Abstract
There are 3.4 million children infected with HIV worldwide, with up to 2.6 million eligible for treatment under current guidelines. However, roughly 70% of infected children are not receiving live-saving HIV care and treatment. Strengthening case finding through improved diagnosis strategies, and actively linking identified HIV-infected children to care and treatment is essential to ensuring that these children benefit from the care and treatment available to them. Without attention or advocacy, the majority of these children will remain undiagnosed and die from complications of HIV. In this article, we summarize the challenges of identifying HIV-infected infants and children, review currently available evidence and guidance, describe promising new strategies for case finding, and make recommendations for future research and interventions to improve identification of HIV-infected infants and children.
Keywords: case finding, HIV testing, pediatric HIV, PITC
Introduction
Over the past 25 years, tremendous progress has been made in the care and treatment of HIV-infected infants and children. Prior to the availability of antiretroviral therapy (ART), the majority of HIV-infected children suffered from rapidly progressive multisystem disease and early death, with 50% mortality before the age of 2 [1]. As availability of cotrimoxazole prophylaxis and ART increased, outcomes for children dramatically improved in both developed and resource-limited countries [2–4]. Evidence that early initiation of ART could decrease mortality in HIV-infected infants by up to 75% [5] led to the 2010 WHO pediatric treatment guidelines expanding treatment eligibility for all HIV-infected infants less than 2 years regardless of immunologic or clinical staging [6]. Simplified ART initiation criteria and child-friendly pediatric formulations facilitated decentralization of pediatric care and treatment, resulting in an over five-fold increase in the number of children on ART since 2006 when less than 100 000 children were accessing treatment (See Treatment 2.0 paper in this series) [7].
Despite these successes, considerable challenges remain. In 2011, an estimated 230 000 deaths in children under the age of 5 were attributable to HIV [8]. Most of these deaths could have been averted, had these children been identified, diagnosed, and initiated on treatment. Many of these children were born to women who did not access, or incompletely accessed prevention of mother to child transmission (PMTCT) programs. These children are at higher risk of infection, and there are currently limited opportunities for diagnosis or entry into care. Consequently, these children do not present, if they present at all, until they have developed serious, AIDS-defining illnesses. When they finally are diagnosed and initiated on ART, mortality and complication rates remain high [9–11].
There are still 3.4 million children infected with HIV, with up to 2.6 million eligible for treatment as the new 2013 WHO treatment guidelines now calls for universal treatment of all children less than 5 years of age [12]. By the end of 2012, in the 21 countries with the highest burden of HIV, only three of 10 or 630 000 eligible children, had been identified and enrolled in HIV treatment. This highlights the fact that 70% of infected children are not receiving live-saving HIV care and treatment. The Global Plan towards Elimination of New HIV infections Among Children and Keeping Their Mothers Alive proposes 100% treatment coverage for eligible HIV-infected children by 2015 [13]. Strengthening case finding through improved diagnosis strategies, and actively linking identified HIV-infected children to care and treatment is essential to ensuring that these 2.6 million children benefit from care and treatment. Without attention or advocacy, the majority of these children will remain undiagnosed and die from complications of HIV.
In this article, we summarize the challenges of identifying HIV-infected infants and children programs, review currently available evidence and guidance, describe promising new strategies for case finding, and make recommendations for future research and interventions to improve identification of HIV-infected infants and children.
Challenges with case finding of HIV-infected infants and children
Case finding of HIV-infected infants and children is challenging on several levels: caregiver/patient-level challenges; provider/facility-level challenges; and policy-level challenges.
Caregiver/patient-level challenges
Although infants born to a known HIV-infected mother should be screened at 4–6 weeks during the first postnatal visit, less than 20% of HIV-exposed infants in resource-limited settings receive an HIV test [14], and there are high rates of loss to follow-up (LTFU) along the PMTCT cascade (see Linkage and Retention paper in this supplement) [15,16]. In the South African prevention of mother-to-child transmission evaluation (SAPMTCTE) study, only 35% of HIV-infected mothers intended to access early infant diagnosis (EID) services [17]. In Malawi’s Option B+ program, of 8700 HIV-exposed infants discharged from maternity, less than half (43%) were enrolled in follow-up before age 2 months [18]. Millions of HIV-exposed and at-risk children have been lost to care as a result of these missed opportunities.
Once children have been lost from the PMTCT/EID system, there are very few opportunities for testing or reentry into care. Although HIV disease in infancy is generally rapidly progressing, up to one third of infected infants have a more slowly progressing disease with survival into their teens without treatment [19–21]. In most settings, such children rely on caregivers to both consent and bring them for testing [22]. Caregivers may not understand or see the need to have their seemingly healthy children tested, or they may desire to ‘protect the child’ from a stigmatizing diagnosis [23]. A mother may also be hesitant to bring her children for testing because doing so is tantamount to disclosing her own status. She may fear blame or feel guilt over the possibility of having transmitted the virus to her child [24–26].
Testing rates are poor even for older children and adolescents who could theoretically consent and pursue HIV testing on their own. A survey conducted in sub-Saharan Africa showed that only 15% of women and 10% of men aged 15–24 years had ever tested for HIV and knew their status [27]. Documented barriers to testing include distance from testing facilities, long wait times, test kit shortages, fear of stigma, and opposition from partners and family [24,28–30]. As a result of these caregiver and client barriers, many children and youth are not tested until clinically ill, compromising treatment outcomes.
Provider/facility-level challenges
Even if HIV-exposed or ill children are brought to a healthcare facility, additional challenges are encountered at the provider and facility level. Healthcare workers may be disincentivized to offer HIV testing and counseling (HTC) to children due to concerns about privacy and disclosure related to maternal HIV status [31], concerns of increased workload related to performing pretest and posttest counseling [32], or a lack of understanding or training in pediatric counseling and handling pediatric blood specimens [33]. Further, unless provider-initiated counseling and testing (PITC) has been routinely implemented or providers have been sensitized to identifying signs and symptoms of HIV, they may treat children for opportunistic infections on multiple occasions without ever conducting an HIV test [34,35]. Finally, limited numbers of test kits and policies that do not allow rapid tests to be administered by certain cadres of staff may also contribute to reluctance of healthcare workers to test children without overt signs or symptoms of HIV disease [32,36].
Policy-level challenges
Age of consent varies from country to country, and while it is considered best to involve older children in the assent of testing, a legal guardian is usually required to provide formal consent [37]. Most countries set the age of consent at 16, though South Africa and Lesotho allow children 12 years and older to test without parental consent, whereas Burundi and some other countries set the age of consent for medical procedures (including HIV testing) at 21 years [38]. If consent is not available, due to caregiver reluctance or unavailability, or not allowed by the child or adolescent, healthcare workers cannot proceed with testing. Significantly, there are very few published international or national policies, guidelines, or tools addressing pediatric HIV case finding. Without unified policies or guidelines, case finding remains uncoordinated and ineffective.
