Summary
Neutrophils are sentinel cells of the innate immune system with a primary role of clearing extracellular pathogens. The release of web-like structures decorated with granular proteins called neutrophil extracellular traps (NETs) has recently been implicated in the pathogenesis of inflammatory and autoimmune diseases. Indeed, NETs may represent an important source of autoantigens and immunostimulatory proteins in systemic lupus erythematosus (SLE). In this chapter, we describe protocols to isolate human peripheral neutrophils, to generate and isolate NETs, and to detect SLE antigens in NETs using immunofluorescence and immunoblot.
Section 1
Neutrophils are terminally differentiated cells that develop in the bone marrow and the most abundant white blood cells in the human circulation(1). They have long been viewed as short-lived effector cells of the innate immune system. They play a critical role in the immune defense by killing pathogens through phagocytosis, degranulation, and the release of web-like structures called neutrophil extracellular traps (NETs)(2, 3). NETs are composed of nuclear components (e.g. DNA and histones) associated to granular proteins from primary [myeloperoxidase (MPO), neutrophil elastase (NE), cathelicidin (LL-37)], secondary (lactoferrin), and tertiary [matrix metalloproteinases (MMPs) granules](3, 4). The molecular mechanisms leading to NET formation are still unraveling. It has been demonstrated that reactive oxygen species (ROS) produced by NADPH oxidase (3), histone citrullination by peptidylarginine deiminase-4 (PAD-4)(5, 6) and translocation of neutrophil elastase (NE) and myeloperoxidase (MPO)(7) appear to be important events leading to NET formation.
Recent evidence implicates externalization of nuclear material bound to neutrophil granular proteins during NET formation as an important event in the pathogenesis of autoimmune disorders including SLE (8,9). Indeed, proteomic and immunofluorescence analyses of NETs have demonstrated the presence of proteins known to be associated with specific autoantibody specificities in SLE (10)(Table 1).
Table 1.
SLE autoantibodies directed to proteins present in NETs.
Here, we describe some basic approaches to isolate NETs from peripheral blood (PB) neutrophils, and to detect autoantigens in NETs using immunofluorescence and Western blot. These approaches should be complemented with more sophisticated techniques such as mass-spectrometry and/or using recombinant proteins combined with in vitro assays.
Section 2
2.1 Neutrophil isolation
25mL of human blood collected in heparin treated tube.
Laminal flow hood
Sterile serological disposable pipettes.
50 mL conical tubes.
15 mL conical tubes.
Hemocytometer.
Ficoll-Paque density gradient medium.
Phosphate-buffered saline (PBS) 1x, pH 7.4 without calcium chloride/magnesium chloride. Store at room temperature
20% (w/v) Dextran: Dissolve 20g of Dextran in deionized water
Filtered 0.2 % (w/v) NaCl solution: Dissolve 0.2g of NaCl in deionized water. Store at room temperature.
Filtered 1.8 % (w/v) NaCl solution: Dissolve 1.8g of NaCl in deionized water. Store at room temperature.
2.2 NETs isolation and protein quantification
Isolated neutrophils.
Microplate reader equipped with filter to detect absorbance 562 nm.
Humidified CO2 incubator.
24-well plate.
96-well plate.
1.5 mL microcentrifuge tubes.
Bicinchoninic acid (BCA) kit (Pierce)
Roswell Park Memorial Institute (RPMI)-1640 medium without supplements.
Microccocal nuclease (10 Units/μL). Store at −20°C.
Lipopolyssacharide (LPS) 1mg/mL. Store at −20°C.
2.3 Immunofluorescence
Isolated neutrophils (1× 106 cells/mL).
Epi-fluorescence or confocal microscope equipped with filters to detect excitation/emission maxima: 350/461 nm (Hoechst), 495/519nm (Alexa Fluor 488), 555/565 nm (Alexa Fluor 555).
Swiss Jewelers Forceps.
12- well plate.
