Abstract
Essential tremor (ET) is a pathologically heterogeneous neurodegenerative disorder with both motor and increasingly recognized non-motor features. It is debated whether the non-motor manifestations in ET result from widespread neurodegeneration or are merely secondary to impaired motor functions and decreased quality of life due to tremor. It is important to review these features to determine how to best treat the non-motor symptoms of patients and to understand the basic pathophysiology of the disease and develop appropriate pharmacotherapies. In this review, retrospective and prospective clinical studies were critically analyzed to identify possible correlations between the severities of non-motor features and tremor. We speculated that if such a correlation existed, the non-motor features were likely to be secondary to tremor. According to the current literature, the deficits in executive function, attention, concentration, and memory often observed in ET are likely to be a primary manifestation of the disease. It has also been documented that patients with ET often exhibit characteristic personality traits. However, it remains to be determined whether the other non-motor features often seen in ET, such as anxiety, depression, and sleep disturbances are primary or secondary to motor manifestations of ET and subsequent poor quality of life. Finally, there is evidence that patients with ET can also have impaired color vision, disturbances of olfaction, and hearing impairments, though there are few studies in these areas. Further investigations of large cohorts of patients with ET are required to understand the prevalence, nature, and true significance of the non-motor features in ET.
Keywords: Tremor, essential tremor, non-motor features, cognition, depression, anxiety
Introduction
Essential tremor (ET) is one of the most common movement disorders in adults and is a classic example of a disorder whose interpretation has shifted from that of a one-dimensional disease with prominent motor features to a multi-dimensional disease, with varied non-motor features. In 2001, Louis et al.1 and Lombardi et al.2 were the first to describe ET as more than a motor disorder. In the past decade, various non-motor aspects of ET have been explored using different tools. With the addition of various non-motor aspects to the ET spectrum, it becomes important to understand disease pathophysiology and correlate these characteristics with the clinical findings observed in patients.
The basic question related to ET is whether it is a primary motor disease with secondary non- motor features or a neurodegenerative disease that simultaneously affects various neural domains. The purpose of this review is to highlight the various non-motor features in patients with ET and attempt to understand whether it should be considered a pure motor disease with secondary features or a neurodegenerative disease. It is important to characterize these features in order to address the various issues (other than motor symptoms) that these patients suffer, to understand basic ET pathophysiology, and to develop appropriate pharmacotherapies.
Methods
This systematic literature review was undertaken according to the relevant criteria of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. We searched the PubMed database through May 1, 2014 for original studies and review articles in patients with ET that evaluated features other than the motor components (tremor, rigidity, bradykinesia, postural instability, imbalance, chorea, dystonia). We applied a broad search strategy including the following terms: (essential tremor AND non-motor), (essential tremor AND cognition), (essential tremor AND non-motor AND depression), (essential tremor AND non-motor AND personality), (essential tremor AND non-motor AND anxiety), (essential tremor AND non-motor AND sleep), (essential tremor AND non-motor AND hearing), (essential tremor AND non-motor AND olfaction), and (essential tremor AND non-motor AND pain). The reference lists of included publications were also searched. Studies were eligible for inclusion if: 1) ET was evaluated; 2) features other than the motor components (tremor, rigidity, bradykinesia, postural instability, imbalance, chorea, dystonia) were studied; 3) patients were compared with controls; and 4) tremor and non-motor features were rated. The exclusion criteria were: 1) a lack of a control group and 2) a lack of appropriate rating scores.
Results
The PubMed database online search results were as follows.
Terms used:
| Key words | No of publications |
|---|---|
| essential tremor AND non-motor | 42 |
| essential tremor AND cognition | 96 |
| essential tremor AND non-motor AND depression | 13 |
| essential tremor AND non-motor AND personality | 6 |
| essential tremor AND non-motor AND anxiety | 10 |
| essential tremor AND non-motor AND sleep | 9 |
| essential tremor AND non-motor AND hearing | 6 |
| essential tremor AND non-motor AND olfaction | 4 |
| essential tremor AND non-motor AND pain | 3 |
Ten publications from the first search, eight from second, nine from the third, three from the fourth, four from the fifth, five from the sixth, two from the seventh, four from the eighth, and one from the ninth met the inclusion criteria. Some publications from the first search were duplicates of those found during subsequent searches; 36 publications were ultimately included in the analysis.
Question to be answered: primary disease feature versus secondary phenomenon
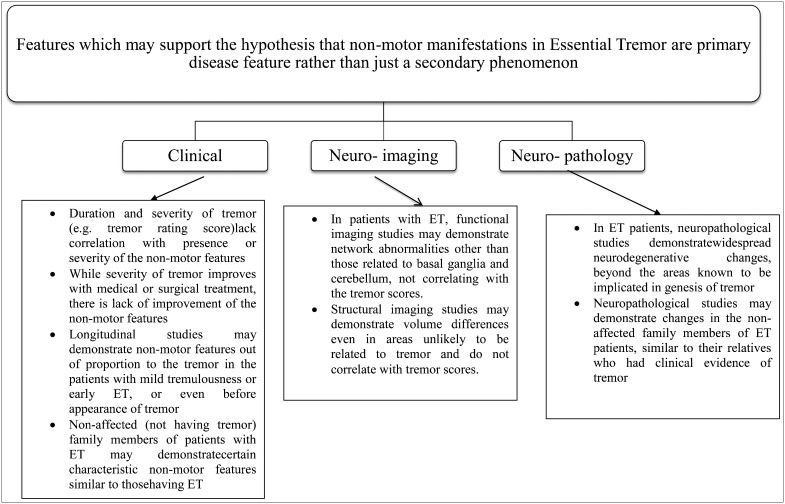
In order to understand whether non-motor features in ET are a primary or secondary feature of the disease, we need to ascertain facts from various clinical, imaging, and pathological studies as shown in Figure 1. Retrospective and prospective clinical studies correlating tremor rating scores with non-motor features should be examined to determine if there is a correlation between the two. If there is a significant correlation, it is likely that non-motor features are an epiphenomenon. Studies assessing non-motor features in non-affected members (who do not present with complaints of tremulousness) of patients with a family history of ET should be reviewed to identify differences in the spectrum of non-motor features between patients with tremor and their asymptomatic family members. A similar spectrum of non-motor features between these groups would clarify which features are primarily related to ET. Longitudinal studies highlighting the severity of non-motor features in patients with mild tremulousness or early ET should be reviewed to provide clues into the pathogenesis of non-motor features.