Guidance on pediatric HIV case finding is limited
Published research, evidence from programs, and guidance around pediatric testing and case finding are limited. The guidance that is available focuses on technical aspects of virologic and antibody-based testing, rather than comprehensive and differentiated strategies to identify undiagnosed children. National HTC guidelines often have limited pediatric content, focusing most on voluntary counseling and testing (VCT) and provider initiated testing and counseling (PITC) and opt-out testing for adults. Though research on pediatric tuberculosis (TB) case finding (including the role of various long-standing and novel diagnostics) is readily available, few studies have specifically looked at multi-faceted strategies for improving case finding of HIV-exposed and HIV-infected children. However, one paper outlines a simple, two-tiered approach to identify children at specific healthcare entry points (Table 1) [14].
Table 1.
Strategy framework to identify previously undiagnosed, HIV-infected children.
| WHERE | Priority areas: can be implemented in existing programming | Second tier: additional resources needed for training, program design |
|---|---|---|
| All settings | Policy of routine testing in inpatient wards, tuberculosis clinics, and malnutrition units Proactive testing of children of adult patients enrolled in HIV care programs |
Testing children in HIV+ social networks and families |
| High-prevalence settings | Testing infants and children at immunization, out-patient settings, or under-5 clinics | Door-to-door testing focused on children |
TB, tuberculosis. Data from [14].
WHO guidance documents offer specific, algorithmic approaches to diagnosing individual children and adolescents, but provide limited guidance on developing or implementing community or population-based case finding strategies for children missed by current testing efforts (Table 2). As case finding and diagnostic strategies are different for children than adults, existing guidelines have limited utility for pediatric providers and programmers.
Table 2.
WHO guidance relating to infant and child HIV testing.
| Title | Recommendations summary | Link |
|---|---|---|
| Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection (2013) |
|
www.who.int/hiv/pub/guidelines/arv2013/en/index.html |
| Planning, Implementing, and Monitoring Home-Based HIV Testing and Counseling (2012) |
|
www.who.int/hiv/pub/vct/home_based_care/en/index.html |
| Service Delivery Approaches to HIV Testing and Counseling (HTC): A Strategic HTC Program Framework (2012) |
|
http://www.who.int/hiv/pub/vct/htc_framework/en/index.html |
| WHO Guidelines on HIV Disclosure and Counseling for Children Up to 12 Years of Age (2011) |
|
www.who.int/hiv/pub/hiv_disclosure/en/index.html |
| HIV Testing for Young Children: Technical Brief (2011) |
|
www.who.int/hiv/pub/vct/WHO_HIV_11_02/en/index.html |
| Operational Guidelines on HIV Testing and Counseling of Infants, Children and Adolescents for Service Providers in the African Region (2011) |
|
www.afro.who.int/en/clusters-a-programmes/dpc/acquired-immune-deficiency-syndrome |
| Policy requirements for HIV testing and counseling of infants and young children in health facilities (2010) |
|
www.who.int/hiv/pub/paediatric/testing_counselling/en/ |
HTC, HIV testing and counseling; PITC, provider-initiated counseling and testing; TB, tuberculosis.
Promising strategies for pediatric case finding
Provider-initiated testing and counseling
PITC refers to the routine offering of HIV testing in which the provider offers testing generally in the context of a health visit. Most PITC programs utilize an opt-out approach, in which all patients are tested unless they decline. The high yield of newly diagnosed infants and children resulting from PITC on inpatient pediatric wards has been well described [35,39,40]. At the main teaching hospital in Lusaka, Zambia, 999 of 3964 (25%) of HIV tests done in children older than 18 months were positive [39]. In Lilongwe, Malawi, PITC in an inpatient pediatric ward led to the identification of 525 of 5465 (8.5%) HIV-infected and 405 of 5465 (6.5%) HIV-exposed children [40]. Based on this evidence, in high prevalence settings, all children admitted to pediatric wards or malnutrition units should be tested for HIV [41]. However, despite the efficiency of PITC in finding previously undiagnosed children, mortality rates remain high among children diagnosed as inpatients and after discharge, likely reflecting late diagnosis [42].
Even in facilities in which PITC is offered, high patient volumes, staffing limitations, shortage of appropriate counseling space, and test kit stockouts have compromised effectiveness [32,34,43,44]. In addition, with current healthcare worker capacity, PITC may be difficult to implement in many settings without overwhelming fragile care delivery systems. In many settings, only one or two nurses are available in high burden primary health clinics and adding PITC responsibilities would be burdensome and unmanageable. Task shifting counseling and testing to lower cadres of health workers staff such as community health workers, discussed below, may be effective in easing that burden.
Integrated management of childhood illness screening
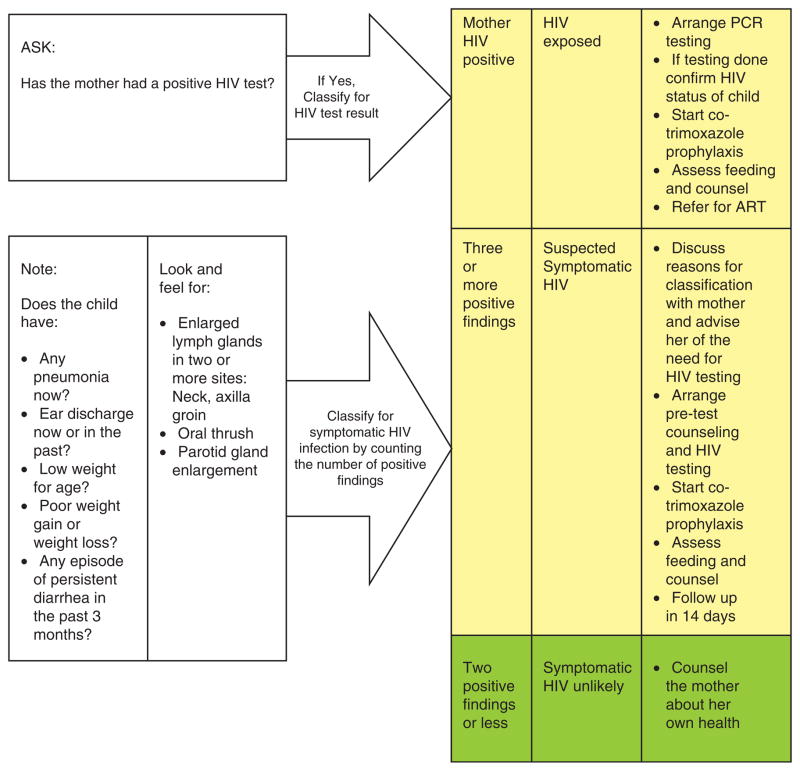
PITC is likely the optimal approach to identifying HIV-infected children in healthcare settings. However, when PITC is not the norm, healthcare workers must be prompted to suggest testing for children. The Integrated Management of Childhood Illness (IMCI) guidelines now provide an algorithm to assess children’s HIV risk, prompting healthcare workers to test children when they present with ‘signs and symptoms suggestive of HIV’ (Fig. 1). However, actual implementation of this algorithm is sporadic. A South African study observing healthcare workers who had been specifically trained on the screening algorithms failed to classify 40% of children with significant risk [45]. Even when the classification system is used correctly and captures children with HIV signs or symptoms, by definition these children are symptomatic and likely have later stage disease and, therefore, worse outcomes [46].
Fig. 1.