12 mm round poly-L-lysine coated glass coverslips.
75 × 25 × 1 mm microscope slides.
1.5 mL microcentrifuge tubes.
PBS 1x, pH 7.4. Store at room temperature.
4 % (w/v) paraformaldehyde (PFA): Dissolve 4 g in 100 mL of PBS. Place the solution in a hotplate and stirrer inside the fume hood. Heat until it becomes clear. Store at 4°C.
0.2% (v/v) Triton-x-100 in PBS.
Blocking buffer 0.2% (w/v) gelatin: Dissolve 0.2g of porcine gelatin in 100 mL of PBS. Place the solution in the microwave and heat until it completely dissolved. Store at −20°C.
Fluorescent mounting medium. Store at −20°C.
Hoechst 33342 (bisBenzimide H33342 trihydrochloride). Store at 4°C.
Human sera from healthy and SLE donors.
Goat anti- human IgG Alexa Fluor 555 secondary antibody (Invitrogen). Store at −20°C.
2.4 Western blot (protein detection)
Protein samples
Forceps
Nitrocellulose or PVDF membrane
Whatman 3MM filter papers
4–20 % gradient gel
Running apparatus
Transfer apparatus with cassettes
Western blot box
Orbital shaker
5x Loading buffer: 60mM Tris-HCl (pH 6.8), 2% Sodium dodecyl sulfate (SDS), 10% lycerol, 0.01% bromophenol blue and 5% β-mercaptoethanol.
SDS-PAGE running buffer: 25 mM Tris, 192 mM glycine, 0.1% SDS, pH 8.3
Transfer buffer: 25 mM Tris, 192 mM glycine, 20% methanol, pH 8.3
Blocking buffer: 10% (w/v) Bovine serum albumin (BSA): Dissolve 1g of BSA in 10 mL of PBS. Store at 4°C.
Wash buffer 0.1% (v/v) PBS-Tween
Human sera from healthy and SLE donors.
Goat anti- human IgG HRP secondary antibody (Invitrogen). Store at −20°C.
Enhanced Chemiluminescence (ECL) substrate
X-ray films
Section 3
3.1 Neutrophil isolation
In a laminal flow hood, add 15 mL of Ficoll-Paque to a 50 mL conical tube.
Carefully add 25 mL of blood on top of the Ficoll.
Centrifuge at 417 xg for 20 min without braking and acceleration 0.
Remove the conical from the centrifuge.
Gently dispose of the plasma and PBMC fractions.
Take 5 mL of the red blood cell layer and add it to a 50 mL conical. (see Note 1)
Add 2.5 mL of 20% Dextran and mix gently. Leave undisturbed for 15 min. (see Note 2)
Add 20 mL of PBS to the conical and mix by inverting the tube several times.
Let the solution to sediment approximately 20–30 min.
Take 15 mL of the cleared supernatant and transfer it to a fresh 50 mL conical.
Add PBS up to 50 mL.
Centrifuge at 515 xg for 10 min at room temperature.
Carefully discard supernatant by decanting.
Resuspend the cell pellet with 20 mL of 0.2% NaCl and mix gently (see Note 3).
After 5 min, add 30 mL of 1.8% NaCl.
Centrifuge at 515 xg for 5 min at 4 C.
Resuspend neutrophils with 10 mL of PBS and transfer suspension to a 15 mL conical.
Centrifuge cells at 515 xg for 5 min.
Discard supernatant and resuspend cells in RPMI.
Count cells using a hemocytometer.
3.2 NETs isolation and protein quantification
In a laminal flow-hood, seed 1× 106 neutrophils/mL per well in a 24-well plate.
Add 1μL of 1 mg/mL of LPS per well.
Place the plate in a humidified CO2 incubator at 37°C for 1 hour (see Note 4)
After incubation, add 10U/mL of microccocal nuclease to each well (see Note 5)
Place the plate back to the humidified CO2 incubator at 37°C for 20 min.
