Abstract
Current teaching and guidelines suggest that aggressive fluid resuscitation is the best initial approach to the patient with hemodynamic instability. The source of this wisdom is difficult to discern, however, Early Goal Directed therapy (EGDT) as championed by Rivers et al. and the Surviving Sepsis Campaign Guidelines appears to have established this as the irrefutable truth. However, over the last decade it has become clear that aggressive fluid resuscitation leading to fluid overload is associated with increased morbidity and mortality across a diverse group of patients, including patients with severe sepsis as well as elective surgical and trauma patients and those with pancreatitis. Excessive fluid administration results in increased interstitial fluid in vital organs leading to impaired renal, hepatic and cardiac function. Increased extra-vascular lung water (EVLW) is particularly lethal, leading to iatrogenic salt water drowning. EGDT and the Surviving Sepsis Campaign Guidelines recommend targeting a central venous pressure (CVP) > 8 mmHg. A CVP > 8 mmHg has been demonstrated to decrease microcirculatory flow, as well as renal blood flow and is associated with an increased risk of renal failure and death. Normal saline (0.9% salt solution) as compared to balanced electrolyte solutions is associated with a greater risk of acute kidney injury and death. This paper reviews the adverse effects of large volume resuscitation, a high CVP and the excessive use of normal saline.
Keywords: Fluid, Fluid balance, Normal saline, Lung water, Extra-vascular lung water, Central venous pressure, ICU, Lactate Ringers Solution, Acute respiratory distress syndrome, Sepsis, Mean circulatory filling pressure, Fluid overload
In the critically ill and injured patient, aggressive supportive measures may be harmful and the ‘less is more’ paradigm appears applicable. In these highly vulnerable patients, more intensive treatments may promote the chances of unwanted adverse effects and hence, iatrogenic injury [1]. Traditional teaching suggests that aggressive fluid resuscitation is the best initial approach for the cardiovascular instability of sepsis. In the Rivers’ Early Goal Directed Therapy (EGDT) study, 4.9 liters of crystalloid were given in the first 6 hours and 13.4 liters in the first 72 hours [2]. The Surviving Sepsis Campaign recommends ‘aggressive fluid resuscitation during the first 24 hours of management’ [3]. The guidelines for hemodynamic support of sepsis published by the American College of Critical Care Medicine state that ‘large fluid deficits exist in patients with septic shock. Up to 6 to 10 liters of crystalloid solutions may be required for initial resuscitation in the first 24 hours’ [4]. Consequently, large volumes of fluid are often infused in the early stages of sepsis. Traditionally, patients undergoing surgery have been managed with a liberal fluid strategy in which fluids are administered to fill the non-existent ‘third space’ [5]–[7]. For patients with traumatic injuries, high volume fluid resuscitation is promoted by the early Advanced Trauma Life Support (ATLS) strategy [8]. There are, however, no human data to show that large volume fluid resuscitation reliably improves organ perfusion [9],[10]. This approach is likely to lead to iatrogenic salt water drowning with the acute respiratory distress syndrome (ARDS), acute kidney injury (AKI), myocardial injury, gastrointestinal dysfunction, poor wound healing with an increased the risk of death [6],[11]–[21]. Aggressive fluid resuscitation is a well-known risk factor for secondary intra-abdominal hypertension which in turn is associated with hepatic and respiratory dysfunction, AKI, multiorgan failure and death [22]–[27]. Current guidelines suggest targeting a central venous pressure (CVP) of 8 to 12 mmHg in patients with severe sepsis and septic shock [28]. However, a high CVP increases venous pressure, increases organ interstitial pressure and reduces organ and microcirculatory flow [29],[30]. A CVP > 8 mmHg has been associated with an increased risk of renal failure and death [13],[16],[31]. Normal saline (0.9% salt solution) is common resuscitation fluid. However, as compared to balanced electrolyte solutions normal saline (NS) is associated with a greater risk of acute kidney injury and death [32]–[35]. This paper reviews the adverse effects of large volume resuscitation, a high CVP and the excessive use of normal saline.
Review
The dangers of large volume resuscitation
From a pathophysiological point of view, large volume fluid resuscitation in patients with sepsis is illogical and may worsen the hemodynamic derangements of sepsis. Sepsis is primarily a vasoplegic state due to increased production of nitric oxide, activation of K ATP channels and vasopressin deficiency [36]. Vasoplegic shock, due to failure of the vascular smooth muscle to constrict, results in arterial and venodilation [36]. Venodilation increases the unstressed vascular compartment, thus decreasing venous return. In patients with septic shock who are fluid responders, vasodilatation with a fall in systematic vascular resistance has been observed following fluid resuscitation [37],[38]. A similar finding has been noted in an experimental sepsis model [21]. Furthermore, it is important to emphasize that the septic heart responds poorly to fluid loading. In patients with sepsis, the Frank-Starling curve is shifted downwards and to the right, with septic patients showing a diminished response to fluid loading. This observation was demonstrated by Ognibene and colleagues over 25 years ago [39]. Patients in septic shock had a minimal increase in left ventricular stroke work index (LVSWI) in response to volume infusion. This suggests that large volume fluid resuscitation will cause a small increase in stroke volume (with further vasodilatation) at the expense of large increases in filling pressures (increase in both the CVP and left atrial pressures). Furthermore, sepsis is characterized by increased endothelial permeability caused by shedding of the endothelial glycocalyx and the development of gaps between endothelial cells (paracellular leak) [40],[41]. An increase in microcirculatory hydrostatic pressure following aggressive fluid resuscitation increases fluid extravasation. Increased cardiac filling pressures following aggressive fluid resuscitation increase the release of natriuretic peptides which act synergistically with nitric oxide causing cGMP mediated vasodilatation [36]. In addition, natriuretic peptides cleave membrane-bound proteoglycans and glycoproteins (most notably syndecan-1 and hyaluronic acid) off the endothelial glycocalyx [42]–[44]. This profoundly increases endothelial permeability. Increased natriuretic peptides inhibit the lymphatic propulsive motor activity reducing lymphatic drainage [45]–[47]. Increased natriuretic peptides following aggressive fluid resuscitation therefore acts to sequestrate fluid into the interstitium. Furthermore, increased filling pressures and a positive fluid balance increase extra-vascular lung water (EVLW) [18]. Increased EVLW impairs gas exchange, reduces lung compliance and increases the work of breathing [18]. Increased EVLW is regarded as a defining feature of acute lung injury/ARDS [48],[49]. Increased EVLW is a strong independent predictor of death [12],[18],[50],[51]. Due to the endothelial injury, capillary leak and increased hydrostatic pressures less than five percent of infused crystalloid remains intravascular within three hours after infusion resulting in further increases in EVLW and tissue edema [52]. Tissue edema impairs oxygen and metabolite diffusion, distorts tissue architecture, impedes capillary blood flow and lymphatic drainage and disturbs cell-cell interactions; these effects contribute to progressive organ dysfunction [14]. These effects are pronounced in encapsulated organs, such as the liver and kidneys, which lack the capacity to accommodate additional volume without an increase in interstitial pressure, resulting in compromised organ blood flow [14]. This may lead to AKI and hepatic congestion with cholestasis and impaired hepatic function [14]. Myocardial edema due to excess fluid administration compounds the myocardial dysfunction common in critically ill patients [21]. In a cohort of patients requiring mechanical ventilation, Cordemans and colleagues reported significantly more respiratory, liver and cardiovascular organ-failure free days in patients’ with a conservative fluid strategy and those whose EVLW fell by more than 2 ml/kg during their ICU stay [18]. Bowel edema results in malabsorption, ileus and bacterial translocation. In patients with pneumonia, large volume fluid resuscitation may result in severe ARDS (see Figure 1). The chest radiograph series presented in Figure 1 represents a typical case of fatal iatrogenic salt water drowning. This patient was resuscitated according to the EGDT and the Surviving Sepsis Campaign bundles, in which fluids are administered until the CVP > 12 mmHg [2],[28].
