Abstract
BH3 interacting-domain death agonist (Bid) is a BH3-only pro-apoptotic member of the Bcl-2 family of proteins. Its function in apoptosis is associated with the proteolytic cleavage to the truncated form tBid, mainly by caspase-8. tBid translocates to mitochondria and assists Bax and Bak in induction of apoptosis. c-Jun N-terminal kinase (JNK)-dependent alternative processing of Bid to jBid was also reported. We have previously shown that the folate stress enzyme 10-formyltetrahydrofolate dehydrogenase (ALDH1L1) activates JNK1 and JNK2 in cancer cells as a pro-apoptotic response. Here we report that in PC-3 prostate cancer cells, JNK1/2 phosphorylate Bid at Thr59 within the caspase cleavage site in response to ALDH1L1. In vitro, all three JNK isoforms, JNK 1–3, phosphorylated Thr59 of Bid with JNK1 being the least active. Thr59 phosphorylation protected Bid from cleavage by caspase-8, resulting in strong accumulation of the full-length protein and its translocation to mitochondria. Interestingly, although we did not observe jBid in response to ALDH1L1 in PC-3 cells, transient expression of Bid mutants lacking the caspase-8 cleavage site resulted in strong accumulation of jBid. Of note, a T59D mutant mimicking constitutive phosphorylation revealed more profound cleavage of Bid to jBid. JNK-driven Bid accumulation had a pro-apoptotic effect in our study: small interfering RNA silencing of either JNK1/2 or Bid prevented Bid phosphorylation and accumulation, and rescued ALDH1L1-expressing cells. As full-length Bid is a weaker apoptogen than tBid, we propose that the phosphorylation of Bid by JNKs, followed by the accumulation of the full-length protein, delays attainment of apoptosis, and allows the cell to evaluate the stress and make a decision regarding the response strategy. This mechanism perhaps can be modified by the alternative cleavage of phospho-T59 Bid to jBid at some conditions.
BH3 interacting-domain death agonist (Bid), a member of BH3-only group of proteins in the Bcl-2 family, functions as a sensor of cellular damage and activator of pro-apoptotic Bax and Bak.1, 2 Bid is a 23 kDa protein localized primarily in the cytosol, but upon apoptotic stimuli it is cleaved to yield a truncated 15 kDa C-terminal fragment tBid. tBid translocates to the mitochondrial membrane, where it interacts with Bax and Bak, enhancing their oligomerization and leading to outer membrane permeabilization, loss of membrane potential and release of mitochondrial apoptogens.3, 4 The canonical example of the activation of Bid cleavage is the FAS-mediated apoptosis, and Bid is viewed as the key molecule in the integration of death receptor and mitochondrial apoptotic pathways.5, 6 The interaction of tBid with Bax or Bak proceeds through the BH3 domain of Bid and occurs only after the protein is localized to mitochondria.7 In the full-length Bid, the BH3 domain can be masked by the N-terminal portion of the protein through the interaction with an α-helical BH-3-like region, the BH3-B domain.5, 8 The caspase-8 cleavage in the middle of the large flexible loop connecting the BH3 and BH3-B domains leads to structural rearrangements of the C-terminal portion of Bid enabling its insertion into mitochondrial membrane.9 The dissociation of the N-terminal fragment in the presence of the mitochondrial membrane and conformational changes of tBid molecule make the BH3 domain accessible for Bax or Bak.10 Other proteolytic enzymes can cleave Bid within the loop but caspase-8 appears to be a major factor generating tBid.8 Full-length Bid can also translocate to mitochondria and induce apoptosis11, 12, 13, 14 but its pro-apoptotic activity is weaker than the activity of tBid.15 It has been hypothesized that in contrast to tBid, the conformational changes enabling the translocation of full-length Bid to mitochondria are reversible.9
Several studies have also indicated the cleavage-independent pro-survival function of Bid in S-phase checkpoint and highlighted the regulation of Bid by phosphorylation at several residues.16, 17 Thus, ATM/ATR protein kinases can phosphorylate Bid at Ser61, Ser64 and Ser78, which protects from caspase-8 cleavage.17 In response to DNA damage, Bid is phosphorylated by ATM protein kinase and translocates to the nucleus to contribute to the decision of cell fate.16, 17 Interestingly, the ablation of phosphorylation at Ser61 and Ser78 ATM sites caused accumulation of full-length Bid in the mitochondria of hematopoetic stem cells and increased cellular proliferation.18 Furthermore, the phosphorylation of murine Bid at Thr58, Ser61 and Ser64 near the caspase-8 cleavage site by casein kinase I and II protected the protein from cleavage, thus making it less active towards the induction of apoptosis.19 Moreover, the pro-survival function of Bid was suggested by the finding that its loss inhibited tumorigenesis of T cells.20 Overall, phosphorylation of Bid can serve as a switch between the pro-apoptotic and pro-survival functions of the protein.
Although phosphorylation of Bid by c-Jun N-terminal kinase (JNK) has not been demonstrated so far, it has been reported that the alternative processing of Bid, which generates jBid, is JNK-dependent.21 Interestingly, the accumulation of full-length Bid and its translocation to mitochondria was observed in HeLa cells in response to staurosporine,22 a known JNK activator.23 Tight relationships between JNK and Bid have been also demonstrated in mouse models of TNFα-induced liver injury.24 This study indicated that Bid is downstream of JNK in TNFα-induced apoptosis and the pro-apoptotic activity of JNK2 is mainly mediated by Bid. Here we report that in PC-3 cells, JNK1/2 phosphorylate Bid at Thr59 in response to folate stress enzyme 10-formyltetrahydrofolate dehydrogenase (ALDH1L1), thus protecting Bid from caspase-8 cleavage. This leads to apoptosis owing to a strong accumulation and mitochondrial translocation of full-length Bid.
Results
Expression of ALDH1L1 in PC-3 cells results in strong elevation of Bid as a pro-apoptotic response
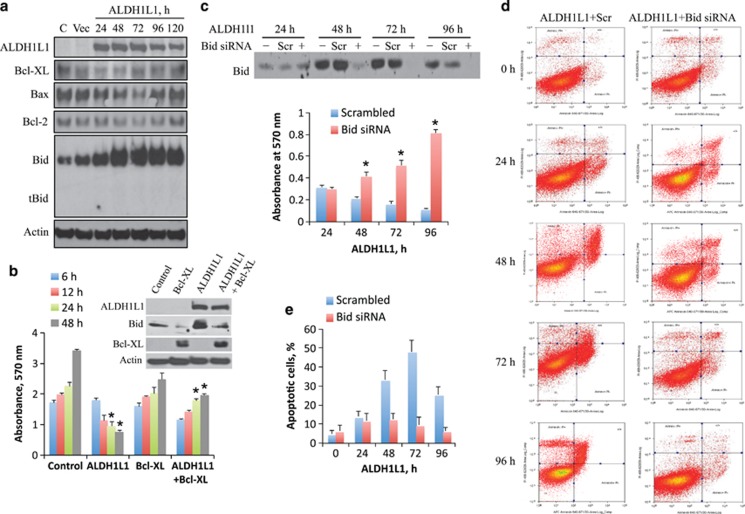
We have previously demonstrated that expression of ALDH1L1 in PC-3 cells induces apoptosis.25 In the present study we observed dramatic and specific elevation of Bid in response to ALDH1L1, whereas levels of several other members of Bcl-2 family were not significantly changed (Figure 1a). Of note, we did not observe tBid formation in these experiments (Figure 1a). We have further investigated whether Bid elevation in response to ALDH1L1 is a pro-apoptotic or pro-survival event. Co-expression of ALDH1L1 with Bcl-XL, which counteracts apoptotic effect of Bid,26 prevented Bid accumulation and protected cells from ALDH1L1-induced cytotoxicity (Figure 1b). Using small interfering RNA (siRNA), we have successfully silenced Bid in PC-3 cells, which prevented its elevation upon ALDH1L1 expression (Figure 1c, inset). MTT assays have confirmed that the elevation of Bid is a pro-death event in ALDH1L1-induced cytotoxicity: the silencing of Bid rescued ALDH1L1-expressing cells (Figure 1c). In agreement with the pro-apoptotic function of Bid, its silencing inhibited ALDH1L1-induced apoptosis (Figures 1d and e).
Figure 1.
