INTRODUCTION
Prostate cancer is the second leading cause of cancer-related death among men in the USA1. Up to 30% of men with clinically localized prostate cancer will progress to metastatic disease, but the genetic and molecular changes involved in prostate cancer progression are still largely unknown. Certain subtypes of clinically significant prostate cancer are remarkably aggressive, rapidly progressing toward metastatic and therapy resistant disease. However, there is also a large proportion of relatively indolent disease. Many experts believe that the vast majority of men will die with prostate cancer rather than from it, and that over-treatment of more indolent prostate cancer with radical therapy is a major source of morbidity. These points demonstrate a critical need for biomarkers of aggressive prostate cancer that correlate with prognosis.
Currently, the only routinely used biomarker for prostate cancer is PSA. However, the value of PSA for the detection of clinically significant prostate cancer is highly controversial2,3. Furthermore, other methods to distinguish aggressive from indolent disease are limited. The identification of biomarkers for aggressive prostate cancer could help to achieve a molecular classification of prostate cancer, distinguishing indolent from aggressive cases – the paradigm of personalized medicine. The advent of next-generation RNA and DNA sequencing provides an unprecedented opportunity for the discovery of novel mutations4 and gene fusions5,6.
The Cancer Genome Atlas (TCGA) program participates in the International Cancer Genome Consortium (www.icgc.org) to discover genetic changes in cancer genome by full-scale genome sequencing. This requires well-characterized and high-quality human biospecimens. Therefore, fresh tissue, as well as blood and urine, must be optimally processed and stored to preserve molecular contents (DNA, RNA, and proteins). Historically, tumor-derived prostate cancer cells are difficult to maintain in culture, leading to a greater dependence on tissue sample quality and availability for research purposes. However, identifying tumor and obtaining large quantities of high-quality specimens at the time of processing while not compromising patient care poses a challenge due to the multifocal and heterogeneous nature of the disease. To address this need, we have modified a previously published protocol developed as a part of the National Cancer Institute funded Prostate SPORE program at the University of Michgian7. The biobanking Standard Operating Procedures (SOPs) are performed within the framework of the Weill Cornell Medical College (WCMC) Institutional Biobank8. We describe SOPs, quality assessment of samples procured, and clinical impact on 105 consecutive patients who underwent radical prostatectomy (RP).
MATERIALS and METHODS
Cohort Description
The clinical cohort consisted of 105 men from WCMC who underwent RP for clinically localized prostate cancer as monotherapy from November 2008 to February 20099–11. The clinico-pathological demographics for the cohort are presented in Table 1.
TABLE 1.
Clinico-Pathological Demographics
| WCMC Patients (n=105) | ||
|---|---|---|
| Median Age (Range) | 60 (40 −82) | |
| Median Preoperative PSA (Range) | 4.9 ng./ml. (1.2–24.23) | |
| Prostatectomy Gleason Score |
6 7 8–9 |
25 (23.4%) 74 (69.2%) 6 (5.6%) |
| Tumor Stage | pT2a–c PT3a pT3b pT4 |
86 (80.4%) 15 (14%) 4 (3.7%) 0 (0%) |
| Surgical Margin Status |
Negative Positive |
95 (90.5%) 10 (9.5%) |
| PSA Biochemical Recurrence |
No Yes |
103 (98.1%) 2 (1.9%) |
| Lymph Node Status |
Negative Positive No LN |
97 (92.4%) 0 (0%) 8 (7.6%) |
Clinico-pathological characteristics of 105 subjects in the study cohort.
Collection Protocol
Subjects provided informed consent for the collection of biomaterial for molecular studies, along with clinical follow-up data11. To make the process ammenable for TCGA program, the informed consent form was adapted to ensure that the participant understood the benefit and risk of the data generated from genomics studies. Table 2 lists elements in the informed consent required for TCGA program. Figure 1 illustrates the workflow of the biobank SOPs – patient consent, specimen collection, processing, and pathology review.
TABLE 2.
Critical Elements in Informed Consent Required for Whole Genome Sequencing
| Informed Consent for The Cancer Genome Atlas |
|---|
| 1. Purpose of genetic studies |
| 2. Voluntary participation |
| 3. Donation of cancer and normal tissues |
| 4. Collection of medical information |
| 5. De-identification of samples and medical information |
| 6. Public access to anonymous information from analyses |
| 7. Medical information and detailed analyses stored in controlled-access database |
| 8. No financial compensation |
| 9. Potential benefits and risks of participation |
| 10. Confidentiality |
| 11. No withdrawal |
| 12. Recontact for additional samples or follow-up information |
Figure 1.
