Abstract
During pregnancy, myometrial phenotype is programmed into three characteristic stages referred to as the early proliferative, the midterm hypertrophic, and the late contractile stage. Increased myometrial growth in the early and midterm of pregnancy involves a complex process of cell proliferation, antiapoptosis and differentiation. We have previously demonstrated that the androgen receptor (AR) is required for myometrial cell proliferation by modulating IGF-1 signaling during early pregnancy. Here, we report that AR also exerts its antiapoptotic function in human myometrial cells. Enhanced AR expression protects, whereas AR silencing sensitizes myometrial cells to both intrinsic and extrinsic apoptotic stimuli. AR agonist inhibits, whereas AR antagonist induces myometrial cells to undergo apoptotic cell death. Gene microarray analysis confirms that the central functions of AR in myometrial cells are to regulate cell cycling and apoptosis through three major gene groups involving the epidermal growth factor (EGF) signaling, RNA splicing and DNA repair processes. AR mediates its antiapoptotic function through two distinct pathways. In the receptor-dependent pathway, AR is required for the expression of several protein factors within the EGF signaling pathway. Through the PI3K/Akt pathway, AR enhances the expression of the antiapoptotic protein Mcl-1. In the ligand-dependent pathway, AR agonist triggers the activation of Src kinase, which in turn phosphorylates STAT3 to increase Mcl-1 expression. We conclude from these results that the AR signaling exerts antiapoptotic function in myometrial cells, further supporting its key role in programming of myometrial phenotype.
The myometrium demonstrates remarkable plasticity during pregnancy, manifest by changes in myometrial phenotype across pregnancy starting from the early proliferative, to the midterm synthetic and the later contractile stages.1, 2 It concludes with postpartum uterine remodeling to complete the reproductive cycle following labor and returns to its nonpregnant receptive state. The proliferative stage of pregnancy involves a complex process of cell proliferation, apoptosis as well as differentiation. At this stage, the myometrial phenotype is characterized by an enhanced proliferation index for example, PCNA and BrdU incorporation.3 In addition, emerging evidence suggests that antiapoptotic mechanisms are also developed to cooperate with myometrial growth. Using a pregnant rat model, we showed high levels of the antiapoptotic proteins, Bcl2 and Bcl2L1, expressed throughout the proliferative stage.3 In addition, although there are several executioner caspases (e.g. caspase 3, 6 and 7) activated during this stage, apoptotic cell death is not observed in myometrial cells.3, 4, 5 As the early proliferative phase of pregnancy is essential to accumulate enough myometrial cell numbers and contractile capacity to engage in parturition, these observations suggest that antiapoptotic mechanisms must be coordinately developed along with cell proliferation during early pregnancy to achieve optimal myometrial growth. Thus, investigation of the antiapoptotic mechanisms of myometrial cells will contribute significantly to our understanding the physiology of uterus and the clinical management of pregnancy and labor progression.
Signaling mediated by estrogen and progesterone receptors had been demonstrated to be important for myometrial growth.6, 7, 8, 9 As transcriptional factors, these steroid receptors regulate expression of several growth factors to modulate myometrial cell growth.10, 11, 12 Growth factors such as insulin-like growth factor (IGF) and epidermal growth factor (EGF) bind their cognate tyrosine kinase receptors and trigger multiple downstream signaling cascades such as PI3K/Akt and Ras-Erk/MAPK.13, 14 Steroid receptors can also exert non-genomic actions to control cell proliferation. Ligand-activated receptors can recruit Src kinase and activate its downstream signaling cascade.15, 16 Therefore, the mutual interplay between steroid receptors and growth factors are vital for myometrial growth.
