Abstract
The Neuronal Ceroid Lipofuscinoses (NCL) are a group of fatal inherited neurodegenerative diseases in humans distinguished by a common clinical pathology, characterized by the accumulation of storage body material in cells and gross brain atrophy. In this study, metabolic changes in three NCL mouse models were examined looking for pathways correlated with neurodegeneration. Two mouse models; motor neuron degeneration (mnd) mouse and a variant model of late infantile NCL, termed the neuronal ceroid lipofuscinosis (nclf) mouse were investigated experimentally. Both models exhibit a characteristic accumulation of autofluorescent lipopigment in neuronal and non neuronal cells. The NMR profiles derived from extracts of the cortex and cerebellum from mnd and nclf mice were distinguished according to disease/wildtype status. In particular, a perturbation in glutamine and glutamate metabolism, and a decrease in γ-amino butyric acid (GABA) in the cerebellum and cortices of mnd (adolescent mice) and nclf mice relative to wildtype at all ages were detected. Our results were compared to the Cln3 mouse model of NCL. The metabolism of mnd mice resembled older (6 month) Cln3 mice, where the disease is relatively advanced, while the metabolism of nclf mice was more akin to younger (1–2 months) Cln3 mice, where the disease is in its early stages of progression. Overall, our results allowed the identification of metabolic traits common to all NCL subtypes for the three animal models.
Keywords: Juvenile Neuronal Ceroid Lipofuscinosis (JNCL), Batten disease, CLN3, NMR, Metabolomics, Neurodegeneration
Introduction
The NCLs are a family of genetically inherited metabolic storage diseases which exhibits a common pathology, manifesting lysosomal accumulation of autofluorescent lipopigment, neurodegeneration and premature death (Mann et al. 1978; Sekhon and Maxwell 1974). The hallmark of the symptoms of the NCLs is impaired vision, mental and motor deterioration often accompanied by ataxia, myoclonus and epilepsy. Traditional diagnosis of NCL gave rise to ‘classical’ NCL subtypes known as: infantile (CLN1), late infantile (CLN2), juvenile (CLN3) and adult (CLN4). Subsequent advances in genetic analyses led to the recognition of four additional NCL subtypes (Goebel and Sharp 1998), variants of the classical late infantile form (CLN2) and mutations in genes designated CLN5, CLN6, CLN7 and CLN8. The corresponding CLN3 protein is targeted to the lysosomes in non-neuronal cells (Jarvela et al. 1998; Kyttala et al. 2004), but is also localized to the presynaptic terminals and synaptosomes in primary neuronal cells (Luiro et al. 2001). The function of CLN3 has been analyzed in two yeast models, and is associated with vacuole function (Gachet et al. 2005; Kim et al. 2003; Pearce et al. 1999). In addition, mammalian studies have suggested a role for CLN3 in cytoskeletal function and membrane trafficking (Luiro et al. 2004).
The Cln3 mouse constitutes a model of juvenile NCL (Batten disease) whereby the mouse Cln3 gene, having 82% sequence identity (Lee et al. 1996) with human Cln3, has been disrupted. Evidence for metabolic dysfunction related to arginine metabolism in Cln3−/− mouse has been previously reported (Chan et al. 2009), as well as similar changes detected in a yeast model (Kim et al. 2003), and in lymphoblasts from patient cell lines bearing mutations in CLN3 (Ramirez-Montealegre and Pearce 2005). Studies carried out on Cln3−/− mice have also demonstrated disruption in the citrulline-Nitric oxide cycle (Chan et al. 2009).
Previously using metabolomic based techniques we demonstrated an increased concentration of glutamine and decreased concentrations of glutamate, N-acetyl aspartate and GABA in Cln3−/− mice (Pears et al. 2005). The reduction in the concentration of GABA appeared to parallel the loss of specific GABAergic inter-neurons reported in affected brains (Oswald et al. 2001), as well as suggesting changes in glutamate/glutamine/GABA cycling.
In addition to the Cln3−/− mouse there is a naturally occurring mouse mutant called the motor neuron degeneration (mnd) mouse, containing a one base pair insertion in the orthologous mouse Cln8 gene (82% homologous to human gene) resulting in a frame shift defect (Ranta et al. 1999). These mice have been shown to exhibit abnormalities akin to those in human NCL patients. Specifically, autofluorescent inclusions in interneuron, the retina and in many somatic organs (Bronson et al. 1998; Pardo et al. 1994). In addition, retinal degeneration is pronounced at 1 month of age with widespread loss of photoreceptor and retinal cells (Chang et al. 1994). Neurological and motor changes have also been documented in the mnd mouse with profound cortical atrophy which is apparent at 9 months of age with progressive loss of hippocampal and cortical GABAergic evident from 5 months of age (Cooper et al. 1999). Up regulation of glial fibrillary acidic protein (GFAP) occurred at 9 months of age, indicative of reactive gliosis. In addition we have previously shown that glutamate/glutamine cycling is altered in these mice (Griffin et al. 2002).
The neuronal ceroid lipofuscinosis (nclf) mouse is a model of late infantile NCL (Bronson et al. 1998). nclf mouse contain a one base pair insertion in the orthologous mouse Cln6 gene (90% homologous to human gene) resulting in a frame shift defect (Wheeler et al. 2002). Similar to the mnd mouse, nclf mouse exhibit a characteristic accumulation of autofluorescent lipopigment in neuronal and non neuronal cells. Cortical atrophy, progressive loss of hippocampal and cortical GABAergic interneurons and the development of cerebral gliosis have all been documented in nclf mice (Bronson et al. 1998; Mitchison et al. 1999). However, these mice have been shown to have a more protracted clinical course than mnd mice, exhibiting comparable symptoms but with a delay of ~2 months. For example, nclf mice are clinically normal until 8 months of age, at which time they develop spastic rear limb paresis which progresses to paralysis and eventually death by 12 months (Bronson et al. 1998).
Relative to the Cln3 mice, mnd and nclf mice display more profound morphological defects. Motor deterioration occurs much earlier with onsets of 4 and 8 months in mnd and nclf mice, respectively, compared to Cln3 mice where there is no obvious motor dysfunction until 16 months of age. Furthermore, retinal function is not compromised in Cln3 mice. It may be that the different genetic background of the Cln3 mouse (129S6) affects the penetrance of the mutation, making it difficult to make direct comparisons. Alternatively, the delayed phenotype of the Cln3 mouse may simply represent the more protracted clinical course of juvenile NCL (Batten disease). Indeed, another Cln3 knockout mouse, on a C57BL/6J background, also exhibits NCL like pathologies later than those in mnd and nclf mice (Katz et al. 1999). Here, we have implemented metabolomic techniques to investigate metabolite profiles in cortex and cerebellum brain regions of the mnd and the nclf mice, both exhibiting abnormalities akin to those in human NCL patients, and compared these changes with results found in the Cln3 mouse.
