Abstract
Background
The skeleton and liver are frequently involved sites of metastasis in patients with metastatic renal cell carcinoma (RCC).
Objective
The purpose of this study was to analyze outcomes based on the presence of bone metastases (BM) and/or liver metastases (LM) in patients with RCC treated with targeted therapy.
Design, Setting and Participants
We conducted a review from the International Metastatic RCC Database Consortium (IMDC) of 2,027 patients with metastatic RCC.
Outcome Measurements and Statistical Analysis
We analyzed the impact of site of metastasis on overall survival (OS) and time-to-treatment failure (TTF). Statistical analyses were performed using multivariable Cox regression.
Results and Limitations
Presence of BM was 34% overall and when stratified by IMDC risk groups was 27%, 33%, and 43% in favorable, intermediate, and poor-risk groups, respectively (pitalic>0.001). Presence of LM was 19% overall and higher in the poor-risk patients (23%) compared to the favorable or intermediate-risk groups (17%) (p=0.003). When patients were classified into four groups based on the presence of BM and/or LM the hazard ratio (HR), adjusted for IMDC risk factors, was 1.4 (95% Confidence Interval (CI) 1.22–1.62) for BM, 1.42 (95% CI 1.17–1.73) for LM, and 1.82 (95% CI 1.47–2.26) for both BM and LM compared to other metastatic sites (pbold>0.0001). The prediction model performance for OS was significantly improved when BM and LM were added to the IMDC prognostic model (likelihood ratio test p<0.0001). Data in this analysis was collected retrospectively.
Conclusions
The presence of BM and LM in patients treated with targeted agents has a negative impact on survival. Patients with BM and/or LM may benefit from earlier inclusion on clinical trials of novel agents or combination-based therapies.
Keywords: Bone metastases, Liver metastases, mTOR inhibitors, Outcome, Renal cell carcinoma, VEGF therapy
Introduction
The most common site of metastasis in patients with RCC is the lung, affecting 45–50% of patients with metastatic disease.[1] Other frequent sites of involvement include the skeleton and liver, with estimates of involvement of 30% and 20%, respectively.[1] BM from RCC cause significant morbidity and are associated with high rates of skeletal complications. Prior to the era of targeted therapy, the rate of skeletal-related events (SREs), defined as pathologic fracture, bone radiotherapy, bone surgery, spinal cord compression, and in some series hypercalcemia, was 74% to upwards of 85%.[2]
Recent advances in our understanding of the pathogenesis of RCC have led to a new treatment paradigm for patients with metastatic RCC. Though studies of patients treated in the cytokine era suggest that the presence of BM and/or LM is associated with poor prognosis, the impact of BM and/or LM on outcomes of patients treated with agents targeting the vascular endothelial growth factor (VEGF) and mammalian target of rapamycin (mTOR) axis is largely unknown. We used the IMDC to determine whether baseline BM and/or LM are associated with worse OS and TTF in patients with metastatic RCC treated with first-line targeted therapy.
Patients and Methods
Study design
The IMDC is a consecutive patient series from institutions in Canada, Denmark, South Korea and the United States. We used the IMDC to identify 2,370 patients from 20 centers treated for metastatic RCC. The database was locked for the current analysis on October 10, 2012. Patient inclusion criteria comprised a diagnosis of metastatic RCC of any histological subtype treated with first-line targeted therapy. Patients who received prior immunotherapy were included in the analysis. Patients were excluded from the analysis if they had missing information regarding baseline number of sites of metastasis, BM, LM, or choice of therapy. For this study, we included 2,027 patients of all ages with metastatic RCC who received first-line targeted therapy between April 7, 2003 and August 8, 2012.
We retrospectively collected baseline demographic, clinical and laboratory data, including those previously found to have prognostic value, on all patients using uniform database templates to ensure consistent data collection.[3] Laboratory values were standardized against institutional upper limits of normal and lower limits of normal values. We collected survival data from patient medical records or publically available records. Institutional review board approval was obtained from each participating center.
