Abstract
Background/Purpose
Neuroblastoma (NB) is the most common extracranial solid tumor of childhood. Preliminary data derived from a human angiogenesis array in NB showed that the bioactive lipid sphingosine-1-phosphate (S1P) induced the secretion of several angiogenesis-related proteins including the important inflammatory factor chemokine (C-C motif) ligand 2 (CCL2). In the present study, we investigated the mechanism of S1P-induced CCL2 expression in NB.
Methods
Quantitative real-time PCR and CCL2 ELISA were conducted to detect the mRNA expression and protein secretion of CCL2 in NB cells. Gain and loss of function studies were performed by using specific S1PR antagonists, adenoviral transduction and siRNA transfection. Macrophage F4/80 receptor in NB xenografts was detected by quantitative real-time PCR and immunohistochemistry staining.
Results
S1P induced CCL2 mRNA expression and protein secretion in a time- and concentration-dependent manner in NB cells. Blockade of S1P2 signaling using the selective S1P2 antagonist JTE-013 inhibited S1P-induced CCL2 expression. Overexpression of S1P2 by adenoviral transduction increased CCL2 secretion while knockdown of S1P2 by siRNA transfection decreased S1P-induced CCL2 secretion in NB cells. Macrophage infiltration, as detected by F4/80 staining, was significantly decreased in JTE-013-treated NB xenografts.
Conclusions
Taken together, our data for the first time demonstrate that S1P induced the macrophage-recruiting factor CCL2 expression in NB cells via S1P2, providing new insights into the complicated functions of S1P2 in cancer.
Keywords: sphingosine 1-phosphate, sphingosine 1-phosphate receptor 2, chemokine (C-C motif) ligand 2, tumor-associated macrophage, neuroblastoma
Introduction
Neuroblastoma (NB) is the most common extracranial solid tumor of childhood and the most frequently diagnosed neoplasm during infancy. It is a highly angiogenic tumor and like many other cancers it benefits from host immune tolerance. The poor outcome in patients with high-risk NB and the significant late adverse effects from radiotherapy and chemotherapy underscore the need for novel therapeutic strategies [1, 2].
Sphingosine-1-phosphate (S1P) is an important bioactive lipid that exerts a wide variety of cellular functions via interaction with its five G protein-coupled receptors (named S1P1-5) [3]. Multiple studies have shown that S1P and its receptors have been implicated in many pathological diseases including cancer. Blockade of S1P signaling has effectively reduced tumor growth and inhibited tumor progression in various cancers [4-6], suggesting that S1P signaling might become a novel therapeutic target in cancer.
Our group and others have demonstrated that S1P regulates various cytokines and chemokines in the tumor microenvironment [7-11]. Our preliminary data obtained from utilizing a human angiogenesis array showed that S1P was able to induce the secretion of several angiogenesis-related proteins such as vascular endothelial growth factor (VEGF) and chemokine (C-C motif) ligand 2 (CCL2) in NB. In a prior publication, we have shown that S1P/S1P2 signaling mediates VEGF expression and thus promotes NB growth [8]. The important inflammatory factor CCL2, also known as monocyte chemoattractant protein 1 (MCP-1), was first identified and purified from human gliomas and myelomonocytic cells in 1989 [12, 13]. It is a small, secreted protein that regulates the recruitment of monocytes, macrophages, and other inflammatory cells to sites of inflammation. A large body of evidence has shown that it plays a critical role in acute and chronic inflammatory responses. Among many chemokines identified, CCL2 is particularly important in cancer development, serving as a key mediator of interactions between tumor and host cells. It is produced by cancer cells and multiple different host cells within the tumor microenvironment and has been shown to mediate tumorigenesis in a variety of cancers [14]. Of note, Expression of CCL2 is positively correlated with the infiltration of tumor-associated macrophages (TAMs), which are increasingly recognized to play a permissive role in cancer progression and metastasis [14]. Surprisingly, little is known about the regulation of CCL2 gene expression in cancer cells. In the present study, we investigated the mechanism of S1P-induced CCL2 expression in NB.
