Abstract
We previously reported that vascular endothelial growth factor induced vascular endothelial (VE)-cadherin tyrosine phosphorylation at Y685 in a Src-dependent manner in vitro. Here, we studied the occurrence of Y685 phosphorylation in vivo in the female reproductive tract because it is a unique model of physiological vascular remodeling dependent on vascular endothelial growth factor. We first developed and characterized an anti-phospho-specific antibody against the site Y685 of VE-cadherin to monitor VE-cadherin phosphorylation along the four phases of mouse estrous cycle, termed proestrus, estrus, metestrus, and diestrus. A dynamic profile of tyrosine phosphorylated proteins was observed in both uterus and ovary throughout mouse estrous cycle, including kinase Src, which was found highly active at the estrus phase. The extent of tyrosine phosphorylated VE-cadherin was low at proestrus but strongly increased at estrus and returned to baseline at metestrus and diestrus, suggesting a potent hormonal regulation of this specific process. Indeed, C57Bl/6 female mice treatment with pregnant mare serum gonadotropin and human chorionic gonadotropin confirmed a significant increase in phosphoY685-VE-cadherin compared with that in untreated mice. These results demonstrate that VE-cadherin tyrosine phosphorylation at Y685 is a physiological and hormonally regulated process in female reproductive organs. In addition, this process was concomitant with the early steps of vascular remodeling taking place at estrus stage, suggesting that phosphoY685-VE-cadherin is a biomarker of endothelial cell activation in vivo.
Keywords: VE-cadherin phosphorylation in vivo, tyrosine kinase, angiogenesis, estrous cycle, vascular remodeling
vascular endothelial (VE)-cadherin is an adhesion molecule exclusively expressed at endothelial adherens junctions. Tyrosine phosphorylation of cadherin complex was described as a mechanism that controls adherens junction integrity (7). Several in vitro studies have demonstrated the involvement of VE-cadherin phosphorylation in the increase of vascular permeability observed upon inflammatory mediator and growth factor challenges (9, 21). Several in vivo studies including one from our laboratory have reported the occurrence of VE-cadherin tyrosine phosphorylation in specifically induced or noninduced processes such as vascular permeability or shear stress (18, 19, 28). The use of phosphomutants and phospho-site targeting antibodies allow the identifying of phosphorylated residues in VE-cadherin cytoplasmic domain during endothelial cell proliferation, leukocyte extravasation, and vascular permeability in vitro (2, 20, 24, 26). Using an in vitro kinase assay, we previously demonstrated that the kinase Src exhibited a higher affinity for the site Y685 than for other tyrosine residues within the VE-cadherin cytoplasmic domain. Consistent with this, the LY685AQV sequence fits the YxxV/I/L motif, which is a consensus site for phosphorylation by Src family kinases (26). Moreover, we identified the site Y685 in endothelial cells upon vascular endothelial growth factor (VEGF) challenge (26). Few studies have investigated VE-cadherin tyrosine phosphorylation in vivo (18), and the identity of the specific phosphorylated tyrosine residues remains unknown. Herein, we aimed to determine the occurrence of VE-cadherin tyrosine phosphorylation at site Y685 in vivo. To that purpose, we developed and characterized an anti-phosphoY685-VE-cadherin antibody and we used the mouse female reproductive tract as a model of physiological vascular remodeling because vascular outgrowth and regression are dependent on VEGF (11).
MATERIALS AND METHODS
Reagents.
Pregnant mare serum gonadotropin (PMSG) and human chorionic gonadotropin (hCG) were from Sigma-Aldrich. Commercially available antibodies were purchased from several sources: the rabbit polyclonal antibody against the extracellular domain of VE-cadherin was produced in the laboratory [see Lambeng et al. (18)], the rat monoclonal antibody to murine VE-cadherin (anti-CD144, 11D.4.1) was from Pharmingen, the monoclonal anti-phosphotyrosine (P-Tyr) antibody (clone 4G10) was purchased at Merck-Millipore, the mouse polyclonal anti-β-actin was from Sigma-Aldrich, the rabbit polyclonal anti-phospho-Src (Y418) was from Invitrogen, and the rabbit polyclonal anti-c-Src was from Santa Cruz Biotechnology. The rabbit polyclonal phosphopeptide-specific antibody (anti-phosphoY685-VE-cadherin) has been produced by Eurogentec and characterized in this study (Fig. 1). Enhanced chemiluminescence detection reagents were purchased from Perkin-Elmer (Courtaboeuf, France). The micro-bicinchoninic acid protein assay reagent kit was from Fischer Scientific.
