Abstract
There is consensus on the benefits for all infants of exclusive breastfeeding for 6 months and introduction of appropriate complementary foods at 6 months, followed by continued breastfeeding. However, guidelines on infant and young child feeding (IYCF) for HIV-positive mothers have changed continually since 2000. This article explores issues and evidence related to IYCF for the prevention and care of paediatric HIV in resource-limited settings in light of new HIV treatment guidelines, implementation challenges and knowledge gaps.
In 2010 the impact of antiretroviral drugs (ARVs) on reducing the risk of mother-to-child transmission of HIV moved WHO to urge countries to endorse either avoidance of all breastfeeding or exclusive breastfeeding for the first 6 months while taking ARVs, depending on which strategy could give their infants the greatest chance of HIV-free survival. Implementation of the 2010 recommendations is challenged by lack of healthcare provider training, weak clinic–community linkages to support mother/infant pairs and lack of national monitoring and reporting on infant feeding indicators.
More evidence is needed to inform prevention and treatment of malnutrition among HIV-exposed and HIV-infected children. Knowledge gaps include the effects of prolonged ARV exposure, the cause of HIV-associated growth faltering, the effects of early infant testing on continuation of breastfeeding and specific nutrition interventions needed for HIV-infected children.
Significant progress has been made toward keeping mothers alive and reducing paediatric HIV infection, but sustained political, financial and scientific commitment are required to ensure meaningful interventions to eliminate postnatal transmission and meet the nutritional needs of HIV-exposed and HIV-infected children.
Keywords: antiretroviral drugs, breastfeeding, HIV, infant feeding options, prevention of mother-to-child transmission, resource-limited settings
Introduction
Evidence-based guidelines in place since 2010 recommend that mothers with HIV in low-resource/high-HIV burden settings receive the same infant and young child feeding (IYCF) advice as HIV-uninfected mothers: early initiation of breastfeeding within 1 hour of birth, exclusive breastfeeding (EBF) for the first 6 months and introduction of appropriate complementary foods at 6 months, followed by continued breastfeeding [1]. Despite this uniformity of guidelines for all infants, factors including rapidly changing global recommendations, slow rollout of updated national guidelines resulting in late training of healthcare providers, unsafe traditions and taboos related to breastfeeding, lack of support for breastfeeding mothers and significant evidence gaps in programmatic experience have led to on-going confusion about and poor adherence to these strategies [2–4]. Changes in guidelines and underlying poor complementary feeding practices among the general population have made it particularly challenging to implement safe weaning and adequate complementary feeding practices.
As remaining questions are answered and recommendations evolve, mothers with HIV need up-to-date, clear messages on and support for optimal IYCF practices. This article is not a systematic review but rather explores issues and evidence related to IYCF practices in resource-limited settings in light of the WHO 2010 HIV and infant feeding guidelines and 2013 Consolidated guidelines, implementation challenges and knowledge gaps that limit optimal care of HIV-exposed and HIV-infected infants and young children, and calls for program implementers to pay attention to critical nutrition issues that are emerging for these children.
Evidence and guidelines on HIV and child nutrition
Evidence suggests that children born to women with HIV are at higher risk of morbidity [5] and mortality [6,7] than their non-exposed peers. There is substantial evidence that women with HIV are more likely to have infants who are small for gestational age [8,9] and that HIV-exposed infants are more likely to be preterm or low birth weight [10].
Although in-utero HIV infection has not been proven to affect foetal growth, HIV affects postnatal growth starting in the early months of life, often before other signs of infection appear, and is strongly associated with both wasting and poor linear growth. Severe wasting is the most visible sign of possible HIV infection, but linear growth faltering is also common in HIV-infected children and may be a better indicator of disease progression than severe wasting [11]. Poor growth can be caused by HIV itself; opportunistic infections or medication side-effects that alter food intake, absorption and metabolism; diarrhoea and other childhood illnesses; poverty and food insecurity.
Unexplained under-nutrition that does not respond to standard treatment may be a sign of advanced HIV disease, and HIV testing should be part of the standard management of malnourished children. Malnourished HIV-infected children lose more muscle and have a higher risk of mortality than their uninfected malnourished peers [12] and, like adults, experience metabolic changes that may increase morbidity.
First 6 months of life
Well documented benefits of breastfeeding include improved infant immunity and thus decreased morbidity and mortality from disease, improved paediatric development, and health benefits for mothers including natural birth spacing, improved maternal-child bonding and economic and environmental benefits [13].
Infants who are fed foods or liquids in addition to breast milk (mixed feeding) or instead of breast milk (replacement feeding) during the first 6 months of life are at increased risk of morbidity and mortality from unsafe water, inadequate formula preparation or storage, unsanitary conditions and formula shortages, particularly in low-resource settings. Programmes have reported that in order to avoid social pressure associated with not breastfeeding, women with HIV often breastfeed in public while also privately feeding formula or other foods and liquids to infants younger than 6 months. This mixed feeding, in addition to the risks mentioned above, increases the risk of vertical transmission [14]. For these reasons, women have long been encouraged to breastfeed exclusively for the first 6 months.
