Abstract
Moss bugs (Coleorrhyncha: Peloridiidae) are members of the order Hemiptera, and like many hemipterans, they have symbiotic associations with intracellular bacteria to fulfill nutritional requirements resulting from their unbalanced diet. The primary endosymbiont of the moss bugs, Candidatus Evansia muelleri, is phylogenetically related to Candidatus Carsonella ruddii and Candidatus Portiera aleyrodidarum, primary endosymbionts of psyllids and whiteflies, respectively. In this work, we report the genome of Candidatus Evansia muelleri Xc1 from Xenophyes cascus, which is the only obligate endosymbiont present in the association. This endosymbiont possesses an extremely reduced genome similar to Carsonella and Portiera. It has crossed the borderline to be considered as an autonomous cell, requiring the support of the insect host for some housekeeping cell functions. Interestingly, in spite of its small genome size, Evansia maintains enriched amino acid (complete or partial pathways for ten essential and six nonessential amino acids) and sulfur metabolisms, probably related to the poor diet of the insect, based on bryophytes, which contains very low levels of nitrogenous and sulfur compounds. Several facts, including the congruence of host (moss bugs, whiteflies, and psyllids) and endosymbiont phylogenies and the retention of the same ribosomal RNA operon during genome reduction in Evansia, Portiera, and Carsonella, suggest the existence of an ancient endosymbiotic Halomonadaceae clade associated with Hemiptera. Three possible scenarios for the origin of these three primary endosymbiont genera are proposed and discussed.
Keywords: genome reduction, endosymbiont, Halomonadaceae, mutualism, metabolic complementation
Introduction
Most members of the hemipteran suborders, Sternorrhyncha (psyllids, whiteflies, aphids, and coccoids), Auchenorrhyncha (Cicadomorpha: Cicadas, spittlebugs, leafhoppers, and treehoppers; Fulgoromorpha: Planthoppers), Heteroptera (lygaeoid, coreoid, and pentatomoid bugs), and Coleorrhyncha (moss bugs), are known to be associated with one or more obligate primary endosymbiont bacteria (Buchner 1965; Baumann 2005). They include representatives from several classes such as Gammaproteobacteria, Betaproteobacteria, Alphaproteobacteria, or Bacteroidetes, with the former being to date the most frequently reported (Moya et al. 2008).
All these hemipteran hosts are nutrient specialists and feed exclusively on restricted diets like xylem/phloem plant sap (Auchenorrhyncha and Sternorrhyncha) or seeds (Heteroptera), which are rich in carbohydrates, mostly sugars, but are deficient in most essential amino acids and many cofactors, like vitamins (Hansen and Moran 2014). Consequently, the lack of essential elements is compensated by the metabolic abilities of the symbionts. The frequent coexistence of obligate primary endosymbionts (P-endosymbionts) with facultative secondary ones may lead to the replacement of the ancestral P-endosymbiont or to the emergence of a coprimary obligate endosymbiosis, with both partners sharing the biosynthesis of the essential compounds required for nutrient provisioning (McCutcheon et al. 2009; Lamelas et al. 2011).
All P-endosymbionts display a large genome reduction, because of their accommodation to an intracellular lifestyle (Moya et al. 2008; McCutcheon and Moran 2012). As a consequence of their small population size and strict vertical transmission (bottleneck effect), they accumulate deleterious mutations, resulting in the loss of gene functions (Moran 1996; Allen et al. 2009), and finally in a complete gene disintegration (Silva et al. 2001). Particularly, functions related to DNA replication, transcription, and translation are subjected to an extreme decline, which in some cases imply that the genome is no longer compatible with its role as a mutualistic endosymbiont and that the endosymbiont has crossed the line between a cell and an organelle (Tamames et al. 2007). Because of the fact that some important differences among extreme symbionts and organelles still remain, the term symbionelle has been proposed recently to name the former (Reyes-Prieto et al. 2013). P-endosymbionts having crossed this critical threshold may include Candidatus Tremblaya princeps in mealybugs (139–171 kb), Candidatus Hodgkinia cicadicola in cicadas (144 kb), Candidatus Carsonella ruddii (158–166 kb) (hereafter referred to as Carsonella) in psyllids or Candidatus Portiera aleyrodidarum (281–358 kb) (hereafter referred to as Portiera) in whiteflies.
As described for the exceptional number of gene losses in Carsonella (Sloan et al. 2014), the loss of essential genes in endosymbionts with extremely reduced genome sizes may be compensated by at least three mechanisms: 1) modification of highly conserved cellular processes or selection of multifunctional proteins (Kelkar and Ochman 2013), 2) complementation by a second endosymbiont (Hansen and Moran 2014), and 3) substitution of the lost functions by host proteins of either eukaryotic or bacterial origins (Husnik et al. 2013).
The enigmatic suborder Coleorrhyncha is represented by only one extant family, called Peloridiidae. The peloridiids or moss bugs, consisting of 17 genera with 36 species, are very small, strange-looking insects (body length 2–5 mm) with a very cryptic life style, resulting in a limited representation in collections and even less information about their biology. However, recent studies have offered some new data in respect of vibrational signaling (Hoch et al. 2006), jumping behavior (Burrows et al. 2007), or cephalic morphology (Spangenberg et al. 2013).
Coleorrhyncha existed 250 Ma, since the late Permian and Cretaceous period, as demonstrated by numerous fossils mostly from the Northern hemisphere (Popov and Shcherbakov 1996). However, the present distribution of Peloridiidae is restricted to the temperate and subantartic rainforests (along with Nothofagus) or sphagnum bogs of the Southern hemisphere (Argentina, Chile, New Zealand, New Caledonia, and eastern Australia from North Queensland to Tasmania), reflecting a typical Gondwana biogeography (Burckhardt 2009). Peloridiids live in wet moss or leaf litter and feed on sap of different mosses and liverworts, preferring bryophyte species that provide a stable wet environment and possess conductive tissue (Hartung V, unpublished data). This kind of nutrition is quite special, in that most animals, especially insects, avoid feeding on mosses because of their poor nutrient content, on the one hand, and the high amount of toxic secondary metabolites, on the other hand (Klavina et al. 2012; Asakawa et al. 2013).
On the basis of this restricted kind of nutrition, moss bugs possess, like many other plant-sap sucking Hemiptera, endosymbiotic microorganisms, which are located in specific organ-like structures called bacteriomes. This observation was first recorded by Evans (1948) in a Hemiodoecus fidelis larva and a more detailed morphological overview was later given by Müller (1951) and Pendergrast (1962). In almost all host species examined, the endosymbionts are harbored inside a type of evolutionarily adapted insect cells called bacteriocytes, located in a pair of slightly orange-colored bacteriomes, subdivided into three partial bacteriomes of spherical shape on each side of the abdomen. Molecular characterization revealed that the P-endosymbionts of the Peloridiidae, called Candidatus Evansia muelleri (Oceanospirillales: Halomonadaceae), are closely related to Carsonella and Portiera, P-endosymbionts of psyllids (Hemiptera: Sternorrhyncha: Psyllidae) and whiteflies (Hemiptera: Sternorrhyncha: Aleyrodidae), respectively (Kuechler et al. 2013). Phylogenetic analysis indicated that the monophyletic group of P-endosymbiont peloridiids is strictly subdivided into well-formed subgroups in accord with their geographical distribution (e.g., endosymbionts of Australia and New Zealand species are clearly separated from each other). Furthermore, a phylogenetic concordance between the P-endosymbionts and their moss bugs hosts exists (Kuechler et al. 2013).
Here, we report the complete genome of the P-endosymbiont “Ca. Evansia muelleri” (hereafter referred to as Evansia) strain Xc1 (Gammaproteobacteria: Oceanospirillales: Halomonadaceae) from Xenophyes cascus strain OF (Hemiptera: Coleorrhyncha: Peloridiidae) (hereafter referred to as Xenophyes OF). Its genome displays an extreme reduction, a low GC content, a high coding density, and the loss of several genes that are essentials for housekeeping functions. Despite its small genome size, Evansia retains one of the most complete gene sets for amino acid biosynthesis and sulfur metabolism. A comparative genomic analysis with the other Halomonadaceae endosymbionts of the order Hemiptera, Portiera and Carsonella, led us to propose a possible scenario for the origin of this new endosymbiotic clade.
Materials and Methods
Sampling and Genome Sequencing
Adult individuals of Xenophyes OF were collected in the field (Otaki Forks, Tararua Ranges, Tararua Forest Park, New Zealand), in January 2013. Subsequently, an ultrastructural analysis using transmission electron microscopy (for experimental procedure see Kuechler et al. 2013) and DNA isolation were carried out. Bacteriomes were dissected from 17 adult females and used for DNA extraction following manufacturer's instructions (PureLink Genomic DNA Mini Kit, Invitrogen). Six independent whole-genome amplifications runs (GenomiPhi v2, GE Healthcare) starting with 8 ng DNA were performed following the manufacturer’s instructions. Reaction was left for 1.5 h at 30 °C and inactivated at 65 °C for 10 min. Because DNA chimeric amplification seems to be a random process, samples were mixed for maintaining possible chimeras at a low ratio in relation to nonchimeric-amplified DNA. Amplified DNA was used for sequencing with Roche 454 GSFLX Titanium (single-end shotgun with 800 bp of average read length) and Illumina HiSeq2000 (350-bp paired-end library and 2 × 100 bp) platforms.
Assembly and Annotation
Sequence read quality filtering was performed with custom python scripts and Perl scripts, whereas quality was checked with FastQC v0.10 after and before filtering. Approximately, 283,000 (454) and 29.3 million (Illumina) reads were generated after filtering.
An initial hybrid assembly with both kinds of reads was performed with MIRA v4.0 (Chevreux et al. 1999), and Evansia contigs were selected based on GC content, read coverage, BLAST similarities, and PhymmBL (Brady and Salzberg 2011). A single contig (150× 454 and 330× Illumina read coverages) containing the mitogenome of Xenophyes OF was also detected by a BLAST search against the published mitogenome of X. cascus (JF323862). Illumina and 454 reads were mapped against the assembled Xenophyes OF mitochondrion and excluded for the subsequent assembly steps. Initial Evansia selected contigs were used for a second round of PhymmBL training, and 454 reads were selected based on GC content, coverage, and PhymmBL results. Illumina reads were mapped against initial Evansia contigs. Selected 454 and Illumina reads were assembled with MIRA, and polisher (Foster et al. 2012) was used to correct the remaining homopolymers also with Illumina reads. Corrected assembly was used as input for SSPACE v2.0 for scaffolding (Boetzer et al. 2011), and gaps were filled with GapFiller v1.0 (Boetzer and Pirovano 2012) using Illumina reads for both programs. Finally, an iterative mapping approach with MIRA v4.0 and manually editing with gap4 (Staden et al. 2000) were used for closing the remaining gaps (Sloan and Moran 2012b). Polisher was run to correct possible homopolymers and indel errors in the finished genome.