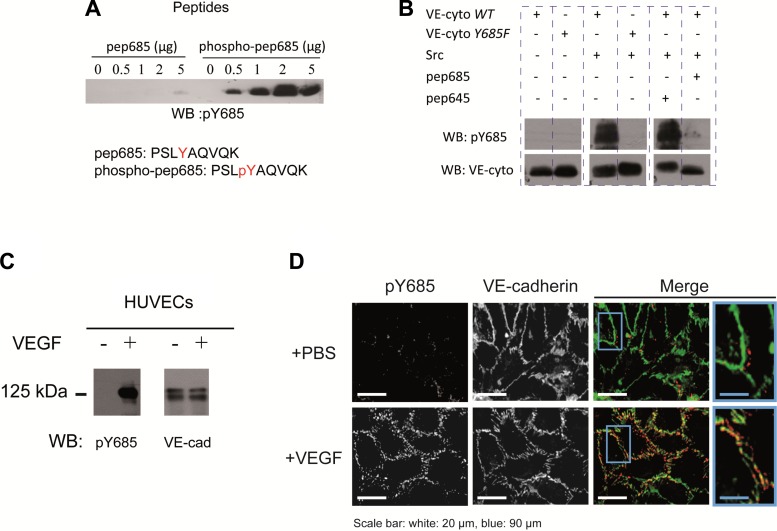
Fig. 1.
Validation of anti-pY685 vascular endothelial-cadherin (VE-Cad) antibody. A: Y685 containing peptide (pep685) was subjected to Src phosphorylation assay or not and resolved by Western blotting (WB), pep685, and phospho (p)-pep685 sequences where indicated; only the phosphorylated peptide was recognized with anti-pY685. WB pictures were cropped to remove unnecessary spaces between bands for the figure clarity. B: recombinant VE-cadherin cytoplasmic domain (VE-cyto) carrying or not carrying the Y685F mutation was phosphorylated or not by Src in presence of either pep685 or pep645 containing, respectively, Y685 and Y645 as indicated; anti-pY685 recognized only the nonmutated VE-cyto-wt when phosphorylated in absence of pep685 as a competitor whereas VE-cyto was detectable wherever it was used. WT, wild-type. C: human umbilical vein endothelial cells (HUVECs) were stimulated or not with VEGF, and cell lysates were analyzed with anti-pY685 and anti-VE-cadherin; only pVE-cadherin (125 kDa) of VEGF-treated cells was recognized. D: immunofluorescence of VEGF-treated HUVECs with anti-pY685 and anti-VE-cadherin: pY685 (red) and VE-cadherin (green); scale bars are indicated; results are representative of at least 3 different experiments.
Animals.
All procedures were approved by ComEth ethical committee. All protocols in this study were conducted in strict accordance with the French guidelines for the care and use of laboratory animals.
Mutation Y685F of VE-cadherin cytoplasmic domain and recombinant protein production.
The DNA sequence coding for the wild-type (WT) VE-cadherin cytoplasmic domain (missing the first 9 amino acids) was inserted into pQE30 (Qiagen), and Y685F mutation was made with the QuickChange kit (Stratagene) according to the manufacturer's protocol. Mutated vector was amplified in DH5α, and recombinant proteins (wt and Y685F) were produced within BL21-CodonPlus Competent Cells (Stratagene) as described in manufacturer's protocol, using an NH2-terminal His tag. The protein produced had a molecular mass of 25 kDa in Coomassie blue staining and was recognized by antibodies against His tag or VE-cadherin COOH-terminus domain.
Estrous cycle-stage examination.
The different stages of estrus cycle were monitored by daily examination of vaginal smears of postpubertal female mice (at least n = 5 per group) as previously described (5). Briefly, vaginal secretions (wet smear) were collected in phosphate-buffered saline with fine tip pipets and observed by phase contrast microscopy with ×10 or ×20 objectives to characterize the different cell types. Mice estrous cycle can be divided into four phases, namely, estrus, proestrus, metestrus, and diestrus, which are defined according to the proportion in three cell types. At proestrus, nucleated epithelial cells are predominant, whereas estrus is distinctively composed of cornified squamous epithelial cells, metestrus is characterized by a mix of the three cell types, and diestrus consists predominantly of leukocytes. In this study we used cycling mice at different estrous stages. At least two consecutive baseline cycles were recorded before experimental manipulation. Mice were injected (intraperitoneally) with peroxovanadate (50 mmol/l in PBS) and deeply anesthetized 5 min later with pentobarbital sodium (50 mg/kg). Ovaries and uterus were collected from mice at different stages of estrus cycle and from mice treated by injection of PMSG and hCG. The ovaries and uterus were carefully dissected from all the adhering extraneous tissue before freezing for biochemical analyses.
Hormone stimulation.
