Abstract
The main aim of this investigation was to determine whether a functional relationship existed between epidermal growth factor (EGF) and voltage-gated sodium channel (VGSC) upregulation, both associated with strongly metastatic prostate cancer cells. Incubation with EGF for 24 h more than doubled VGSC current density. Similar treatment with EGF significantly and dose-dependently enhanced the cells’ migration through Transwell filters. Both the patch clamp recordings and the migration assay suggested that endogenous EGF played a similar role. Importantly, co-application of EGF and tetrodotoxin, a highly selective VGSC blocker, abolished 65% of the potentiating effect of EGF. It is suggested that a significant portion of the EGF-induced enhancement of migration occurred via VGSC activity.
Keywords: Voltage-gated sodium channel, prostate cancer, metastasis, Mat-LyLu cells, epidermal growth factor, nerve growth factor, migration
INTRODUCTION
Ionic activity plays a significant role in intracellular homeostasis under both physiological and pathophysiological conditions. Abnormally high levels of voltage-gated Na+ channels (VGSCs) have been detected in rat and human metastatic prostate cancer (PCa) in vitro and in vivo (Grimes et al., 1995; Laniado et al., 1997; Smith et al., 1998; Bennett et al., 2004; Diss et al., 2005) and also occurs in human glioblastomas (Labrakakis et al., 1997), oligodendrogliomas (Patt et al., 1996), melanomas (Allen et al., 1997), lung cancer (Blandino et al., 1995; Onganer and Djamgoz, 2005), medullary thyroid carcinomas (Klugbauer et al., 1995), neoplastic mesothelia (Fulgenzi et al., 2006), cervical cancer (Diaz et al., 2007) and breast cancer (Roger et al., 2003; Fraser et al., 2005). As regards the functional consequences of these VGSCs, most work has been done on PCa and it has been shown that VGSC activity enhances a range of metastatic cellular behaviours, including directional motility (Djamgoz et al., 2001; Fraser et al., 2003) and invasiveness (Grimes et al., 1995; Laniado et al., 1997). However, the mechanism(s) responsible for the VGSC upregulation in PCa is not known. VGSC expression generally is well known to be dynamic (Diss et al., 2004). In PCa, VGSC plasticity has been demonstrated by the sensitivity to external serum concentration, although the serum factor(s) responsible was not determined (Ding and Djamgoz, 2004). VGSC regulation by growth factors has been shown for a variety cells, including pheochromocytoma PC12 cells (Toledo-Aral et al., 1995) and neurones (Blum et al., 2002). The role of growth factors has also been emphasised in PCa, as regards both androgen sensitivity (Culig et al., 1996) and progression from invasive to metastatic carcinoma (Culig et al., 1994). We have shown previously that nerve growth factor (NGF) upregulated functional VGSC expression and transwell migration in Mat-LyLu rat PCa cells but the two effects were not connected (Brackenbury and Djamgoz, 2007).
In the present study, we aimed to evaluate the role of epidermal growth factor (EGF) in this regard. EGF has recently been shown to potentiate VGSC currents in guinea pig ventricular myocytes via tyrosine phosphorylation (Liu et al., 2007). Expressed prostatic fluid contains the highest concentration of EGF in the body (175.5 ng/ml; Russell et al., 1998; Gann et al., 1999), and EGF has been demonstrated to enhance cellular invasiveness, thus suggesting that it could also play a role in metastatic PCa (Turner et al., 1996; Kim et al., 1999; Montano and Djamgoz, 2004). These data collectively would suggest that EGF could underlie the VGSC upregulation in PCa. In the present study, we tested this hypothesis, again, using the Mat-LyLu rat Dunning cell model of PCa. These cells induce metastases in >90% of cases when injected into syngeneic rats (Isaacs et al., 1986) and express ~1,800× more VGSC/Nav1.7 mRNA, compared with their weakly/non-metastatic counterparts (Diss et al., 2001), and generate functional VGSCs (Grimes et al., 1995).
MATERIALS AND METHODS
Cell culture
Mat-LyLu cells were maintained in RPMI medium containing 1% heat-inactivated foetal bovine serum (FBS; Invitrogen/Gibco, Paisley, UK), supplemented with 250 nM dexamethasone. Medium was supplemented with 2 mM glutamine, 1 mM sodium pyruvate, and 100 IU/ml penicillin/streptomycin (Invitrogen/Gibco) (Grimes et al., 1995).