Figure 1. Features that May Support the Hypothesis that Non-motor Manifestations in ET are Primary Disease Features Rather than Secondary Phenomena. Features that may support the hypothesis that non-motor manifestations in ET are primary disease features rather than secondary phenomena.
Structural and functional neuroimaging studies have demonstrated network abnormalities and volume changes in ET patients; whether these changes correlate with non-motor features will clarify whether non-motor features in ET are a primary part of the disease or just a secondary phenomenon.
In this review, we highlight the various non-motor features of ET and attempt to understand the primary bases of these features to determine whether non-motor features in ET are a primary or secondary disease event.
Neuropathology
Lines of evidence from various neuroimaging,3 animal,4 and clinical studies5 have highlighted the role of the cerebellum and its connections in the pathogenesis of ET. In 2003, the Essential Tremor Centralized Brain Repository (ETCBR) was established to study ET brains.6 Post-mortem studies from the ETCBR have demonstrated two different types of patients with ET: the first is characterized by degenerative cerebellar changes (cerebellar ET), and the second features brainstem Lewy bodies with a relatively preserved cerebellum (LBVET).6 These post-mortem studies have highlighted the pathological heterogeneity of ET and its degenerative nature. This heterogeneity may account for the varied motor and non-motor features that are currently being explored.
Neuropathological studies in ET indicate that it is a neurodegenerative disorder, and like Parkinson's disease (PD), it includes a varied spectrum of manifestations that may be due to its degenerative process rather than a secondary phenomenon.
Cognition
Background
Tröster et al.7 were the first to evaluate the short-term effects of unilateral thalamic deep brain stimulation (DBS) on cognition, mood, and quality of life in patients with ET. The authors found that patients in their study improved in tasks of visuoperceptual and constructional ability, visual attention, delayed word list recognition, and prose recall, but lexical verbal fluency declined significantly after surgery.7 Due to the absence of a control group in this study, definite cognitive involvement could not be established. Lucas et al.8 described the effects of “ON” and “OFF” bilateral thalamic DBS on cognitive abilities and mood in an 80-year-old male with ET 3 months after electrode implantation. They reported reductions in verbal fluency and recall in the OFF state compared to when the stimulators were ON. In both of the above studies, there were clinical improvements and better cognitive profiles except for verbal fluency and recall. This provides evidence suggesting that non-motor cognitive involvement in ET is a secondary phenomenon, as improved tremor scores were associated with significant cognitive improvement, except for verbal fluency scores.
ET and PD have always been compared due to their phenomenological overlap. Gasparini et al.9 were first to explore frontal lobe dysfunction in patients with ET. Although their sample size was small (27 patients), their findings indicated that cognitive impairment in ET is similar to that observed in PD. They suggested that ET could represent an oligo-symptomatic cognitive variation of PD.9 Lombardi et al.2 published a study of 18 ET patients and reported deficits in verbal fluency, naming, recent memory, working memory, and mental set shifting. They attributed these findings to changes in the frontocerebellar circuits and stated that similar cognitive deficits were observed in patients with cerebellar lesions. Gasparini et al.9 did not evaluate tremor scores in patients with ET, making it difficult to interpret the primary or secondary involvement of these cognitive features in patients with ET. The authors reported similar cognitive impairment in patients with ET compared to PD, as highlighted by Gasparini et al.,9 which may suggest that cognitive involvement is a primary component of ET.
Lacritz et al.10 published a small clinical series of 13 non-demented ET patients that further confirmed the cognitive deficits in patients with ET. ET with cognitive dysfunction was only described in small clinical series until 2002, when a larger clinical study of 101 patients by Tröster et al.11 confirmed cognitive deficits in patients with ET and attributed these findings to cerebello-thalamo-cortical tract involvement. They did not find a significant relationship between patients' neuropsychological test scores and tremor severity (Fahn-Tolosa-Marin [FTM] Tremor Rating Scale), indicating that cognitive deficits in ET are a primary phenomenon. A large population-based study was needed to generalize the findings and actually understand the basis of cognitive involvement in patients with ET. This was published by the Neurological Disorders in Central Spain (NEDICES) group, which performed a cohort study of 5,278 individuals that surveyed common and important neurological disorders in the elderly.12 ET was one of the disorders analyzed in detail by this group.12,13 In their subsequent analysis, they performed a neuropsychological assessment that included tests of global cognitive performance, frontal executive function, verbal fluency, and memory in 232 patients with ET and 696 matched controls.14 They reported that cases performed more poorly on formal neuropsychological testing than controls, but tremor duration did not correlate with the tremor scores.14 This group also found increases in mild cognitive impairment and dementia in patients with ET, which was later corroborated by studies of a community-based cohort in New York.15,16 Passamonti et al.17 used functional magnetic resonance imaging (fMRI) to examine verbal memory dysfunction in patients with ET and attributed this deficit to altered cortico-cerebellar pathway alterations. Ceresa et al.18 administered an fMRI Stroop task and demonstrated that patients with ET must exert additional cognitive effort to achieve comparable performance levels on tests of attention and execution.
Below, we review each cognitive domain and elucidate the origins of non-motor symptoms in ET.
Deficits in executive dysfunction
The term “executive function” includes cognitive processes and behavioral competencies that include verbal reasoning, problem solving, planning, sequencing, the ability to sustain attention, resistance to interference, utilization of feedback, multitasking, cognitive flexibility, and the ability to deal with novelty.19 Table 1A highlights the tests used to assess executive functions in various clinical and population-based studies that assessed both patient and control populations. Results of the Wisconsin Card Sorting Test (WCST), Stroop test, and Trail Making Test (TMT) were found to be abnormal in many studies (Table 1A). Very few studies have correlated tremor severity scores with cognitive abnormalities in patients with ETs.2,11,20,21 Most studies failed to find any significant correlation between the tremor score (FTM Tremor Rating Scale) and executive dysfunction.2,11,21 Kim et al.21 performed a prospective study of 47 ET patients and reported a weak association between frontal executive functions and tremor severity scores. Their study was more robust because they accounted for various confounding factors. Recently, Bhalsing et al.22 performed structural imaging in ET patients with and without cognitive deficits. They reported a significant positive correlation between neuropsychological scores and gray matter volumes in various brain structures but did not find a significant correlation between tremor score and gray matter volume or neuropsychological scores. These clinical and neuroimaging studies support the hypothesis that cognitive abnormalities in ET may be a primary phenomenon related to the disease rather than a secondary effect of the disease processes. Conversely, Sahin et al.20 assessed 16 young ET patients and reported a significant inverse correlation between WCST scores and tremor scores (Clinical Tremor Rating Score). They also found a significant inverse correlation between cerebral blood flow in the frontal cortices and tremor severity scores.20 This study may have reached different conclusions due to the small sample size, its use of the Clinical Tremor Rating Scale compared to other studies that used the FTM Tremor Rating Scale, and the younger age of the patients. In an fMRI study, Passamonti et al.17 reported that the FTM scores were negatively correlated with functional connectivity between the source or seed and ventrolateral prefrontal cortex suggesting executive dysfunction to be a secondary phenomenon due to tremor in ET.