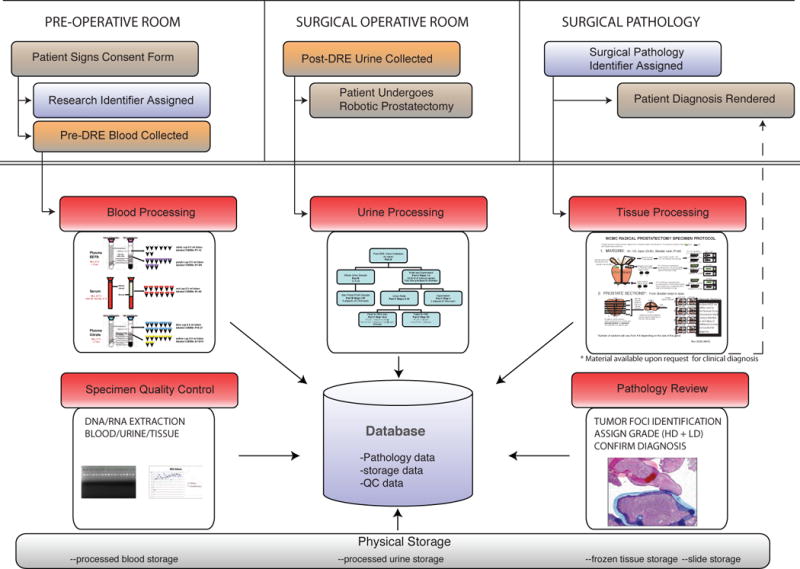
Biobank Workflow: Illustrates the Standard Operating Procedures of the Weill Cornell Medical College Biobank for patient consent, specimen collection, specimen processing, pathology examination, data collection, and storing samples and slides. The workflow is designed to provide the best clinical care with concurrent collection of well-annotated biospecimens for future research.
Processing Protocol
Blood and urine samples were collected from consented patients pre- and perioperatively. The blood and urine samples were processed and stored according to SOPs of a sponsored National Cancer Institute (NCI) program – Early Detection Research Network (EDRN)12 (Detailed EDRN protocols are in the supplementary data).
Prostatectomy specimens
Radical prostatectomy specimens10 were transported from the operating room to Surgical Pathology within 30 +/− 15 minutes to be accessioned. Under pathologist supervision, the prostatectomy specimen was grossed and processed with recorded start and end times by a trained histology technician. The prostate gland was weighed, measured, and inked with green and black ink to identify the right and left margins, respectively. The seminal vesicles and vas deferens were removed at the prostate base. The base and apical margins were resected from the prostate gland and cut into perpendicular sections7. The remaining prostate was sectioned into levels at a thickness of 5 +/− 1 mm depending on the size of the individual prostate, along a transverse plane perpendicular to the posterior surface from apex to base. Each level was quartered individually, keeping the orientation (e.g., right anterior, etc.). Alternating levels, including the apical/base margins and seminal vesicles were submitted for clinical diagnosis as formalin-fixed, paraffin-embedded (FFPE) blocks. The remaining levels were quartered and placed into separate cryomolds. Fresh tissue in the cryomold was completely embedded in Optimal Cutting Temperature (OCT) media and covered by a circular disc of cork. Then the tissue was frozen in the mixture of dry ice and methanol and placed in −80°C tumor banking freezer. The processing time to gross, procure, and freeze a RP specimen ranges between 40–60 minutes. Figure 2 illustrates the schematic of the fresh tissue procurement process.
Figure 2.