Pharmacology
Mat-LyLu cells were plated for 24 h, serum starved for another 24 h and then treated with pharmacological agent(s) for a further 24 h. The agents used, their working concentrations and suppliers were as follows: EGF, 100 ng/ml (Calbiochem, Nottingham, UK); AG1478, 1 μM (Calbiochem), an inhibitor of EGF receptor (EGFR) tyrosine kinase (e.g. Liu et al., 1999); tetrodotoxin (TTX), 500 nM (Alomone, Jerusalem, Israel); and EGFR antibody, 1 μg/ml (Oncogene Research Products/Calbiochem, Nottingham, UK).
Electrophysiology
Patch-clamp recordings were performed as detailed before (e.g. Grimes and Djamgoz, 1998; Ding and Djamgoz, 2004). Patch pipettes were pulled from borosilicate glass capillaries (Clarke Electromedical GC100F) and typically had resistances of 5–10 MΩ when filled with intracellular solution containing: 145 mM CsCl; 5 mM NaCl; 2 mM MgCl2; 1 mM CaCl2; 11 mM EGTA and 10 mM HEPES (pH 7.2 adjusted with 1 M CsOH). The extracellular solution contained 144 mM NaCl; 5.4 mM KCl; 1 mM MgCl2; 5 mM CaCl2; 5 mM HEPES and 5.6 mM glucose (pH 7.2 adjusted with 1 M NaOH). VGSC currents were recorded by pulsing membrane potentials from −50 to +70 mV in 10 mV increments, from a holding potential of −100 mV.
Migration assay
Details of this were described before (Fraser et al., 2005). Briefly, cells were seeded in multi-well dishes in tissue culture medium. Following drug treatment, cells were re-suspended using trypsin-EDTA and put onto 12 μm-pore Transwell filters (Corning, MA) at a density of 2 × 105 cells/well. Following 6 h incubation, MTT assay was performed to determine the number of migrated cells. The optical density of the coloured reaction was measured at 570 nm on a plate reader. These measurements were plotted as a percentage of the fluorescence readings for migrated cells/original cell number plated in the upper chamber, giving migration index (MiI). In the text, MiI values given were normalized with respect to the control value (corresponding to untreated cells in 0% FBS) as 100%.
Data analysis
All data were analysed as means ± standard errors (SEM). For statistical comparisons, Student’s t-test or ANOVA with Newman–Keuls post hoc analysis were used, as appropriate (Brackenbury and Djamgoz, 2006).
RESULTS
Initial observations suggested that Mat-LyLu cells grown in 0% FBS were viable for at least 24 h, the monolayer appearing flat and most cells having extended pseudopodia (Ding and Djamgoz, 2004). Treatment with EGF (100 ng/ml) for 24 h presented a more rounded and refractive form.
EGF increased VGSC current amplitude
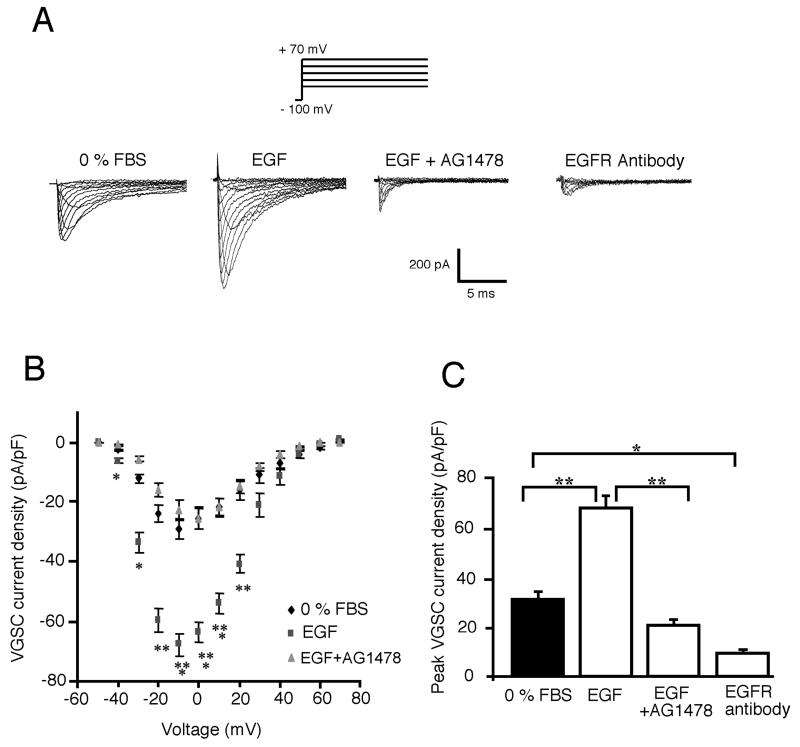
Whole-cell patch clamp recordings showed that treatment of cells with EGF for 24 h increased the VGSC current amplitude (Fig. 1). Similar treatment for only 5 min had no effect. The effect of EGF was blocked completely by co-incubation with AG1478, an inhibitor of EGFR tyrosine kinase (Fig. 1). The current–voltage (I–V) relationships showed that activation voltages and voltages for peak were similar, around −40 and −10 mV, respectively, under all three conditions tested: 0% FBS, EGF- and AG1478-treated (Fig. 1B). However, the mean peak current density of cells grown in the presence of EGF (68.1 ± 4.7 pA/pF; n = 19) was significantly (two- to sevenfold) greater than in 0% FBS (31.3 ± 2.8 pA/pF; n = 18; P < 0.01), anti-EGFR antibody (9.4 ± 2.3 pA/pF; n = 5; P < 0.001) or EGF + AG1478 (20.6 ± 2.2 pA/pF; n = 9; P < 0.001; Fig. 1C). Thus, EGF upregulated VGSC functional expression and this was dependent on tyrosine kinase activity. Importantly, EGF + AG1478, or application of the anti-EGFR antibody by itself, reduced the VGSC current amplitude to levels significantly less than the control (Fig. 1A,C). The latter effects were consistent with biochemical data (not shown) in suggesting that some basal EGFR activity occurred.