Statistical methods
The primary outcome of this study was OS, which was defined as the time from initiation of first-line targeted therapy to death from any cause or was censored at the date of last follow-up. The secondary outcome was TTF, which was defined as the time from initiation of first-line targeted therapy to date of progression, drug discontinuation, death or was censored at last follow-up. Distributions of OS and TTF were calculated using the Kaplan-Meier method. Median OS and TTF along with 95% CIs were reported. Associations between OS and TTF and site of metastasis were assessed using the log-rank test in univariate analysis or Wald chi-square test from multivariable Cox regression adjusted for IMDC prognostic factors.[3] For each of these analyses, two models were undertaken. In model 1, BM (yes versus no) and LM (yes versus no) were evaluated as two individual factors. In model 2, patients were classified into four groups based on the combination of BM and LM (presence of both BM and LM, presence of either BM or LM, or other metastases). The likelihood ratio test was also conducted to test the improvement in prediction performance that was gained by addition of BM and LM to the IMDC prognostic model.[4]
Subgroup analyses were performed in those with: 1) single and multiple sites of metastasis, 2) IMDC favorable, intermediate, and poor-risk groups, and 3) different types of targeted therapy (sunitinib, sorafenib, and other). All statistical computations were performed using SAS 9.2 (SAS Institute Inc.) and a p-value (two-sided) < 0.05 was considered significant.
Results
Patient and disease characteristics and clinical outcomes
Patient and disease characteristics at initiation of targeted therapy are displayed in Table 1. Most patients were men greater than 60 years of age with good performance status and clear-cell histology. In total, 97.6% (n=1,978) received first-line VEGF targeted therapy, whereas 2.4% (n=49) received first-line mTOR targeted therapy. The majority of patients had a previous nephrectomy and less than one third of patients received previous immunotherapy.
Table 1.
Patient and disease characteristics at initiation of targeted therapy (n=2,027).
| Characteristic | n (%) |
|---|---|
|
| |
| Age at initiation of therapy | |
| < 60 years | 945 (47%) |
| ≥60 years | 1,082 (53%) |
|
| |
| Karnofsky performance score | |
| ≥ 80% | 1,465 (72%) |
| < 80% | 445 (22%) |
| Unknown | 117 (6%) |
|
| |
| Sex | |
| Male | 1,494 (74%) |
| Female | 524 (26%) |
| Unknown | 9 (<1%) |
|
| |
| Pathology | |
| Clear cell | 1,661 (82%) |
| Non-clear cell | 238 (12%) |
| Unknown | 128 (6%) |
|
| |
| Sarcomatoid features | |
| Yes | 185 (9%) |
| No | 1,599 (79%) |
| Unknown | 243 (12%) |
|
| |
| Previous nephrectomy | |
| Yes | 1,570 (78%) |
| No | 455 (22%) |
| Unknown | 2 (<1%) |
|
| |
| Previous immunotherapy | |
| Yes | 444 (22%) |
| No | 1,583 (78%) |
|
| |
| Type of targeted agent | |
| Sunitinib | 1491 (74%) |
| Sorafenib | 357 (18%) |
| Bevacizumab | 80 (4%) |
| Pazopanib | 40 (2%) |
| Tivazonib | 7 (<1%) |
| Axitinib | 3 (<1%) |
| Temsirolimus | 42 (2%) |
| Everolimus | 7 (<1%) |
|
| |
| Number of metastases > 1 | 1,529 (75%) |
|
| |
| Metastasis site | |
| Lung | 1,390 (69%) |
| Lymph node | 864 (43%) |
| Bone | 693 (34%) |
| Liver | 381 (19%) |
| Brain | 165 (8%) |
| Other | 713 (35%) |
|
| |
| IMDC risk group | |
| Favorable | 321 (16%) |
| Intermediate | 969 (48%) |
| Poor | 504 (25%) |
| Unknown | 233 (11%) |
Most patients had greater than one site of metastasis. Stratified by the IMDC risk groups, more patients with poor-risk disease (82%) had greater than one site of metastasis compared to favorable or intermediate-risk groups (74%) (p=0.001). The presence of BM was 34% overall and 27%, 33%, and 43% in the favorable, intermediate, and poor-risk groups, respectively (p<0.001). The presence of LM was 19% overall and higher in poor-risk patients (23%) compared to favorable or intermediate-risk groups (17%) (p=0.002).