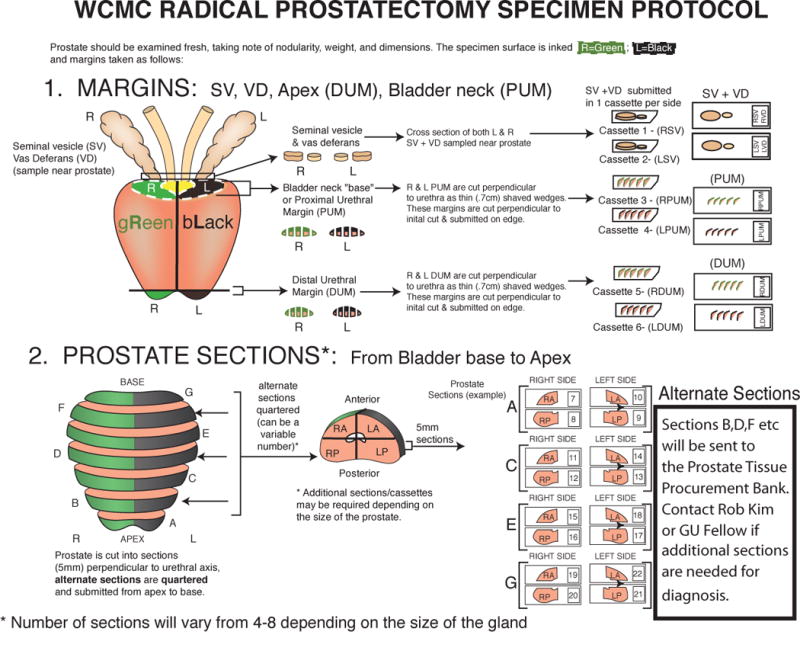
Illustrates the grossing protocol for prostate gland from radical prostatectomy. The seminal vesicles and vas deferens are removed at the prostate base. The base and apical margins are resected from the prostate gland and cut into perpendicular sections. The remaining prostate is sectioned perpendicular to urethral axis and each level is quartered retaining glandular orientation.
The FFPE material was processed according to standard protocols within Surgical Pathology. The complimentary frozen blocks were sectioned and stained with Hematoxylin and Eosin (H&E) immediately after being frozen. Frozen H&E stained slides were available to the surgical pathologist as additional diagnostic material upon request. If requested by the surgical pathologist, frozen tissue could be converted into FFPE material for further submission of diagnostic specimens. Once the surgical pathology report was finalized, the biobanked samples were made available for research studies.
Additional Pathology Review
All the frozen H&E slides of 105 consecutive RP specimens were reviewed after clinical diagnosis. Each frozen H&E slide was assessed for Gleason score, margin status and stage (presence or absence of extraprostatic extension), independent of the previously reviewed FFPE blocks. Differences in pathological findings between the clinically reviewed specimens and procured specimens were annotated and reviewed.
For research purposes, each tumor focus was outlined, percentage of tumor area in overall tissue area was calculated, and the total number of tumor foci and zone of origin for each tumor focus were recorded. The outline method entails marking high-density tumor area with <10% non-tumor contamination in blue, low-density tumor area with >10% non-tumor contamination in red, benign epithelial glands in green, and stroma in black framings. All tumors were classified as high-density (HD) or low-density (LD) cell population for various downstream applications (Figure 3). High-density tumor foci from frozen tissue were used for DNA- and RNA-sequencing while low-density tumor foci from matching FFPE tissue were utilized for tissue microarray (TMA) construction and other studies such as fluorescent in situ hybridization (FISH) and immunohistochemistry (IHC). Benign prostate tissues from the RP specimen were processed for somatic copy number variation (CNV) studies13.
Figure 3.
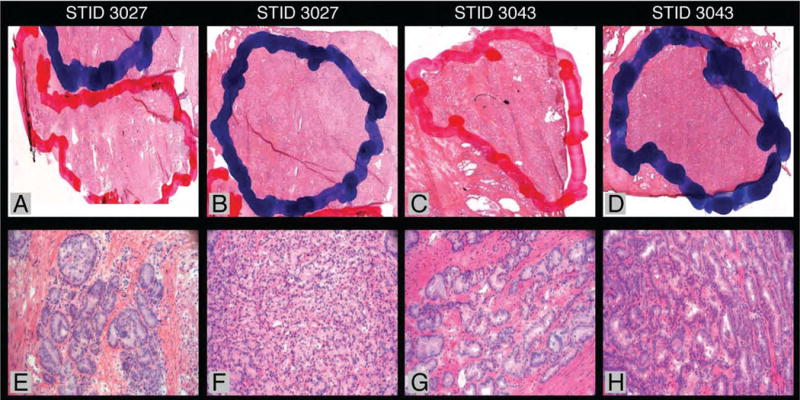
Digital images by Ariol Platform (Genetix, San Jose, CA) demonstrate how blue and red framings are marked on frozen H&E slides. Corresponding 20X photomicrographs show tumor foci with >90% tumor area and <90% tumor area, respectively. DNA samples from STID 3027 and 3043 were sequenced at the Broad Institute (Cambridge, MA).
DNA-RNA extraction
For DNA and RNA extractions, high-density tumor foci outlined on the H&E slides were matched with the corresponding areas on the frozen tissue blocks. Extracted nucleic acids were quantified with NanoDrop 8000 Spectophotometer (Thermo Scientific, Wilmington, DE), and the absorbance ratios at 260nm/280nm and 260nm/230nm were evaluated for purity5 (Extraction method is in the supplementary data).