PC-3 cells strongly elevate Bid as a pro-apoptotic response to ALDH1L1. (a) Levels of Bcl-2 family proteins in PC-3 cells transiently expressing ALDH1L1. (b) Bcl-XL prevents Bid accumulation and rescues cells from ALDH1L1-induced cell death (MTT assays; inset, Western blot assays). siRNA silencing of Bid rescues PC-3 cells from ALDH1L1-induced cytotoxicity (c) by preventing apoptosis (d and e). Number of apoptotic cells (e) was calculated from annexin V assays shown in (d); average of two independent experiments is shown. MTT assays (b and c) were performed using six replicates for each time point. Statistically significant differences within each time point (b and c) between ALDH1L1-transfected and ALDH1L1/Bcl-XL or ALDH1L1/Bid siRNA-transfected cells are indicated by (*); P<0.0001 (48 h in b and 72 h and 96 h in c); P<0.05 (24 h in b and 48 h in c)
Elevation of Bid in response to ALDH1L1 is associated with the prevention of its degradation and is JNK-dependent
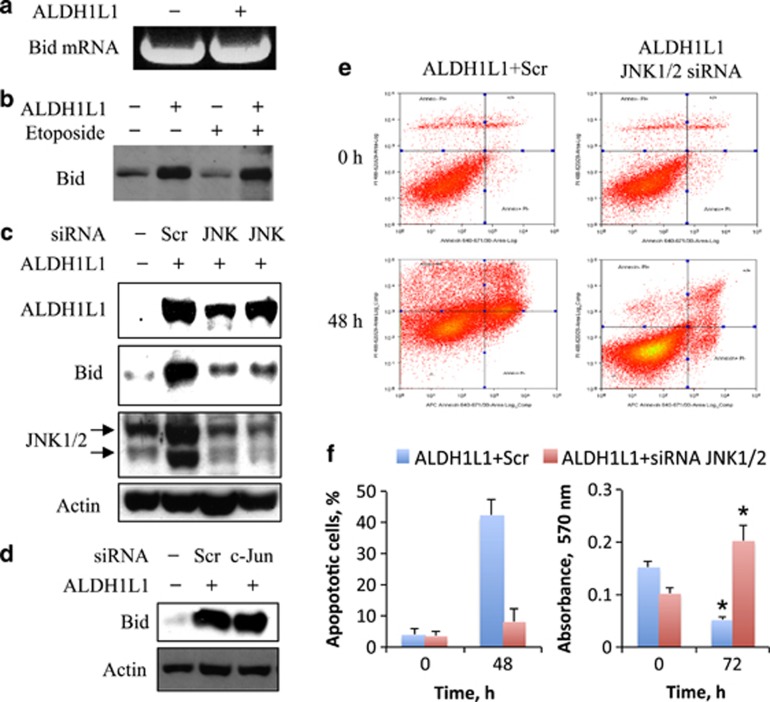
Levels of Bid mRNA evaluated by RT-PCR were not changed after expression of ALDH1L1 in PC-3 cells (Figure 2a), whereas at the same time Bid protein was not degraded in response to etoposide treatment (Figure 2b). These experiments suggested that Bid accumulation was likely due to the decreased protein degradation. ALDH1L1-induced apoptosis in PC-3 cells requires the activation of JNK/c-Jun pathway.25 Therefore, we evaluated whether c-Jun or JNKs are required for Bid accumulation in response to ALDH1L1. The simultaneous siRNA knockdown of JNK1/2 abrogated Bid accumulation in response to ALDH1L1 (Figure 2c). In contrast, siRNA silencing of c-Jun did not prevent Bid elevation (Figure 2d; in this experiment, we have reanalyzed samples generated in our previous study;25 the efficiency of c-Jun silencing is shown in that publication). These data indicated that the effect of ALDH1L1 on Bid requires JNKs but not c-Jun. In agreement with the role of JNKs in mediating ALDH1L1 effects, the silencing of JNK1/2 inhibited ALDH1L1-induced apoptosis and rescued cells (Figures 2e and f), further indicating that the JNK-dependent accumulation of Bid is a pro-apoptotic event.
Figure 2.
Elevation of Bid in response to ALDH1L1 is associated with JNK-dependent protection from degradation. (a) Levels of Bid mRNA are not changed in response to ALDH1L1. (b) Bid is not degraded upon etoposide treatment in ALDH1L1-expressing PC-3 cells. siRNA silencing of JNK1/2 (c), but not the canonical JNK1/2 downstream target c-Jun (d), prevents Bid accumulation in response to ALDH1L1 (in this experiment, samples generated in the previous study25 were reanalyzed; the efficiency of c-Jun silencing is shown in that publication). (e and f) JNK1/2 silencing by siRNA inhibits ALDH1L1-induced apoptosis and protects PC-3 cells from the ALDH1L1 cytotoxic effect. Average of two independent experiments is shown for apoptotic cells; MTT assays were performed with six replicates. Difference between numbers of live cells at 72 h is highly statistically significant (P<0.0001, indicated by *)
JNKs phosphorylate Bid at the caspase-8 cleavage site preventing its degradation
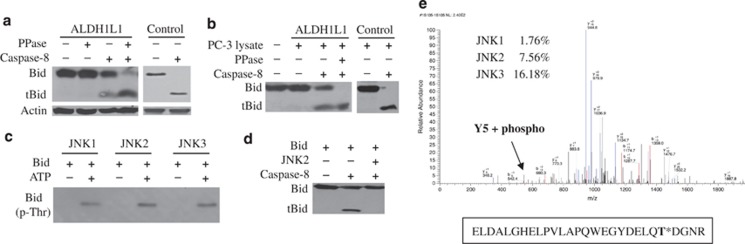
We hypothesized that JNKs promote Bid accumulation by the phosphorylation at the caspase-8 cleavage site that prevents Bid degradation. To study this mechanism, we treated lysate of ALDH1L1-expressing cells, accumulating high levels of Bid, with recombinant caspase-8. We observed that only small fraction of Bid was cleaved to tBid (Figure 3a), the finding indicating that most of the protein is protected from degradation. Pretreatment of the lysate with λ-phosphatase allowed more complete cleavage yielding an intense band of tBid (Figure 3a). In contrast, in cells transfected with ‘empty' vector Bid was fully susceptible to caspase-8 cleavage (Figure 3a, Control). In a similar experiment, recombinant Bid was pretreated with the lysate from ALDH1L1-expressing PC-3 cells, which have activated JNKs.25 This pretreatment partially protected Bid cleavage by caspase-8, but this protective effect was eliminated after pretreatment with λ-phosphatase (Figure 3b). Of note, there was no protection from cleavage in the case of Bid pretreatment with lysate from vector-transfected cells (Figure 3b, Control). We suggested that JNK phosphorylates Bid at Thr59 within the caspase-8 cleavage site. In support of this hypothesis, the phosphotylation of Bid at a threonine residue by purified recombinant JNKs was demonstrated using phospho-threonine antibody (Figure 3c). In agreement with our previous findings, JNK-phosphorylated Bid was not cleaved by caspase-8 (Figure 3d). LC/MS-MS analysis of the tryptic digest of Bid after in vitro kinase assays with JNK1-3 revealed that all three kinases phosphorylate Bid at Thr59 (Figure 3e). The abundance of the phosphopeptide indicated that JNK3 is the most capable of three kinases to use Bid as a substrate, whereas JNK1 was the least efficient (Figure 3e).
Figure 3.