IYCF guidance for HIV-exposed children has continued to evolve since WHO issued its first infant feeding technical consultation consensus statement in 2000 [15]. The risk of HIV transmission from breastfeeding up to 18–24 months is 15–20%, but studies in low-resource environments have concluded that not breastfeeding or stopping breastfeeding early increased mortality and reduced HIV-free survival [16,17]. Additional studies have found that safer practices such as exclusive breastfeeding for the first 6 months, proper breast health management, and antiretroviral therapy (ART) for the mother and infant during the breastfeeding period, significantly lowers the risk of HIV transmission [18,19].
In 2006, WHO recommended that mothers with HIV choose between 1) exclusive breastfeeding for 6 months or 2) exclusive replacement feeding for 6 months if acceptable, feasible, affordable, sustainable and safe (AFASS) [20]. Mothers who chose EBF were advised to continue breastfeeding until they could provide a safe and adequate replacement diet. By 2010 data on the impact of antiretroviral drugs (ARVs) on reducing the risk of mother-to-child transmission of HIV and increased all-cause mortality among infants who were not exclusively breastfed moved WHO to update its recommendations again: this time urging countries to endorse one nationwide infant feeding recommendation for women with HIV – either avoid all breastfeeding, or breastfeed while taking ARVs. In countries that adopted breastfeeding as the policy, women with HIV are encouraged to continue breastfeeding for at least 1 year and until a nutritionally adequate and safe diet without breast milk can be provided. Even where ARVs are not available, WHO recommends breastfeeding of HIV-exposed infants [1].
The rapid change in global recommendations for infant feeding in the context of HIV only partly explains confusion about what to advise mothers with HIV. Countries are often slow to adopt new global guidelines, and face challenges in retraining healthcare providers and addressing norms among mothers. However, the latest WHO guidelines provide an opportunity for HIV and child survival communities to harmonize and promote common feeding messages for all children, including those who are HIV-exposed, during the first year of life.
Second 6 months of life
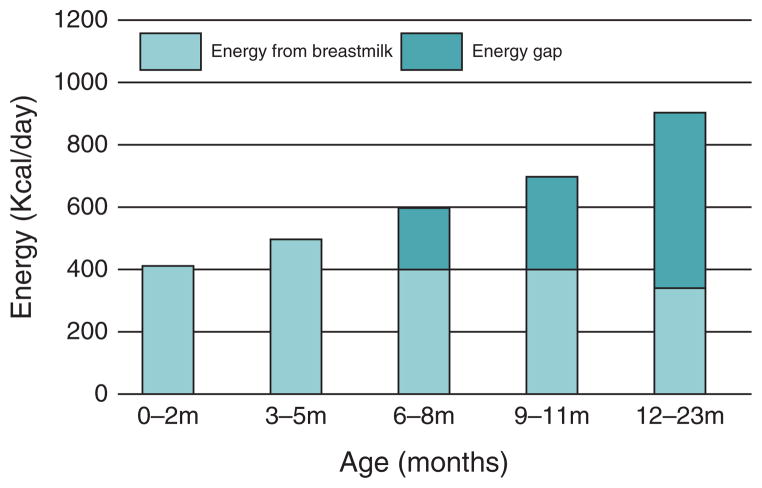
For the first 12 months of life, the high levels of nutrients in breast milk protects against mortality from diarrhoea, pneumonia and malnutrition. However, around the age of 6 months, breast milk alone can no longer meet all nutritional requirements of the infant, and complementary feeding – the transition from exclusive breastfeeding to family foods – is necessary. Although complementary foods provide energy and nutrients to help meet the growing child’s needs, breastfeeding continues to provide at least half of a child’s nutritional requirements between the ages of 6 to 12 months (Fig. 1). Changes in advice on how long women with HIV should breastfeed beyond 6 months and changes in ARV prophylaxis and treatment regimens have been a source of significant confusion for healthcare providers. As recently as 10 years ago, mothers with HIV were advised to stop breastfeeding at 4–6 months [21]. However, evidence of growth failure, increased morbidity from diarrhoea and all-cause mortality among these infants demonstrated that it was not advisable for mothers with HIV to stop breastfeeding at that time [20,22]. The lack of safe alternative milks, the continued burden of infectious diseases and the risk of growth faltering in children 6–12 months of age underscore the on-going value of breastfeeding. Breast-milk contains growth factors such as epidermal growth factor and transforming growth factor β, which may enhance the maturation of the gut epithelial barrier by strengthening its integrity and hindering passage of the virus [23]. Because the risk of HIV transmission decreases as a child’s gut matures, mixed feeding does not carry the same risk after the age of 6 months.
Fig. 1. Energy required by age and provided by breast milk.
Source: WHO. Infant and Young Child Feeding: Model Chapter for Textbooks for Medical Students and Allied Health Professionals. Geneva: WHO; 2009.
ARVs that reduce the risk of HIV transmission through breastfeeding have shifted the risk/benefit analysis in favour of breastfeeding. Several important trials with differing ARV approaches have demonstrated that mothers with HIV can breastfeed safely for longer durations and that this can be lifesaving for their infants. Although longer-term exposure to ARVs may have undiscovered consequences, the best available evidence suggests that the risks of infants dying from other diseases if breastfeeding stops before the age of 1 are greater than the potential side-effects of prolonged drug exposure.