RAST (Aziz et al. 2008) was used for a first step annotation. InterProScan (Quevillon et al. 2005) was used for annotation refinement followed by a manual curation of the genome. Rfam (Burge et al. 2013) was used to predict noncoding RNA genes, and TFAM (Ardell and Andersson 2006) was employed to predict tRNA genes. Cluster of orthologous clusters (COG) (Tatusov et al. 2000) were assigned with a set of custom Perl scripts (BLASTP e-value cutoff of 1e−03), and Circos (Krzywinski et al. 2009) was used to plot the genome overview. Xenophyes OF mitochondrion was annotated with MITOS (Bernt et al. 2013) and manually curated.
Pathway tools (Karp et al. 2002) in combination with BioCyc (Karp et al. 2005) and BRENDA (Schomburg et al. 2013) databases were used to infer and curate Evansia metabolic capabilities. The curated metabolism of Evansia muelleri Xc1 in pathway tools (Ocelot format) can be supplied under request. Metabolism graph was done with yEd Graph Editor.
Phylogenomics Analyses
Proteomes from completely sequenced Halomonadaceae were downloaded from GenBank database, including three Carsonella (DC, HC, and PV strains) and three Portiera genomes (BT-QVLC, BT-B, and TV strains). Pseudomonas aeruginosa B136-33 was also downloaded and used as outgroup. Proteomes were fed into PhyloPhlAn (Segata et al. 2013) for a preliminary phylogenomic reconstruction. Because symbionts lack most of the 400 markers genes used by PhyloPhlAn, only 23 of these genes that were present in all the Halomonadaceae genomes (including symbionts) were selected for a curated phylogenomic reconstruction. The 23 proteins were aligned with MAFFT (L-INS-i algorithm) (Katoh et al. 2002) and concatenated. Gblocks (Castresana 2000) was used to prune the concatenated alignment and used as input for ProtTest3 (Darriba et al. 2011). The Improved General Amino Acid Replacement Matrix, with gamma distributed rates across sites using empirical base frequencies (LG+G+F) was scored as the best evolutionary model by ProtTest3. RaxML (Stamatakis 2006) with branch length optimization and 1,000 rapid bootstrap replicates was used for maximum likelihood (ML) tree reconstruction. PhyloBayes3 (Lartillot et al. 2009) was used to perform Bayesian analysis of the ML tree under the specified model for the concatenated proteins. PhyloBayes3 was left to run 2,868 cycles (328,604 trees) in five simultaneous chains, and 250 cycles were discarded as “burn in,” giving a final number of 2,618 trees (with sample frequency of 500). Effective sizes for all inferred parameters were higher than 1,300, and convergence of the chains was checked. A majority rule consensus tree was recovered from selected trees.
Phylogenetic analyses under ML were also performed for each separate protein alignment as described above, but adjusting the evolutionary model for each case.
Proteomes of complete mitochondria from Heteroptera, Coleorrhyncha, Auchenorrhyncha, and Sternorrhyncha were downloaded from GenBank database. Proteins encoded by ten genes (COX1-3, ND1-4, ND5, cytB, and atp6) for all mitochondria, including Xenophyes OF, were selected, and the best evolutionary model for each one was predicted with ProtTest3. MtArt model with gamma distribution was the best model for all the proteins under the Bayesian Information Criterion. RaxML and PhyloBayes3 were run as described above, adjusting the evolutionary model and partitions for each protein. PhyloBayes3 was run with five independent chains and stopped at 1,000 cycles (228,952 trees). Convergence of the chains and valid effective sizes were checked. First 250 cycles were discarded as “burn in.” Finally, a majority rule consensus tree was obtained.
COG Clustering Analysis
COG (cluster of orthologous groups) profiles for different symbionts were downloaded from the IMG (Markowitz et al. 2014) database (supplementary table S1, Supplementary Material online). COG categories for Portiera TV strain were assigned as described above. A count matrix was generated from COG profiles, and clustering for different COG categories was performed with R (R Development Core Team 2014), under a binary distance model and a complete clustering method. Heatmap and clustering dendograms were plotted with gplots (Warnes et al. 2013).
Orthologous Gene Identification
Chromohalobacter salexigens DSM 3043, Halomonas elongata DSM 2581, Carsonella (strains DC, HC, and PV), Portiera (strains BT-B, BT-QVLC, and TV), and Evansia proteomes were used as input for OrthoMCL (Li et al. 2003) and run as described previously (Manzano-Marín et al. 2012). OrthoMCL output clusters were manually inspected and refined due to the failure to assign some proteins from Carsonella to the correct cluster (based on BLASTP results and gene name or protein function). An Euler diagram for orthologous clusters was plotted with gplots package from R.
Genome Rearrangements
A total of 150 orthologous clusters shared by all genomes were selected for rearrangement analysis. MGR (Bourque and Pevzner 2002) was used to calculate the minimum number of rearrangements needed to explain the differences in the genomic architecture between the selected genomes. Phylogenetic reconstruction based on rearrangements were made with TIBA (Lin et al. 2012), using a neighbor joining method with 100 bootstrap replicates.
Results
Genomic Features
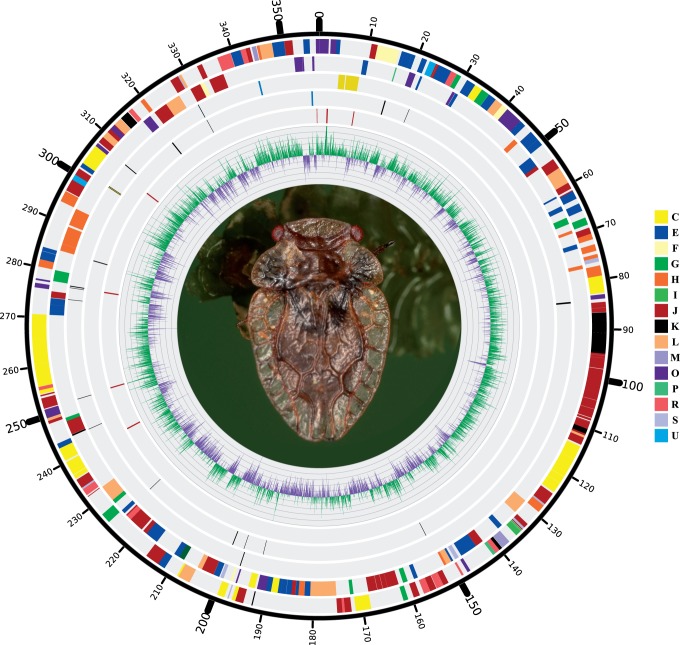
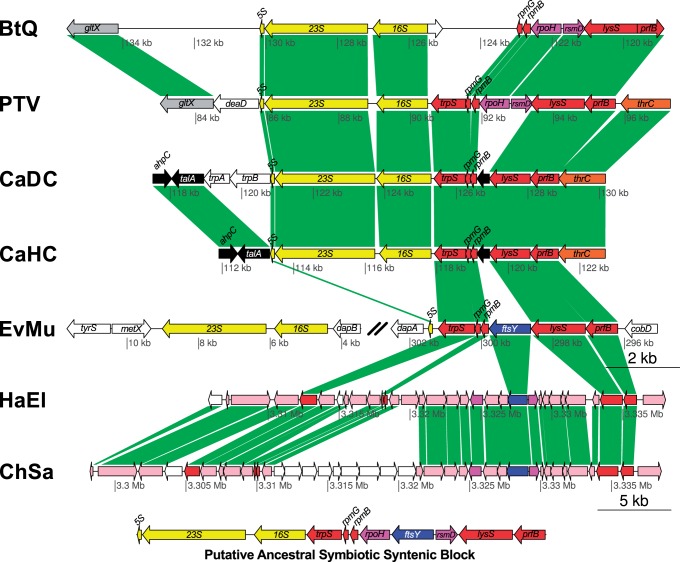
After quality filtering, symbiont read selection, assembly (253× 454 and 557× Illumina coverages), and correction of homopolymers, a single contig for the Evansia chromosome was obtained. Evansia presents a small genome of 357,498 bp composed of a single circular chromosome, a low GC content, close to 25%, and a coding density of 93.7% (fig. 1, table 1). A total of 369 genes were detected (330 protein coding and 39 noncoding RNA genes), which is a higher number than those of its closest symbiont relatives Portiera and Carsonella (table 1 and supplementary table S1, Supplementary Material online). The genome contains a complete set of tRNA genes (able to translate all codons, which include the initiator formyl methionine [MET] and the lysylated isoleucine [ILE] tRNA), three noncoding RNA genes (rnpB, a putative sRNA sX4, and ssrA), and one ribosomal RNA (rRNA) gene of each type. In contrast to Portiera and Carsonella, the rRNA operon was split in two segments, one containing the 16S and 23S rRNAs and the other the 5S rRNA. The rRNA operons of Carsonella and Portiera, as well as the 5S rRNA from Evansia, seem to be orthologous because they have the same upstream genomic context, suggesting an ancestral syntenic block, which includes the trpS, rpmG, rpmB, rpoH, ftsY, rsmD, lysS, and prfB genes (fig. 2).
Fig. 1.—
Circular view of Candidatus Evansia muelleri genome. From inner to outer tracks: (I) Positive (green) and negative (purple) GC skew across the genome. (II) Tandem repeats (red lines). (III) Complementary strand noncoding RNA genes: rRNA genes (yellow), transfer RNA genes (black), other RNA genes (blue). (IV) Direct strand noncoding RNA genes: rRNA genes (yellow), transfer RNA genes (black), other RNA genes (blue). (V) Complementary strand CDS. (VI) Direct strand CDS. CDS were colored according to cluster of orthologous groups (COG) classification. The background image is a picture of Evansia's host Xenophyes cascus.
Table 1.
Main Genomic Features of Halomonadaceae Endosymbionts
| Symbiont | Genome Size (bp) | GC (%) | Genes | CDS | Coding Density (%) | rRNA | tRNA | Other RNA | Pseudo |
|---|---|---|---|---|---|---|---|---|---|
| Candidatus Carsonella ruddii PV | 159,662 | 17 | 213 | 182 | 97 | 3 | 28 | 0 | 0 |
| Ca. Carsonella ruddii HC | 166,163 | 14 | 223 | 192 | 98 | 3 | 28 | 0 | 0 |
| Ca. Portiera aleyrodidarum TV | 280,663 | 25 | 307 | 269 | 92 | 3 | 34 | 1 | 0 |
| Ca. Portiera aleyrodidarum BT-QVLC | 357,472 | 26 | 284 | 246 | 68 | 3 | 33 | 2 | 8 |
| Ca. Evansia muelleri Xc1 | 357,498 | 25 | 369 | 330 | 94 | 3 | 33 | 3 | 0 |
| Buchnera aphidicola Cca | 422,434 | 20 | 403 | 365 | 87 | 3 | 31 | 4 | 3 |
| Bu. aphidicola 5A | 642,122 | 27 | 592 | 555 | 87 | 3 | 32 | 2 | 7 |
Note.—For comparative purposes, two strains of the endosymbiont of aphids, Bu. aphidicola were included.
aPlasmid pLeu-BCc is included in the summary statistics.
