Abstract
Introduction
Hirschsprung’s disease is characterized by a developmental arrest of neural crest cell migration, causing distal aganglionosis. Transplanted cells derived from the neural crest may regenerate enteric ganglia in this condition. We investigated the potential of skin-derived precursor cells (SKPs) to engraft and to differentiate into enteric ganglia in aganglionic rat intestine in vivo.
Methods
Adult Lewis rat jejunal segments were separated from intestinal continuity and treated with benzalkonium chloride to induce aganglionosis. Ganglia were identified via immunohistochemical stains for S100 and β-III tubulin (TUJ1). SKPs were procured from neonatal Lewis rats expressing enhanced green fluorescent protein (GFP) and cultured in neuroglial-selective media. SKP cell line expansion was quantified, and immunophenotypes were assessed by immunocytochemistry. Aganglionic segments underwent SKP transplantation 21–79 days after benzalkonium chloride treatment. The presence of GFP+ cells, mature neurons, and mature glia was evaluated at post-transplant days 1, 6, and 9.
Results
Benzalkonium chloride-induced aganglionosis persisted for at least 85 days. Prior to differentiation, SKPs expressed S100, denoting neural crest lineage, and nestin, a marker of neuronal precursors. Differentiated SKPs in vitro expressed GFAP, a marker of glial differentiation, as well as TUJ1 and several enteric neurotransmitters. After transplantation, GFP+ structures resembling ganglia were identified between longitudinal and circular smooth muscle layers.
Conclusion
SKPs are capable of engraftment, migration, and differentiation within aganglionic rodent intestine in vivo. Differentiated SKPs generate structures that resemble enteric ganglia. Our observations suggest that SKPs represent a potential gangliogenic therapeutic agent for Hirschsprung’s disease.
Keywords: Hirschsprung’s disease, Skin -derived precursor cells, Regenerative medicine, Stem cell, Cell-based therapy, Neural crest, Aganglionosis
Introduction
In normal development, elements of the neural crest migrate aborally along the gut to generate the enteric nervous system. In Hirschsprung’s disease, intestinal neural crest migration is arrested, resulting in aganglionosis caudal to the site of arrest. Nearly 1 in 5000 live births are diagnosed with Hirschsprung’s disease annually in the United States.1,2 Definitive therapy for this condition is surgical, and it entails intestinal diversion and resection of aganglionic bowel. Intestinal resection for Hirschsprung’s disease in children carries a 58% risk of overall morbidity and 1–5% risk of late mortality.1 Complications of bowel resections in children include enterostomal retraction and prolapse, anal excoriation, bowel obstruction, permanent fecal incontinence, enterocolitis, reoperation, and death.2–9
Stem cell transplantation therapy for Hirschsprung’s disease is an auspicious subject of scientific investigation. In recent years, investigators have transplanted neural crest stem cells (NCSCs) into rodent intestine with promising results.10,11 Transplanted NCSCs have generated enteric neurons that synapse with native enteric ganglia.12 In a recent study, NCSCs that underwent genetic modification in vitro were successfully transplanted into aganglionic gut in vivo.13 These studies entailed NCSC harvest either from the intestine or from the central nervous system. Stem cells derived from the neural crest are also found in postnatal skin. These cells may be harvested in a significantly less invasive fashion than intestinal or brain cells. Skin-derived precursor cells (SKPs) are found in the perivascular dermis, they persist into adulthood, and they represent a potentially autologous source of transplanted cells.14,15 Clonal analyses and in vitro studies have demonstrated that SKPs are capable of generating glial cells, smooth muscle cells, chondrocytes, adipocytes, melanocytes, and electrophysiologically active neurons.16–18 Kwok and colleagues recently demonstrated successful SKP engraftment upon transplantation into aganglionic gut explants.19
In the present study, we transplanted SKPs into aganglionic rat intestine in vivo. This study is the first to investigate SKP transplantation into aganglionic intestine in vivo. Furthermore, no prior study has investigated cell transplantation in live mammalian intestine using a model of induced aganglionosis.