Materials and methods
Mice sample collection
Tissue from wildtype, mnd (Chang et al. 1994) and nclf (Bronson et al. 1998) mice on a C57/BL background were taken from stable mouse colonies at Rochester University, NY, USA (Table 1). Colonies were housed in micro isolator cages on Lab diet 5010 (PMI Nutritional International, Brentwood, MO, USA) under a strict 12 h light/dark cycle, at 22°C with a relative humidity of 70–75%. Tissue was taken rapidly (within 30 s) after sacrifice by cervical dislocation, snap-frozen in liquid nitrogen and stored at −80°C. All procedures conformed to the National Institutes of Health guidelines and the University of Rochester Animal Care and Use Committee Guidelines. The cortex and cerebellum (20–50 mg wet weight) were dissected on ice and the individual samples stored in micro-centrifuge tubes at −80 °C.
Table 1.
mnd and nclf brain tissues phenotyped by metabolomic analyses
| Genotype | Age (months) | n number |
|---|---|---|
| C57BL/6J control | 6 | 7 |
| 9 | 4 | |
| 19 | 6 | |
| mnd | 6 | 7 |
| nclf | 6 | 8 |
| 9 | 4 | |
| 18 | 6 |
Tissues are defined by age and n number
Extraction and preparation of aqueous metabolites
Frozen cortex and cerebellum brain tissue samples (~50 and 20 mg, respectively) were ground on dry ice using a pestle and mortar, and methanol and chloroform in a ratio of 2:1 (v/v; 1 ml) added (Le Belle et al. 2002). Homogenates were sonicated for 15 min, after which 200 μl each of chloroform and distilled water were added. The phases were separated by centrifugation, and the upper aqueous phase was removed and dried in an evacuated centrifuge (Eppendorf, Hamburg, Germany). Subsequently, the dried tissue extracts were reconstituted in 600 μl of 50 mM sodium phosphate buffer in D2O (pH 7.4), which contained 0.25 mM sodium (3-trimethylsilyl)-2,2,3,3-tetra-deuteriopropionate (TSP) (Cambridge Isotope Laboratories, Inc, USA) as a chemical shift standard, and placed into 5 mm NMR tubes (Wilmad-Lab Glass, New Jersey, USA).
1D 1H NMR data acquisition
One-dimensional spectra were acquired using a conventional pre-saturation pulse sequence for water suppression based on the first increment of the nuclear Overhauser effect spectroscopy (NOESY) pulse program (relaxation delay-90°-t1-90°-tm-90°-acquire) (Macura and Huang 1981), whereby the water resonance was irradiated during the relaxation delay. Spectra for C57BL/6J control, mnd and nclf mice aged 6 months were recorded on DRX500 Bruker (Bruker BioSpin GmbH, Rheinstetten, Germany) at a proton frequency of 500 MHz at 27°C (relaxation delay = 1.3 s, mixing time of tm = 150 ms, t1 = 3 s). Samples were analyzed using 3 mm NMR tubes, 196 scans with a spectral width of 12.00 ppm. For the C57BL/6J control, mnd and nclf mice aged 9 and above spectra were recorded on a Bruker Avance III spectrometer, equipped with a 5 mm TXI ATMA probe (Bruker BioSpin GmbH, Rheinstetten, Germany) using 5 mm tubes at a proton frequency of 500.3 MHz, temperature of 27°C (relaxation delay = 2.0 s, tm = 50 ms, with t1 fixed at 4 μs). Each spectrum was acquired with 128 scans collected into 64 k data points with an acquisition time of 4.09 s and spectral width of 16.00 ppm.
All spectra were processed using 1D NMR Manager software (Advanced Chemistry Development Inc., Toronto, Canada). Spectra were multiplied by an exponential weighting function corresponding to a line broadening of 0.3 Hz and Fourier transformed from the time to the frequency domain. Spectra were phased, baseline corrected and referenced to the TSP singlet at 0.00 ppm. Spectra were segmented into 0.04 ppm chemical shift bins between 0.2 and 9.8 ppm (excluding water and TSP resonances) using a fixed bucket size of 0.04 and the Intelligent Bucketing facility within ACD NMR Manager (projection method and 50% looseness factor implemented). To account for differences in sample volumes, each integrated region was normalised to the total spectral area for all integral regions in that spectrum, excluding water and TSP resonances. Spectra were assigned by comparison with assignment tables (Lindon et al. 1999; Govindaraju et al. 2000; Pears et al. 2005; Salek et al. 2008; Salek et al. 2010) and on-line databases (http://www.bmrb.wisc.edu/metabolomics and http://mmcd.nmrfam.wisc.edu) such that metabolites contributing to each bin were defined.
Chemometric analyses
Multivariate data analysis was carried out using SIMCA-P + 12.0 (Umetrics AB, Umeå, Sweden) with the data mean centred and Pareto scaled prior to analysis. Pareto scaling augments the representation of low concentration metabolites in statistical models by dividing each integral region or variable by the square root of the standard deviation of the variable, without increasing the contribution spectral noise makes to the model. Three different multivariate statistical techniques were used within the SIMCA package; principal components analysis (PCA) and projection to latent structures by partial least squares discriminant analysis (PLS-DA) and Orthogonal PLS-DA (OPLS-DA). PCA is an unsupervised technique that describes observations (i.e. spectra from mouse brain tissue) with regards to one or more latent variables termed principal components (PCs) which are linear combinations of the original variables (NMR buckets). PLS-DA and OPLS are supervised techniques separating observations based on class-membership. In OPLS-DA the objective is to remove the variation in the model that is orthogonal to response and this can produce more easily interpretable models where the first component represents the major changes that distinguish the two groups (Trygg and Wold 2002). The weights given to each original NMR variable within any given principle component describe how influential that particular variable is and the relation (or correlation) of the variables to each other. To determine which of the original variables are responsible for this separation the loading scores and correlation coefficients of the loadings for each model was analysed (Jackson 1991; Wold et al. 1984). The first component of the loading plots was plotted against NMR chemical shifts to complement the scores plot. The coefficient values were multiplied by a factor to represent both their contribution to the loadings scores and their significance. The significance of the contribution was determined by multiplying (1-p-value) against the coefficient. The p-value itself was calculated using a Student’s t test on the coefficients for a jack-knifing routine used to calculate the contribution from each variable made to a given component. The parameters R2 and Q2 were used to evaluate the performance of each model. The R2 score indicates how much of the total variation in the dataset is described by a particular component and indicates the variation described by all the components in the model. Q2 estimates the predictive ability of the model by leaving out observations (1/7th) from the building of the model and then predicting their class membership or trend variable. All PLS-DA and OPLS-DA models were assessed for the risk of being spurious i.e., the model fits the training set well but does not accurately predict Y for new observations (Eriksson et al. 1999) using the validate function within the SIMCA package.
