesion (f) hemisphere. Error bars indicate mean ± standard error of the mean. (c,g) Population average of normalized V4 LFP gamma power averaged over 50-90 Hz in the control (c) and lesion (g) hemisphere. (d,h) Mean LFP gamma power averaged 200-300 ms following array onset in the four trial types in the control (d) and lesion (h) hemisphere. n.s.d not significantly different.
By contrast, in the lesion-affected hemisphere, responses in the four trial types reflected the location of the vertical stimulus regardless of the cue/target color. Responses in INcr and INincr did not differ significantly and the same was the case for OUTcr and OUTincr (Fig. 7e-h, Tukey-Kramer test p > 0.08 in both comparisons for both firing rate and LFP gamma power). Unfortunately, due to the small number of error trials we were not able to address the same question for coherence the calculation of which requires a large number of trials for a reliable estimate. However, the behavioral data also suggested increased distractibility in the absence of PFC showing a significantly higher incidence of incorrect releases to vertical distracters in the lesion-affected compared to the intact hemifield (intact: median 12%[monkey 1:10%, monkey 2: 13%], lesion: median 17%, [monkey 1: 20%, monkey 2: 17%], Wilcoxon rank-sum test p < 0.01). These results indicate that in the absence of PFC, the monkeys' ability to use the cue to guide their attention was compromised and they were more easily distracted by stimuli associated with a response.
Discussion
As a strong test of the idea that PFC is necessary for the top-down attentional modulation of neuronal responses and synchrony in V4, we abolished PFC inputs to extrastriate cortex in one hemisphere and recorded activity in area V4 in the intact and lesion-affected hemispheres during an attention task. The absence of PFC input, resulted in reduced attentional modulation of firing rates in V4 and in a delayed onset of attention effects on neuronal responses. This finding is consistent with human studies showing that perturbations of frontal cortex function in healthy subjects as well as unilateral focal lesions in dorsolateral PFC of stroke patients diminish attentional modulation of ERP responses on posterior sites25-27. Our results establish a necessary role of PFC in the modulation of neuronal responses with attention – the earliest effects of attention on V4 responses (those occurring 150-200 ms following the onset of a stimulus) seem to be entirely dependent on PFC.
A selective enhancement of the representation of the attended stimulus can be achieved by modulating the gain of firing rates in visual cortex as well as by modulating the synchronization of activity. A role of synchrony in selective attention has been suggested by studies showing increases in local gamma synchronization with attention in extrastriate cortex7,16,18,20,21 and correlation of these enhancements with behavior21,28. More importantly, long range synchronization of activities between areas is enhanced with attention and it has been proposed that these oscillatory interactions with the appropriate phase relationship could provide a temporal structure to facilitate communication across selected populations of neurons in distant areas5,7,20,29-31. However, the brain structures responsible for the initiation of the oscillatory activity in extrastriate cortex have been unknown.
The reduction we found in gamma power modulation and gamma coherence on the lesion-affected V4 as well as the longer attentional latencies in gamma power demonstrate that PFC is one source of modulation of gamma synchrony in V4. If increases in gamma synchrony between selected neuronal populations facilitate neuronal communication and processing of attended stimuli over unattended ones as previously suggested, the reduction of gamma synchronization in the lesion-affected hemisphere could result in less efficient selection of relevant stimuli and inadequate filtering of distracters. Although the modulation of gamma synchronization we found with attention extended to somewhat higher frequencies compared to those found in one previous study7,19 it remained within the frequency range of attentional modulation reported in other studies18,21. The small difference in the frequency range could be due to differences in the size or contrast of the stimuli across studies32,33.
Similar reductions were found in modulation of synchrony in lower frequencies in the lesion-affected hemisphere indicating that PFC is also critical for the suppression of beta frequency synchronization as well as for the reduction in noise correlation. A decrease in noise correlation has been suggested to improve neural coding by increasing the signal-to-noise ratio24. Beta band oscillations have been suggested to signal the status quo and a decrease of this activity is thought to allow processing of novel events34. Our data are consistent with this theory. A suppression of beta power and SFC in the intact hemisphere with attention could facilitate processing of the orientation of the grating that determined the required response. The diminished modulation found in the lesion-affected hemisphere could reflect maintenance of a cognitive set (e.g. perseverance or search for the vertical stimulus regardless of the cue identity). The exact mechanisms through which PFC inputs can induce a selective suppression of beta synchrony and how this allows for a change of the current state remain open questions.