Hormone stimulation was performed as previously described (5). Briefly, mice were given an intraperitoneal injection of 10 IU of PMSG in 0.75 ml of 0.9% NaCl on day 1, followed by 5 IU of hCG (both from Sigma-Aldrich) in 0.4 ml of 0.9% NaCl, 48 h later. Animals were euthanized 6 h after second injection by cervical dislocation after peroxovanadate administration.
Tissues extractions, immunoprecipitation, and Western blot analysis.
Tissues extractions, immunoprecipitation, and Western blot analysis were performed as previously described (5).
Data analysis.
Unless stated elsewhere, Student's t-test was used to assess differences in Western blot band quantity. The results highlighting P values < 0.05 were considered significantly different. At least three mice per group were used in each set of experiments. The experiments were performed at least three times under identical conditions with similar results.
RESULTS
Anti-pY685 antibody recognizes specifically VE-cadherin phosphorylated at Tyr685.
To study VE-cadherin Y685 phosphorylation in vivo, we first developed a rabbit polyclonal anti-phospho-Y685 (anti-pY685). The specificity of the antibody was tested by Western blot analysis using the nonphosphorylated and the phosphorylated synthetic peptide spanning Y685 residue. As shown in Fig. 1A, anti-pY685 antibody recognized the phosphorylated peptide but not the nonphosphorylated one. Using the recombinant VE-cadherin cytoplasmic domain carrying or not carrying Y685F mutation, we demonstrated that only the phosphorylated WT cytoplasmic tail was detectable with the anti-pY685. When Y685 or Y645 peptides were used as competitors in the tyrosine phosphorylation assay, this signal disappeared only with Y685 peptide (Fig. 1B). The anti-pY685 was then tested for its ability to recognize specifically tyrosine phosphorylated VE-cadherin in human umbilical vein endothelial cells (HUVECs) upon VEGF challenge. The Western blot analysis of cell lysates demonstrated the presence of pY685 VE-cadherin in VEGF-treated HUVECs (Fig. 1C). The anti-pY685 antibody was next tested in immunofluorescence experiments. As shown in Fig. 1D, anti-pY685 VE-cadherin staining was located at cell junctions and colocalized with VE-cadherin staining in VEGF-treated HUVECs. Altogether, these results demonstrate the specificity of anti-pY685 toward VE-cadherin phosphorylated at Y685.
VE-cadherin tyrosine phosphorylation in vivo in the female reproductive tract.
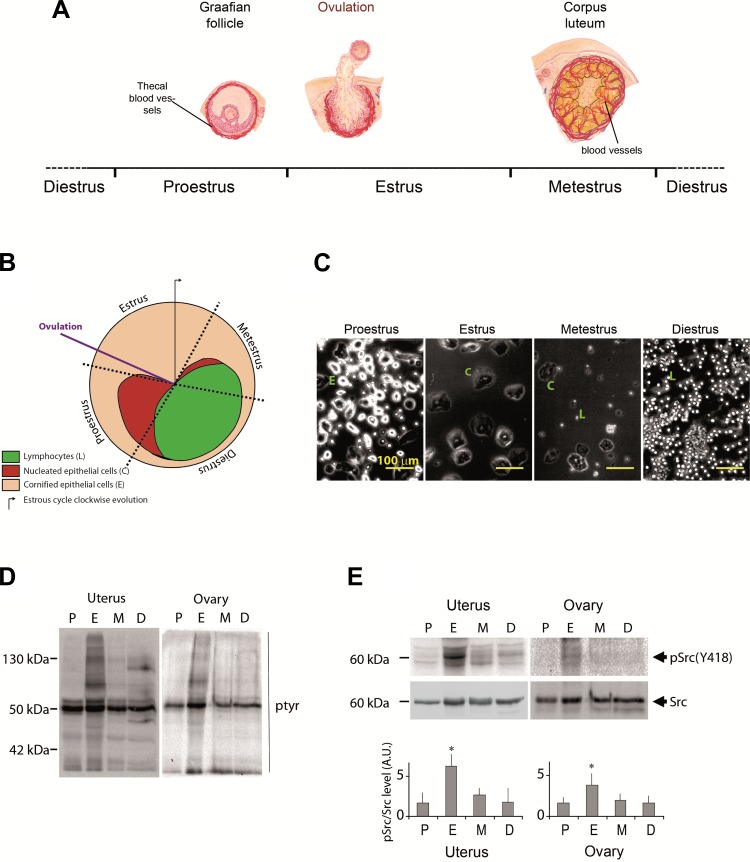
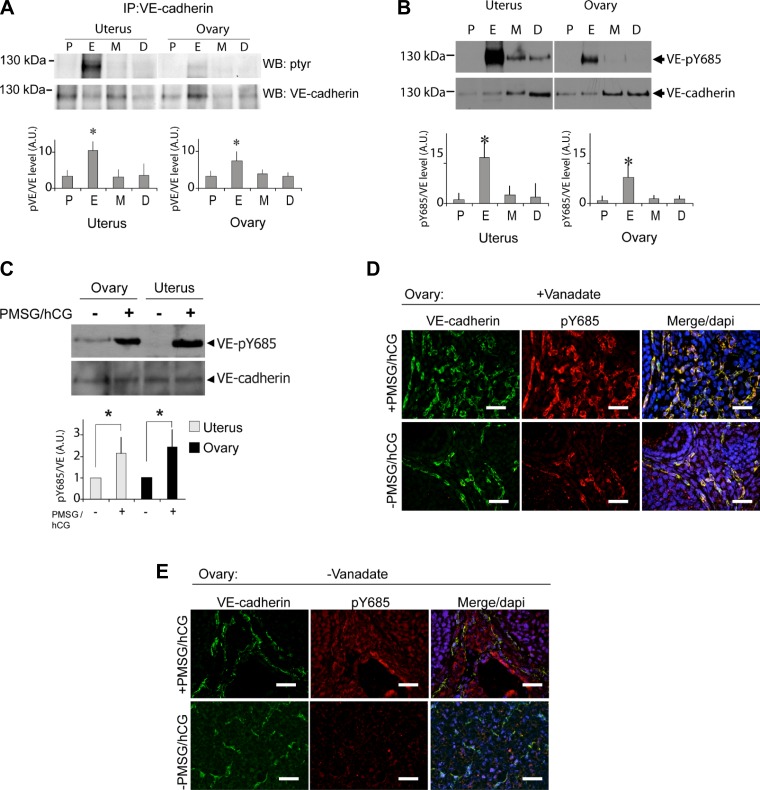
Murine estrous cycle is divided into four phases termed proestrus, estrus, metestrus, and diestrus that can be identified by daily assessment of the relative ratio of nucleated epithelial cells, cornified squamous epithelial cells, and leukocytes present in vaginal smears (Fig. 2, A–C). The analysis of the status of protein tyrosine phosphorylation throughout mouse estrous cycle showed that numerous proteins including the kinase Src were highly tyrosine phosphorylated at the estrus phase in both uterus and ovary (Fig. 2, D and E). VE-cadherin tyrosine phosphorylation