Materials and Methods
Materials
S1P was purchase from Biomol (Plymouth Meeting, PA) and JTE-013 was from Tocris Bioscience (Ellisville, MO). Fatty-acid free BSA was purchased from Sigma (Saint Louis, MO).
Cell culture, adenoviral transduction and siRNA transfection
SK-N-AS cell line was obtained from the American Type Culture Collection (ATCC) and cultured in DMEM (Sigma, Saint Louis, MO) supplemented with 10% FBS (Hyclone, Logan, UT), 1x MEM NEAA (Gibco, Grand Island, NY) and penicillin-streptomycin (Gibco) at 37 °C in a humidified chamber of 5% CO2. SK-N-BE(2) cell line, originally from ATCC, was kindly provided by Dr. Nehal Parikh (Connecticut Children's Medical Center) and cultured in 1:1 MEM/F12 (Gibco) contained with 10% FBS, 1x MEM NEAA and antibiotics. For adenoviral transduction, cells were infected with adenovirus containing GFP or S1P2 for 16-24 h (100 multiplicity of infection, MOI) before different assays were done. Transfection of small interfering RNA (siRNA) oligonucleotide duplexes to block S1P2 expression was done using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) according to manufacturer's instructions. Briefly, NB cells were transfected with 100 pmol of S1P2 siRNA or non-specific (NS) siRNA per 6-well (Dharmacon Lafayette, CO; S1P2 siRNA: UACCUUGCUCUCUGGCUCU; NS siRNA: UUCUCCGAACGUGUCACGUUU) for 24 h followed by different assays.
Quantitative real-time PCR
SYBR Green-based quantitative real-time PCR was carried out as described [9]. Primers were designed using Primer Express™ 2.0 (Applied Biosystems). Sequences were as follows: 5′-CCATTGTGGCCAAGGAGATC-3′ (forward) and 5′-TGCTTGTCCAGGTGGTCCAT-3′ (reverse) for the CCL2 gene and 5′- GCACATCCAGCCAAAGCAG-3′ (forward) and 5′-CCATCTCCCATCCTCCACAT-3′ (reverse) for the mouse F4/80 gene. Results were expressed relative to the internal control gene GAPDH.
CCL2 ELISA analysis of conditioned media
NB cells (1×106 cells/well) were seeded into 6-well plates. After attachment, they were serum starved for 24 h and treated with or without S1P in 1ml/well serum-free (SF) media under different conditions for different time. The conditioned media was collected, spin down at 1000 g for 5 min at 4°C and analyzed for CCL2 production using Human MCP-1 ELISA Development Kit (PeproTech, Rocky Hill, NJ) according to the manufacturer's instructions.
F4/80 immunohistochemistry staining
The previously obtained paraffin-embedded blocks from NB xenografts treated with or without JTE-013 [8] were cut for 5 μm sections followed by F4/80 immunohistochemistry staining (MCA497R, 1:200, AbD Serotec) as previously described [15]. Sections were examined under light microscope and 5-10 random fields in viable tumor area per sample were chosen and photos were taken under 20x field. Data were presented as the number of positive stained cells per field.
Statistical Analysis
All experiments on cell lines were performed at least twice on separate occasions. Data are presented as means ± SE from representative experiments. Statistical significance of differences between two groups was determined by two-tailed homoscedastic Student's t-test using Microsoft Excel software.
Results
S1P induced CCL2 expression in NB cells
To determine the effect of S1P on CCL2 expression in NB, the SK-N-AS cell line was utilized since it has an S1P receptor (S1PR) expression profile consistent with that of human NB specimens, as demonstrated in our previous findings [8]. Quantitative real-time PCR and CCL2 ELISA were performed to detect the mRNA expression and protein secretion of CCL2 induced by S1P. We found that S1P at a concentration as low as 10 nM, was able to significantly induce CCL2 mRNA expression and protein secretion (Fig. 1A and 1B). Time-course effect further showed that this effect began at as early as 1 h, and was maintained up to 24 h of incubation (Fig. 1C and 1D). MYCN gene amplification is the best characterized genetic aberration in NB [16]. The induction of CCL2 expression by S1P was also observed in a MYCN-amplified cell line SK-N-BE(2) [17] (Fig. 1E and 1F). All the above data indicate that the effect of S1P on CCL2 induction is likely, a general phenomenon in NB, regardless of MYCN status.