Beta band modulation did not correlate with behavioral performance as it was not significantly different in correct and error trials supporting the view that the magnitude of beta synchrony modulation reflects the strategy adopted by the monkey. By contrast, modulation of gamma synchrony, firing rates and alpha power reflected attentional performance in that all three measures showed a larger modulation in correct compared to error trials.
We also assessed how the absence of PFC affects attentional guidance. Firing rates and LFP gamma power in the lesion-affected V4 but not V4 on the intact side reflected the position of the vertical grating and not the location of the cued stimulus. Moreover, on the lesion-affected hemifield monkeys tended to make more errors and respond to vertical distracters when present in the array. These findings strongly point to two alternative interpretations that are not mutually exclusive. One is that the loss of PFC forced the monkeys to adopt a new strategy on the lesion-affected side, which allowed them to look for the same type of stimulus in all trials i.e. a vertical grating ignoring the cue. We have previously shown that PFC is not critical for this behavior1. Alternatively, the monkeys may have been distracted by the vertical grating and could not inhibit a response to it. Consistent with this interpretation previous studies have reported impairments in distracters' suppression and increased distractibility in patients with focal PFC lesions35. Recent electrophysiological and inactivation data have also suggested a role of PFC in suppressing the representation of distracters36.
Although the current findings clearly demonstrate that PFC is one source of modulatory signals that influence attention-related responses in V4, we can only speculate on the exact origins of such influence based on the known anatomical connections between PFC regions and V4. Besides the direct FEF projections to V4, signals from prefrontal areas 9, 12, 45, and 46 can reach V4 through FEF37. Signals from 46, FEF and 12 can also influence V4 activity through the lateral intraparietal area (LIP) and the adjacent parietal convexity37-40. Finally, FEF, area 12, ventral 46 and 45 can also exert their effect on V4 via projections to visual areas TE and TEO in the inferior temporal cortex37,41,42. Unfortunately, although the data from large permanent lesions of PFC can directly determine the necessity of PFC in top-down control they cannot narrow down the critical PFC sources and the specific circuits involved. Future studies employing spatially restricted inactivations within PFC could address this question.
Despite the extended PFC lesion we performed, attentional modulation of neuronal responses and synchrony in V4 was not completely abolished as one would expect if PFC was the only source of top-down signals to V4. Moreover, the monkeys were still able to perform the task although reaction times and accuracy were affected by the lesion. There are several possible sources of the remaining modulation. Parts of the prefrontal and orbitofrontal cortex that were spared by the lesion might have contributed to the residual modulation, although it is not clear that these areas have the spatial resolution to separate targets from distracters. These include area 10, the spared part of area 12, mesial prefrontal areas, as well as orbitofrontal areas 11, 12, 13 and 14. The orbitofrontal cortex shares anatomical connections with the temporal cortex and could conceivably affect visual processing in V4 through area TE37. Similarly, mesial prefrontal areas 9, 10 and 32 are directly connected to orbitofrontal cortical areas37 and could therefore indirectly influence visual processing in the ipsilateral temporal lobe. Although there is no evidence of an orbitofrontal cortex involvement in the top-down control of attention it is possible that plasticity mechanisms allowed nearby cells in the spared tissue to assume the function of the removed PFC as previously shown in the somatosensory system43. It is also possible that the PFC in the intact hemisphere may have contributed attention-related signals to the contralateral superior collliculus (SC) (through the ipsilateral SC and the posterior commissure), which may have in turn influenced V4 responses through the tecto-pulvinar pathway. This seems unlikely given that reversible inactivation of SC causes visual selection deficits but does not affect attentional modulation in MT and MST44. However, it is possible that long-term plasticity mechanisms utilized an unusual anatomical route. Moreover, we cannot rule out the possibility that the monkeys adopted a strategy that did not require the PFC on the lesion-affected hemifield. In fact, our results indicate that on the lesion-affected side monkeys tended to respond to the vertical grating irrespective of whether this was the target or a distracter. It is possible that this strategy depended on structures outside the PFC.