Figure 1. Upregulation of VGSC activity in Mat-LyLu cells by treatment for 24 h with exogenous EGF.
A: Typical VGSC current traces recorded from Mat-LyLu cells in different culture conditions: 0% FBS, EGF (100 ng/ml), EGF + AG1478 (1 μM), and anti-EGFR antibody (1 μg/ml). VGSC currents were recorded by pulsing membrane potentials from −50 to +70 mV in 10 mV increments, from a holding potential of −100 mV. B: Effects of EGF and EGF + AG1478 (as in A) on current–voltage relationship. Peak values of VGSC current density were plotted against membrane potential, showing EGF-stimulated VGSC functional activity. C: Histograms showing mean values of peak VGSC current density recorded in different conditions (as in A). All data points shown are mean and SEM. Significance: *P < 0.05; **P < 0.01; ***P < 0.001.
EGF enhanced cellular migration via VGSC activity
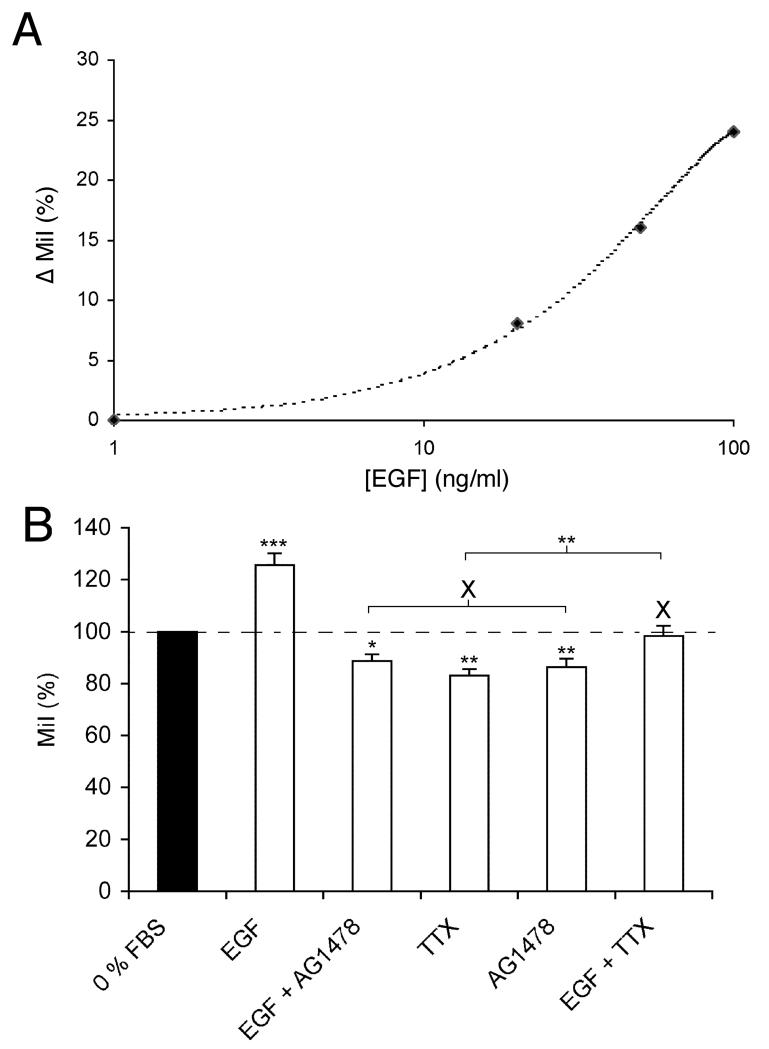
Pre-treatment with 100 ng/ml EGF for 24 h significantly increased Mat-LyLu cell migration by 26 ± 4% (n = 14; P < 0.001; Fig. 2). This effect was dose-dependent (Fig. 2A). Interestingly, following treatment with AG1478 (alone or with EGF), migration was significantly less than in 0% FBS, the control value (P < 0.01 and P < 0.05, respectively). These results would indicate possible involvement of endogenous EGF in migration, also apparent from the electrophysiology. There was no difference in the values of MiI for AG1478 and EGF + AG1478 (86 ± 3% and 89 ± 3%, respectively; P = 0.28; n = 14; Fig. 2B). This suggested that AG1478 blocked completely the effect of exogenous (and endogenous) EGF. TTX (500 nM) applied by itself during the assay, suppressed migration by 18 ± 3% (P < 0.001; n = 14; Fig. 2B). Importantly, in the presence of TTX (500 nM), exogenous EGF (100 ng/ml) still caused an increase in migration but this was ~65% less than the effect of EGF alone (P < 0.05; n = 14; Fig. 2B). This result suggested that a significant portion of EGF signalling operated upstream of the VGSC in the same pathway controlling migration.
Figure 2. Effects of EGF, AG1478 and TTX on Transwell migration of Mat-LyLu cells.
Migration index (MiI) values were expressed as percentages of the basal value in 0% FBS. A: Dose–response relationship for the effect of EGF (1–100 ng/ml) on percentage increase in MiI (ΔMiI). B: Effects of EGF (100 ng/ml), AG1478 (1 μM) and TTX (500 nM), and their specific combinations on MiI. Data are shown relative to the control value for 0% FBS (100%). Bars represent means and SEMs (n = 14). These data were consistent with the following: (1) Both EGF/EGFR and VGSC activity were involved in potentiating Mat-LyLu cell migration. (2) The EGF-stimulated enhancement of motility occurred partially via VGSC activity.
DISCUSSION