Results
A metabolomics approach was used to define biochemical abnormalities associated with two mouse models of NCL subtypes, mnd and nclf mice, associated with late variant infantile NCL (CLN8) and CLN6 NCL subtypes, respectively. Our objective was to acquire metabolic changes across two NCL subtypes at different ages and to compare the above models to the previously described Cln3 mouse model (Pears et al. 2005). The 1H NMR spectral profiles from the cortex (Fig. 1) and cerebellum of wildtype, mnd and nclf mice were examined for metabolic abnormalities.
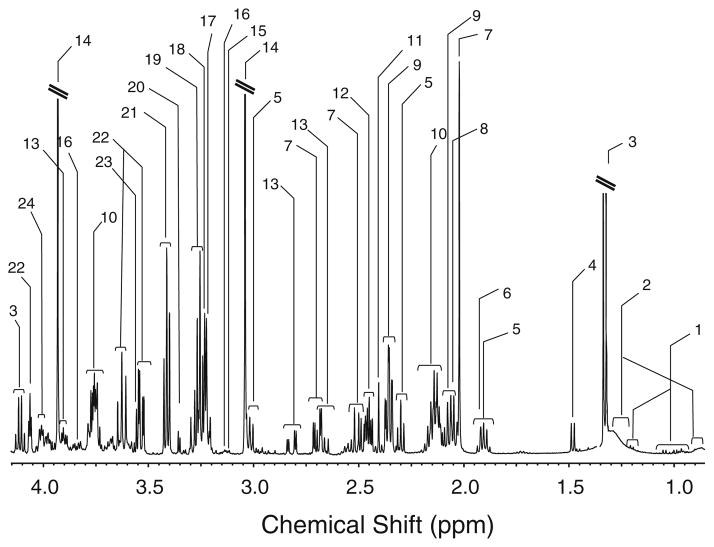
Fig. 1.
A high resolution solution 1H NMR spectrum of an aqueous extract of cortex tissue taken from a mnd mice, recorded at a proton frequency of 500.13 MHz. Peak 1, valine, leucine, isoleucine; peak 2, fatty acids; peak 3, lactate; peak 4, alanine; peak 5, γ-amino butyric acid (GABA); peak 6, acetate; peak 7, N-acetyl-L-aspartate (NAA); peak 8, N-acetyl-aspartyl-glutamate (NAAG); peak 9, glutamate; peak 10, glutamate and glutamine; peak 11, glutamine; peak 12, succinate; peak 13, aspartate; peak 14, phospho-creatine; peak 15, malonate; peak 16, ethanolamine; peak 17, choline; peak 18, phosphocholine; peak 19, taurine and myo-inositol; peak 20, scyllo-inositol; peak 21, taurine; peak 22, myo-inositol; peak 23, glycine peak 24 phosphoethanolamine
Young (6 months) mnd and nclf mice both exhibit distinct cerebral metabolic profiles relative to control mice
Wildtype and mnd 1H NMR spectral profiles were readily separated by PLS-DA when the cortex and cerebellum brain regions were considered separately (Fig. 2a, b). Analysis of the variable coefficients for these models identified several metabolic perturbations responsible for each separation (Table 2). Lactate, glutamine, choline, phosphocholine (PC) and glycerophosphocholine (GPC) were increased in concentration in mnd cortex and cerebellum tissues relative to wildtype, whilst creatine, γ-aminobutyric acid (GABA), taurine and myo-inositol were decreased. In addition, N-acetyl aspartate (NAA) and aspartate were decreased in the mnd cortex relative to wildtype, whilst isoleucine/valine/leucine, scyllo-inositol and ethanolamine were increased in the cortex. Glutamate was increased in the cortex, but decreased in the cerebellum.
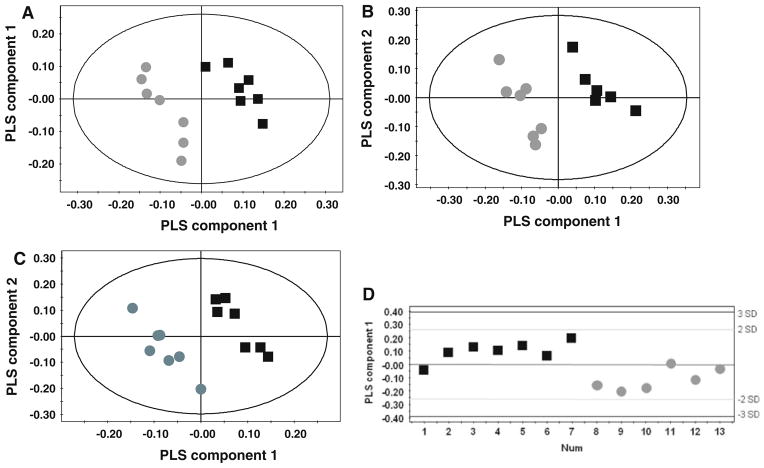
Fig. 2.
Pattern recognition models of metabolic changes identified in the brains of mnd, nclf and wildtype mice. PLS-DA score plots distinguishing wildtype (black square) and mnd (dark grey circle) 1H NMR metabolic profiles derived from a the cortex [R2Y = 98%; Q2 = 77%], and b cerebellum [R2Y = 95%; Q2 = 68%]. Two components used for each model. PLS-DA score plots distinguishing wildtype (black square) and nclf (dark grey circle) 1H NMR metabolic profiles derived from c cortex [R2Y = 97%; Q2 = 78%] using two component and d cerebellum brain tissue [R2Y = 67%; Q2 = 33%], one component
Table 2.