In spite of the possible complications of long term plastic changes following the PFC lesion, we favor the view that PFC normally shares its role in attentional control with other structures, such as the parietal cortex. Microstimulation, lesion and inactivation studies suggest that similar to FEF, the parietal cortex, and LIP in particular, which has direct connections with V438,45, plays a role in the covert deployment of attention4,46,47. The pulvinar is another possible source of attentional modulation in V430,48 through its widespread connections with visual cortical areas49. The ventral part of the pulvinar, which receives input from temporal visual areas and the SC49,50 is likely to relay tectal and cortical input from temporal and parietal areas to V4 and/or regulate synchrony of activities across different areas to subserve attentional selection. The lateral pulvinar, which has direct projections to V450 may also relay the relevant information.
In conclusion, the present findings establish that the lateral PFC is one source of attention related signals to V4, which are responsible for the modulation of responses and oscillatory activity. Loss of PFC results in long-lasting effects on attentional measures, including firing rate responses and gamma synchrony in V4. However, the loss of PFC-induced modulation is compensated to some degree by inputs from other areas. Future studies should reveal the areas necessary for the control of attention and elucidate their distinct contributions.
Online Methods
Subjects and lesions
Two male rhesus monkeys (Macaca mulatta) weighing 8-10 kg were used. Lesions of prefrontal cortex were made as previously described1. Briefly, unilateral lesions of the lateral surface of prefrontal cortex of the right hemisphere were made by aspiration of the gray matter. The caudal border of the lesion coincided with the depth of the arcuate sulcus, and the rostral border extended to the frontal pole. The lesion extended dorsomedially to the midline and ventromedially to the orbital gyrus. As a result it included area 8, dorsolateral areas 9 and 46, and ventrolateral areas 45 and 12 sparing the mesial and orbital prefrontal cortices. The extent of the prefrontal lesion was verified postsurgically by a reconstruction of the remaining cortical structures from coronal slices obtained with magnetic resonance imaging (MRI) (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK; 1.5 T; 1 mm thick slices; 256x256 pixel resolution; 11 cm field of view). In addition to the prefrontal lesion, the anterior commissure and corpus callosum were transected so that visual processing in only the left hemisphere could be affected by feedback from PFC. This allowed us to have one intact control hemisphere and thus, to compare recording results from the two hemispheres in the same monkey. Naturally, by transecting the corpus callosum and the anterior commissure we also eliminated the interhemispheric connections between the two prefrontal lobes. Thus, it is not unlikely that PFC function on the non-lesioned side was affected by this manipulation. Although we cannot rule out this possibility the size of the attentional effects we measured in V4 on the non-lesioned side are comparable to those reported in previous studies7,15. This fact suggests that the non-lesioned hemisphere was a valid control. A reconstruction of the lesion is illustrated in Fig. 1.
The monkeys were implanted with a post to fix the head, with a scleral search coil for monitoring eye position51, and with a recording chamber over area V4. All procedures and animal care were in accordance with the NIH guidelines and were approved by the National Institute of Mental Health Institutional Animal Care and Use Committee.
Behavioral Task
The same two monkeys were previously used in a study, which examined the behavioral effects of PFC removal in an attention task1. The monkeys were trained before the lesion to discriminate the orientation of a target grating among distractors in a task in which no color cue was presented and there was no fixation control. Five to nine months after the lesion, following a recovery period and additional training, the monkeys were tested in different versions of an orientation discrimination task using a staircase procedure. The training protocol and time frame of training in these tasks have been described in more detail previously1. The task used in the current study during V4 recordings was very similar to one of the tasks employed in our previous study (experiment 1) to test the monkeys' ability to switch top-down control in a cued attention task. During the task the monkeys were seated in front of a computer monitor (resolution 800x600 pixels and refresh rate 100Hz) at a distance of 57cm with their heads fixed. Behavioral parameters and presentation of visual stimuli were controlled by the CORTEX software package. Receptive fields (RFs) were mapped by flashing stimuli while the monkeys were fixating centrally.