Carefully collect the supernatant from each well in a 1.5 mL microcentrifuge tubes.
Centrifuge supernatants at 300 xg for 5 min at 4°C.
Transfer supernatant to a fresh 1.5 mL microcentrifuge tubes (see Note 6).
Using a BCA kit, quantify NETs proteins (see Note 7).
Take an aliquot of 10 μL and place it in a 96-well plate.
Mix reagents A and B in a proportion 1:50.
Add 200 μL of the mixture to the well containing your NET sample.
Incubate the plate for 30 min at 37°C.
Read the plate using microplate reader equipped with filter to detect absorbance 562 nm.
Calculate NETs concentration.
3.3 Immunofluorescence
Place one poly-L-lysine coated glass coverslip into each well of the 12-well plate.
Add 1μL of LPS (1mg/mL) to 1mL of isolated neutrophils (1× 106 cells/mL) (see Note 8)
Pipette 50 uL of the suspension onto the center of the poly-L-lysine coated coverslips.
Incubate for 1 h in a humidified incubator (37°C, 5% CO2)
Transfer the cell plate to a fume hood and fix cells by adding 500 μL of 4% PFA (see Note 9).
Gently aspirate the PFA and add 500 μL of PBS.
Permeabilize cells by adding 500 μL of 0.2% Triton X-100 to each well and incubate for 10 min at room temperature.
Aspirate the solution and wash coverslips with PBS for 5 min.
Block nonspecific sites with 500 μL of 0.2% gelatin for 30 min at room temperature (see Note 10)
Prepare two 10 % dilutions containing serum from healthy or SLE donors in 0.2% gelatin.
Place a 45 μL drop of the diluted serum in the humid chamber.
Using Swiss Jewelers Forceps, transfer coverslip from the plate to the humid chamber. Place the coverslip upside down.
Place the humid chamber inside the incubator (37°C) for 1 hour.
Place coverslips back to the 12-well plate containing PBS.
Wash coverslips with 500 μL of PBS for 5 min 3 times.
Prepare goat anti- human IgG-Alex fluor 555 (1:400 dilution) in 0.2% gelatin (see Note 11).
Place a 45 μL drop of the diluted secondary antibodies in the humid chamber.
Using Swiss Jewelers Forceps, transfer coverslip from the plate to the humid chamber. Place the coverslip upside down.
Place the humid chamber inside the incubator (37°C) for 30 min.
Place coverslips back to the 12-well plate containing PBS.
Wash coverslips with 500 μL of PBS for 5 min 3 times.
To counterstain the DNA, prepare DNA staining solution, Hoechst (1:1000 dilution in PBS).
Aspirate PBS and add 1 mL of DNA staining dilution to each coverslips.
Incubate for 10 min at room temperature.
Aspirate DNA staining solution and wash coverslips for 5 min with PBS 3 times at room temperature.
Mount coverslips using a 6 μL drop of anti-fade ProLong gold mounting medium per microscope slide. Allow the medium to dry overnight protected from light at room temperature.
Visualize staining on a confocal or epi-fluorescence microscope.
3.4 Western blot (protein detection)
Mix the NETs proteins with 5 μL of loading buffer.
Heat samples at 100°C for 5 minutes.
Meanwhile, fit the gradient gel plate within the running apparatus. Pour SDS- PAGE running buffer.
Clean the wells and load the samples inside the wells.
Run gel at 100 volts until the bromophenol blue frontline reaches about 5 mm near the bottom.
Turn off the power supply and remove the plate.
Cut the nitrocellulose or PVDF membrane equal to the size of the gel.
Cut 6 Whatman 3MM filter paper equal to the size of the gel.
Separate the two plates of the gradient gel with a spatula.
-
Open a transfer cassette and make the “sandwich” in the following order, starting from the black side of the cassette. Wet filter paper and membrane in transfer buffer before assemble the “sandwich”:
Foam
3 Whatman filter papers
Gel up side-down
Membrane
3 Whatman filter papers
Foam
Close the cassette and place it inside the transfer apparatus.