Figure 1.
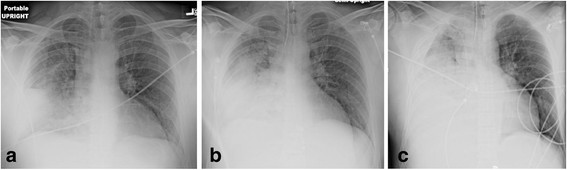
Fourty-four-year old male with Pneumococcal pneumonia. (a) Initial chest radiograph (CXR) in emergency department. (b) CXR four hours later after four liters of crystalloid (patient now intubated). (c) CXR 12 hours after admission, after 9 liters of crystalloid, central venous pressure (CVP) = 10 mmHg. Patient died six hours later of refractory hypoxemia.
The harmful effects of aggressive fluid resuscitation on the outcome of sepsis are supported by experimental studies as well as data accumulated from clinical trials [15],[20],[21],[31],[51],[53]–[58]. Multiple clinical studies have demonstrated an independent association between an increasingly positive fluid balance and increased mortality in patient with sepsis [15],[18],[31],[51],[53]–[58]. In a secondary analysis of the Vasopressin in Septic Shock Trial (VASST), Boyd and colleagues demonstrated that a greater positive fluid balance at both 12 hours and 4 days were independent predictors of death [31]. Furthermore, a number of studies have demonstrated that a positive fluid balance was associated with an increased risk for AKI [15]. Bouchard et al. demonstrated that in patients with AKI, fluid overload was independently associated with mortality [58]. In the Fluid and Catheter Treatment Trial (FACTT), the conservative fluid strategy was associated with a trend towards a reduced requirement for renal replacement therapy [59]. The most compelling data that fluid loading in sepsis is harmful come from The Fluid Expansion as Supportive Therapy (FEAST) study performed in 3,141 sub-Saharan children with severe sepsis [60]. In this randomized study, aggressive fluid loading was associated with a significantly increased risk of death. Furthermore, there was no subgroup of patients that benefited from aggressive fluid resuscitation [61].
Emerging data suggest that less than 50% of septic patients who present to the emergency department (and are fluid naive) will be fluid responsive [62]. In some patients, hypotension and tachycardia do resolve with limited fluid resuscitation. However, fluids alone will not reverse the hemodynamic instability of patients with more severe sepsis; in these patients’ fluids alone are likely to exacerbate the vasodilatory shock and increase the capillary leak, tissue edema and organ dysfunction [21]. In the Australasian Resuscitation of Sepsis Evaluation (ARISE) study which used the same entry criteria as the EGDT study, 2.2 ± 1.9 liters of fluid were given in the first 6 hours [63]. This compares to 4.9 liters in the intervention arm of the EGDT study [2]. The hospital mortality was 23% in the ARISE study compared to 30% in the intervention arm of the EGDT study. This difference in the prescription of fluid is likely due to variability in the management style of severe sepsis between countries. In a survey conducted by Reade et al., intensivists from Australia tended to give less fluid than Americans with only 15% of Australians targeting a CVP of 8 to 12 mmHg [64]. In the VASST study optimal survival occurred with a positive fluid balance of approximately three liters at twelve hours [31]. Recently, the Protocolized Care for Early Septic Shock (ProCESS) study was published which compared protocol-based EGDT (Rivers’ protocol) and protocol-based standard therapy with usual care [65]. It is noteworthy that the amount of fluid given in the first 6 hours and from 7 to 72 hours was significantly less in the ProCESS EGDT patients than in the Rivers’ EGDT patients (see Table 1). As patients were resuscitated according to the same protocol this would appear to be anomalous. However, it should be noted that the mean CVP at 6 hours was 13.8 ± 4.4 mmHg in the Rivers’ EGDT group. Assuming a normal distribution, 50% of patients in the Rivers’ EGDT would have achieved CVPs greater than the mean value of 13.8 mmHg. Thus most patients in the Rivers’ EGDT study had CVPs outside the stated goal (>8 to 12 mmHg). Furthermore, the use of vasopressors in the first six hours of EGDT was significantly greater in ProCESS than in the Rivers’ study (see Table 1). We hypothesize that targeting a lower mean CVP in the EGDT arm of the ProCESS trial resulted in less fluid being given and the earlier use of vasopressors. It is therefore possible that the large amount of fluid administered in the Rivers’ study partly accounted for the mortality difference between the EGDT arms of the Rivers’ and ProCESS studies.
Table 1.
Contrasting use of fluids and vasopressors (and mortality) in the Early Goal Directed Therapy (EGDT) arms of the Rivers’ and ProCESS studies
| Study | Fluid 0 to 6 hours (ml) | Fluid 7 to 72 hours (ml) | Fluid 0 to 72 hours (ml) | Vasopressors (%) 0 to 6 hours | 60-day mortality (%) |
|---|---|---|---|---|---|
| Rivers’ EGDT |
4,981 |
8,625 |
13,443 |
27.4 |
44.3 |
| ProCESS EGDT | 2,805 | 4,428 | 7,220 | 54.9 | 21 |
Multiple RCT’s and cohort studies have demonstrated that a conservative fluid strategy in patients undergoing elective non-cardiac surgery is associated with significantly fewer complications with a lower mortality (in the high risk patients) than patients managed with the traditional liberal fluid strategy in which fluids are administered to fill the non-existent ‘third space’ [5]–[7]. For patients with traumatic injuries, high volume fluid resuscitation as promoted by the early Advanced Trauma Life Support (ATLS) strategy [8], has given way to a ‘damage control’ resuscitation strategy [66]. This approach has seen a fall in the volume of crystalloid delivered in the emergency department and an associated fall in mortality. In a prospective analysis of 3,137 trauma patients treated in the Emergency Department, fluid volumes of 1.5 liters or more were significantly associated with mortality [67]. This observation is supported by a meta-analysis which demonstrated in both RCTs and cohort studies that a conservative fluid strategy was associated with a lower mortality in trauma patients [68]. Similarly, an aggressive fluid strategy in the resuscitation of patients with acute pancreatitis has been associated with an increased risk of complications [69].