Quality assessment of samples
Gel electrophoresis was performed to assess the integrity of genomic DNA. The quality of the RNA was determined using Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) (Full method is in the supplementary data).
Biobank Database
All pertinent information for each case, from molecular to pathological characteristics, was maintained in a previously described database called Profiler14. Clinical follow-up data after RP is continuously updated by the Department of Urology as new information becomes available15 (Details are in the supplementary data).
Sequencing Data
RNA samples were sequenced using Illumina Genome Analyzer II at WCMC and the Broad Institute (Cambridge, MA) sequenced DNA samples provided by WCMC. Authorized researchers can access the database for genetic data generated (Details are in the supplementary data).
RESULTS
Comparison study
One hundred and five consecutive prostates were histologically evaluated by two independent pathologists (RE, MAR) on frozen H&E slides and compared to the pathology review from FFPE H&E slides. We identified four cases with higher focal Gleason score within the frozen H&E slides, although this did not affect the overall Gleason score. One case was identified with equivocal extraprostatic extension and another three cases exhibited equivocal positive margins. The frozen blocks from these cases were recut and after further review with the original pathologist of record (MS), the pT stage was not altered. Finally, the frozen H&E slides were used to confirm the diagnosis of two clinical cases where minute prostate cancer foci were observed on the complimentary FFPE slides.
Quality of the samples
Ninety percent of the frozen samples contained cancer and 15% of all the reviewed cases had sufficiently large high-density tumor areas for nucleic acid extraction. Among the 105 cases, 22 cases were used for DNA and RNA extraction.
After stringent quality control (Figure 4), 18 samples from the cohort were used for RNA-sequencing at WCMC and sent to the Broad Institute as candidates for DNA-sequencing. To date, DNA from 7 samples have been sequenced and 2 of these (STID 3027 and 3043) were included in a full prostate cancer genome sequencing study conducted in collaboration by the Broad Institute and WCMC (a total of 7 samples were prepared using this protocol and sequenced in this study). Estimated by the ABSOLUTE algorithm that infers the tumor purity and average ploidy from the allele-specific copy number levels (Carter S.L. et al., manuscript in preparation), STID 3027 had a tumor purity of 74% and a mean ploidy of 2.12 and STID 3043 had 68% and 1.956, respectively4. Despite the strict protocol, the extracted DNA from high-density areas (>90% tumor) had more contamination than expected.
Figure 4.
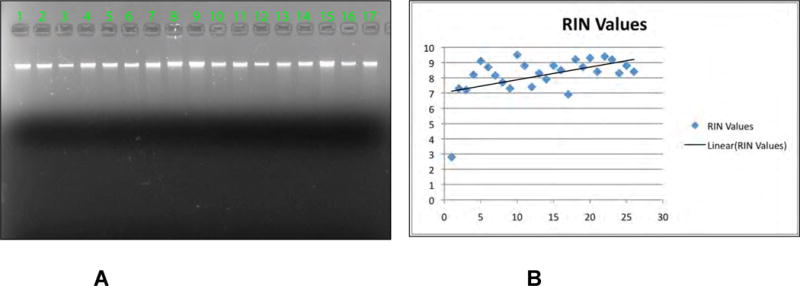
A
To assess the integrity of the genomic DNA, gel electrophoresis is performed with 50ng of DNA. Only the samples with DNA of high molecular weight as the large majority were selected as candidates for SNP arrays and whole genome sequencing assays.
B
The quality of the RNA is determined using Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) with Agilent RNA 6000 Nano Kit. RIN above 7 is required to be considered for gene expression studies.
The quality of RNA extracted from high-density tumor area was excellent with a mean RNA Integrity Number (RIN) value of 8.4. Therefore, samples could be preselected based on high-density tumor and deemed suitable for RNA-sequencing analysis. Table 3 lists all the individual cases that were used for nucleic acid extraction.
TABLE 3.