Materials and Methods
The following experimentation was conducted under UCLA protocol #2006-061 with the approval of the Institutional Animal Care and Use Committee.
SKP Procurement and Culture
Neonatal Lewis rat pups (3 to 5 days old) expressing enhanced green fluorescent protein (GFP) on the ubiquitin promoter were used for SKP proliferation and transplantation.20 Native GFP fluorescence was confirmed by visualizing tail clippings from euthanized pups under ultraviolet light microscopy (excitation λ = 490 nm). SKPs from non-GFP neonatal Lewis rat pups were harvested to characterize in vitro neuronal differentiation potential. SKPs were harvested according to the Biernaske protocol.21 A 1.5×1.5 cm2 patch of dorsal skin was harvested and mechanically stripped of subdermal connective tissue and epidermis using a #10 scalpel blade. The dermis was diced into 1–3 mm2 fragments and digested in 500 mg collagenase IV (Sigma-Aldrich, St. Louis, MO) dissolved in 10 mL Hank’s Balanced Salt Solution (HBSS) (Invitrogen, Carlsbad, CA) for 1 hour at 37°C. The dissociated cells were isolated and suspended in NeuroCult® NS-A Basal Medium (Rat) (StemCell Technologies, Vancouver, Canada). Cells were filtered through a 40-μm cell strainer (BD Falcon, San Jose, CA) and plated at a density of 5 × 105 cells per T75 flask in the following proliferation media: NeuroCult® NS-A Basal Medium (Rat) with 10% Proliferation Supplement, 0.0002% heparin solution (StemCell Technologies), 1X Antibiotic Antimycotic (ABAM) solution (Invitrogen), 20 ng/ml epidermal growth factor (EGF) (Peprotech, Rocky Hill, NJ), and 20 ng/ml basic fibroblast growth factor (bFGF) (Peprotech). SKPs were allowed to proliferate to confluency. Cells intended for transplantation were cultured in differentiation media composed of NeuroCult® NS-A Basal Medium (Rat) with 10% Differentiation Supplement (StemCell Technologies) and 1X ABAM (Invitrogen) for 7–14 days. Cells in culture were incubated at 37°C with 5% ambient CO2. Subsets of each SKP line were fixed for immunocytochemistry (ICC) to characterize immunophenotype and differentiation potential in vitro.
Aganglionosis Model
Adult female Lewis rats (n=18) were purchased from Charles River Laboratories (Wilmington, MA) for the purpose of enteric nervous ablation and SKP transplantation. Isoflurane anesthesia was administered, midline laparotomy performed, and the small bowel eviscerated. A 1-cm segment of jejunum was isolated from continuity, preserving its vascular supply, approximately 10–15cm distal to the ligament of Treitz. The jejunal segment was wrapped with sterile gauze saturated with benzalkonium chloride (BAC, 0.2% w/v, Sigma-Aldrich) for 20 minutes. A jejunojejunostomy was performed with 6-0 polypropylene suture to restore intestinal continuity, excluding the isolated segment. The treated segment was then irrigated with sterile 0.9% normal saline and the ends sutured closed with 6-0 polypropylene. The omentum was wrapped around the isolated segment, and the viscera were replaced into the peritoneal cavity. The abdomen was closed using 3-0 polyglactin braided suture, and the skin closed with 3-0 nylon suture. Rats were administered trimethoprim sulfa (TMS, 1% v/v) for 14 days, postoperatively. For comparison, two additional adult female Lewis rats underwent jejunal segment isolation without BAC-treatment.