The androgen receptor (AR), a nuclear steroid receptor, is also expressed in the myometrium. Serum androgen levels start to increase progressively during the pregnant luteal phase, initiated by the luteinizing hormone surge.17 AR expression levels in the myometrium are also maintained at higher levels during early proliferative and synthetic phases of pregnancy.18 We have shown that while positively regulating IGF-1 receptor protein stability, AR is an important regulator of myometrial cell cycling and cell proliferation.18 These findings are further supported by studies on female AR knockout mice.19 Loss of function of AR results in a subinfertile phenotype with a thinner uterine myometrial wall and reduced myometrial cell numbers when compared with wild-type mice, indicating that AR is also a regulator of myometrial growth during uterine development. As the balance between cell proliferation and cell death determines myometrium tissue growth, we propose that AR may also have an antiapoptotic role in myometrial cells.
Results
AR protein levels determine the responses of human myometrial smooth muscle cells to apoptotic stimuli
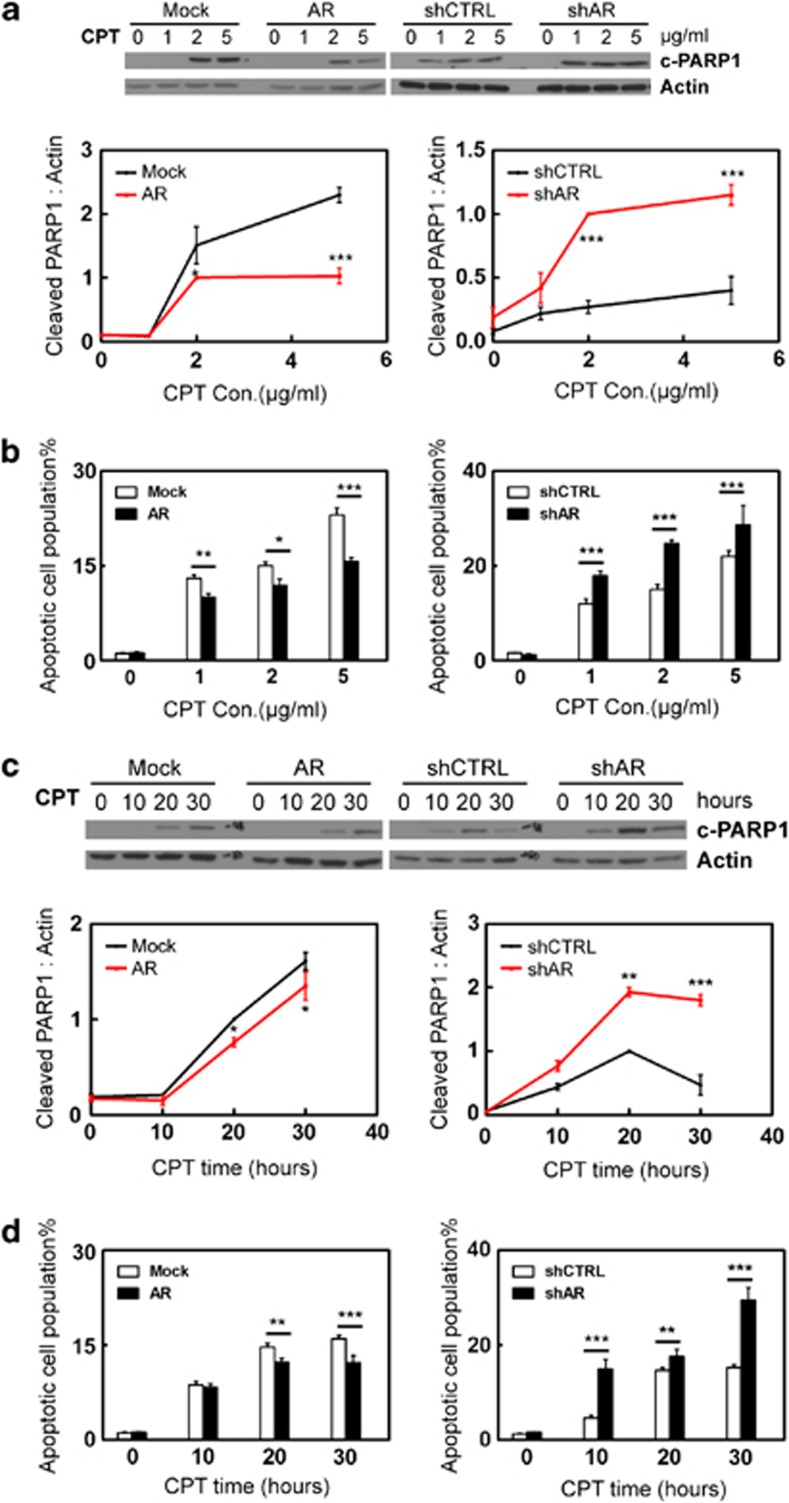
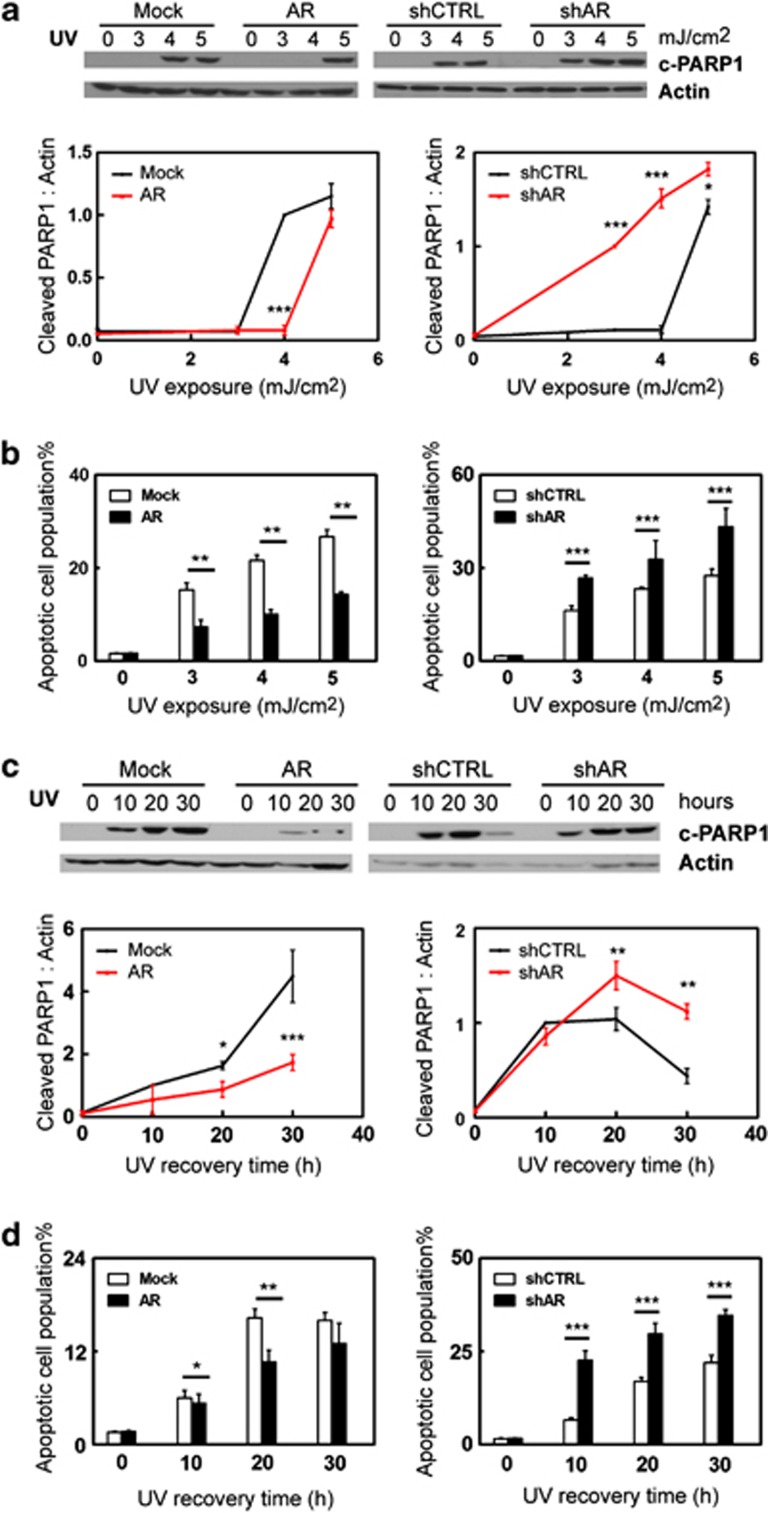
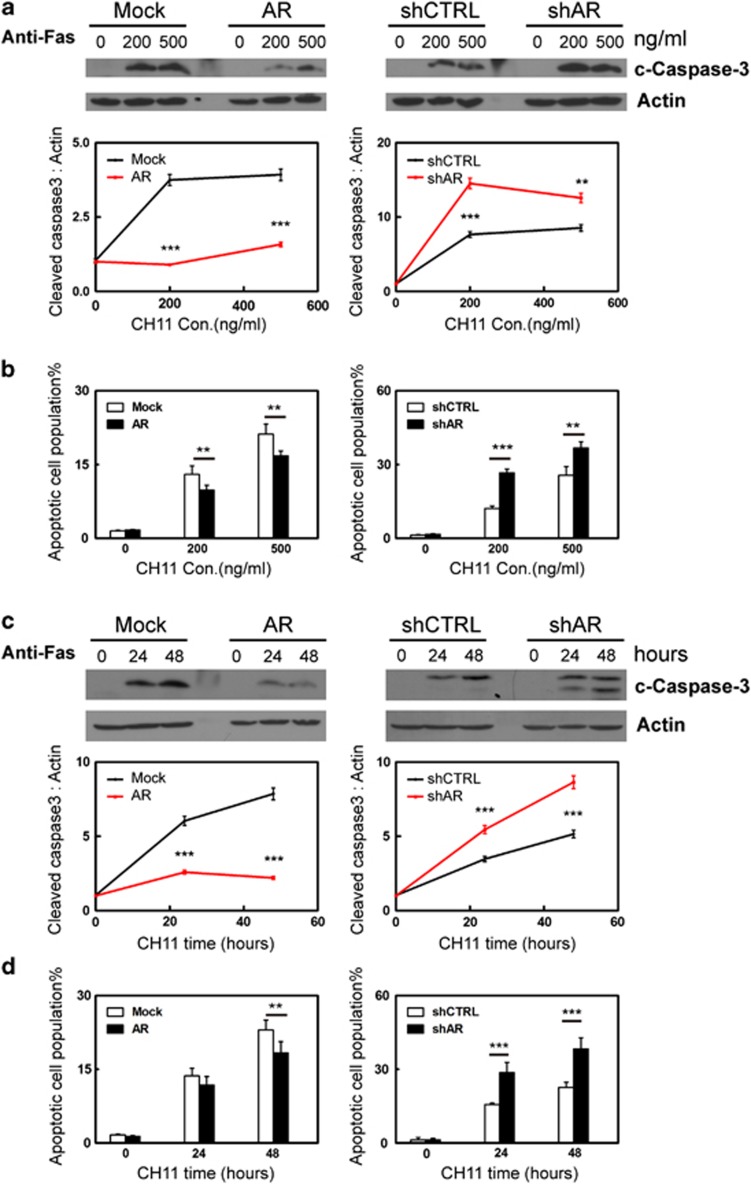
To test the potential role of AR in myometrial cell apoptosis, we used human myometrial cells with either overexpression or RNA silencing of AR as described.18 These cells are referred to as hTERT(AR), hTERT(Mock), hTERT(shAR) and hTERT(shCTRL). Their AR protein expression levels were shown in Supplementary Figure S1. These myometrial cells were exposed to Camptothecin (CPT) or UV light as intrinsic apoptosis stimuli or Anti-Fas antibody as an extrinsic stimulus. Cell apoptosis were detected by two independent methods, which are the levels of cleaved PARP1 (c-PARP1) or cleaved caspase 3 (c-CASP3) detected by western blotting and cell populations that bind Annexin V measured by fluorescence-activated cell sorting (FACS) assay. By measuring c-PARP1 levels, we showed that CPT induced myometrial cell apoptosis in a dose-dependent manner (Figure 1a). Enhanced AR expression reduced the cellular apoptotic response, whereas AR knockdown rendered myometrial cells more sensitive to CPT-induced apoptosis. These results are consistent with the cell apoptosis assay measuring the cell population that bind Annexin V (Figure 1b). We also used 2.5 μg/ml of CPT to induce cell apoptosis in a 0–30 h time course. Both c-PARP1 levels and Annexin V binding showed that AR antagonized CPT-induced apoptotic cell death (Figures 1c and d). To further confirm the protective role of AR, we also induced apoptosis by UV light. Human myometrial cells were exposed to 0–5 mJ/cm2 followed by 16-hour recovery (Figures 2a and b and Supplementary Figure S2), or to 3.5 mJ/cm2 of UV light followed by 0–30 h recovery (Figures 2c and d). We observed that AR antagonized cellular apoptosis induced by UV light. Furthermore, we assess the impact of triggering the extrinsic apoptotic pathway in myometrial cells by the anti-Fas antibody. Myometrial cells were treated with 0–500 ng/ml of anti-Fas antibody (clone CH11) for 24 h (Figures 3a and b), or with 300 ng/ml of Fas antibody for 0–48 h (Figures 3c and d).20 Both c-CASP3 levels and Annexin V binding were measured. Cells with enhanced AR expression were more resistant, whereas cells with AR silencing were more sensitive to anti-Fas antibody induced apoptosis. In summary, these results indicate that AR has a protective role to prevent myometrial cells from apoptotic cell death.