Metabolic changes detected by multivariate data analysis in mnd and nclf cerebral tissue
| Tissue | Relative increase in mnd | Relative decrease in mnd |
|---|---|---|
| Cortex | Choline/PC | Creatine |
| GPC | GABA | |
| Lactate | Taurine | |
| Glutamine | NAA | |
| Glutamate | Aspartate | |
| Ethanolamine | Myo-inositol | |
| Isoleucine/valine/leucine | ||
| Scyllo-inositol | ||
| Cerebellum | Lactate | Creatine |
| Choline | Taurine | |
| PC and GPC | Glutamate | |
| Glutamine | Myo-inositol | |
| GABA |
| Relative increase in nclf | Relative decrease in nclf | |
|---|---|---|
| Cortex (1 and 6 month) | Glutamate | Choline/PC |
| Glutamine (less) | Creatine | |
| NAA | Myo-inositol | |
| Asp | GABA | |
| Succinate | GPC | |
| Cerebellum (1 and 6 month) | Lactate | Creatine |
| Glutamine | Myo-inositol | |
| Asp | GABA | |
| Choline/PC | ||
| GPC | ||
| Glutamate | ||
| Taurine | ||
| Cortex (18 month) | Choline/PC | Glutamine |
| GPC | Lys-Leu/Ile-Val | |
| Glutamate | ||
| Cerebellum (18 month) | Taurine | Choline/PC |
| Glutamate | GPC | |
| Lys-Leu/Ile-Val | Myo-inositol | |
| GABA | ||
| NAA | ||
| Asp | ||
| Creatine |
Changes are listed for each of the brain regions examined. For the nclf mice there are two age groups of young (6 months) and older mice (18 months)
Similarly, wildtype and nclf 1H NMR profiles (mice aged 6 months) were separated by PLS-DA when the cortex and cerebellum were considered separately (Fig. 2c, d) and several metabolic changes identified (Table 2). In the nclf mice, glutamate, glutamine, succinate, aspartate and NAA were increased in the cortex, while lactate, glutamine and aspartate were relatively increased in the cerebellum compared to wildtype. In both the cortex and cerebellum, choline/PC, GPC, creatine, myo-inositol and GABA were decreased in nclf mice relative to wildtype. In addition glutamate and taurine were also decreased in the cerebellum.
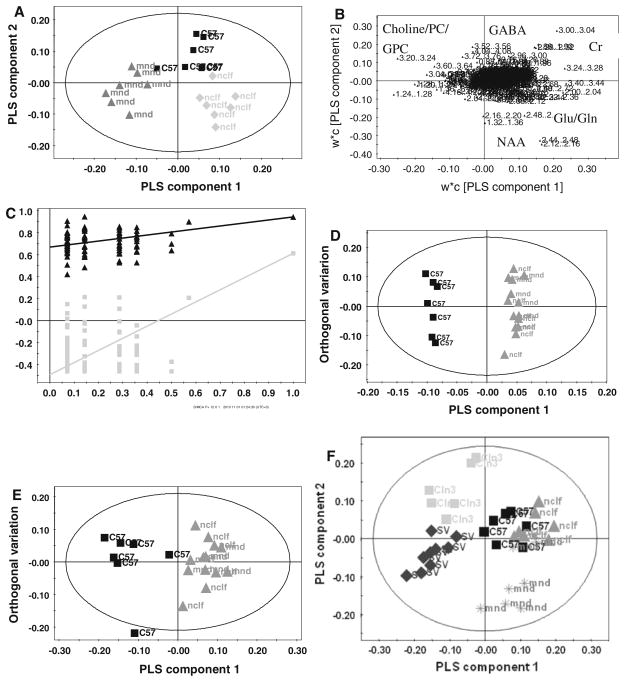
1H NMR profiles from mnd, nclf and control mice were examined together to identify metabolite changes dominant for each mouse model. Separation, by PLS-DA between wildtype, mnd and nclf profiles was apparent in the cortex (Fig. 3a, with the relevant loading and validation plot shown in Fig. 3b–c) and cerebellum (Data not shown). In the cortex model, PLS component 1 described the variation associated with mouse strain while PLS component 2 represented disease associated variation common to both mnd and nclf mice. Analysis of component 1 and 2 loadings, revealed glutamine, glutamate and NAA were relatively increased in the nclf mouse cortex whereas creatine, GABA and taurine were increased in wildtype controls. Additionally choline/PC, aspartate and myo-inositol were relative increased in the mnd mice compared to the wild-type and nclf mice. An OPLS-DA model (Fig. 3d) combining both models of NCL against the control mice revealed common metabolic traits. The metabolic changes were an increase in glutamate, glutamine and NAA while GABA, creatine and myo-inositol decreased in the mouse models of NCL.
Fig. 3.
Comparison of different mouse models of NCL. a PLS-DA of 1H NMR profiles derived from the cortex of wildtype (black square), mnd (grey triangle) and nclf (light grey diamond) mice [R2Y = 95%; Q2 = 69%] using 4 components. b The relevant loading plot, with important metabolites for classification highlighted and c the validation plot for model (a) as an example, assessing validity and the degree of overfit for the model. The validation plot for the first PLS-DA component of the PLS-DA model in A is shown. The validation plot displays the correlation coefficient between the original classification variables (Y-matrix) and the randomly permuted classification variables versus the cumulative R2(black triangle) and Q2(light grey square) for these models and draws the regression line. The intercept is a measure of the over fit. d An OPLS-DA score plot from cortex brain region separating the NCL mice model (dark grey triangle mnd and dark grey triangle nclf) from control mice (black square)[R2Y = 98%; Q2 = 67%] e OPLS-DA score plot separating the cerebellum of NLC mutants (dark grey triangle mnd and dark grey triangle nclf) from the wildtype (black square)[R2Y = 85%; Q2 = 38%]. f PLS-DA score plot of 1H NMR profiles derived from the cortex of wildtype—c57 background (black square), wildtype—sv 129 (SV-black diamond) mnd (dark grey asterisks), nclf (dark grey triangle) and Cln3 (light grey square) mice [R2Y = 40%; Q2 = 30%] using 2 components
The PLS-DA model for extracts of the cerebellum from wildtype, mnd and nclf mice was less predictable than the model for the cortex (Q2 of 30% compared with 69%; data not shown). The disease effect dominated component 1, which separated mainly the wildtype mice from the two mouse models of NCL, while component 2 mainly separated the mnd from nclf mice. An OPLS-DA model comparing the combined grouping of the two NCL mouse models against their wildtype controls (Fig. 3e) highlighted metabolites contributing to the disease separation; these were relative increases in lactate, glutamate and glutamine and decreases in creatine, NAA, myo-inositol, taurine and GABA in the cerebellum of mutant mice. Adding the Cln3 age matched data set and its control group to the dataset, and building a PLS-DA model for the young mice allowed the separation of the mnd, nclf and their wildtype (C57BL background) mice from Cln3 and its wildtype mice (sv129 background) along component 1 (Fig. 3f). The separation along component 1 was mostly associated with strain difference while component 2 separated wildtype from the Cln3 mice as well as mnd from nclf and control mice. Analysis of the loading plot and correlation coefficients of the loading (data not shown) indicated that lactate was increased in the sv129 wildtype, GABA, NAA, myo-inositol and glutathione in the Cln3 mice, glutamate and taurine in the nclf mice and glutamine, creatine and choline/PC were increased in the mnd mice. However, in this model there was no clear separation between the wildtype (C57BL background) and the nclf mice.