Figure 1.
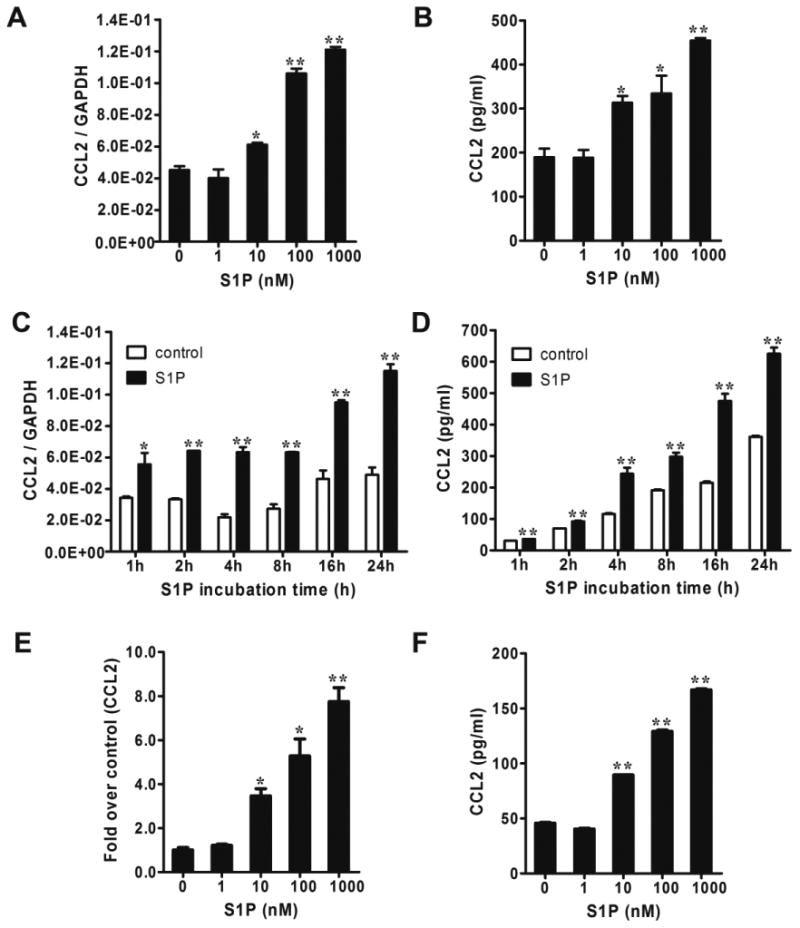
S1P induced CCL2 expression in NB cells. (A and B) SK-N-AS cells were serum starved for 24 h and treated with different concentrations of S1P for 4 h followed by quantitative real-time PCR (A) and CCL2 ELISA (B). (C and D) Serum-starved SK-N-AS cells were treated with 1μM of S1P for 1-24 h followed by quantitative real-time PCR (C) and CCL2 ELISA (D). (E and F) SK-N-BE(2) cells were serum starved for 24 h and treated with S1P for 2 h (E) or 4 h (F) followed by quantitative real-time PCR (E) and CCL2 ELISA (F). *, P < 0.05, **, P < 0.01 versus without S1P treatment.