Fill out with transfer buffer.
Connect the transfer apparatus to a power supply.
Run transfer at 360 mA for 60 minutes.
Disassemble the “sandwich”.
Take the membrane with forceps and place it in western blot box containing PBS (see Note 12)
Wash the membrane for 5 min at room temperature.
Block the membrane with 10% BSA for 30 min at room temperature (see Note 13)
Prepare two 1:250 dilutions containing serum from healthy or SLE donors in 5% BSA.
Cut the membrane with scissors in two halves.
Incubate half of the membrane with control serum and the other half with SLE serum overnight at 4°C on an orbital shaker.
Discard serum dilutions and wash the membranes with PBS-Tween for 5 min at room temperature 3 times in an orbital shaker.
Prepare a dilution of 1: 20,000 of the secondary antibody in 5% BSA.
Incubate membrane with secondary antibody for 2 hours at room temperature on an orbital shaker.
Discard secondary antibody and wash the membrane with PBS-Tween for 5 min at room temperature 3 times in an orbital shaker.
Prepare a 1:1 dilution of ECL substrates.
Incubate the membrane with ECL substrate for 1 min at room temperature with gentle agitation.
Place the membrane between two sheets of transparency using forceps.
Place it in an X-ray cassette.
In a dark room, place an X-ray film on top of the membrane to capture chemiluminicent signal.
Place the X-ray film in a developing machine.
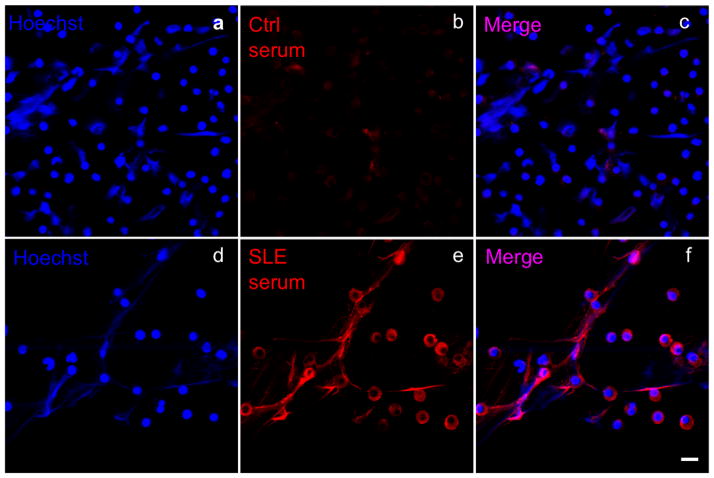
Figure 1.
Visualization of human peripheral blood (PB)-derived NETs by immunofluorescence microscopy. PB neutrophils were stimulated with LPS 1μg for 1 h at 37°C. Cells were fixed and immunostained with healthy donor (Ctrl; b) or SLE-sera (red; e) and for DNA (Hoechst, blue; a,d). Scale bar 10μm.
Figure 2.
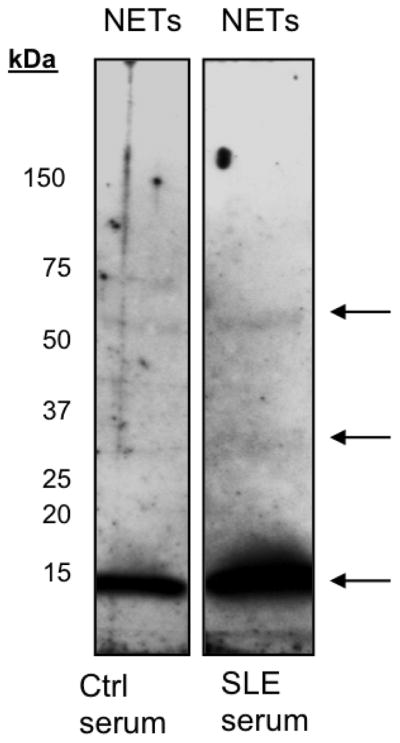
Detection of SLE autoantigens in NETs by Western blot. NETs were resolved in a 4–20% gradient gel. Proteins were transferred onto a nitrocellulose membrane. Nitrocellulose was cut in half and incubated with 1:250 dilution of healthy donor or SLE-sera. Horseradish peroxidase-conjugated secondary antibodies were used to detect control (Ctrl) and SLE – IgGs. Arrows indicate the antigens recognized in the NETs by SLE autoantibodies.