Table 1. Literature Review of Cognitive Functions in Patients with ET.
| A) Executive Dysfunction Deficits | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gasparini et al.9 | Lombardi et al.2 | Tröster et al.11 | Sahin et al.20 | Duane et al.25 | León et al.14 | Higginson et al.23 | Kim et al.24 | |
| WCST | ++ | ++ | NS | NS | NS | NP | NP | NP |
| Verbal fluency test (FAS) | ++ | NP | NP | NP | NP | NP | NS | NP |
| Verbal fluency (letters and animals) | NP | ++ | ++ | NP | NP | ++ | NP | NP |
| Stroop test | ++ | NP | + | ++ | NP | NP | NP | NP |
| Towers of London and Hanoi | NS | NP | NP | NS | NP | NP | NP | NP |
| Matrix reasoning | NP | ++ | NP | NP | NP | NP | NS | NP |
| TMT A | ++ | NP | NP | NS | NP | ++ | NP | NP |
| TMT B | NP | NP | NP | NS | NP | NP | NP | NP |
| Controlled oral word-association test | NP | NP | NP | NP | NP | NP | NP | ++ |
| B) Deficits in Attention and Concentration | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gasparini et al.9 | Lombardi et al.2 | Tröster et al.11 | Sahin et al.20 | Duane et al.25 | León et al.14 | Higginson et al.23 | Kim et al.24 | |
| WAIS-R DS-forward | NP | ++ | NP | NS | NS | NP | ++ | ++ |
| WAIS-R DS-backward | NP | ++ | NP | NS | NS | NP | ++ | ++ |
| Verbal fluency K-A-S | NP | NP | NP | ++ | NP | NP | NP | NP |
| Symbol search | NP | NP | NP | NP | NP | NP | ++ | NP |
| Brief test of attention | NP | NP | ++ | NP | NP | NP | NP | NP |
| Spatial span | NP | NS | NP | NP | NP | NP | NP | NP |
| Letter–number sequencing | NP | ++ | NP | NP | NP | NP | NP | NP |
| Letter cancellation task | NP | NP | NP | NP | ++ | NP | NP | NP |
| C) Memory Deficits | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gasparini et al.9 | Lombardi et al.2 | Tröster et al.11 | Sahin et al.20 | Duane et al.25 | León et al.14 | Higginson et al.23 | Kim et al.24 | |
| CVLT (total) | NP | ++ | ++ | ++ | NP | NP | NP | NP |
| CVLT (short delay free recall) | NP | ++ | ++ | NS | NP | ++ | NP | NP |
| CVLT (long delay free recall) | NP | ++ | ++ | NS | NP | ++ | NP | NP |
| CVLT (semantic clustering) | NP | NS | ++ | NS | NP | NP | NP | NP |
| CVLT (serial clustering) | NP | NS | ++ | NS | NP | NP | NP | NP |
| CVLT (slope) | NP | NS | NS | NS | NP | NP | NP | NP |
| CVLT (recognition discrimination) | NP | ++ | ++ | ++ | NP | NP | NP | NP |
| WMS (figural memory) | NP | NP | NS | NP | NP | NP | ++ | NP |
| WMS (logical memory I) | NP | NP | NS | NP | NP | ++ | NP | |
| WMS (logical memory II) | NP | NP | NS | NP | NP | ++ | NP | |
| WMS (logical memory percent retention) | NP | NP | NS | NP | NP | NP | NP | NP |
| ROF | NP | NP | NP | NP | NS | NP | NS | ++ (recognition) |
| Three-word registration | NP | NP | NP | NP | NP | NP | NP | NS |
| Three-word recall | NP | NP | NP | NP | NP | NP | NP | NS |
| HVLT | NP | NP | NP | NP | NP | NP | NP | ++ (recall) |
| D) Visuospatial Function Deficits | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gasparini et al.9 | Lombardi et al.2 | Tröster et al.11 | Sahin et al.20 | Duane et al.25 | León et al.14 | Higginson et al.23 | Kim et al.24 | |
| Benton Facial Recognition | NP | NS | ++ | ++ | NP | NP | NP | NP |
| Benton line Orientation | NP | NP | NP | ++ | NP | NP | NP | NP |
| Block design | NP | NP | NP | NS | NP | NP | NP | NP |
| HVOT | NP | NS | NS | ++ | NP | NP | NP | NP |
Abbreviations: +, Significant Difference Between the Patients and Controls; ++, XXX; CVLT, California Verbal Learning Test; FAS, XXX; HVLT, Hopkins Verbal Learning Test; HVOT, Hooper Visual Organization Test; K-A-S, test with (K.A and S letters); NP, Not Performed; NS, No Significant Difference Between Patients and Controls; ROF, Rey-Osterreith Complex Figure; TMT, Trail Making Test; WAIS-R DS, Wechsler Adult Intelligence Scale Digit Span; WCST, Wisconsin Card Sorting Test; WMS, Wechsler Memory Scale.
Attention and concentration deficits
Most published literature has highlighted the presence of impaired attention and concentration in patients with ET.2,23,24 Different neuropsychological batteries were performed by various studies, with digit span forward and backward being the most frequently used tests. These test results were abnormal in most studies,2,23,24 except those reported by Duane et al.25 and Sahin et al.20 These differences may be due to the fact that younger patients were included in these studies. The results of other less commonly used tests, such as the brief test of attention11 and symbol search test23 were reported to be abnormal in ET (Table 1B). Overall, most studies demonstrated attention deficits in ET, with digit span, TMT A, and the brief test of attention consistently showing abnormal tests.15 None of the studies found a significant correlation between attention and concentration deficits and tremor scores, but large prospective studies are essential to systematically analyze all of these domains. Therefore, the available evidence supports the view that attention deficits may be a primary manifestation of the disease rather than secondary to tremor.