Fig. 2.—
Genome context of rRNA operons. The single complete rRNA operon of Portiera strains BT-QVLC (BtQ) and TV (PTV) and Carsonella strains DC (CaDC) and HC (CaHC), and the two rRNA transcription units in Evansia (EvMu) are shown. The putative ancestral symbiotic syntenic block that includes the rRNA operon retained in Carsonella and Portiera is shown at the bottom. The genome context of the upstream genes in Chromohalobacter salexigens (ChSa) and Halomonas elongata (HaEl) is also shown. Homologous genes are tagged with several colors. White genes represent genes without homologs in the displayed chromosomal region. The chromosomal positions of the displayed segments are shown under each drawing.
Evansia also contains eight tandem repeats, ranging from 100 to 330 bp, distributed along the genome (fig. 1). This type of repeat has also been reported and proposed as a possible explanation for the genomic instability of Portiera from Bemisia tabaci (Sloan and Moran 2013).
The genome of Evansia does not encode all the proteins required for the basic DNA and RNA metabolisms (Gil et al. 2004). It lacks the DNA polymerase subunit encoding genes holA, holB, and dnaX from the basic replication machinery and the argS gene from the aminoacyl-tRNA synthetases. It also lacks asnS (asparaginyl-tRNA synthetase), but the synthesis of Asn-tRNA could be performed by a nondiscriminating aspartyl tRNA synthetase (aspS) (Charron et al. 2003) coupled to GatABC aspartyl/glutamyl-tRNA amidotransferase reaction.
Cell Morphology
Evansia cells are large, pleomorphic, and has nucleoid-like masses (electron-dense granules) similar to Carsonella and Portiera (Costa et al. 1993; Baumann 2005; Kuechler et al. 2013) occupying most of the bacteriocyte cytosol. Interestingly, although Evansia does not encode genes for the fatty acid metabolism (only fabF is present), it shows a three-membrane system (fig. 3), one derived from the host and the two typical Gram-negative bacterial membranes (inner and outer membranes).
Fig. 3.—
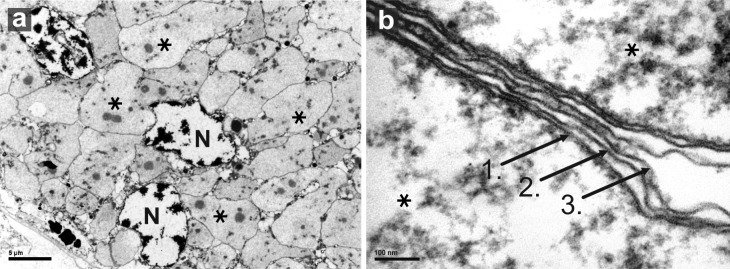
Transmission electron microscopy micrographs of Candidatus Evansia muelleri membranes. (a) Bacteriome overview. (b) Three membrane system in Evansia: 1. inner membrane; 2. outer membrane; and 3. host-derived membrane. Evansia cells (*). Host nuclei (N).
Phylogenomic Reconstruction
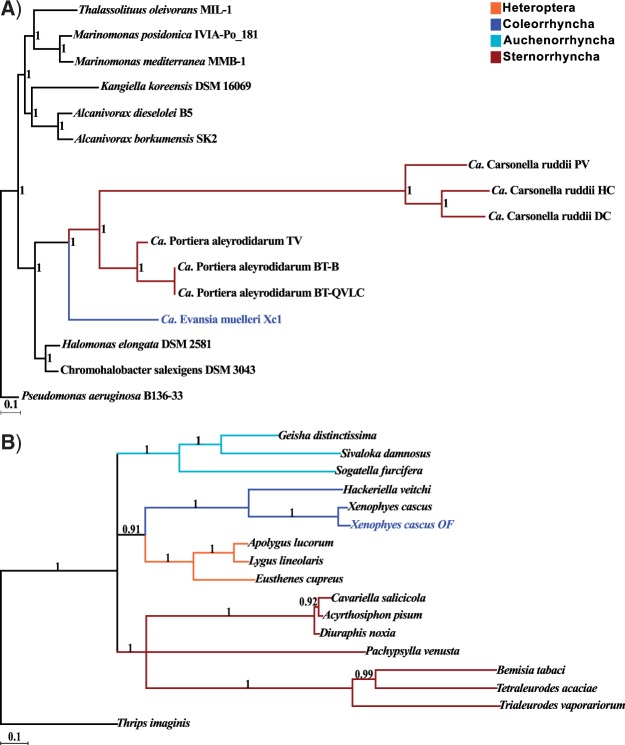
Initial ML phylogenomic reconstruction performed with PhyloPhlAn included Evansia within a symbiont clade with Portiera and Carsonella as recently proposed based on a 16S rRNA phylogeny (Kuechler et al. 2013). The phylogenomic reconstruction gave a different topology, with Evansia as the first divergent lineage and Portiera and Carsonella more closely related (data not shown). To solve the disagreement with the previously published phylogeny, ML phylogeny reconstructions were performed with a subsample of 23 groups of orthologous proteins and with its concatenate alignment (supplementary file 1: supplementary fig. S1, Supplementary Material online). Sixteen single-protein phylogenies placed Evansia outside the Portiera/Carsonella cluster (100% median bootstrap), whereas seven located Evansia as the closest relative of Carsonella (76% median bootstrap confidence) (as in the 16S rRNA phylogeny) (see supplementary file 2, Supplementary Material online). Concatenated protein ML phylogeny supported also the most frequent gene phylogeny with a 100% bootstrap value (supplementary file 1: supplementary fig. S1, Supplementary Material online). This phylogeny was also reconstructed with a Bayesian analysis performed on the concatenated alignment with same results (fig. 4A). The fact that two topologies were obtained using 23 genes could suggest that Evansia has some genome heterogeneity. The presence of ancestral duplicated genes and the differential loss of paralogs in each lineage could be a reason for this heterogeneity, but an artifactual topology due to the rapid sequence evolution in extreme reduced genomes could not be ruled out. Indeed, rRNA genes in Evansia seem to derive from different rRNA clusters if we look at their genomic contexts (fig. 2). The above considerations could be the reason our updated phylogeny differs from the previously published one. Also, a phylogenetic Bayesian inference was performed for the mitochondrial proteomes of different Heteroptera, Coleorrhyncha, Auchenorrhyncha, and Sternorrhyncha (fig. 4B). Although only three rooted topologies are possible with equal probabilities, it should be noted that the Evansia, Portiera, and Carsonella topology is concordant with the one of their hosts.
Fig. 4.—
Host and symbiont phylogenies. (A) Phylogenomic reconstruction for sequenced Halomonadaceae genomes. Majority rule consensus tree for the 23 concatenated genes from Bayesian analysis is displayed. Posterior distributions are displayed at each node. Pseudomonas aeruginosa was used as outgroup. Evansia is displayed in blue. (B) Phylogenomic reconstruction for different hemipteran mitochondrial genomes. Majority rule consensus tree for the 23 concatenated genes from Bayesian analysis is displayed. Posterior distributions are displayed at each node. Thrips imaginis (Thysanoptera) was used as outgroup. Evansia host (Xenophyes cascus OF) is displayed in blue.
Comparative Analysis of Evansia Proteins Based on COG Functional Categories
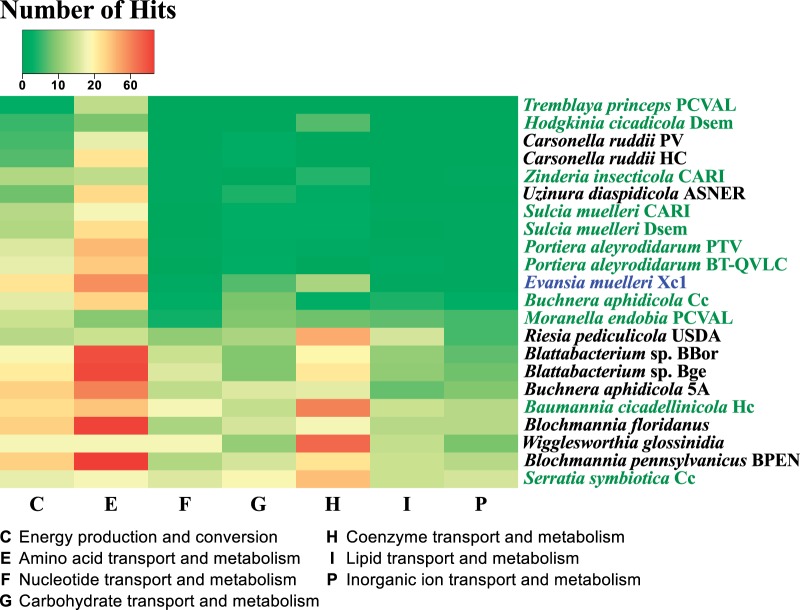
The distribution of proteins in COG categories was compared with those of other insect P-endosymbionts (fig. 5). Evansia falls into the category of P-endosymbionts that do not require another coprimary symbiont for its mutualistic action. The C (energy production) category showed more hits in Evansia than in other endosymbionts with small genome sizes like Portiera, Buchnera aphidicola BCc, Moranella, or Hodgkinia. In fact, clustering analysis for this category showed that Evansia was closer to Blochmannia spp., the endosymbionts of carpenter ants with genomes of 700–800 kb (supplementary file 1: supplementary fig. S2, Supplementary Material online). E (amino acids biosynthesis) category also showed a slight increase in Evansia when compared with endosymbionts with similar genome size and the clustering analysis confirmed that Evansia was closer to Blattabacterium, Bu. aphidicola strain 5A, and Blochmannia that are able to almost completely synthesize all the essential amino acids (supplementary file 1: supplementary fig. S3, Supplementary Material online). Finally, H (coenzyme metabolism) category also showed an increase when it was compared with the extreme reduced endosymbionts with the exception of Hodgkinia. In this case, Hodgkinia and Evansia clustered together, pointing to a similar role in vitamin biosynthesis (supplementary file 1: supplementary fig. S4, Supplementary Material online). The remaining COG categories did not show clear differences with the endosymbionts of similar genome sizes. These data suggest that even though Evansia possesses a much reduced genome, it has some interesting features similar to endosymbionts with larger genomes.
Fig. 5.—
Heatmap of selected COG categories from different endosymbionts. Endosymbionts are sorted by genome size from the smallest (Tremblaya princeps PCVAL) to the biggest (Serratia symbiotica Cc). For each genome, the numbers of hits in each COG category are shown. P-endosymbionts living with another co-P-endosymbiont are displayed in green. Evansia muelleri Xc1, which has no co-P-endosymbiont, is displayed in blue. Serratia symbiotica Cc is an example of a coprimary symbiont of large genome. COG descriptions are showed in the bottom.