The changes in the relative concentrations of GABA, NAA, glutamate and glutamine identified by the multivariate statistics were considered as ratios of GABA/Glu, Gln/Glu and NAA/Glu for the cortex (Table 3). The Gln/Glu ratio was decreased for all models of NCL compared to the wildtype, with changes being more significant for the mnd and Cln3 mouse models. However, the ratios of GABA/Glu and NAA/Glu were only significantly different in the mnd and Cln3 mouse models.
Table 3.
Metabolic changes for GABA, NAA, glutamate and glutamine represented as ratios from the NMR spectra for the cortex brain region without any overlapping peaks
| C57 vs. mnd | C57 vs. nclf | 129 vs. Cln3 young | 129 vs. Cln3 old | |
|---|---|---|---|---|
| GABA/Glu | ↑0.04 | – | – | ↑0.05 |
| Gln/Glu | ↓0.03 | ↓0.05 | ↓0.002 | ↓0.02 |
| NAA/Glu | ↑0.008 | – | – | ↑0.05 |
A Student t test was carried out to determine the significance of the changes (reported for significant changes)
Metabolic changes in older nclf mice (18 months)
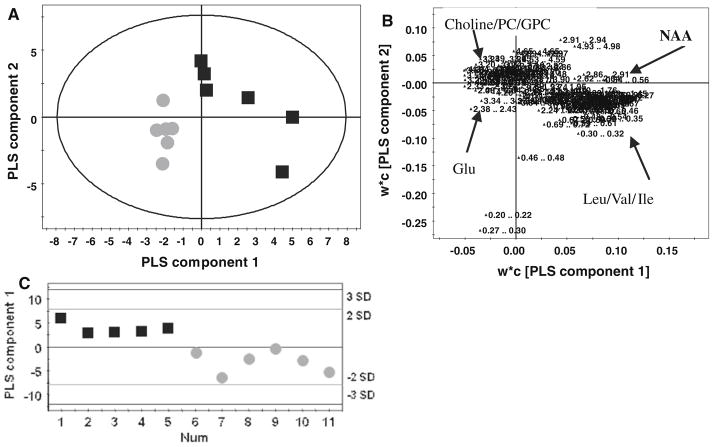
The young nclf group (6 month) for both the cortex and cerebellum spectra were readily separated from the spectra for the 9 and 18 months old animals. Studying the correlation coefficient of variables (data not shown) for the PLS-DA model of 9 versus 18 months old nclf mice identified several metabolites relevant to the difference associated with aging in mice. The mice aged 18 month had relatively decreased concentrations of taurine and GABA and increased concentrations of NAA, aspartate, myo-inositol and creatine compared to mice at 9 months. The cortex of the nclf mice at 18 months compared to wildtype (19 months) had increased concentrations of choline/PC, GPC and glutamate while glutamine, leucine, isoleucine and valine were decreased (Fig. 4a–b). The cerebellum of 18 month wildtype mice were also separated from the aged match nclf mice by PCA and PLS-DA analysis. Taurine, glutamate, leucine/isoleucine and valine were increased while choline/PC, GPC, myo-inositol, scyllo-inositol, GABA, aspartate and creatine were decreased in the nclf mice compared to wildtype (Fig. 4c). For the mnd mice aged 6 months and the nclf mice aged 9 months the n number was not sufficient (n = 4—Table 1) to produce reliable and valid models.
Fig. 4.
a PLS-DA score plot separating the cortex of the 19 month control mice (black square) from the (dark grey circle) 18 months nclf mice [R2Y = 61%; Q2 = 47%] b The relevant loadings plot for model a with important metabolites for classification highlighted. c PLS-DA of 1H NMR profiles derived from the cerebellum of wildtype (black square) and nclf (dark grey circle) mice [R2Y = 79%; Q2 = 63%]
Discussion
The systematic analysis of several NCL mouse models, encompassing an array of NCL subtypes, has enabled the definition of a catalogue of neuropathological landmarks for each NCL subtype (Cooper 2003). It is apparent that many of these mice share several degenerative features, including regional atrophy and loss of specific interneuronal populations in the cortex and hippocampus (Bible et al. 2004; Cooper 2003; Cooper et al. 1999; Mitchison et al. 2004; Pontikis et al. 2004). In this study, the metabolomic analysis using 1H NMR spectroscopy of the mnd and nclf mouse models, in addition to the Cln3 mouse, has enabled a comparative study of these models in terms of their metabolism in the cortex and cerebellum.
Similar metabolic traits were apparent in each NCL mouse model suggesting a common metabolic trend. In particular, glutamate was increased and GABA, creatine and myo-inositol were decreased in the cortices of mnd and nclf mice relative to wildtype. These changes were defined in models considering mnd and nclf profiles individually, as well in a model where the profiles were combined and analysed. Other metabolic changes were different between the two mouse models; choline and phosphocholine were decreased in the cortex of nclf mice and NAA and aspartate concentrations were decreased in the mnd mice. Further changes were observed which were specific to the individual mouse models. Therefore, while common metabolic trends were apparent between models of different NCL subtypes, unique metabolic perturbations also existed.
The cerebellum tissue from each NCL model was less predictable for genotype than the cortex which suggests that the cerebellum suffers a less severe phenotype than the cortex brain regions, in keeping with what is observed histologically. Metabolite changes identified in the cerebellum of both NCL models were similar to the cortex and included a relative increase in glutamine and lactate whilst glutamate, creatine, myo-inositol, GABA and taurine were decreased relative to wildtype. The differences between the NCL models included an increased choline/PC and GPC concentrations in mnd mice whereas the latter was reduced in young nclf but increased as mice aged. A similar increase in choline metabolites was also reported in the Cln3 mice cortex (Pears et al. 2005). While a direct comparison between the Cln3 mouse and the other two models is confounded by differences in strain backgrounds, the changes in phosphocholine are common across all three models. This in turn is suggestive of deregulated phospholipid metabolism. Perturbed phospholipid metabolism has also been previously documented in both infantile and juvenile NCL patients (Kakela et al. 2003). In addition, in the cortex of the aged nclf mice, the relative concentration of glutamine changed from being increased to decreased compared with wildtype controls. These changes detected in the aged nclf cortex are similar to that found in the mnd and Cln3 mice. Other changes in the cerebellum of older nclf mice are an increase in glutamate and taurine and a decrease in aspartate in contrast to the young nclf mice. One caveat of the results from older nclf mice is that it was not possible to get perfectly matched aged controls at 18 months due to the complications and costs associated with breeding aged mice. However, both the wildtype and nclf transgenic mice would be classed as aged mice and we would expect only minor changes as the mice aged from 18 to 19 months.