Memory deficits
Most of the studies exploring the non-motor features in ET have evaluated patients for explicit memory and described significant abnormalities.2,11,20 Various neuropsychological tests were employed to test this domain (Table 1C). Lombardi et al.2 were the first to report deficits in short- and long-term recall and semantic and series clustering by using the California Verbal Learning Test (CVLT). This observation was later supported by Benito et al.14 and Tröster et al.11 On the contrary, Sahin et al.20 did not find any significant differences in CVLT scores between patients with ET and controls, which again may be attributed to the small, young cohort of ET patients.
Again, there were conflicting reports when memory was assessed using a different neuropsychological battery, such as the complete Wechsler Memory Scale (WMS) and its subscales.15 Higginson et al.23 assessed a cohort of 18 patients with ET and found significant impairment on the WMS. However, Tröster et al.11 reported significant memory impairment using the CVLT in 101 patients with ET but failed to replicate this finding using the WMS test in the same subjects. This implies that the tools used to assess memory can significantly influence the results.
Visual memory was investigated with the Rey-Osterreith Complex (ROF) test in three studies. There were no significant differences between ET patients and controls in two studies,23,25 whereas, Kim et al.24 reported significant impairment in the recognition subscale of the ROF.
Kronenbuerger et al.26 tested implicit memory in patients with ET using the eye blink reflex, which is a well-established paradigm for assessing motor learning. They reported abnormal implicit memory in patients with ET but did not find a significant correlation between conditioned reflex scores and tremor severity.
Overall, the available evidence suggests that patients with ET have deficits in explicit and working memory, with the most abnormal results on the CVLT. However, none of the studies described any significant correlation between the neuropsychological scores and clinical tremor scores, again suggesting that memory deficits are likely to be a primary phenomenon in ET.
Visuospatial function deficits
There is a dearth of information regarding visuospatial functions in ET.2,11,20 Lombardi et al.,2 Tröster et al.,11 and Sahin et al.20 used the Benton facial recognition test, Benton line orientation, and the Hooper visual organization test to explore these deficits in patients (Table 1D). In addition the latter group20 also used a block design to further evaluate the patients' deficits. While Lombardi et al.2 did not find significant visuospatial abnormalities in ET, Sahin et al.20 reported visuospatial abnormalities in most of the tests except the block design. Tröster et al.11 reported mixed results.
Overall, visuospatial domain impairment still needs to be explored in patients with ET to determine whether it is a primary or secondary non-motor feature of ET.
Personality
The hypothesis that ET is more than a motor disorder has become established in recent years, with many series highlighting cognitive changes in patients. Personality changes have been reported as a prominent non-motor feature in several other neurodegenerative disorders, including Huntington's disease and PD.27 Therefore, the possibility that personality changes occur in patients with ET needs to be explored. Chatterjee et al.27 used the tridimensional personality questionnaire (TPQ) in 55 patients with ET to explore their personality profiles. It was for the first time reported that patients with ET were assessed for harm avoidance (HA). However, HA subscale scores did not correlate with tremor severity or subjective and objective disability scales, suggesting that the personality profile observed may be a primary disorder in ET, as seen in many other neurodegenerative disorders. The above observations were further validated in a subsequent study by Thenganatt et al.28 in 60 patients using the same TPQ questionnaire. They also did not find any significant difference between the HA sub scores and tremor severity and duration of the tremor. A large study of 105 patients by Lorenz et al.29 employed the revised version of the Eysenck Personality Questionnaire (EPQ-R) in a German population. The EPQ-R measures three dimensions of personality: extraversion (E), neuroticism (N), and psychoticism (P).29 They reported a significantly lower score on the P-scale of the EPQ-R, suggesting that ET patients are kinder, more tender-minded, and less aggressive than the normal population.29 There was no significant correlation between EPQ scores and tremor severity or duration reported by Lorenz et al.29
All the studies exploring personality profile in ET patients have reported HA personality without correlations with tremor scores or duration (Table 2), suggesting that personality changes may be a primary feature of the disease. Larger follow-up studies are required to characterize ET patient personality to ascertain whether this particular personality profile is a pre-morbid or co-morbid feature of the illness.
Table 2. Review of Literature on Personality Changes in Patients with ET.
| Chatterjee et al.27 | Lorenz et al.29 | Thenganatt et al.28 | |
|---|---|---|---|
| No. of Patients | 55 | 105 | 60 |
| Scale used | TPQ | EPQ-R | TPQ |
| Results | Personality profile characterized by greater HA | ET patients of this study are kinder, more tender-minded, and less aggressive than the normal population | Personality profile characterized by greater HA |
| Comments | ET patients are pessimistic, fearful, shy, anxious, and easily fatigued. There were a lot of non-responders in their study, which was a major limitation | ET patients are kinder, tender minded and less aggressive | ET patients are pessimistic worriers who tend to anticipate harm and failure and they have difficulties getting over humiliating and embarrassing experiences |
Abbreviations: EPQ-R, Eysenck Personality Questionnaire; ET, Essential Tremor; HA, Harm Avoidance; TPQ, Tridimensional Personality Questionnaire.
Neuropsychiatric manifestations
Anxiety
Tröster et al.7 studied 40 patients with ET who underwent unilateral thalamic DBS and exhibited reduced anxiety levels (performed by measures of mood state) 3 and 12 months after surgery.30 This improvement of anxiety post-surgery may have been due to the improvement of the tremor or improving secondary thalamic connections. Louis et al.1 assessed correlates of functional disability in 37 patients with ET and determined whether psychological factors were associated with functional disability in these patients. They reported that anxiety was associated with greater self-reported disability and with more difficulty on a performance-based test.1 Their findings were independent of tremor severity.1 Dogu et al.31 carried out a door-to-door survey in the Mersey province of Turkey and identified 89 individuals with ET. They compared these cases with healthy controls and found significantly higher anxiety levels in patients with ET compared to controls, and this finding correlated with tremor severity. Tan et al.32 reported ET patients in the Asian population to be more anxious, and this correlated with tremor severity but not duration. A similar study performed by Chandran et al.33 in 50 Asian subjects with ET used the Hamilton Anxiety Rating Scale (HARS) and reported higher anxiety scores in patients, but tremor severity scores did not correlate with HARS scores.