Monkeys were trained to fixate a central cue and discriminate the orientation of a colored target grating presented among distracter gratings of different colors. Specifically, at the beginning of each trial the monkeys had to hold a lever for a central cue to appear. Successful fixation of the cue for 400 ms was followed by the appearance of three or four sinusoidal gratings of different color (red, green, blue and white) for 350 ms. The gratings were arranged on one hemifield (contralateral to the recorded V4), at equal distances from the central cue, at the appropriate eccentricity so that one or two gratings were positioned in the lower quadrant inside the RF of the recorded neurons and two gratings were positioned in the upper quadrant outside the RF. All stimuli were matched for luminance. The central cue was present throughout the trial and its color identified the target. For example, a red cue indicated that attention should be directed to the red grating, a blue cue to the blue grating etc. The color of the cue and thus the identity of the target changed randomly from trial to trial. The relative positions of the colored gratings were randomly assigned on each trial (see Fig. 1d). Monkeys were required to release the lever within 850 ms following the gratings onset if the target grating was vertical and keep holding the bar for 850 ms if the grating was non vertical. Non vertical orientations were confined to orientations 30, 45, −30, −45 deg away from vertical. The monkeys had to maintain fixation of the cue throughout the trial. The probability of the cued grating being vertical was 50%. Moreover, to ensure that the monkeys would not just release the bar every time a vertical grating was present in the display we included a vertical distractor (with the other gratings being non-vertical) in 20% of the trials.
Recording
Recordings started 4.5-5 years after the lesion in both monkeys. Spikes and local field potentials (LFPs) were recorded from area V4 with up to four tungsten microelectrodes using a Multichannel Acquisition Processor system (Plexon Inc). Electrodes were spaced 650 or 900 μm apart. Spike data were obtained after filtering between 250 Hz-8 kHz, amplifying and digitizing the signal at 40 kHz. Spikes were selected offline to include multi-unit activity on each electrode and were sorted offline using the Offline Sorter software (Plexon, Inc) to isolate spike trains from single units. For the LFP, the signals were filtered between 0.7-170 Hz, amplified and digitized at 1 kHz. Training (and recordings) were carried out first in the intact hemifield (left hemisphere) and later in the lesion-affected hemifield (right hemisphere) in both monkeys. We selected this design to ensure that the monkeys would be motivated to perform the task when stimuli were presented in the contralesional hemifield.
Data Analysis
All statistical analyses were assessed by two-tailed tests in MATLAB (MathWorks). We did not run any statistical test to determine sample sizes (number of signals) a priori. The sample sizes included in the analyses are similar to those reported in previous publications. Our experimental design did not have multiple experimental groups and as a result no randomization or blinding was necessary.
Firing Rates
For analysis, spike data were downsampled to 1kHz. Analysis was restricted to neurons with statistically significant visual responses relative to baseline (Wilcoxon sign-rank test, p < 0.01, visual response 80-250 ms following the visual array onset, baseline activity: −200-0 ms relative to stimuli onset).
To assess selectivity and the effect of sensory interactions for the two stimuli in the RF we first measured firing rates to each stimulus presented alone inside the RF with the monkey's attention directed away from the RF i.e. with the color of the cue matching the color of a grating positioned outside the RF. Selectivity was calculated as the difference between the normalized responses to each of the two stimuli presented alone in the RF within a window 150-300 ms following stimulus onset (SE = FRnormstim1−FRnormstim2). We then measured the response when these two stimuli (stim1 and stim2) were presented together in the lower quadrant inside the RF and the monkey's attention was directed in the upper quadrant away from the RF. Sensory interaction was quantified by an index SI defined as SI = FRnormpair – FRnormstim2 where FRnormpair corresponds to the normalized response to the pair and FRnormstim2 to the normalized response to stimulus 2 when this was presented alone in the RF. Subsequently, we directed the monkeys' attention to each of the two stimuli in the RF and measured how responses to the pair were affected by attention.