Bid is protected from caspase-8 cleavage to tBid by phosphorylation at a threonine residue. (a) Cleavage of endogenous Bid (the lysate of PC-3 cells expressing ALDH1L1) to tBid by recombinant caspase-8 before and after pretreatment with λ protein phosphatase (PPase). Control, lysate from PC-3 cells transfected with ‘empty' vector. (b) Cleavage of recombinant Bid exposed to lysate from ALDH1L1-expressing PC-3 cells by caspase-8 with and without PPase pretreatment. Control, recombinant Bid exposed to lysate from PC-3 cells transfected with ‘empty' vector. (c) In vitro phosphorylation of recombinant Bid by JNK1-3 (band corresponding to Bid is detected by phospho-threonine antibody). (d) Cleavage of recombinant Bid before and after in vitro kinase reaction in the presence of JNK2. (e) Mass-spectrometry identification of phosphorylated Bid peptides after in vitro kinase assay with JNK1-3 (numbers on the plot indicate the abundance of the phosphopeptide upon phosphorylation with each JNK)
Identification of Bid phosphorylation by specific antibody
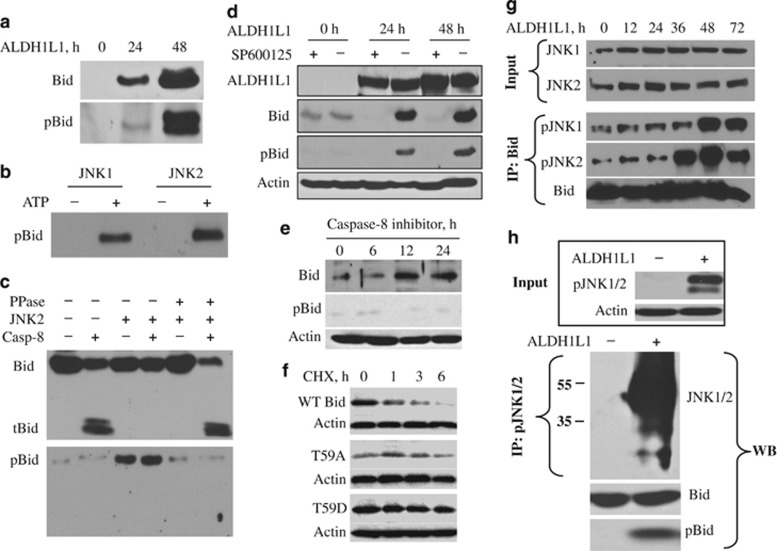
To further confirm Bid phosphorylation by JNK and to get insight into the cellular role of this process, we have generated a specific antibody recognizing Bid phosphorylated at Thr59. We have first confirmed that ALDH1L1 induces Bid phosphorylation at Thr59 (Figure 4a). We have also demonstrated that this antibody recognizes phospho-Bid after phosphorylation by JNK1 and JNK2 in an in vitro kinase assay (Figure 4b). JNK3 was excluded from these experiments because its expression is limited to brain, heart and testis.27, 28 Using the phospho-Bid-specific antibody we have shown that only phospho-Bid was protected from cleavage by caspase-8, and the effect is abrogated by the treatment with λ-phosphatase (Figure 4c). We have previously reported that treatment with the JNK-specific inhibitor SP600125 protects ALDH1L1-expressing cells from apoptosis.25, 29 Likewise, this treatment prevented Bid phosphorylation at Thr59 and its accumulation (Figure 4d). In agreement with the proposed mechanism of Bid accumulation, through the inactivation of caspase-8 cleavage, the treatment of PC-3 cells with caspase-8 inhibitor resulted in Bid accumulation in the absence of phosphorylation (Figure 4e). Further support of caspase-8-dependent degradation of Bid and the role of T59 in this process came from the evaluation of half-life of wild-type Bid and its T59A and T59D mutants resistant to the caspase-8 cleavage (these mutants will be discussed below). A strong decline in levels of transiently expressed wild-type Bid upon treatment of PC-3 cell with 50 μM cycloheximide was seen as early as 1 h with almost complete disappearance of the protein after 6 h (Figure 4f). In contrast, both mutants have shown much slower degradation rate (Figure 4f).
Figure 4.
JNK1/2 phosphorylate Bid at Thr59 both in vitro and in vivo. (a) Levels of Bid and p-Bid (Thr59) in PC-3 cells in response to ALDH1L1. (b) Phosphorylation of Bid at Thr59 by JNK1 and JNK2 (in vitro kinase assay). (c) The phosphorylation of recombinant Bid by JNK2 (in vitro kinase assay) prevents its cleavage by caspase-8; the treatment of p-Bid (Thr59) with λ-phosphatase dephosphorylates Thr59 and enables the cleavage by caspase-8. (d) Treatment of PC-3 cells with specific JNK inhibitor SP600125 prevents both Bid phosphorylation at Thr59 and its accumulation in response to ALDH1L1. (e) Cell-permeable caspase-8 inhibitor increases levels of Bid in the absence of the protein phosphorylation. (f) Levels of transiently expressed wild-type (WT) Bid and its mutants in PC-3 cells treated with 50 μM cycloheximide (CHX). (g) Co-immunoprecipitation of activated JNK1 and JNK2 (pJNK1 and pJNK2) using Bid-specific antibody at different times of ALDH1L1 expression. (h) Activated JNK1/2, pulled down from ALDH1L1-expressing PC-3 cells using phospho-JNK-specific antibody, phosphorylates recombinant Bid at Thr59
In ALDH1L1-expressing cells activated (phosphorylated) JNK1 and JNK2 co-immunoprecipitate with Bid indicating the direct interaction between these proteins (Figure 4g). We have directly demonstrated that activated (phosphorylated) endogenous JNKs phosphorylate Bid. In these experiments, we have pulled down JNK1/2 from the lysate of ALDH1L1-deficient or ALDH1L1-expressing PC-3 cells using JNK1/2-specific antibody and used it in the kinase assay with recombinant Bid. We observed that only JNK from ALDH1L1-expressing cells (phosphorylated kinases) produce PT59 Bid (Figure 4h).
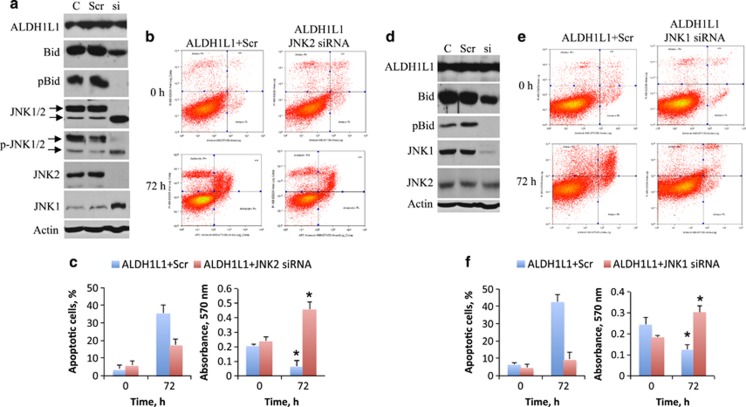
Both JNK1 and JNK2 contribute to Bid phosphorylation
Simultaneous silencing of JNK1 and 2 by siRNA prevented Bid accumulation in response to ALDH1L1 and inhibited apoptosis (Figure 2). We further observed that the silencing of either kinase prevented phosphorylation of Bid at Thr59 and decreased Bid accumulation (Figures 5a and d). In agreement with a pro-apoptotic function of Bid, in these experiments we also observed partial suppression of apoptosis and cell rescue (Figures 5b, c, e and f). However, stronger effects were observed upon silencing of JNK2 than JNK1 (Figure 5). Interestingly, a significant increase in JNK1 levels was seen in JNK2-silenced cells (Figure 5a), whereas JNK2 was not elevated upon JNK1 silencing (Figure 5d). This phenomenon could be an indication of a compensatory effect in response to the loss of JNK2. This effect, however, was unable to restore Bid phosphorylation and apoptosis. Overall, these findings indicate a more essential role for JNK2 with regard to Bid phosphorylation and apoptosis in response to ALDH1L1.
Figure 5.
Silencing of either JNK1 or JNK2 abolishes Bid phosphorylation in response to ALDH1L1 preventing both Bid accumulation and antiproliferative effects. (a) Levels of JNK1/2, Bid and their phosphorylated forms in PC-3 cells with silenced JNK2 upon ALDH1L1 expression. Silencing of JNK2 prevents ALDH1L1-induced cytotoxicity (b and c) and rescues PC-3 cells (c). Number of apoptotic cells (c) was calculated from annexin V assays shown in (b). (d) Levels of JNK1/2, Bid and their phosphorylated forms in PC-3 cells with silenced JNK1 upon ALDH1L1 expression. Silencing of JNK1 prevents ALDH1L1-induced cytotoxicity (e and f) and rescues PC-3 cells (f). Number of apoptotic cells in (f) was calculated from annexin V assays shown in (e). Differences between number of live cells at 72 h were highly statistically significant (indicated by *, P<0.0001 and <0.001 for c and f, respectively). Average of two independent experiments is shown for apoptotic cells; all MTT assays were performed with six replicates
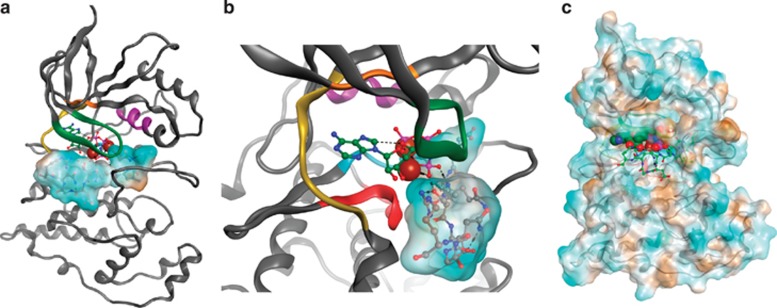
Computational docking of Bid peptide to JNK2
Using computer simulations, we modeled the interaction of a 9-mer Bid peptide (D55-R63, from PDB entry 2BID30) into JNK2 (PDB entry 3E7O31) using molecular docking. Simulations were focused on the ATP site and allowed for induced fit of JNK2. Although the Bid peptide has the freedom in the simulations to adopt any pose and orientation, the best predicted pose (final total energy −7.1 kcal) indicated a clear interaction between T59 and the terminal (gamma) phosphate of the ATP analog ANP (Figure 6). The 9-mer Bid peptide also interacted with the G-loop and the HRD domain, that is consistent with substrate recognition and subsequent catalytic chemistry. These other interactions, likely critical for substrate recognition, were hydrogen bonds between: Bid D55 and JNK2 residues R107/K191/N194, and Mg2+; Bid L57 (backbone) and V225/T226; Bid Q58 and K191/S193/T226; and Bid G61 (backbone) and R63 to C154.