The results of multiple studies across diverse patient populations have clearly demonstrated that aggressive fluid resuscitation is associated with an increased risk of complications and death. The only published study conducted in adult patients that has demonstrated that early aggressive fluid resuscitation improves outcome is the EGDT study by Rivers et al. [2]. However, the results of this study do not appear to be biologically plausible (implausible effect size) [70], the elements of the protocol were not based on evidence-based interventions [71], the analysis of the results of study has come under scrutiny [72], and the results were not validated in a large randomized controlled trial [65]. While hypovolemia will result in decreased cardiac output (and blood pressure) with inadequate organ perfusion leading to organ dysfunction, overzealous fluid resuscitation and hypervolemia induces a cascade of events that similarly results in organ dysfunction. From an evolutionary point of view, humans have evolved to deal with hypovolemia and not hypervolemia. Hypervolemia is a recent (last 20 years) and largely iatrogenic phenomenon. The argument is no longer ‘wet or dry’ but ‘just the right amount of fluid’ [73],[74].
The dangers of a high CVP
Not only has the CVP failed as a useful measure for the assessment of preload and fluid responsiveness [75], but a CVP > 8 mmHg is independently associated with a higher mortality and increased risk of AKI in patients with sepsis and heart failure [13],[31],[76]. This suggests that the CVP component of the six-hour resuscitation bundle as widely promoted by the Surviving Sepsis Campaign may lead to harm [28]. It is important to note that a normal CVP is close to zero and not 8 to 12 mmHg as the Surviving Sepsis Campaign might lead one to believe [28]. The mean circulatory filling pressure (MCFP) is regarded as the driving pressure that determines venous return and is considered synonymous with the effective circulatory blood volume [77]–[81]. The MCFP is conceptualized as the pressure distending the vasculature when the heart is stopped (zero flow) and the pressures in all segments of the circulatory system have equalized [78]–[80]. The MCFP in humans is normally in the range of 8 to 10 mmHg [80]. According to Guyton, venous return is determined by the gradient between MCFP and CVP [78]. An increase in the CVP or a fall in the MCFP will reduce venous return, stroke volume and cardiac output. Cecconi and colleagues investigated the relationship between the changes in MCFP with the change in CVP and stroke volume following a fluid challenge in postsurgical ICU patients [82]. In this study MCFP increased equally in fluid responders and non-responders (3.1 ± 1.9 versus 3.1 ± 1.8 mmHg). However, the increase in the CVP was greater in the non-responders than the responders. This study emphasizes that a disproportionate increase in the CVP will impede venous return and cardiac output [81].
In addition to influencing venous return, a high CVP is transmitted backwards increasing venous pressure. The increase in venous pressure has a profound effect on microcirculatory flow and organ function. The kidney is particularly affected by congestion and increased venous pressure, which leads to increased renal subcapsular pressure and lowered renal blood flow and glomerular filtration rate (GFR) [83]. Furthermore, increased renal interstitial pressure may collapse intrarenal collecting lymphatics which compromise lymph flow [84]. The detrimental effect of high venous pressure on renal function was established by FR Winton in an elegant set of experiments performed in 1930s [29]. This investigator attached the kidneys of a dog to a heart-lung circulation by means of cannulae inserted into the artery and veins of the kidneys and then independently altered venous and arterial pressure. Dr Winton demonstrated that increasing venous pressure dramatically decreased urine production. More recently, Legrand and colleagues investigated the association between hemodynamic variables and AKI in patients with sepsis [13]. In this study, the CVP was the only hemodynamic variable associated with the development of AKI; cardiac output, mixed venous oxygen saturation (ScvO2) and mean arterial pressure (MAP) were unable to predict the development of AKI. These authors noted a linear relationship between increasing CVP and AKI; there was a trend for higher CVP to be associated with worse renal outcome for all levels of CVP above 4 mmHg, with a CVP of 15 mmHg being associated with an 80% risk of new or persistent AKI, compared to approximately 30% at a CVP of 6 mmHg. Van Biesen and colleagues demonstrated that septic patients developing AKI had a higher positive fluid balance, a higher CVP and worse oxygenation than septic patients without AKI [16]. It is important to point out that these are observational data, and that it is likely that other factors play a role in the pathophysiology of AKI in sepsis. In patients with acute decompensated heart failure, Mullens et al. demonstrated a near linear relationship between increasing CVP and worsening renal function [76]. In this study, worsening renal function occurred significantly less frequently in patients with a CVP < 8 mmHg. Furthermore, similar to the findings of Legrand and colleagues, the CVP was the only hemodynamic parameter that predicted worsening renal failure, with the cardiac index, systolic blood pressure and pulmonary capillary wedge pressure being similar between those patients who maintained renal function as compared to those with worsening renal function. In a subanalysis of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness trial (ESCAPE), Nohria et al. demonstrated a significant correlation between baseline renal function and the CVP, there was, however no correlation between baseline renal function and cardiac index, pulmonary capillary wedge pressure or systemic vascular resistance [85]. These data suggest that a high CVP independently increases the risk for ‘congestive kidney failure’. Most clinicians fluid load patients with oliguria; this intervention sets into motion a vicious cycle, with fluid loading further increasing renal venous pressure with a further decline in renal function and urine output [16].