Figure 1.
Human myometrial cells hTERT(Mock), hTERT(AR), hTERT(shCTRL) or hTERT(shAR) cells were cultured in DMEM plus 5% charcoal-stripped serum. Cells were treated with 0, 1, 2, 5 μg/ml of camptothecin (CPT) for 24 h (a and b) or with 2.5 μg/ml of CPT for 0, 10, 20, 30 h (c and d). Representative western blot and densitometry analysis on data from triplicate experiments on c-PARP1 protein levels relative to actin were expressed as mean±S.E.M (a and c). FACS assays measured cell populations that bind Annexin V from triplicate experiments (n=2/repeat) and were expressed as mean±S.E.M. (b and d). Student's t-test was performed with * as P<0.05; ** as P<0.01 and *** as P<0.001
Figure 2.
Human myometrial cells hTERT(Mock), hTERT(AR), hTERT(shCTRL) or hTERT(shAR) cells were cultured in DMEM plus 5% charcoal-stripped serum. Cells were exposed to 0, 3, 4, 5 mJ/cm2 UV light and allowed to recover for 24 h (a and b) or to 3.5 mJ/cm2 and allowed to recover for 0, 10, 20, 30 h (c and d). Representative western blot and densitometry analysis on data from triplicate experiments on c-PARP1 protein levels relative to actin were expressed as mean±S.E.M. (a and c). FACS assays measured cell populations that bind Annexin V from triplicate experiments (n=2/repeat) and were expressed as mean±S.E.M. (b and d). Student's t-test was performed with * as P<0.05; ** as P<0.01 and *** as P<0.001
Figure 3.
Human myometrial cells hTERT(Mock), hTERT(AR), hTERT(shCTRL) or hTERT(shAR) cells were cultured in DMEM plus 5% charcoal-stripped serum. Cells were treated with 0, 200 or 500 ng/ml anti-Fas antibody for 24 h (a and b) or with 300 ng/ml of anti-Fas antibody for 0, 24 or 48 h (c and d). Representative western blot and densitometry analysis on data from triplicate experiments on c-CASP3 protein levels relative to actin were expressed as mean±S.E.M. (a and c). FACS assays measured cell population that bind Annexin V from triplicate experiments (n=2/repeat) and were expressed as mean±S.E.M. (b and d). Student's t-test was performed with * as P<0.05; ** as P<0.01 and *** as P<0.001
AR agonist protects human myometrial smooth muscle cells from apoptotic stimuli
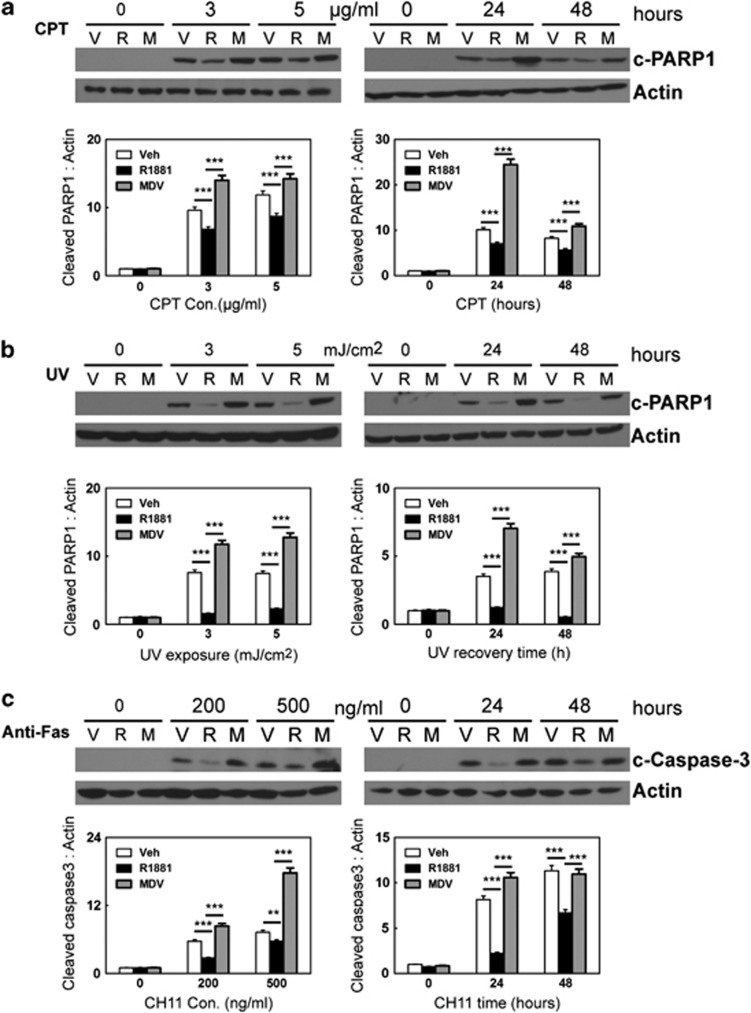
The function of AR can be mediated through either receptor-dependent or ligand-dependent pathways.21 To study the hormonal regulation of AR in myometrial cell apoptosis, we treated parental hTERT-HM cells with CPT, UV light or anti-Fas antibody in the presence of vehicle, synthetic AR agonist R1881 or AR antagonist MDV3100 (Figures 4a–c) for 0–48 h. Cell apoptosis was again measured by the levels of c-PARP1 or c-CASP3. We showed that R1881 significantly reduced the levels of c-PARP1 under CPT and UV light treatments (Figures 4a and b). In addition, MDV3100 treatment resulted in higher levels of c-PARP1 when compared with cells under R1881 treatment. We also showed that R1881 decreased, whereas MDV3100 increased c-CASP3 levels in myometrial cells, when cells were challenged by anti-Fas antibody (Figure 4c). These data were further confirmed by FACS assays measuring Annexin V binding, when myometrial cells were exposed to CPT, UV light and the anti-Fas antibody (Supplementary Figure S3). These results demonstrated that in addition to AR protein, the antiapoptotic function of AR can also be modulated by AR ligands.