The Integrated Management of Childhood Illness assessment and classification for HIV infection.
Presumptive diagnosis of HIV in infants
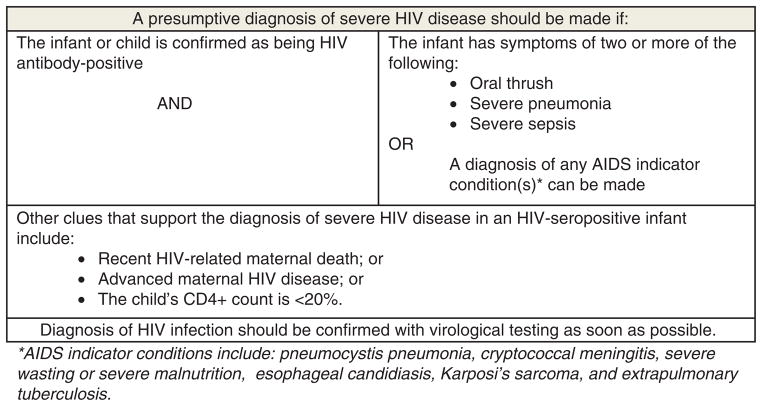
Because access to virologic testing and timely return of results is still limited or problematic in many settings, alternative diagnostic algorithms are necessary to identify infected infants and children. Studies evaluating the currently available WHO clinical criteria to make a presumptive diagnosis of HIV infection in the absence of virologic testing (Fig. 2) have suggested that although the approach may have limited sensitivity (ranging from 19 to 23%), specificity is reasonably high (ranging from 88 to 94%) [47–49]. Though presumptive diagnosis using this algorithm may miss quite a few HIV-infected children and would be inadequate as a general screening tool, in the absence of virologic testing, it does provide clinicians with reassurance when initiating ART in HIV-exposed infants. At the least, presumptive diagnosis strategies should be individualized and discussed with caregivers, because although the risk of ART-associated toxicity is low, it is not insignificant. Sensitivity of this approach may be improved to 70–80% by combining antibody testing with clinical criteria and CD4+ cell measurements [48,50]. However, in settings in which virologic testing is absent, CD4+ cell measurement is often also unavailable.
Fig. 2. WHO clinical algorithm to identify severe HIV infection needing antiretroviral therapy.
*AIDS indicator conditions include the following: pneumocystis pneumonia, cryptococcal meningitis, severe wasting or severe malnutrition, esophageal candidiasis, Kaposi’s sarcoma, and extrapulmonary tuberculosis.
HIV screening at immunization clinics
Routine HIV screening for all infants attending immunization or under-5 clinics is a promising strategy for identification of HIV-exposed and HIV-infected infants in high-burden areas with insufficient PMTCT, or for those children whose mothers seroconverted late in pregnancy or during breastfeeding. In a pilot anonymous surveillance program at immunization clinics in Kwazulu Natal, 188 of 2489 (7.5%) of infants screened by DNA PCR were HIV-infected [51]. In a follow-up study in which results were communicated to patients, 54 of 584 (9.2%) of infants had positive DNA PCRs, but only 332 of 584 (56.8%) returned to receive their results [52]. At two clinics in Malawi, 1757 infants were screened to identify 101 exposed children eligible for PCR; five of 70 (7.1%) tested at immunization clinic and nine of 28 (32.1%) tested at under-5 clinic were HIV-infected [53]. In a population-based seroprevalence survey of infants presenting for their first immunization visit in Malawi, HIV prevalence among children was 1.2% [54]. The effectiveness and additional yield depends on how many mothers and infants were missed by PMTCT. As the integration and strengthening of PMTCT and EID programs improve, the efficacy of this type of screening will likely decrease [55,56]. Also, with immunization clinic screening, identification takes place after opportunities for effective prevention interventions have already passed, so from a prevention-focused perspective, it remains unsatisfactory (See PMTCT paper in this series). Finally, with extended breastfeeding, immunization clinic screening is less effective in capturing children during their entire exposure period, that is, until weaning from breastfeeding occurs. Ultimately, the utility of immunization clinic screening for case finding may be effective to evaluate HIV-free survival, and thus the effectiveness of current PMTCT programming, as was recently done in Malawi [54].
School-based testing campaigns
Primary school-based HIV testing campaigns with development of school-based ART clinics have proven effective in some settings. Mass HIV testing campaigns conducted in Kenya, Tanzania, and Malawi are acceptable and effective for identifying HIV-infected older children [57,58]. In Kenya, 47 173 people were tested for HIV and received their results, with identification of 1964 (4%) positive cases [59]. School-based national testing campaigns for children may have similar impact given that after immunization clinics, schools are the only other venue where large numbers of children congregate [60,61]. Recent studies suggest a considerable proportion of unidentified, untreated HIV-infected children may survive beyond 10 years of age [19–21], and school-based testing may be effective in identifying children and adolescents infected through horizontal transmission as well [26]. Further, such campaigns may provide opportunities for HIV education and can help reduce HIV-related stigma and discrimination as has been found with home-based VCT campaigns [62,63]. A criticism of mass testing campaigns is the lack of focus on linkage to care of those newly diagnosed [64], but establishment of school-based HIV/ART clinics could facilitate such linkage, improve retention in care, and reduce absenteeism from school [65].
Community-based case finding
Innovative community-based models for HTC are feasible, reduce disparities in access to testing services, and are cost-effective for increasing HIV testing among previously unreached populations [66–69]. Home-based HIV testing, compared with VCT and PITC can identify symptomatic and asymptomatic patients earlier in their disease [70]. While the majority of these programs have focused on adults [63,68,70–75], pilot projects focusing on children are demonstrating similar efficacy. The Tingathe Program in Malawi utilizes community health workers to conduct community-based HIV testing and case finding resulting in more than 2500 HIV-exposed and HIV-infected children enrolled in care from 2008 to 2011. A 10-fold increase in enrollment of HIV-infected children into care from 3.2/month preintervention to 32.7/month postintervention was observed [55,76,77]. In Tanzania, a community-based testing campaign focused on children called Know Your Child’s Status conducted 2994 tests in children with 108 new cases identified (3.6%) [78]. In Cote d’Ivoire, the use of community counselors led to an increase in exposed infants receiving prophylaxis rise from 30 to 100% within 5 months of implementation [79]. There are several limitations to this approach as well. Community-based case finding generally has a lower yield than health facility-based testing [80], activities are often difficult to supervise, quality assurance of testing can be challenging in the field, and linkage to care after community-based diagnosis is often suboptimal [77].
Family testing and counseling
Testing the children of index adult cases is a particularly powerful intervention. A survey conducted in Malawi reported greater than 80% of children of adult patients on ART had not been tested for HIV [81]. Targeted family testing of all family members of known HIV-infected adults can provide early case finding for HIV-exposed and HIV-infected children [81]. This may be extended to include family testing for adults presenting with TB or sexually transmitted infections in settings in which coinfection rates are high. Given that these children are at high risk of HIV exposure, ensuring that children of HIV-infected adults are tested may be a targeted, cost-effective strategy.
