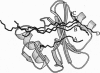Abstract
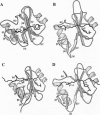
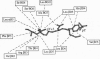
She is a widely expressed adapter protein that plays an important role in signaling via a variety of cell surface receptors and has been implicated in coupling the stimulation of growth factor, cytokine, and antigen receptors to the Ras signaling pathway. She interacts with several tyrosine-phosphorylated receptors through its C-terminal SH2 domain, and one of the mechanisms of T-cell receptor-mediated Ras activation involves the interaction of the Shc SH2 domain with the tyrosine-phosphorylated zeta chain of the T-cell receptor. Here we describe a high-resolution NMR structure of the Shc SH2 domain complexed to a phosphopeptide (GHDGLpYQGLSTATK) corresponding to a portion of the zeta chain of the T-cell receptor. Although the overall architecture of the protein is similar to other SH2 domains, distinct structural differences were observed in the smaller beta-sheet, BG loop, (pY + 3) phosphopeptide-binding site, and relative position of the bound phosphopeptide.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bax A., Ikura M. An efficient 3D NMR technique for correlating the proton and 15N backbone amide resonances with the alpha-carbon of the preceding residue in uniformly 15N/13C enriched proteins. J Biomol NMR. 1991 May;1(1):99–104. doi: 10.1007/BF01874573. [DOI] [PubMed] [Google Scholar]
- Blaikie P., Immanuel D., Wu J., Li N., Yajnik V., Margolis B. A region in Shc distinct from the SH2 domain can bind tyrosine-phosphorylated growth factor receptors. J Biol Chem. 1994 Dec 23;269(51):32031–32034. [PubMed] [Google Scholar]
- Burns L. A., Karnitz L. M., Sutor S. L., Abraham R. T. Interleukin-2-induced tyrosine phosphorylation of p52shc in T lymphocytes. J Biol Chem. 1993 Aug 25;268(24):17659–17661. [PubMed] [Google Scholar]
- Clore G. M., Gronenborn A. M. Structures of larger proteins, protein-ligand and protein-DNA complexes by multi-dimensional heteronuclear NMR. Prog Biophys Mol Biol. 1994;62(2):153–184. doi: 10.1016/0079-6107(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Eck M. J., Shoelson S. E., Harrison S. C. Recognition of a high-affinity phosphotyrosyl peptide by the Src homology-2 domain of p56lck. Nature. 1993 Mar 4;362(6415):87–91. doi: 10.1038/362087a0. [DOI] [PubMed] [Google Scholar]
- Giorgetti S., Pelicci P. G., Pelicci G., Van Obberghen E. Involvement of Src-homology/collagen (SHC) proteins in signaling through the insulin receptor and the insulin-like-growth-factor-I-receptor. Eur J Biochem. 1994 Jul 1;223(1):195–202. doi: 10.1111/j.1432-1033.1994.tb18983.x. [DOI] [PubMed] [Google Scholar]
- Ikura M., Kay L. E., Bax A. A novel approach for sequential assignment of 1H, 13C, and 15N spectra of proteins: heteronuclear triple-resonance three-dimensional NMR spectroscopy. Application to calmodulin. Biochemistry. 1990 May 15;29(19):4659–4667. doi: 10.1021/bi00471a022. [DOI] [PubMed] [Google Scholar]
- Kavanaugh W. M., Williams L. T. An alternative to SH2 domains for binding tyrosine-phosphorylated proteins. Science. 1994 Dec 16;266(5192):1862–1865. doi: 10.1126/science.7527937. [DOI] [PubMed] [Google Scholar]
- Logan T. M., Olejniczak E. T., Xu R. X., Fesik S. W. Side chain and backbone assignments in isotopically labeled proteins from two heteronuclear triple resonance experiments. FEBS Lett. 1992 Dec 21;314(3):413–418. doi: 10.1016/0014-5793(92)81517-p. [DOI] [PubMed] [Google Scholar]
- Marengere L. E., Pawson T. Identification of residues in GTPase-activating protein Src homology 2 domains that control binding to tyrosine phosphorylated growth factor receptors and p62. J Biol Chem. 1992 Nov 15;267(32):22779–22786. [PubMed] [Google Scholar]
- Marion D., Driscoll P. C., Kay L. E., Wingfield P. T., Bax A., Gronenborn A. M., Clore G. M. Overcoming the overlap problem in the assignment of 1H NMR spectra of larger proteins by use of three-dimensional heteronuclear 1H-15N Hartmann-Hahn-multiple quantum coherence and nuclear Overhauser-multiple quantum coherence spectroscopy: application to interleukin 1 beta. Biochemistry. 1989 Jul 25;28(15):6150–6156. doi: 10.1021/bi00441a004. [DOI] [PubMed] [Google Scholar]
- Neri D., Szyperski T., Otting G., Senn H., Wüthrich K. Stereospecific nuclear magnetic resonance assignments of the methyl groups of valine and leucine in the DNA-binding domain of the 434 repressor by biosynthetically directed fractional 13C labeling. Biochemistry. 1989 Sep 19;28(19):7510–7516. doi: 10.1021/bi00445a003. [DOI] [PubMed] [Google Scholar]