S1P-induced CCL2 expression is mediated by S1P2 in NB cells
Five S1PRs have been identified to bind specifically to S1P [3]. Our previous findings have shown that NB mainly expresses S1P1-3, and that S1P2 mediates S1P-induced VEGF expression [8]. To determine which S1PR is responsible for S1P-induced CCL2 expression, the selective S1P2 antagonist JTE-013 [18, 19] was utilized. Interestingly, blockade of S1P2 signaling by JTE-013 significantly inhibited S1P-induced CCL2 mRNA expression and protein secretion in both SK-N-AS and SK-N-BE(2) cell lines (Fig. 2A-2D), suggesting that S1P2 might be responsible for S1P-induced CCL2 expression. To substantiate this notion, we altered S1P2 expression level in both NB cell lines by adenoviral transduction and siRNA transfection. Consistent with our hypothesis, overexpression of S1P2 by adenoviral transduction, a technique we have previously successfully performed in SK-N-AS cells [8], dramatically increased the secretion of CCL2 protein in both cell lines (Fig. 3A and 3B). In addition, using the same siRNA knockdown strategy previously applied in SK-N-AS cells [8], downregulation of S1P2 in both cell lines significantly inhibited S1P-induced CCL2 protein secretion (Fig. 4A and 4B). Taken together, all the above data demonstrate that S1P-induced CCL2 expression is mediated by S1P2.
Figure 2.
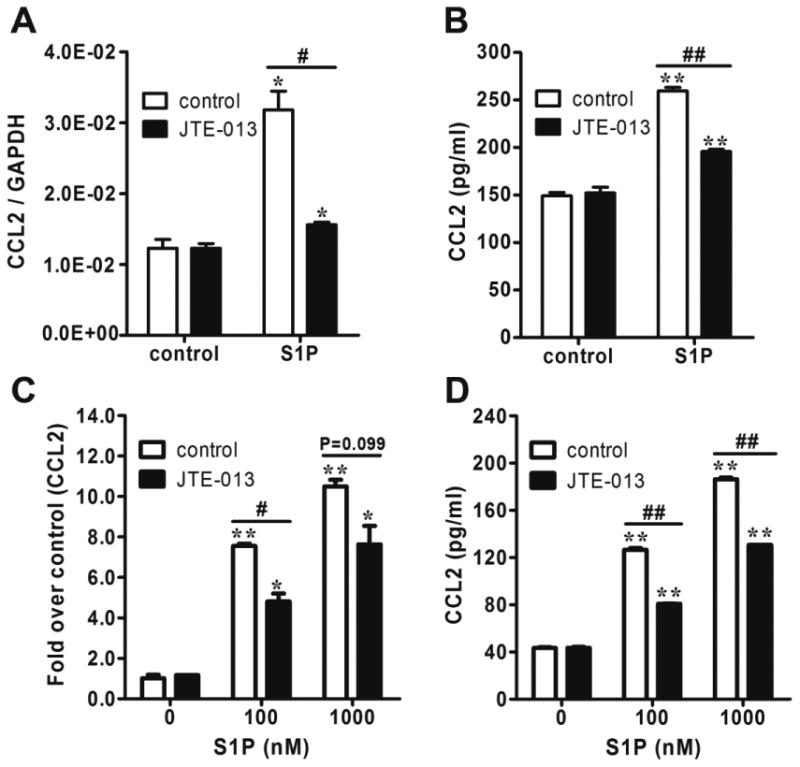
S1P2 antagonist JTE-013 inhibited S1P-induced CCL2 expression in NB cells. (A and B) Serum-starved SK-N-AS cells were pretreated with JTE-013 (1μM) for 0.5 h and incubated with S1P (1μM) for 2 h (A) or 4 h (B) followed by quantitative real-time PCR (A) and CCL2 ELISA (B). (C and D) Serum-starved and JTE-013-pretreated SK-N-BE(2) cells were incubated with different concentrations of S1P for 2 h (C) or 4 h (D) followed by quantitative real-time PCR (C) and CCL2 ELISA (D). *, P< 0.05, **, P< 0.01 versus non S1P-treated controls. #, P < 0.01, ##, P < 0.01 as indicated.
Figure 3.

Overexpression of S1P2 increased CCL2 secretion in NB cells. SK-N-AS cells (A) or SK-N-BE(2) cells (B) were infected with S1P2 or GFP adenovirus with MOI 100, serum starved and stimulated with 1μM (A) or 100nM (B) of S1P in 1 ml SF media for 4 h followed by CCL2 ELISA. *, P< 0.05, **, P< 0.01 versus non S1P-treated controls. ##, P < 0.01 as indicated.