Adherence to complementary feeding guidelines is generally poor in most low-resource contexts [24]. Complementary foods are often introduced too early or too late, with little variety and inadequate portions, and food safety is often poor. As a result, growth faltering often increases dramatically during the complementary feeding period, along with the risk of stunting. Providing complementary foods to HIV-exposed and HIV-infected children is even more challenging as they may lack appetite or suffer from other factors that reduce food intake or increase nutrient losses [8].
Second year of life
Although experts agree that mothers with HIV should breastfeed for at least 1 year, the optimal duration of breastfeeding beyond 12 months is uncertain. Some question the need for any limitation. For the first 12 months of life, the high levels of nutrients and immunologic benefits of breast milk protects against mortality from diarrhoea, pneumonia and malnutrition [Table 1]. While breast milk continues to provide significant health benefits after 12 months, there is a less compelling benefit in terms of reduced mortality because older children are better able to recover from childhood illnesses. Moreover, after 12 months of age, a child’s nutritional needs can be met more easily by family foods that include an age-appropriate amount of cow’s milk.
Table 1.
Composition of breastmilk.
| Component Fats: 3.5 g per 100 ml [docosahexaenoic acid (DHA) and arachidonic acid (ARA)] | Benefit Neurological development |
| Carbohydrates: 7 g per 100 ml (lactose and oligosaccharides) | Energy and protection against infection |
| Protein: 0.9 g per 100 ml (casein, α-lactalbumin) | Growth and development |
| Vitamins: All except K | Optimal growth and development |
| Minerals: Sodium, potassium, calcium, magnesium, phosphorus, chlorine | Optimal growth and development |
| Anti-infective factors: IgA, white blood cells | Immunity and protection against inflammation, bacteria and other pathogens |
| Other bioactive factors: bile-salt stimulated lipase, epidermal growth factor | Digestion, maturity of the infant’s intestinal lining |
Mothers with HIV should be advised to stop breastfeeding only when they can provide ‘a nutritionally adequate and safe diet’ without breast milk after 12 months. Otherwise, they should continue breastfeeding beyond 12 months while remaining on (and adhering to) ARVs. Whenever they decide to stop breastfeeding, weaning should be done gradually, within 1 month, although the optimal approach and length of the cessation process is not known. Weaning abruptly is not advisable, as some studies found it to be is associated with mastitis and elevated viral load in breast milk [25–27]. HIV-infected children can continue breastfeeding for up to 2 years or longer, as breastfeeding increases their chance of survival [1].
Antiretroviral therapy and breastfeeding
To prevent postnatal vertical transmission of HIV, mothers should take ARVs as prophylaxis or as therapy for their own health throughout the duration of breastfeeding to reduce viral load, and their infants should receive ARVs for 4–6 weeks after birth. The 2010 WHO Guidelines on HIV and Infant Feeding reflect evidence of the benefits of both breastfeeding and the ability of ARVs for either mothers or infants during breastfeeding to reduce the risk of postnatal HIV transmission significantly. With these recommendations, ARV prophylaxis during breastfeeding became part of the public health approach to prevention of mother-to-child transmission of HIV (PMTCT). With changes in CD4 eligibility and the introduction of life-long ART for pregnant women regardless of CD4 cell count (Option B+), more pregnant and lactating women with HIV are eligible for ART. These advances can not only prevent postnatal HIV transmission through breastfeeding, but can also reduce transmission risk in future pregnancies while sustaining the health of mothers.
The 2013 WHO Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection maintain that the primary aim of the HIV and infant feeding recommendations is to improve HIV-free survival by reducing the risk of transmission through breastmilk using ARVs, while avoiding malnutrition and the increased risk of serious infections in infants and children from unsafe feeding practices [28]. The efficacy of ARVs to prevent postnatal transmission depends largely on whether women with HIV take ARVs consistently throughout breastfeeding. If treatment is interrupted for any reason, virologic failure and drug resistance may result and lead to increased transmission, morbidity and mortality [29]. Supporting mothers with HIV to remain on ART is essential, given numerous individual and health system challenges. The 2013 guidelines include a chapter on retention across the continuum of care and build the case for investing in improved health systems to provide a quality of care commensurate with the needs of mothers and children. For mothers without access to ARVs, optimal infant feeding remains critical to minimize the risk of vertical transmission.
Antiretroviral therapy and child nutrition
Many recent studies show that ART can lead to significant improvement in both weight and height of HIV-infected children. Its effect on growth appears to be most pronounced in younger children before chronic under-nutrition (stunting) has become permanent, and in children with less severe growth deficiencies at initiation of treatment [30–32]. However, ART alone does not appear to help children achieve full growth recovery or sustain their growth over the long-term [33]. Moreover, a recent study found that children who were underweight when they began ART had worse immunological outcomes [34], highlighting the importance of aggressive case-finding of unrecognized HIV among malnourished children and non-medical strategies such as IYCF counselling and improved water, sanitation and hygiene to avoid growth faltering and improve treatment outcomes.