Figure 6.
Theoretical molecular model of JNK2 substrate recognition of the Bid peptide. (a) Ribbon diagram of JNK2-ANP homology model with docked Bid peptide. The Bid peptide is rendered as a hydrophobic surface with brown being the most hydrophobic and blue being hydrophilic. Ribbon is colored according to kinase domains with green as the G-loop, HRD in red, hinge in yellow and alphaC in purple. (b) Close-up view of (a) highlighting the bonding of ANP with the Bid peptide. (c) JNK2 surface rendering colored according to hydrophobicity (the Bid peptide, sticks; ANP space fill mode)
Full-length Bid translocates to mitochondria of PC-3 cells
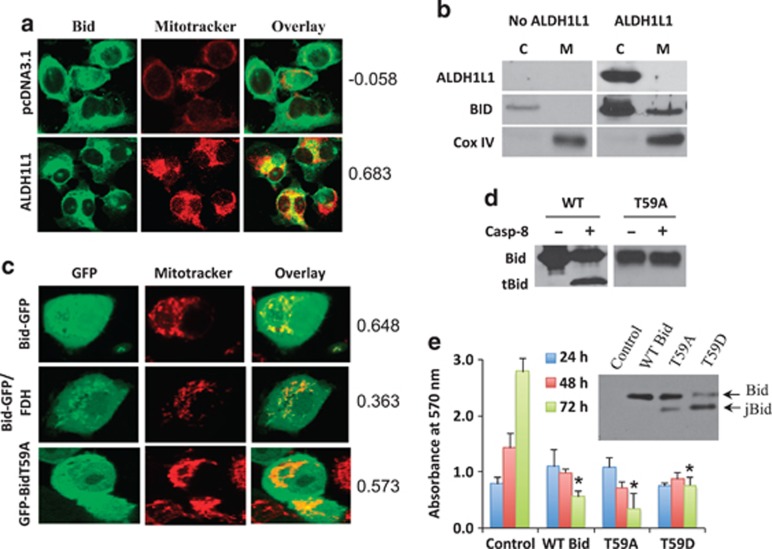
We have observed that in response to ALDH1L1, endogenous Bid accumulates in the cytosol and translocates to mitochondria (Figure 7a, Pearson's correlation coefficient was 0.683 for ALDH1L1-expressing cells versus −0.058 for control cells). Western blot assays revealed that in both compartments Bid was present as the full-length protein and tBid was not detected (Figure 7b). To investigate whether full-length Bid translocates to mitochondria of PC-3 cells simply because of its elevation, we expressed GFP fusion of wild-type and T59A mutant Bid by transient transfection. Confocal microscopy has shown that both proteins colocalized with mitochondria (Figure 7c, Pearson's correlation coefficient 0.648 and 0.573, respectively). These experiments confirmed the translocation of full-length Bid to mitochondria, as the introduction of the T59A mutation prevented Bid cleavage by caspase-8 (Figure 7d). They also indicated that the phosphorylation at T59 is not required for the mitochondrial localization. Co-expression of ALDH1L1 and GFP-Bid did not change significantly in the distribution of the fluorescent construct between cytosol and mitochondria (Figure 7c). However, the translocation of GFP-Bid in this case should be accompanied by the translocation of endogenous Bid, which could compete with the fluorescent construct preventing its stronger accumulation. Both wild-type Bid and the T59A mutant induced cell death upon expression in PC-3 cell line (Figure 7e). Interestingly, upon the expression of T59A or T59D mutants, the accumulation of jBid was observed, which was much stronger in the case of T59D mutant mimicking constitutive phosphorylation (Figure 7e, inset). Of note, at a later time point (72 h) T59D mutant produced lesser number of dead cell, though this difference was not statistically significant (Figure 7e). It could be speculated, however, that such effect, if real, is a result of higher levels of jBid than full-length Bid upon T59D mutant expression.
Figure 7.
Full-length Bid translocates to mitochondria of PC-3 cells and induces cell death. (a) Confocal microscopy shows colocalization of endogenous Bid in mitochondria in response to ALDH1L1. (b) Western blot analysis indicates the accumulation of full-length endogenous Bid in both cytosol and mitochondria. (c) Transiently expressed fusion of full-length Bid with GFP is localized in cytosol and mitochondria (wild-type or caspase-8 non-cleavable T59A mutant tagged with GFP were expressed in these experiments). (d) Caspase-8 does not cleave T59A Bid mutant in contrast to the wild-type (WT) protein. (e) Expression of wild-type (WT) Bid as well as T59A or T59D mutants induces cell death (inset shows levels of respective proteins). In experiments described in (d) and (e) non-tagged Bid proteins were expressed. Difference for live-cell count at 72 h between control and Bid expressing cells was highly statistically significant for all three proteins (*P <0.0001). Numbers in (a) and (c) indicate Pearson's correlation coefficient
Phosphorylation and accumulation of full-length Bid is a common phenomenon in cancer cell lines
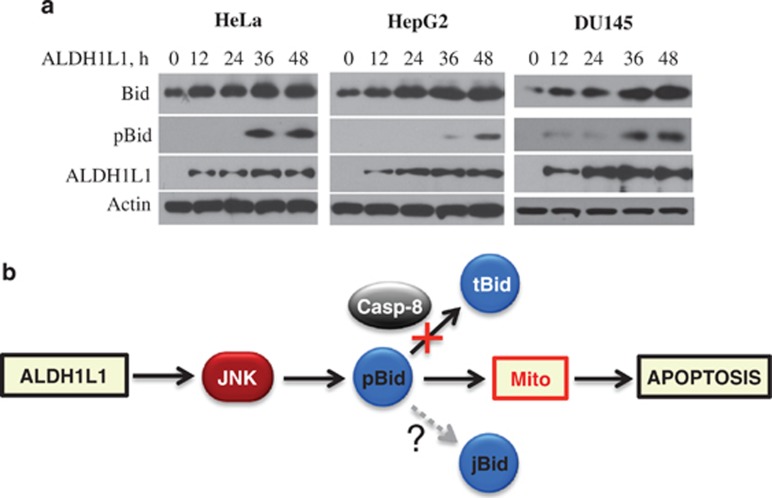
To investigate whether the phosphorylation of Bid at Thr59 and the protein accumulation is a common response to ALDH1L1, we have screened a panel of cancer cell lines transfected for ALDH1L1 expression. We observed that this phenomenon is not limited to PC-3 cell lines or prostate cancer cell lines: HeLa, HepG2 and DU145 demonstrated Bid phosphorylation and accumulation of the full-length protein in these experiments (Figure 8a). Of note, in several cell lines, including A549, H1299, Tsu-Pr, and HCT116 the response in terms of Bid phosphorylation and elevation was not evident (data not shown).
Figure 8.
(a) Levels of full-length Bid and pT59 Bid assessed by western blot assays in cancer cells in response to ALDH1L1. (b) Proposed mechanism of JNK-mediated Bid accumulation. In response to certain stimuli (ALDH1L1 folate stress enzyme in this case) the activation of JNK1/2 results in Bid phosphorylation at T59 within the caspase-8 cleavage site. This prevents cleavage of Bid to tBid and leads to the accumulation of full-length Bid. Full-length Bid translocates to mitochondria and induces apoptosis. It is proposed that the phosphorylation at T59 also allows the cleavage to jBid by yet unidentified proteases
Discussion
Pro-apoptotic Bid integrates intrinsic and extrinsic apoptotic pathways and its function is associated with the mitochondrial translocation of the truncated protein tBid.5 In our study we observed a remarkably strong accumulation of full-length Bid in both cytosol and mitochondria in response to folate stress enzyme ALDH1L1. Such pronounced effect should be associated with either a dramatic increase in protein production or diminished protein degradation. The lack of changes in Bid mRNA in our experiments suggested that the increase in Bid was unlikely caused by altered transcription or translation but pointed towards protein degradation as a major mechanism increasing Bid. Bid degradation, however, is not well studied. Though Bid can be cleaved by several proteases, this process was mainly investigated from the angle of functional activation of the protein.32, 33, 34, 35 The finding that Bid can be cleaved by cathepsin33 suggests a possibility for its lysosomal degradation. This, however, can be the nonspecific result of the lysosome damage and release of lysosomal proteases.36, 37 At the same time, the degradation of Bid through the proteasomal pathway has been demonstrated.38 The ubiquitin ligase Itch promotes the ubiquitylation and degradation of the truncated C-terminal portion of Bid, thus mediating the anti-apoptotic activity of epidermal growth factor.39 Itch did not interact with full-length Bid, suggesting that it cannot be degraded through this pathway.39 Of note, the N-terminal fragment of Bid generated by caspase cleavage can be also degraded in an ubiquitylation-dependent manner.40
These studies indicate the importance of Bid cleavage by caspase-8 as the initial step in the protein degradation,38, 39, 40 and the protection from caspase cleavage could be expected to prevent Bid degradation. We have experimentally confirmed such mechanism: treatment with a cell-permeable caspase-8 inhibitor elevated Bid in PC-3 cells. Interestingly, the caspase-8 cleavage site overlaps with the CK1/2 phosphorylation site (Thr59), and its phosphorylation protects Bid from cleavage.19, 41 We have further established Bid as a substrate for JNKs and demonstrated that the phosphorylation regulates Bid accumulation. In cancer cells, the JNK activation by ALDH1L1 leads to phosphorylation of either the canonical JNK target c-Jun or the less common target p53.25, 29 The ALDH1L1-induced phosphorylation of p53 is accomplished by JNK2, which is activated through phosphorylation by JNK1.29 Thus, both kinases are required in the process. In the present study, in vitro phosphorylation of recombinant Bid by either JNK1 or JNK2 was possible, but in cells, silencing of any of the two kinases prevented this process. This observation implies that two kinases work in concert in Bid phosphorylation. Mechanisms of such cooperation could be associated with a cascade similar to that observed in p53 phosphorylation or could be attributed to a threshold effect. Of note, the phosphorylation site for JNK in Bid molecule, Thr59, is the same as for CK1/2.19 This site locates in the middle of a long unstructured loop connecting N- and C-terminal domains of Bid, a conformational arrangement suggesting easy accessibility to a kinase catalytic center. In agreement with this notion, our docking simulations indicated a good fit for the part of the loop adjacent to T59 into JNK2 peptide-binding site with the threonine side chain being within hydrogen bond distance to the γ-terminal phosphate of ATP (Figure 6). These data also implicated Bid residues D55 and Q58 upstream of T59 as being important in JNK recognition of Bid.