SKP Transplantation
pH-neutralized rat tail collagen was prepared using our laboratory’s previously-described protocol.11 SKPs were liberated and suspended in differentiation media with 15% v/v neutralized rat tail collagen in differentiation media and 2% v/v India ink (Becton, Dickinson and Company, Franklin Lakes, NJ) at a density of 5 × 105 cells/mL. SKPs were transplanted at days 24, 41, or 94 after initial plating. Recipient rats (n=9) underwent a second laparotomy under isoflurane anesthesia at post-BAC treatment day 21–79. The isolated BAC-treated segments were identified and meticulous adhesiolysis was performed. The SKP suspension was injected subserosally in a fanning linear fashion using a micro-injector with a 0.5-inch long 33-gauge needle (Hamilton, Reno, NV). Approximately 100–200 μL of suspended SKPs were injected into each segment. After injection, the isolated segment was wrapped with mobilized omentum, replaced into the peritoneal cavity, and the abdomen and skin were closed. At 1, 6, or 9 days post-transplant, the animals were euthanized and the injected segments collected and fixed for immunohistochemistry (IHC). Recipient muscularis thickness and the presence of native ganglia were compared among segments injected at earlier versus later time points after BAC treatment.
SKP Immunocytochemistry
A subset of SKPs in culture was fixed overnight in 10% formalin (Fisher Scientific, Pittsburgh, PA) at 4°C. Cells were washed with phosphate-buffered saline (PBS). SKPs were incubated overnight with the following primary antibodies: anti-S100 (1:200; Dako, Carpinteria, CA), anti-nestin (1:200; Abcam, Cambridge, MA), anti-neuron specific β-III-tubulin (1:200 Abcam), anti-glial fibrillary acid protein (1:400; Sigma-Aldrich), and anti-GFP IgY (1:400; Invitrogen).
A subset of GFP-negative SKPs was cultured in differentiation media for 7 days prior to formalin fixation as above. These SKPs were incubated overnight with the following primary antibodies: anti-vasointestinal peptide (1:400; Abcam), anti-neural nitric oxide synthase (nNOS) (1:50; Abcam), and anti-dopamine-β-hydroxylase (1:400; Abcam), anti-serotonin (1:50; Dako), and anti-choline acetyltransferase (ChAT; 1 μg/μL; Abcam).
Cells were washed three times with PBS, and fluorophore-tagged secondary antibodies were administered to the cells for 30 minutes. Secondary antibodies included goat anti-rabbit Alexa Fluor® 488, goat anti-mouse Alexa Fluor® 488, goat anti-rabbit Alexa Fluor® 546, donkey anti-mouse Alexa Fluor® 555, goat anti-rabbit Alexa Fluor® 594, goat anti-mouse Alexa Fluor® 594 (all 1:250; Invitrogen), and goat anti-chicken IgY Alexa Fluor® 488 (1:200; Aves Labs, Tigard, OR). Cell plates were visualized via fluorescence microscopy (Leica Microsystems, Bannockburn, IL, and Zeiss, Thornwood, NY).
Jejunal Immunohistochemistry
After tissue procurement, retrieved jejunal segments were fixed in 10% formalin (Fisher Scientific) for 24 hours at 4°C. Fixed tissue was embedded in paraffin wax and cut into 5-μm sections. Paraffin was dissolved in xylenes for 10 minutes and rehydrated in serial washes for 2 minutes each in 100%, 95%, 70% and 0% ethanol (Fisher Scientific). Antigen retrieval was performed in citrate buffer (Biogenex, San Ramon, CA) for 20 minutes at 95°C. Slides were cooled in a running water bath for 30 minutes. A hydrophobic barrier was applied to each section with a PAP pen (Vector Laboratories, Burlingame, CA). Non-specific secondary antibody binding was blocked by incubating the slides in a solution of 5% normal goat serum (Vector Laboratories) and 2% bovine serum albumin in PBS with 0.05% Tween-20 (PBS/T) for one hour at room temperature. Primary antibodies were anti-GFP IgY (1:400; Invitrogen), anti-S100 (1:200; Dako), anti-neuron specific β-III-tubulin (5 ug/ml; Abcam), and anti-glial fibrillary acid protein (1:400; Sigma-Aldrich). These primary antibodies were selected to maximize the sensitivity of glial and neuronal cell detection. Antibodies diluted in PBS/T were incubated with slides overnight at 4°C in a humidified slide chamber. Slides were washed for 30 minutes in PBS/T three times. Secondary antibodies were diluted in PBS/T and exposed to the tissue sections for 30 minutes at room temperature. Slides were washed again for 30 minutes with PBS/T three times. Secondary antibodies included goat anti-rabbit Alexa Fluor® 488, goat anti-mouse Alexa Fluor® 488, goat anti-rabbit Alexa Fluor® 546, donkey anti-mouse Alexa Fluor® 555, goat anti-rabbit Alexa Fluor® 594, goat anti-mouse Alexa Fluor® 594 (all 1:200; Invitrogen), and goat anti-chicken IgY Alexa Fluor® 488 (1:200; Aves Labs, Tigard, OR). Each section was treated with Prolong Gold with DAPI (Invitrogen) and covered with a glass coverslip. Histological slides were visualized under fluorescence microscopy (Leica Microsystems and Zeiss).