Food supplementation and fortification for HIV-infected children
HIV-infected children have higher energy needs than HIV-negative children. It is recommended that HIV-infected children consume 10% more energy than HIV-negative children if asymptomatic, 20–30% more energy if they have opportunistic infections and 50–100% more energy during and after episodes of severe acute malnutrition to recover lost weight [35]. Eating more energy-rich food can help HIV-infected children to gain weight. However, poor linear growth has an even stronger association with HIV viral load. The relationship between viral load and growth is not well understood but could involve several disease-related endocrine and metabolic changes. HIV-infected children are also especially vulnerable to the diarrhoeal disease and gastrointestinal infections that are common causes of malnutrition in the general population. Diarrhoea-related nutrient losses, malabsorption and inadequate feeding practices for catch-up growth may all contribute to malnutrition in these children.
A 2010 Cochrane review concluded that vitamin A supplementation is well tolerated and is beneficial for HIV-infected children and that zinc is as well tolerated and is possibly as beneficial for HIV-infected children as for uninfected children. Further trials of other supplements (vitamin D, zinc and selenium) are needed to advance the evidence base and evaluate long-term benefits, adverse effects and optimal formulations of multiple micronutrient supplements [36].
Operational and programmatic challenges to consider
Given the historical confusion about HIV and infant feeding messages, high rates of malnutrition and poor IYCF practices in many settings, programme managers need to consider operational and programmatic challenges at each stage of service delivery to ensure effective, uninterrupted patient-centred care.
At community level
To strengthen the adoption of IYCF counselling recommendations by mothers with HIV and ensure nutrition care and follow-up of HIV-infected children, HIV and nutrition services must reach beyond clinics into communities. Social norms, stigma, gender-based inequities and socioeconomic conditions profoundly influence the ability of women to put messages from healthcare providers into practice. Consistently, poor infant feeding practices in the general population and high rates of nonadherence to drug regimens are evidence of a poor enabling environment and the challenge associated with changing behaviours. In low-resource/high-HIV-burden countries that recommend breastfeeding of HIV-exposed infants, communities must be mobilized to support exclusive breastfeeding and optimal complementary feeding for all infants. Women need support to answer their questions about IYCF, adhere to ART, maintain their own health and provide optimal care to their children. Common questions and knowledge gaps around optimal infant feeding for women with HIV are listed below:
Is it safe for a woman with HIV to breastfeed?
Why is it safe to feed infants a mix of breast milk and other foods after 6 months but not before 6 months?
How long should women with HIV breastfeed?
Do ARVs cause problems during breastfeeding?
Does breastfeeding cause health problems such as too much weight loss for women on ART?
What does ‘gradual weaning’ mean?
When syrup medicines are not available for infants younger than 6 months, should medicine be mixed with breast milk or water?
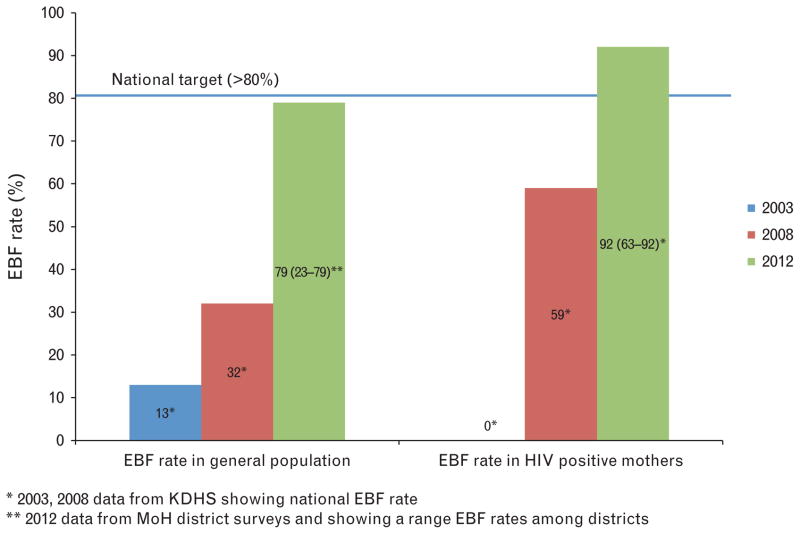
There is programmatic evidence that it is possible to improve EBF rates among mothers with HIV. Exclusive breastfeeding rates increased with intentional acceleration and multi-pronged system strengthening efforts that focused on health facility and community level point of care support (Fig. 2). Such improvement could positively influence infant feeding practices in the general population.
Fig. 2. Rates of exclusive breastfeeding (EBF) in the general population and for HIV-positive mothers in Kenya, 2003–2012.
Source: Kenya Demographic and Health Survey (KDHS) 2003 and 2008; Kenya Ministry of Health (MOH) district surveys, 2012; Kenya National PMTCT evaluation, 2010; and Nyanza Programme data.