We determined the color that elicited the largest response (preferred color) for each neuron by considering trials in which attention was directed outside the RF in the upper quadrant while there was one stimulus inside the RF. For the “Attend Inside RF” condition we included trials in which attention was directed inside the RF to the stimulus with the preferred color. In the “Attend Outside RF” condition we included trials in which attention was directed outside the RF with the preferred stimulus inside the RF. We did not observe any baseline shift in the two conditions, thus, the difference in the cued color in the two conditions (preferred vs other) has not confounded our results. The attentional effect for each neuron was quantified by computing an attention index as AIFR = (Response in Attend In − Response in Attend Out)/(Response in Attend In + Response in Attend Out). Responses were averaged within a window 150-300 ms following stimuli onset. Data from both release and hold trials were included in the analyses. To calculate the population average of firing rates we normalized responses of individual neurons to the mean activity −200 to 400 ms relative to stimuli onset across both attention conditions. The latency (onset) of attentional effects was estimated for each neuron as follows. First, for each attention condition we convolved the spiking signal in each trial with a Gaussian kernel with a standard deviation of 10ms. We next compared the values in the two conditions across trials and asked for at least 15 consecutive 1ms bins to be significantly different (unpaired t-test, p < 0.05). The first of the 15 bins signified the onset of attentional effect.
Noise correlation was computed as the Pearson's correlation of spike counts over trials, within a window 150-300 ms post stimulus, between pairs of simultaneously recorded neurons. We used trials in which the stimuli inside the receptive field were the same in both attention conditions to avoid any confounding effect of selectivity in our noise correlation calculation.
LFP power
The time course of the LFP power spectra was calculated using the Hilbert-Huang Transform (HHT)52 which employs the Empirical Mode Decomposition (EMD) method and the Hilbert transform. First, we calculated the Hilbert spectrum for each trial in 2 ms bins and smoothed the three dimensional time-frequency spectra with a 2D Gaussian filter (sigma=[4ms, 2Hz], size=[10ms, 5Hz]). To compute the population average we normalized LFP power within the frequency range of interest per condition for each LFP signal by dividing with the average power within the frequency range of interest across both conditions in a 200 ms window before stimuli onset. To obtain latency estimates of the attentional modulation in gamma power in each signal we averaged LFP power between 50 and 90 Hz. The attentional latency was determined as the time corresponding to the first out of 8 consecutive 2 ms bins that were significantly different in the two conditions (t-test, p< 0.05). Attentional modulation of LFP power in the various frequencies of interest (gamma, beta, alpha) was quantified by calculating a modulation index for each frequency range of interest (foi) AIfoi = (LFP power in freq of interest in Attend In − LFP power in freq of interest in Attend Out) / (LFP power in freq of interest in Attend In + LFP power in freq of interest in Attend Out).
Coherence Analysis
Coherence spectra between spikes (multi-units) and LFPs were calculated using the following formula for the calculation of coherency between two signals x and y:
Where Sx(f), and Sy(f) represent the auto-spectra and Sxy(f) the cross-spectrum of the two signals x and y averaged across trials. We calculated coherence within a window 200 to 400 ms following stimuli onset and we used multi-taper methods to achieve optimal spectral concentration. An optimal family of orthogonal tapers given by the discrete prolate spheroid sequences (Slepian functions) was employed as described before7,18, 53 providing an effective smoothing of ± 10Hz. Only spike signals showing a significant visual response and a mean firing rate of at least 10 spikes/sec were included in the coherence analysis. To avoid spurious correlations that could arise from contamination of LFP signals from spikes recorded on the same electrode we included in the analysis only multi-unit activity and LFPs recorded on different electrodes. To avoid any sample size bias we selected an equal number of trials for each attention condition. To ensure that firing rate differences did not account for any differences measured in coherence between conditions, we equated firing rates across attention conditions using a procedure that has been described in detail before7. Results were similar with and without this firing rate correction. We report results without the correction.
Attentional modulation of coherence in the frequency bands of interest was quantified by computing an attention index AIcoh = (Coherence in Attend In− Coherence in Attend Out)/(Coherence in Attend In + Coherence in Attend Out) where coherence was averaged within the frequency range of interest.