Both pro-survival and pro-apoptotic Bcl-2 proteins are known targets for JNKs. Depending on the targeted protein, the phosphorylation can produce either pro-apoptotic or pro-survival effect. Thus, the phosphorylation of anti-apoptotic Bcl-2, Bcl-XL or Mcl-1 prevents their pro-survival functions, whereas phosphorylation of pro-apoptotic Bad disables it as a death-mediating protein.42, 43, 44, 45 In contrast to Bad, the phosphorylation of pro-apoptotic Bim by JNK results in its release from microtubules and enables its function in mediating apoptosis.46 Of note, the phosphorylation of Bad by JNK also increases its mitochondrial localization, thus enhancing apoptosis.47 So far, phosphorylation of Bid by JNK has not been reported. However, several studies indicated that the activation of both JNK and Bid is involved in TNFα-induced apoptosis.48, 49 For example, in TNFα-induced liver injury, Bid is downstream of JNK, and pro-apoptotic activity of JNKs is mediated by Bid.24, 50 Furthermore, the activation of JNK-dependent Bid processing has been reported.21 Thus, upon TNFα-induced apoptosis, the activation of JNK enables caspase-8-independent cleavage of Bid at amino-acid residue 25 to produce jBid, which translocated to mitochondria and led to apoptosis through the release of Smac/DIABLO, but not cytochrome c.21 More recently, jBid formation was also observed upon silencing of a C2H2-type zinc finger transcription factor in a hepatocellular carcinoma cell line.51
Our study also demonstrated that the accumulation of full-length Bid in the absence of the protein cleavage is required for the pro-apoptotic response. Although the cleavage of Bid to tBid followed by the translocation of tBid to mitochondria is viewed as the main mechanism of pro-apoptotic function, numerous reports have demonstrated that full-length Bid also translocates to mitochondria and induces apoptosis. For example, the accumulation of full-length Bid in mitochondria, leading to apoptosis, was observed in Jurkat cells upon simultaneous activation of Fas-receptor and the inhibition of caspase-8, in neuronal cells as part of the excitotoxic response, or in epithelial cells during anoikis.12, 14, 52 Interestingly, the accumulation of full-length Bid in mitochondria takes place in hematopoetic cells upon DNA damage, preserving the quiescence of stem cells in the bone marrow.18 In line with the latter report, the pro-survival role of Bid in the DNA-damage response appears associated with the full-length protein.16, 17 This function, however, involved the translocation of Bid to the nucleus,16, 17 a process not observed in our studies.
Apoptosis induced by full-length Bid versus tBid is likely to proceed through different mechanisms. In support of this possibility, the release of cytochrome c by tBid is mitochondria permeability transition (MPT)-independent, whereas such release by full-length Bid is MPT-dependent.52, 53, 54, 55 This effect could be associated with the fact that full-length Bid can insert specific lysolipids into the membrane surface, thereby priming mitochondria for the release of apoptogenic factors.56 It is not clear at present what mechanism targets full-length Bid to mitochondria. It is likely that the lipid composition of mitochondrial membrane can affect full-length Bid versus tBid translocation. In particular, phospholipids such as phosphatidic acid and phosphatidyl glycerol induce the accumulation of full-length Bid in mitochondria.57 In further support of the differential effect of lipids on Bid translocation, it has been shown that tBid binds very efficiently to cardiolipin-containing vesicles but full-length Bid was unable of such binding.58 The recruitment of tBID to mitochondria is facilitated by the interaction with mitochondrial carrier homolog 2, an integral membrane protein exposed on the mitochondria surface.59, 60 It is not clear at present whether full-length Bid interacts with this protein or whether Bid phosphorylation changes the conformation of Bid molecule to enable its mitochondrial translocation.
It has been previously suggested that phosphorylation of Bid could be an additional apoptosis-controlling mechanism.19 We hypothesize that the phosphorylation of Bid by JNK followed by the accumulation of the full-length protein is a slower apoptotic response, which allows the cell time to evaluate the stress and make a decision whether the survival is feasible (Figure 8b). It is also possible that the JNK phosphorylation of Bid at Thr59, associated with the inability to produce tBid, drives Bid cleavage to a different site yielding jBid, which would explain JNK dependence of the latter process (schematically depicted in Figure 8b). Interestingly, although we did not observe the accumulation of jBid in response to ALDH1L1, the transient expression of Bid mutants lacking the caspase-8 cleavage site resulted in significant accumulation of jBid (Figure 7e). Of note, the cleavage of Bid to jBid was much more profound in the case of T59D mutant mimicking constitutive phosphorylation than in T59A mutant. Future studies should address the question of whether the phosphorylation of Bid at Thr59 by JNKs is the mechanism of jBid generation and which stress stimuli can evoke such effect.
Materials and Methods
Reagents
MAPK inhibitor (SP600125) was from Sigma (St. Louis, MO, USA). Recombinant human caspase-8 and λ protein phosphatase (PPase) were from R&D Systems (Minneapolis, MN, USA). Active recombinant JNK1/2/3 enzymes were from SignalChem (Richmond, Canada). All chemicals were from Sigma unless otherwise specified. Cell-permeable caspase-8 inhibitor (EMD Millipore, San Diego, CA, USA) was dissolved in DMSO and used at 20 μM.
Cell culture and transfection
Cell media and reagents were purchased from Life Technologies (Carlsbad, CA, USA). All cell lines were obtained from ATCC (Manassas, VA, USA). Transfections were carried out using Neon nucleofection system from Life Technologies. Cell viability was assessed by trypan blue exclusion or by MTT cell proliferation assay using CellTiter 96 kit from Promega (Madison, WI, USA).
Plasmids
pcDNA3-Bcl-XL was a kind gift from Dr Matsuzawa (Sanford-Burnham Medical Institute). Human Bid cDNA was amplified by PCR (corresponding primers are shown in Supplementary Table S1) from liver cDNA library (Clontech Laboratories, Mt. View, CA, USA), cloned into PCR2.1 plasmid (Life Technologies) according to the manufacturer's protocol, and then sub-cloned to pcDNA3.1(−), pRSET-C (both from Life Technologies) and pEGFP-N1 (Clontech) expression vectors. Bid T59A and T59D mutants were generated using QuickChange Mutagenesis Kit (Agilent Technologies, Santa Clara, CA, USA) and primers shown in Supplementary Table S1. All constructs were confirmed by DNA sequencing.