status in uterus and ovary was then analyzed according to the estrous cycle stage (Fig. 3A). VE-cadherin was found tyrosine phosphorylated to a low extent at proestrus but strongly increased at estrus and returned to baseline at metestrus and diestrus. Because Src was found activated during estrus cycle and is responsible of VE-cadherin phosphorylation at Y685 residue upon VEGF challenge, we evaluated the phosphorylation of Y685 in vivo throughout mouse estrous cycle using our newly developed anti-pY685. The extent of Y685 phosphorylation followed the same profile than VE-cadherin global phospho-tyrosine status during estrous cycle, suggesting a potent hormonal regulation of this specific tyrosine phosphorylation process (Fig. 3B). To confirm this hypothesis, C57Bl/6 female mice were hormonally treated with PMSG and hCG to induce female reproductive organ maturation as previously described (5). Interestingly, pY685-VE-cadherin signal was barely detectable in ovaries and uterus from untreated mice, whereas it was significantly increased in hormone-treated mice (ovary P = 0.03; uterus P = 0.023) (Fig. 3C). VE-cadherin phosphorylation at Y685 is then hormonally modulated during estrus cycle in female reproductive organs. Ovarian vasculature was also analyzed by immunostaining of VE-cadherin and VE-pY685 status in C57Bl/6 female mice treated or not with PMSG/hCG (Fig. 3, D and E). Images were collected on ovary cross sections in mice pretreated (Fig. 3D) or not (Fig. 3E) with vanadate. The appearance of VE-pY685 was strongly detected and colocalized with VE-cadherin in PMSG/hCG-treated mice in the presence of tyrosine phosphatases inhibitor, when compared with hormonally untreated mice (Fig. 3D). Furthermore, the effect of hormone treatment in the absence of vanadate is still detectable but to a lesser extent than in its presence, confirming the basal level of phosphorylation in this specific angiogenic organ (Fig. 3E). Altogether, these data demonstrate the hormonal regulation of VE-cadherin tyrosine phosphorylation at site Y685 either during physiological estrous cycle or upon PMSG/hCG challenge.
Fig. 2.
Female reproductive system is a unique model for studying the regulation of tyrosine phosphorylation processes. A: illustrative scheme of the 4 stages [proestrus (P), estrus (E), metestrus (M), and diestrus (D)] of mouse estrous cycle. B: clockwise scheme of cell composition of mouse vaginal secretion during estrous cycle: purple line indicates the ovulation time within the estrous cycle. C: phase contrast microscopy analysis of mouse vaginal smear cell composition to identify the 4 stages of estrous cycle. Nucleated epithelial cells (round shaped), cornified epithelial cells, and lymphocytes as indicated in the scheme. D: WB analysis of protein tyrosine phosphorylation (pTyr) processes during estrous cycle in ovary and uterus. E: analysis of Src kinase activation status throughout estrous cycle using anti-pSrc (Y418) by WB (top) and its quantification (bottom). AU, arbitrary units. *P < 0.05. All the results are representative of at least 3 independent experiments.