Footnotes
To avoid contamination with the interphase between the Ficoll and redblood cell layer, insert the pipette to the bottom of the tube and aspirate the sample.
Do not leave samples without processing for more than 15 min since the neutrophil recovering yield will decrease.
While typically red-blood cells will be lysed within a minute, sometimes longer incubation period are required to get rid of all red blood cells.
Incubation periods range from 1–4 hours depending on the condition utilized, the source of neutrophils (e.g. healthy donor, SLE patient, mouse) and the tyope of stimulation (e.g. LPS, PMA, IL-8).
DNase I or MNase can be used in this protocol. However, it is important to note that DNAse I will completely degrade the DNA, while MNase will generate nucleosomal fragments.
When transfer the supernatant, do not disturb the bottom where intact cells and debris are present. If not used immediately, store the NETs at −20°C and quantify them later.
Follow BCA kit instructions, which include preparation of standards.
Under experimental conditions, a non-treated control should be added.
Samples can be fixed overnight at 4°C or for 20 min at room temperature.
To detect specific proteins within the NETs, double staining can be performed. Non-specific staining (false-positive signal) can occur. Therefore, controls should be included accordingly and results should be confirmed using a different biochemical approach such as Western blot.
To ensure protein transfer, stain the membrane with Ponceau S solution, a red dye that can be washed out with buffer and will not interfere with protein detection.
Non-fat dry milk can be used instead BSA.
References
- 1.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 3.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 4.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, Rubin CJ, Zhao W, Olsen SH, Klinker M, Shealy D, Denny MF, Plumas J, Chaperot L, Kretzler M, Bruce AT, Kaplan MJ. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, Allis CD, Coonrod SA. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–213. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knight JS, Zhao W, Luo W, Subramanian V, O’Dell AA, Yalavarthi S, Hodgin JB, Eitzman DT, Thompson PR, Kaplan MJ. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J Clin Invest. 2013 doi: 10.1172/JCI67390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, Gilliet M. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahlenberg JM, Carmona-Rivera C, Smith CK, Kaplan MJ. Neutrophil Extracellular Trap-Associated Protein Activation of the NLRP3 Inflammasome Is Enhanced in Lupus Macrophages. J Immunol. 2012 doi: 10.4049/jimmunol.1202388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knight JS, Carmona-Rivera C, Kaplan MJ. Proteins derived from neutrophil extracellular traps may serve as self-antigens and mediate organ damage in autoimmune diseases. Front Immunol. 2012;3:380. doi: 10.3389/fimmu.2012.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamiya H, Tani K, Miyata J, Sato K, Urata T, Lkhagvaa B, Otsuka S, Shigekiyo S, Sone S. Defensins- and cathepsin G-ANCA in systemic lupus erythematosus. Rheumatol Int. 2006;27:147–152. doi: 10.1007/s00296-006-0173-9. [DOI] [PubMed] [Google Scholar]