Whereas advanced PCa initially responds to androgen ablation therapy, most patients eventually develop androgen-independent cancer, which often leads to metastatic disease (Kreis, 1995). The transition of androgen-dependent to independent status could be associated with increased EGF signalling (Limonta et al., 1995; Sherwood and Lee, 1995). Indeed, it is well established that EGF promotes migration of PCa cells (Zolfaghari and Djakiew, 1996; Kim et al., 1999). Also, in primary corneal epithelial cells disoriented in an external direct-current electric field when grown in serum free medium, directional motility was restored by addition of EGF (Zhao et al., 1999). Thus, EGF and VGSC activity could both contribute to directional movement (Djamgoz et al., 2001; Fraser et al., 2003). The present study is the first to provide evidence in support of a functional relationship between EGF signalling and VGSC activity in PCa cells. Thus, adding EGF to Mat-LyLu cells serum-starved for 24 h increased VGSC current density and this was strongly EGFR-mediated. These effects were seen at a concentration of EGF, ca. 100 ng/ml, very similar to that found in expressed prostatic fluid (Gann et al., 1999). The somewhat limited increase in migration caused by EGF application may have been caused by the presence of endogenously secreted EGF (Fig. 2B). In agreement with this, and consistent with the electrophysiology (Fig. 1A,B), AG1478 alone also slightly but significantly reduced migration. The effect of TTX was also less than previously reported value of 40–50% for Mat-LyLu cells (Grimes et al., 1995). A likely cause of this is the serum-free condition used, which could have limited the involvement of VGSC activity in migration. Nevertheless, co-application of TTX with EGF blocked >50% of the EGF-induced increase in migration, suggesting that the enhancing effect of EGF occurred significantly via VGSC activity.
Upregulation of VGSC activity by EGF may be through a direct interaction with channel protein, for example tyrosine phosphorylation (Liu et al., 2007). It is also possible that the effect may be indirect and involve mechanisms in addition to VGSC. Indeed, EGF is likely to regulate a multiplicity of cellular components in metastatic PCa cells, which in turn may also influence migration. For example, EGF has been reported to cause system-wide changes in actin cytoskeleton extracellular matrix, Ca2+ signalling, pH, and transcription factor expression (Schalkwijk et al., 1995; Citri and Yarden, 2006; Lopez-Perez and Salazar, 2006; Mimura et al., 2006; Neumann-Giesen et al., 2007). Further work is required to evaluate these possibilities and to determine the signal transduction pathway involved in the EGF-induced VGSC upregulation. There are two other issues worthy of discussion.