Effective mechanisms for tracking mother/infant pairs after they leave the clinic are essential to increase service uptake and retention, improve adherence to ART and IYCF recommendations and monitor infant nutritional and HIV status. Community counselling and follow-up are particularly critical during times of transition in order to avoid confusion about introduction of complementary foods or weaning. Determining whether an adequate diet can be provided without breast milk requires individual support and linkage to food security interventions, particularly when family foods cannot meet the nutritional needs of growing children with additional energy needs caused by HIV.
At health facility level
Healthcare providers need training, job aids and guidance to provide consistent, accurate and culturally acceptable IYCF counselling and services to support pregnant women and new mothers with HIV. Irregular and poor-quality training and supervision can result in mixed or incorrect feeding messages. Healthcare providers also need guidance to counsel and support clients whose situation may need individualized care. Training and implementation plans should address referral linkages between facility services and community care to avoid gaps in support and follow-up.
Growth faltering of HIV-infected infants begins early [37], but few countries have been successful in scaling up early infant testing or keeping infants in appropriate care and treatment for the duration of exposure. Weak health systems, inadequately trained staff and ineffective follow-up mechanisms make it difficult to track infants at risk. Early HIV testing and initiation of ARVs can help infants and young children recover lost weight and height to improve their chances of survival. Countries should urgently scale up efforts to undertake active case- finding of children whose mothers may have been be missed during the antenatal period or were infected during the postnatal period, improve testing and follow-up of all HIV-exposed infants through the weaning period, and ensure that HIV-infected children remain in treatment. Nutrition assessment should be part of all paediatric care and treatment, guided by country contexts and local epidemiology of both malnutrition and HIV. Healthcare providers and volunteers should trained to refer children identified with failure to thrive and severe growth faltering promptly for HIV testing in high-prevalence areas.
At national level
Countries need costed and sufficiently detailed implementation plans to operationalize IYCF guidelines for women with HIV. Plans should be guided by technical experts, stakeholder consensus at all service delivery levels, and collaboration among ministries of health, education and finance to build political will and enable appropriate resource allocation. In 2012, UNICEF assessed implementation of the 2010 WHO HIV and infant feeding guidelines in 25 countries in Africa. The assessment found that 24 of the 25 countries had a national policy on HIV and infant feeding but only 12 had a related implementation plan and only six of those 12 plans were costed [38]. Planning must consider in which settings to reach mothers, how to make services appealing and accessible and how much it will cost to deliver them.
An integrated, real-time monitoring and evaluation (M&E) system for collecting, analyzing and using breastfeeding, replacement feeding and mixed feeding data is a critical component of a national IYCF implementation plan. Such a system is lacking in many countries with high HIV burden. Countries do not routinely report infant feeding practices in their annual reports. Current estimates come from general population surveys such as multiple indicator cluster surveys (MICS) and demographic and health surveys (DHS), which are done every 3 or 5 years and often do not provide sufficient information to identify service gaps or improve programming. National M&E systems also do not adequately document qualitative information such as provision of breastfeeding counselling. IYCF programmes should make more use of qualitative or mixed method studies to obtain reliable data to inform policy and advice programme improvements.
Preliminary data from a WHO-funded pilot study showed that routine data collection on infant feeding practices using 24-hour recall is feasible and reliable [39]. Indicators included the percentage of HIV-exposed infants who received exclusive breastfeeding, exclusive replacement feeding or mixed feeding at 3 months of age and ARV uptake among HIV-exposed breastfed infants. Results showed that infant feeding practices for HIV-exposed infants during the first 6 months can be reported as a national indicator.
Data collection and reporting become more challenging during the complementary feeding period, and effective recording systems are lacking. Proposed IYCF indicators should be integrated into strengthened maternal and child health M&E systems, with the aim of consolidating them into one harmonized system instead of working in parallel.
At global level
Neither the Global AIDS Response Progress Reporting (GARPR) nor President’s Emergency Plan for AIDS Relief (PEPFAR) require implementing partners to share data on infant nutritional status or feeding practices. The UNAIDS Indicator Registry includes four indicators related to IYCF. (The current UNAIDS Indicator Registry includes two WHO indicators on infant feeding: distribution of feeding practices (exclusive breastfeeding, replacement feeding, mixed feeding/other) for infants born to women with HIV and women receiving counseling on infant feeding at the first infant follow-up visit. UNICEF indicators in the registry include distribution of feeding practices as well as the comparative nutritional status of orphans and vulnerable children (OVC) and non-OVC at 4 years of age.) A joint consultative group including WHO, UNAIDS, UNI-CEF, the Global Fund to Prevent AIDS, Tuberculosis and Malaria; the World Bank, WFP, PEPFAR and others, has proposed globally harmonized indicators on HIV-free survival at 12 months of age; maternal and infant nutritional status; and infant feeding status at 3, 6, and 9 months of age. Countries must be encouraged and supported to report annually on these indicators.
IYCF service delivery challenges will become more acute with efforts to roll out lifelong ART to all pregnant women with HIV, regardless of CD4 cell count. As countries review and amend their national PMTCT guidelines, the time is ripe to address implementation challenges by ensuring that IYCF plans, M&E and training are developed in tandem.