Figure 4.
hTERT-HM cells were cultured in DMEM with 5% charcoal-stripped serum and treated with vehicle, 10 nM of R1881 or 20 uM of MDV3100. (a) Cells were also co-treated with 0, 3 or 5 μg/ml of CPT for 24 h or with 2.5 μg/ml for 0, 24 or 48 h. (b) Cells were co-treated with 0, 3, or 5 mJ/cm2 UV light and allowed to recover for 24 h or with 3.5 mJ/cm2 and allowed to recover for 0, 24 or 48 h. (c) Cells were co-treated with 0, 200 or 500 ng/ml anti-Fas antibody for 24 h or with 300 ng/ml of anti-Fas antibody for 0, 24 or 48 h. Representative western blot and densitometry analysis on data from triplicate experiments on c-PARP1 or c-CASP3 protein levels relative to actin were expressed as mean±S.E.M. Student's t-test was performed with * as P<0.05; ** as P<0.01 and *** as P<0.001
Gene microarray analyses of AR-regulated genes
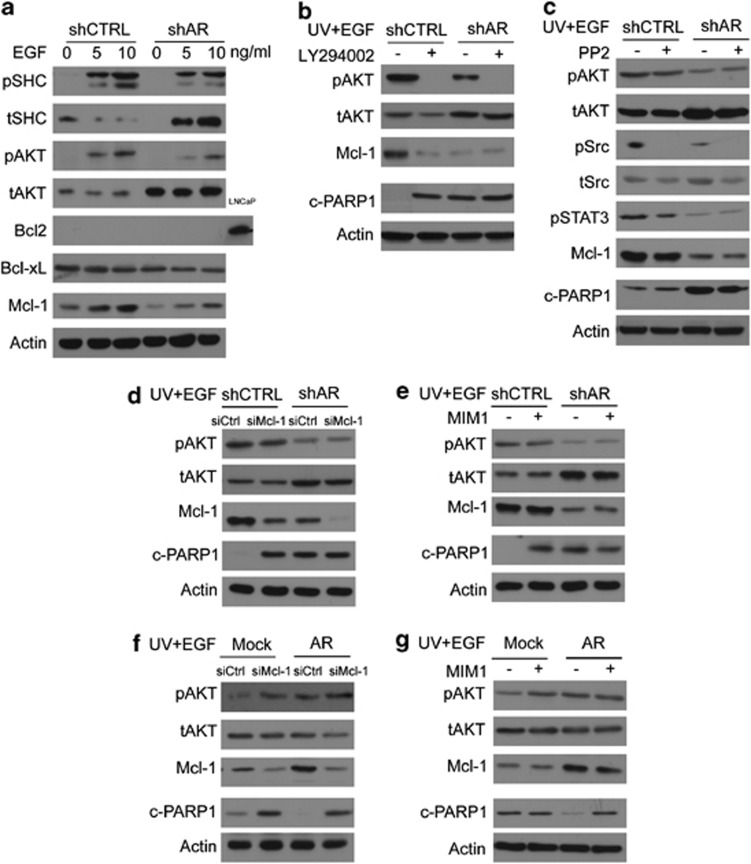
To identify AR-targeted genes that are responsible for its antiapoptotic action, we performed gene microarray analyses. In total, 1567 genes were significantly changed by AR knockdown (>1.5 fold). Gene ontology (GO) analyses by The Database for Annotation, Visualization and Integrated Discovery (DAVID) version 6.7 further characterized the key cellular processes regulated by AR. The top two ranked GO terms are cell cycling and apoptosis, which involve a total of 201 genes (Figure 5a). These results further confirmed that the main functions of AR in myometrial cells are to regulate cell proliferation and apoptosis. Information of the top-ranked 30 genes by q-value either up- or downregulated by AR knockdown are also listed in Figure 5b. Using the IPA software (Ingenuity Systems), we further identified three major signaling cascades that are regulated by AR and may mediate AR antiapoptotic function (Figure 5c). They are protein factors within the EGF signaling (EGF, SHC, PKC, GRB2 and RASA), RNA splicing factors (SRSF1, SRSF2, SRSF4, hnRNPM and hnRNPU) and proteins involving in DNA repair (XRCC5, XRCC6, PARP1, NONO). The EGF signaling is particularly interesting as its downstream effector, Akt, is well known for its antiapoptotic function. Using real-time PCR to validate microarray findings, we showed that mRNA levels of EGF, SHC, PKC, GRB2 and RASA genes were significantly reduced in the presence of AR knockdown (Figure 5d), and upregulated by enhanced AR expression. It should be noted that mRNA levels of these genes were not altered by R1881 treatment, indicating that AR regulation of EGF signaling is receptor-dependent.
Figure 5.
(a) Go-term analyses by DAVID (version 6.7) identified top 15 ranked gene groups regulated by AR. (b) Information on top 30 ranked genes that either upregulated or downregulated by AR were listed. (c) IPA identified canonical connection of signaling pathways regulated by AR. (d) Real-time PCR validate mRNA levels of components of EGF signaling in hTERT(shCTRL), hTERT(shAR), hTERT(mock) and hTERT(AR) cells or hTERT-HM cells treated with vehicle or 10 nM R1881
Antiapoptotic action of the AR is mediated through a receptor-dependent mechanism
AR regulates several protein components within the EGF signaling, suggesting that AR may exert its antiapoptotic function by modulating EGF signaling. To test this hypothesis, we first treated hTERT(shCTRL) and hTERT(shAR) cells with 0, 5 and 10 ng/ml of recombinant EGF for 30 min. Activation of EGF signaling led to increased phospho-SHC (p-SHC) and phospho-Akt (p-Akt), whereas AR knockdown attenuated these changes (Figure 6a). Within the antiapoptotic Bcl2 family, very low levels of Bcl2 protein was expressed in the myometrial cells. There were no changes of Bcl-xL protein levels in response to EGF treatment or to AR silencing. However, a robust upregulation of Mcl-1 was induced by EGF, concurrent with the activation of Akt signaling, suggesting that the antiapoptotic function of AR may be mediated through the Akt signaling to upregulate Mcl-1 protein levels. To test this possibility, we applied the PI3K/Akt inhibitor, LY294002 to the myometrial cells. Blocking Akt activity dramatically reduced Mcl-1 protein levels (even in the presence of EGF treatment) accompanied by enhanced cellular apoptosis as measured by c-PARP1 levels (Figure 6b). These results indicate that in myometrial cells, AR utilizes EGF/Akt signaling to control Mcl-1 expression and exert an antiapoptotic function.
Figure 6.