Metabolic Capabilities of Evansia
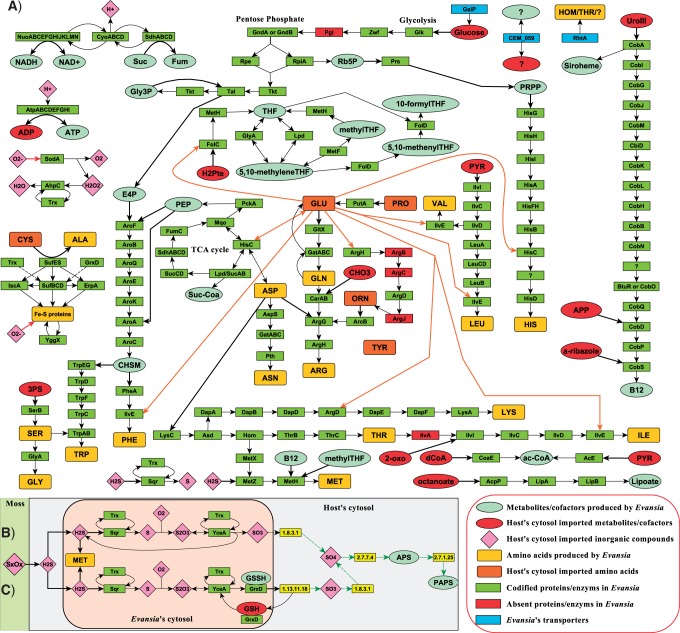
The full metabolism of Evansia was inferred, and its biosynthetic capabilities were represented as a simplified graph (fig. 6A–C). Evansia showed an incomplete glycolytic pathway and an almost complete pentose phosphate pathway (the function of the absent pgl gene could be complemented by the host). The presence of a functional pentose phosphate pathway would permit Evansia to produce different metabolites, which are used as input for other pathways and reducing power (NADPH). Evansia also possesses a reduced TCA cycle, able to produce all necessary metabolites, with HisC producing a bypass from oxalacetate to 2-oxoglutarate and making dispensable the three first steps of the cycle (encoded by gltA, acnAB, and icd) as it occurs in some Blattabacterium strains (González-Domenech et al. 2012).
Fig. 6.—
Biosynthetic capabilities of Evansia. (A) Simplified graph showing the metabolic reconstruction for Evansia. (B) Putative H2S recycling pathway. (C) Putative H2S detoxification pathway. Amino acids are identified by the standard three letter code. Black arrows point the direction of the reaction. Black thick arrows represent inputs for a reaction. Orange thick arrows indicate reactions that need glutamate as input. Red arrows represent ROS interactions. Green arrows denote reactions produced by the host. Dashed arrows indicate poorly characterized or putative reactions. “?” denotes an unidentified protein/metabolite/compound. APP, (R)-1-amino-2-propanol O-2-phosphate; UroII, uroporphyrinogen-III; CHO3, bicarbonate; PYR, pyruvate; CHSM, chorismate; E4P, D-erythrose 4-phosphate; Rb5P, D-ribose 5-phosphate; PRPP, 5-phospho-α-D-ribose 1-diphosphate; Gly3P, D-glyceraldehyde 3-phosphate- H2Pte, 7,8-dihydropteroate; THF, tetrahydrofolate; CoA, coenzyme A; dCoA, 3'-dephospho-CoA; Ac-CoA, acetyl-CoA; Suc-Coa, succinyl-CoA; PEP, phosphoenolpyruvate; 3PS, 3-phospho-L-serine; O2−, reactive oxygen species; 2-oxo, 2-oxoglutarate; GSH, glutathione; GSSH, glutathione sulfide; APS, adenosine 5'-phosphosulfate; PAPS, phosphoadenosine-5'-phosphosulfate; SxOx, Sulfur oxidized forms.
Regarding the synthesis of amino acids, Evansia is able to produce alone, or complemented by the host, the ten essential ones. It harbors the full pathways for the seven essential amino acids leucine, valine, lysine, tryptophan, phenylalanine, threonine (THR), and arginine (ARG). It contains almost complete pathways for ILE and histidine. In the case of ILE, the pathway lacks IlvA and the regulatory subunit IlvH, but IlvI maintains a reduced catalytic activity in its absence (Vyazmensky et al. 1996). Finally, the MET biosynthetic pathway is very unusual regarding other P-endosymbionts. The synthesis is carried out by a sulfhydrylation step (encoded by metX and metZ) and finished by the cobalamin-dependent MET synthase (encoded by metH). This alternative pathway is probably of ancestral origin because it is also found in species of the genus Pseudomonas (Alaminos and Ramos 2001). Evansia needs to import S-adenosyl-MET from the host. Concerning nonessential amino acids, Evansia is able to synthesize alanine, asparagine, aspartate, glutamine, glycine, and serine, whereas it needs to import glutamate, proline, ornithine, cysteine (CYS), and tyrosine from the host.
Evansia can also produce different cofactors and intermediate metabolites importing different compounds from the host's cytosol. Among these cofactors, we can found acetyl-CoA, lipoate, and different folate forms. Evansia almost maintains the full cobalamin (B12) biosynthesis pathway, but although one enzyme was not identified, the presence of the remaining enzymes points to this enzyme activity is encoded in the genome. The missing enzyme, which produces the reduction from Cob(II)yrinic acid a,c-diamide to Cob(I)yrinic acid a,c-diamide, is still under some controversy (revised in Mera and Escalante-Semerena 2010), and some candidates could be present in Evansia.
Reductive power (NADH) and energy (ATP) can be produced by the NADH-quinone oxidoreductase and the ATP synthase complexes, respectively. In contrast, Evansia is not able to produce purines and pyrimidines, so they would have to be imported from the host. Evansia only conserves the ribH gene from the flavin biosynthesis pathway, so all its derivatives (riboflavin, FMN, and FAD) would also have to be imported from the host cytosol. In addition, Evansia also needs to import NAD.
Regarding the sulfur metabolism, Evansia seems to have the ability to transform hydrogen sulfide (H2S) to intracellular sulfur (S) and vice versa by a sulfide:quinone oxidoreductase (SQR; encoded by sqr) coupled to thioredoxin (encoded by trx). Fe-S proteins could be assembled by the A-type carriers (encoded by erpA and iscA), the CYS desulfurase (SufES), and the SufBCD complex, whereas YggX is in charge of repair Fe-S proteins from superoxide damages (Gralnick and Downs 2001).
Reactive oxygen species (ROS) and their derivatives (O2-) can be reduced to water by the combination of sodA, ahpC, and trx gene products (Parsonage et al. 2008).
Finally, Evansia genome encodes two transporters involved in the metabolism: GalP that is involved in the glucose import and RhtA, a homoserine/theonine efflux transporter that can also export other amino acids (Hernández-Montalvo et al. 2003; Livshits et al. 2003). A third transporter (encoded by CEM_059), which seems to be distantly related to DitE transporter from P. abietaniphila, was identified (Martin and Mohn 2000). This transporter may have a different function, because no diterpenoids degrading enzymes were found in Evansia. As it belongs to the major facilitator superfamily, it could act on a wide number of substrates but its function remains unknown.
Comparative Genomics between Evansia and Its Relatives
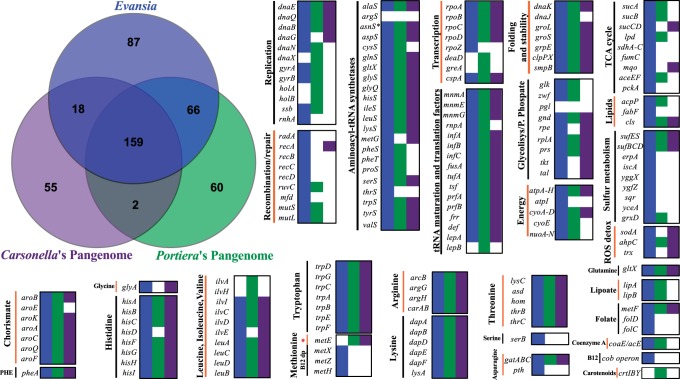
Orthologous clusters were generated for C. salexigens DSM 3043, H. elongata DSM 2581, Carsonella pangenome (genes present in any of the strains DC, HC, and PV), Portiera pangenome (genes present in any of the strains BT-B, BT-QVLC, and TV), and Evansia. If the free-living relatives (Chromohalobacter and Halomonas) were included, the Euler diagram subspaces of the orthologous clusters gave a core genome of 158 clusters (supplementary file 1: supplementary fig. S5, Supplementary Material online). Chromohalobacter and Halomonas showed 884 and 1,026 strain-specific genes, respectively, and 1,978 shared genes. Based on the Euler diagram, it seems that endosymbiont gene repertoires are just a reduced subset from the free-living ones without new genes acquired after the divergence of symbiotic lineages. Although Carsonella pangenome contains 54 strain-specific genes (most of them hypothetical proteins), this number could be an artifact due to the problematic assignment to orthologous clusters of several Carsonella genes owing to its high evolutionary divergence. Similarly, in Portiera pangenome, 16 genes encoding small hypothetical proteins could be artifacts (supplementary file 1: supplementary fig. S5, Supplementary Material online). The specific carotenoid biosynthetic genes, although absent in Chromohalobacter and Halomonas, are encoded in the genomes of other free-living Halomonadaceae, ruling out a case of horizontal gene transfer (HGT) in the symbiotic lineage. Finally, also discarding some hypothetical proteins without recognizable domains in Evansia, the two other remaining genes were probably of ancestral origin because they are present in other Oceanospirillales species.
An Euler diagram was also generated for the three endosymbionts (fig. 7). In this case, the core genome is composed of 159 genes: Replication machinery, transcription machinery, part of the Fe-S cluster assembly proteins (and other chaperones/protein turnover), energy production, most of the essential amino acid metabolism, ribosomal genes (45 genes), and aminoacyl-tRNA synthetases, among other genes (fig. 7). Carsonella's pangenome and Evansia present a more complete TCA cycle and pentose phosphate pathway than Portiera, whereas Evansia has retained a more complex energy production machinery. Evansia shows a different pathway for MET synthesis (metHXZ and cobalamin biosynthetic genes) than Portiera and Carsonella (metE). Although Carsonella is not able to produce cofactors, Evansia and Portiera are able to synthesize some of them. In contrast, only Portiera is able to synthesize carotenoids. The homologous recombination machinery is only complete, and probably active as can be seen by the absence of a clear GC skew, in Evansia (fig. 1) that is an unusual characteristic in extreme reduced endosymbionts (fig. 7). Although the three symbionts maintain membrane proteins such as the ATPase machinery and some transporters, related to the Fe-S cluster assembly (sufBC), or chaperones (clpX), Evansia presents three additional transporters, two shared with Portiera (GalP and the DitE related transporter CEM_059) and one related to the extrusion of different amino acids (encoded by rthA). Carsonella does not present any other transporter. Evansia also conserves three Sec-dependent pathway proteins (encoded by ftsY, ffh, and secE, the latter perhaps a pseudogene) and a protein in charge to remove signal peptides (lspA). Two of them are also present in Portiera (secE and lspA).
Fig. 7.—
Orthologous coding gene clusters from Evansia, Carsonella's Pangenome, and Portiera's Pangenome. Euler diagram displaying the number of clusters found on each subspace of the pangenome (top left). Gene contents for the most relevant cell functions, and metabolic capabilities are plotted for each endosymbiont. Filled squares indicate the presence of genes in Evansia (blue), Carsonella’s pangenome (purple), and Portiera’s pangenome (green). Absent genes (white). Carsonella's and Portiera's pangenomes include the CDS from Carsonella strains DC, HC, and PV and Portiera strains BT-B, BT-QVLC, and TV, respectively. B12: cobalamin. Red star denotes the MET cobalamin-independent pathway. B12 dp denotes the MET cobalamin-dependent pathway. The asterisk besides asnS denotes that, although none of the endosymbiont genomes harbors this gene, they may produce l-asparaginyl-tRNA with a nondiscriminating aspS in combination with gatABC. Each gene was plotted only once, although it was involved in two or more categories.