At the time of analysis, 1,722 (85%) of patients had stopped their first-line therapy. Median OS after initiation of targeted therapy was 20.5 months with 763 (38%) patients remaining alive at the time of data analysis. The median time on first-line therapy was 6.7 months (range 0–91). Median follow-up in living patients was 20.9 months.
The impact of bone and liver metastases on clinical outcomes
We evaluated BM and LM as two individual prognostic factors (Table 2, Model 1). Patients with BM had a significantly shorter median OS (14.9 versus 25.1 months, p<0.0001) and TTF (5.7 versus 7.6 months, p<0.0001) than those without BM. Similarly, patients with LM compared to those without LM had a worse median OS (14.3 versus 22.2 months, p<0.001) and TTF (5.5 versus 7.3 months, p=0.013). The associations remained in the multivariable analyses. When patients were further classified into four groups based on the combination of BM and LM (Table 2, Model 2), the HR was 1.40 for BM, 1.42 for LM, and 1.82 for both BM and LM when compared to other metastatic sites (p<0.0001). A similar trend was observed for TTF analysis.
Table 2.
Univariate and multivariable analysis of BM and LM on OS and TTF.
| OS | TTF | |||||
|---|---|---|---|---|---|---|
| N | Mediana (months) | HRb (95% CI) | N | Mediana (months) | HRb (95% CI) | |
| Model 1c | ||||||
| Bone Metastasis | ||||||
| Yes | 693 | 14.9 | 1.38 (1.22, 1.56) | 678 | 5.7 | 1.19 (1.07, 1.33) |
| No | 1334 | 25.1 | reference | 1319 | 7.6 | reference |
| P-value | <0.0001 | <0.0001 | <0.0001 | 0.001 | ||
| Liver Metastasis | ||||||
| Yes | 381 | 14.3 | 1.37 (1.18, 1.58) | 371 | 5.5 | 1.15 (1.01, 1.32) |
| No | 1646 | 22.2 | reference | 1626 | 7.3 | reference |
| P-value | <0.0001 | <0.0001 | 0.013 | 0.032 | ||
| Model 2d | ||||||
| Bone + Liver +/− Othere | 147 | 10.9 | 1.82 (1.47, 2.26) | 140 | 4.2 | 1.45 (1.19, 1.78) |
| Bone +/− Othere | 546 | 16.2 | 1.40 (1.22, 1.62) | 538 | 6.4 | 1.16 (1.03, 1.32) |
| Liver +/− Othere | 234 | 18.2 | 1.42 (1.17, 1.73) | 231 | 6.6 | 1.10 (0.93, 1.30) |
| Othere | 1100 | 27.1 | reference | 1088 | 7.8 | reference |
| P-value | <0.0001 | <0.0001 | <0.0001 | 0.001 | ||
Log rank test.
Wald chi-square test from multivariable Cox regression adjusted for the IMDC risk factors, including time from diagnosis to treatment < 1 year, Karnofsky performance status <80, hemoglobin < upper limit of normal, neutrophilia, thrombocytosis, and hypercalcemia.
In model 1, BM (yes versus no) and LM (yes versus no) were evaluated as two individual factors.
In model 2, patients were classified into four groups based on the combination of BM and LM (presence of both BM and LM, presence of either BM or LM, or other metastases).
Other is defined as sites of metastasis excluding bone and liver.
The likelihood ratio tests comparing the IMDC prognostic model alone to the alternative models with the addition of BM and LM were highly significant (p values<0.001), suggesting that inclusion of BM and LM to the IMDC model enhances the prediction performance of the IMDC prognostic model.