Evidence gaps
Existing evidence-based nutrition interventions can improve the nutrition and well being of HIV-infected children, but there has been little research on the impact of specific nutrition interventions on HIV-infected children on ARVs. In 2009, WHO released Guidelines for an Integrated Approach to Nutrition Care of HIV-Infected Children (6 Months–14 Years) to help assess and classify nutritional status and develop nutrition care plans. This was an important step in mainstreaming nutrition into HIV care. WHO needs to update guidance on nutritional support for people with HIV in light of the expanded availability of ARVs and document more evidence on how to reach HIV-infected children successfully in low-resource settings with these proven interventions. Many women drop out of PMTCT programmes during the postnatal period resulting in missed opportunities to test infants for HIV; provide nutrition assessment, counselling and support; and initiate ART before severe growth failure or immune suppression reduces the efficacy of interventions and the odds of survival.
More evidence is needed to inform not only implementation but also HIV prevention, starting in pregnancy, and treatment of malnutrition in HIV-exposed and HIV-infected children. There is little evidence on whether or how nutrition-related care for HIV-infected children should differ from that for HIV-negative children. More scientific evidence is needed to understand the cause of HIV-associated growth faltering and to develop cost-effective interventions to promote adequate height and weight. Programme-based studies on supplementary feeding and nutritional rehabilitation of HIV-positive children are needed to identify practical options to improve linear growth and body composition, and slow disease progression in resource-constrained settings. Evidence is also lacking on the safety and effect on infant growth and development of longer-term exposure to ARVs. Continued pharmacovigilance is necessary to inform the on-going risk-benefit analysis.
Implementation questions will continue to arise as protocols change and trends in lifelong treatment uptake emerge. Women’s ART adherence needs to be tracked throughout the breastfeeding period and beyond. The effects of early infant testing on continuation of breastfeeding also need to be carefully monitored.
Conclusion
It is critical to promote optimal growth, health and development for all infants from birth to 2 years of age. Sub-optimal IYCF and caring practices and high rates of infections have a detrimental impact on health and growth during these important years. For the best possible infant health and development outcomes, all mothers, including those living with HIV must adopt optimal infant feeding practices that maximize protection against early childhood illnesses and lower the risk of HIV infection. Nutrition assessment, counselling and support should be a central part of the care and treatment of HIV-infected children to improve their health and survival outcomes.
Despite the availability of evidence-based recommendations and guidelines, misconceptions and confusion about safer IYCF practices persist in developing countries. Lack of national data coupled with implementation challenges limits the ability of programmes to make appropriate improvements. Governments and implementers should focus on practical and effective approaches to training and supervising healthcare providers; increasing PMTCT and nutrition service uptake; and strengthening health systems, including mechanisms to track mother/infant pairs and national monitoring and reporting of globally agreed infant feeding indicators.
The success of ARVs in lowering HIV transmission and improving maternal treatment is limited by knowledge gaps about optimal maternal regimens, duration of infant prophylaxis, and the short-term and long-term effects on children of prolonged ARV exposure through breast milk. To inform global recommendations, new ART regimens and possibly vaccines, which enable women with HIV to breastfeed without consideration of duration should be identified, and health system interventions that improve adherence to postnatal ARVs and breastfeeding should be tested.
Despite significant progress toward keeping mothers alive and reducing paediatric HIV, challenges remain for operationalizing evidence-based recommendations. Sustained political, financial and scientific commitments are key to eliminate mother-to-child HIV transmission while protecting the health and well being of all mothers and children.
Acknowledgments
The authors would like to acknowledge the contribution of Nazia Akhtar in incorporating the different components of this article.
Footnotes
Conflicts of interest
There are no conflict of interest.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the World Health Organization or the U.S. government including the U.S. Centers for Disease Control and Prevention and Agency for Toxic Substances Disease Registry and the United States Agency for International Development. The authors acknowledge the support of UNICEF and the Canadian International Development Agency (CIDA) whose financial assistance made this series possible and the U.S. President’s Emergency Plan for AIDS Relief for support of contributing staff time.