Among the outstanding gene sets of Evansia are those involved in sulfur metabolism, including the common Fe-S cluster assembly proteins SufBCES and the SufD protein shared with Portiera. Also, Evansia encodes four proteins directly related to the Fe-S cluster assembly (iscA, yggX, ygfZ, and erpA) and three proteins probably involved in sulfur mobilization (sqr, yceA, and grxD).
Lastly, Evansia and Carsonella seem to be able to reduce ROS to water (sodA and ahpC). See supplementary table S2, Supplementary Material online, for a detailed list of the endosymbiont Euler diagram (fig. 7).
Genome Rearrangements Analysis
The analysis performed with 150 core clusters showed a total of 27–33 rearrangements required to explain the changes in the genomic architecture from the ancestral free-living relative (the most recent common ancestor with H. elongata and C. salexigens) to the three endosymbionts (fig. 8). The lineage of Portiera from B. tabaci was excluded from the analysis because the high number of rearrangements occurred in this strain after its divergence from Portiera of Trialeurodes vaporariorum (Sloan and Moran 2013). Our rearrangement-based phylogeny is congruent with the sequence-based phylogeny (fig. 4), thus corroborating the topology of the symbiotic clade with other phylogenetic approach.
Fig. 8.—
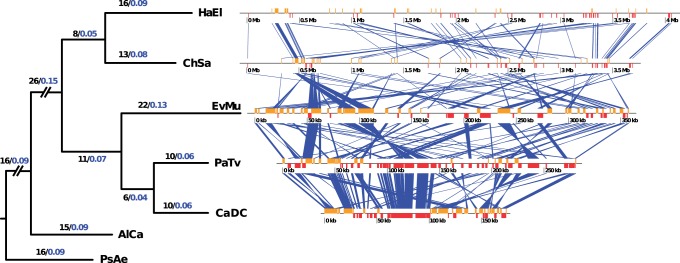
Evolution of genome rearrangements in Evansia and its relatives. Rooted tree showing the neighbor joining rearrangement phylogeny (TIBA) (left). Black numbers at each node are the minimum number of rearrangements needed for explaining the genomic order at each node (MGR), whereas blue numbers are relative values (the number of rearrangements in each branch/total number of rearrangements in the tree). Branches out of the study scope were shortened (slashes). Branch lengths are proportional to the number of rearrangements. On the right, blue bands connect orthologous genes between analyzed genomes. HaEl, Halomonas elongata DSM 2581; ChSa, Chromohalobacter salexigens DSM 3043; EvMu, Candidatus Evansia muelleri Xc1; PaTv, Ca. Portiera aleyrodidarum strain TV; CaDC, Ca. Carsonella ruddii strain DC; AlCa, Alcanivorax borkumensis SK2; PsAe, Pseudomonas aeruginosa B136-33.
During the same evolutionary time, the numbers of rearrangements in the branches leading to the free-living C. salexigens and H. elongata were only slightly smaller than those leading to the symbiotic clade, revealing that in comparison with some other symbionts such as Bu. aphidicola or Wigglesworthia glossinidia (Belda et al. 2005), there was not a high rearrangement rate during the initial steps of symbiosis.
Discussion
A Common Origin for Halomonadaceae Endosymbionts
The close phylogenetic relationship of P-endosymbionts of whiteflies and psyllids was previously observed by several authors (Thao and Baumann 2004; Sloan and Moran 2012b; Sloan and Moran 2013). In this work, we report the first genome of a Peloridiidae (Coleorrhyncha) P-endosymbiont, Ca. Evansia muelleri, which was recently proposed as a close relative of Portiera and Carsonella (Kuechler et al. 2013). Our phylogenomic reconstruction showed that the three endosymbionts form a well-supported monophyletic group included in the family Halomonadaceae (Gammaproteobacteria) (fig. 4A) as reported in a previous study based on 16S rRNA gene sequences (Kuechler et al. 2013). However, the relative position of the three endosymbionts was different with Evansia being the basal lineage when a phylogenomic approach was performed as explained above.
Although the obtained endosymbiont topology is concordant with the topology of their insect hosts (Coleorrhyncha, Psylloidea, and Aleyrodoidea) (fig. 4B), it is necessary to take this result with caution because the low number of host and endosymbiont taxa included. Moreover, the phylogenetic relationships with other members of the order Hemiptera are still under discussion (Cryan and Urban 2012; Song et al. 2012). However, paleontological evidence has served to propose the group Psyllinea, which would include superfamilies Psylloidea and Aleyrodoidea in opposition to Aphidinea (including Aphidoidea and Coccoidea) (Shcherbakov 2000).
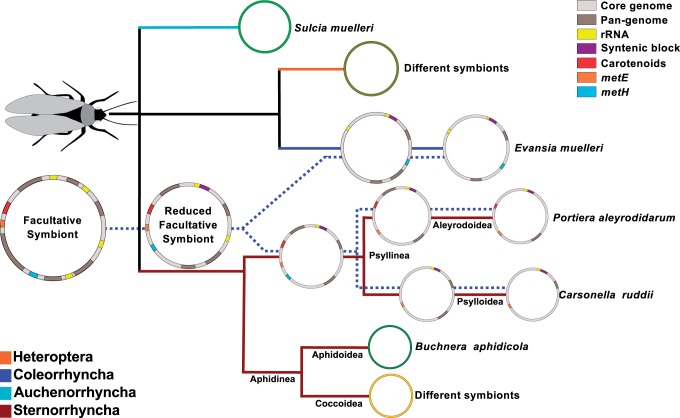
When reconciling the evolutionary histories of P-endosymbionts and their hosts, three scenarios are possible: 1) a single event of host–symbiont association in the ancestor of all Hemiptera (>250 Ma); 2) two infection events, one at the base of Psyllinea clade and another at the base of the Peloridiidae; 3) three infection events, one for Psylloidea, one for Aleyrodoidea, and one for Peloridiidae followed by the coevolution of host and endosymbiont in each lineage
There are some clues that can help us to understand the origin of this endosymbiotic clade: 1) The concordance between the host and P-endosymbiont phylogenies (fig. 4), 2) the retention of the same rRNA operon in Portiera and Carsonella and partially in Evansia (fig. 2), 3) the extremely reduced genomes of these P-endosymbionts, indicative of a very long endosymbiotic relationship (table 1), 4) the ancient origin of all the endosymbiont retained genes (discarding those encoding for hypothetical proteins). They are subsets of the Halomonadaceae free-living bacteria, and HGT events are not found in the symbiotic lineages (supplementary fig. S5, Supplementary Material online), and 5) the fixation of different MET biosynthetic pathways in their reduced genomes.
As free-living bacteria usually harbor multiple rRNA operons (four and five in Halomonas and Chromohalobacter, respectively), the observation that the only rRNA operon retained in Carsonella and Portiera is ortholog suggests that their most recent common ancestor already had a partially reduced genome (fig. 2). To explain the formation of this syntenic block, a chromosomal rearrangement moving an rRNA operon adjacent to the trpS/prfB block, besides several gene losses during the process of genome reduction, is required (fig. 2). This rearrangement took place before the divergence of Evansia from Carsonela/Portiera as shown by the presence of the 5S rRNA gene in Evansia. The distribution of rRNA genes in two transcription units in Evansia may be explained by one or more rearrangement events moving the 16S and 23S rRNA genes outside the rRNA/prfB block or by the presence of two rRNA operons at the time when Evansia and Portiera-Carsonella lineages diverged. None of the two hypotheses may be discarded. However, both would suggest the idea of a facultative symbiont with a genome at the beginning steps of the genome reduction process, because to explain the present genomic features of the three endosymbionts, either rRNA gene losses and/or rearrangements are required. Because a genomic stability is commonly obtained once a stable obligate endosymbiont has evolved (Tamas et al. 2002; Silva et al. 2003), the common origin of Portiera and Carsonella implies that their last common ancestor, although reduced in genome size, was already able to rearrange its genes (figs. 8 and 9). Although genomic architecture favors two infection events as the most likely scenario, the possibility of the single or the three infection events scenarios cannot be fully discarded.
Fig. 9.—
Evolutionary histories of P-endosymbionts and insect hosts. Model of reconciliation of host and P-endosymbiont phylogenies based on the hypothesis of two events of host–symbiont association followed by coevolution. Each Hemiptera suborder is marked by a different solid colored line. Endosymbiont phylogeny is shown with a dotted line. In Sternorrhyncha suborder, phylogenetic relationships are based in Shcherbakov (2000). Parallel dotted and solid lines mean host–symbiont coevolution.
The metabolism of the three endosymbionts also would support the two infection events hypothesis. Although Carsonella and Portiera (as many other endosymbionts) have maintained the cobalamin-independent MET biosynthesis pathway (metE) (Hansen and Moran 2014), Evansia has retained the dependent one (metH). Because the genes encoding these functions were present in the symbiotic ancestor, we can speculate that, at the time of the divergence of Evansia from the two other symbionts, the ancestral genome was partially reduced, and the fixation of different MET biosynthetic systems took place during the last steps of the reductive process together with the events of obligate associations of Evansia by one way, and the ancestor of Portiera/Carsonella by the other.
The single-event hypothesis would have implied the replacement of the ancestral symbiont in several lineages (Heteroptera, Auchenorrhyncha, and Aphidoidea), because some hemipterans harbor other primary endosymbionts (Baumann 2005; Hansen and Moran 2014). However, the replacement of an ancient obligate symbiont by a new more efficient one has been reported several times in insects (Conord et al. 2008; Koga et al. 2013; Oakeson et al. 2014). Overall, the single-event hypothesis seems less plausible than the two infection events from a parsimonious point of view.
In support of the alternative three event hypothesis, it could be argued that concordant phylogenies and retention of the same rRNA operon could have been produced by chance, and that, although extremely reduced genomes require a long coevolutionary relationship with their hosts, they might be produced in a shorter period of time (i.e., <200 Myr). In this three event hypothesis, the bacteria that started the obligate associations with the ancestors of each of the three insect lineages would come from a facultative endosymbiont clade able to infect different Hemiptera (or arthropod) lineages, as it has been reported for Wolbachia, Hamiltonella, Rickettsia, Cardinium, etc. (White 2011). This reasoning leads us to consider the two infection events hypothesis as the more plausible (fig. 9).
In conclusion, there is no doubt that an ancestral insect endosymbiont clade was able to infect ancestral Hemiptera, but the remaining question is whether the shift to only vertical transmission and to the obligate association took place one, two, or three times. To distinguish between them, the sequencing of the endosymbionts of other lineages and a better understanding of the phylogeny of Hemiptera will be required.