Fig. 3.
Dynamic profile of VE-cadherin phosphorylation at Y685 along with estrous cycle. A: C57BL/6 female mice were euthanized at 1 of the 4 stages of estrous cycle, VE-cadherin was immunoprecipitated (IP) from uterus and ovaries, and its tyrosine phosphorylation status was then determined along with estrous cycle by WB (top) and its relative quantification (bottom); VE-cadherin tyrosine phosphorylation extent was highest at the estrus phase in both organs. B: examination of pTyr in VE-cadherin with antibody specific to pY685 by WB (top) and its relative quantification (bottom); Y685 was phosphorylated in the same manner than the global tyrosine phosphorylation of VE-cadherin. C: C57BL/6 female mice were treated with pregnant mare serum gonadotropin (PMSG)/human chorionic gonadotropin (hCG) to stimulate the ovulation, and the tissue extracts from uterus and ovaries were resolved by WB with anti-pY685 and anti-VE-cadherin (left), VE-cadherin appeared phosphorylated at Y685 in both organs of hormonally stimulated females, quantification of the extent of VE-cadherin phosphorylation at Y685 induced by hormonal stimulation in uterus and ovaries is shown (right). Standard deviations were high because of the variability of pY685 level in nontreated mice. *P < 0.05, significant difference; n = 4 per group (Mann and Whitney rank test). D and E: C57BL/6 female mice were treated or not with PMSG/hCG in presence (D) or absence (E) of vanadate. Ovarian vasculature features was observed by VE-cadherin (green) and pY685-VE-cadherin immunodetection (red). Scale bar = 220 μm. DAPI, 4′,6-diamidino-2-phenylindole. All the results are representative of at least 3 independent experiments.
VE-cadherin phosphorylation is associated with ovarian vascular remodeling.
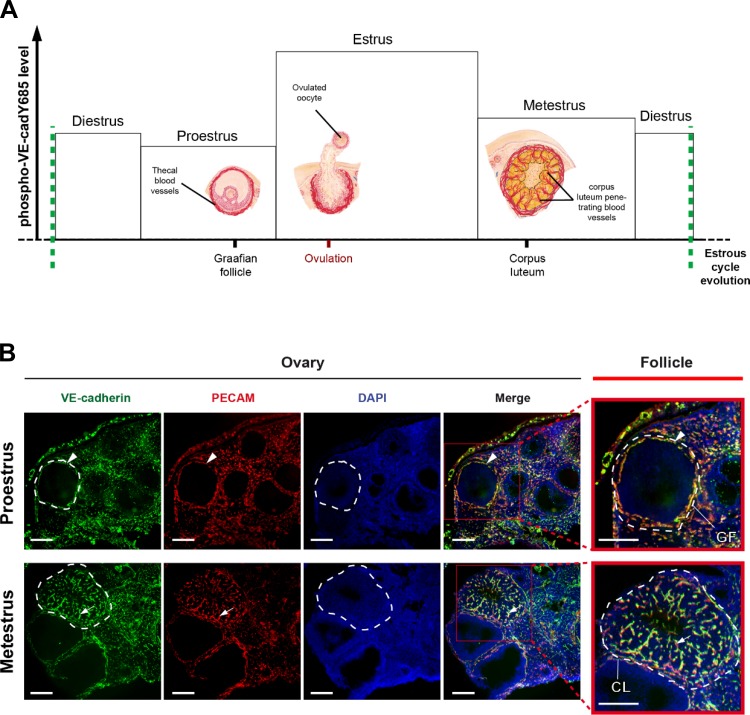
Extensive vascular remodeling in developing ovarian follicles occurs at the estrus phase where pY685-VE-cadherin level is high (Fig. 4A). We analyzed the ovarian vasculature in tissue sections by immunostaining with platelet endothelial cell adhesion molecule-1 and VE-cadherin antibodies at proestrus and metestrus stages to highlight differences in the vasculature before and after VE-cadherin phosphorylation, respectively. We found that peripheral vessels surround follicles at both stages, whereas the blood vessel density was very high in the central zone of corpus luteum only at metestrus stage (Fig. 4B). As VE-cadherin tyrosine phosphorylation was very high at estrus stage but low at proestrus and metestrus, these data demonstrate that the process is associated with early physiological steps of vascular remodeling taking place at estrus stage.
Fig. 4.