First, although both NGF and EGF upregulated VGSC expression, their effects upon migration were different, the EGF-induced effect involved VGSC activity (this study), the NGF effect did not (Brackenbury and Djamgoz, 2007). This is a clear demonstration of the growth factor multiplicity and diversity of metastatic cell behaviour control, in part involving upregulation of VGSC expression/activity. Such a situation could have consequences for treatment modes for metastatic disease (Onganer et al., 2005).
Second, since (i) VGSC activity was shown earlier to control secretory membrane activity in PCa cells (Abdul and Hoosein, 2001; Mycielska et al., 2003; Krasowska et al., 2004) and (ii) PCa cells have been reported to secrete EGF (Connolly and Rose, 1990, 1991; also inferred in the present study from the effects of AG1478), it is possible that there is a positive feed-back loop between VGSC activity/upregulation and EGF release (Montano and Djamgoz, 2004). Such a mechanism could have a significant accelerating effect upon metastatic PCa progression.
Finally, EGF-induced upregulation of VGSC activity could also occur in other cancers, especially metastatic breast cancer which is known to be associated with both expression of EGF/EGFR (Atalay et al., 2003) and VGSC upregulation (Fraser et al., 2005; Brackenbury et al., 2007). Hence, suppressing VGSC expression/activity, alongside the EGF system could have added therapeutic value in clinical management of metastatic disease.
ACKNOWLEDGEMENTS
This work was supported by the Medical Research Council (UK), South of England Prostate Cancer Collaborative (MBAD and XM) and a Priority Area—Prostate Cancer PhD studentship (WJB). Additional support was provided by the Pro Cancer Research Fund (PCRF).
ABBREVIATIONS
- I-V
current-voltage
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- FBS
foetal bovine serum
- MiI
migration index
- NGF
nerve growth factor
- PCa
prostate cancer
- SEM
standard error
- TTX
tetrodotoxin
- VGSC
voltage-gated Na+ channel
REFERENCES
- Abdul M, Hoosein N. Inhibition by anticonvulsants of prostate-specific antigen and interleukin-6 secretion by human prostate cancer cells. Anticancer Res. 2001;21:2045–2048. [PubMed] [Google Scholar]
- Allen DH, Lepple-Wienhues A, Cahalan MD. Ion channel phenotype of melanoma cell lines. J Membr Biol. 1997;155:27–34. doi: 10.1007/s002329900155. [DOI] [PubMed] [Google Scholar]
- Atalay G, Cardoso F, Awada A, Piccart MJ. Novel therapeutic strategies targeting the epidermal growth factor receptor (EGFR) family and its downstream effectors in breast cancer. Ann Oncol. 2003;14:1346–1363. doi: 10.1093/annonc/mdg365. [DOI] [PubMed] [Google Scholar]
- Bennett ES, Smith BA, Harper JM. Voltage-gated Na(+) channels confer invasive properties on human prostate cancer cells. Pflugers Arch. 2004;447:908–914. doi: 10.1007/s00424-003-1205-x. [DOI] [PubMed] [Google Scholar]
- Blandino JK, Viglione MP, Bradley WA, Oie HK, Kim YI. Voltage-dependent sodium channels in human small-cell lung cancer cells: Role in action potentials and inhibition by Lambert-Eaton syndrome IgG. J Membr Biol. 1995;143:153–163. doi: 10.1007/BF00234661. [DOI] [PubMed] [Google Scholar]
- Blum R, Kafitz KW, Konnerth A. Neurotrophin-evoked depolarization requires the sodium channel Na(V)1.9. Nature. 2002;419:687–693. doi: 10.1038/nature01085. [DOI] [PubMed] [Google Scholar]
- Brackenbury WJ, Djamgoz MB. Activity-dependent regulation of voltage-gated Na(+) channel expression in Mat-LyLu rat prostate cancer cell line. J Physiol. 2006;573:343–356. doi: 10.1113/jphysiol.2006.106906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury WJ, Djamgoz MB. Nerve growth factor enhances voltage-gated Na(+) channel activity and transwell migration in Mat-LyLu rat prostate cancer cell line. J Cell Physiol. 2007;210:602–608. doi: 10.1002/jcp.20846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury WJ, Chioni AM, Diss JK, Djamgoz MB. The neonatal splice variant of Nav1.5 potentiates in vitro metastatic behaviour of MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat. 2007;101:149–160. doi: 10.1007/s10549-006-9281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A, Yarden Y. EGF-ERBB signalling: Towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- Connolly JM, Rose DP. Production of epidermal growth factor and transforming growth factor-alpha by the androgen-responsive LNCaP human prostate cancer cell line. Prostate. 1990;16:209–218. doi: 10.1002/pros.2990160304. [DOI] [PubMed] [Google Scholar]