- Nilges M., Clore G. M., Gronenborn A. M. Determination of three-dimensional structures of proteins from interproton distance data by dynamical simulated annealing from a random array of atoms. Circumventing problems associated with folding. FEBS Lett. 1988 Oct 24;239(1):129–136. doi: 10.1016/0014-5793(88)80559-3. [DOI] [PubMed] [Google Scholar]
- Obermeier A., Bradshaw R. A., Seedorf K., Choidas A., Schlessinger J., Ullrich A. Neuronal differentiation signals are controlled by nerve growth factor receptor/Trk binding sites for SHC and PLC gamma. EMBO J. 1994 Apr 1;13(7):1585–1590. doi: 10.1002/j.1460-2075.1994.tb06421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal S. M., Singer A. U., Gish G., Yamazaki T., Shoelson S. E., Pawson T., Kay L. E., Forman-Kay J. D. Nuclear magnetic resonance structure of an SH2 domain of phospholipase C-gamma 1 complexed with a high affinity binding peptide. Cell. 1994 May 6;77(3):461–472. doi: 10.1016/0092-8674(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Pawson T., Schlessingert J. SH2 and SH3 domains. Curr Biol. 1993 Jul 1;3(7):434–442. doi: 10.1016/0960-9822(93)90350-w. [DOI] [PubMed] [Google Scholar]
- Pelicci G., Lanfrancone L., Grignani F., McGlade J., Cavallo F., Forni G., Nicoletti I., Grignani F., Pawson T., Pelicci P. G. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992 Jul 10;70(1):93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- Pelton J. G., Torchia D. A., Meadow N. D., Roseman S. Tautomeric states of the active-site histidines of phosphorylated and unphosphorylated IIIGlc, a signal-transducing protein from Escherichia coli, using two-dimensional heteronuclear NMR techniques. Protein Sci. 1993 Apr;2(4):543–558. doi: 10.1002/pro.5560020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran K. S., Burakoff S. J. The adapter protein Shc interacts with the interleukin-2 (IL-2) receptor upon IL-2 stimulation. J Biol Chem. 1994 Jan 21;269(3):1599–1602. [PubMed] [Google Scholar]
- Ravichandran K. S., Lee K. K., Songyang Z., Cantley L. C., Burn P., Burakoff S. J. Interaction of Shc with the zeta chain of the T cell receptor upon T cell activation. Science. 1993 Nov 5;262(5135):902–905. doi: 10.1126/science.8235613. [DOI] [PubMed] [Google Scholar]
- Ruff-Jamison S., McGlade J., Pawson T., Chen K., Cohen S. Epidermal growth factor stimulates the tyrosine phosphorylation of SHC in the mouse. J Biol Chem. 1993 Apr 15;268(11):7610–7612. [PubMed] [Google Scholar]
- Sato N., Sakamaki K., Terada N., Arai K., Miyajima A. Signal transduction by the high-affinity GM-CSF receptor: two distinct cytoplasmic regions of the common beta subunit responsible for different signaling. EMBO J. 1993 Nov;12(11):4181–4189. doi: 10.1002/j.1460-2075.1993.tb06102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit L., de Vries-Smits A. M., Bos J. L., Borst J. B cell antigen receptor stimulation induces formation of a Shc-Grb2 complex containing multiple tyrosine-phosphorylated proteins. J Biol Chem. 1994 Aug 12;269(32):20209–20212. [PubMed] [Google Scholar]
- Songyang Z., Shoelson S. E., McGlade J., Olivier P., Pawson T., Bustelo X. R., Barbacid M., Sabe H., Hanafusa H., Yi T. Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol Cell Biol. 1994 Apr;14(4):2777–2785. doi: 10.1128/mcb.14.4.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. M., Loeb D. M., Copeland T. D., Pawson T., Greene L. A., Kaplan D. R. Trk receptors use redundant signal transduction pathways involving SHC and PLC-gamma 1 to mediate NGF responses. Neuron. 1994 Mar;12(3):691–705. doi: 10.1016/0896-6273(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Waksman G., Shoelson S. E., Pant N., Cowburn D., Kuriyan J. Binding of a high affinity phosphotyrosyl peptide to the Src SH2 domain: crystal structures of the complexed and peptide-free forms. Cell. 1993 Mar 12;72(5):779–790. doi: 10.1016/0092-8674(93)90405-f. [DOI] [PubMed] [Google Scholar]
- Wüthrich K., Billeter M., Braun W. Pseudo-structures for the 20 common amino acids for use in studies of protein conformations by measurements of intramolecular proton-proton distance constraints with nuclear magnetic resonance. J Mol Biol. 1983 Oct 5;169(4):949–961. doi: 10.1016/s0022-2836(83)80144-2. [DOI] [PubMed] [Google Scholar]
- Yokote K., Mori S., Hansen K., McGlade J., Pawson T., Heldin C. H., Claesson-Welsh L. Direct interaction between Shc and the platelet-derived growth factor beta-receptor. J Biol Chem. 1994 May 27;269(21):15337–15343. [PubMed] [Google Scholar]