Stratified by single or multiple sites of metastasis
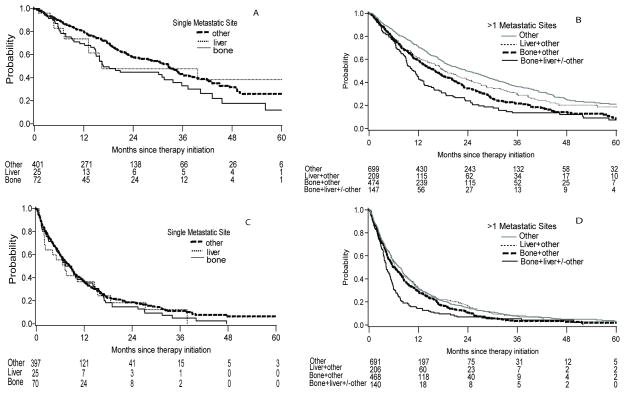
Compared to patients with a single site of metastasis, patients with multiple metastatic sites had shorter OS (18.2 versus 31.4 months, p<0.0001) and TTF (6.2 versus 8.3 months, p=0.005). We stratified patients by either a single site of metastasis or two or more metastatic sites to evaluate the significance of BM and LM on OS and TTF (Table 3, Figure 1). In patients with a single site of metastatic disease, those with BM or LM had shorter median OS but similar TTF compared to other single sites. There was no significant difference in multivariable analysis adjusted for IMDC risk factors.
Table 3.
OS and TTF according to numbers and sites of metastasis.
| OS | TTF | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | Mediana (months) | HRb (95% CI) | N | Mediana (months) | HRb (95% CI) | |
|
| ||||||
| Single metastasis | 498 | 492 | ||||
|
| ||||||
| Bone | 72 | 16.8 | 1.18 (0.81–1.71) | 70 | 8.1 | 1.08 (0.79–1.47) |
|
| ||||||
| Liver | 25 | 16.4 | 1.06 (0.51–2.17) | 25 | 7.2 | 1.09 (0.64–1.84) |
|
| ||||||
| Otherc | 401 | 32.6 | Reference | 397 | 8.3 | Reference |
| Lung | 256 | 33.7 | 254 | 8.6 | ||
| Lymph node | 75 | 23.5 | 74 | 7.5 | ||
| Brain | 5 | - | 5 | - | ||
| Otherd | 65 | 34.7 | 64 | 9.0 | ||
|
| ||||||
| P-value | 0.032 | 0.696 | 0.553 | 0.861 | ||
|
| ||||||
| Two or more metastatic sites | 1,529 | 1,505 | ||||
|
| ||||||
| Bone + Liver +/− Otherc | 147 | 10.9 | 1.70 (1.36–2.12) | 140 | 4.2 | 1.42 (1.15–1.74) |
|
| ||||||
| Bone + Otherc | 474 | 16.1 | 1.38 (1.18–1.61) | 468 | 6.1 | 1.16 (1.01–1.33) |
|
| ||||||
| Liver + Otherc | 209 | 18.2 | 1.39 (1.14–1.71) | 206 | 6.4 | 1.08 (0.90–1.30) |
|
| ||||||
| Otherc | 699 | 23.5 | Reference | 691 | 7.3 | Reference |
| Lung | 562 | 22.2 | 555 | 7.6 | ||
| Lymph node | 410 | 19.0 | 407 | 6.6 | ||
| Brain | 96 | 20.0 | 95 | 9.4 | ||
| Otherd | 349 | 26.0 | 346 | 7.8 | ||
|
| ||||||
| P-value | <0.0001 | <0.0001 | <0.0001 | 0.006 | ||
Log rank test. Patients without BM and LM are combined as one group in the comparison (labeled as otherc).
Wald chi-square test from multivariable Cox regression adjusted for the IMDC risk factors, including time from diagnosis to treatment < 1 year, Karnofsky performance status <80, hemoglobin < upper limit of normal, neutrophilia, thrombocytosis, and hypercalcemia. Patients without BM and LM are combined as one group in the comparison (labeled as otherc).
Other is defined as sites of metastasis excluding bone and liver.
Other is defined as sites of metastasis excluding bone, liver, brain, lymph nodes and lung.
Figure 1.
Kaplan-Meier plots of OS by site of metastasis in patients with a single metastatic site (A) and in patients with > 1 metastatic sites (B). Kaplan-Meier plots of TTF by site of metastasis in patients with a single metastatic site (C) and in patients with > 1 metastatic sites (D).
For patients with multiple metastatic sites, median OS was 23.5 months in those without BM and LM, and was worse in patients with the presence of either BM (16.1 months) or LM (18.2 months). The combination of both BM and LM had the shortest median OS (10.9 months) (p<0.0001). A similar trend was observed for TTF. The association was retained in multivariable analysis.