- Connolly JM, Rose DP. Autocrine regulation of DU145 human prostate cancer cell growth by epidermal growth factor-related polypeptides. Prostate. 1991;19:173–180. doi: 10.1002/pros.2990190210. [DOI] [PubMed] [Google Scholar]
- Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54:5474–5478. [PubMed] [Google Scholar]
- Culig Z, Hobisch A, Cronauer MV, Radmayr C, Hittmair A, Zhang J, Thurnher M, Bartsch G, Klocker H. Regulation of prostatic growth and function by peptide growth factors. Prostate. 1996;28:392–405. doi: 10.1002/(SICI)1097-0045(199606)28:6<392::AID-PROS9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Diaz D, Delgadillo D, Hernandez-Gallegos E, Ramirez-Dominguez M, Hinojosa L, Ortiz C, Berumen J, Camacho J, Gomora J. Functional expression of voltage-gated sodium channels in primary cultures of human cervical cancer. J Cell Physiol. 2007;210:469–478. doi: 10.1002/jcp.20871. [DOI] [PubMed] [Google Scholar]
- Ding Y, Djamgoz MB. Serum concentration modifies amplitude and kinetics of voltage-gated Na+ current in the Mat-LyLu cell line of rat prostate cancer. Int J Biochem Cell Biol. 2004;36:1249–1260. doi: 10.1016/j.biocel.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Diss JK, Archer SN, Hirano J, Fraser SP, Djamgoz MB. Expression profiles of voltage-gated Na(+) channel alpha-subunit genes in rat and human prostate cancer cell lines. Prostate. 2001;48:165–178. doi: 10.1002/pros.1095. [DOI] [PubMed] [Google Scholar]
- Diss JK, Fraser SP, Djamgoz MB. Voltage-gated Na+ channels: Multiplicity of expression, plasticity, functional implications and pathophysiological aspects. Eur Biophys J. 2004;33:180–193. doi: 10.1007/s00249-004-0389-0. [DOI] [PubMed] [Google Scholar]
- Diss JK, Stewart D, Pani F, Foster CS, Walker MM, Patel A, Djamgoz MB. A potential novel marker for human prostate cancer: Voltage-gated sodium channel expression in vivo. Prostate Cancer Prostatic Dis. 2005;8:266–273. doi: 10.1038/sj.pcan.4500796. [DOI] [PubMed] [Google Scholar]
- Djamgoz MBA, Mycielska M, Madeja Z, Fraser SP, Korohoda W. Directional movement of rat prostate cancer cells in direct-current electric field: Involvement of voltage gated Na+ channel activity. J Cell Sci. 2001;114:2697–2705. doi: 10.1242/jcs.114.14.2697. [DOI] [PubMed] [Google Scholar]
- Fraser SP, Salvador V, Manning EA, Mizal J, Altun S, Raza M, Berridge RJ, Djamgoz MB. Contribution of functional voltage-gated Na(+) channel expression to cell behaviors involved in the metastatic cascade in rat prostate cancer. I. lateral motility. J Cell Physiol. 2003;195:479–487. doi: 10.1002/jcp.10312. [DOI] [PubMed] [Google Scholar]
- Fraser SP, Diss JK, Chioni AM, Mycielska M, Pan H, Yamaci RF, Pani F, Siwy Z, Krasowska M, Grzywna Z, Brackenbury WJ, Theodorou D, Koyuturk M, Kaya H, Battaloglu E, De Bella MT, Slade MJ, Tolhurst R, Palmieri C, Jiang J, Latchman DS, Coombes RC, Djamgoz MB. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res. 2005;11:5381–5389. doi: 10.1158/1078-0432.CCR-05-0327. [DOI] [PubMed] [Google Scholar]