Further, several studies have demonstrated that couples counseling, in which spouses and partners are simultaneously tested with immediate disclosure of results facilitates disclosure, enhances communication about HIV within couples, and reduces high-risk behavior [82–84]. Family-based HIV testing may have similar impact. Several studies have demonstrated that children play a vital role within households for coping with the impact of HIV-AIDS. Campaigns utilizing family counseling may yield increased case finding of pediatric HIV infection while facilitating disclosure and communication within the family [85–87].
Social network and orphan and vulnerable children HIV testing and counseling
Variations on social network testing, in which HIV-infected persons identify others in their peer group for prevention interventions may be an innovative approach to identifying at-risk children. In one US study, this method resulted in HIV-positivity rates of 5.6 compared with 1% prevalence from traditional outreach strategies [88]. Such targeted outreach or clinic-based testing can be used for children at higher risk such as orphaned, homeless, and other vulnerable children and their peers.
Task shifting
Task shifting, which delegates tasks to the least costly healthcare worker, is especially suited to settings with limited human resource capacity [89–92]. Several pilot programs have demonstrated the efficacy of using community health workers and lay counselors for HIV testing in inpatient and community settings resulting in significant increases in identification of HIV-infected children [40,42,55,77]. Potential barriers to this approach include training and supervision needs, remuneration and retention of this cadre, and how best to integrate these workers into existing healthcare systems [93,94]. In addition, it is known that the reliability of HIV rapid tests can be significantly affected by the user, so assurance of quality of care by lay counselors is essential [95–97]. In Malawi, where community health worker level counselors conduct a majority of testing, the Ministry of Health is proactively addressing the issue with a broad retraining of HIV counselors and development of strict quality control procedures [98]. Other countries considering task shifting may need to similarly develop standardized HIV testing guidelines, national training and supervision programs, and stringent quality assurance systems.
Considerations for low-prevalence settings and concentrated epidemics
The approaches discussed thus far are most relevant for high HIV-prevalence settings. However, case finding for HIV-infected children is still important in low burden settings. The de facto strategy of waiting to test only those children presenting with advanced clinical disease is inadequate.
While routine testing of pregnant women in antenatal clinic [99] and expanded testing for adults [100–102] is cost effective even in low-prevalence settings, there has been little discussion of designing a similar program for infants and children. Extrapolating from adult experience, opt-out PITC should be routinely offered to children of known HIV-infected adults, those admitted for inpatient care, presenting at TB clinics, malnutrition clinics, or for orphan and vulnerable (OVC) services. In outpatient clinics, testing may be targeted toward children who are symptomatic or who present with a history of possible or known exposure [51].
Many concentrated epidemics are associated with marginalized populations (e.g. intravenous drug users, commercial sex workers) who may be less likely to access routine healthcare or fear stigmatization when they do present for care [103–105]. Children of members of marginalized populations are at much higher risk for infection and like their parents, unlikely to be enrolled in care. Programs that aim to reach marginalized populations must include the testing of children, as they may not be reached through routine health services.
Finally, screening tools that use clinical and epidemiological data may be useful in regions with concentrated HIV epidemics. One program in India developed a weighted scoring system utilizing common clinical presentations as well as local epidemiology and was able to achieve high sensitivity and specificity (95.7 and 98.6%, respectively) in detecting children with HIV infection [106].
Conclusion
Despite expanded scale up of PMTCT services in recent years, millions of children living with HIV remain undiagnosed or present late in the course of their disease. Current case finding efforts for these children, particularly outside of the PMTCT continuum, are inadequate with no clear strategy from international agencies, national ministries of health, and implementers. Steps to improve case finding for HIV-infected children are necessary; it is unconscionable to simply wait for this hidden epidemic to die off.
Global funding agencies should support clinical trials, operational research, program development, and cost–effectiveness studies focused on novel strategies for case finding, and linkage and retention in care of HIV-infected children. A formal consultation by international organizations and agencies to develop guidelines for case finding, linkage and retention in care for affected children is also needed. These guidelines would provide up-to-date knowledge about case finding strategies for HIV-infected children in both high and low-prevalence settings and practical guides and tools for program managers. They would not only highlight the need for case finding efforts, but also assist national programs in implementation of HTC, VCT, PITC, immunization clinic screening, and community-based case finding.
On the basis of current evidence and best practices, we recommend the following five key steps toward immediately improving pediatric HIV case finding:
Operationalizing ‘opt-out’, PITC in all high-risk pediatric healthcare settings focusing on in-patient wards, malnutrition treatment centers, TB clinics, and OVC programs. HTC in these settings should be routine, either through a set of standing clinician orders, and/or as part of the facility standard operating procedures.
Targeted family testing and social network testing to identify high-risk infants, children, and youth.
Active tracing and enrollment of all HIV-infected infants and children into HIV care and treatment.
Advocating for national Ministries of Health to update policies to facilitate pediatric case finding. This includes revisiting the age of consent for HIV testing to allow youth 12 years of age or older to give consent for testing, as has been done in South Africa and Lesotho. Additionally, facilitating task shifting for HIV counseling and testing to all cadres of healthcare workers in affected countries would allow more access to expanded pediatric testing.
In high-prevalence settings, implementing universal HIV screening at immunization clinics, and supporting community and home-based HIV testing programs addressing children as well as adults.
If these five steps can be taken, we will make a major impact on identifying, linking, and initiating HIV-infected infants and children.
Acknowledgments
The authors would like to thank Katharine Yen for assistance with literature review and Henry Miller for expert editorial support.
Footnotes
Conflicts of interest
There are no conflicts of interest.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the World Health Organization or the U.S. government including the U.S. Centers for Disease Control and Prevention and Agency for Toxic Substances Disease Registry and the United States Agency for International Development. The authors acknowledge the support of UNICEF and the Canadian International Development Agency (CIDA) whose financial assistance made this series possible and the U.S. President’s Emergency Plan for AIDS Relief for support of contributing staff time.