References
- 1.WHO, UNICEF, UNAIDS, UNFPA. . Guidelines on HIV and infant feeding: Principles and recommendations for infant feeding in the context of HIV and a summary of evidence. Geneva: WHO; 2010. [PubMed] [Google Scholar]
- 2.Adler MR, Brewinski M, Heap AN, Bolu O. The role of the President’s Emergency Plan for AIDS Relief in infant and young child feeding guideline development and program implementation. Adv Exp Med Biol. 2012;743:247–260. doi: 10.1007/978-1-4614-2251-8_18. [DOI] [PubMed] [Google Scholar]
- 3.Desclaux A, Alfieri C. Counseling and choosing between infant-feeding options: Overall limits and local interpretations by healthcare providers and women living with HIV in resource-poor countries (Burkina Faso, Cambodia, Cameroon) Soc Sci Med. 2009;69:821–829. doi: 10.1016/j.socscimed.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Sibeko L, Coutsoudis A, Nzuza S, et al. Mothers’ infant feeding experiences: constraints and supports for optimal feeding in an HIV-impacted urban community in South Africa. Public Health Nutr. 2009;12:1983–1990. doi: 10.1017/S1368980009005199. [DOI] [PubMed] [Google Scholar]
- 5.Filteau S. The HIV-exposed, uninfected African child. Trop Med Int Health. 2009;14:276–287. doi: 10.1111/j.1365-3156.2009.02220.x. [DOI] [PubMed] [Google Scholar]
- 6.Marinda E, Humphrey JH, Iliff PJ, Mutasa K, Nathoo KJ, Piwoz EG, et al. Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J. 2007;26:519–526. doi: 10.1097/01.inf.0000264527.69954.4c. [DOI] [PubMed] [Google Scholar]
- 7.Landes M, van Lettow M, Chan AK, Mayuni I, Schouten EJ, Bedell RA. Mortality and health outcomes of HIV exposed and unexposed children in a PMTCT cohort in Malawi. PloS One. 2012;7:1–7. doi: 10.1371/journal.pone.0047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taha TE, Dallabetta GA, Canner JK, Chiphangwi JD, Liomba G, Hoover DR, et al. The effect of human immunodeficiency virus infection on birthweight and infant and child mortality in urban Malawi. Int J Epidemiol. 1995;24:1022–1029. doi: 10.1093/ije/24.5.1022. [DOI] [PubMed] [Google Scholar]
- 9.Ndirangu J, Newell ML, Bland RM, Thorne C. Maternal HIV infection associated with small-for-gestational age infants but not preterm births: Evidence from rural South Africa. Hum Reprod. 2012;27:1846–1856. doi: 10.1093/humrep/des090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arpadi SM. Growth Failure in HIV-Infected Children. WHO Consultation on Nutrition and HIV/AIDS in Africa: Evidence, Lessons and Recommendations for Action, Durban, South Africa, 10–13 April 2005. Geneva: WHO; 2005. [Google Scholar]
- 11.Benjamin DK, Jr, Miller WC, Benjamin DK, Ryder RW, Weber DJ, Walter E, et al. A comparison of height and weight velocity as part of the composite endpoint in pediatric HIV. AIDS. 2003;17:2331–2336. doi: 10.1097/00002030-200311070-00007. [DOI] [PubMed] [Google Scholar]
- 12.Callens SF, Shabani N, Lusiama J, Lelo P, Kitetele F, Colebunders R, et al. Mortality and associated factors after initiation of pediatric antiretroviral treatment in the Democratic Republic of the Congo. Pediatr Infect Dis J. 2009;28:35–40. doi: 10.1097/INF.0b013e318184eeb9. [DOI] [PubMed] [Google Scholar]
- 13.American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–e841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 14.UNFPA/UNICEF/WHO/UNAIDS. Inter-Agency task Team on Mother-to-Child Transmission of HIV. New Data on the Prevention of Mother-to-Child Transmission of HIV and Their Policy Implications. WHO Technical Consultation; Geneva. 11–13 October 2000; Geneva: WHO; 2001. [Google Scholar]
- 15.WHO. New data on the prevention of mother-to-child transmission of HIV and their policy implications: conclusions and recommendations. Geneva: WHO; 2000. [Google Scholar]
- 16.Homsy J, Moore D, Barasa A, Were W, Likicho C, Waiswa B, et al. Breastfeeding, mother-to-child HIV transmission, and mortality among infants born to HIV-infected women in highly active antiretroviral therapy in rural Uganda. J Acquir Immune Defic Syndr. 2010;53:28–35. doi: 10.1097/QAI.0b013e3181bdf65a. [DOI] [PubMed] [Google Scholar]
- 17.Thior I, Lockman S, Smeaton LM, Shapiro RL, Wester C, Heymann J, et al. Breastfeeding plus infant Ziduvodine prophylaxis for 6 months vs formula feeding plus infant Ziduvodine for 1 month to reduce mother-to-child HIV transmission in Botswana. A randomized trial: The Mashi Study. J Am Med Assoc. 2006;296:794–805. doi: 10.1001/jama.296.7.794. [DOI] [PubMed] [Google Scholar]
- 18.Siegfried N, van der Merwe L, Brocklehurst P, Sint TT. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database of Syst Rev. 2011:7. doi: 10.1002/14651858.CD003510.pub3. [DOI] [PubMed] [Google Scholar]
- 19.Horvath T, Madi BC, Iuppa IM, Kennedy GE, Rutherford GW, Read JS. Interventions for preventing late postnatal mother-to-child transmission of HIV. Cochrane Database of Syst Rev. 2009:1. doi: 10.1002/14651858.CD006734.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO, UNICEF, UNAIDS, UNFPA. HIV and Infant Feeding: New Evidence and Programmatic Experience. Report of a technical consultation held on behalf of the Inter-agency Task Team (IATT) on Prevention of HIV infections in Pregnant Women, Mothers and Their Infants; Geneva, Switzerland. 25–27 October 2006; Geneva: WHO; 2007. [Google Scholar]