Differences and Similarities between Halomonadaceae Endosymbionts
The genomic features of Evansia, Portiera, and Carsonella reveal the consequences of a long obligate endosymbiosis. Recently, the term symbionelle (Reyes-Prieto et al. 2013) was proposed to describe bacteria that fail to reach the minimal gene set (Gil et al. 2004). Although the number of genes in Portiera (circa 290) and Evansia (369) was a little higher that the threshold proposed for symbionelle, both may be considered symbionelles because they have lost some of the gene products required for a minimal cell to be autonomous. In the case of Evansia, the loss of argS, the gene encoding ARG-tRNA synthetase, but the retention of the noncoding gene tRNA-Arg suggests that a nuclear-encoded protein of unknown origin should be targeted to the bacterial cytosol to charge with ARG the tRNA-Arg. This argS gene could have been acquired through an HGT event either from Evansia or from a secondary endosymbiont. It could also be that the protein imported by mitochondria could also be imported by Evansia. HGT events from multiple bacteria to the nuclear genome have been recently observed in the mealybug Planococcus citri (Husnik et al. 2013). Also, recently, the import of several proteins of the photosystem I into the nascent photosynthetic organelles of the amoeba Paulinella chromatophora has been demonstrated (Nowack and Grossman 2012) suggesting that these systems may evolve frequently.
The cell structure of the three endosymbionts displays some important differences. Although Carsonella and Evansia present the usual three-membrane system (host–outer membrane-inner membrane) (fig. 3), Portiera lacks the outer membrane (Costa et al. 1993; Baumann 2005). In contrast, Portiera pangenome presents the largest number of transporters (11), whereas Evansia only presents three and Carsonella pangenome none. According to the Transporter Classification Database (www.tcdb.org, last accessed April 20, 2014), four (PalTV_015, 099, 174, and 204) out of the nine transporters found in Portiera TV are usually localized in both parts of the membrane (outer and inner). Thus, a closer look to Portiera cell structure needs to be revisited, because even Carsonella, the most reduced endosymbiont, also presents the three-membrane system. In addition, the way of membrane assembly in Evansia and Carsonella is still poorly understood. However, it is possible that these genes have been transferred to the host genome (Husnik et al. 2013; Sloan et al. 2014) and that the cytoplasm-synthesized proteins were incorporated to the bacterial membrane.
The most relevant characteristic of the metabolism of Evansia is that, in spite of its small genome size, it has retained, comparatively to other endosymbionts of similar or bigger sizes, a large set of the genes required for the synthesis of amino acids and cofactors, the metabolism of sulfur, and the production of energy (fig. 6A). This feature could be related to the fact that no other obligate endosymbiont partner was detected in the host (X. cascus) bacteriome or surrounding tissues, and all the amino acid biosynthetic roles rely on Evansia (Kuechler et al. 2013). Because Evansia, Portiera, and Carsonella seem to hold a subset of the gene repertoire of their free-living relatives (supplementary fig. S5, Supplementary Material online), evolution of their respective gene repertories could be related to the diet of each insect. Thus, psyllids could have access to a more enriched diet than other sap-sucking insects, and this could explain that some Carsonella strains have lost some amino acid biosynthetic pathways without an endosymbiotic partner. In contrast, whiteflies feed on phloem and usually harbor one or more secondary symbionts that share the same bacteriocyte with Portiera and probably complement the lost biosynthetic pathways (Gottlieb et al. 2008; Santos-Garcia et al. 2012; Sloan and Moran 2012a). In this context, X. cascus (and Peloridiidae in general) feeds on bryophytes (mosses and liverworts) that appear much poorer in nitrogen and sulfur compounds when compared with tracheophytes (higher plants) (Huneck 1983; Klavina et al. 2012). The analysis of Evansia metabolism showed that it has a large ability to synthetize most of proteinogenic amino acids, including ten essentials, six nonessentials, and N-formyl-MET (figs. 6 and 7). This seems to be related with the poor nitrogen content found in bryophytes (Klavina et al. 2012). There are also other gene sets whose retention in the small Evansia genome seems to be associated with the maintenance of any metabolic piece required for the synthesis of amino acids. For example, the pentose phosphate pathway, TCA cycle, and respiratory machinery are connected with the requirement of this huge amino acid biosynthetic machinery. The retention of the cobalamin biosynthetic pathway was also associated with the synthesis of amino acids, because this cofactor is required for the last step of MET synthesis. In contrast to Carsonella and Portiera and other primary endosymbionts, Evansia has maintained the MetH enzyme (cobalamin dependent) in opposition to MetE (cobalamin independent). The cobalamin biosynthetic pathway in Evansia lacks a nitroreductase that is present in H. elongata (and other Halomonadaceae) but not in several Pseudomonas (both able to synthesize cobalamin), suggesting that another enzyme can supply this step in Evansia. The retention of the ability to synthesize 5-methyltetrahydrofolate is also associated to the synthesis of MET (fig. 6A). The retention of gene rthA, which encodes a transporter able to export THR outside the bacterial cell, seems to be associated with the insect-endosymbiont shared biosynthesis of ILE. The interrupted pathway from THR to ILE may be bypassed in a similar way at the one observed in the aphid-Bu. aphidicola system (Wilson et al. 2010) by exporting THR to the insect cell, which will convert it in 2-oxobutanoate, compound that would enter the bacterium to continue the ILE biosynthetic pathway. However, because this enzyme is absent in the family Halomonadaceae, and a D-alpha,beta-D-heptose 1,7-bisphosphate phosphatase has been proposed to fill this gap by the gapfiller tool from Ecocyc. Nevertheless, it is possible that another phosphatase (i.e., serB) could do the function as well. Finally, in contrast to Bu. aphidicola, the gene encoding the last step (ilvE) is present in Evansia.
Sulfur Metabolism in Evansia
Sulfur metabolism is another intriguing feature in Evansia when it is compared with other endosymbionts. Most of the extreme reduced endosymbionts usually retain the sufBCDES operon, which is required for the formation of the Fe-S proteins. This system has been proposed as an indicative of an oxidative stress environment in endosymbionts because it is more resistant to ROS than the isc operon (Lee et al. 2004; Lill 2009; Py and Barras 2010) but seems more an adaptation of the first stages of pathogenesis/endosymbiosis (Toft and Andersson 2010). In these stages, the host's immune system are fighting against the symbiont/pathogen, and one known reaction is to increase the oxidative stress, and it is reasonable that pathogenic/endosymbiotic bacteria have retained a Fe-S machinery that can work under these conditions (Huet et al. 2005). In addition, Evansia has retained two related A-type carriers, IscA and ErpA (Lill 2009; Py and Barras 2010). These proteins have been proposed as alternative routes for Fe-S assembly or as scaffolder proteins that carry the formed Fe-S cluster to the target apoprotein (Lill 2009; Py and Barras 2010). It also contains a glutaredoxin 4 homolog (grxD) that has been recently involved in an alternative Fe-S pathway (Li and Outten 2012) and YggX protein that is involved in Fe-S cluster repair after ROS damage. This suggests an oxidative environment potentially related to some toxins present in bryophytes (revised in Asakawa et al. 2013) although it could also be related to the endogenous detox pathway because Evansia seems to have a very active metabolism. It is also possible that the low disposition of sulfur in bryophytes is the clue for the maintenance of this enriched sulfur machinery, including the recycling and protection of the Fe-S clusters.
Surprisingly, an SQR, which allows the oxidations of H2S to sulfur or polysulfide chains, is present in Evansia (Marcia et al. 2010) (fig. 6A–C). This enzyme has been described in phototrophic sulfur bacteria and in symbionts from marine vent hosts and armored snails, but the sulfur oxidation machinery is much more complex in these organisms than in Evansia (Frigaard and Dahl 2009; Harada et al. 2009; Nakagawa et al. 2014). Likewise, SQR may be part of a mitochondrial detoxification machinery against H2S in combination with a rhodanese (RHOD) and a sulfur dioxygenase (SDO) (Hildebrandt and Grieshaber 2008). This system also seems to be a common mechanism for sulfur oxidation in a wide range of heterotrophic bacteria (Liu et al. 2014). Because of the low amount of inorganic sulfur (usually uptaken from the soil in the form of sulfate or sulfite) in bryophytes, it is possible that the only source of organic sulfur for the system Xenophyes–Evansia comes from Evansia. Oxidized sulfur, present in the insect diet, could be reduced by the insect to H2S. This reduced form is used by Evansia to produce MET that could be exported to the host's cytosol where it would be used for the synthesis of CYS, one of the organic forms of sulfur. If some H2S could not be used for MET biosynthesis, SQR could be involved in the storage of sulfur (S) for its use when required. Intracellular sulfur (S) is then oxidized spontaneously to thiosulfate. At this point, two hypotheses arise: 1) SQR and YceA proteins are part of a recycling mechanism used to ensure maximum usage of H2S or 2) a combination of SQR, YceA, and GrxD transfers the leftover H2S to the host's cytosol as a detoxifying mechanism (fig. 6B and C).
If the SQR is working in a recycling manner, it is possible that the YceA protein (as other rhodaneses) could regenerate one H2S and to produce a sulfite molecule to be exported to the host for excretion because no sulfite oxidase has been detected in Evansia (Cheng et al. 2008) (fig. 6B). Furthermore, and taking into account that sulfur is a limiting factor, it is possible that a sulfite oxidase (present in most of the sequenced arthropods) could use the sulfite to produce other intermediate metabolites as a way of Evansia's “waste recycling” like nitrogen metabolism in Blattabacterium cuenotti (López-Sánchez et al. 2009; González-Domenech et al. 2012) (fig. 6B). On the other hand, H2S is a toxic compound (similar to cyanide) that, even at low concentrations, can interfere with the electron transport chain. In this context, and in combination with the rhodanese YceA and the glutaredoxin 4 GrxD, SQR could be involved in the elimination of the remaining hydrogen sulphide, which is not used in the MET biosynthesis (fig. 6C). In this case, SQR would oxidize H2S to thiosulfate that is combined with glutathione (GSH) by YceA to form glutathione sulfide (GSSH). GSSH could be transferred to the host's cytosol by GrxD where SDO would oxidize it, releasing a glutathione molecule and a sulfite. Finally, sulfite could be excreted or used in the host metabolism (PAPS biosynthesis). Because both pathways share most of the enzymes, it is possible that both are co-occurring in Evansia–Xenophyes system to ensure the optimization of sulfur utilization.
Conclusion
In summary, in spite of the long-term endosymbiotic life associated with moss bugs, Evansia has retained almost any gene required for the biosynthesis of amino acids including those involved in the synthesis of the enzyme cofactors, sulfur metabolism, and central metabolism. No special gene for detoxification of biologically active compounds (e.g., sesquiterpenoids) could be found in Evansia, so far. This indicates that moss bugs possibly do not come into contact with any of these toxic secondary metabolites by sucking only on the conductive tissue (that should contain only a minor amount of antifeedant) or that possible toxic metabolites will be degraded by gut microbiota or the host detoxification system (Hansen and Moran 2014), for example, via the extremely huge Malpighian tubules in moss bugs. Further studies about the insect (symbiont)–plant interaction will bring more light into this fascinating system.
Supplementary Material
Supplementary tables S1 and S2, figures S1–S5 (Supplementary file 1), and Supplementary file 2 are available at Genome Biology and Evolution online (http://www.gbe.oxfordjournals.org/).