Control for behavioral performance differences in the two hemifields
We have previously shown that the ability of the monkeys to use the cue to discriminate the orientation of the target grating among distracters was compromised on the lesion-affected hemifield1. To avoid any confounding effect that differences in performance might have on attentional modulation in the two hemispheres we tried to eliminate the possibility of such differences during recordings. To this end, we used orientations that were well above the discrimination threshold that was previously determined with a staircase procedure1. Moreover, given that performance for one of the monkeys was still somewhat lower on the lesion-affected hemifield we carried out an additional analysis. We selected a subset of trials that belonged to conditions with similar performance in the two hemifields. We first calculated for each condition and for each animal the percent correct performance per recording session. Subsequently, for each condition we tested whether there was a significant difference in performance between the two hemifields taking into account all recording sessions. For each animal we selected those conditions for which no statistically significant difference was found in performance between the two hemifields (Wilcoxon rank-sum test, p < 0.05). This manipulation resulted in a considerable reduction in the number of trials included in the analyses (e.g. for the attend in RF condition we ended up with only 20-30% of the trials originally included). The results of the analyses using this subset of trials were identical to those using the entire dataset and are shown in Supplementary Figure 6.
Limitations due to sample size differences
Because in some cases we found significant differences with attention in the control hemisphere but not in the lesion-affected hemisphere and given that our sample size was smaller in the latter, we wanted to rule out the possibility that the smaller sample size alone was responsible for the difference between the two hemispheres. To this end, for these particular cases (i.e. modulation of beta power, beta coherence and noise correlation) we down-sampled the data from the control hemisphere by randomly selecting a number of signals equal to those included in the analysis of the lesion-affected hemisphere. Had the absence of a significant effect in the lesion-affected hemisphere been due to the smaller sample size alone, we should have found no significant effect of attention in the control hemisphere for this reduced dataset. On the contrary, we found that this manipulation did not change the results of the statistical tests for the control hemisphere. This indicates that the effects were indeed larger on the control side. Although the smaller sample size alone does not explain the absence of effects on beta and noise correlation in the lesion hemisphere, we cannot rule out the possibility that we might have found a small, significant attentional effect with a larger sample size. Therefore, to address this concern we report 95% confidence intervals, which give a direct measure of the effect size for those cases.
Supplementary Material
Acknowledgments
We thank Grant Pielli for help with the animal training and Richard Saunders for assistance with the surgical procedures. This work was supported by the National Institute of Mental Health Intramural Research Program and National Institutes of Health Grant R01EY017292 (R.D.). G.G. was partially supported by the European Community's Seventh Framework Programme (Grant PIRG05-GA-2009-246761)
Footnotes
Author Contributions: G.G.G., A.F.R, L.G.U and R.D designed the experiment, G.G.G and A.F.R acquired the data, G.G.G. analyzed the data, G.G.G and R.D wrote the paper.
A supplementary methods checklist is available.