(a) hTERT(shCTRL) and hTERT(shAR) cells were treated with 0, 5, 10 ng/ml recombinant EGF peptide for 30 min. Cell lyses were collected. (b–e) hTERT(shCTRL) and hTERT(shAR) cells were treated with vehicle, 50 μM of LY294002 (b), 4 μM of PP2 (c), siRNA for Mcl-1 (d) or 20 μM of MIM1 (e) for 16 h, after which cells were co-treated with 10 ng/ml of EGF and exposed to 3.5 mJ/cm2 of UV light. After recovery for 24 h, cell lyses were collected. hTERT(mock) and hTERT(AR) cells were transfected with siRNA for Mcl-1 (f) or MIM1 (g) for 24 h and then subjected to10 ng/ml of EGF and exposed to 3.5 mJ/cm2 of UV light. After recovery for 24 h, cell lyses were collected. Western blotting assays were performed with antibodies as indicated. Representative blots from triplicate experiments were presented. Note: Bcl2 is expressed in extremely low levels in myometrial cells as shown in Figure 6a. Protein lysate from LNCaP cells was used as a positive control for immunoreactivity to Bcl2 antibody
It is known that EGF treatment can also activate Src kinase to exert antiapoptotic function.22 To test whether this mechanism also exists in myometrial cells, we co-treated hTERT(shCTRL) and hTERT(shAR) cells with EGF plus PP2, a Src inhibitor (Figure 6c). We observed that although phosphorylation of Src is efficiently blocked, Mcl-1 and c-PARP1 protein levels were not altered, unless RNA silencing of AR was present. These results indicate that the AR-dependent antiapoptotic function is dominant through the EGF-Akt signaling pathway.
To further confirm that Mcl-1 serves as the downstream effector for the antiapoptotic function of AR, we treated myometrial cells with siRNA for Mcl-1 (Figure 6d). RNA silencing of Mcl-1 resulted in increased c-PARP1 levels in hTERT(shCTRL) cells regardless of the phospho-Akt status. As Mcl-1 protein levels were lower and c-PARP1 level were higher in hTERT(shAR) cells, Mcl-1 knockdown did not show any impact on c-PARP1 levels in hTERT(shAR) cells. We also treated cells with MIM1, a specific inhibitor of Mcl-1 that selectively targets the BH3 domain of Mcl-1 protein.23 MIM1 treatment increased c-PARP1 levels, even when the myometrial cells expressed high levels of phospho-Akt. Although enhanced AR expression resulted in elevated protein levels of Mcl-1, treatment of siRNA to Mcl-1 or MIM1 can antagonize AR's impact, resulting in higher levels of c-PARP1 (Figure 6e). Together, these results led us to conclude that by modulating the EGF-Akt-Mcl-1 signal pathway, AR exerts an antiapoptotic function in an AR-dependent manner.
Antiapoptotic action of the AR can also be mediated through a ligand-dependent mechanism
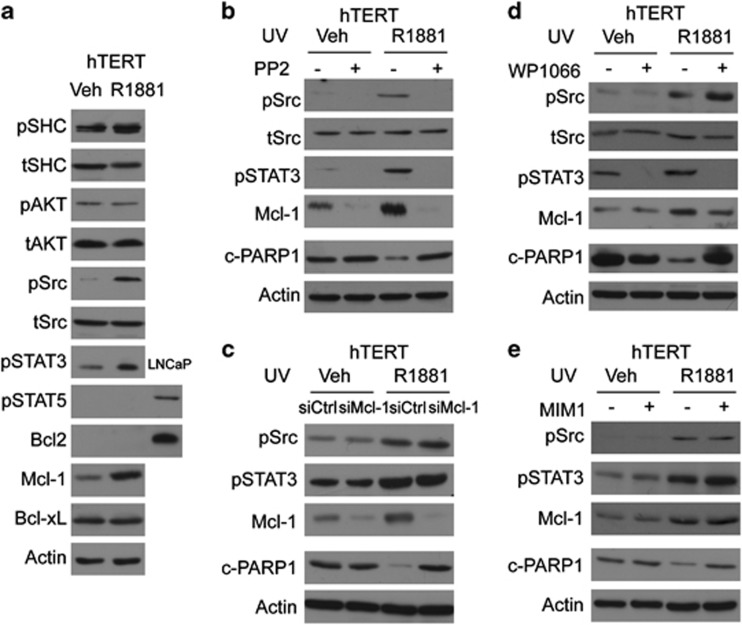
Ligand-activated AR can recruit and activate Src kinase, a potential mechanism for the ligand-dependent antiapoptotic action of the AR. To test this hypothesis, we first treated parental hTERT-HM cells with either vehicle or R1881. Although the EGF-Akt pathway is not altered (no changes in p-SHC and p-Akt levels), R1881 stimulated phosphorylation of Src and its downstream effector STAT3, but not STAT5 (Figure 7a and Supplementary Figure S5). As one of the downstream targeted genes of STAT3, Mcl-1 expression was also upregulated by R1881 treatment. By contrast, there were no changes of Bcl2 and Bcl-xL, two other antiapoptotic Bcl2 family members. We further demonstrated that R1881-induced activation of Src was effectively inhibited by PP2, resulting in decreased phosphorylation of STAT3. We also observed subsequent downregulation of Mcl-1 expression and increased levels of c-PARP1 protein even in the presence of R1881 (Figure 7b). We have also applied the STAT3 inhibitor, WP1066, to show that transcriptional activity of STAT3 induced by R1881 was directly associated with Mcl-1 protein levels (Figure 7c). Blocking the activation of STAT3 resulted in decreased protein levels of Mcl-1 and higher levels of c-PARP1 in the presence of R1881 treatment. We further showed that Mcl-1 is the downstream effector of STAT3. Mcl-1 knockdown or inhibition of Mcl-1 activity by MIM1 resulted in higher levels of c-PARP1 protein, even in the presence of Src and STAT3 activations by R1881 (Figure 7d). We conclude from this set of experiments that AR can also exert its antiapoptotic function in a ligand-dependent manner through the Src-STAT3-Mcl-1 signaling pathway.
Figure 7.
Human myometrial cells, hTERT-HM, were cultured in DMEM medium containing 5% charcoal-stripped serum for 48 h. (a) Cells were treated with vehicle or 10 nM R1881 for 16 h. Protein lyses were collected. (b–e) Cells were exposed to 3.5 mJ/cm2 UV light in the presence of ± 10 nM of R1881. Cells were also treated with vehicle, 4 μM of PP2 (b), 5 μM of WP1066 (c), siRNA for Mcl-1 (d) or 20 μM of MIM1 (e) for 16 h. Protein lyses were collected and immunoblotted with antibodies as indicated. Representative blots from triplicate experiments were presented. Note: STAT5 and Bcl2 are expressed in extremely low levels in myometrial cells as shown in Figure 7a. Protein lysis from LNCaP cells was used as a positive control for immunoreactivity to phosphor-STAT5 and Bcl2 antibodies
Discussion
The functions of myometrium during pregnancy and labor are not only to provide an environment for the fetus to fully develop, but also to contract at labor to expel the fetus into the extrauterine environment. Owing to the complexity of these multiple tasks, the myometrium has to adapt into different phenotypes to achieve these goals in a coordinated fashion with the progression of pregnancy. Therefore, we have proposed the myometrial phenotype programming concept to address the phenotypic plasticity of this organ. Our findings presented here for the first time characterized the antiapoptotic function of the AR signaling in human myometrial cells. By revealing the molecular mechanisms by which AR signaling mediates such functions by both receptor-dependent and ligand-dependent pathways, we further strengthen the notion that the AR signaling is a critical component of myometrial phenotype programming.