Results
SKP Cell Culture
Five out of six SKP cell lines were successfully cultured for characterization and transplantation. One SKP cell line yielded insufficient cells to sustain proliferation. The average SKP doubling time in culture was 16.6 days. In proliferation media, GFP expression was ubiquitous in SKPs; however, native GFP expression was considerably attenuated upon fixation (Figure 1.A–C). Increased time in culture was associated with less intense anti-GFP immunofluorescence (data not shown). Most (>85%) SKPs in proliferation media expressed S100, a marker of neural crest lineage, and approximately 15% expressed nestin, a marker of neural precursor cells (Figure 1.D–F).
Figure 1.
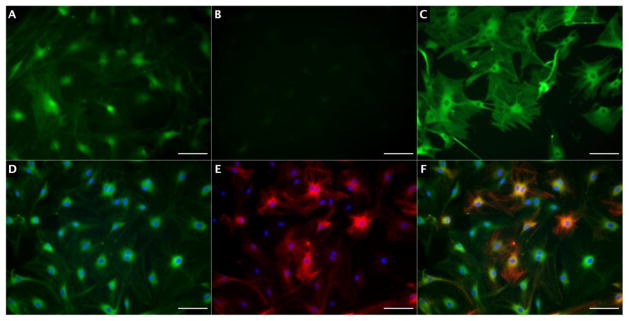
SKPs in proliferation media. A) Native GFP fluorescence in live SKPs; B) GFP+ SKPs demonstrating attenuated native fluorescence upon fixation; C) anti-GFP immunofluorescence in fixed SKPs; D) S100 expression (green); E) Nestin expression (red); F) Merged S100 and Nestin expression. Nuclei in panes 4–6 appear blue from DAPI, and scale bars represent 100 μm.
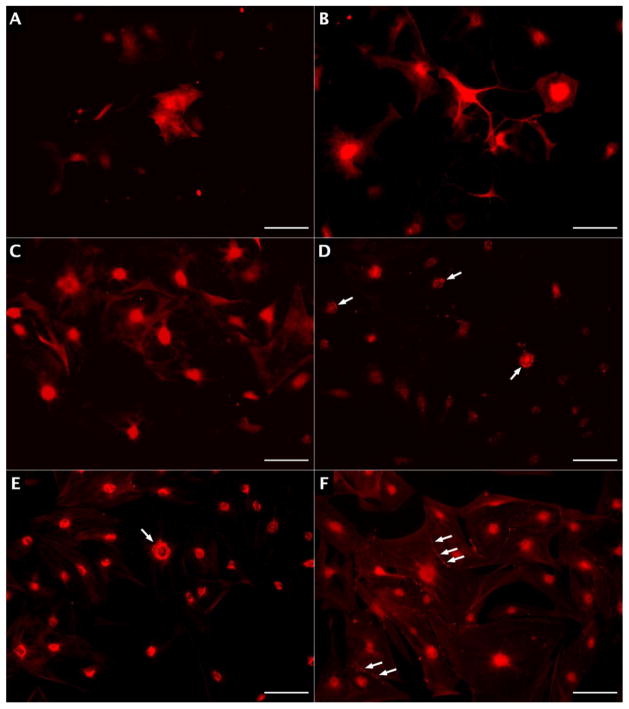
After 7–14 days in differentiation media, approximately 20% of SKP cells expressed GFAP and 10% expressed neuron specific β-III-tubulin (TUJ1) (Figure 2.A–B), indicating glial and neuronal differentiation, respectively. SKP-derived neurons expressed ChAT, dopamine-β-hydroxylase, VIP, and nNOS, markers of enteric neurotransmitter synthesis (Figure 2.C–F). SKPs in differentiation media expressing serotonin (5-HT) were not visualized.