Figure 4.
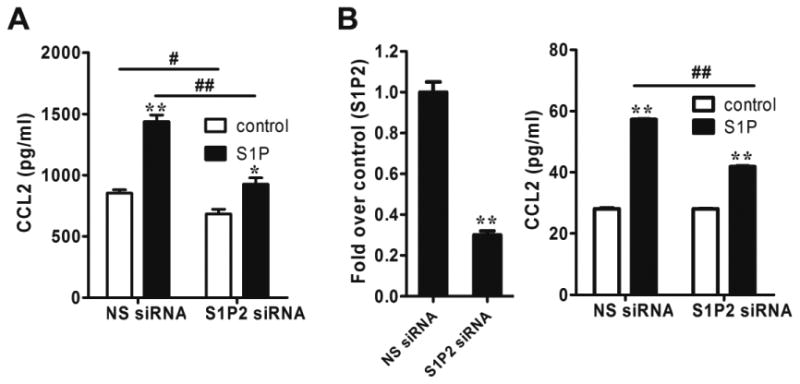
Downregulation of S1P2 decreased CCL2 expression in NB cells. (A) SK-N-AS cells were transfected with S1P2 siRNA or NS siRNA, serum-starved and stimulated with S1P (1μM) in 1 ml SF media for 24 h followed by CCL2 ELISA. (B) SK-N-BE(2) cells were transfected with S1P2 siRNA or NS siRNA, serum-starved and stimulated with S1P (100nM) in 1 ml SF media for 4 h followed by quantitative real-time PCR to check the S1P2 knockdown efficiency (left) and CCL2 ELISA (right). *, P< 0.05, **, P< 0.01 versus non S1P-treated controls or NS siRNA (B, left). #, P < 0.05, ##, P < 0.01 as indicated.
Macrophage infiltration was significantly decreased in JTE-013-treated NB xenografts
Having confirmed the role of S1P2 in S1P-induced CCL2 expression in vitro, we were interested in knowing whether this pathway was operational in vivo. In our previous findings, we have shown that S1P2 antagonist JTE-013 significantly inhibited the growth of NB xenografts [8]. Therefore, in this study, the mRNA expression of the macrophage recruiting factor CCL2 and the murine macrophage marker F4/80 was detected in these NB xenografts. Quantitative real-time PCR noted a decrease of their mRNA expression in these JTE-013-treated NB xenografts, however this did not achieve significance (Fig. 5A and 5B). Interestingly, the immunohistochemistry staining revealed a significant decrease of F4/80 positive cells in the viable tumor area of these samples (Fig. 5C), suggesting reduced macrophage infiltration in these JTE-013-treated NB xenografts. All the above data indicate that S1P/S1P2/CCL2 pathway also exists and is functional in vivo.
Figure 5.

Macrophage infiltration was decreased in JTE-013-treated NB xenografts. (A and B) Quantitative real-time PCR was performed in NB xenografts treated with or without S1P2 antagonist JTE-013 to detect the mRNA expression of CCL2 (A) and murine F4/80 (B). Data was normalized to that of the housekeeping gene GAPDH and presented as means ± SE. (C) Immunohistochemistry staining was performed to detect the F4/80 positive cells in control and JTE-013-treated NB xenografts and photos were taken under the light microcrope (20x). A representative picture from each group (left) and the quantitative result (right) were shown. *, P < 0.05, **, P < 0.01 versus the corresponding control.