A decrease in GABA was observed in all NCL models. It has previously been suggested that the decrease in concentration of GABA detected in Cln3 mice is due to the inhibitive action of autoantibodies for glutamic acid decarboxylase (GAD65) without compensation by the second GAD isoform, GAD76 (Pears et al. 2005; Chattopadhyay et al. 2002). However, such autoantibodies have not been detected in mnd and nclf mice. Therefore, given that all the mouse models display a loss of GABAergic neurons, it is likely that the decrease in GABA, at least in mnd and nclf mice, is reflective of GABAergic neuronal cell loss (Bronson et al. 1998; Cooper et al. 1999; Mitchison et al. 1999; Pontikis et al. 2004).
Changes in glutamate and glutamine metabolism were apparent in all NCL models. Collective changes in glutamate, glutamine and GABA are suggestive of a neurotransmitter cycling perturbation, as has been previously proposed in the Cln3 mouse (Pears et al. 2005). Given that such cycling impairments have been observed in other neurological disorders, including epilepsy and Huntington’s disease (Behrens et al. 2002; Burbaeva et al. 2003; Petroff et al. 2002), it is not surprising that this is a common trait across a range of NCL subtypes. Dysfunction of neurotransmitter cycling is likely to have a significant impact on the underlying NCL pathophysiology. The increase in glutamate concentration relative to GABA is consistent with, and extends the temporal scope of, a previous investigation, which revealed a similar increase in glutamate concentration at 3 months of age (Chattopadhyay et al. 2002). Since glutamate is the principle excitatory neurotransmitter, if this increased glutamate accumulates extracellularly it may result in increased stimulation of specific neurons leading to excitotoxicity. From an overall comparison of the cortex of mouse models of NCL, we detected that the highest perturbation in glutamate and glutamine metabolism was in the aged nclf and mnd mice as well as the largest perturbation in choline metabolism being found in mnd mice.
The metabolic profiles (Tables 2, 3) of mnd mice were more akin to older Cln3 mice where the disease is relatively advanced, with an increase in choline/PC, glutamate and glutamine and decreases in the ratio of glutamine to glutamate, GABA, taurine and NAA (cortex only), relative to wildtype. Conversely, nclf mice were more akin to younger Cln3 mice where the disease is in its early stages of progression (Table 3). These mice had an increase in glutamate and NAA within the cortex, and a decrease in GABA and choline/PC. This is consistent with mnd mice having an earlier clinical onset than nclf mice (Bronson et al. 1998; Chang et al. 1994). NAA has been suggested to be a precursor in myelin production by oligodendrocytes, providing a link between NAA degradation, lipid metabolism/transport, and metabolism within glia cells (Chakraborty et al. 2001). A decrease in the concentration of NAA in mnd mice suggest a potential impaired transportation of NAA from the mitochondrial production site to where it is metabolized. In addition, reductions in NAA have been linked to neuronal cell loss or thought to reflect changes in energy status of neurones in a number of neurodegenerative disorders. In addition, the PLS-DA model built using mnd, nclf, Cln3 and their matched wildtype mice showed the nclf metabolic profile was most similar to that of control mice (close proximity of the clusters) suggesting a metabolic profile closer to that of wildtype rather than the mnd mice.
The results for the mnd mouse extend the temporal scope of a previous 1H NMR metabolomic investigation, where the metabolic deficits associated with mnd mice aged 6 months were defined (Griffin et al. 2002). Glutamate was found to be increased, and glutamine and GABA decreased, in mnd mice relative to wildtype. Whilst a decrease in GABA concentration is consistent with the results of the current study, changes in glutamate and glutamine conflict. This may be because of differences in age as the relative proportions of glutamate and glutamine vary markedly with age as in Cln3 mice (Pears et al. 2005).
Using a solution state 1H NMR based metabolomic approach, profound metabolic changes have been identified in specific brain regions of mnd and nclf mice. Moreover, a comparative analysis of three NCL mouse models has enabled metabolic similarities and differences to be extracted. In the brain of the effected animal, a common metabolic trait comprises changes in glutamate, glutamine and GABA that is suggestive of impaired neurotransmitter cycling.
Acknowledgments
The authors would like to thank Dr. ChunHung Chan for help preparing the mice. This work was partially supported by Merck Sharp Dohme, NIH R21NS060185, NIH R21DK070288-01 and the Batten Disease Support and Research Association.