Figure 2.
SKPs in differentiation media. A) GFAP expression; B) TUJ1 expression; C) ChAT expression; D) dopamine-β-hydroxylase expression; E) VIP expression; F) nNOS expression. Arrows indicate clusters of fluorescent granules, and scale bars represent 100 μm.
Aganglionosis model
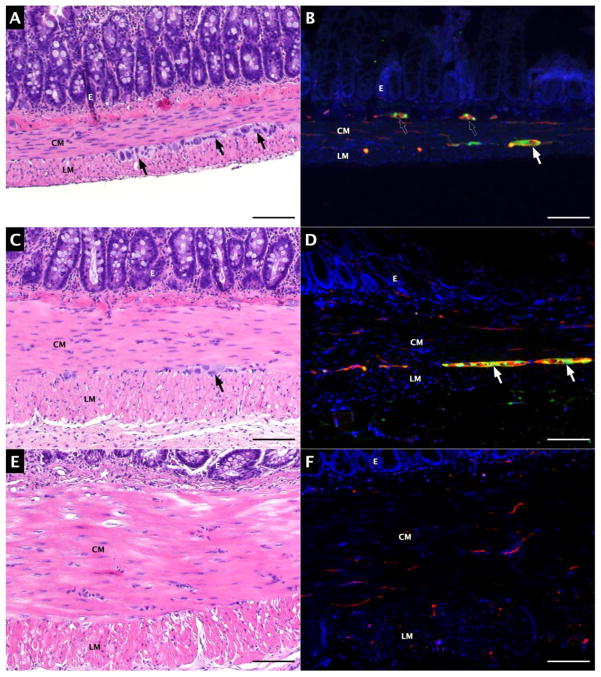
Sixteen out of eighteen adult Lewis rats were successfully treated with BAC. Two rats succumbed to respiratory insufficiency at the time of BAC treatment. Histologic sections of normal, isolated, and BAC-treated isolated jejunal segments are shown in Figure 3. Segmental isolation alone (without BAC treatment) resulted in muscularis propria hypertrophy with easily detectable myenteric and submucosal ganglia (Figure 3.C–D). BAC treatment obliterated enteric ganglia at all time points observed. Muscular hypertrophy and aganglionosis persisted for at least 85 days after BAC-treatment (Figure 3.E–F).
Figure 3.
Jejunal aganglionosis model. A) Hematoxylin and eosin (H&E) staining of normal jejunum; B) S100 (green) and TUJ1 (red) expression; C) H&E staining of isolated jejunum; D) S100 (green) and TUJ1 (red) expression in isolated jejunum; E) H&E staining of isolated, BAC-treated jejunum; F); S100 (green) and TUJ1 (red) expression in isolated, BAC-treated jejunum. Arrows indicate myenteric ganglia, and scale bars represent 100 μm.