Acknowledgments
This work was supported by grants BFU2012-39816-C02-01 (cofinanced by FEDER funds and Ministerio de Economía y Competitividad, Spain) and Prometeo/2009/092 (Conselleria d’Educació, Generalitat Valenciana, Spain). D.S.-G. was recipient of a Prometeo 92/2009 contract. The authors gratefully acknowledge EcoCyc team (SRI International) and specially to Suzanne Paley for her support with Evansia's metabolic database and to Josep Antoni Rosselló for advising about moss biology. They also acknowledge the SCSIE from the Universitat de València for sequencing and microscopy support. Finally, they thank Ekkehard Wachmann for providing a photo of Xenophyes cascus.
Literature Cited
- Alaminos M, Ramos J. The methionine biosynthetic pathway from homoserine in Pseudomonas putida involves the metW, metX, metZ, metH and metE gene products. Arch Microbiol. 2001;176:151–154. doi: 10.1007/s002030100293. [DOI] [PubMed] [Google Scholar]
- Allen JM, Light JE, Perotti MA, Braig HR, Reed DL. Mutational meltdown in primary endosymbionts: selection limits Muller’s ratchet. PLoS One. 2009;4:e4969. doi: 10.1371/journal.pone.0004969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardell DH, Andersson SGE. TFAM detects co-evolution of tRNA identity rules with lateral transfer of histidyl-tRNA synthetase. Nucleic Acids Res. 2006;34:893–904. doi: 10.1093/nar/gkj449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa Y, Ludwiczuk A, Nagashima F. Phytochemical and biological studies of bryophytes. Phytochemistry. 2013;91:52–80. doi: 10.1016/j.phytochem.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Aziz RK, et al. The RAST server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P. Biology bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu Rev Microbiol. 2005;59:155–189. doi: 10.1146/annurev.micro.59.030804.121041. [DOI] [PubMed] [Google Scholar]
- Belda E, Moya A, Silva FJ. Genome rearrangement distances and gene order phylogeny in gamma-Proteobacteria. Mol Biol Evol. 2005;22:1456–1467. doi: 10.1093/molbev/msi134. [DOI] [PubMed] [Google Scholar]
- Bernt M, et al. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 2013;69:313–319. doi: 10.1016/j.ympev.2012.08.023. [DOI] [PubMed] [Google Scholar]
- Boetzer M, Henkel CV, Jansen HJ, Butler D, Pirovano W. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics. 2011;27:578–279. doi: 10.1093/bioinformatics/btq683. [DOI] [PubMed] [Google Scholar]
- Boetzer M, Pirovano W. Toward almost closed genomes with GapFiller. Genome Biol. 2012;13:R56. doi: 10.1186/gb-2012-13-6-r56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque G, Pevzner PA. Genome-scale evolution: reconstructing gene orders in the ancestral species. Genome Res. 2002;12:26–36. [PMC free article] [PubMed] [Google Scholar]
- Brady A, Salzberg S. PhymmBL expanded: confidence scores, custom databases, parallelization and more. Nat Methods. 2011;8:367. doi: 10.1038/nmeth0511-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner P. Endosymbiosis of animals with plant microorganisms. New York: John Wiley and Sons; 1965. [Google Scholar]
- Burckhardt D. Taxonomy and phylogeny of the Gondwanan moss bugs or Peloridiidae (Hemiptera, Coleorrhyncha) Dtsch Entomol Z. 2009;56:173–235. [Google Scholar]
- Burge SW, et al. Rfam 11.0: 10 years of RNA families. Nucleic Acids Res. 2013;41:D226–D232. doi: 10.1093/nar/gks1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows M, Hartung V, Hoch H. Jumping behaviour in a Gondwanan relict insect (Hemiptera: Coleorrhyncha: Peloridiidae) J Exp Biol. 2007;210:3311–3318. doi: 10.1242/jeb.007914. [DOI] [PubMed] [Google Scholar]
- Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 2000;17:540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- Charron C, Roy H, Blaise M, Giegé R, Kern D. Non-discriminating and discriminating aspartyl-tRNA synthetases differ in the anticodon-binding domain. EMBO J. 2003;22:1632–1643. doi: 10.1093/emboj/cdg148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Donahue JL, Battle SE, Ray WK, Larson TJ. Biochemical and genetic characterization of PspE and GlpE, two single-domain sulfurtransferases of Escherichia coli. Open Microbiol. J. 2008;2:18–28. doi: 10.2174/1874285800802010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevreux B, Wetter T, Suhai S. Genome sequence assembly using trace signals and additional sequence information. Comput Sci Biol Proc Ger Conf Bioinform. 1999;99:45–56. [Google Scholar]
- Conord C, et al. Long-term evolutionary stability of bacterial endosymbiosis in curculionoidea: additional evidence of symbiont replacement in the dryophthoridae family. Mol Biol Evol. 2008;25:859–868. doi: 10.1093/molbev/msn027. [DOI] [PubMed] [Google Scholar]
- Costa HS, Westcot DM, Ullman DE, Johnson MW. Ultrastructure of the endosymbionts of the whitefly, Bemisia tabaci and Trialeurodes vaporariorum. Protoplasma. 1993;176:106–115. [Google Scholar]
- Cryan JR, Urban JM. Higher-level phylogeny of the insect order Hemiptera: is Auchenorrhyncha really paraphyletic? Syst Entomol. 2012;37:7–21. [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics. 2011;27:1164–1165. doi: 10.1093/bioinformatics/btr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JW. Some former inhabitants of Antarctica. The illustrated London News, February 21. 1948;1948:218. [Google Scholar]
- Foster B, et al. POLISHER: a tool for using ultra short reads in genome sequence improvement. 2012 Available from: http://jgi.doe.gov/data-and-tools/polisher/ (last accessed July 5, 2014) [Google Scholar]
- Frigaard NU, Dahl C. Sulfur metabolism in phototrophic sulfur bacteria. Adv Microb Physiol. 2009;54:103–200. doi: 10.1016/S0065-2911(08)00002-7. [DOI] [PubMed] [Google Scholar]
- Gil R, Silva FJ, Peretó J, Moya A. Determination of the Core of a Minimal Bacterial Gene Set. Microbiol Mol Biol Rev. 2004;68:518–537. doi: 10.1128/MMBR.68.3.518-537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Domenech CM, et al. Metabolic stasis in an ancient symbiosis: genome-scale metabolic networks from two Blattabacterium cuenoti strains, primary endosymbionts of cockroaches. BMC Microbiol. 2012;12(Suppl 1):S5. doi: 10.1186/1471-2180-12-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb Y, et al. Inherited intracellular ecosystem: symbiotic bacteria share bacteriocytes in whiteflies. FASEB J. 2008;22:2591–2599. doi: 10.1096/fj.07-101162. [DOI] [PubMed] [Google Scholar]
- Gralnick J, Downs D. Protection from superoxide damage associated with an increased level of the YggX protein in Salmonella enterica. Proc Natl Acad Sci U S A. 2001;98:8030–8035. doi: 10.1073/pnas.151243198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AK, Moran NA. The impact of microbial symbionts on host plant utilization by herbivorous insects. Mol Ecol. 2014;23:1473–1496. doi: 10.1111/mec.12421. [DOI] [PubMed] [Google Scholar]
- Harada M, et al. Expression of genes for sulfur oxidation in the intracellular chemoautotrophic symbiont of the deep-sea bivalve Calyptogena okutanii. Extremophiles. 2009;13:895–903. doi: 10.1007/s00792-009-0277-8. [DOI] [PubMed] [Google Scholar]
- Hernández-Montalvo V, et al. Expression of galP and glk in a Escherichia coli PTS mutant restores glucose transport and increases glycolytic flux to fermentation products. Biotechnol Bioeng. 2003;83:687–694. doi: 10.1002/bit.10702. [DOI] [PubMed] [Google Scholar]
- Hildebrandt TM, Grieshaber MK. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008;275:3352–3361. doi: 10.1111/j.1742-4658.2008.06482.x. [DOI] [PubMed] [Google Scholar]
- Hoch H, Deckert J, Wessel A. Vibrational signalling in a Gondwanan relict insect (Hemiptera: Coleorrhyncha: Peloridiidae) Biol Lett. 2006;2:222–224. doi: 10.1098/rsbl.2006.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet G, Daffé M, Saves I. Identification of the Mycobacterium tuberculosis SUF machinery as the exclusive mycobacterial system of [Fe-S] cluster assembly: evidence for its implication in the pathogen’s survival. J Bacteriol. 2005;187:6137–6146. doi: 10.1128/JB.187.17.6137-6146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huneck S. Chemistry and biochemistry of bryophytes. In: Schuster RM, editor. New manual of bryology. 1983. Vol. I. Nichinan (Japan): The Hattori Botanical Laboratory. p. 1–116. [Google Scholar]
- Husnik F, et al. Horizontal gene transfer from diverse bacteria to an insect genome enables a tripartite nested mealybug symbiosis. Cell. 2013;153:1567–1578. doi: 10.1016/j.cell.2013.05.040. [DOI] [PubMed] [Google Scholar]
- Karp PD, Paley S, Romero P. The pathway tools software. Bioinformatics. 2002;18(Suppl 1):S225–S232. doi: 10.1093/bioinformatics/18.suppl_1.s225. [DOI] [PubMed] [Google Scholar]
- Karp PD, et al. Expansion of the BioCyc collection of pathway/genome databases to 160 genomes. Nucleic Acids Res. 2005;33:6083–6089. doi: 10.1093/nar/gki892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelkar YD, Ochman H. Genome reduction promotes increase in protein functional complexity in bacteria. Genetics. 2013;193:303–307. doi: 10.1534/genetics.112.145656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klavina L, Bikovens O, Steinberga I, Maksimova V, Eglite L. Characterization of chemical composition of some bryophytes common in Latvia. Environ Exp Biol. 2012;10:27–34. [Google Scholar]
- Koga R, Bennett GM, Cryan JR, Moran NA. Evolutionary replacement of obligate symbionts in an ancient and diverse insect lineage. Environ Microbiol. 2013;15:2073–2081. doi: 10.1111/1462-2920.12121. [DOI] [PubMed] [Google Scholar]
- Kuechler SM, Gibbs G, Burckhardt D, Dettner K, Hartung V. Diversity of bacterial endosymbionts and bacteria-host co-evolution in Gondwanan relict moss bugs (Hemiptera: Coleorrhyncha: Peloridiidae) Environ Microbiol. 2013;15:2031–2042. doi: 10.1111/1462-2920.12101. [DOI] [PubMed] [Google Scholar]
- Krzywinski M, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamelas A, et al. Serratia symbiotica from the aphid Cinara cedri: a missing link from facultative to obligate insect endosymbiont. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1002357. e1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lartillot N, Lepage T, Blanquart S. PhyloBayes 3: a Bayesian software package for phylogenetic reconstruction and molecular dating. Bioinformatics. 2009;25:2286–2288. doi: 10.1093/bioinformatics/btp368. [DOI] [PubMed] [Google Scholar]