References
- 1.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 2.Chintu C, Bhat GJ, Walker AS, Mulenga V, Sinyinza F, Lishimpi K, et al. Co-trimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomised placebo-controlled trial. Lancet. 2004;364:1865–1871. doi: 10.1016/S0140-6736(04)17442-4. [DOI] [PubMed] [Google Scholar]
- 3.Kabue MM, Buck WC, Wanless SR, Cox CM, McCollurg ED, Caviness AC, et al. Mortality and clinical outcomes in HIV-infected children on antiretroviral therapy in Malawi, Lesotho, and Swaziland. Pediatrics. 2012;130:e591–e599. doi: 10.1542/peds.2011-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brady MT, Oleske JM, Williams PL, Elgie C, Mofenson LM, Dankner WM, et al. Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J Acquir Immune Defic Syndr. 2010;53:86–94. doi: 10.1097/QAI.0b013e3181b9869f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Antiretroviral therapy for HIV infection in infants and children: towards universal access. Recommendations for a public health approach. Geneva, Switzerland: World Health Organization; 2010. [PubMed] [Google Scholar]
- 7.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global report: UNAIDS Report on the Global AIDS Epidemic. 2012. [Google Scholar]
- 8.Joint United Nations Programme on HIV/AIDS (UNAIDS) 2013 progress report on the global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. 2013. [Google Scholar]
- 9.Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8:477–489. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- 10.Charlebois ED, Ruel TD, Gasasira AF, Achan J, Kateera F, Akello C, et al. Short-term risk of HIV disease progression and death in Ugandan children not eligible for antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;55:330–335. doi: 10.1097/QAI.0b013e3181e583da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newell ML, Patel D, Goetghebuer T, Thorne C. CD4 cell response to antiretroviral therapy in children with vertically acquired HIV infection: is it associated with age at initiation? J Infect Dis. 2006;193:954–962. doi: 10.1086/500842. [DOI] [PubMed] [Google Scholar]
- 12.Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. WHO; 2013. [accessed 20 September 2013]. http://www.who.int/hiv/pub/guidelines/arv2013/download/en/index.html. [PubMed] [Google Scholar]
- 13.Joint United Nations Programme on HIV/AIDS (UNAIDS) Countdown to zero: global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. 2011 [Google Scholar]
- 14.Kellerman S, Essajee S. HIV testing for children in resource-limited settings: what are we waiting for? PLoS Med. 2010;7:e1000285. doi: 10.1371/journal.pmed.1000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wettstein C, Mugglin C, Egger M, Blaser N, Vizcaya LS, Estill J, et al. Missed opportunities to prevent mother-to-child-transmission: systematic review and meta-analysis. AIDS. 2012;26:2361–2373. doi: 10.1097/QAD.0b013e328359ab0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gourlay A, Birdthistle I, Mburu G, Iorpenda K, Wringe A. Barriers and facilitating factors to the uptake of antiretroviral drugs for prevention of mother-to-child transmission of HIV in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2013;16:18588. doi: 10.7448/IAS.16.1.18588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goga AE, Dinh TH, Jackson DJ for the SAPMTCTE study group. Evaluation of the Effectiveness of the National Prevention of Mother-to-Child Transmission (PMTCT) Programme Measured at Six Weeks Postpartum in South Africa 2010. South African Medical Research Council, National Department of Health of South Africa and PEPFAR/US Centers for Disease Control and Prevention; 2012. [Google Scholar]
- 18.Malawi Ministry of Health. Integrated HIV program Report; July-September 2012; Lilongwe, Malawi. 2012. [Google Scholar]
- 19.Ferrand RA, Corbett EL, Wood R, Hargrove J, Ndhlovu CE, Cowan FM, et al. AIDS among older children and adolescents in southern Africa: projecting the time course and magnitude of the epidemic. AIDS. 2009;23:2039–2046. doi: 10.1097/QAD.0b013e32833016ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrand RA, Luethy R, Bwakura F, Mujuru H, Miller RF, Corbett EL. HIV infection presenting in older children and adolescents: a case series from Harare, Zimbabwe. Clin Infect Dis. 2007;44:874–878. doi: 10.1086/511873. [DOI] [PubMed] [Google Scholar]
- 21.Ferrand RA, Munaiwa L, Matsekete J, Bandason T, Nathoo K, Ndhlovu CE, et al. Undiagnosed HIV infection among adolescents seeking primary healthcare in Zimbabwe. Clin Infect Dis. 2010;51:844–851. doi: 10.1086/656361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donahue MC, Dube Q, Dow A, Umar E, Van Rie A. ’They have already thrown away their chicken’: barriers affecting participation by HIV-infected women in care and treatment programs for their infants in Blantyre, Malawi. AIDS care. 2012;24:1233–1239. doi: 10.1080/09540121.2012.656570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vreeman RC, Nyandiko WM, Braitstein P, et al. Acceptance of HIV testing for children ages 18 months to 13 years identified through voluntary, home-based HIV counseling and testing in western Kenya. J Acquir Immune Defic Syndr. 2010;55:e3–e10. doi: 10.1097/QAI.0b013e3181f0758f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vernooij E, Hardon A. ’What mother wouldn’t want to save her baby?’ HIV testing and counselling practices in a rural Ugandan antenatal clinic. Cult Health Sex. 2013:1–14. doi: 10.1080/13691058.2012.758314. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Yeap AD, Hamilton R, Charalambous S, Dwadwa T, Churchyard GJ, Geissler PW, Grant AD. Factors influencing uptake of HIV care and treatment among children in South Africa: a qualitative study of caregivers and clinic staff. AIDS Care. 2010;22:1101–1107. doi: 10.1080/09540121003602218. [DOI] [PubMed] [Google Scholar]
- 26.Bandason T, Langhaug LF, Makamba M, Laver S, Hatzold K, Mahere S, et al. Burden of HIV among primary school children and feasibility of primary school-linked HIV testing in Harare, Zimbabwe: a mixed methods study. AIDS Care. 2013:1–7. doi: 10.1080/09540121.2013.780120. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.UNICEF, UNESCO, UNFPA, ILO, WHO and World Bank. Opportunity in crisis: preventing HIV from early adolescence to early adulthood. New York: UNICEF; 2011. [Google Scholar]
- 28.Nkuoh GN, Meyer DJ, Nshom EM. Women’s attitudes toward their partners’ involvement in antenatal care and prevention of mother-to-child transmission of HIV in Cameroon, Africa. J Midwifery Womens Health. 2013;58:83–91. doi: 10.1111/j.1542-2011.2012.00208.x. [DOI] [PubMed] [Google Scholar]