CM: circular smooth muscle, LM: longitudinal smooth muscle, E: mucosal epithelium
SKP Transplantation
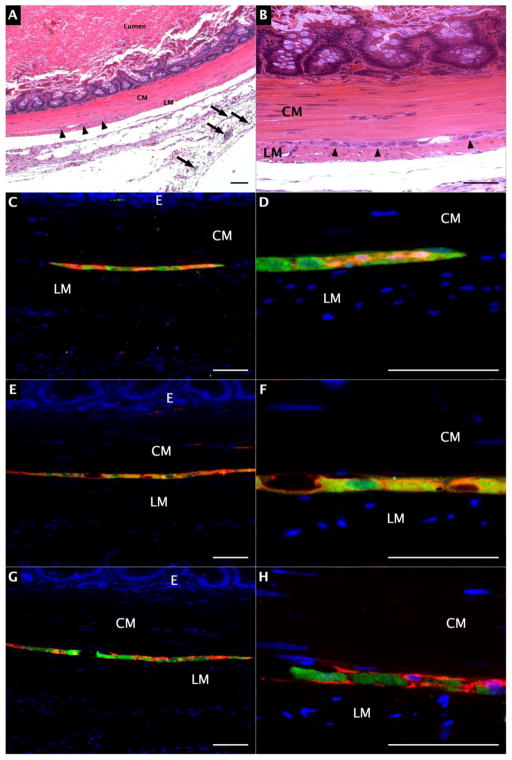
Nine adult Lewis rats received SKP cell injections at 21–79 days post-BAC treatment. Smooth muscle hypertrophy was insufficient to accommodate intestinal injection prior to 21 days after BAC treatment. There was greater hypertrophy at later time points (data not shown). India ink-stained collagen aggregates indicated injection sites on hematoxylin and eosin (H&E) staining (Figure 4.A). India ink was present within the submucosa, smooth muscle, serosa, and extraserosal fat layers of several sections. Well-circumscribed, elongated structures comprised of GFP+ cells co-expressing GFAP, S100, and TUJ1 were identified in the intermuscular layer of 7 rats after transplantation (Figure 4.C–H). These structures were evident at all post-transplant intervals, and with all SKP cell lines transplanted. These structures were not evident in other bowel layers. Differences in SKP age were not associated with observable changes in post-transplant tissue morphology or immunophenotype.
Figure 4.
Post-transplantation jejunum. A) H&E staining of BAC-treated jejunum, 100X magnification. Arrowheads indicate myenteric neuroglial structure, arrows indicate India ink in extraserosal tissue; B) H&E staining of BAC-treated jejunum, 200X magnification. Arrowheads indicate myenteric neuroglial structure; C) GFP (green) and S100 (red) expression, 200X magnification; D) GFP and S100 expression, 630X magnification; E) GFP (green) and GFAP (red) expression, 200X magnification; F) GFP and GFAP expression, 630X magnification; G) GFP (green) and TUJ1 (red) expression, 200X magnification; H) GFP and TUJ1 expression, 630X magnification. Scale bars represent 100 μm.
CM: circular smooth muscle, LM: longitudinal smooth muscle, E: mucosal epithelium
Discussion
SKPs are capable of migration, differentiation, and engraftment within living aganglionic recipient intestine. We observed the presence of ganglia composed of GFP-expressing mature neurons and glial cells in recipient aganglionic jejunum following SKP transplantation. Our findings suggest that SKPs comprise a potential source of autologous cell therapy to reconstitute the enteric nervous system.
When cultured in differentiation media, our SKPs recapitulated the observations of Kwok and colleagues, who demonstrated S100, GFAP, TUJ1, VIP, NOS, and ChAT expression among human SKPs cultured in differentiation media for 14 days.19 These results are promising, in that SKPs are capable of differentiating into glial and neuronal subtypes common to enteric ganglia. These in vitro results also signify that our SKPs represent a mixture of cell types. The clinical yield of a mixed-cell culture may be superior to that of a clonal culture. Liebmann and colleagues observed that SKP-derived neurons proliferate more efficiently when co-cultured with glial cells.18 While the growth characteristics and immunophenotypes of each cell line in our study were somewhat variable, we observed little dissimilarity in post-transplant outcomes. The intercellular activity within a mixed culture may be instrumental in translating cell harvest to cell therapy. Nevertheless, SKP gene expression profiles and clonal characterization over time and in growth factor-enriched media should be the subject of further experimentation.