Vascular remodeling taking place after ovulation in the corpus luteum and VE-cadherin tyrosine phosphorylation. A: scheme of the profile of VE-cadherin tyrosine phosphorylation at Y685 (in ordinate axis) throughout estrous cycle (in abscissa) and the concomitant vascular remodeling events occurring in ovarian follicles; the level of VE-cadherin phosphorylation at Y685 was highest at estrus, the stage at which the post ovulatory angiogenesis occurs in the forming corpus luteum. B: ovarian vasculature features at proestrus and metestrus by staining VE-cadherin (green) and platelet endothelial cell adhesion molecule-1 (PECAM; red) and Hoescht (blue). The merge allowed to better visualize the vasculature only in theca folliculi at proestrus (before ovulation) while newly formed blood vessels were penetrating in the corpus luteum at metestrus. Follicles are indicated with white dashed lines (scale bar = 200 μm); red frames are magnifications of follicles within ovary sections; black arrowhead indicates thecal blood vessels, black arrow indicates corpus luteum new formed blood vessels. GF, Graphian follicle; CL, corpus luteum.
DISCUSSION
In the present study, we aimed to investigate the occurrence of VE-cadherin tyrosine phosphorylation in vivo in the female reproductive tract (ovary, uterus), as it undergoes physiological continuous and dynamic changes with regular intervals of vascularization and vessel breakdown (14, 22). Herein, we provide the first evidence for a physiological dynamic profile of VE-cadherin tyrosine phosphorylation processes at site Y685 concomitant with Src activation (Src-pY418) in vivo in ovary and uterus throughout the physiological mouse estrus cycle. The observed correlation between Src activation and the detection of pY685-VE-cadherin strongly suggest the involvement of Src kinase in the physiological modulation of VE-cadherin phosphorylation at Y685 in vivo as previously demonstrated in vitro (26). This phosphorylation level was highest at the estrus stage, the phase just after the ovulation, suggesting a role for this VE-cadherin tyrosine phosphorylation in early postovulatory events including angiogenesis in uterus and ovary. On the basis of the present data, the observed cyclic variations in VE-cadherin tyrosine phosphorylation mainly detected at estrus phase are likely to depend on physiological changes in mice hormonal status as demonstrated by exogenous stimulation by PMSG/hCG. The effect of these hormones on VE-cadherin tyrosine phosphorylation might presumably be due to a combination of simultaneously acting angiogenic signals including VEGF. VEGF is a potent inducer of VE-cadherin tyrosine phosphorylation in vitro and was reported as being essential for angiogenesis in corpus luteum (11). Others confirmed the requirement of VEGF and VEGFR2 for correct angiogenesis in ovary and uterus, emphasizing the importance of VEGF/Flk-1 pathway in ovarian angiogenesis and vascular permeability (10, 13, 29). Therefore, VE-cadherin tyrosine phosphorylation throughout estrous cycle might be mediated by VEGF in uterus and ovary. Moreover, cycling reproductive hormones have been reported to upregulate VEGF expression in vivo. Indeed, several lines of evidences have demonstrated estrogen effect on the increase in VEGF expression in various contexts (1, 4, 15, 17). VEGF gene contains two estrogen response elements that can mediate estrogen-induced VEGF expression and uterine vascular permeability (6, 16). Many studies have reported controversial effects of progesterone on VEGF expression. Nevertheless, angiogenesis and associated vascular permeability are necessary for preparing the uterine endometrium for implantation and successful reproduction (12). Strikingly, estrogen were reported to reach a peak at proestrus whereas other hormones including follicle-stimulating hormone (FSH), luteinizing hormone or progesterone have their peaks during ovulation, just at the beginning of the estrus phase (3, 23). Consistent with all these data, we found VE-cadherin to get its highest tyrosine phosphorylation state at estrus stage, supporting thereby the idea of an early postovulatory effect of VEGF on endothelial cell activation. Whether steroid hormone can induce VE-cadherin tyrosine phosphorylation in a direct manner or through other angiogenic factors and then be accountable for this along estrous cycle, remain to be investigated. A