In addition to increasing renal venous and interstitial pressure, a high CVP will result in an increase in hepatic and intestinal venous pressure causing hepatic and intestinal congestion and impaired microcirculatory flow. Indeed, in a study of 70 patients with sepsis, Vellinga and colleagues demonstrated that the sublingual microvascular flow index (MFI) and percentage of perfused vessels (PPV) was significantly lower with a patients with a high CVP (>12 mmHg) than a low CVP: 1.44 ± 0.94 versus 1.89 ± 0.91, P = 0.006; and 88 ± 21 versus 95 ± 8%, P = 0.006) [30]. The cardiac index, MAP and perfusion pressure (MAP-CVP) did not differ significantly between the high and low CVP groups. In a multivariate logistic regression analysis, the only significant predictor for an abnormal MFI was a CVP > 12 mmHg. Because microcirculatory driving pressure is the difference between post-arteriolar and venular pressure, a relatively mild increase in CVP may considerably influence the capillary perfusion pressure and microcirculatory flow [30]. As the pressure drop in the vascular system occurs upstream at the level of small arterioles (resistance vessels), the microcirculation is considered a low pressure compartment. Therefore, mean capillary pressure is much closer to venous than to arterial pressure. Therefore, as long as the MAP is within an organ’s autoregulatory range, the CVP becomes the major determinant of capillary blood flow. This suggests that venous pressure has a much greater effect on microcirculatory flow than the MAP. The adverse consequences of volume overload and a high CVP are summarized listed below
Consequences of volume overload
Pulmonary edema and increased extra-vascular lung water
Impaired oxygenation
Altered pulmonary and chest wall mechanics
Increased work of breathing
Myocardial edema
Decreased contractility
Diastolic dysfunction
Conduction defects
Increased intraabdominal pressure
Acute kidney injury
Hepatic dysfunction
Decreased lung volumes
Ileus
Gastrointestinal
Ileus
Malabsorption
Bacterial translocation
Hepatic congestion
Decreased wound healing
Consequences of a high central venous pressure
Decreased venous return and stroke volume
Acute kidney injury
Hepatic congestion
Decreased splanchnic microcirculatory flow
The dangers of a normal saline
Despite differences in composition between normal saline (0.9% NaCl) and Lactated Ringer’s (LR), Normosol, Isolyte and Plasmalyte solutions, they are frequently considered physiologically equivalent. Whilst no body fluid has an electrolyte composition similar to that of normal saline (NS), this fluid is frequently referred to as ‘physiologic salt solution (PSS)’. However, 0.9% NaCl is more correctly known as ‘Unphysiologic Salt Solution’. Experimental and clinical data have clearly demonstrated that these fluids are not physiologically equivalent. Only LR, Normosol, Isolyte and Plasmalyte solutions are balanced salt solutions. Due to the calcium content of LR (and not Plasmalyte), it has been traditionally taught that LR should not be infused in the same venous line as blood (may activate clotting). However, this is not true [86].
Renal failure
The increased chloride load delivered to the macula densa results in afferent arterial constriction [87]. In health volunteers, NS significantly reduces renal arterial flow and renal cortisol tissue perfusion as compared to Plasmalyte [88]. In a sequential cohort study Yunos and colleagues demonstrated that a chloride liberal fluid (NS) was associated with a much higher incidence of renal failure than critically patients resuscitated with a chloride restrictive fluid (LR and Plasmalyte) [32].
Hyperchloremic metabolic acidosis and death
Numerous studies have demonstrated the development of a hyperchloremic metabolic acidosis in human volunteers and patients resuscitated with NS [89]–[92]. The additional loss (renal) of HCO3 in the setting of reduced buffering capacity only adds to the acid–base burden characteristic of hypoperfused states [90]. Furthermore, resuscitation with NS may produce a ‘dilutional acidosis’. In both experimental and clinical (pancreatitis) studies, hyperchloremic acidosis has been demonstrated to increase the release of inflammatory mediators [93],[94]. In a prospective cohort study, Boniatti et al. demonstrated that hyperchloremia was an independent predictor of death [95]. Similarly, in two large cohorts of patients undergoing non-cardiac surgery, hyperchloremia was an independently associated with increased morbidity and mortality [33],[34]. In these studies the risk of death increased with increasing chloride levels. Using a large multi-institutional database, Raghunathan et al. performed propensity score matching in patients with severe sepsis comparing resuscitation with balanced solutions versus NS [35]. In this study, resuscitation with a balanced solution was associated with a lower in-hospital mortality (19.6% versus 22.8%; relative risk (RR) 0.86; 95% CI, 0.78 to 0.94).
Coagulopathy
Studies in surgical patients have demonstrated that as compared to LR, volume replacement with NS results in greater blood loss with a greater need for blood transfusion [91]. The cause of the coagulopathy is unclear, and is only partly explained by the difference in calcium between the two solutions.
Lactate as a metabolic fuel
It is not an accidental quirk of nature that the body produces lactate during stress states. The proportion of lactate uptake by the myocardium and its use a metabolic fuel increases during exercise, β-adrenergic stimulation and shock [96]–[98]. During shock the heart undergoes a major shift in substrate utilization such that it oxidizes lactate for the majority of its energy needs [98]. Revelly and coworkers demonstrated that an infusion of sodium lactate increased cardiac performance in patients with both cardiogenic and septic shock [99]. Similarly, during increased demand on brain metabolism, lactate is increasingly utilized as an energy substrate [100]–[102]. LR may therefore have additional advantages in shocked patients with the lactate being oxidized and serving as a source of energy [103],[104].
Conclusions
A liberal fluid resuscitation strategy, a CVP > than 8 mmHg and the use of 0.9% NaCl as the predominant resuscitation fluid are all associated with an increased risk of renal failure, respiratory failure, gastrointestinal dysfunction and death across a broad spectrum of clinical disorders. These three treatment strategies probably act synergistically to harm patients, forming the ‘Deadly Trio’.
Abbreviations
AKI: acute kidney injury
ARDS: acute respiratory distress syndrome
ARISE: Australasian Resuscitation of Sepsis Evaluation trial
ATLS: Advanced Trauma Life Support
CVP: central venous pressure
CXR: chest radiograph
EGDT: Early Goal Directed Therapy
ESCAPE: Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness trial
EVLW: extra-vascular lung water
FACTT: Fluid and Catheter Treatment Trial
FEAST: The Fluid Expansion as Supportive Therapy study
GFR: glomerular filtration rate
LR: Lactated Ringer’s solution
MAP: mean arterial pressure
MCFP: mean circulatory filling pressure
NS: normal saline
ProCESS: Protocolized Care for Early Septic Shock trial
RR: relative risk
ScvO2: mixed venous oxygen saturation
VASST: Vasopressin in Septic Shock Trial
Competing interest
The author has no real or perceived conflicts of interest or a financial interest in any of the products mentioned in this paper.
Authors’ contribution
The author solely contributed to this paper and assumes full responsibility for the content of the paper.