- Fulgenzi G, Graciotti L, Faronato M, Soldovieri MV, Miceli F, Amoroso S, Annunziato L, Procopio A, Taglialatela M. Human neoplastic mesothelial cells express voltage-gated sodium channels involved in cell motility. Int J Biochem Cell Biol. 2006;38:1146–1159. doi: 10.1016/j.biocel.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Gann PH, Klein KG, Chatterton RT, Ellman AE, Grayhack JT, Nadler RB, Lee C. Growth factors in expressed prostatic fluid from men with prostate cancer, BPH, and clinically normal prostates. Prostate. 1999;40:248–255. doi: 10.1002/(sici)1097-0045(19990901)40:4<248::aid-pros6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Grimes JA, Djamgoz MB. Electrophysiological characterization of voltage-gated Na(+) current expressed in the highly metastatic Mat-LyLu cell line of rat prostate cancer. J Cell Physiol. 1998;175:50–58. doi: 10.1002/(SICI)1097-4652(199804)175:1<50::AID-JCP6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Grimes JA, Fraser SP, Stephens GJ, Downing JE, Laniado ME, Foster CS, Abel PD, Djamgoz MB. Differential expression of voltage-activated Na(+) currents in two prostatic tumour cell lines: Contribution to invasiveness in vitro. FEBS Lett. 1995;369:290–294. doi: 10.1016/0014-5793(95)00772-2. [DOI] [PubMed] [Google Scholar]
- Isaacs JT, Isaacs WB, Feitz WF, Scheres J. Establishment and characterization of seven Dunning rat prostatic cancer cell lines and their use in developing methods for predicting metastatic abilities of prostatic cancers. Prostate. 1986;9:261–281. doi: 10.1002/pros.2990090306. [DOI] [PubMed] [Google Scholar]
- Kim HG, Kassis J, Souto JC, Turner T, Wells A. EGF receptor signaling in prostate morphogenesis and tumorigenesis. Histol Histopathol. 1999;14:1175–1182. doi: 10.14670/HH-14.1175. [DOI] [PubMed] [Google Scholar]
- Klugbauer N, Lacinova L, Flockerzi V, Hofmann F. Structure and functional expression of a new member of the tetrodotoxin-sensitive voltage-activated sodium channel family from human neuroendocrine cells. EMBO J. 1995;14:1084–1090. doi: 10.1002/j.1460-2075.1995.tb07091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasowska M, Grzywna ZJ, Mycielska ME, Djamgoz MB. Patterning of endocytic vesicles and its control by voltage-gated Na(+) channel activity in rat prostate cancer cells: Fractal analyses. Eur Biophys J. 2004;33:535–542. doi: 10.1007/s00249-004-0394-3. [DOI] [PubMed] [Google Scholar]
- Kreis W. Current chemotherapy and future directions in research for the treatment of advanced hormone-refractory prostate cancer. Cancer Invest. 1995;13:296–312. doi: 10.3109/07357909509094465. [DOI] [PubMed] [Google Scholar]
- Labrakakis C, Patt S, Weydt P, Cervos-Navarro J, Meyer R, Kettenmann H. Action potential-generating cells in human glioblastomas. J Neuropathol Exp Neurol. 1997;56:243–254. doi: 10.1097/00005072-199703000-00003. [DOI] [PubMed] [Google Scholar]
- Laniado ME, Lalani EN, Fraser SP, Grimes JA, Bhangal G, Djamgoz MB, Abel PD. Expression and functional analysis of voltage-activated Na(+) channels in human prostate cancer cell lines and their contribution to invasion in vitro. Am J Pathol. 1997;150:1213–1221. [PMC free article] [PubMed] [Google Scholar]
- Limonta P, Dondi D, Marelli MM, Moretti RM, Negri-Cesi P, Motta M. Growth of the androgen-dependent tumor of the prostate: Role of androgens and of locally expressed growth modulatory factors. J Steroid Biochem Mol Biol. 1995;53:401–405. doi: 10.1016/0960-0760(95)00086-f. [DOI] [PubMed] [Google Scholar]
- Liu W, Akhand AA, Kato M, Yokoyama I, Miyata T, Kurokawa K, Uchida K, Nakashima I. 4-Hydroxynonenal triggers an epidermal growth factor receptor-linked signal pathway for growth inhibition. J Cell Sci. 1999;112:2409–2417. doi: 10.1242/jcs.112.14.2409. [DOI] [PubMed] [Google Scholar]
- Liu H, Sun H-Y, Lau C-P, Li G-R. Regulation of voltage-gated cardiac sodium current by epidermal growth factor receptor kinase in guinea pig ventricular myocytes. J Mol Cell Cardiol. 2007;42:760–768. doi: 10.1016/j.yjmcc.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Lopez-Perez M, Salazar EP. A role for the cytoskeleton in STAT5 activation in MCF7 human breast cancer cells stimulated with EGF. Int J Biochem Cell Biol. 2006;38:1716–1728. doi: 10.1016/j.biocel.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Mimura Y, Ihn H, Jinnin M, Asano Y, Yamane K, Tamaki K. Epidermal growth factor affects the synthesis and degradation of type I collagen in cultured human dermal fibroblasts. Matrix Biol. 2006;25:202–212. doi: 10.1016/j.matbio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Montano X, Djamgoz MB. Epidermal growth factor, neurotrophins and the metastatic cascade in prostate cancer. FEBS Lett. 2004;571:1–8. doi: 10.1016/j.febslet.2004.06.088. [DOI] [PubMed] [Google Scholar]