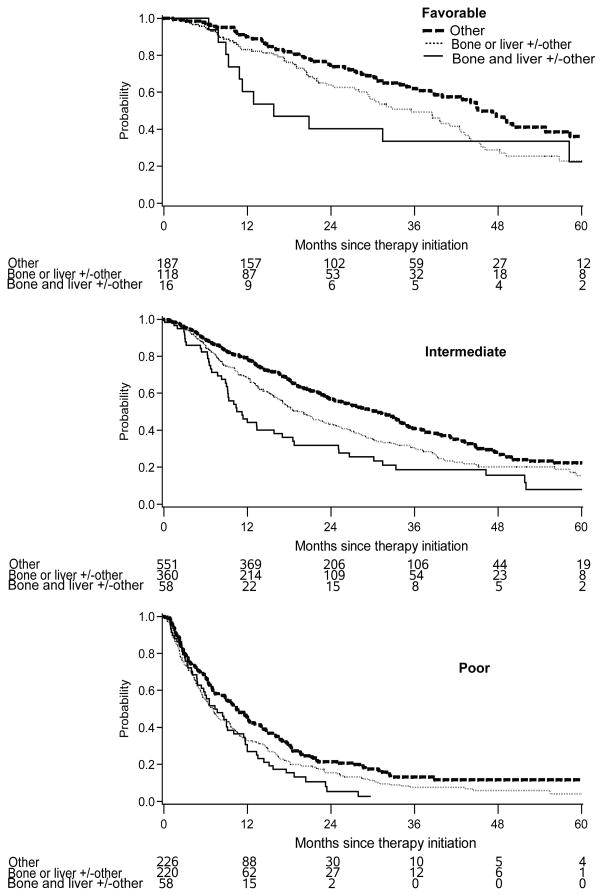
Stratified by the IMDC risk groups
When patients were stratified by the IMDC risk groups (Table 4, Figure 2), those with other sites of metastasis had the best median OS, while patients with both BM and LM had the shortest OS in the IMDC favorable and intermediate-risk groups (p<0.05). In the poor-risk group, there was no difference between bone only, liver only, or the combination of both, but they were all worse than patients with other metastases. A similar trend was observed for TTF analysis.
Table 4.
OS and TTF according to the IMDC risk groups and sites of metastasis.
| Favorable | Intermediate | Poor | ||||
|---|---|---|---|---|---|---|
| N | Median (months) | N | Median (months) | N | Median (months) | |
| OS | 321 | 969 | 504 | |||
| Bone + Liver +/− Other | 16 | 15.8 | 58 | 11.2 | 58 | 7.4 |
| Bone or Liver +/− Other | 118 | 35.5 | 360 | 20.1 | 220 | 7.1 |
| Othera | 187 | 45.1 | 551 | 30.6 | 226 | 10.4 |
| P-value | 0.016 | <0.0001 | 0.005 | |||
| TTF | 320 | 960 | 497 | |||
| Bone + Liver +/− Other | 16 | 5.0 | 57 | 5.3 | 55 | 3.1 |
| Bone or Liver +/− Other | 118 | 11.3 | 355 | 7.4 | 218 | 3.8 |
| Othera | 186 | 12.3 | 548 | 8.3 | 224 | 4.2 |
| P-value | 0.073 | 0.009 | 0.100 | |||
Other is defined as sites of metastasis excluding bone and liver.
Figure 2.
Kaplan-Meier plots of OS by site of metastasis in patients with IMDC favorable, intermediate, and poor risk group, respectively.
Stratified by type of targeted therapy
In this cohort, the majority of patients received first-line treatment with sunitinib (74%). For OS, results were similar regardless of first-line therapy. The HR among patients receiving sunitinib, sorafenib and other therapies was 1.40 (95% CI 1.21–1.62), 1.56 (95% CI 1.16–2.11) and 1.50 (95% CI 0.91–2.47), respectively, for the presence of either BM or LM, and was 1.87 (95% CI 1.45–2.43), 2.14 (95% CI 1.29–3.55) and 1.63 (95% CI 0.87–3.08), respectively, for the presence of both BM and LM compared to patients with other sites of metastases. For TTF, presence of both BM and LM had a poorer TTF in all treatment groups compared to other sites of metastasis. There was no difference between presence of BM or LM compared to other metastases in patients who received sorafenib or other therapies (data not shown).