We find the migration and differentiation behavior of transplanted SKPs particularly interesting. We observed that, whether injected into the extraserosal, subserosal, intramuscular, or submucosal layers, SKP-derived neurons and glia localize to the precise layer normally occupied by the myenteric plexus within days. In one study, neural crest-derived cells transplanted into the peritoneal cavity were detected within the intestinal wall.22 Within this context, our observations support the notion that transplanted precursor cells are impelled to migrate by the recipient tissue milieu. Other studies have also demonstrated the capacity of SKPs to transdifferentiate into peripheral nervous tissue, corneal epithelium, and skin, depending in large part upon recipient factors.23–25 Specific factors within the intestinal wall microenvironment that contribute to SKP migration and differentiation remain indistinct. In Hirschsprung’s disease, the unidentified elements that precipitate neural crest migratory arrest may also impede the migration of transplanted cells; however, allogeneic myenteric neuronal growth has been demonstrated in Hirschsprung’s colon.26
The function of recipient intestinal tissue after SKP cell transplantation necessitates further investigation. In addition to differentiation, migration, and engraftment, transplanted SKPs must undergo physiologic orientation, synaptogenesis, and target-innervation to generate coordinated intestinal motility and glandular stimulation. Specific subjects of future studies should include diagnostic indicators of human Hirschsprung’s disease such as pre- and post-transplant expression of calretinin and acetylcholinesterase, SKP-derived neuron neurotransmitter production, in vivo action potential conduction, enteric plexus architecture, amplitude and direction of peristaltic muscle contraction, and alimentary function of recipient tissue.
Acknowledgments
We thank Elvin Chiang for technical assistance and the California Institute of Regenerative Medicine and the Eli and Edythe Broad Stem Cell Research Center at UCLA for support. Additional support was provided by NIH R01 DK083319 and the Sun West Company.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Georgeson KE. Hirschsprung’s Disease. In: Holcomb GW, Murphy JP, Ostlie DJ, editors. Ashcraft’s Pediatric Surgery. Elsevier Health Sciences; 2010. pp. 456–467. [Google Scholar]
- 2.Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nature Rev Neurosci. 2007;8:466–79. doi: 10.1038/nrn2137. [DOI] [PubMed] [Google Scholar]
- 3.Bai Y, Chen H, Hao J, et al. Long-term outcome and quality of life after the Swenson procedure for Hirschsprung’s disease. J Pediatr Surg. 2002;37:639–642. doi: 10.1053/jpsu.2002.31625. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff A, Levitt MA, Peña A. Total colonic aganglionosis: A surgical challenge. How to avoid complications? Pediatr Surg Int. 2011;27:1047–1052. doi: 10.1007/s00383-011-2960-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dasgupta R, Langer JC. Evaluation and management of persistent problems after surgery for Hirschsprung disease in a child. J Pediatr Gastroenterol Nutr. 2008;46:13–19. doi: 10.1097/01.mpg.0000304448.69305.28. [DOI] [PubMed] [Google Scholar]
- 6.Ludman L, Spitz L, Tsuji H, et al. Hirschsprung’s disease: functional and psychological follow up comparing total colonic and rectosigmoid aganglionosis. Arch Dis Child. 2002;86:348–351. doi: 10.1136/adc.86.5.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuji H, Spitz L, Kiely E, et al. Management and long-term follow-up of infants with total colonic aganglionosis. J Pediatr Surg. 1999;34:158–162. doi: 10.1016/s0022-3468(99)90248-8. [DOI] [PubMed] [Google Scholar]
- 8.Baillie CT, Kenny SE, Rintala RJ, et al. Long-term outcome and colonic motility after the Duhamel procedure for Hirschsprung’s disease. J Pediatr Surg. 1999;34:325–9. doi: 10.1016/s0022-3468(99)90201-4. [DOI] [PubMed] [Google Scholar]