- Mycielska ME, Fraser SP, Szatkowski M, Djamgoz MB. Contribution of functional voltage-gated Na(+) channel expression to cell behaviors involved in the metastatic cascade in rat prostate cancer. II. Secretory membrane activity. J Cell Physiol. 2003;195:461–469. doi: 10.1002/jcp.10265. [DOI] [PubMed] [Google Scholar]
- Neumann-Giesen C, Fernow I, Amaddii M, Tikkanen R. Role of EGF-induced tyrosine phosphorylation of reggie-1/flotillin-2 in cell spreading and signaling to the actin cytoskeleton. J Cell Sci. 2007;120:395–406. doi: 10.1242/jcs.03336. [DOI] [PubMed] [Google Scholar]
- Onganer PU, Djamgoz MB. Small-cell lung cancer (human): Potentiation of endocytic membrane activity by voltage-gated Na(+) channel expression in vitro. J Membr Biol. 2005;204:67–75. doi: 10.1007/s00232-005-0747-6. [DOI] [PubMed] [Google Scholar]
- Onganer PU, Seckl MJ, Djamgoz MB. Neuronal characteristics of small-cell lung cancer. Br J Cancer. 2005;93:1197–1201. doi: 10.1038/sj.bjc.6602857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patt S, Labrakakis C, Bernstein M, Weydt P, Cervos-Navarro J, Nisch G, Kettenmann H. Neuron-like physiological properties of cells from human oligodendroglial tumors. Neuroscience. 1996;71:601–611. doi: 10.1016/0306-4522(95)00468-8. [DOI] [PubMed] [Google Scholar]
- Roger S, Besson P, Le Guennec JY. Involvement of a novel fast inward sodium current in the invasion capacity of a breast cancer cell line. Biochim Biophys Acta. 2003;1616:107–111. doi: 10.1016/j.bbamem.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Russell PJ, Bennett S, Stricker P. Growth factor involvement in progression of prostate cancer. Clin Chem. 1998;44:705–723. [PubMed] [Google Scholar]
- Schalkwijk CG, Spaargaren M, Defize LH, Verkleij AJ, van den Bosch H, Boonstra J. Epidermal growth factor (EGF) induces serine phosphorylation-dependent activation and calcium-dependent translocation of the cytosolic phospholipase A2. Eur J Biochem. 1995;231:593–601. doi: 10.1111/j.1432-1033.1995.tb20737.x. [DOI] [PubMed] [Google Scholar]
- Sherwood ER, Lee C. Epidermal growth factor-related peptides and the epidermal growth factor receptor in normal and malignant prostate. World J Urol. 1995;13:290–296. doi: 10.1007/BF00185972. [DOI] [PubMed] [Google Scholar]
- Smith P, Rhodes NP, Shortland AP, Fraser SP, Djamgoz MB, Ke Y, Foster CS. Sodium channel protein expression enhances the invasiveness of rat and human prostate cancer cells. FEBS Lett. 1998;423:19–24. doi: 10.1016/s0014-5793(98)00050-7. [DOI] [PubMed] [Google Scholar]
- Toledo-Aral JJ, Brehm P, Halegoua S, Mandel G. A single pulse of nerve growth factor triggers long-term neuronal excitability through sodium channel gene induction. Neuron. 1995;14:607–611. doi: 10.1016/0896-6273(95)90317-8. [DOI] [PubMed] [Google Scholar]
- Turner T, Chen P, Goodly LJ, Wells A. EGF receptor signaling enhances in vivo invasiveness of DU-145 human prostate carcinoma cells. Clin Exp Metastasis. 1996;14:409–418. doi: 10.1007/BF00123400. [DOI] [PubMed] [Google Scholar]
- Zhao M, Dick A, Forrester JV, McCaig CD. Electric field-directed cell motility involves up-regulated expression and asymmetric redistribution of the epidermal growth factor receptors and is enhanced by fibronectin and laminin. Mol Biol Cell. 1999;10:1259–1276. doi: 10.1091/mbc.10.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolfaghari A, Djakiew D. Inhibition of chemomigration of a human prostatic carcinoma cell (TSU-pr1) line by inhibition of epidermal growth factor receptor function. Prostate. 1996;28:232–238. doi: 10.1002/(SICI)1097-0045(199604)28:4<232::AID-PROS4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]