potential experimental approach to demonstrate the involvement of Src or VEGF in vivo in the observed phosphorylation of VE-cadherin, would be to inhibit their signaling pathway in vivo using specific tyrosine kinase inhibitors. However, in the model of female reproductive tract, cyclical phases are regulated by hormones such as FSH, which was shown to induce a Src family kinase-dependent Granulosa cell differentiation (27). Thus the use of tyrosine kinase inhibitors during mouse estrus cycle model is then problematic as they might impair FSH action and change the physiological development of the estrous cycle. The cyclic hormonal regulation of VEGF expression lead to a tight regulation of tyrosine phosphorylation processes through a balance between the activities of protein tyrosine kinases and phosphotyrosine phosphatases (PTPases). The pattern of VE-cadherin phosphorylation throughout estrus cycle is consistent with the possibility of a hormonal activation of PTPases that may regulate VE-cadherin phosphorylation level at the different stages including metestrus and diestrus phases. Known mammalian cytoplasmic PTPases possess Src homology-2 domains, which allow them to associate with molecules phosphorylated at tyrosine residues. Several PTPs have been shown to associate with components of the VE-cadherin complex, maintaining low levels of tyrosine phosphorylation and thereby promoting endothelial barrier function. VE-cadherin-associated PTPs include PTP-μ, VE-PTP, PTP1B, and Src homology 2-domain containing tyrosine phosphatase (8). It will be of great interest in future studies to identify the VE-cadherin-associated phosphatases in vivo to better understand the physiological regulation of these processes throughout the mouse estrus cycle. We recently published that VE-cadherin was tyrosine phosphorylated at site Y685 in human brain tumors (25). The antibody used in that study was the same as the one described here. Identification of this phosphorylated site in in ovary and uterus in addition to angiogenic tumors such as human glioma, strongly suggests a major role for Y685 phosphorylation in vascular remodeling occurring in tumor and physiological angiogenesis. The high level of tyrosine phosphorylation at site Y685 in tumors suggests that in contrast to ovary and uterus, the regulation by tyrosine phosphatases is missing in tumors because the balance between kinases and phosphatases is largely in favor of tyrosine kinases in cancers. Female reproductive tract is thus a nice model to study the regulation of the balance of phosphorylation of the site Y685 VE-cadherin as it is detected mostly at the estrus phase.
Taken together, these news findings demonstrate VE-cadherin phosphorylation at site Y685 in adult tissues undergoing vascular remodeling and open a new era in studying this process in vivo, as mouse estrous cycle represents a new physiological model for studying VE-cadherin phosphorylation dynamic in vivo.
GRANTS
This work was supported by the French National Institute of Health and Medical Research INSERM (UMRS 1036, U836), the French Atomic Energy and Alternative Energies Commission (CEA), Life Science division (DSV)/Institute of life sciences research and technologies (iRTSV)/Endothelial cell junctions and Angiogenesis team/Grenoble University Hospital, Institut National du Cancer (INCA 07/3D1616/PL-96–031/NG-NC.), French Association against cancer (ARC Foundation), Federation Nationale des Centres de Lutte contre le Cancer (GEFLUC Grenoble-Dauphiné-Savoie). A. Sidibé and T. Mannic received grants from Courtin Arthritis Foundation and Helena Polena from ARC Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.S. and I.Y.V. conception and design of research; A.S., H.P., J.R., T.M., N.C., S.B., and I.M. performed experiments; A.S., T.M., P.H., L.B., and I.Y.V. analyzed data; A.S., P.H., D.G.-D., L.B., and I.Y.V. interpreted results of experiments; A.S., H.P., J.R., T.M., and I.Y.V. prepared figures; A.S., H.P., T.M., and I.Y.V. drafted manuscript; A.S., H.P., and I.Y.V. edited and revised manuscript; A.S., H.P., J.R., T.M., N.C., S.B., I.M., P.H., D.G.-D., L.B.; and I.Y.V. approved final version of manuscript.
ACKNOWLEDGMENTS
We are indebted to Laurie Godart for technical and Hervé Pointu for administrative assistances respectively.
Present address of A. Sidibé and T. Mannic: University of Geneva, CH-1211, Geneva, Switzerland.