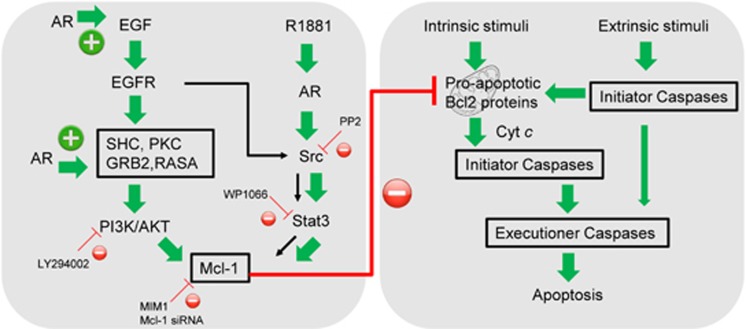
Our studies support the dual roles of AR in regulating myometrial cell proliferation and resistance to apoptotic stimuli. This conclusion is supported by the AR gestational profile. High levels of AR protein were observed during the proliferative phase of pregnancy, but decreased dramatically as labor was approached.18 This expression pattern is controlled by the mechanical stretch induced by growing fetus as well as estrogen/progesterone signaling.18 Furthermore, AR utilizes different signaling pathways to control proliferation and apoptosis of myometrial cells. The proliferative action of AR is ligand-independent. We have shown that AR protein is required for IGF-1R protein stability and regulates downstream phosphorylation of Akt.18 High levels of AR during early pregnancy are associated with enhanced activation of IGF-1 downstream effectors, including IRS-1, phospho-mTOR and phospho-S6K1 in the pregnant rat myometrium.24 However, the antiapoptotic function of AR is both ligand-dependent and receptor-dependent (Figure 8). In the receptor-dependent pathway, AR but not androgen controls transcription of several components of the EGF signaling including EGF, RASA, GRB2 and SHC (Figure 6). EGF signaling cannot be effectively activated in the presence of AR knockdown. In the ligand-dependent pathway, AR agonist R1881 does not affect PI3K/Akt pathway. Rather, it triggers the activation of Src kinase and the transcription factor STAT3, resulting in an increase of Mcl-1 expression (Figure 7). Interestingly, EGF signaling also activates Src kinase and STAT3 (Supplementary Figure S4), suggesting that Src and STAT3 may possibly induce Mcl-1 protein levels. However, our further investigation shows that although EGF induced-Src activation can be blocked by PP2, it does not effectively decrease Mcl-1 levels. In contrast, PI3K/Akt inhibitor LY294002 dramatically abolishes Mcl-1 levels and induces c-PARP1. These results indicate that under EGF signaling, Akt activation has the dominant role over Src-STAT3 in mediating AR antiapoptotic function (Figure 8).
Figure 8.
Proposed mechanisms by which AR exert its antiapoptotic function in human myometrial cells
AR signaling involves both intrinsic and extrinsic pathways of cellular apoptosis. The common feature of both pathways is that apoptotic stimuli activate initiator caspases 8/10 for the extrinsic pathway or initiator caspase 9 for intrinsic pathway.25 The initiator caspases then activate the executioner caspases (e.g. caspases 3, 6 and 7) to cleave death substrate. Our studies indicate that AR controls Mcl-1 expression through either EGF/Akt or Src/STAT3 pathways. Mcl-1 is known to prevent the activation of the pro-apoptotic proteins, Bax and BAK.26 Furthermore, Akt can phosphorylate and inactivate the pro-apoptotic BAD protein.14 Therefore, the AR/Mcl-1 axis inhibits activation of caspase 9 activation and subsequent executioner caspases to supress the intrinsic apoptosis pathway. The intrinsic pathway can also serve as an enhancer for the extrinsic pathway and facilitate cell apoptosis by the extrinsic pathway. This mechanism had been demonstrated in studies showing that enhanced expression of antiapoptotic Bcl2 family members blocked death receptor-mediated activation of caspase 8 and downstream executioner caspases.27 The same principle is also applied to myometrial cells. AR agonist, through upregulation of Mcl-1 expression, can inhibit the activation of executioner caspases that are triggered by the extrinsic apoptotic pathway.
Our findings support the critical role of Mcl-1 in protecting myometrial cells from apoptotic cell death. Activation of caspase 3 is required to maintain uterine quiescence through fragmentation of uterine myocyte contractile proteins.28 Upregulation of Mcl-1 was demonstrated to be used by myometrial cells to neutralize the apoptotic property of caspase 3.28 In our studies, we also find Mcl-1 has a unique role in mediating AR antiapoptotic function in myometrial cells, as other antiapoptotic the expression of Bcl2 family members, such as Bcl2 and Bcl-xL, is not affected by the AR signaling. This phenomenon is consistent with reports in human macrophages, where the antiapoptotic action of Akt is specifically mediated by Mcl-1, but not by Bcl2 or Bcl-xL.29
We report that AR utilizes multiple signaling cascades (e.g. Akt and Src/STAT3) to induce Mcl-1 expression in myometrial cells. As the expression of Mcl-1 is regulated by both transcriptional and posttranslational levels, Akt can trigger its downstream mTOR pathway to enhance Mcl-1 protein translation.30, 31 Akt can also enhance Mcl-1 protein stability through modulating phosphorylation of GSK3.32 Furthermore, Akt can rapidly induce Mcl-1 gene transcription by regulating Akt-dependent transcription factors.33 Ligand-activated AR can recruit and activate Src kinase,34 which in turn enhance the transcriptional activity of STAT3 to upregulate Mcl-1 gene transcription.35 Other reported pathways that induce Mcl-1 levels in myometrial cells are the NFkB pathway, endoplasmic reticulum stress response and its adaptive unfolded protein response.4 Through different mechanisms, these pathways add to the contribution of AR signaling to sustain high levels of Mcl-1 present in myometrial cells and to ensure an antiapoptotic environment through this phase of pregnancy.
Our studies suggest that the AR may interact with other steroid receptors to control myometrial cells in response to apoptosis. AR protein levels were relatively high in the proliferation phase and low during the late secretory phase of the menstrual cycle, implying that estradiol and progesterone regulate AR gene expression.36 Using ovariectomized mice, AR mRNA levels were shown to be mildly upregulated by estradiol in the myometrium.37 However, no impressive increases of AR protein levels were apparent.18, 38 As these studies were performed using whole-uterine tissues, it is possible that this represents a paracrine action involving the endometrium, stroma and myometrium. In our myometrial cell model, microarray combined with real-time PCR assays did not show any AR regulation of ER and PR expression. These findings do not exclude the possibility that AR may alter ER or PR function by cross talk with IGF or EGF signal pathways, as the downstream effectors, Akt and Src kinase are important to transduce steroid hormone signaling. Akt and Src are thus attractive therapeutic targets to block myometrial cell proliferation and induce apoptotic cell death. Recent studies have shown that Akt inhibitors can reduce leiomyoma cell viability and leiomyoma tumor volume.39 It is of interest to test whether combining AKT blocker and AR antagonist (e.g. MDV3100) would result in synergistic suppression of leiomyoma tumor growth.
Though our results on the antiapoptotic actions of AR were collected from in vitro myometrial hTERT-HM cell model, further studies shall apply in vivo animal models (e.g. the female AR knockout mice). These animal models have the privilege to study the paracrine regulation of AR signaling by growth hormones and other sex steroids among myometrium, stroma and endometrium.
In summary, our studies demonstrated that the AR exerts antiapoptotic functions in human myometrial cells and the AR signaling is an important component of myometrium phenotype programming during pregnancy.