Contributor Information
Reza M. Salek, Department of Biochemistry and the Cambridge Systems Biology Centre, University of Cambridge, 80 Tennis Court Road, Cambridge, CB2 1GA, UK
Michael R. Pears, Department of Biochemistry and the Cambridge Systems Biology Centre, University of Cambridge, 80 Tennis Court Road, Cambridge, CB2 1GA, UK
Jonathan D. Cooper, Pediatric Storage Disorders Laboratory, Department of Neuroscience, Institute of Psychiatry, King’s College London, London, UK
Hannah M. Mitchison, Department of Paediatrics and Child Health, Royal Free and University College Medical School, London, UK
David A. Pearce, Department of Pediatrics, Sanford School of Medicine of the University of South Dakota, 2301 East 60th Street North, Sioux Falls, SD 57104-0589, USA
Russell J. Mortishire-Smith, Johnson & Johnson PR & D, Turnhoutsweg 30, 2340 Beerse, Belgium
Julian L. Griffin, Email: jlg40@mole.bio.cam.ac.uk, Department of Biochemistry and the Cambridge Systems Biology Centre, University of Cambridge, 80 Tennis Court Road, Cambridge, CB2 1GA, UK. Department of Biochemistry, University of Cambridge, Building O, Downing Site, Tennis Court Road, Cambridge CB2 1QW, UK
References
- Behrens PF, Franz P, Woodman B, Lindenberg KS, Landwehrmeyer GB. Impaired glutamate transport and glutamate-glutamine cycling: downstream effects of the Huntington mutation. Brain. 2002;125(Pt 8):1908–1922. doi: 10.1093/brain/awf180. [DOI] [PubMed] [Google Scholar]
- Bible E, Gupta P, Hofmann SL, Cooper JD. Regional and cellular neuropathology in the palmitoyl protein thioesterase-1 null mutant mouse model of infantile neuronal ceroid lipofuscinosis. Neurobiol Dis. 2004;16(2):346–359. doi: 10.1016/j.nbd.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Bronson RT, Donahue LR, Johnson KR, Tanner A, Lane PW, Faust JR. Neuronal ceroid lipofuscinosis (nclf), a new disorder of the mouse linked to chromosome 9. Am J Med Genet. 1998;77(4):289–297. doi: 10.1002/(sici)1096-8628(19980526)77:4<289::aid-ajmg8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Burbaeva G, Boksha IS, Turishcheva MS, Vorobyeva EA, Savushkina OK, Tereshkina EB. Glutamine synthetase and glutamate dehydrogenase in the prefrontal cortex of patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(4):675–680. doi: 10.1016/s0278-5846(03)00078-2. [DOI] [PubMed] [Google Scholar]
- Chakraborty G, Mekala P, Yahya D, Wu G, Ledeen RW. Intraneuronal N-acetylaspartate supplies acetyl groups for myelin lipid synthesis: evidence for myelin-associated aspartoacylase. J Neurochem. 2001;78(4):736–745. doi: 10.1046/j.1471-4159.2001.00456.x. [DOI] [PubMed] [Google Scholar]
- Chan CH, Ramirez-Montealegre D, Pearce DA. Altered arginine metabolism in the central nervous system (CNS) of the Cln3(−/−) mouse model of juvenile Batten disease. Neuropath Appl Neuro. 2009;35(2):189–207. doi: 10.1111/j.1365-2990.2008.00984.x. [DOI] [PubMed] [Google Scholar]
- Chang B, Bronson RT, Hawes NL, Roderick TH, Peng C, Hageman GS, Heckenlively JR. Retinal degeneration in motor neuron degeneration: a mouse model of ceroid lipofuscinosis. Invest Ophthalmol Vis Sci. 1994;35(3):1071–1076. [PubMed] [Google Scholar]
- Chattopadhyay S, Ito M, Cooper JD, Brooks AI, Curran TM, Powers JM, Pearce DA. An autoantibody inhibitory to glutamic acid decarboxylase in the neurodegenerative disorder Batten disease. Hum Mol Genet. 2002;11(12):1421–1431. doi: 10.1093/hmg/11.12.1421. [DOI] [PubMed] [Google Scholar]
- Cooper JD. Progress towards understanding the neurobiology of Batten disease or neuronal ceroid lipofuscinosis. Curr Opin Neurol. 2003;16(2):121–128. doi: 10.1097/01.wco.0000063762.15877.9b. [DOI] [PubMed] [Google Scholar]
- Cooper JD, Messer A, Feng AK, Chua-Couzens J, Mobley WC. Apparent loss and hypertrophy of interneurons in a mouse model of neuronal ceroid lipofuscinosis: evidence for partial response to insulin-like growth factor-1 treatment. J Neurosci. 1999;19(7):2556–2567. doi: 10.1523/JNEUROSCI.19-07-02556.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson L, Johansson E, Kettaneh-Wold N, Wold S. Introduction to multi- and megavariate data analysis using projection methods (PCA and PLS) Umetrics; Umea: 1999. [Google Scholar]
- Gachet Y, Codlin S, Hyams JS, Mole SE. btn1, the Schizosaccharomyces pombe homologue of the human Batten disease gene CLN3, regulates vacuole homeostasis. J Cell Sci. 2005;118(Pt 23):5525–5536. doi: 10.1242/jcs.02656. [DOI] [PubMed] [Google Scholar]
- Goebel HH, Sharp JD. The neuronal ceroid-lipofuscinoses. Recent advances. Brain Pathol. 1998;8(1):151–162. doi: 10.1111/j.1750-3639.1998.tb00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13(3):129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Griffin JL, Muller D, Woograsingh R, Jowatt V, Hindmarsh A, Nicholson JK, Martin JE. Vitamin E deficiency and metabolic deficits in neuronal ceroid lipofuscinosis described by bioinformatics. Physiol Genomics. 2002;11(3):195–203. doi: 10.1152/physiolgenomics.00100.2002. [DOI] [PubMed] [Google Scholar]
- Jackson JE. A user’s guide to principal components. Wiley; New York: 1991. [Google Scholar]
- Jarvela I, Sainio M, Rantamaki T, Olkkonen VM, Carpen O, Peltonen L, Jalanko A. Biosynthesis and intracellular targeting of the CLN3 protein defective in Batten disease. Hum Mol Genet. 1998;7(1):85–90. doi: 10.1093/hmg/7.1.85. [DOI] [PubMed] [Google Scholar]
- Kakela R, Somerharju P, Tyynela J. Analysis of phospholipid molecular species in brains from patients with infantile and juvenile neuronal-ceroid lipofuscinosis using liquid chromatography-electrospray ionization mass spectrometry. J Neurochem. 2003;84(5):1051–1065. doi: 10.1046/j.1471-4159.2003.01602.x. [DOI] [PubMed] [Google Scholar]
- Katz ML, Shibuya H, Liu PC, Kaur S, Gao CL, Johnson GS. A mouse gene knockout model for juvenile ceroid-lipofuscinosis (Batten disease) J Neurosci Res. 1999;57(4):551–556. [PubMed] [Google Scholar]