- 12.Mosca M, Chimenti D, Pratesi F, Baldini C, Anzilotti C, Bombardieri S, Migliorini P. Prevalence and clinico-serological correlations of anti-alpha-enolase, anti-C1q, and anti-dsDNA antibodies in patients with systemic lupus erythematosus. J Rheumatol. 2006;33:695–697. [PubMed] [Google Scholar]
- 13.Mansour RB, Lassoued S, Gargouri B, El Gaid A, Attia H, Fakhfakh F. Increased levels of autoantibodies against catalase and superoxide dismutase associated with oxidative stress in patients with rheumatoid arthritis and systemic lupus erythematosus. Scand J Rheumatol. 2008;37:103–108. doi: 10.1080/03009740701772465. [DOI] [PubMed] [Google Scholar]
- 14.Zhao MH, Liu N, Zhang YK, Wang HY. Antineutrophil cytoplasmic autoantibodies (ANCA) and their target antigens in Chinese patients with lupus nephritis. Nephrol Dial Transplant. 1998;13:2821–2824. doi: 10.1093/ndt/13.11.2821. [DOI] [PubMed] [Google Scholar]
- 15.Manolova I, Dancheva M, Halacheva K. Antineutrophil cytoplasmic antibodies in patients with systemic lupus erythematosus: prevalence, antigen specificity, and clinical associations. Rheumatol Int. 2001;20:197–204. doi: 10.1007/s002960100108. [DOI] [PubMed] [Google Scholar]
- 16.Nassberger L, Jonsson H, Sjoholm AG, Sturfelt G, Heubner A. Circulating anti-elastase in systemic lupus erythematosus. Lancet. 1989;1:509. doi: 10.1016/s0140-6736(89)91420-7. [DOI] [PubMed] [Google Scholar]
- 17.Monestier MDP, Briand JP, Gabriel JL, Muller S. Molecular and structural properties of three autoimmune IgG monoclonal antiboies to histone H2B. J Biol Chem. 2000:13558–13563. doi: 10.1074/jbc.275.18.13558. [DOI] [PubMed] [Google Scholar]
- 18.Robinson WH, DiGennaro C, Hueber W, Haab BB, Kamachi M, Dean EJ, Fournel S, Fong D, Genovese MC, de Vegvar HE, Skriner K, Hirschberg DL, Morris RI, Muller S, Pruijn GJ, van Venrooij WJ, Smolen JS, Brown PO, Steinman L, Utz PJ. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat Med. 2002;8:295–301. doi: 10.1038/nm0302-295. [DOI] [PubMed] [Google Scholar]
- 19.van Bavel CC, Dieker JW, Kroeze Y, Tamboer WP, Voll R, Muller S, Berden JH, van der Vlag J. Apoptosis-induced histone H3 methylation is targeted by autoantibodies in systemic lupus erythematosus. Ann Rheum Dis. 2011;70:201–207. doi: 10.1136/ard.2010.129320. [DOI] [PubMed] [Google Scholar]
- 20.Lee SS, Lawton JW, Chan CE, Li CS, Kwan TH, Chau KF. Antilactoferrin antibody in systemic lupus erythematosus. Br J Rheumatol. 1992;31:669–673. doi: 10.1093/rheumatology/31.10.669. [DOI] [PubMed] [Google Scholar]
- 21.Caccavo D, Rigon A, Picardi A, Galluzzo S, Vadacca M, Ferri GM, Amoroso A, Afeltra A. Anti-lactoferrin antibodies in systemic lupus erythematosus: isotypes and clinical correlates. Clin Rheumatol. 2005;24:381–387. doi: 10.1007/s10067-004-1040-2. [DOI] [PubMed] [Google Scholar]
- 22.Nassberger L, Sjoholm AG, Jonsson H, Sturfelt G, Akesson A. Autoantibodies against neutrophil cytoplasm components in systemic lupus erythematosus and in hydralazine-induced lupus. Clin Exp Immunol. 1990;81:380–383. doi: 10.1111/j.1365-2249.1990.tb05342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cambridge G, Wallace H, Bernstein RM, Leaker B. Autoantibodies to myeloperoxidase in idiopathic and drug-induced systemic lupus erythematosus and vasculitis. Br J Rheumatol. 1994;33:109–114. doi: 10.1093/rheumatology/33.2.109. [DOI] [PubMed] [Google Scholar]