In summary, the current evidence supports the hypothesis that ET patients are more anxious compared to controls, and anxiety significantly affects their ability to perform daily activities (Table 3). However, there have been conflicting reports regarding the correlation of anxiety with tremor scores. These conflicting results make it difficult to say whether anxiety is primarily related to ET or if it is a secondary phenomenon.
Table 3. Review of Literature on Anxiety in Patients with ET.
| Louis et al.1 | Dogu et al.31 | Tan et al.32 | Chandran et al.33 | |
|---|---|---|---|---|
| No. of Patients | 37 | 89 | 84 | 50 |
| Scale used | HARS | HARS | SCL-90R | HARS |
| Result | ++ | ++ | ++ | ++ |
| Correlation with tremor | — | ++ | ++ | — |
Abbreviations: ++, Significant Difference Between Patients and Controls with Patients Having More Anxiety Than Controls; —, No Significant Difference Between Patients and Controls; ET, Essential Tremor; HARS, Hamilton Anxiety Rating Scale; SCL-90R, Symptom Checklist-90R.
Depression
Depression is an important non-motor feature in patients with ET and has been extensively studied (Table 4). Various reports, ranging from small case-control studies2 to large door-to-door surveys34 have highlighted that depression is a part of ET. Lombardi et al.2 were among the first to systematically evaluate depression in 18 patients with ET using the Geriatric Depression Scale (GDS). They found significant depression in patients that did not correlate with tremor severity scores.2 During the same period, Lacritz et al.10 performed a small study of 13 patients and reported mild to moderate depression in 4 of the 13 patients using the Beck Depression Inventory (BDI) but did not find a correlation of these findings with the tremor severity score. Dogu et al.31 and Louis et al.34 conducted two large door-to-door surveys and reported significant depression in patients with ET. Chandran et al.33 carried out a survey of 89 patients and reported that Hamilton Depression Rating Scale (HDRS) scores correlated with the patients' tremor scores, which contradicted the report by Lombardi et al.2 Louis et al.35 along with the NEDICES Study Group studied morale in ET patients by applying the Philadelphia Geriatric Center Morale Scale (PGCMS) and reported a significant difference. In their study, PGCMS score and Agitation and Lonely Dissatisfaction subscores were lower in ET patients compared to controls, but PGCMS score did not correlate with the tremor duration or tremor severity scores.35 According to them, lower morale could in part be a proxy for mild, untreated depression.35
Table 4. Review of Literature on Depression in Patients with ET.
| Study | Lombardi et al.2 | Lacritz et al.10 | Louis et al.1 | Dogu et al.31 | Miller et al.*12 | Louis et al.34 | Louis et al.35 | Li et al.**36 | Chandran et al.33 |
|---|---|---|---|---|---|---|---|---|---|
| No. of patients | 18 | 13 | 89 | 89 | 53 | 235 | 187 | 61 | 50 |
| Scale used | GDS | BDI | Depression module of the Structured Clinical Interview for DSM-IV | HDRS | BDI | Self-reported depression | PGCMS | MADRS | HDRS |
| Results | Significantly increased (p<0.02) depression scores in patients compared to controls | 4 in 13 (∼30%) patients had depression (p values not reported) | Found that current major depression was associated with greater self-reported disability | Significantly increased HDRS scores (p = 0.003) in patients compared to controls | No significant difference in depression scores between PD, dystonia and ET | Significantly increased self reported depression (p<0.001) in patients compared to controls | Significantly increased (p = 0.006) PGCMS score in patients compared to controls | Depressive symptoms in ET patients possess distinct characteristics compared to those in depressed patients without ET | Significantly increased (p = 0.009) HDRS scores in patients compared to controls |
| Significant correlation of depression scores with tremor severity | No | INA | No | Yes | Yes | INA | INA | Yes | Yes |
Abbreviations: *, Comparison Among PD, ET, and dystonia; **, Comparison Between ET Patients with Depression and Major Depressive Patients Without ET; BDI, Beck Depression Inventory; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders-IV; ET, Essential Tremor; GDS, Geriatric Depression Scale; HDRS, Hamilton Depression Rating Scale; INA, Information Not Available; MADRS, Montgomery-Asberg Depression Rating Scale; PD, Parkinson's Disease; PGCMS, Philadelphia Geriatric Center Morale Scale.
Li et al.36 assessed 61 depressed patients with ET and 112 depressed patients without ET with the Montgomery-Asberg Depression Rating Scale (MADRS) and reported distinct characteristics of depression in ET patients. They also found that ET patients had more problems in concentrating; experienced lassitude; and reported less on items such as “reported sadness,” “inability to feel,” and “pessimistic thoughts” compared to patients with depression without ET.36
Therefore, the existing evidence indicates that anxiety and depression are also important non-motor manifestations of ET. There are conflicting reports on its correlation with tremor severity, thereby keeping the debate open as to whether these mood changes are an integral part of ET or merely a secondary phenomenon.
Sleep
In their autopsy study of 33 patients with ET and 21 controls, Louis et al.37 highlighted the presence of Lewy bodies in the brainstem, mainly in the locus coeruleus. Among its various functions, locus coeruleus is known to play an active role in sleep regulation. Adler et al.38 compared the prevalence of rapid-eye movement (REM) sleep behavior disorder (RBD) and excessive daytime sleepiness (EDS) in patients with ET, PD and restless legs syndrome (RLS) with that in control subjects. They failed to find any significant difference in the Epworth Sleepiness Scale (ESS) score between ET patients (mean score of 5.6±3.7) and controls (with mean score of 5.2±3.7), whereas there was a significant difference in ESS scores between patients with PD and RLS compared to the controls.38 Gerbin et al.39 assessed sleep using ESS and the Pittsburgh Sleep Quality Index (PSQI) in 120 ET cases and 40 PD cases. They found that sleep scores were in between the PD patients and controls, but there no was significant difference in adjusted ESS and PSQI scores between patients with ET and controls. Although Chandran et al.33 did not find a significant difference in ESS scores in their study of 50 patients with ET and controls; they observed a significant difference in PSQI scores between the two groups. These results were different than the findings reported by Gerbind et al.39 These differences may be due to the adjusted ESS and PSQI scores used by Gerbind et al.,39 who considered various factors, such as gender, work status, smoking, and caffeine intake, medications with sleep effects, disease duration, and depression.