- Lee JH, Yeo WS, Roe JH. Induction of the sufA operon encoding Fe-S assembly proteins by superoxide generators and hydrogen peroxide: involvement of OxyR, IHF and an unidentified oxidant-responsive factor. Mol Microbiol. 2004;51:1745–1755. doi: 10.1111/j.1365-2958.2003.03946.x. [DOI] [PubMed] [Google Scholar]
- Li H, Outten CE. Monothiol CGFS glutaredoxins and BolA-like proteins: [2Fe-2S] binding partners in iron homeostasis. Biochemistry. 2012;51:4377–4389. doi: 10.1021/bi300393z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Stoeckert CJ, Roos DS. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13:2178–2189. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill R. Function and biogenesis of iron-sulphur proteins. Nature. 2009;460:831–838. doi: 10.1038/nature08301. [DOI] [PubMed] [Google Scholar]
- Lin Y, Rajan V, Moret BM. TIBA: a tool for phylogeny inference from rearrangement data with bootstrap analysis. Bioinformatics. 2012;28:3324–3325. doi: 10.1093/bioinformatics/bts603. [DOI] [PubMed] [Google Scholar]
- Liu H, Xin Y, Xun L. Distribution, diversity, and activities of sulfur dioxygenases in heterotrophic bacteria. Appl Environ Microbiol. 2014;80:1799–1806. doi: 10.1128/AEM.03281-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livshits VA, Zakataeva NP, Aleshin VV, Vitushkina MV. Identification and characterization of the new gene rhtA involved in threonine and homoserine efflux in Escherichia coli. Res Microbiol. 2003;154:123–135. doi: 10.1016/S0923-2508(03)00036-6. [DOI] [PubMed] [Google Scholar]
- López-Sánchez MJ, et al. Evolutionary convergence and nitrogen metabolism in Blattabacterium strain Bge, primary endosymbiont of the cockroach Blattella germanica. PLoS Genet. 2009;5:e1000721. doi: 10.1371/journal.pgen.1000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano-Marín A, Lamelas A, Moya A, Latorre A. Comparative genomics of Serratia spp.: two paths towards endosymbiotic life. PLoS One. 2012;7:e47274. doi: 10.1371/journal.pone.0047274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcia M, Ermler U, Peng G, Michel H. A new structure-based classification of sulfide:quinone oxidoreductases. Proteins. 2010;78:1073–1083. doi: 10.1002/prot.22665. [DOI] [PubMed] [Google Scholar]
- Markowitz VM, et al. IMG 4 version of the integrated microbial genomes comparative analysis system. Nucleic Acids Res. 2014;42:D560–D567. doi: 10.1093/nar/gkt963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin VJ, Mohn WW. Genetic investigation of the catabolic pathway for degradation of abietane diterpenoids by Pseudomonas abietaniphila BKME-9. J Bacteriol. 2000;182:3784–3793. doi: 10.1128/jb.182.13.3784-3793.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JP, McDonald BR, Moran NA. Convergent evolution of metabolic roles in bacterial co-symbionts of insects. Proc Natl Acad Sci U S A. 2009;106:15394–15399. doi: 10.1073/pnas.0906424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JP, Moran NA. Extreme genome reduction in symbiotic bacteria. Nat Rev Microbiol. 2012;10:13–26. doi: 10.1038/nrmicro2670. [DOI] [PubMed] [Google Scholar]
- Mera PE, Escalante-Semerena JC. Dihydroflavin-driven adenosylation of 4-coordinate Co(II) corrinoids: are cobalamin reductases enzymes or electron transfer proteins? J Biol Chem. 2010;285:2911–2917. doi: 10.1074/jbc.M109.059485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran NA. Accelerated evolution and Muller’s rachet in endosymbiotic bacteria. Proc Natl Acad Sci U S A. 1996;93:2873–2878. doi: 10.1073/pnas.93.7.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya A, Peretó J, Gil R, Latorre A. Learning how to live together: genomic insights into prokaryote-animal symbioses. Nat Rev Genet. 2008;9:218–229. doi: 10.1038/nrg2319. [DOI] [PubMed] [Google Scholar]
- Müller HJ. Über die intrazellulare symbiose der peloridiidae Hemiodoecus fidelis evans und ihre stellung unter den homopterensymbiosen. Zool Anz. 1951;146:150–167. [Google Scholar]
- Nakagawa S, et al. Allying with armored snails: the complete genome of gammaproteobacterial endosymbiont. ISME J. 2014;8:40–51. doi: 10.1038/ismej.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowack ECM, Grossman AR. Trafficking of protein into the recently established photosynthetic organelles of Paulinella chromatophora. Proc Natl Acad Sci U S A. 2012;109:5340–5345. doi: 10.1073/pnas.1118800109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakeson KF, et al. Genome degeneration and adaptation in a nascent stage of symbiosis. Genome Biol Evol. 2014;6:76–93. doi: 10.1093/gbe/evt210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonage D, Karplus PA, Poole LB. Substrate specificity and redox potential of AhpC, a bacterial peroxiredoxin. Proc Natl Acad Sci U S A. 2008;105:8209–8214. doi: 10.1073/pnas.0708308105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergrast JG. The internal anatomy of the Peloridiidae. Ecol Entomol. 1962;114:49–65. [Google Scholar]
- Popov YA, Shcherbakov DE. Origin and evolution of the Coleorrhyncha as shown by the fossil record. In: Schaefer CW, editor. Studies on Hemipteran phylogeny. Lanham (MD): Entomological Society of America; 1996. pp. 9–30. [Google Scholar]
- Py B, Barras F. Building Fe-S proteins: bacterial strategies. Nat Rev Microbiol. 2010;8:436–446. doi: 10.1038/nrmicro2356. [DOI] [PubMed] [Google Scholar]
- Quevillon E, et al. InterProScan: protein domains identifier. Nucleic Acids Res. 2005;33:W116–W120. doi: 10.1093/nar/gki442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing [Internet] 2014 [cited 2014 Jul 5]. Available from: http://www.r-project.org/ [Google Scholar]
- Reyes-Prieto M, Latorre A, Moya A. Scanty microbes, the “symbionelle” concept. Environ Microbiol. 2013;16:335–338. doi: 10.1111/1462-2920.12220. [DOI] [PubMed] [Google Scholar]
- Santos-Garcia D, et al. Complete genome sequence of “Candidatus Portiera aleyrodidarum” BT-QVLC, an obligate symbiont that supplies amino acids and carotenoids to Bemisia tabaci. J Bacteriol. 2012;194:6654–6655. doi: 10.1128/JB.01793-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomburg I, et al. BRENDA in 2013: integrated reactions, kinetic data, enzyme function data, improved disease classification: new options and contents in BRENDA. Nucleic Acids Res. 2013;41:D764–D772. doi: 10.1093/nar/gks1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N, Börnigen D, Morgan XC, Huttenhower C. PhyloPhlAn is a new method for improved phylogenetic and taxonomic placement of microbes. Nat Commun. 2013;4:2304. doi: 10.1038/ncomms3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcherbakov D. The most primitive whiteflies (Hemiptera; Aleyrodidae; Bernaeinae subfam. nov.) from the Mesozoic of Asia and Burmese amber, with an overview of Burmese amber hemipterans. Bull Nat Hist Mus Lond (Geol.). 2000;56:29–37. [Google Scholar]
- Silva FJ, Latorre A, Moya A. Genome size reduction through multiple events of gene disintegration in Buchnera APS. Trends Genet. 2001;17:615–618. doi: 10.1016/s0168-9525(01)02483-0. [DOI] [PubMed] [Google Scholar]
- Silva FJ, Latorre A, Moya A. Why are the genomes of endosymbiotic bacteria so stable? Trends Genet. 2003;19:176–180. doi: 10.1016/S0168-9525(03)00041-6. [DOI] [PubMed] [Google Scholar]
- Sloan DB, Moran NA. Endosymbiotic bacteria as a source of carotenoids in whiteflies. Biol Lett. 2012a;8:986–989. doi: 10.1098/rsbl.2012.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DB, Moran NA. Genome reduction and co-evolution between the primary and secondary bacterial symbionts of psyllids. Mol Biol Evol. 2012b;29:3781–3792. doi: 10.1093/molbev/mss180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DB, Moran NA. The evolution of genomic instability in the obligate endosymbionts of whiteflies. Genome Biol Evol. 2013;5:783–793. doi: 10.1093/gbe/evt044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DB, et al. Parallel Histories of horizontal gene transfer facilitated extreme reduction of endosymbiont genomes in sap-feeding insects. Mol Biol Evol. 2014;31:857–871. doi: 10.1093/molbev/msu004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song N, Liang AP, Bu CP. A molecular phylogeny of Hemiptera inferred from mitochondrial genome sequences. PLoS One. 2012;7:e48778. doi: 10.1371/journal.pone.0048778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangenberg R, et al. The cephalic morphology of the Gondwanan key taxon Hackeriella (Coleorrhyncha, Hemiptera) Arthropod Struct Dev. 2013;42:315–337. doi: 10.1016/j.asd.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Staden R, Beal KF, Bonfield JK. The Staden package, 1998. Methods Mol Biol. 2000;132:115–130. doi: 10.1385/1-59259-192-2:115. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Tamames J, et al. The frontier between cell and organelle: genome analysis of Candidatus Carsonella ruddii. BMC Evol Biol. 2007;7:181. doi: 10.1186/1471-2148-7-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas I, et al. 50 Million years of genomic stasis in endosymbiotic bacteria. Science. 2002;296:2376–2379. doi: 10.1126/science.1071278. [DOI] [PubMed] [Google Scholar]
- Tatusov RL, Galperin MY, Natale DA, Koonin EV. The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000;28:33–36. doi: 10.1093/nar/28.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thao MLL, Baumann P. Evolutionary relationships of primary prokaryotic endosymbionts of whiteflies and their hosts. Appl Environ Microbiol. 2004;70:3401–3406. doi: 10.1128/AEM.70.6.3401-3406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft C, Andersson SGE. Evolutionary microbial genomics: insights into bacterial host adaptation. Nat Rev Genet. 2010;11:465–475. doi: 10.1038/nrg2798. [DOI] [PubMed] [Google Scholar]
- Vyazmensky M, Sella C, Barak Z, Chipman DM. Isolation and characterization of subunits of acetohydroxy acid synthase isozyme III and reconstitution of the holoenzyme. Biochemistry. 1996;35:10339–10346. doi: 10.1021/bi9605604. [DOI] [PubMed] [Google Scholar]
- Warnes GR, et al. gplots: various R programming tools for plotting data. 2013 Available from: http://cran.r-project.org/package=gplots (last accessed July 5, 2014) [Google Scholar]
- White JA. Caught in the act: rapid, symbiont-driven evolution. BioEssays. 2011;33:823–829. doi: 10.1002/bies.201100095. [DOI] [PubMed] [Google Scholar]
- Wilson ACC, et al. Genomic insight into the amino acid relations of the pea aphid, Acyrthosiphon pisum, with its symbiotic bacterium Buchnera aphidicola. Insect Mol Biol. 2010;19(Suppl 2):249–258. doi: 10.1111/j.1365-2583.2009.00942.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.