Discussion
Our preliminary data obtained from human angiogenesis array assay showed that the bioactive lipid S1P, which we have shown to be an important regulator of VEGF expression [8], also induces the secretion of the chemokine CCL2. Quantitative real-time PCR and CCL2 ELISA confirmed that S1P induced CCL2 mRNA expression and protein secretion in both MYCN amplified and non-amplified NB cells (Fig. 1). The induction is an early phase and continued event. Five S1PRs have been reported to bind specifically to S1P [3]. To delineate which S1PR is responsible for this effect, different methodologies have been utilized including S1PR antagonist, overexpression by adenoviral transduction and downregulation by siRNA transfection, which we have successfully applied in our previous studies [8]. The data clearly demonstrate that S1P2 was responsible for S1P-induced CCL2 expression (Fig. 2-4), further extending the biological function of S1P2 in cancer.
There has been some controversy in the literature regarding the role of CCL2 in cancer. A number of reports from the 1990's in cancers such as colon [20], renal [21] and pancreas [22] have suggested that CCL2 negatively regulates tumor growth and metastasis. One recent study supporting this theory comes from Takahashi et al who reported that CCL2 negatively regulates metastasis in a mouse breast cancer model [23]. Conversely, in prostate cancer, it has been shown that CCL2 has multiple roles in promoting prostate cancer growth and metastasis by stimulating cancer cell proliferation, migration, invasion, and modulating TAMs migration as well as promoting osteoclast maturation. It also protects prostate cancer cells from autophagic death by activating survivin [24]. Therefore, anti-CCL2 strategy has been tested as a therapeutic option in prostate cancer [25, 26] and recently has entered into clinical trials. In breast cancer, CCL2 expression has been found to correlate significantly with TAM accumulation, tumor angiogenesis and poor prognosis [27, 28]. More recently, Qian et al further showed that CCL2 was able to recruit metastasis-associated macrophages to facilitate breast cancer metastasis, providing a mechanistic link between CCL2 expression and macrophage infiltration [29]. Therefore, the role of CCL2 on tumor progression is complex and may be context-dependent in different cancer models.
In NB, prior studies from Metelitsa's group found that invariant natural killer T (iNKT) cells that are potentially important for antitumor immunity migrate toward NB cells in a CCL2-dependent manner [30]. Subsequently, they reported that oncogene MYCN, the hallmark of aggressive NB, repressed CCL2 expression in a STAT-3-independent manner [31], indicating that CCL2, by regulation of iNKT cells, may negatively regulate NB tumor progression. On the other hand, a recent study showed that metastatic NB had higher infiltration of TAMs compared to the locoregional tumors. Further, by using the 14-gene tumor classification score, which includes 5 inflammation related genes that represent TAMs, they found the metastatic NB with high-risk score had worse progression-free survival compared to those with low-risk score, suggesting that TAMs contribute to tumor progression and are associated with the poor clinical outcome in NB [32]. However, as a macrophage recruiting factor, the role of CCL2 on TAMs in NB remains to be defined. Interestingly, when we analyzed NB xenografts treated with S1P2 antagonist JTE-013 [8], we noted that both VEGF and CCL2 mRNA levels were decreased, accompanied by tumor growth inhibition. Aware of CCL2's important role in influencing the tumor microenvironment through recruitment of TAMs, we performed quantitative real-time PCR and immunohistochemistry staining and found that F4/80 positive cells in the viable tumor area were significantly decreased in the JTE-013-treated groups, along with the decrease of CCL2 and F4/80 mRNA expression (Fig. 5). These findings suggested that CCL2, regulated by S1P2 pathway, might be responsible for macrophage infiltration in NB, which is consistent with the findings in other cancers [28]. Given CCL2's documented role in TAMs regulation and its ability to induce M2-type macrophage polarization [33], further characterization of macrophage phenotype will be required to fully understand the role of CCL2 in NB. These studies however could prove very relevant to future therapeutic approaches, as Weigert et al has recently shown that the failure of polarizing macrophage towards M2 phenotype in NB significantly inhibits tumor growth [34].
In summary, our study for the first time clearly demonstrates that S1P induces the macrophage recruiting factor CCL2 expression in NB cells and this effect is mediated by S1P2. These findings further extend the known biological function of S1P2 in cancer and highlight our incomplete understanding of CCL2 regulation and function in NB. Our previous findings [8-10, 35], together with this report, indicate that further in-depth work is needed to allow us to better understand the role of S1P2 in tumor microenvironment and develop a rational approach to targeted S1P2 therapy.