Materials and Methods
Tissue culture and western blotting
Paternal human myometrial cells were received from Dr. Condon (University of Pittsburgh) and referred to as hTERT-HM. As described in our previous study,18 hTERT-HM cells stably expressing control shRNA or shRNA against AR were designated as hTERT(shAR) and hTERT(shCTRL), whereas cells overexpressing mock or AR cDNA were designated as hTERT(AR), hTERT(Mock). When steroid hormone treatments were used, cells were first cultured in phenol red-free DMEM medium containing 5% charcoal-stripped serum (Hyclone, Logan, UT, USA) for 48 h. Protein was extracted in lysis buffer containing 50 mM Tris (pH 8.0), 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate. Western blotting assays were performed using antibodies listed in the Supplementary Table. The density of protein bands was measured using Image J software analysis (NIH, Bethesda, MD, USA). The values of each sample were expressed as a ratio of protein of interest to the housekeeping protein. R1881, CPT, LY294002, WP1066, PP2 and EGF were purchased from Cedarlane (Burlington, ON, Canada). MDV3100 was from Haoyuan Chemexpress (Shanghai, China). MIM1 was from Tocris (Bristol, UK). Detailed information of antibodies and primers are listed in Supplementary Table.
UV light and anti-Fas antibody induced apoptosis
cells were serum starved for 12 h before exposured to UV light (UV Stratalinker 1800) for various doses. Cells were allowed to recover for the indicated periods of time. To induce apoptosis through the extrinsic pathway, cells were treated with anti-Fas antibody (clone CH110) at various doses and time points.20 Cells were harvested and the percentage of cells with Annexin V binding was determined by flow cytometry.20 Protein lysates was extracted for western blotting of c-PARP1 and c-CASP3.
Flow cytometry
Annexin V-PE/7-AAD Apoptosis Detection Kit (BD Pharmingen, San Diego, CA, USA) were used to detect apoptotic cell populations according to manufacturer's protocol. Briefly, the cells (1 × 105 cells) were suspended in 100 μl binding buffer, and then treated with 5 μl of Annexin V-PE (BD Pharmingen) and 5 μl 7-AAD before analyzed on FACSCanto II flow cytometer and BD FACSDiva software v5.0.3 (Becton Dickinson, Franklin, NJ, USA). Ten thousand events are acquired for statistical analysis. Detection of apoptotic population was performed according to the manufacturer's instructions (https://www.bdbiosciences.com/external_files/pm/doc/tds/cell_bio/live/web_enabled/6900KK_559763.pdf).
Microarray and Real-time PCR
Gene microarray analyses were performed as described before.40 Briefly, Total RNA was extracted from hTERT(shAR) and hTERT(shCTRL) cells by the mirVana RNA Isolation Kit (Ambion, Austin, TX, USA) from three independently repeated experiments. The quality and quantity of RNA were assessed with an Agilent 2100 Bioanalyzer (Caliper Technologies Corp., CA, USA). Amplified and Alexa Fluor 3 labeled RNA samples from hTERT(shCTRL) and hTERT(shAR) cells were hybridized onto the Human Agilent 4 × 44k (Agilent Technologies, Santa Clara, CA, USA), along with Alexa Fluor 5 labeled human reference RNA. Hybridization signals were analyzed following the manufacturer's instruction. Statistical Analysis of Microarray (SAM) program (http://www-stat.stanford.edu/tibs/SAM/) was used to analyze expression differences between RNA samples from hTERT(shAR) and hTERT(shCTRL) cells. Unpaired t-tests were calculated for all probes passing filters and controlled for multiple testing by estimation of q-values using the false discovery rate method.41 IPA software (Ingenuity Systems) was used to analyze AR-regulated gene groups and signaling pathways.
Real-time PCR assays
Total RNA was extracted using Purelink RNA mini kit (Invitrogen, Burlington, ON, Canada) according to the manufacturer's instructions. Two micrograms of total RNA was subjected to a random-primed reverse transcription using M-MLV reverse transcriptase (Invitrogen). Real-time qPCR was conducted in triplicates using Applied Biosystems7900HT with 5 ng of cDNA, 1 μM of each primer pair and SYBR Green PCR master mix (Roche, Mississauga, ON, Canada). The sequences of primers were shown below. Relative mRNA levels were normalized to GAPDH.
Statistics
Results are expressed as the mean±S.E.M. To determine differences between two groups, student t-test was carried out using GraphPad Prism (version 4) with the level of significance set at P<0.05 as *, P<0.01 as ** and P<0.001 as ***.
Acknowledgments
This work is supported by Canadian Institutes of Health Research Operating Grant (MOP-111148) to XSD and SL and by a Rising Star Award (RS2013-58) from Prostate Cancer Canada to XSD.
Glossary
- AR
androgen receptor
- EGF
epidermal growth factor
- PI3K
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- Akt
protein kinase B
- Mcl-1
induced myeloid leukemia cell differentiation protein
- Src
proto-oncogene tyrosine-protein kinase
- STAT3/5
signal transducer and activator of transcription 3/5
- Bcl2
B-cell lymphoma 2
- Bcl-xL
B-cell lymphoma-extra-large
- PARP1
poly [ADP-ribose] polymerase 1
- CASP3
caspase 3
- FACS
fluorescence-activated cell sorting
- CPT
camptothecin
- SHC
Src homology 2 domain containing transforming protein
- PKC
protein kinase C
- Grb2
growth factor receptor-bound protein 2
- RASA
Ras GTPase-activating protein
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by A Stephanou
Supplementary Material
References
- Lye SJ, Tsui P, Dong X, Mitchell J, Dorogin A, MacPhee D, et al. Myometrial programming: a new concept underlying the regulation of myometrial function during pregnancyIn: Petraglia F, Strauss III JF, Gabbe SG, Weiss G, (ed)Preterm birth—mechanisms, mediators, prevention and interventions Informa UK Ltd: London, UK; 20071–18. [Google Scholar]
- Shynlova O, Tsui P, Jaffer S, Lye SJ. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol. 2009;144:S2–10. doi: 10.1016/j.ejogrb.2009.02.044. [DOI] [PubMed] [Google Scholar]
- Shynlova O, Oldenhof A, Dorogin A, Xu Q, Mu J, Nashman N, et al. Myometrial apoptosis: activation of the caspase cascade in the pregnant rat myometrium at midgestation. Biol Reprodn. 2006;74:839–849. doi: 10.1095/biolreprod.105.048124. [DOI] [PubMed] [Google Scholar]
- Jeyasuria P, Subedi K, Suresh A, Condon JC. Elevated levels of uterine anti-apoptotic signaling may activate NFKB and potentially confer resistance to caspase 3-mediated apoptotic cell death during pregnancy in mice. Biol Reprod. 2011;85:417–424. doi: 10.1095/biolreprod.111.091652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh A, Subedi K, Kyathanahalli C, Jeyasuria P, Condon JC. Uterine endoplasmic reticulum stress and its unfolded protein response may regulate caspase 3 activation in the pregnant mouse uterus. PLoS One. 2013;8:e75152. doi: 10.1371/journal.pone.0075152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010;151:2433–2442. doi: 10.1210/en.2009-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura Y, Matsuo H, Samoto T, Maruo T. Up-regulation by progesterone of proliferating cell nuclear antigen and epidermal growth factor expression in human uterine leiomyoma. J Clin Endocrinol Metab. 1998;83:2192–2198. doi: 10.1210/jcem.83.6.4879. [DOI] [PubMed] [Google Scholar]
- Burroughs KD, Fuchs-Young R, Davis B, Walker CL. Altered hormonal responsiveness of proliferation and apoptosis during myometrial maturation and the development of uterine leiomyomas in the rat. Biol Reprod. 2000;63:1322–1330. doi: 10.1095/biolreprod63.5.1322. [DOI] [PubMed] [Google Scholar]