References
- 1.Rossi AF, Bichot NP, Desimone R, Ungerleider LG. Top down attentional deficits in macaques with lesions of lateral prefrontal cortex. J Neurosci. 2007;27:11306–11314. doi: 10.1523/JNEUROSCI.2939-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duncan J. Disorganization of Behavior after Frontal-Lobe Damage. Cognitive Neuropsychology. 1986;3:271–290. [Google Scholar]
- 3.Heilman KM, Valenstein E. Frontal lobe neglect in man. Neurology. 1972;22:660–664. doi: 10.1212/wnl.22.6.660. [DOI] [PubMed] [Google Scholar]
- 4.Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- 5.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 6.Everling S, Tinsley CJ, Gaffan D, Duncan J. Filtering of neural signals by focused attention in the monkey prefrontal cortex. Nat Neurosci. 2002;5:671–676. doi: 10.1038/nn874. [DOI] [PubMed] [Google Scholar]
- 7.Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High frequency long range coupling between prefrontal cortex and visual cortex during attention. Science. 2009;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson KG, Bichot NP, Schall JD. Dissociation of visual discrimination from saccade programming in macaque frontal eye field. J Neurophysiol. 1997;77:1046–1050. doi: 10.1152/jn.1997.77.2.1046. [DOI] [PubMed] [Google Scholar]
- 9.Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- 10.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 11.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 12.Ekstrom LB, Roelfsema PR, Arsenault JT, Bonmassar G, Vanduffel W. Bottom-up dependent gating of frontal signals in early visual cortex. Science. 2008;321:414–417. doi: 10.1126/science.1153276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- 14.Noudoost B, Moore T. Control of visual cortical signals by prefrontal dopamine. Nature. 2011;474:372–375. doi: 10.1038/nature09995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. J Neurosci. 1999;19:1736–1753. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bichot NP, Rossi AF, Desimone R. Parallel and serial neural mechanisms for visual search in macaque area V4. Science. 2005;308:529–534. doi: 10.1126/science.1109676. [DOI] [PubMed] [Google Scholar]
- 17.Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 18.Fries P, Womelsdorf T, Oostenveld R, Desimone R. The effects of visual stimulation and selective visual attention on rhythmic neuronal synchronization in macaque area V4. J Neurosci. 2008;28:4823–4835. doi: 10.1523/JNEUROSCI.4499-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregoriou GG, Gotts SJ, Desimone R. Cell-type specific synchronization of neural activity in FEF with V4 during attention. Neuron. 2012;73:581–594. doi: 10.1016/j.neuron.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saalmann YB, Pigarev IN, Vidyasagar TR. Neural mechanisms of visual attention: how top-down feedback highlights relevant locations. Science. 2007;316:1612–1615. doi: 10.1126/science.1139140. [DOI] [PubMed] [Google Scholar]
- 21.Taylor K, Mandon S, Freiwald WA, Kreiter AK. Coherent oscillatory activity in monkey area v4 predicts successful allocation of attention. Cereb Cortex. 2005;15:1424–1437. doi: 10.1093/cercor/bhi023. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell JF, Sundberg KA, Reynolds JH. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron. 2009;63:879–888. doi: 10.1016/j.neuron.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen MR, Maunsell JH. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci. 2009;12:1594–1600. doi: 10.1038/nn.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zohary E, Shadlen MN, Newsome WT. Correlated neuronal discharge rate and its implications for psychophysical performance. Nature. 1994;370:140–143. doi: 10.1038/370140a0. [DOI] [PubMed] [Google Scholar]
- 25.Zanto TP, Rubens MT, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat Neurosci. 2011;14:656–661. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor PC, Nobre AC, Rushworth MF. FEF TMS affects visual cortical activity. Cereb Cortex. 2007;17:391–399. doi: 10.1093/cercor/bhj156. [DOI] [PubMed] [Google Scholar]
- 27.Barcelo F, Suwazono S, Knight RT. Prefrontal modulation of visual processing in humans. Nat Neurosci. 2000;3:399–403. doi: 10.1038/73975. [DOI] [PubMed] [Google Scholar]
- 28.Womelsdorf T, Fries P, Mitra PP, Desimone R. Gamma-band synchronization in visual cortex predicts speed of change detection. Nature. 2006;439:733–736. doi: 10.1038/nature04258. [DOI] [PubMed] [Google Scholar]
- 29.Gregoriou GG, Gotts SJ, Zhou H, Desimone R. Long-range neural coupling through synchronization with attention. Prog Brain Res. 2009;176:35–45. doi: 10.1016/S0079-6123(09)17603-3. [DOI] [PubMed] [Google Scholar]