Acknowledgments
This work was supported by NIH grant R01CA168903 (F.F), the Seraph Foundation and the Burr Curtis Surgical Endowment.
Abbreviations
- NB
neuroblastoma
- S1P
sphingosine-1-phosphate
- CCL2
chemokine (C-C motif) ligand 2
- TAMs
tumor-associated macrophages
- VEGF
vascular endothelial growth factor
- S1PR
S1P receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Park JR, Eggert A, Caron H. Neuroblastoma: biology, prognosis, and treatment. Pediatr Clin North Am. 2008;55:97–120. x. doi: 10.1016/j.pcl.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Perwein T, Lackner H, Sovinz P, et al. Survival and late effects in children with stage 4 neuroblastoma. Pediatr Blood Cancer. 2011;57:629–35. doi: 10.1002/pbc.23036. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–22. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 4.French KJ, Zhuang Y, Maines LW, et al. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J Pharmacol Exp Ther. 2010;333:129–39. doi: 10.1124/jpet.109.163444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LaMontagne K, Littlewood-Evans A, Schnell C, et al. Antagonism of sphingosine-1-phosphate receptors by FTY720 inhibits angiogenesis and tumor vascularization. Cancer Res. 2006;66:221–31. doi: 10.1158/0008-5472.CAN-05-2001. [DOI] [PubMed] [Google Scholar]
- 6.Visentin B, Vekich JA, Sibbald BJ, et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–38. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 7.Bryan L, Paugh BS, Kapitonov D, et al. Sphingosine-1-phosphate and interleukin-1 independently regulate plasminogen activator inhibitor-1 and urokinase-type plasminogen activator receptor expression in glioblastoma cells: implications for invasiveness. Mol Cancer Res. 2008;6:1469–77. doi: 10.1158/1541-7786.MCR-08-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li MH, Hla T, Ferrer F. Sphingolipid modulation of angiogenic factor expression in neuroblastoma. Cancer Prev Res (Phila) 2011;4:1325–32. doi: 10.1158/1940-6207.CAPR-11-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li MH, Sanchez T, Milne GL, et al. S1P/S1P2 signaling induces cyclooxygenase-2 expression in Wilms tumor. J Urol. 2009;181:1347–52. doi: 10.1016/j.juro.2008.10.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li MH, Sanchez T, Pappalardo A, et al. Induction of antiproliferative connective tissue growth factor expression in Wilms' tumor cells by sphingosine-1-phosphate receptor 2. Mol Cancer Res. 2008;6:1649–56. doi: 10.1158/1541-7786.MCR-07-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz BM, Hong G, Morrison BH, et al. Lysophospholipids increase interleukin-8 expression in ovarian cancer cells. Gynecol Oncol. 2001;81:291–300. doi: 10.1006/gyno.2001.6124. [DOI] [PubMed] [Google Scholar]
- 12.Matsushima K, Larsen CG, DuBois GC, et al. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989;169:1485–90. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshimura T, Robinson EA, Tanaka S, et al. Purification and amino acid analysis of two human glioma-derived monocyte chemoattractants. J Exp Med. 1989;169:1449–59. doi: 10.1084/jem.169.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Patel L, Pienta KJ. Targeting chemokine (C-C motif) ligand 2 (CCL2) as an example of translation of cancer molecular biology to the clinic. Prog Mol Biol Transl Sci. 2010;95:31–53. doi: 10.1016/B978-0-12-385071-3.00003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li MH, Sanchez T, Yamase H, et al. S1P/S1P1 signaling stimulates cell migration and invasion in Wilms tumor. Cancer Lett. 2009;276:171–9. doi: 10.1016/j.canlet.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–16. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 17.Megison ML, Stewart JE, Nabers HC, et al. FAK inhibition decreases cell invasion, migration and metastasis in MYCN amplified neuroblastoma. Clinical & experimental metastasis. 