- 29.Nkuoh GN, Meyer DJ, Tih PM, Nkfusai J. Barriers to men’s participation in antenatal and prevention of mother-to-child HIV transmission care in Cameroon, Africa. J Midwifery Womens Health. 2010;55:363–369. doi: 10.1016/j.jmwh.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Abrams EJ, Simonds RJ, Modi S, Rivadeneira E, Vaz R, Kankasa C, et al. PEPFAR scale-up of pediatric HIV services: innovations, achievements, and challenges. J Acquir Immune Defic Syndr. 2012;60 (Suppl 3):S105–S112. doi: 10.1097/QAI.0b013e31825cf4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bharat S, Mahendra VS. Meeting the sexual and reproductive health needs of people living with HIV: challenges for health-care providers. Reprod Health Matters. 2007;15 (Suppl 29):93–112. doi: 10.1016/S0968-8080(07)29030-5. [DOI] [PubMed] [Google Scholar]
- 32.Ntuli AK, Kabengula JS, Msuya SE. Perceived barriers and attitudes of healthcare providers towards Provider-Initiated HIV Testing and Counseling in Mbeya region, southern highland zone of Tanzania. Pan Afr Med J. 2011;8:17. doi: 10.4314/pamj.v8i1.71070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horwood C, Voce A, Vermaak K, Rollins N, Qazi S. Routine checks for HIV in children attending primary healthcare facilities in South Africa: attitudes of nurses and child care-givers. Soc Sci Med. 2010;70:313–320. doi: 10.1016/j.socscimed.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 34.MacPherson P, Lalloo DG, Choko AT, Mann GH, Squire SB, Mwale D, et al. Suboptimal patterns of provider initiated HIV testing and counselling, antiretroviral therapy eligibility assessment and referral in primary health clinic attendees in Blantyre, Malawi. Trop Med Int Health. 2012;17:507–517. doi: 10.1111/j.1365-3156.2011.02946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mutanga JN, Raymond J, Towle MS, Mutemb S, Fubisha RC, Lule F, Muhe L. Institutionalizing provider-initiated HIV testing and counselling for children: an observational case study from Zambia. PloS One. 2012;7:e29656. doi: 10.1371/journal.pone.0029656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rujumba J, Tumwine JK, Tylleskar T, Neema S, Heggenhougen HK. Listening to health workers: lessons from Eastern Uganda for strengthening the programme for the prevention of mother-to-child transmission of HIV. BMC Health Services Res. 2012;12:3. doi: 10.1186/1472-6963-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.WHO. HIV testing for young children: technical brief (2011) 2011. [Google Scholar]
- 38.Global Commission on HIV and the Law. Regional issues brief: children, HIV and the law. For the Africa Regional Dialogue of the Global Commission on HIV and the Law; 11 April 2011; Pretoria, South Africa. 2011. [Google Scholar]
- 39.Kankasa C, Carter RJ, Briggs N, Bulterys M, Chama E, Cooper ER, et al. Routine offering of HIV testing to hospitalized pediatric patients at university teaching hospital, Lusaka, Zambia: acceptability and feasibility. J Acquir Immune Defic Syndr. 2009;51:202–208. doi: 10.1097/qai.0b013e31819c173f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCollum ED, Preidis GA, Kabue MM, Singogo EB, Mwansambo C, Kazembe PN, Kline MW. Task shifting routine inpatient pediatric HIV testing improves program outcomes in urban Malawi: a retrospective observational study. PloS One. 2010;5:e9626. doi: 10.1371/journal.pone.0009626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Rie A, Sabue M, Jarrett N, Westreich D, Behets F, Kokolomani J, Bahati ER. Counseling and testing TB patients for HIV: evaluation of three implementation models in Kinshasa, Congo. Int J Tuberc Lung Dis. 2008;12 (3 Suppl 1):73–78. [PubMed] [Google Scholar]
- 42.McCollum ED, Preidis GA, Golitko CL, Siwande LD, Mwansambo C, Kazembe PN, et al. Routine inpatient human immunodeficiency virus testing system increases access to pediatric human immunodeficiency virus care in sub-Saharan Africa. Pediatr Infect Dis J. 2011;30:e75–e81. doi: 10.1097/INF.0b013e3182103f8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sibanda EL, Hatzold K, Mugurungi O, Ncube G, Dupwa B, Siraha P, et al. An assessment of the Zimbabwe ministry of health and child welfare provider initiated HIV testing and counselling programme. BMC Health Services Res. 2012;12:131. doi: 10.1186/1472-6963-12-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dalal S, Lee CW, Farirai T, Schilsky A, Goldman T, Moore J, Bock NN. Provider-initiated HIV testing and counseling: increased uptake in two public community health centers in South Africa and implications for scale-up. PloS One. 2011;6:e27293. doi: 10.1371/journal.pone.0027293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horwood C, Vermaak K, Rollins N, Haskins L, Nkosi P, Qazi S. Paediatric HIV management at primary care level: an evaluation of the integrated management of childhood illness (IMCI) guidelines for HIV. BMC Pediatr. 2009;9:59. doi: 10.1186/1471-2431-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitescarter J, Miotti P, Bazin B, Blesson S, Arrivé E, Marquis B, et al. Low risk of death, but substantial program attrition, in pediatric HIV treatment cohorts in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2008;49:523–531. doi: 10.1097/QAI.0b013e31818aadce. [DOI] [PubMed] [Google Scholar]
- 47.Jones SA, Sherman GG, Coovadia AH. Can clinical algorithms deliver an accurate diagnosis of HIV infection in infancy? Bull World Health Organ. 2005;83:559–560. [PMC free article] [PubMed] [Google Scholar]
- 48.Grundmann N, Iliff P, Stringer J, Wilfert C. Presumptive diagnosis of severe HIV infection to determine the need for antiretroviral therapy in children less than 18 months of age. Bull World Health Organ. 2011;89:513–520. doi: 10.2471/BLT.11.085977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inwani I, Mbori-Ngacha D, Nduati R, Obimbo E, Wamalwa D, John-Stewart G, Farquhar C. Performance of clinical algorithms for HIV-1 diagnosis and antiretroviral initiation among HIV-1-exposed children aged less than 18 months in Kenya. J Acquir Immune Defic Syndr. 2009;50:492–498. doi: 10.1097/QAI.0b013e318198a8a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mutesu-Kapembwa K, Andrews B, Kapembwa K, Chi BH, Banda Y, Mulenga V, Kankasa C. Performance of modified WHO presumptive criteria for diagnosis of HIV infection in children <18 months admitted to University Teaching Hospital in Lusaka. Med J Zambia. 2010;37:64–70. [PMC free article] [PubMed] [Google Scholar]
- 51.Rollins N, Little K, Mzolo S, Horwood C, Newell ML. Surveillance of mother-to-child transmission prevention programmes at immunization clinics: the case for universal screening. AIDS. 2007;21:1341–1347. doi: 10.1097/QAD.0b013e32814db7d4. [DOI] [PubMed] [Google Scholar]
- 52.Rollins N, Mzolo S, Moodley T, Esterhuizen T, van Rooyen H. Universal HIV testing of infants at immunization clinics: an acceptable and feasible approach for early infant diagnosis in high HIV prevalence settings. AIDS. 2009;23:1851–1857. doi: 10.1097/QAD.0b013e32832d84fd. [DOI] [PubMed] [Google Scholar]