References
- Knox M, Pickkers P. ‘Less is More’ in critically ill patients. Not too intensive. JAMA Intern Med. 2013;4:1369–1372. doi: 10.1001/jamainternmed.2013.6702. [DOI] [PubMed] [Google Scholar]
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;4:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, Ramsay G, Zimmerman JL, Vincent JL, Levy MM. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;4:858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- Hollenberg SM, Ahrens TS, Annane D, Astiz ME, Chalfin DB, Dasta JF, Heard SO, Martin C, Napolitano LM, Susla GM, Totaro R, Vincent JL, Zanotti-Cavazzoni S. Practice parameters for hemodynamic support of sepsis in adult patients: 2004 update. Crit Care Med. 2004;4:1928–1948. doi: 10.1097/01.ccm.0000139761.05492.d6. [DOI] [PubMed] [Google Scholar]
- Cecconi M, Corredor C, Arulkumaran N, Abuella G, Ball J, Grounds RM, Hamilton M, Rhodes A. Clinical review: goal-directed therapy-what is the evidence in surgical patients? The effect on different risk groups. Crit Care. 2013;4:209. doi: 10.1186/cc11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran T, Rhodes JE, Clarke S, Myles PS, Ho KM. Perioperative fluid management strategies in major surgery: a stratified meta-analysis. Anesth Analg. 2012;4:640–651. doi: 10.1213/ANE.0b013e318240d6eb. [DOI] [PubMed] [Google Scholar]
- Jacob M, Chappell D, Rehm M. The ‘third space’ - fact or fiction? Best practice research Clin Anesthesiol. 2009;4:145–157. doi: 10.1016/j.bpa.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Advanced Trauma Life Support for Doctors; Student Course Manual. American College of Surgeons, Chicago; 1997. [Google Scholar]
- Hilton AK, Bellomo R. A critique of fluid bolus resuscitation in severe sepsis. Crit Care. 2012;4:302. doi: 10.1186/cc11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton AK, Bellomo R. Totem and taboo: fluids in sepsis. Crit Care. 2011;4:164. doi: 10.1186/cc10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeTourneau JL, Pinney J, Phillips CR. Extravascular lung water predicts progression to acute lung injury in patients with increased risk. Crit Care Med. 2012;4:847–854. doi: 10.1097/CCM.0b013e318236f60e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozwiak M, Silva S, Persichini R, Anguel N, Osman D, Richard C, Teboul JL, Monnet X. Extravascular lung water is an independent prognostic factor in patients with acute respiratory distress syndrome. Crit Care Med. 2013;4:472–480. doi: 10.1097/CCM.0b013e31826ab377. [DOI] [PubMed] [Google Scholar]
- Legrand M, Dupuis C, Simon C, Gayat E, Mareo J, Lukaszewicz AC, Payen D. Association between systemic hemodynamics and septic kidney injury in critically ill patients: a retrospective observational study. Crit Care. 2013;4:R278. doi: 10.1186/cc13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prowle JR, Echeverri JE, Ligabo EV, Ronco C, Bellomo R. Fluid balance and acute kidney injury. Nat Rev Nephrol. 2010;4:107–115. doi: 10.1038/nrneph.2009.213. [DOI] [PubMed] [Google Scholar]
- Payen D, De Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;4:R74. doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Biesen W, Yegenaga I, Vanholder R, Verbeke F, Hoste E, Colardyn F, Lameire N. Relationship between fluid status and its management on acute renal failure (ARF) in intensive care unit (ICU) patients with sepsis: a prospective analysis. J Nephrol. 2005;4:54–60. [PubMed] [Google Scholar]
- Wang CH, Hsieh WH, Chou HC, Huang YS, Shen JH, Yeo YH, Chang HE. Liberal versus restricted fluid resuscitation strategies in trauma patients: a systematic review and meta-analysis of randomized controlled trials and observational studies. Crit Care Med. 2014;4:954–961. doi: 10.1097/CCM.0000000000000050. [DOI] [PubMed] [Google Scholar]
- Cordemans C, De Laet I, Van Regenmortel N, Schoonheydt K, Dits H, Huber W, Malbrain ML. Fluid management in critically ill patients: the role of extravascular lung water, abdominal hypertension, capillary leak, and fluid balance. Ann Intensive Care. 2012;4:S1. doi: 10.1186/2110-5820-2-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arulkumaran N, Corredor C, Hamilton MA, Ball J, Grounds RM, Rhodes A, Cecconi M. Cardiac complications associated with goal-directed therapy in high-risk surgical patients: a meta-analysis. Br J Anaesth. 2014;4:648–659. doi: 10.1093/bja/aet466. [DOI] [PubMed] [Google Scholar]
- Brandt S, Regueira T, Bracht H, Porta F, Djafarzadeh S, Takala J, Gorrasi J, Borotto E, Krejci V, Hiltebrand LB, Bruegger LE, Beldi G, Wilkens L, Lepper PM, Kessler U, Jakob SM. Effect of fluid resuscitation on mortality and organ function in experimental sepsis models. Crit Care. 2009;4:R186. doi: 10.1186/cc8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehberg S, Yamamoto Y, Sousse L, Bartha E, Jonkam C, Hasselbach AK, Traber LD, Cox RA, Westphal M, Enkhbaatar P, Traber DL. Selective V(1a) agonism attenuates vascular dysfunction and fluid accumulation in ovine severe sepsis. Am J Physiol Heart Circ Physiol. 2012;4:H1245–H1254. doi: 10.1152/ajpheart.00390.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malbrain ML, Chiumello D, Pelosi P, Bihari D, Innes R, Ranieri VM, Del TM, Wilmer A, Brienza N, Malcangi V, Cohen J, Japiassu A, De Keulenaer BL, Daelemans R, Jacquet L, Laterre PF, Frank G, De Souza P, Cesana B, Gattinoni L. Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: a multiple-center epidemiological study. Crit Care Med. 2005;4:315–322. doi: 10.1097/01.ccm.0000153408.09806.1b. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick AW, Roberts DJ, De WJ, Jaeschke R, Malbrain ML, De KB, Duchesne J, Bjorck M, Leppaniemi A, Ejike JC, Sugrue M, Cheatham M, Ivatury R, Ball CG, Reintam BA, Regli A, Balogh ZJ, D’Amours S, Debergh D, Kaplan M, Kimball E, Olvera C. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;4:1190–1206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalfino L, Tullo L, Donadio I, Malcangi V, Brienza N. Intra-abdominal hypertension and acute renal failure in critically ill patients. Intensive Care Med. 2008;4:707–713. doi: 10.1007/s00134-007-0969-4. [DOI] [PubMed] [Google Scholar]