Immunoblot assays
Cells were lysed in RIPA buffer containing protease inhibitor cocktail (Sigma) and phosphatase inhibitors (Roche Applied Bioscience, Indianapolis, IN, USA). Cell lysates were subjected to SDS-PAGE followed by immunoblot with corresponding antibodies. Polyclonal antibodies against JNK1, JNK2, pJNK, Bcl-XL, Bcl-2, BAX, phospho threonine and COX IV were from Cell Signaling Technology (Beverly, MA, USA; all used at 1:1000). Actin monoclonal antibody (1:5000) was from Sigma. ALDH1L1 and Bid were detected using in-house polyclonal antibodies (1:20 000) generated against full-length proteins. Rabbit polyclonal phospho-Bid-specific (pT59) antibody was generated by AnaSpec (Freemont, CA, USA) against ELQ(pT)DGNRSSHSRLG peptide (1:3000). In all experiments, a Hybond TM-ECL nitrocellulose membrane (GE Healthcare Life Sciences, Pittsburg, PA, USA) and Pierce ECL detection kit (Thermo Scientific, Waltham, MA, USA) were used.
siRNA
Stealth siRNA duplexes for JNKs and c-Jun were from Invitrogen. On-TARGET plus hBid siRNA duplexes were from Thermo Scientific. Experiments were performed in PC-3 cells (1.4 × 106) essentially as we previously described for JNK1/2.29 The silencing was performed 6 h after ALDH1L1 transfection using 65 nM siRNA and Lipofectamine RNAiMAX Transfection Reagent (Life Technologies). Cells were collected 24–96 h later, lysed and analyzed by conventional PCR and immunoblot assays for levels of targeted proteins. In all silencing experiments, Scrambled Stealth RNAi was used as a negative control.
Apoptosis assay
Cells were labeled with annexin V and PI using annexin V APC and PI labeling kit (Affymetrix eBioscience, San Diego, CA, USA). All cells (floating and attached) were used in the assay. Flow cytometry was carried out in the MUSC core facility on a Becton Dickinson FACSCalibur. Data analysis was performed using CellQuest and Mod Fit software (Becton Dickinson, Franklin Lakes, NJ, USA). The settings and gates were established daily based on the negative control cells.
Pull-down assays
Cells were washed with ice-cold PBS and lysed in RIPA buffer, supplemented with protease inhibitor cocktail and phosphatase inhibitors, at 4 °C for 30 min. After centrifugation at 14 000 × g for 15 min at 4 °C, supernatant (250 μg of total protein) was pre-cleared with protein G Sepharose 4 fast flow (GE Healthcare Life Sciences) for 1 h, and incubated with Bid-specific antibody (5 μg) overnight and then with 50 μl of protein G Sepharose 4 fast flow for an additional 3 h (all steps were carried out at 4 °C). Beads were pelleted and washed with cold PBS containing 1% NP-40 and 2 mM sodium orthovanadate (three times) and lysis buffer. The pulled down material was eluted with 100 mM Glycine buffer, pH 3.0 and analyzed by SDS-PAGE/immunoblotting.
Subcellular fractionation
Cells (20–100 × 106) were disrupted in hypotonic buffer (10 mM HEPES, pH 7.5, 10 mM NaCl, 1.5 mM MgCl2 1 mM Na3VO4, 4 mM NaF and protease cocktail) using a Dounce homogenizer on ice, then NaCl was added (150 mM final concentration), and homogenates were spun down for 5 min at 700 × g to remove nuclei and cell debris. The supernatant was centrifuged at 13 000 × g to precipitate mitochondria. The cytosolic fraction was obtained after further ultracentrifugation of supernatant at 100 000 × g for 30 min. Equivalent amounts (total protein) of cytosolic and mitochondrial fractions were analyzed by SDS-PAGE/immunoblot.
Confocal microscopy
Cells were seeded in Lab-Tek II Chamber (Nulg Nunc International, Rochester, NY, USA), fixed with 3.7% methanol-free formaldehyde for 10 min, permeabilized with 0.1% Triton X-100 for 5 min and incubated with 10% pre-immune goat serum in PBS for 45 min. Slides were stained with Bid-specific antibody (1 : 500) at 4 oC overnight and then with secondary goat anti-rabbit antibody conjugated with Alexa Fluor 488 (Life Technologies) in dark chamber for 1 h. For live-cell imaging, cells transfected with pEGFP-N1-Bid were grown on MatTek Chamber Slides (MatTek corporation, Ashland, MA, USA). In both approaches, cells were co-stained with MitoTracker Red CMXRos (Life Technologies) according to the manufacturer's instructions. Images were captured using Olympus FV10 laser scanning confocal microscope using identical confocal settings for all samples and processed using Olympus FV10 1.7a viewer (Olympus, Center Valley, CA, USA) and Photoshop CS2 (Adobe Systems, San Jose, CA, USA). Pearson's correlation coefficients were calculated using ImageJ software with the Just Another Co-localization Plugin.
In vitro kinase assays
Kinase reactions were performed in 100 μl of kinase assay buffer (Cell Signaling Technology) using 5 μg activated recombinant JNKs or 500 μl of lysate from ALDH1L1-transfected cells, and 2 μg purified human recombinant Bid. Reaction was initiated by 1 mM ATP. Phosphorylation of Bid at T59 was evaluated by immunoblot assays with a specific antibody after SDS-PAGE. For the LC/MS-MS analysis of phosphorylated Bid peptides, after the kinase reaction with pure JNKs reaction mixture has been subjected to SDS-PAGE. In the case of kinase reaction with cell lysates, Bid was pulled down on Ni-beads and eluted with 250 mM imidazole prior to SDS-PAGE.
Bid expression and purification
E. coli BL21 (DE3) cells transformed with pRSET/Bid expression construct were grown on an LB/ampicillin plate overnight, one colony was transferred into 5 ml LB medium with 50 μg/ml ampicillin, and the culture was grown overnight at 37 °C with shaking. The 500 ml of medium containing ampicillin was inoculated with the overnight culture and incubated at 37 °C with shaking until OD600 reached 0.8 (about 6 h) followed by induction with IPTG (0.5 mM final concentration). Three hours after induction, the cells were harvested by centrifugation (5000 × g, 10 min), resuspended in 10 ml 50 mM NaH2PO4 buffer, pH 8.0 containing 300 mM NaCl and incubated for 30 min with 0.2 mg/ml lysozyme at 4 °C. The suspension was chilled on ice, sonicated (six 30 s pulses) and spun down at 13 000 × g for 20 min). The supernatant was loaded onto PrepEase Ni–NTA high specificity agarose column (USB Corporation, Cleveland, OH, USA) equilibrated with 20 mM phosphate buffer, pH 8.0 containing 300 mM NaCl, the column was washed with the same buffer, and then with the buffer containing 20 mM imidazole. Recombinant Bid was eluted in 250 mM imidazole, pH 8.0 and further purified on Sephacryl S300 column followed by FPLC on Milli-Q column (both from GE Healthcare Life Sciences).
LC/MS-MS
After SDS-PAGE, Bid was excised from gel and processed for tryptic digestion according to standard procedures. The extracted peptides were dried down to about 1 μl, reconstituted in 7 μl of mobile phase A (95% water, 5% acetonitrile, and 0.2% formic acid) and loaded on a trap column. Eluted peptides were separated on a 75 μM × 20 cm fused-silica column packed in-house with C18 reversed-phase resin (YMC-ODS-AQ; 5-μm particles; 200 Å pore; Waters, Milford, MA, USA) using an acetonitrile gradient of 5–50% in 120 min containing 0.2% formic acid on a LC Packings U3000 nano LC system at flow rate of 200 nl/min. The sample was introduced via nano-electrospray ionization to a hybrid dual-pressure linear ion trap-orbitrap mass spectrometer (Thermo Fisher Scientific). Mass spectra were acquired in data-dependent mode using a TOP20 method, which acquires one FTMS survey MS scan in the mass range of m/z 400–2000 followed by tandem mass spectra (MS/MS) of the 20 most intense ions in the LTQ Velos. The automatic gain control target value in the Orbitrap was 106 for the survey MS scan at a resolution of 60 000 at m/z 400. Fragmentation in the LTQ was performed by collision induced dissociation with a target value of 5000 ions and a threshold of 500 counts. Dynamic exclusion was enabled with a repeat count of 3, repeat duration of 30 s, and exclusion duration of 180 s.
Database searching and data filtering
The MS/MS spectra were analyzed using MASCOT (Version 2.3.02) and SEQUEST search algorithms utilizing Proteome Discoverer 1.3 (Thermo Scientific) and the human IPI protein sequence database and a custom built database containing only Bid. For both algorithms, the search parameters allowed for two missed cleavages, precursor mass tolerances of ±10 ppm, fragment mass tolerances ±0.8 Da, and dynamic modifications of phosphorylation on Ser, Thr, Tyr, oxidation of Met and carbamidomethyl on cysteine. Results were filtered by Sequest score versus charge state (≥1.5, 2.0, 2.5 for +1, +2, and +3 ions) with Mascot significance threshold of ≥0.05 medium confidence peptides and the false discovery rate set at 1% of the peptide level. Confirmation of the localization of phosphorylation sites was performed using Phospho RS in Proteome Discoverer. All confirmed sites of phosphorylation contained a localization probability >75% and were reviewed manually.