The impact of other metastatic sites on clinical outcomes
Table 3 describes the OS and TTF estimates for patients with lung, lymph node, or brain metastases without BM and LM. In patients with a single metastatic site, lung, lymph node, brain, or other metastasis sites had improved OS compared to either BM or LM. In patients with two or more metastatic sites, patients with lung, lymph node, brain, or other metastasis sites without BM or LM, has improved OS compared those with either BM, LM or both.
Discussion
In our study, the presence of BM and LM was associated with a clinically significant negative impact on survival. Our series is currently the largest to date, with over 2,000 patients, evaluating the effect of not only BM but also LM on outcomes of patients with RCC. Additionally, in our series, we included RCC patients of all histological subtypes. As the treatment paradigm for patients with metastatic RCC is rapidly evolving, prognostic factors need to reflect changes in systemic therapy. Our series includes patients treated in the current era of targeted therapy, receiving a broad range of first-line agents (eight in total), differentiating it from other analyses evaluating the impact of BM and LM in patients with RCC.
Many prognostic factors have been investigated in RCC and multiple prognostic models have been developed. The presence of BM and/or LM has been evaluated and shown to be prognostic in some models, but not others given the correlation with factors associated with increased disease burden.[5–8] In our analysis, the significance of the likelihood ratio tests demonstrates that the addition of site of metastasis to the current IMDC prognostic model improves the predictive ability of the model.
In addition to providing prognostic information, data regarding site of metastasis may have therapeutic implications. Additionally, site of metastasis may inform the use of newer agents currently under investigation in RCC which may have preferential activity in bone, such as cabozantinib, and osteoclast-targeted agents. Other novel agents currently in preclinical development, including polymer-targeted angiogenesis inhibitors, which target angiogenesis-dependent BM, are of particular interest in patients with BM from RCC.[9]
In regards to BM, our findings are consistent with growing evidence that the presence of BM in metastatic RCC is associated with an adverse effect on outcome (Table 5). In a retrospective study of 223 patients with clear-cell metastatic RCC treated with first-line sunitinib, BM was associated with a shorter progression-free survival (PFS) (8.2 versus 19.1 months, p<0.0001) and OS (19.5 versus 38.5 months, p<0.0001).[10] In multivariable analysis, BM was the independent variable most significantly associated with poor PFS and OS. There are limited data regarding the impact of BM in patients treated with mTOR targeted therapy. In a phase III study of 416 patients with metastatic clear-cell RCC treated with everolimus after progression on VEGF-targeted therapy, presence of BM was an independent risk factor for shorter PFS and OS.[11] In our study, in multivariable analyses adjusting for IMDC risk factors, the presence of BM independently predicted worse survival for the overall cohort and when stratified by IMDC risk groups.
Table 5.
Summary of studies describing the impact of BM in RCC.
| Study | Patients | Agent | Response Rate | Median PFS (months) | Median OS (months) |
|---|---|---|---|---|---|
| Negrier et al (2002)[8] | 782 | Cytokine- based | NR | NR | 8.8 vs. 15.7a |
| Choueiri et al (2007)[19] | 120 | Anti-VEGF therapy | NR | 10.7 vs. 16.5 | NR |
| Donskov et al (2006)[5] | 120 | Cytokine- based | NR | NR | 12 vs. 19a |
| Riechelmann et al (2008)[20] | 58 | Sorafenib | NR | 4.7 vs. 11.2a | NR |
| Beuselinck et al (2011)[10] | 223 | Sunitinib | 35% vs. 55%a | 8.2 vs. 19.1a | 19.5 vs. 38.5a |
| Patil et al (2011)[21] | 375 | Sunitinib | NR | NR | BM independent risk factor for worse OS |
| Motzer et al (2010)[11] | 416 | Everolimus | NR | BM independent risk factor for worse PFS | BM independent risk factor for worse OS |
Signifies statistical significance.
Not recorded (NR).