- Diebel LN, Wilson RF, Dulchavsky SA, Saxe J. Effect of increased intra-abdominal pressure on hepatic arterial, portal venous, and hepatic microcirculatory blood flow. J Trauma. 1992;4:279–282. doi: 10.1097/00005373-199208000-00019. [DOI] [PubMed] [Google Scholar]
- El D, Liang H, Taichman D, Hansen-Flaschen J, Fuchs BD. Abdominal compartment syndrome is common in medical intensive care unit patients receiving large-volume resuscitation. J Intensive Care Med. 2007;4:294–299. doi: 10.1177/0885066607305247. [DOI] [PubMed] [Google Scholar]
- Malbrain ML, Viaene D, Kortgen A, De Laet I, Dits H, Van RN, Schoonheydt K, Bauer M. Relationship between intra-abdominal pressure and indocyanine green plasma disappearance rate: hepatic perfusion may be impaired in critically ill patients with intra-abdominal hypertension. Ann Intensive Care. 2012;4(Suppl 1):S19. doi: 10.1186/2110-5820-2-S1-S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock: 2012. Crit Care Med. 2013;4:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- Winton FR. The influence of venous pressure on the isolated mammalian kidney. J Physiol. 1931;4:49–61. doi: 10.1113/jphysiol.1931.sp002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellinga NA, Ince C, Boerma EC. Elevated central venous pressure is associated with impairment of microcirculatory blood flow in sepsis. BMC Anesthesiol. 2013;4:17. doi: 10.1186/1471-2253-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd JH, Forbes J, Nakada T, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure increase mortality. Crit Care Med. 2011;4:259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- Yunos NM, Bellomo R, Hegarty C, Stoty D, Ho L, Bailey M. Association between a chloride-liberal versus chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;4:1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- McCluskey SA, Karkouti K, Wijeysundera D, Minkovich L, Tait G, Beattie WS. Hyperchloremia after noncardiac surgery is independently associated with increased morbidity and mortality: a propensity-matched cohort study. Anesth Analg. 2013;4:412–421. doi: 10.1213/ANE.0b013e318293d81e. [DOI] [PubMed] [Google Scholar]
- Shaw AD, Bagshaw SM, Goldstein SL, Scherer LA, Duan M, Schermer CR, Kellum JA. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg. 2012;4:821–829. doi: 10.1097/SLA.0b013e31825074f5. [DOI] [PubMed] [Google Scholar]
- Raghunathan K, Shaw A, Nathanson B, Sturmer T, Brookhart A, Stefan MS, Setoguchi S, Beadles C, Lindenauer PK. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis. Crit Care Med. 2014;4:1585–1591. doi: 10.1097/CCM.0000000000000305. [DOI] [PubMed] [Google Scholar]
- Landry DW, Oliver JA. Pathogenesis of vasodilatory shock. N Engl J Med. 2001;4:588–595. doi: 10.1056/NEJMra002709. [DOI] [PubMed] [Google Scholar]
- Pierrakos C, Velissaris D, Scolletta S, Heenen S, De BD, Vincent JL. Can changes in arterial pressure be used to detect changes in cardiac index during fluid challenge in patients with septic shock? Intensive Care Med. 2012;4:422–428. doi: 10.1007/s00134-011-2457-0. [DOI] [PubMed] [Google Scholar]
- Monnet X, Chemla D, Osman D, Anguel N, Richard C, Pinsky MR, Teboul JL. Measuring aortic diameter improves accuracy of esophageal Doppler in assessing fluid responsiveness. Crit Care Med. 2007;4:477–482. doi: 10.1097/01.CCM.0000254725.35802.17. [DOI] [PubMed] [Google Scholar]
- Ognibene FP, Parker MM, Natanson C, Shelhamer JH, Parrillo JE. Depressed left ventricular performance: response to volume infusion in patients with sepsis and septic shock. Chest. 1988;4:903–910. doi: 10.1378/chest.93.5.903. [DOI] [PubMed] [Google Scholar]
- Goldenberg NM, Steinberg BE, Slutsky AS, Lee WL. Broken barriers: a new take on sepsis pathogenesis. Sci Transl Med. 2011;4:88ps25. doi: 10.1126/scitranslmed.3002011. [DOI] [PubMed] [Google Scholar]
- London NR, Zhu W, Bozza FA, Smith MC, Greif DM, Sorensen LK, Chen L, Kaminoh Y, Chan AC, Passi SF, Day CW, Barnard DL, Zimmerman GA, Krasnow MA, Li DY. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci Transl Med. 2010;4:23ra19. doi: 10.1126/scitranslmed.3000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruegger D, Jacob M, Rehm M, Loetsch M, Welsch U, Conzen P, Becker BF. Atrial natriuretic peptide induces shedding of endothelial glycocalyx in coronary vascular bed of guinea pig hearts. Am J Physiol Heart Circ Physiol. 2005;4:H1993–H1999. doi: 10.1152/ajpheart.00218.2005. [DOI] [PubMed] [Google Scholar]
- Berg S, Golster M, Lisander B. Albumin extravasation and tissue washout of hyaluronan after plasma volume expansion with crystalloid or hypooncotic colloid solutions. Acta Anaesthesiol Scand. 2002;4:166–172. doi: 10.1034/j.1399-6576.2002.460207.x. [DOI] [PubMed] [Google Scholar]
- Bruegger D, Schwartz L, Chappell D, Jacob M, Rehm M, Vogeser M, Christ F, Reichart B, Becker BF. Release of atrial natriuretic peptide precedes shedding of the endothelial glycocalyx equally in patients undergoing on- and off-pump coronary artery bypass surgery. Basic Res Cardiol. 2011;4:1111–1121. doi: 10.1007/s00395-011-0203-y. [DOI] [PubMed] [Google Scholar]
- Atchison DJ, Johnston MG. Atrial natriuretic peptide attenuates flow in an isolated lymph duct preparation. Eur J Physiol. 1996;4:618–624. doi: 10.1007/BF02191911. [DOI] [PubMed] [Google Scholar]
- Anderson WD, Kulik TJ, Mayer JE, Anderson WD, Kulik TJ, Mayer JE. Inhibition of contraction of isolated lymphatic ducts by atrial natriuretic peptide. Am J Physiol. 1991;4:R610–R614. doi: 10.1152/ajpregu.1991.260.3.R610. [DOI] [PubMed] [Google Scholar]
- Ohhashi T, Watanabe N, Kawai Y, Ohhashi T, Watanabe N, Kawai Y. Effects of atrial natriuretic peptide on isolated bovine mesenteric lymph vessels. Am J Physiol. 1990;4:H42–H47. doi: 10.1152/ajpheart.1990.259.1.H42. [DOI] [PubMed] [Google Scholar]