Estimated percentages of phosphorylation occupancy were determined by calculating the ratio of the sum of the areas under the curve (AUC) for all phosphorylated T59 peptides detected to the sum of the AUCs for all peptides containing the T59 amino acid in the same run: (% Occupancy =((AUC pT59)/(AUC pT59+AUC T59)) × 100).
This approach provides an estimate of the percent phosphorylation occupancy within a single sample or LC-MS/MS analysis. The estimated percent occupancy of T59 BID phosphorylation was calculated in this manner after treatment with JNK1, JNK2 and JNK3. Each sample was analyzed separately; the estimated percent occupancies were calculated based on the individual runs; internal control was not included in the samples. An unmodified BID peptide present in all samples served as a quality control across experiments. Run-to-run variability would not affect the estimated percent occupancies calculated in this manner. Although the phosphorylated versions of the T59 peptides may have lower ionization efficiencies compared with the non-phosphorylated counterpart, this deviation would be consistent across all samples and occupancy calculations.
Molecular modeling
Modeling, simulations and visualizations were performed using Molecular Operating Environment Version 2012.10 (Chemical Computing Group Inc., Montreal, Canada). Docking simulations were performed on a Dell E8500 with an Intel Core 2 Duo @ 3.16 GHz using Windows XP OS. All other computational procedures were performed using a Dell Latitiude E6420 with an Intel COREi5 processor 2540 @ 2.60 GHz with 4GB RAM using Windows 7 OS. 2BID and 3E7O pdb structural files were used as input for analysis and docking simulations. Before simulations the main chain protein and the Bid peptide (9-mer – D55-R63) were protonated at pH 7.0 with a salt concentration of 0.1 M and the structures energy minimized using the OPLS-AA forcefield and Born solvation model. The apo JNK2 structure was modified to contain ATP by superposing with JNK3-ANP (PDB entry 1JNK61), the main chain of JNK3 was deleted, and the new system minimized. Initial placement calculated 60 poses per molecule using triangle-matching placement with London dG scoring, the top 30 poses were then refined using forcefield placement and Affinity dG scoring.
Acknowledgments
This work was supported by the NIH grants CA095030 and DK054388 to SK. Flow Cytometry and Cell and Molecular Imaging Core Facilities were supported in part by Cancer Center Grant P30 CA138313 to the Hollings Cancer Center, Medical University of South Carolina. LC/MS-MS experiments were carried out using Proteomics Mass Spectrometry Core Facility, MUSC. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Glossary
- ALDH1L1
10-formyltetrahydrofolate dehydrogenase
- Bid
BH3 interacting-domain death agonist
- JNK
c-Jun N-terminal kinase
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by A Oberst
Supplementary Material
References
- Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- Zinkel SS, Yin XM, Gross A. Rejuvenating Bi(d)ology. Oncogene. 2013;32:3213–3219. doi: 10.1038/onc.2012.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, et al. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Kantari C, Walczak H. Caspase-8 and bid: caught in the act between death receptors and mitochondria. Biochim Biophys Acta. 2011;1813:558–563. doi: 10.1016/j.bbamcr.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Billen LP, Shamas-Din A, Andrews DW. Bid: a Bax-like BH3 protein. Oncogene. 2008;27 (Suppl 1:S93–S104. doi: 10.1038/onc.2009.47. [DOI] [PubMed] [Google Scholar]
- Shamas-Din A, Bindner S, Zhu W, Zaltsman Y, Campbell C, Gross A, et al. tBid undergoes multiple conformational changes at the membrane required for Bax activation. J Biol Chem. 2013;288:22111–22127. doi: 10.1074/jbc.M113.482109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarig R, Zaltsman Y, Marcellus RC, Flavell R, Mak TW, Gross A. BID-D59A is a potent inducer of apoptosis in primary embryonic fibroblasts. J Biol Chem. 2003;278:10707–10715. doi: 10.1074/jbc.M210296200. [DOI] [PubMed] [Google Scholar]
- Valentijn AJ, Gilmore AP. Translocation of full-length Bid to mitochondria during anoikis. J Biol Chem. 2004;279:32848–32857. doi: 10.1074/jbc.M313375200. [DOI] [PubMed] [Google Scholar]
- Ward MW, Rehm M, Duessmann H, Kacmar S, Concannon CG, Prehn JH. Real time single cell analysis of Bid cleavage and Bid translocation during caspase-dependent and neuronal caspase-independent apoptosis. J Biol Chem. 2006;281:5837–5844. doi: 10.1074/jbc.M511562200. [DOI] [PubMed] [Google Scholar]
- Konig HG, Rehm M, Gudorf D, Krajewski S, Gross A, Ward MW, et al. Full length Bid is sufficient to induce apoptosis of cultured rat hippocampal neurons. BMC Cell Biol. 2007;8:7. doi: 10.1186/1471-2121-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin XM. Bid, a BH3-only multi-functional molecule, is at the cross road of life and death. Gene. 2006;369:7–19. doi: 10.1016/j.gene.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Zinkel SS, Hurov KE, Ong C, Abtahi FM, Gross A, Korsmeyer SJ. A role for proapoptotic BID in the DNA-damage response. Cell. 2005;122:579–591. doi: 10.1016/j.cell.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Kamer I, Sarig R, Zaltsman Y, Niv H, Oberkovitz G, Regev L, et al. Proapoptotic BID is an ATM effector in the DNA-damage response. Cell. 2005;122:593–603. doi: 10.1016/j.cell.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Maryanovich M, Oberkovitz G, Niv H, Vorobiyov L, Zaltsman Y, Brenner O, et al. The ATM-BID pathway regulates quiescence and survival of haematopoietic stem cells. Nat Cell Biol. 2012;14:535–541. doi: 10.1038/ncb2468. [DOI] [PubMed] [Google Scholar]
- Desagher S, Osen-Sand A, Montessuit S, Magnenat E, Vilbois F, Hochmann A, et al. Phosphorylation of bid by casein kinases I and II regulates its cleavage by caspase 8. Mol Cell. 2001;8:601–611. doi: 10.1016/s1097-2765(01)00335-5. [DOI] [PubMed] [Google Scholar]
- Biswas S, Shi Q, Wernick A, Aiello A, Zinkel SS. The loss of the BH3-only Bcl-2 family member Bid delays T-cell leukemogenesis in Atm-/- mice. Cell Death Differ. 2013;20:869–877. doi: 10.1038/cdd.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Ren X, Yang L, Lin Y, Wu X. A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell. 2003;115:61–70. doi: 10.1016/s0092-8674(03)00757-8. [DOI] [PubMed] [Google Scholar]
- Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, et al. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Vollmer M, De Geyter M, Durrenberger M, De Geyter C. Apoptosis and differentiation induced by staurosporine in granulosa tumor cells is coupled with activation of JNK and suppression of p38 MAPK. Int J Oncol. 2005;26:1575–1580. [PubMed] [Google Scholar]
- Ni HM, Chen X, Shi YH, Liao Y, Beg AA, Fan J, et al. Genetic delineation of the pathways mediated by bid and JNK in tumor necrosis factor-alpha-induced liver injury in adult and embryonic mice. J Biol Chem. 2009;284:4373–4382. doi: 10.1074/jbc.M807259200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose S, Oleinik NV, Krupenko NI, Krupenko SA. 10-formyltetrahydrofolate dehydrogenase-induced c-Jun-NH2-kinase pathways diverge at the c-Jun-NH2-kinase substrate level in cells with different p53 status. Mol Cancer Res. 2009;7:99–107. doi: 10.1158/1541-7786.MCR-08-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Mohit AA, Martin JH, Miller CA. p493F12 kinase: a novel MAP kinase expressed in a subset of neurons in the human nervous system. Neuron. 1995;14:67–78. doi: 10.1016/0896-6273(95)90241-4. [DOI] [PubMed] [Google Scholar]
- Martin JH, Mohit AA, Miller CA. Developmental expression in the mouse nervous system of the p493F12 SAP kinase. Brain Res Mol Brain Res. 1996;35:47–57. doi: 10.1016/0169-328x(95)00181-q. [DOI] [PubMed] [Google Scholar]
- Oleinik NV, Krupenko NI, Krupenko SA. Cooperation between JNK1 and JNK2 in activation of p53 apoptotic pathway. Oncogene. 2007;26:7222–7230. doi: 10.1038/sj.onc.1210526. [DOI] [PubMed] [Google Scholar]
- Chou JJ, Li H, Salvesen GS, Yuan J, Wagner G. Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell. 1999;96:615–624. doi: 10.1016/s0092-8674(00)80572-3. [DOI] [PubMed] [Google Scholar]