- Kim Y, Ramirez-Montealegre D, Pearce DA. A role in vacuolar arginine transport for yeast Btn1p and for human CLN3, the protein defective in Batten disease. Proc Natl Acad Sci USA. 2003;100(26):15458–15462. doi: 10.1073/pnas.2136651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyttala A, Ihrke G, Vesa J, Schell MJ, Luzio JP. Two motifs target Batten disease protein CLN3 to lysosomes in transfected nonneuronal and neuronal cells. Mol Biol Cell. 2004;15(3):1313–1323. doi: 10.1091/mbc.E03-02-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Belle JE, Harris NG, Williams SR, Bhakoo KK. A comparison of cell and tissue extraction techniques using high-resolution 1H-NMR spectroscopy. NMR Biomed. 2002;15(1):37–44. doi: 10.1002/nbm.740. [DOI] [PubMed] [Google Scholar]
- Lee RL, Johnson KR, Lerner TJ. Isolation and chromosomal mapping of a mouse homolog of the Batten disease gene CLN3. Genomics. 1996;35(3):617–619. doi: 10.1006/geno.1996.0410. [DOI] [PubMed] [Google Scholar]
- Lindon JC, Nicholson JK, Everett JR. NMR spectroscopy of biofluids. Annual Reports on Nmr Spectroscopy. 1999;38:1–88. [Google Scholar]
- Luiro K, Kopra O, Lehtovirta M, Jalanko A. CLN3 protein is targeted to neuronal synapses but excluded from synaptic vesicles: new clues to Batten disease. Hum Mol Genet. 2001;10(19):2123–2131. doi: 10.1093/hmg/10.19.2123. [DOI] [PubMed] [Google Scholar]
- Luiro K, Yliannala K, Ahtiainen L, Maunu H, Jarvela I, Kyttala A, Jalanko A. Interconnections of CLN3, Hook1 and Rab proteins link Batten disease to defects in the endocytic pathway. Hum Mol Genet. 2004;13(23):3017–3027. doi: 10.1093/hmg/ddh321. [DOI] [PubMed] [Google Scholar]
- Macura S, Huang Y. Two-dimensional chemical exchange and cross-relaxation spectroscopy of coupled nuclear spins. J Magn Reson. 1981;43(2):259–281. [Google Scholar]
- Mann DM, Yates PO, Stamp JE. The relationship between lipofuscin pigment and ageing in the human nervous system. J Neurol Sci. 1978;37(1–2):83–93. doi: 10.1016/0022-510x(78)90229-0. [DOI] [PubMed] [Google Scholar]
- Mitchison HM, Bernard DJ, Greene ND, Cooper JD, Junaid MA, Pullarkat RK, de Vos N, Breuning MH, Owens JW, Mobley WC, Gardiner RM, Lake BD, Taschner PE, Nussbaum RL. Targeted disruption of the Cln3 gene provides a mouse model for Batten disease. The Batten Mouse Model Consortium [corrected] Neurobiol Dis. 1999;6(5):321–334. doi: 10.1006/nbdi.1999.0267. [DOI] [PubMed] [Google Scholar]
- Mitchison HM, Lim MJ, Cooper JD. Selectivity and types of cell death in the neuronal ceroid lipofuscinoses. Brain Pathol. 2004;14(1):86–96. doi: 10.1111/j.1750-3639.2004.tb00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald MJ, Kay GW, Palmer DN. Changes in GABAergic neuron distribution in situ and in neuron cultures in ovine (OCL6) Batten disease. Eur J Paediatr Neurol. 2001;5(Suppl A):135–142. doi: 10.1053/ejpn.2000.0450. [DOI] [PubMed] [Google Scholar]
- Pardo CA, Rabin BA, Palmer DN, Price DL. Accumulation of the adenosine triphosphate synthase subunit C in the mnd mutant mouse. A model for neuronal ceroid lipofuscinosis. Am J Pathol. 1994;144(4):829–835. [PMC free article] [PubMed] [Google Scholar]
- Pearce DA, Ferea T, Nosel SA, Das B, Sherman F. Action of BTN1, the yeast orthologue of the gene mutated in Batten disease. Nat Genet. 1999;22(1):55–58. doi: 10.1038/8861. [DOI] [PubMed] [Google Scholar]
- Pears MR, Cooper JD, Mitchison HM, Mortishire-Smith RJ, Pearce DA, Griffin JL. High resolution 1H NMR-based metabolomics indicates a neurotransmitter cycling deficit in cerebral tissue from a mouse model of Batten disease. J Biol Chem. 2005;280(52):42508–42514. doi: 10.1074/jbc.M507380200. [DOI] [PubMed] [Google Scholar]
- Petroff OA, Errante LD, Rothman DL, Kim JH, Spencer DD. Glutamate-glutamine cycling in the epileptic human hippocampus. Epilepsia. 2002;43(7):703–710. doi: 10.1046/j.1528-1157.2002.38901.x. [DOI] [PubMed] [Google Scholar]
- Pontikis CC, Cella CV, Parihar N, Lim MJ, Chakrabarti S, Mitchison HM, Mobley WC, Rezaie P, Pearce DA, Cooper JD. Late onset neurodegeneration in the Cln3−/− mouse model of juvenile neuronal ceroid lipofuscinosis is preceded by low level glial activation. Brain Res. 2004;1023(2):231–242. doi: 10.1016/j.brainres.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Ramirez-Montealegre D, Pearce DA. Defective lysosomal arginine transport in juvenile Batten disease. Hum Mol Genet. 2005;14(23):3759–3773. doi: 10.1093/hmg/ddi406. [DOI] [PubMed] [Google Scholar]
- Ranta S, Zhang Y, Ross B, Lonka L, Takkunen E, Messer A, Sharp J, Wheeler R, Kusumi K, Mole S, Liu W, Soares MB, Bonaldo MF, Hirvasniemi A, de la Chapelle A, Gilliam TC, Lehesjoki AE. The neuronal ceroid lipofuscinoses in human EPMR and mnd mutant mice are associated with mutations in CLN8. Nat Genet. 1999;23(2):233–236. doi: 10.1038/13868. [DOI] [PubMed] [Google Scholar]
- Salek RM, Colebrooke RE, Macintosh R, Lynch PJ, Sweatman BC, Emson PC, Griffin JL. A metabolomic study of brain tissues from aged mice with low expression of the vesicular monoamine transporter 2 (VMAT2) gene. Neurochem Res. 2008;33(2):292–300. doi: 10.1007/s11064-007-9542-3. [DOI] [PubMed] [Google Scholar]
- Salek RM, Xia J, Innes A, Sweatman BC, Adalbert R, Randle S, McGowan E, Emson PC, Griffin JL. A metabolomic study of the CRND8 transgenic mouse model of Alzheimer’s disease. Neurochem Int. 2010;56(8):937–947. doi: 10.1016/j.neuint.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Sekhon SS, Maxwell DS. Ultrastructural changes in neurons of the spinal anterior horn of ageing mice with particular reference to the accumulation of lipofuscin pigment. J Neurocytol. 1974;3(1):59–72. doi: 10.1007/BF01111932. [DOI] [PubMed] [Google Scholar]
- Trygg J, Wold S. Orthogonal projections to latent structures (O-PLS) J Chemometr. 2002;16(3):119–128. [Google Scholar]
- Wheeler RB, Sharp JD, Schultz RA, Joslin JM, Williams RE, Mole SE. The gene mutated in variant late-infantile neuronal ceroid lipofuscinosis (CLN6) and in nclf mutant mice encodes a novel predicted transmembrane protein. Am J Hum Genet. 2002;70(2):537–542. doi: 10.1086/338708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold S, Albano C, Dunn WJ, Edlund U, Esbensen K, Geladi P, Hellberg S, Johansson E, Lindberg W, Sjöström M. Multivariate data analysis in chemistry. Chemometrics: mathematics and statistics in chemistry. D. Reidel Publishing Company; Holland: 1984. [Google Scholar]