- 53.McCollum ED, Johnson DC, Chasela CS, Siwande LD, Kazembe PN, Olson D, et al. Superior uptake and outcomes of early infant diagnosis of HIV services at an immunization clinic versus an ‘under-five’ general pediatric clinic in Malawi. J Acquir Immune Defic Syndr. 2012;60:e107–e110. doi: 10.1097/QAI.0b013e31825aa721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sinunu MASE, Wadonda N, Kajowa E, Eliya M, Moyo K, Chimbwandira F, Strunin L, Kellerman SE. Evaluating the impact of prevention of mother-to-child transmission of HIV in Malawi through immunization clinic-based surveillance. International AIDS Conference; Kuala Lumpur. 2013; p. abstract no. TUPE277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim MH, Ahmed S, Buck WC, Preidis GA, Hosseinipour MC, Bhalakia A, et al. The Tingathe programme: a pilot intervention using community health workers to create a continuum of care in the prevention of mother to child transmission of HIV (PMTCT) cascade of services in Malawi. J Int AIDS Soc. 2012;15 (Suppl 2):17389. doi: 10.7448/IAS.15.4.17389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ciampa PJ, Burlison JR, Blevins M, Sidat M, Moon TD, Rothman RL, Vermund SH. Improving retention in the early infant diagnosis of HIV program in rural Mozambique by better service integration. J Acquir Immune Defic Syndr. 2011;58:115–119. doi: 10.1097/QAI.0b013e31822149bf. [DOI] [PubMed] [Google Scholar]
- 57.Chamie G, Kwarisiima D, Clark TD, Kabami J, Jain V, Geng E, et al. Leveraging rapid community-based HIV testing campaigns for non-communicable diseases in rural Uganda. PloS One. 2012;7:e43400. doi: 10.1371/journal.pone.0043400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Granich R, Muraguri N, Doyen A, Garg N, Williams BG. Achieving universal access for human immunodeficiency virus and tuberculosis: potential prevention impact of an integrated multidisease prevention campaign in Kenya. AIDS Res Treat. 2012;2012:412643. doi: 10.1155/2012/412643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lugada E, Millar D, Haskew J, Grabowsky M, Garg N, Vestergaard M, et al. Rapid implementation of an integrated large-scale HIV counseling and testing, malaria, and diarrhea prevention campaign in rural Kenya. PloS One. 2010;5:e12435. doi: 10.1371/journal.pone.0012435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haddison EC, Nguefack-Tsague G, Noubom M, Mbatcham W, Ndumbe PM, Mbopi-Keou FX. Voluntary counseling and testing for HIV among high school students in the Tiko health district, Cameroon. Pan Afr Med J. 2012;13:18. [PMC free article] [PubMed] [Google Scholar]
- 61.Kharsany AB, Mlotshwa M, Frohlich JA, Zuma NY, Samsunder N, Karim SS, Karim QA. HIV prevalence among high school learners: opportunities for school-based HIV testing programmes and sexual reproductive health services. World Health Popul. 2012;13:43–50. doi: 10.12927/whp.2012.22966. [DOI] [PubMed] [Google Scholar]
- 62.Fylkesnes K, Sandoy IF, Jurgensen M, Chipimo PJ, Mwangala S, Michelo C. Strong effects of home-based voluntary HIV counselling and testing on acceptance and equity: a cluster randomised trial in Zambia. Soc Sci Med. 2013;86:9–16. doi: 10.1016/j.socscimed.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 63.Jurgensen M, Sandoy IF, Michelo C, Fylkesnes K. Effects of home-based voluntary counselling and testing on HIV-related stigma: findings from a cluster-randomized trial in Zambia. Soc Sci Med. 2013;81:18–25. doi: 10.1016/j.socscimed.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 64.Heywood M. Putting HIV testing to the test: progress, gaps and opportunities. International AIDS Conference; Vienna. 2010. session THBS02. [Google Scholar]
- 65.Souza E, Santos N, Valentini S, Silva G, Falbo A. Long-term follow-up outcomes of perinatally HIV-infected adolescents: infection control but school failure. J Trop Pediatr. 2010;56:421–426. doi: 10.1093/tropej/fmq008. [DOI] [PubMed] [Google Scholar]
- 66.Bateganya M, Abdulwadud OA, Kiene SM. Home-based HIV voluntary counselling and testing (VCT) for improving uptake of HIV testing. Cochrane Database Syst Rev. 2010:CD006493. doi: 10.1002/14651858.CD006493.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Negin J, Wariero J, Mutuo P, Jan S, Pronyk P. Feasibility, acceptability and cost of home-based HIV testing in rural Kenya. Trop Med Int Health. 2009;14:849–855. doi: 10.1111/j.1365-3156.2009.02304.x. [DOI] [PubMed] [Google Scholar]
- 68.Helleringer S, Kohler HP, Frimpong JA, Mkandawire J. Increasing uptake of HIV testing and counseling among the poorest in sub-Saharan countries through home-based service provision. J Acquir Immune Defic Syndr. 2009;51:185–193. doi: 10.1097/QAI.0b013e31819c1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lugada E, Levin J, Abang B, Mermin J, Mugalanzi E, Namara G, et al. Comparison of home and clinic-based HIV testing among household members of persons taking antiretroviral therapy in Uganda: results from a randomized trial. J Acquir Immune Defic Syndr. 2010;55:245–252. doi: 10.1097/QAI.0b013e3181e9e069. [DOI] [PubMed] [Google Scholar]
- 70.Wachira J, Kimaiyo S, Ndege S, Mamlin J, Braitstein P. What is the impact of home-based HIV counseling and testing on the clinical status of newly enrolled adults in a large HIV care program in Western Kenya? Clin Infect Dis. 2012;54:275–281. doi: 10.1093/cid/cir789. [DOI] [PubMed] [Google Scholar]
- 71.Menzies N, Abang B, Wanyenze R, Nuwaha F, Muqisha B, Coutinho A, et al. The costs and effectiveness of four HIV counseling and testing strategies in Uganda. AIDS. 2009;23:395–401. doi: 10.1097/QAD.0b013e328321e40b. [DOI] [PubMed] [Google Scholar]
- 72.Grabbe KL, Menzies N, Taegtmeyer M, Emukule G, Angala P, Mwega I, et al. Increasing access to HIV counseling and testing through mobile services in Kenya: strategies, utilization, and cost-effectiveness. J Acquir Immune Defic Syndr. 2010;54:317–323. doi: 10.1097/QAI.0b013e3181ced126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolff B, Nyanzi B, Katongole G, Ssesanga D, Ruberantwari A, Whitworth J. Evaluation of a home-based voluntary counselling and testing intervention in rural Uganda. Health Policy Plan. 2005;20:109–116. doi: 10.1093/heapol/czi013. [DOI] [PubMed] [Google Scholar]
- 74.Fylkesnes K, Siziya S. A randomized trial on acceptability of voluntary HIV counselling and testing. Trop Med Int Health. 2004;9:566–572. doi: 10.1111/j.1365-3156.2004.01231.x. [DOI] [PubMed] [Google Scholar]
- 75.Sweat M, Morin S, Celentano D, Mulawa M, Singh B, Mbwambo J, et al. Community-based intervention to increase HIV testing and case detection in people aged 16–32 years in Tanzania, Zimbabwe, and Thailand (NIMH Project Accept, HPTN 043): a randomised study. Lancet Infect Dis. 2011;11:525–532. doi: 10.1016/S1473-3099(11)70060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahmed S. Using Community Health workers to improve identification, enrollment into care and outcomes for HIV-exposed infants at the Kawale Health Centre in Lilongwe Malawi. AIDS; Vienna: 2010. [Google Scholar]
- 77.Ahmed S, Kanjelo D, Nanthuru D, Cox C, Kautzman M, Golitko C, et al. The Tingathe Program: community health workers improving case finding, enrollement, and care of HIV-exposed and infected children in Malawi. 6th International AIDS Conference; Rome, Italy. 2011. p. Abstract no. MOPE460. [Google Scholar]