- Shaw D, Wang SM, Villasenor AG, Tsing S, Walter D, Browner MF, et al. The crystal structure of JNK2 reveals conformational flexibility in the MAP kinase insert and indicates its involvement in the regulation of catalytic activity. J Mol Biol. 2008;383:885–893. doi: 10.1016/j.jmb.2008.08.086. [DOI] [PubMed] [Google Scholar]
- Barry M, Heibein JA, Pinkoski MJ, Lee SF, Moyer RW, Green DR, et al. Granzyme B short-circuits the need for caspase 8 activity during granule-mediated cytotoxic T-lymphocyte killing by directly cleaving Bid. Mol Cell Biol. 2000;20:3781–3794. doi: 10.1128/mcb.20.11.3781-3794.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoka V, Turk B, Schendel SL, Kim TH, Cirman T, Snipas SJ, et al. Lysosomal protease pathways to apoptosis. Cleavage of bid, not pro-caspases, is the most likely route. J Biol Chem. 2001;276:3149–3157. doi: 10.1074/jbc.M008944200. [DOI] [PubMed] [Google Scholar]
- Mandic A, Viktorsson K, Strandberg L, Heiden T, Hansson J, Linder S, et al. Calpain-mediated Bid cleavage and calpain-independent Bak modulation: two separate pathways in cisplatin-induced apoptosis. Mol Cell Biol. 2002;22:3003–3013. doi: 10.1128/MCB.22.9.3003-3013.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Q, Sun Y, Wei T, Zhao X, Zhao K, Yan L, et al. Chymotrypsin B cached in rat liver lysosomes and involved in apoptotic regulation through a mitochondrial pathway. J Biol Chem. 2008;283:8218–8228. doi: 10.1074/jbc.M709789200. [DOI] [PubMed] [Google Scholar]
- Reiners Jr, JJ, Caruso JA, Mathieu P, Chelladurai B, Yin XM, Kessel D. Release of cytochrome c and activation of pro-caspase-9 following lysosomal photodamage involves Bid cleavage. Cell Death Differ. 2002;9:934–944. doi: 10.1038/sj.cdd.4401048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirman T, Oresic K, Mazovec GD, Turk V, Reed JC, Myers RM, et al. Selective disruption of lysosomes in HeLa cells triggers apoptosis mediated by cleavage of Bid by multiple papain-like lysosomal cathepsins. J Biol Chem. 2004;279:3578–3587. doi: 10.1074/jbc.M308347200. [DOI] [PubMed] [Google Scholar]
- Breitschopf K, Zeiher AM, Dimmeler S. Ubiquitin-mediated degradation of the proapoptotic active form of bid. A functional consequence on apoptosis induction. J Biol Chem. 2000;275:21648–21652. doi: 10.1074/jbc.M001083200. [DOI] [PubMed] [Google Scholar]
- Azakir BA, Desrochers G, Angers A. The ubiquitin ligase Itch mediates the antiapoptotic activity of epidermal growth factor by promoting the ubiquitylation and degradation of the truncated C-terminal portion of Bid. FEBS J. 2010;277:1319–1330. doi: 10.1111/j.1742-4658.2010.07562.x. [DOI] [PubMed] [Google Scholar]
- Tait SW, de Vries E, Maas C, Keller AM, D'Santos CS, Borst J. Apoptosis induction by Bid requires unconventional ubiquitination and degradation of its N-terminal fragment. J Cell Biol. 2007;179:1453–1466. doi: 10.1083/jcb.200707063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen BB, Petersen J, Issinger OG. BID, an interaction partner of protein kinase CK2alpha. Biol Chem. 2006;387:441–449. doi: 10.1515/BC.2006.059. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol. 1999;19:8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharbanda S, Saxena S, Yoshida K, Pandey P, Kaneki M, Wang Q, et al. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-x(L) in response to DNA damage. J Biol Chem. 2000;275:322–327. doi: 10.1074/jbc.275.1.322. [DOI] [PubMed] [Google Scholar]
- Inoshita S, Takeda K, Hatai T, Terada Y, Sano M, Hata J, et al. Phosphorylation and inactivation of myeloid cell leukemia 1 by JNK in response to oxidative stress. J Biol Chem. 2002;277:43730–43734. doi: 10.1074/jbc.M207951200. [DOI] [PubMed] [Google Scholar]
- Datta SR, Ranger AM, Lin MZ, Sturgill JF, Ma YC, Cowan CW, et al. Survival factor-mediated BAD phosphorylation raises the mitochondrial threshold for apoptosis. Dev Cell. 2002;3:631–643. doi: 10.1016/s1534-5807(02)00326-x. [DOI] [PubMed] [Google Scholar]
- Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci USA. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan N, Becker EB, Konishi Y, Bonni A. JNK phosphorylation and activation of BAD couples the stress-activated signaling pathway to the cell death machinery. J Biol Chem. 2002;277:40944–40949. doi: 10.1074/jbc.M206113200. [DOI] [PubMed] [Google Scholar]
- Gabai VL, Mabuchi K, Mosser DD, Sherman MY. Hsp72 and stress kinase c-jun N-terminal kinase regulate the bid-dependent pathway in tumor necrosis factor-induced apoptosis. Mol Cell Biol. 2002;22:3415–3424. doi: 10.1128/MCB.22.10.3415-3424.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Jakob S, Corazza N, Badmann A, Sidler D, Stuber-Roos R, Keogh A, et al. Synergistic induction of cell death in liver tumor cells by TRAIL and chemotherapeutic drugs via the BH3-only proteins Bim and Bid. Cell Death Dis. 2010;1:e86. doi: 10.1038/cddis.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Ding WX, Ni HM, Gao W, Shi YH, Gambotto AA, et al. Bid-independent mitochondrial activation in tumor necrosis factor alpha-induced apoptosis and liver injury. Mol Cell Biol. 2007;27:541–553. doi: 10.1128/MCB.01166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigematsu S, Fukuda S, Nakayama H, Inoue H, Hiasa Y, Onji M, et al. ZNF689 suppresses apoptosis of hepatocellular carcinoma cells through the down-regulation of Bcl-2 family members. Exp Cell Res. 2011;317:1851–1859. doi: 10.1016/j.yexcr.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Tafani M, Karpinich NO, Hurster KA, Pastorino JG, Schneider T, Russo MA, et al. Cytochrome c release upon Fas receptor activation depends on translocation of full-length bid and the induction of the mitochondrial permeability transition. J Biol Chem. 2002;277:10073–10082. doi: 10.1074/jbc.M111350200. [DOI] [PubMed] [Google Scholar]
- Karpinich NO, Tafani M, Schneider T, Russo MA, Farber JL. The course of etoposide-induced apoptosis in Jurkat cells lacking p53 and Bax. J Cell Physiol. 2006;208:55–63. doi: 10.1002/jcp.20638. [DOI] [PubMed] [Google Scholar]
- Eliseev RA, Malecki J, Lester T, Zhang Y, Humphrey J, Gunter TE. Cyclophilin D interacts with Bcl2 and exerts an anti-apoptotic effect. J Biol Chem. 2009;284:9692–9699. doi: 10.1074/jbc.M808750200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamzami N, El Hamel C, Maisse C, Brenner C, Munoz-Pinedo C, Belzacq AS, et al. Bid acts on the permeability transition pore complex to induce apoptosis. Oncogene. 2000;19:6342–6350. doi: 10.1038/sj.onc.1204030. [DOI] [PubMed] [Google Scholar]
- Goonesinghe A, Mundy ES, Smith M, Khosravi-Far R, Martinou JC, Esposti MD. Pro-apoptotic Bid induces membrane perturbation by inserting selected lysolipids into the bilayer. Biochem J. 2005;387 (Pt 1:109–118. doi: 10.1042/BJ20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposti MD, Erler JT, Hickman JA, Dive C. Bid, a widely expressed proapoptotic protein of the Bcl-2 family, displays lipid transfer activity. Mol Cell Biol. 2001;21:7268–7276. doi: 10.1128/MCB.21.21.7268-7276.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalmar O, Garcia-Saez AJ, Berland L, Gonzalvez F, Petit PX. Giant unilamellar vesicles (GUVs) as a new tool for analysis of caspase-8/Bid-FL complex binding to cardiolipin and its functional activity. Cell Death Dis. 2010;1:e103. doi: 10.1038/cddis.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinberg M, Schwarz M, Zaltsman Y, Eini T, Niv H, Pietrokovski S, et al. Mitochondrial carrier homolog 2 is a target of tBID in cells signaled to die by tumor necrosis factor alpha. Mol Cell Biol. 2005;25:4579–4590. doi: 10.1128/MCB.25.11.4579-4590.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaltsman Y, Shachnai L, Yivgi-Ohana N, Schwarz M, Maryanovich M, Houtkooper RH, et al. MTCH2/MIMP is a major facilitator of tBID recruitment to mitochondria. Nat Cell Biol. 2010;12:553–562. doi: 10.1038/ncb2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Gu Y, Fox T, Coll JT, Fleming MA, Markland W, et al. Crystal structure of JNK3: a kinase implicated in neuronal apoptosis. Structure. 1998;6:983–991. doi: 10.1016/s0969-2126(98)00100-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.