- Tagami T, Sawabe M, Kushimoto S, Marik PE, Mieno MN, Kawaguchi T, Kusakabe T, Tosa R, Yokota H, Fukuda Y. Quantitative diagnosis of diffuse alveolar damage using extravascular lung water. Crit Care Med. 2013;4:2144–2150. doi: 10.1097/CCM.0b013e31828a4643. [DOI] [PubMed] [Google Scholar]
- Michard F, Fernandez-Mondejar E, Kirov M, Malbrain M, Tagami T. A new and simple definition for acute lung injury. Crit Care Med. 2012;4:1004–1006. doi: 10.1097/CCM.0b013e31823b97fd. [DOI] [PubMed] [Google Scholar]
- Sakka SG, Klein M, Reinhart K, Meier-Hellmann A. Prognostic value of extravascular lung water in critically ill patients. Chest. 2002;4:2080–2086. doi: 10.1378/chest.122.6.2080. [DOI] [PubMed] [Google Scholar]
- Chung FT, Lin SM, Lin SY, Lin HC. Impact of extravascular lung water index on outcomes of severe sepsis patients in a medical intensive care unit. Respir Med. 2008;4:956–961. doi: 10.1016/j.rmed.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Bark BP, Persson J, Grande PO. Importance of the infusion rate for the plasma expanding effect of 5% albumin, 6% HES 130/0.4, 4% gelatin and 0.9% NaCl in the septic rat. Crit Care Med. 2013;4:857–866. doi: 10.1097/CCM.0b013e318274157e. [DOI] [PubMed] [Google Scholar]
- Rosenberg AL, Dechert RE, Park PK, Bartlett RH. Review of a large clinical series: association of cumulative fluid balance on outcome in acute lung injury: a retrospective review of the ARDSnet tidal volume study cohort. J Intensive Care Med. 2009;4:35–46. doi: 10.1177/0885066608329850. [DOI] [PubMed] [Google Scholar]
- Micek SC, McEnvoy C, McKenzie M, Hampton N, Doherty JA, Kollef MH. Fluid balance and cardiac function in septic shock as predictors of hospital mortality. Crit Care. 2013;4:R246. doi: 10.1186/cc13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;4:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- Alsous F, Khamiees M, De Girolamo A, Moateng-Adjepong Y, Manthous CA. Negative fluid balance predicts survival in patients with septic shock: a retrospective pilot study. Chest. 2000;4:1749–1754. doi: 10.1378/chest.117.6.1749. [DOI] [PubMed] [Google Scholar]
- Murphy CV, Schramm GE, Doherty JA, Reichley RM, Gajic O, Afessa B, Micek ST, Kollef MH. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;4:102–109. doi: 10.1378/chest.08-2706. [DOI] [PubMed] [Google Scholar]
- Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;4:422–427. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;4:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, Mtove G. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;4:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- Maitland K, George EC, Evans JA, Kiguli S, Olupot-Olupot P, Akech SO, Opoka RO, Engoru C, Nyeko R, Mtove G, Reyburn H, Brent B, Nteziyaremye J, Mpoya A, Prevatt N, Dambisya CM, Semakula D, Ddungu A, Okuuny V, Wokulira R, Timbwa M, Otii B, Levin M, Crawley J, Babiker AG, Gibb DM. Exploring mechanisms of excess mortality with early fluid resuscitation: insights from the FEAST trial. BMC Med. 2013;4:68. doi: 10.1186/1741-7015-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B, Phillips R, Madigan V, West M. Decreased mortality, morbidity and emergency transport in septic shock: a new protocol based on advanced noninvasive haemodynamics and early antibiotics [abstract] Crit Care Med. 2013;4:ᅟ. doi:10.1097/01.ccm.0000425236.44389.af. [Google Scholar]
- Peake SL, Bailey M, Bellomo R, Cameron PA, Cross A, Delaney A, Finfer S, Higgins A, Jones DA, Myburgh JA, Syres GA, Webb SA, Williams P. Australasian resuscitation of sepsis evaluation (ARISE): a multi-centre, prospective, inception cohort study. Resuscitation. 2009;4:811–818. doi: 10.1016/j.resuscitation.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Reade MC, Huang DT, Bell D, Coats TJ, Cross AM, Moran JL, Peake SL, Singer M, Yealy DM, Angus DC. Variability in management of early severe sepsis. Emerg Med J. 2010;4:110–115. doi: 10.1136/emj.2008.070912. [DOI] [PubMed] [Google Scholar]
- A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;4:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T, Thomas GO, Brohi K. Early fluid resuscitation in severe trauma. BMJ. 2012;4:e5752. doi: 10.1136/bmj.e5752. [DOI] [PubMed] [Google Scholar]
- Ley EJ, Clond MA, Srour MK, Barnajian M, Mirocha J, Margulies DR, Salim A. Emergency department crystalloid resuscitation of 1.5 L or more is associated with increased mortality in elderly and nonelderly trauma patients. J Trauma. 2011;4:398–400. doi: 10.1097/TA.0b013e318208f99b. [DOI] [PubMed] [Google Scholar]
- Wang CH, Hsieh WH, Chou HC, Huang YS, Shen JH, Yeo YH, Chang HE. Liberal versus restricted fluid resuscitation strategies in trauma patients: a systematic review and meta-analysis of randomized controlled trials and observational studies. Crit Care Med. 2014;4:954–962. doi: 10.1097/CCM.0000000000000050. [DOI] [PubMed] [Google Scholar]
- de-Madaria E, Soler-Sala G, Sanchez-Paya J, Lopez-Font I, Martinez J, Gomez-Escolar L, Sempere L, Sanchez-Fortun C, Perez-Mateo M. Influence of fluid therapy on the prognosis of acute pancreatitis: a prospective cohort study. Am J Gastroenterol. 2011;4:1843–1850. doi: 10.1038/ajg.2011.236. [DOI] [PubMed] [Google Scholar]
- Bellomo R, Warrillow SJ, Reade MC. Why we should be wary of single-center trials. Crit Care Med. 2009;4:3114–3119. doi: 10.1097/CCM.0b013e3181bc7bd5. [DOI] [PubMed] [Google Scholar]
- Marik PE. Surviving sepsis: going beyond the guidelines. Ann Intensive Care. 2011;4:17. doi: 10.1186/2110-5820-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton TM. New therapy for sepsis infection raises hope but many questions (lead article) Wall St J. 2008;4:ᅟ. Page 1, August 14th 2008. [Google Scholar]
- Marik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Ann Crit Care. 2011;4:1. doi: 10.1186/2110-5820-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marik PE, Lemson J. Fluid responsiveness: an evolution of our understanding. Br J Anaesth. 2014;4:620–622. doi: 10.1093/bja/aet590. [DOI] [PubMed] [Google Scholar]