- 30.Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337:753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Gieselmann MA, Thiele A. Comparison of spatial integration and surround suppression characteristics in spiking activity and the local field potential in macaque V1. Eur J Neurosci. 2008;28:447–459. doi: 10.1111/j.1460-9568.2008.06358.x. [DOI] [PubMed] [Google Scholar]
- 33.Ray S, Maunsell JH. Differences in gamma frequencies across visual cortex restrict their possible use in computation. Neuron. 2010;67:885–896. doi: 10.1016/j.neuron.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engel AK, Fries P. Beta-band oscillations--signalling the status quo? Curr Opin Neurobiol. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Chao LL, Knight RT. Human prefrontal lesions increase distractibility to irrelevant sensory inputs. Neuroreport. 1995;6:1605–1610. doi: 10.1097/00001756-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki M, Gottlieb J. Distinct neural mechanisms of distractor suppression in the frontal and parietal lobe. Nat Neurosci. 2013;16:98–104. doi: 10.1038/nn.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeterian EH, Pandya DN, Tomaiuolo F, Petrides M. The cortical connectivity of the prefrontal cortex in the monkey brain. Cortex. 2012;48:58–81. doi: 10.1016/j.cortex.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersen RA, Asanuma C, Essick G, Siegel RM. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J Comp Neurol. 1990;296:65–113. doi: 10.1002/cne.902960106. [DOI] [PubMed] [Google Scholar]
- 39.Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- 40.Ninomiya T, Sawamura H, Inoue K, Takada M. Segregated pathways carrying frontally derived top-down signals to visual areas MT and V4 in macaques. J Neurosci. 2012;32:6851–6858. doi: 10.1523/JNEUROSCI.6295-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webster MJ, Bachevalier J, Ungerleider LG. Connections of inferior temporal areas TEO and TE with parietal and frontal cortex in macaque monkeys. Cereb Cortex. 1994;4:470–483. doi: 10.1093/cercor/4.5.470. [DOI] [PubMed] [Google Scholar]
- 42.Rempel-Clower NL, Barbas H. The laminar pattern of connections between prefrontal and anterior temporal cortices in the Rhesus monkey is related to cortical structure and function. Cereb Cortex. 2000;10:851–865. doi: 10.1093/cercor/10.9.851. [DOI] [PubMed] [Google Scholar]
- 43.Jenkins WM, Merzenich MM. Reorganization of neocortical representations after brain injury: a neurophysiological model of the bases of recovery from stroke. Prog Brain Res. 1987;71:249–266. doi: 10.1016/s0079-6123(08)61829-4. [DOI] [PubMed] [Google Scholar]
- 44.Zenon A, Krauzlis RJ. Attention deficits without cortical neuronal deficits. Nature. 2012;489:434–437. doi: 10.1038/nature11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ungerleider LG, Galkin TW, Desimone R, Gattass R. Cortical connections of area V4 in the macaque. Cereb Cortex. 2008;18:477–499. doi: 10.1093/cercor/bhm061. [DOI] [PubMed] [Google Scholar]
- 46.Cutrell EB, Marrocco RT. Electrical microstimulation of primate posterior parietal cortex initiates orienting and alerting components of covert attention. Exp Brain Res. 2002;144:103–113. doi: 10.1007/s00221-002-1032-x. [DOI] [PubMed] [Google Scholar]
- 47.Wardak C, Olivier E, Duhamel JR. A deficit in covert attention after parietal cortex inactivation in the monkey. Neuron. 2004;42:501–508. doi: 10.1016/s0896-6273(04)00185-0. [DOI] [PubMed] [Google Scholar]
- 48.Petersen SE, Robinson DL, Keys W. Pulvinar nuclei of the behaving rhesus monkey: visual responses and their modulation. J Neurophysiol. 1985;54:867–886. doi: 10.1152/jn.1985.54.4.867. [DOI] [PubMed] [Google Scholar]
- 49.Shipp S. The brain circuitry of attention. Trends Cogn Sci. 2004;8:223–230. doi: 10.1016/j.tics.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Gattass R, Galkin TW, Desimone R, Ungerleider LG. Subcortical connections of area V4 in the macaque. J Comp Neurol. 2013 doi: 10.1002/cne.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurements of eye position: An improved method. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- 52.Huang NE, et al. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proceedings of the Royal Society of London Series a-Mathematical Physical and Engineering Sciences. 1998;454:903–995. [Google Scholar]
- 53.Jarvis MR, Mitra PP. Sampling properties of the spectrum and coherency of sequences of action potentials. Neural Comput. 2001;13:717–749. doi: 10.1162/089976601300014312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