- Yin XJ, Wang G, Khan-Dawood FS. Requirements of phosphatidylinositol-3 kinase and mammalian target of rapamycin for estrogen-induced proliferation in uterine leiomyoma- and myometrium-derived cell lines. Am JObstet Gynecol. 2007;196:176 e171–175. doi: 10.1016/j.ajog.2006.09.037. [DOI] [PubMed] [Google Scholar]
- Adesanya OO, Zhou J, Bondy CA. Sex steroid regulation of insulin-like growth factor system gene expression and proliferation in primate myometrium. J Clin Endocrinol Metab. 1996;81:1967–1974. doi: 10.1210/jcem.81.5.8626866. [DOI] [PubMed] [Google Scholar]
- Rossi MJ, Chegini N, Masterson BJ. Presence of epidermal growth factor, platelet-derived growth factor, and their receptors in human myometrial tissue and smooth muscle cells: their action in smooth muscle cells in vitro. Endocrinology. 1992;130:1716–1727. doi: 10.1210/endo.130.3.1311246. [DOI] [PubMed] [Google Scholar]
- Matsuo H, Kurachi O, Shimomura Y, Samoto T, Maruo T. Molecular bases for the actions of ovarian sex steroids in the regulation of proliferation and apoptosis of human uterine leiomyoma. Oncology. 1999;57:49–58. doi: 10.1159/000055275. [DOI] [PubMed] [Google Scholar]
- Yu L, Saile K, Swartz CD, He H, Zheng X, Kissling GE, et al. Differential expression of receptor tyrosine kinases (RTKs) and IGF-I pathway activation in human uterine leiomyomas. Mol Med. 2008;14:264–275. doi: 10.2119/2007-00101.Yu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, et al. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO Jl. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, et al. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- Castracane VD, Stewart DR, Gimpel T, Overstreet JW, Lasley BL. Maternal serum androgens in human pregnancy: early increases within the cycle of conception. Hum Reprod. 1998;13:460–464. doi: 10.1093/humrep/13.2.460. [DOI] [PubMed] [Google Scholar]
- Liu L, Li Y, Xie N, Shynlova O, Challis JR, Slater D, et al. Proliferative action of the androgen receptor in human uterine myometrial cells--a key regulator for myometrium phenotype programming. J Clin Endocrinol Metab. 2013;98:218–227. doi: 10.1210/jc.2012-2451. [DOI] [PubMed] [Google Scholar]
- Walters KA, Allan CM, Jimenez M, Lim PR, Davey RA, Zajac JD, et al. Female mice haploinsufficient for an inactivated androgen receptor (AR) exhibit age-dependent defects that resemble the AR null phenotype of dysfunctional late follicle development, ovulation, and fertility. Endocrinology. 2007;148:3674–3684. doi: 10.1210/en.2007-0248. [DOI] [PubMed] [Google Scholar]
- Hayashi N, Peacock JW, Beraldi E, Zoubeidi A, Gleave ME, Ong CJ. Hsp27 silencing coordinately inhibits proliferation and promotes Fas-induced apoptosis by regulating the PEA-15 molecular switch. Cell Death Differ. 2012;19:990–1002. doi: 10.1038/cdd.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- Kitagawa D, Tanemura S, Ohata S, Shimizu N, Seo J, Nishitai G, et al. Activation of extracellular signal-regulated kinase by ultraviolet is mediated through Src-dependent epidermal growth factor receptor phosphorylation. Its implication in an anti-apoptotic function. J Biol chemistry. 2002;277:366–371. doi: 10.1074/jbc.M107110200. [DOI] [PubMed] [Google Scholar]
- Cohen NA, Stewart ML, Gavathiotis E, Tepper JL, Bruekner SR, Koss B, et al. A competitive stapled peptide screen identifies a selective small molecule that overcomes MCL-1-dependent leukemia cell survival. Chem Biol. 2012;19:1175–1186. doi: 10.1016/j.chembiol.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffer S, Shynlova O, Lye S. Mammalian target of rapamycin is activated in association with myometrial proliferation during pregnancy. Endocrinology. 2009;150:4672–4680. doi: 10.1210/en.2009-0419. [DOI] [PubMed] [Google Scholar]
- Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyasuria P, Wetzel J, Bradley M, Subedi K, Condon JC. Progesterone-regulated caspase 3 action in the mouse may play a role in uterine quiescence during pregnancy through fragmentation of uterine myocyte contractile proteins. Biol Reprod. 2009;80:928–934. doi: 10.1095/biolreprod.108.070425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Perlman H, Pagliari LJ, Pope RM. Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages. Role of Mcl-1, independent of nuclear factor (NF)-kappaB, Bad, or caspase activation. J Exp Med. 2001;194:113–126. doi: 10.1084/jem.194.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JR, Hippo Y, Robert F, Chen SM, Malina A, Lin CJ, et al. mTORC1 promotes survival through translational control of Mcl-1. Proc Natl Acad Sci USA. 2008;105:10853–10858. doi: 10.1073/pnas.0804821105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coloff JL, Macintyre AN, Nichols AG, Liu T, Gallo CA, Plas DR, et al. Akt-dependent glucose metabolism promotes Mcl-1 synthesis to maintain cell survival and resistance to Bcl-2 inhibition. Cancer Res. 2011;71:5204–5213. doi: 10.1158/0008-5472.CAN-10-4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Wang JM, Chao JR, Chen W, Kuo ML, Yen JJ, Yang-Yen HF. The antiapoptotic gene mcl-1 is up-regulated by the phosphatidylinositol 3-kinase/Akt signaling pathway through a transcription factor complex containing CREB. Mol Cell Biol. 1999;19:6195–6206. doi: 10.1128/mcb.19.9.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castoria G, Lombardi M, Barone MV, Bilancio A, Di Domenico M, Bottero D, et al. Androgen-stimulated DNA synthesis and cytoskeletal changes in fibroblasts by a nontranscriptional receptor action. J Cell Biol. 2003;161:547–556. doi: 10.1083/jcb.200211099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epling-Burnette PK, Liu JH, Catlett-Falcone R, Turkson J, Oshiro M, Kothapalli R, et al. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J Clin Invest. 2001;107:351–362. doi: 10.1172/JCI9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens HJ, Heineman MJ, Theunissen PH, de Jong FH, Evers JL. Androgen, estrogen and progesterone receptor expression in the human uterus during the menstrual cycle. Eur J Obstet Gynecol Reprod Biol. 2001;98:58–65. doi: 10.1016/s0301-2115(00)00554-6. [DOI] [PubMed] [Google Scholar]
- Pelletier G, Luu-The V, Li S, Labrie F. Localization and estrogenic regulation of androgen receptor mRNA expression in the mouse uterus and vagina. J Endocrinol. 2004;180:77–85. doi: 10.1677/joe.0.1800077. [DOI] [PubMed] [Google Scholar]
- Weihua Z, Ekman J, Almkvist A, Saji S, Wang L, Warner M, et al. Involvement of androgen receptor in 17beta-estradiol-induced cell proliferation in rat uterus. Biol Reprod. 2002;67:616–623. doi: 10.1095/biolreprod67.2.616. [DOI] [PubMed] [Google Scholar]
- Sefton EC, Qiang W, Serna V, Kurita T, Wei JJ, Chakravarti D, et al. MK-2206, an AKT inhibitor, promotes caspase-independent cell death and inhibits leiomyoma growth. Endocrinology. 2013;154:4046–4057. doi: 10.1210/en.2013-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Liu L, Xie N, Xue H, Fazli L, Buttyan R, et al. Expression and function of the progesterone receptor in human prostate stroma provide novel insights to cell proliferation control. J Clin Endocrinol Metab. 2013;98:2887–2896. doi: 10.1210/jc.2012-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson SR, Zhang X, Dumpit R, Coleman I, Lakely B, Roudier M, et al. Characterization of osteoblastic and osteolytic proteins in prostate cancer bone metastases. Prostate. 2013;73:932–940. doi: 10.1002/pros.22639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.