Leon et al.40 performed a prospective, population-based study of individuals >65 years old and found a significant relation between short sleep duration and an increased risk of ET. Their findings suggest that decreased sleep duration may be a risk for ET.
Evidence from several studies has revealed sleep dysregulation is present in ET similar to that present in PD.41 However, large follow-up studies are required to validate these findings.
Color vision
Dopamine localized in the amacrine and interplexiform retinal cells modulates the center-surround antagonism of the receptive visual field.42 Though patients with PD do not complain of blurring, impaired color vision is a well-documented non-motor feature of PD.42 A few studies have attempted to determine whether there are color vision abnormalities in ET as seen in patients with PD, due to the overlap of some symptoms between the two diseases.43,44 Oh et al.43 studied 36 patients with ET and reported no significant difference in the total error scores between patients and controls using the Farnsworth-Munsell 100 Hue test. Louis et al.44 described similar results in 55 patients with ET.
Although there are overlapping features between PD and ET, color vision is an isolated non-motor feature of PD and probably is not affected in patients with ET. However, more prospective studies are needed to validate these findings.
Hyperhidrosis
Hyperhidrosis as a manifestation of ET was initially reported in the early 20th century and later by Edmund Critchley in 1972.45 Diamond et al.46 described a case of hyperhidrosis as a complication of DBS in a patient with ET. However, since these reports, there has been no published literature regarding an association between hyperhidrosis and ET.
Pain
Chandran et al.33 used the Brief Pain Inventory (BPI) scale and reported increases in pain severity and interference in patients with ET. However, they did not find a correlation between BPI scores and tremor severity score or duration. These findings indicate that pain may be a primary feature of ET, but further evidence from larger studies is needed to provide conclusive evidence.
Olfaction
With growing evidence that ET is a neurodegenerative disorder, neuroscientists have explored olfactory functions in ET because they are altered in other neurodegenerative diseases, such as PD. Louis et al.47 assessed 37 ET patients and reported significantly low mean University of Pennsylvania Smell Identification Test (UPSIT) scores compared to controls, but these scores did not correlate with tremor severity or duration. The same group of authors further extended their study in 87 ET patients and reported similar low UPSIT scores that also were not correlated with tremor severity.48 Conversely, Busenbark et al.49 reported no significant differences in UPSIT scores between 16 ET patients and controls, and Shah et al.50 failed to find any significant difference in UPSIT scores and olfactory event-related potentials (OERPs) between 59 ET patients and controls.
In summary, these contradictory reports are not sufficient to support the conclusion that olfactory dysfunction is a primary non-motor feature in patients with ET.
Hearing
To date, only two large studies have evaluated hearing function in patients with ET, and both suggested that auditory pathways may be affected in ET. Ondo et al.51 were the first to assess hearing in 250 patients with ET using the Nursing Home Hearing Handicap Index (NHHI). They reported that patients with ET had greater hearing disability compared to controls, and this impairment correlated with tremor severity. In a large population-based case-control study in 248 ET patients, Leon et al.52 reported a significant hearing impairment in ET patients compared to the large population control. They did not report the patients' tremor severity scores.
Conclusions
ET is a pathologically heterogeneous neurodegenerative disorder with a spectrum of motor and non-motor features. It is debated whether non-motor manifestations in ET result from widespread neurodegeneration or are merely secondary to impaired motor functions and reduced quality of life resulting from tremor. There is literature supporting the hypothesis that ET patients have characteristic personality traits; impairment in several domains of cognition; mood changes (increased anxiety and depression); and altered sleep, color vision, olfaction, and hearing. Several studies have reported that the severity of some of these non-motor features (mainly impaired cognition) is not correlated with tremor duration or severity, thereby indicating that these non-motor features are probably a primary feature in ET.
Longitudinal studies of large cohorts of patients with ET are required to validate the available evidence. Further information is required on the prevalence, evolution, natural history, and prognosis of various non-motor-symptoms in ET; their correlation (if any) with tremor duration and severity; and the effect of tremor treatment (both medical and surgical). Finally, studies assessing impairment in attention and memory and the utility of cholinesterase inhibitors, such as donepezil, galantamine, and rivastigminein, in patients with ET may provide useful information.53
Since ET can develop at any stage of life and there are no confirmed biomarkers or genetic markers to make a pre-clinical diagnosis, the apparently healthy controls in various studies may not truly represent a cohort who will not develop ET in the future. Pending useful biomarkers for ET, a detailed family history and a meticulous examination for the presence of tremor in the family members of the controls should be considered in future studies.
In addition, most studies are cross sectional. Follow-up studies are essential for determining the new occurrence of non-motor features or progression of existing non-motor features in ET patients, as well as the occurrence of ET in “healthy” controls.
Finally, it needs to be determined whether treatment of the motor symptoms (tremor) simultaneously improves the non-motor features, which would indicate that the non-motor features are secondary. It is likely that the patients with ET in these various studies who present to the hospital represent a small proportion of patients in the community. For this reason, more emphasis should be placed on population-based studies.
Footnotes
Funding: None.
Financial Disclosures: None.
Conflict of Interests: The authors report no conflict of interest.