- 78.Shea SRM, Makungu S, Sultan I, Minde M, Anosike B, Sanders J, et al. Know your child?s status testing events: a targeted strategy for paediatric HIV case identification in the lake zone of Tanzania. 7th International AIDS Conference; Kuala Lumpur. 2013. p. Abstract no. A-581-0103-03018. [Google Scholar]
- 79.UNAIDS. Promising practices in community engagement for elimination of new HIV infections among children by 2015 and keeping their mothers alive. Geneva: UNAIDS; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ahmed S, Kim MH, Dave AC, Kanjelo K, Kim El-Mallawany N, Kazembe PN. Active case finding: a comparison of home-based testing and health center-based testing for identifying HIV-infected children in Lilongwe, Malawi. 7th Internatinoal AIDS Conference; Kuala Lumpur. 2013. p. Abstract No. TUPE412. [Google Scholar]
- 81.Cohen D, Lungu M, van Oosterhout JJ. HIV testing coverage of family members of adult antiretroviral therapy patients in Malawi. AIDS Care. 2010;22:1346–1349. doi: 10.1080/09540121003720986. [DOI] [PubMed] [Google Scholar]
- 82.Allen S, Karita E, Chomba E, Roth DL, Telfair J, Zulu I, et al. Promotion of couples’ voluntary counselling and testing for HIV through influential networks in two African capital cities. BMC Public Health. 2007;7:349. doi: 10.1186/1471-2458-7-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kelley AL, Karita E, Sullivan PS, Katangulia F, Chomba E, Carael M, et al. Knowledge and perceptions of couples’ voluntary counseling and testing in urban Rwanda and Zambia: a cross-sectional household survey. PloS One. 2011;6:e19573. doi: 10.1371/journal.pone.0019573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wall KM, Kilembe W, Nizam A, Vwalika C, Kautzman M, Chomba E, et al. Promotion of couples’ voluntary HIV counselling and testing in Lusaka, Zambia by influence network leaders and agents. BMJ Open. 2012;2:e001171. doi: 10.1136/bmjopen-2012-001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bond V, Chilikwela L, Simwinga M, Reade Z, Ayles H, Godfrey-Faussett P, Hunleth J. Children’s role in enhanced case finding in Zambia. Int J Tuberc Lung Dis. 2010;14:1280–1287. [PubMed] [Google Scholar]
- 86.Onyango-Ouma W, Aagaard-Hansen J, Jensen BB. The potential of schoolchildren as health change agents in rural western Kenya. Soc Sci Med. 2005;61:1711–1722. doi: 10.1016/j.socscimed.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 87.Ansell N. Children’s migration as a household/family strategy: coping with AIDS in Lesotho and Malawi. J South Afr Stud. 2004;30:673–690. [Google Scholar]
- 88.Kimbrough LW, Fisher HE, Jones KT, Johnson W, Thadiparthi S, Dooley S. Accessing social networks with high rates of undiagnosed HIV infection: the social networks demonstration project. Am J Public Health. 2009;99:1093–1099. doi: 10.2105/AJPH.2008.139329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dalal W, Feikin DR, Amolloh M, Ransom R, Burke H, Lugalia F, et al. Home-based HIV testing and counseling in rural and urban Kenyan communities. J Acquir Immune Defic Syndr. 2013;62:e47–e54. doi: 10.1097/QAI.0b013e318276bea0. [DOI] [PubMed] [Google Scholar]
- 90.McPake B, Mensah K. Task shifting in healthcare in resource-poor countries. Lancet. 2008;372:870–871. doi: 10.1016/S0140-6736(08)61375-6. [DOI] [PubMed] [Google Scholar]
- 91.Callaghan M, Ford N, Schneider H. A systematic review of task- shifting for HIV treatment and care in Africa. Hum Resour Health. 2010;8:8. doi: 10.1186/1478-4491-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zachariah R, Ford N, Philips M, Lynch S, Massaquoi M, Janssens V, Harries AD. Task shifting in HIV/AIDS: opportunities, challenges and proposed actions for sub-Saharan Africa. Trans R Soc Trop Med Hyg. 2009;103:549–558. doi: 10.1016/j.trstmh.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 93.Yakam JC, Gruenais ME. Involving new actors to achieve ART scaling-up: difficulties in an HIV/AIDS counselling and testing centre in Cameroon. Int Nurs Rev. 2009;56:50–57. doi: 10.1111/j.1466-7657.2008.00680.x. [DOI] [PubMed] [Google Scholar]
- 94.Hermann K, Van Damme W, Pariyo GW, Schouten E, Assefa Y, Cirera A, Massavon W. Community health workers for ART in sub-Saharan Africa: learning from experience – capitalizing on new opportunities. Hum Resour Health. 2009;7:31. doi: 10.1186/1478-4491-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Greenwald JL, Burstein GR, Pincus J, Branson B. A rapid review of rapid HIV antibody tests. Curr Infect Dis Rep. 2006;8:125–131. doi: 10.1007/s11908-006-0008-6. [DOI] [PubMed] [Google Scholar]
- 96.Gray RH, Makumbi F, Serwadda D, Lutalo T, Nalugoda F, Opendi P, et al. Limitations of rapid HIV-1 tests during screening for trials in Uganda: diagnostic test accuracy study. BMJ. 2007;335:188. doi: 10.1136/bmj.39210.582801.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moodley D, Moodley P, Ndabandaba T, Esterhuizen T. Reliability of HIV rapid tests is user dependent. South Afr Med J. 2008;98:707–709. [PubMed] [Google Scholar]
- 98.Malawi Ministry of Health. Integrated HIV Program Report; April – June 2012; Lilongwe, Malawi. 2012. [Google Scholar]
- 99.Lee PM, Wong KH. Universal antenatal human immunodeficiency virus (HIV) testing programme is cost-effective despite a low HIV prevalence in Hong Kong. Hong Kong Med J. 2007;13:199–207. [PubMed] [Google Scholar]
- 100.Long EF, Brandeau ML, Owens DK. The cost-effectiveness and population outcomes of expanded HIV screening and anti-retroviral treatment in the United States. Ann Int Med. 2010;153:778–789. doi: 10.1059/0003-4819-153-12-201012210-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lucas A, Armbruster B. The cost-effectiveness of expanded HIV screening in the United States. AIDS. 2013;27:795–801. doi: 10.1097/QAD.0b013e32835c54f9. [DOI] [PubMed] [Google Scholar]
- 102.Paltiel AD, Walensky RP, Schackman BR, Seage GR, 3rd, Mercincavage LM, Weinstein MC, Freedberg KA. Expanded HIV screening in the United States: effect on clinical outcomes, HIV transmission, and costs. Ann Int Med. 2006;145:797–806. doi: 10.7326/0003-4819-145-11-200612050-00004. [DOI] [PubMed] [Google Scholar]
- 103.Beyrer C, Wirtz AL, Baral S, Peryskina A, Sifakis F. Epidemiologic links between drug use and HIV epidemics: an international perspective. J Acquir Immune Defic Syndr. 2010;55 (Suppl 1):S10–S16. doi: 10.1097/QAI.0b013e3181f9c0c9. [DOI] [PubMed] [Google Scholar]
- 104.Singh K, Brodish P, Mbai F, Kingola N, Rinyura A, Njeru C, et al. A venue-based approach to reaching MSM, IDUs and the general population with VCT: a three study site in Kenya. AIDS Behav. 2012;16:818–828. doi: 10.1007/s10461-011-0103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.WHO, UNICEF, UNAIDS. Global update on HIV treatment 2013: results, impact and opportunities. WHO: 2013. [Google Scholar]
- 106.Bandyopadhyay A, Bhattacharyya S, Banerjee A. Clinicoepidemiological scoring system for early diagnosis of pediatric HIV. Indian Pediatr. 2009;46:512–515. [PubMed] [Google Scholar]