The reason for worse clinical outcomes associated with BM has yet to be elucidated. A possible explanation includes interactions between tumor cells and the bone microenvironment which results in a vicious cycle of bone destruction and tumor growth.[12] Another possible explanation for worse clinical outcomes associated with BM may include the limited distribution of targeted agents to bone. Given that BM warrant special consideration for measurability, bone response to targeted agents has not been evaluated in patients on clinical trials. Additionally, patients with bone-only metastases are often excluded from clinical trials given lack of measurable disease by response criteria.
Given the relationship between BM and the bone microenvironment, it is logical to investigate the use of osteoclast-targeted therapy in metastatic RCC. Currently approved agents for the prevention of SREs in these patients include zoledronic acid, a potent bisphosphonate, and denosumab, a monoclonal antibody against the receptor activator of nuclear factor-κβ ligand, a cytokine important in osteoclast differentiation, activation, and survival.[13] Retrospective data suggest that bisphosphonate therapy combined with VEGF-targeted therapy may improve survival in patients with metastatic RCC, however may be associated with an increased risk of osteonecrosis of the jaw.[14, 15] Additionally, treatment with zoledronic acid was found to potentiate the effects of mTOR inhibition in preclinical studies.[16]
In our series, the presence of LM was also associated with a negative impact on outcomes. Limited studies have suggested worse outcomes in patients with metastatic RCC with LM. In a retrospective review of patients with metastatic RCC treated with first-line sunitinib, presence of LM was associated with a shorter PFS (11.3 versus 16.1 months, p=0.3) and OS (23.6 versus 30.7 months, p=0.3), which was not statistically significant.[10] In a study of patients with metastatic clear-cell RCC treated with everolimus after progressing on VEFG-targeted therapy, the presence of LM was an independent risk factor for shorter PFS and OS (HR 1.42, p=0.016 and HR 1.64, p<0.001, respectively).[11]
The rational for worse outcomes in patients with LM is uncertain. Most targeted agents are metabolized in the liver and it is possible that patients with LM have some degree of liver dysfunction. Therapy with targeted agents in this setting could lead to dose reductions and delays, thus compromising efficacy. Additionally, given significant intratumor heterogeneity in patients with metastatic RCC, the unique organ microenvironment of the liver may be selective for a more aggressive clinical phenotype.[17, 18] Molecular characterization of both BM and LM in patients with RCC may help inform the development of improved prognostic and predictive biomarkers.
There are several limitations to our study. Data was collected retrospectively. Although the included patients are consecutive patients, there is the probability of a selection bias. Our data did not capture information regarding tumor volume, though we did perform a subset analysis based on single or multiple sites of disease. Additionally, we did not focus on second-line therapies and use of osteoclast-targeted therapies. Given that we did not have information regarding the reason for treatment discontinuation, we were unable to calculate PFS estimates.
Conclusions
In conclusion, the presence of BM and LM in patients with metastatic RCC treated with targeted therapy has a negative impact on survival. Site of metastasis may possibly be used for risk-stratification of patients with metastatic RCC. In addition to providing prognostic information, data regarding site of metastasis may have therapeutic implications in guiding clinical decision making.
Acknowledgments
Funding: None.
Footnotes
Financial Disclosures: J. Lee has received honoraria from Novartis, Bayer, and Pfizer and has received research funding from Bayer. J. J. Knox has been a consultant and played an advisory role at Aveo and has received research funding from Pfizer. G. A. Bjarnason has been a consultant and played an advisory role at Pfizer and received honoraria and research funding from Pfizer. M. J. MacKenzie has an advisory role at Novartis and Pfizer and has received research funding from both. L. Wood has an advisory role at Pfizer and Novartis and has received research funding from Pfizer, Novartis, and GlaxoSmithKline. U. N. Vaishamayan has received honoraria and research funding from Pfizer, Novartis, and GlaxoSmithKline. S. Y. Rha has an advisory role at Novartis, Pfizer, and GlaxoSmithKline and has received research funding from Novartis and Bayer Korea. F. Donskov has received research funding from Novartis. B. I. Rini has an advisory role at Pfizer, GlaxoSmithKline, Aveo, Bayer, and Onyx and has received research funding from GlaxoSmithKline and Pfizer. D. Y. C. Heng has an advisory role at Aveo, Pfizer, Novartis, and Bayer. T. K. Choueiri has received research funding from Pfizer and has an advisory role at Aveo, Pfizer, Novartis, GlaxoSmithKline, Genentech, Bayer, and Onyx. All remaining authors declared no conflicts of interest.