- Marik PE, Cavallazzi R. Does the Central Venous Pressure (CVP) predict fluid responsiveness: an update meta-analysis and a plea for some common sense. Crit Care Med. 2013;4:1774–1781. doi: 10.1097/CCM.0b013e31828a25fd. [DOI] [PubMed] [Google Scholar]
- Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;4:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss WM, Starling EH. Observations on venous pressures and their relationship to capillary pressures. J Physiol. 1894;4:159–318. doi: 10.1113/jphysiol.1894.sp000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton AC. Determination of cardiac output by equating venous return curves with cardiac response curves. Physiol Rev. 1955;4:123–129. doi: 10.1152/physrev.1955.35.1.123. [DOI] [PubMed] [Google Scholar]
- Peters J, Mack GW, Lister G. The importance of the peripheral circulation in critical illnesses. Intensive Care Med. 2001;4:1446–1458. doi: 10.1007/s001340101034. [DOI] [PubMed] [Google Scholar]
- Gelman S. Venous function and central venous pressure: a physiologic story. Anesthesiol. 2008;4:735–748. doi: 10.1097/ALN.0b013e3181672607. [DOI] [PubMed] [Google Scholar]
- Funk DJ, Jacobsohn E, Kumar A. The role of venous return in critical illness and shock - part I: physiology. Crit Care Med. 2013;4:250–257. doi: 10.1097/CCM.0b013e3182772ab6. [DOI] [PubMed] [Google Scholar]
- Cecconi M, Aya HD, Geisen M, Ebm C, Fletcher N, Grounds RM, Rhodes A. Changes in the mean systemic filling pressure during a fluid challenge in postsurgical intensive care patients. Intensive Care Med. 2013;4:1299–1305. doi: 10.1007/s00134-013-2928-6. [DOI] [PubMed] [Google Scholar]
- Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol. 2014;4:37–47. doi: 10.1038/nrneph.2013.232. [DOI] [PubMed] [Google Scholar]
- Rohn DA, Stewart RH, Elk JR, Laine GA, Drake RE. Renal lymphatic function following venous pressure elevation. Lymphology. 1996;4:67–75. [PubMed] [Google Scholar]
- Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;4:1268–1274. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- Lorenzo M, Davis JW, Negin S, Kaups K, Parks S, Brubaker D, Tyroch A. Can Ringer’s lactate be used safely with blood transfusions? Am J Surg. 1998;4:308–310. doi: 10.1016/s0002-9610(98)00011-7. [DOI] [PubMed] [Google Scholar]
- Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;4:726–735. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury AH, Cox EF, Francis S, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;4:18–24. doi: 10.1097/SLA.0b013e318256be72. [DOI] [PubMed] [Google Scholar]
- Scheingraber S, Rehm M, Sehmisch C, Finsterer U. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiol. 1999;4:1265–1270. doi: 10.1097/00000542-199905000-00007. [DOI] [PubMed] [Google Scholar]
- Kellum JA, Bellomo R, Kramer DJ, Pinsky MR. Etiology of metabolic acidosis during saline resuscitation in endotoxemia. Shock. 1998;4:364–368. doi: 10.1097/00024382-199805000-00009. [DOI] [PubMed] [Google Scholar]
- Waters JH, Gottlieb A, Schoenwald P, Popovich MJ, Sprung J, Nelson DR. Normal saline versus lactated Ringer’s solution for intraoperative fluid management in patients undergoing abdominal aortic aneurysm repair: an outcome study. Anesth Analg. 2001;4:817–822. doi: 10.1097/00000539-200110000-00004. [DOI] [PubMed] [Google Scholar]
- Reid F, Lobo DN, Williams RN, Rowlands BJ, Allison SP. (Ab)normal saline and physiological Hartmann’s solution: a randomized double-blind crossover study. Clin Sci. 2003;4:17–24. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Kellum JA, Song M, Almasri E. Hyperchloremic acidosis increases circulating inflammatory molecules in experimental sepsis. Chest. 2006;4:962–967. doi: 10.1378/chest.130.4.962. [DOI] [PubMed] [Google Scholar]
- Wu BU, Hwang JQ, Gardner TH, Repas K, Delee R, Yu S, Smith B, Banks PA, Conwell DL. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;4:710–717. doi: 10.1016/j.cgh.2011.04.026. [DOI] [PubMed] [Google Scholar]
- Boniatti MM, Cardoso PR, Castilho RK, Vieira SR. Is hyperchloremia associated with mortality in critically ill patients? A prospective cohort study. J Crit Care. 2011;4:175–179. doi: 10.1016/j.jcrc.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Stanley WC, Stanley WC. Myocardial lactate metabolism during exercise. Med Sci Sports Exerc. 1991;4:920–924. [PubMed] [Google Scholar]
- Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;4:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- Kline JA, Thornton LR, Lopaschuk GD, Barbee RW, Watts JA. Lactate improves cardiac efficiency after hemorrhagic shock. Shock. 2000;4:215–221. doi: 10.1097/00024382-200014020-00023. [DOI] [PubMed] [Google Scholar]
- Revelly JP, Tappy L, Martinez A, Bollmann M, Cayeux MC, Berger MM, Chiolero RL. Lactate and glucose metabolism in severe sepsis and cardiogenic shock. Crit Care Med. 2005;4:2235–2240. doi: 10.1097/01.ccm.0000181525.99295.8f. [DOI] [PubMed] [Google Scholar]
- Van Hall G, Stromstad M, Rasmussen P, Jans O, Zaar M, Gam C, Quistorff B, Secher NH, Nielsen HB. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab. 2009;4:1121–1129. doi: 10.1038/jcbfm.2009.35. [DOI] [PubMed] [Google Scholar]
- Quistorff B, Secher NH, Van Lieshout JJ. Lactate fuels the human brain during exercise. FASEB J. 2008;4:3443–3449. doi: 10.1096/fj.08-106104. [DOI] [PubMed] [Google Scholar]
- Wyss MT, Jolivet R, Buck A, Magistretti PJ, Weber B. In vivo evidence for lactate as a neuronal energy source. J Neurosci. 2011;4:7477–7485. doi: 10.1523/JNEUROSCI.0415-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alvarez M, Marik PE, Bellomo R. Stress hyperlactemia. Lancet Endo Diabetes. 2013;4:ᅟ. doi: 10.1016/S2213-8587(13)70154-2. doi:org/10.1016/S2213-8587(13)70154-2. [DOI] [PubMed] [Google Scholar]
- White SA, Goldhill DR, White SA, Goldhill DR. Is Hartmann’s the solution? Anaesthesia. 1997;4:422–427. doi: 10.1111/j.1365-2044.1997.090-az0082.x. [DOI] [PubMed] [Google Scholar]