References
- 1.Louis ED, Barnes L, Albert SM, et al. Correlates of functional disability in essential tremor. Mov Disord. 2001;16:914–920. doi: 10.1002/mds.1184. [DOI] [PubMed] [Google Scholar]
- 2.Lombardi WJ, Woolston DJ, Roberts JW, Gross RE. Cognitive deficits in patients with essential tremor. Neurology. 2001;57:785–790. doi: 10.1212/wnl.57.5.785. [DOI] [PubMed] [Google Scholar]
- 3.Bucher SF, Seelos KC, Dodel RC, Reiser M, Oertel WH. Activation mapping in essential tremor with functional magnetic resonance imaging. Ann Neurol. 1997;41:32–40. doi: 10.1002/ana.410410108. [DOI] [PubMed] [Google Scholar]
- 4.Martin FC, Thu Le A, Handforth A. Harmaline-induced tremor as a potential preclinical screening method for essential tremor medications. Mov Disord. 2005;20:298–305. doi: 10.1002/mds.20331. [DOI] [PubMed] [Google Scholar]
- 5.Deuschl G, Wenzelburger R, Loffler K, Raethjen J, Stolze H. Essential tremor and cerebellar dysfunction clinical and kinematic analysis of intention tremor. Brain. 2000;123:1568–1580. doi: 10.1093/brain/123.8.1568. [DOI] [PubMed] [Google Scholar]
- 6.Louis ED, Vonsattel JP. The emerging neuropathology of essential tremor. Mov Disord. 2008;23:174–182. doi: 10.1002/mds.21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tröster AI, Fields JA, Pahwa R, et al. Neuropsychological and quality of life outcome after thalamic stimulation for essential tremor. Neurology. 1999;53:1774–1780. doi: 10.1212/wnl.53.8.1774. [DOI] [PubMed] [Google Scholar]
- 8.Lucas JA, Rippeth JD, Uitti RJ, Shuster EA, Wharen RE. Neuropsychological functioning in a patient with essential tremor with and without bilateral VIM stimulation. Brain Cogn. 2000;42:253–267. doi: 10.1006/brcg.1999.1103. [DOI] [PubMed] [Google Scholar]
- 9.Gasparini M, Bonifati V, Fabrizio E, et al. Frontal lobe dysfunction in essential tremor: a preliminary study. J Neurol. 2001;248:399–402. doi: 10.1007/s004150170181. [DOI] [PubMed] [Google Scholar]
- 10.Lacritz LH, Dewey R, Jr, Giller C, Cullum CM. Cognitive functioning in individuals with “benign” essential tremor. J Int Neuropsychol Soc. 2002;8:125–129. doi: 10.1017/s1355617702001121. [DOI] [PubMed] [Google Scholar]
- 11.Tröster AI, Woods SP, Fields JA, et al. Neuropsychological deficits in essential tremor: an expression of cerebello-thalamo-cortical pathophysiology? Eur J Neurol. 2002;9:143–151. doi: 10.1046/j.1468-1331.2002.00341.x. [DOI] [PubMed] [Google Scholar]
- 12.Morales JM, Bermejo FP, Benito-Leon J, et al. Methods and demographic findings of the baseline survey of the NEDICES cohort: a door-to-door survey of neurological disorders in three communities from Central Spain. Public Health. 2004;118:426–433. doi: 10.1016/j.puhe.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Benito-León J, Bermejo-Pareja F, Louis ED, Neurological Disorders in Central Spain (NEDICES) Study Group Incidence of essential tremor in three elderly populations of central Spain. Neurology. 2005;64:1721–1725. doi: 10.1212/01.WNL.0000161852.70374.01. [DOI] [PubMed] [Google Scholar]
- 14.Benito-León J, Louis ED, Bermejo-Pareja F, Neurological Disorders in Central Spain (NEDICES) Study Group Population-based case-control study of cognitive function in essential tremor. Neurology. 2006;66:69–74. doi: 10.1212/01.wnl.0000192393.05850.ec. [DOI] [PubMed] [Google Scholar]
- 15.Bermejo-Pareja F, Puertas-Martín V. Cognitive features of essential tremor: a review of the clinical aspects and possible mechanistic underpinnings. Tremor Other Hyperkinet Mov (N Y) 2012;2 doi: 10.7916/D89W0D7W. pii: 02-74-541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thawani SP, Schupf N, Louis ED. Essential tremor is associated with dementia: prospective population-based study in New York. Neurology. 2009;73:621–625. doi: 10.1212/WNL.0b013e3181b389f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Passamonti L, Novellino F, Cerasa A, et al. Altered cortical-cerebellar circuits during verbal working memory in essential tremor. Brain. 2011;134:2274–2286. doi: 10.1093/brain/awr164. [DOI] [PubMed] [Google Scholar]
- 18.Cerasa A, Passamonti L, Novellino F, et al. Fronto-parietal overactivation in patients with essential tremor during Stroop task. Neuroreport. 2010;21:148–151. doi: 10.1097/WNR.0b013e328335b42c. [DOI] [PubMed] [Google Scholar]
- 19.Chan RC, Shum D, Toulopoulou T, Chen EY. Assessment of executive functions: review of instruments and identification of critical issues. Arch Clin Neuropsychol. 2008;23:201–216. doi: 10.1016/j.acn.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Sahin HA, Terzi M, Ucak S, Yapici O, Basoglu T, Onar M. Frontal functions in young patients with essential tremor: a case comparison study. J Neuropsychiatry Clin Neurosci. 2006;18:64–72. doi: 10.1176/jnp.18.1.64. [DOI] [PubMed] [Google Scholar]
- 21.Kim JS, Song IU, Shim YS, et al. Impact of tremor severity on cognition in elderly patients with essential tremor. Neurocase. 2010;16:50–58. doi: 10.1080/13554790903193216. [DOI] [PubMed] [Google Scholar]
- 22.Bhalsing KS, Upadhyay N, Kumar KJ, et al. Association between cortical volume loss and cognitive impairments in essential tremor. Eur J Neurol. 2014;21:874–883. doi: 10.1111/ene.12399. [DOI] [PubMed] [Google Scholar]
- 23.Higginson CI, Wheelock VL, Levine D, King DS, Pappas CT, Sigvardt KA. Cognitive deficits in essential tremor consistent with frontosubcortical dysfunction. J Clin Exp Neuropsychol. 2008;30:760–765. doi: 10.1080/13803390701754738. [DOI] [PubMed] [Google Scholar]
- 24.Kim JS, Song IU, Shim YS, et al. Cognitive impairment in essential tremor without dementia. J Clin Neurol. 2009;5:81–84. doi: 10.3988/jcn.2009.5.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duane DD, Vermilion KJ. Cognitive deficits in patients with essential tremor. Neurology. 2002;58:1706. doi: 10.1212/wnl.58.11.1706. author reply. [DOI] [PubMed] [Google Scholar]
- 26.Kronenbuerger M, Gerwig M, Brol B, Block F, Timmann D. Eyeblink conditioning is impaired in subjects with essential tremor. Brain. 2007;130:1538–1551. doi: 10.1093/brain/awm081. [DOI] [PubMed] [Google Scholar]