References
- 1.Bianchi M, Sun M, Jeldres C, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23:973–80. doi: 10.1093/annonc/mdr362. [DOI] [PubMed] [Google Scholar]
- 2.Woodward E, Jagdev S, McParland L, et al. Skeletal complications and survival in renal cancer patients with bone metastases. Bone. 2011;48:160–6. doi: 10.1016/j.bone.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–9. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 4.Pepe MS, Kerr KF, Longton G, Wang Z. Testing for improvement in prediction model performance. Stat Med. 2013;32:1467–82. doi: 10.1002/sim.5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donskov F, von der Maase H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J Clin Oncol. 2006;24:1997–2005. doi: 10.1200/JCO.2005.03.9594. [DOI] [PubMed] [Google Scholar]
- 6.Mekhail TM, Abou-Jawde RM, Boumerhi G, et al. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol. 2005;23:832–41. doi: 10.1200/JCO.2005.05.179. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Bukowski RM, Figlin RA, et al. Prognostic nomogram for sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2008;113:1552–8. doi: 10.1002/cncr.23776. [DOI] [PubMed] [Google Scholar]
- 8.Negrier S, Escudier B, Gomez F, et al. Prognostic factors of survival and rapid progression in 782 patients with metastatic renal carcinomas treated by cytokines: a report from the Groupe Francais d’Immunotherapie. Ann Oncol. 2002;13:1460–8. doi: 10.1093/annonc/mdf257. [DOI] [PubMed] [Google Scholar]
- 9.Segal E, Pan H, Ofek P, et al. Targeting angiogenesis-dependent calcified neoplasms using combined polymer therapeutics. PLoS One. 2009;4:e5233. doi: 10.1371/journal.pone.0005233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beuselinck B, Oudard S, Rixe O, et al. Negative impact of bone metastasis on outcome in clear-cell renal cell carcinoma treated with sunitinib. Ann Oncol. 2011;22:794–800. doi: 10.1093/annonc/mdq554. [DOI] [PubMed] [Google Scholar]
- 11.Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116:4256–65. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 12.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–93. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 13.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 14.Beuselinck B, Wolter P, Karadimou A, et al. Concomitant oral tyrosine kinase inhibitors and bisphosphonates in advanced renal cell carcinoma with bone metastases. Br J Cancer. 2012;107:1665–71. doi: 10.1038/bjc.2012.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keizman D, Ish-Shalom M, Pili R, et al. Bisphosphonates combined with sunitinib may improve the response rate, progression free survival and overall survival of patients with bone metastases from renal cell carcinoma. Eur J Cancer. 2012;48:1031–7. doi: 10.1016/j.ejca.2012.02.050. [DOI] [PubMed] [Google Scholar]
- 16.Moriceau G, Ory B, Mitrofan L, et al. Zoledronic acid potentiates mTOR inhibition and abolishes the resistance of osteosarcoma cells to RAD001 (Everolimus): pivotal role of the prenylation process. Cancer Res. 2010;70:10329–39. doi: 10.1158/0008-5472.CAN-10-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 19.Choueiri TK, Garcia JA, Elson P, et al. Clinical factors associated with outcome in patients with metastatic clear-cell renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. Cancer. 2007;110:543–50. doi: 10.1002/cncr.22827. [DOI] [PubMed] [Google Scholar]
- 20.Riechelmann RP, Chin S, Wang L, et al. Sorafenib for metastatic renal cancer: the Princess Margaret experience. Am J Clin Oncol. 2008;31:182–7. doi: 10.1097/COC.0b013e3181574084. [DOI] [PubMed] [Google Scholar]
- 21.Patil S, Figlin RA, Hutson TE, et al. Prognostic factors for progression-free and overall survival with sunitinib targeted therapy and with cytokine as first-line therapy in patients with metastatic renal cell carcinoma. Ann Oncol. 2011;22:295–300. doi: 10.1093/annonc/mdq342. [DOI] [PubMed] [Google Scholar]