- 21.WHO, UNICEF, UNAIDS, UNFPA. . HIV and Infant Feeding: Guidelines for Decision-Makers. Geneva: WHO; 2004. [Google Scholar]
- 22.WHO, UNICEF, UNAIDS, UNFPA. HIV and Infant Feeding: Update based on the technical consultation held on behalf of the Inter-Agency Team (IATT) on Prevention of HIV Infections in Pregnant Women, Mothers and Their Infants; Geneva. 25–27 2006; Geneva: WHO; 2007. [Google Scholar]
- 23.Planchon SM, Martins CAP, Guerrant RL, Roche JK. Regulation of intestinal epithelial barrier function. J Immunol. 1994;153:5730–5739. [PubMed] [Google Scholar]
- 24.Dewey KG, Adu-Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern Child Nutr. 2008;4:24–85. doi: 10.1111/j.1740-8709.2007.00124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willumsen JF, Filteau SM, Coutsoudis A, Newell ML, Rollins NC, Coovadia HM, et al. Breastmilk RNA viral load in HIV-infected South African women: effects of subclinical mastitis and infant feeding. AIDS. 2003;17:407–414. doi: 10.1097/00002030-200302140-00015. [DOI] [PubMed] [Google Scholar]
- 26.Thea DM, Aldrovandi F, Kankasa C, Kasonde P, Decker WD, Semrau K, et al. Postweaning breast milk HIV-1 viral load, blood prolactin levels and breast milk volume. AIDS. 2006;20:1539–1547. doi: 10.1097/01.aids.0000237370.49241.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.John GC, Nduati RW, Mbori-Ngacha DA, Richardson BA, Panteleeff D, Mwata A, et al. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: Association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding and breast infections. J Infect Dis. 2001;183:206–212. doi: 10.1086/317918. [DOI] [PubMed] [Google Scholar]
- 28.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: WHO; 2013. [PubMed] [Google Scholar]
- 29.Sherr L, Lampe FC, Clucas C, Johnson M, Fisher M, Leake Date H, et al. Self-reported nonadherence to ART and virologicaloutome in a multiclinic UK study. AIDS Care. 2010;22:939–945. doi: 10.1080/09540121.2010.482126. [DOI] [PubMed] [Google Scholar]
- 30.Musoke PM, Mudiope P, Barlow-Mosha LN, Ajuna P, Bagenda D, Mubiru MM, et al. Growth, immune, and viral responses in HIV-infected African children receiving highly active antiretroviral therapy: a prospective cohort study. BMC Pediatr. 2010;10:56. doi: 10.1186/1471-2431-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feinstein L, Yotebieng M, Moultrie H, Meyers T, Van Rie A. Effect of baseline immune suppression on growth recovery in HIV-positive South African children receiving antiretroviral treatment. J Acquir Immune Defic Syndr. 2012;61:235–242. doi: 10.1097/QAI.0b013e3182634e09. [DOI] [PubMed] [Google Scholar]
- 32.Aurpibul L, Puthanakit T, Taecharoenkul S, Sirisanthana T, Sirisanthana V. Reversal of growth failure in HIV-infected Thai children treated with nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy. AIDS Patient Care and STDs. 2009;23:1067–1071. doi: 10.1089/apc.2009.0093. [DOI] [PubMed] [Google Scholar]
- 33.Gsponer T, Weigel R, Davies MA, Bolton C, Moutrie H, Vaz P, et al. Variability of growth in children starting anti-retroviral treatment in Southern Africa. Pediatrics. 2012;130:e966–e977. doi: 10.1542/peds.2011-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Omoni AO, Christian PS, Sadoh WE, Okechukwa A, Olateju E, Omoigberale A, et al. Immunologic outcomes of antiretroviral therapy among HIV-infected Nigerian children and its association with early infant feeding and nutritional status at treatment initiation. Pediatr Infect Dis J. 2013;32:e291–e297. doi: 10.1097/INF.0b013e31828b2a2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO. Report of a Technical Consultation, 13–15 May 2003. Geneva: WHO; 2003. Nutrient Requirements for People Living with HIV/AIDS. [Google Scholar]
- 36.Irlam JH, Visser ME, Rollins NN, Siegfried N. Micronutrient supplementation in children and adults with HIV infection. Cochrane Database of Syst Rev. 2010:12. doi: 10.1002/14651858.CD003650.pub3. [DOI] [PubMed] [Google Scholar]
- 37.Webb L, Manji L, Fawzi WF, Villamor E. Time-independent maternal and infant factors and time-dependent infants morbidities including HIV infection contribute to infant growth faltering during the first 2 years of life. J Trop Pediatr. 2009;55:83–90. doi: 10.1093/tropej/fmn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sint TT, Liu M. Progress Tracking of Country Roll-Out of the 2010 WHO HIV and Infant Feeding Guidelines. Presentation at the regional workshop on infant feeding in the context of HIV: The implementation of the 2010 WHO recommendations on infant feeding and HIV; Nairobi, Kenya. 6–9 November 2012. [Google Scholar]
- 39.Elizabeth Glaser Pediatric AIDS Foundation and WHO. Pilot Test of HIV and Infant feeding indicators in Lesotho (draft) 2013. [Google Scholar]