REFERENCES
- 1.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 451: 1008–1012, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Baumeister U, Funke R, Ebnet K, Vorschmitt H, Koch S, Vestweber D. Association of Csk to VE-cadherin and inhibition of cell proliferation. EMBO J 24: 1686–1695, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17β throughout the 4-day estrous cycle of the rat. Endocrinology 94: 1704–1708, 1974 [DOI] [PubMed] [Google Scholar]
- 4.Buteau-Lozano H, Ancelin M, Lardeux B, Milanini J, Perrot-Applanat M. Transcriptional regulation of vascular endothelial growth factor by estradiol and tamoxifen in breast cancer cells: a complex interplay between estrogen receptors α and β. Cancer Res 62: 4977–4984, 2002 [PubMed] [Google Scholar]
- 5.Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci Appendix 4: Appendix 4I, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakraborty I, Das SK, Dey SK. Differential expression of vascular endothelial growth factor and its receptor mRNAs in the mouse uterus around the time of implantation. J Endocrinol 147: 339–352, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Daniel JM, Reynolds AB. Tyrosine phosphorylation and cadherin/catenin function. Bioessays 19: 883–891, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Dejana E, Vestweber D. The role of VE-cadherin in vascular morphogenesis and permeability control. Prog Mol Biol Transl Sci 116: 119–144, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol 140: 947–959, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan X, Krieg S, Kuo CJ, Wiegand SJ, Rabinovitch M, Druzin ML, Brenner RM, Giudice LC, Nayak NR. VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. FASEB J 22: 3571–3580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrara N, Chen H, Davis-Smyth T, Gerber HP, Nguyen TN, Peers D, Chisholm V, Hillan KJ, Schwall RH. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat Med 4: 336–340, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1: 27–31, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Fraser HM, Dickson SE, Lunn SF, Wulff C, Morris KD, Carroll VA, Bicknell R. Suppression of luteal angiogenesis in the primate after neutralization of vascular endothelial growth factor. Endocrinology 141: 995–1000, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Gómez R, Simón C, Remohí J, Pellicer A. Vascular endothelial growth factor receptor-2 activation induces vascular permeability in hyperstimulated rats, and this effect is prevented by receptor blockade. Endocrinology 143: 4339–4348, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Hervé MA, Meduri G, Petit FG, Domet TS, Lazennec G, Mourah S, Perrot-Applanat M. Regulation of the vascular endothelial growth factor (VEGF) receptor Flk-1/KDR by estradiol through VEGF in uterus. J Endocrinol 188: 91–99, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Hyder SM, Nawaz Z, Chiappetta C, Stancel GM. Identification of functional estrogen response elements in the gene coding for the potent angiogenic factor vascular endothelial growth factor. Cancer Res 60: 3183–3190, 2000 [PubMed] [Google Scholar]
- 17.Kazi AA, Koos RD. Estrogen-induced activation of hypoxia-inducible factor-1α, vascular endothelial growth factor expression, and edema in the uterus are mediated by the phosphatidylinositol 3-kinase/Akt pathway. Endocrinology 148: 2363–2374, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Lambeng N, Wallez Y, Rampon C, Cand F, Christe G, Gulino-Debrac D, Vilgrain I, Huber P. Vascular endothelial-cadherin tyrosine phosphorylation in angiogenic and quiescent adult tissues. Circ Res 96: 384–391, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, Ristagno G, Maddaluno L, Koh GY, Franco D, Kurtcuoglu V, Poulikakos D, Baluk P, McDonald D, Grazia Lampugnani M, Dejana E. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun 3: 1208, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potter MD, Barbero S, Cheresh DA. Tyrosine phosphorylation of VE-cadherin prevents binding of p120- and beta-catenin and maintains the cellular mesenchymal state. J Biol Chem 280: 31906–31912, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Rabiet MJ, Plantier JL, Rival Y, Genoux Y, Lampugnani MG, Dejana E. Thrombin-induced increase in endothelial permeability is associated with changes in cell-to-cell junction organization. Arterioscler Thromb Vasc Biol 16: 488–496, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Reynolds LP, Killilea SD, Redmer DA. Angiogenesis in the female reproductive system. FASEB J 6: 886–892, 1992 [PubMed] [Google Scholar]
- 23.Staley K, Scharfman H. A woman's prerogative. Nat Neurosci 8: 697–699, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Turowski P, Martinelli R, Crawford R, Wateridge D, Papageorgiou AP, Lampugnani MG, Gamp AC, Vestweber D, Adamson P, Dejana E, Greenwood J. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J Cell Sci 121: 29–37, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vilgrain I, Sidibé A, Polena H, Cand F, Mannic T, Arboleas M, Boccard S, Baudet A, Gulino-Debrac D, Bouillet L, Quesada JL, Mendoza C, Lebas JF, Pelletier L, Berger F. Evidence for post-translational processing of vascular endothelial (VE)-cadherin in brain tumors: towards a candidate biomarker. PLoS One 8: e80056, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallez Y, Cand F, Cruzalegui F, Wernstedt C, Souchelnytskyi S, Vilgrain I, Huber P. Src kinase phosphorylates vascular endothelial-cadherin in response to vascular endothelial growth factor: identification of tyrosine 685 as the unique target site. Oncogene 26: 1067–1077, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Wayne CM, Fan HY, Cheng X, Richards JS. Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol Endocrinol 21: 1940–1957, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Weis S, Shintani S, Weber A, Kirchmair R, Wood M, Cravens A, McSharry H, Iwakura A, Yoon YS, Himes N, Burstein D, Doukas J, Soll R, Losordo D, Cheresh D. Src blockade stabilizes a Flk/cadherin complex, reducing edema and tissue injury following myocardial infarction. J Clin Invest 113: 885–894, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmermann RC, Hartman T, Kavic S, Pauli SA, Bohlen P, Sauer MV, Kitajewski J. Vascular endothelial growth factor receptor 2-mediated angiogenesis is essential for gonadotropin-dependent follicle development. J Clin Invest 112: 659–669, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]