- 9.Menezes M, Corbally M, Puri P. Long-term results of bowel function after treatment for Hirschsprung’s disease: a 29-year review. Pediatr Surg Int. 2006;22:987–90. doi: 10.1007/s00383-006-1783-8. [DOI] [PubMed] [Google Scholar]
- 10.Schafer KH, Micci MA, Pasricha PJ. Neural stem cell transplantation in the enteric nervous system: roadmaps and roadblocks. Neurogastroenterol Motil. 2009;21:103–12. doi: 10.1111/j.1365-2982.2008.01257.x. [DOI] [PubMed] [Google Scholar]
- 11.Geisbauer CL, Wu BM, Dunn JCY. Transplantation of enteric cells into the aganglionic rodent small intestines. J Surg Res. 2012;176:20–8. doi: 10.1016/j.jss.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Hotta R, Stamp LA, Foong JPP, et al. Transplanted progenitors generate functional enteric neurons in the postnatal colon. J Clin Invest. 2013;123:1182–91. doi: 10.1172/JCI65963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shu X, Meng Q, Jin H, et al. Treatment of aganglionic megacolon in mice via neural stem cell transplantation. Mol Neurobiol. 2013 doi: 10.1007/s12035-013-8430-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Fernandes KJL, Kobayashi NR, Gallagher CJ, et al. Analysis of the neurogenic potential of multipotent skin-derived precursors. Exp Neurol. 2006;201:32–48. doi: 10.1016/j.expneurol.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Achilleos A, Trainor PA. Neural crest stem cells: discovery, properties and potential for therapy. Cell Res. 2012;22:288–304. doi: 10.1038/cr.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandes KJ, McKenzie IA, Mill P, et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol. 2004;6:1082–1093. doi: 10.1038/ncb1181. [DOI] [PubMed] [Google Scholar]
- 17.Toma JG, McKenzie IA, Bagli D, et al. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–37. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- 18.Liebmann L, Beetz C, Thorwarth M, et al. Morphological and electrophysiological features of mature neurons in differentiated skin-derived precursor cells. J Stem Cells Regen Med. 2012;8(1):35–6. doi: 10.46582/jsrm.0801006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwok CK, Tam PK, Ngan ES. Potential use of skin-derived precursors (SKPs) in establishing a cell-based treatment model for Hirschsprung’s disease. J Pediatr Surg. 2013;48(3):619–28. doi: 10.1016/j.jpedsurg.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 20.van den Brandt J, Wang D, Kwon SH, et al. Lentivirally generated eGFP-transgenic rats allow efficient cell tracking in vivo. Genesis. 2004;39:94–9. doi: 10.1002/gene.20037. [DOI] [PubMed] [Google Scholar]
- 21.Biernaske JA, McKenzie IA, Toma JG, et al. Isolation of skin-derived precursors (SKPs) and differentiation and enrichment of their Schwann cell progeny. Nature Protocols. 2006;1(6):2803–12. doi: 10.1038/nprot.2006.422. [DOI] [PubMed] [Google Scholar]
- 22.Tsai YH, Murakami N, Gariepy CE. Postnatal intestinal engraftment of prospectively selected enteric neural crest stem cells in a rat model of Hirschsprug disease. Neurogastroenterol Motil. 2011;23(4):362–9. doi: 10.1111/j.1365-2982.2010.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saichanma S, Bunyaratvej A, Sila-Asna M. In vitro transdifferentiation of corneal epithelial-like cells from human skin-derived precursor cells. Int J Ophthalmol. 2012;5(2):158–63. doi: 10.3980/j.issn.2222-3959.2012.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruetze M, Knauer T, Gallinat S, et al. A novel niche for skin derived precursors in non-follicular skin. J Dermatol Sci. 2013;69(2):132–9. doi: 10.1016/j.jdermsci.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Zong Z, Li N, Ran X, et al. Isolation and characterization of two kinds of stem cells from the same human skin back sample with therapeutic potential in spinal cord injury. PLoS One. 2012;7(11):e50222. doi: 10.1371/journal.pone.0050222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagl CI, Rauch U, Klotz M, et al. The microenvironment in the Hirschsprung’s disease gut supports myenteric plexus growth. Int J Colorectal Dis. 2012;27(6):817–29. doi: 10.1007/s00384-012-1411-0. [DOI] [PubMed] [Google Scholar]