- 27.Chatterjee A, Jurewicz EC, Applegate LM, Louis ED. Personality in essential tremor: further evidence of non-motor manifestations of the disease. J Neurol Neurosurg Psychiatry. 2004;75:958–961. doi: 10.1136/jnnp.2004.037176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thenganatt MA, Louis ED. Personality profile in essential tremor: a case-control study. Parkinsonism Relat Disord. 2012;18:1042–1044. doi: 10.1016/j.parkreldis.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenz D, Schwieger D, Moises H, Deuschl G. Quality of life and personality in essential tremor patients. Mov Disord. 2006;21:1114–1118. doi: 10.1002/mds.20884. [DOI] [PubMed] [Google Scholar]
- 30.Fields JA, Tröster AI, Woods SP, et al. Neuropsychological and quality of life outcomes 12 months after unilateral thalamic stimulation for essential tremor. J Neurol Neurosurg Psychiatry. 2003;74:305–311. doi: 10.1136/jnnp.74.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dogu O, Louis ED, Sevim S, Kaleagasi H, Aral M. Clinical characteristics of essential tremor in Mersin, Turkey—a population-based door-to-door study. J Neurol. 2005;252:570–574. doi: 10.1007/s00415-005-0700-8. [DOI] [PubMed] [Google Scholar]
- 32.Tan EK, Fook-Chong S, Lum SY, et al. Non-motor manifestations in essential tremor: use of a validated instrument to evaluate a wide spectrum of symptoms. Parkinsonism Relat Disord. 2005;11:375–380. doi: 10.1016/j.parkreldis.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Chandran V, Pal PK, Reddy JY, Thennarasu K, Yadav R, Shivashankar N. Non-motor features in essential tremor. Acta Neurol Scand. 2012;125:332–337. doi: 10.1111/j.1600-0404.2011.01573.x. [DOI] [PubMed] [Google Scholar]
- 34.Louis ED, Benito-León J, Bermejo-Pareja F, Neurological Disorders in Central Spain (NEDICES) Study Group Self-reported depression and anti-depressant medication use in essential tremor: cross-sectional and prospective analyses in a population-based study. Eur J Neurol. 2007;14:1138–1146. doi: 10.1111/j.1468-1331.2007.01923.x. [DOI] [PubMed] [Google Scholar]
- 35.Louis ED, Benito-León J, Bermejo-Pareja F, Neurological Disorders in Central Spain (NEDICES) Study Group Philadelphia Geriatric Morale Scale in essential tremor: a population-based study in three Spanish communities. Mov Disord. 2008;23:1435–1440. doi: 10.1002/mds.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li ZW, Xie MJ, Tian DS, et al. Characteristics of depressive symptoms in essential tremor. J Clin Neurosci. 2011;18:52–56. doi: 10.1016/j.jocn.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 37.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 38.Adler CH, Hentz JG, Shill HA, et al. Probable RBD is increased in Parkinson's disease but not in essential tremor or restless legs syndrome. Parkinsonism Relat Disord. 2011;17:456–458. doi: 10.1016/j.parkreldis.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerbin M, Viner AS, Louis ED. Sleep in essential tremor: a comparison with normal controls and Parkinson's disease patients. Parkinsonism Relat Disord. 2012;18:279–284. doi: 10.1016/j.parkreldis.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benito-Leon J, Louis ED, Bermejo-Pareja F. Short sleep duration heralds essential tremor: a prospective, population-based study. Mov Disord. 2013;28:1700–1707. doi: 10.1002/mds.25590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Driver-Dunckley ED, Adler CH. Movement disorders and sleep. Neurol Clin. 2012;30:1345–1358. doi: 10.1016/j.ncl.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 42.Pieri V, Diederich NJ, Raman R, Goetz CG. Decreased color discrimination and contrast sensitivity in Parkinson's disease. J Neurol Sci. 2000;172:7–11. doi: 10.1016/s0022-510x(99)00204-x. [DOI] [PubMed] [Google Scholar]
- 43.Oh YS, Kim JS, Chung SW, et al. Color vision in Parkinson's disease and essential tremor. Eur J Neurol. 2011;18:577–583. doi: 10.1111/j.1468-1331.2010.03206.x. [DOI] [PubMed] [Google Scholar]
- 44.Louis ED, Gerbin M, Viner AS. Color vision: a study of essential tremor cases versus normal controls. Eur J Neurol. 2012;19:1136–1139. doi: 10.1111/j.1468-1331.2012.03688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Critchley E. Clinical manifestations of essential tremor. J Neurol Neurosurg Psychiatry. 1972;35:365–372. doi: 10.1136/jnnp.35.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diamond A, Kenney C, Almaguer M, Jankovic J. Hyperhidrosis due to deep brain stimulation in a patient with essential tremor. Case report. J Neurosurg. 2007;107:1036–1038. doi: 10.3171/JNS-07/11/1036. [DOI] [PubMed] [Google Scholar]
- 47.Louis ED, Bromley SM, Jurewicz EC, Watner D. Olfactory dysfunction in essential tremor: a deficit unrelated to disease duration or severity. Neurology. 2002;59:1631–1633. doi: 10.1212/01.wnl.0000033798.85208.f2. [DOI] [PubMed] [Google Scholar]
- 48.Applegate LM, Louis ED. Essential tremor: mild olfactory dysfunction in a cerebellar disorder. Parkinsonism Relat Disord. 2005;11:399–402. doi: 10.1016/j.parkreldis.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Busenbark KL, Huber SJ, Greer G, Pahwa R, Koller WC. Olfactory function in essential tremor. Neurology. 1992;42:1631–1632. doi: 10.1212/wnl.42.8.1631. [DOI] [PubMed] [Google Scholar]
- 50.Shah M, Muhammed N, Findley LJ, Hawkes CH. Olfactory tests in the diagnosis of essential tremor. Parkinsonism Relat Disord. 2008;14:563–568. doi: 10.1016/j.parkreldis.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 51.Ondo WG, Sutton L, Dat Vuong K, Lai D, Jankovic J. Hearing impairment in essential tremor. Neurology. 2003;61:1093–1097. doi: 10.1212/01.wnl.0000086376.40750.af. [DOI] [PubMed] [Google Scholar]
- 52.Benito-León J, Louis ED, Bermejo-Pareja F, Neurological Disorders in Central Spain (NEDICES) Study Group Reported hearing impairment in essential tremor: a population-based case-control study. Neuroepidemiology. 2007;29:213–217. doi: 10.1159/000112463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janicki SC, Cosentino S, Louis ED. The cognitive side of essential tremor: what are the therapeutic implications? Ther Adv Neurol Disord. 2013;6:353–368. doi: 10.1177/1756285613489591. [DOI] [PMC free article] [PubMed] [Google Scholar]