2013;30:555–68. doi: 10.1007/s10585-012-9560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salomone S, Waeber C. Selectivity and specificity of sphingosine-1-phosphate receptor ligands: caveats and critical thinking in characterizing receptor-mediated effects. Front Pharmacol. 2011;2:9. doi: 10.3389/fphar.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez T, Skoura A, Wu MT, et al. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol. 2007;27:1312–8. doi: 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- 20.Huang S, Singh RK, Xie K, et al. Expression of the JE/MCP-1 gene suppresses metastatic potential in murine colon carcinoma cells. Cancer Immunol Immunother. 1994;39:231–8. doi: 10.1007/BF01525986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang S, Xie K, Singh RK, et al. Suppression of tumor growth and metastasis of murine renal adenocarcinoma by syngeneic fibroblasts genetically engineered to secrete the JE/MCP-1 cytokine. J Interferon Cytokine Res. 1995;15:655–65. doi: 10.1089/jir.1995.15.655. [DOI] [PubMed] [Google Scholar]
- 22.Monti P, Leone BE, Marchesi F, et al. The CC chemokine MCP-1/CCL2 in pancreatic cancer progression: regulation of expression and potential mechanisms of antimalignant activity. Cancer Res. 2003;63:7451–61. [PubMed] [Google Scholar]
- 23.Takahashi M, Miyazaki H, Furihata M, et al. Chemokine CCL2/MCP-1 negatively regulates metastasis in a highly bone marrow-metastatic mouse breast cancer model. Clin Exp Metastasis. 2009;26:817–28. doi: 10.1007/s10585-009-9281-8. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Lu Y, Pienta KJ. Multiple roles of chemokine (C-C motif) ligand 2 in promoting prostate cancer growth. J Natl Cancer Inst. 2010;102:522–8. doi: 10.1093/jnci/djq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Loberg R, Liao J, et al. A destructive cascade mediated by CCL2 facilitates prostate cancer growth in bone. Cancer Res. 2009;69:1685–92. doi: 10.1158/0008-5472.CAN-08-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loberg RD, Ying C, Craig M, et al. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res. 2007;67:9417–24. doi: 10.1158/0008-5472.CAN-07-1286. [DOI] [PubMed] [Google Scholar]
- 27.Saji H, Koike M, Yamori T, et al. Significant correlation of monocyte chemoattractant protein-1 expression with neovascularization and progression of breast carcinoma. Cancer. 2001;92:1085–91. doi: 10.1002/1097-0142(20010901)92:5<1085::aid-cncr1424>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 28.Ueno T, Toi M, Saji H, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6:3282–9. [PubMed] [Google Scholar]
- 29.Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breasttumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metelitsa LS, Wu HW, Wang H, et al. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J Exp Med. 2004;199:1213–21. doi: 10.1084/jem.20031462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song L, Ara T, Wu HW, et al. Oncogene MYCN regulates localization of NKT cells to the site of disease in neuroblastoma. J Clin Invest. 2007;117:2702–12. doi: 10.1172/JCI30751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asgharzadeh S, Salo JA, Ji L, et al. Clinical significance of tumor-associated inflammatory cells in metastatic neuroblastoma. J Clin Oncol. 2012;30:3525–32. doi: 10.1200/JCO.2011.40.9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roca H, Varsos ZS, Sud S, et al. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. 2009;284:34342–54. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weigert A, Schiffmann S, Sekar D, et al. Sphingosine kinase 2 deficient tumor xenografts show impaired growth and fail to polarize macrophages towards an anti-inflammatory phenotype. Int J Cancer. 2009;125:2114–21. doi: 10.1002/ijc.24594. [DOI] [PubMed] [Google Scholar]
- 35.Lepley D, Paik JH, Hla T, et al. The G protein-coupled receptor S1P2 regulates Rho/Rho kinase pathway to inhibit tumor cell migration. Cancer Res. 2005;65:3788–95. doi: 10.1158/0008-5472.CAN-04-2311. [DOI] [PubMed] [Google Scholar]