Follow-up of 22 Cases with Nucleic Acids Extraction
| Sample | RNA Integrity Number | RNA (ug) | RNA-seq | Sent to the Broad Institute | DNA-seq completed to date |
|---|---|---|---|---|---|
| STID3023_B62 | 7.3 | 23.0 | Y | Y | N |
| STID3026_D56 | 7.0 | 17.3 | Y | Y | N |
| STID3027_B57 | 8.2 | 47.9 | Y | Y | Y |
| STID3034_C51 | 9.1 | 19.5 | Y | Y | N |
| STID3035_B53 | 8.7 | 19.9 | Y | Y | N |
| STID3036_B51 | 8.2 | 25.6 | N | Y | N |
| STID3042_H51 | 7.7 | 22.0 | Y | Y | Y |
| STID3043_B56 | 7.3 | 32.4 | Y | Y | Y |
| STID3048_B50 | 9.5 | 18.2 | Y | Y | N |
| STID3050_B51 | 8.8 | 19.1 | Y | N | N |
| STID3051_B51 | 7.4 | 19.0 | Y | Y | N |
| STID3071_B51 | 8.3 | 43.4 | Y | N | N |
| STID3076_B51 | 7.9 | 16.4 | N | N | N |
| STID3080_C55 | 8.8 | 8.7 | N | Y | N |
| STID3084_D58 | 8.5 | 33.6 | Y | Y | N |
| STID3085_B57 | 6.9 | 68.9 | Y | Y | Y |
| STID3105_C55 | 9.2 | 10.9 | Y | Y | Y |
| STID3127_B56 | 8.7 | 53.0 | Y | Y | Y |
| STID3129_C52 | 9.3 | 8.7 | N | Y | N |
| STID3131_B51 | 8.4 | 31.9 | Y | N | N |
| STID3132_B51 | 9.4 | 18.6 | Y | Y | N |
| STID3134_B58 | 9.2 | 18.1 | Y | Y | Y |
| Total | 18 | 18 | 7 |
Y=Yes
N=No
RNA and DNA were extracted from 22 cases out of 105 cases in the study cohort. The samples were subjected to strict quality control before next-generation sequencing.
To further elucidate differences between high-density and low-density tumors, we compared clinico-pathological features of the two groups as presented in Table 4. The cases with high-density tumor foci were associated with higher Gleason scores (generalized Fisher’s exact test, p-value<0.0001) and higher PSA (Wilcoxon’s rank sum test, p-value=0.0401). Differences in age, surgical margin status, tumor stage, and subsequent development of biochemical recurrence were not statistically significant between the two groups.
TABLE 4.
Comparison of Clinico-Pathological Features between Cases with High-Density and Low-Density tumors
| High Density | Low Density | P-Value | |
|---|---|---|---|
| Age: | 0.0642 | ||
| Max Age Min Age Median |
72 44 62 |
82 40 58 |
|
| PSA: | 0.0401 | ||
| Max PSA Min PSA Median PSA PSA<10 PSA=10–20 PSA>20 |
24.23 1.65 5.35 35 8 1 |
22 1.2 4.60 48 2 1 |
|
| Gleason Score: | 1.571e-06 | ||
| <7 =7 >7 |
1 37 6 |
19 32 0 |
|
| Tumor stage: | 0.1746 | ||
| pT2 pT3 pT4 |
31 13 0 |
45 6 0 |
|
| Surgical margin: | 0.1793 | ||
| Negative Positive |
37 7 |
48 3 |
|
| Biochemical recurrence: | 1 | ||
| No Yes |
43 1 |
50 1 |
These clinico-pathological features could potentially influence the quality of extracted RNA and help characterize and pre-select prospective high-quality tumor samples. In Table 5, we assessed the distribution of RIN values of the 22 cases with extraction according to clinico-pathological characteristics. There was no correlation found in the two parameters, possibly due to the sample size and selection bias – all the samples were from cases with high-density tumor areas and 21/22 samples had RIN above 7.
TABLE 5.
Assessment of Clinico-Pathological Features in regards to RIN Values from 22 Cases with RNA Extraction.
| RIN < 7 | RIN > 7 | |
|---|---|---|
| Number of patients | 1 | 21 |
| Age at diagnosis median, years (range) | 68 | 64 (47–70) |
| Diagnostic PSA median, ng/mL (range) | 4.9 | 5 (1.65–24.23) |
| Prostate weight median, grams (range) | 52.2 | 44 (30–92.2) |
| Diagnostic PSA value | ||
| 0 – 9.9 | 1 | 15 |
| 10 – 20 | 0 | 5 |
| ≥ 20 | 0 | 1 |
| Gleason score | ||
| 6 or less | 0 | 0 |
| 7 | 1 | 17 |
| 8 or more | 0 | 4 |
| Seminal vesicles (SV) status | ||
| SV free | 1 | 18 |
| SV invaded | 0 | 3 |
| Tumor stage | ||
| pT2 | 0 | 14 |
| pT3 | 1 | 7 |
| pT4 | 0 | 0 |
| Surgical margins | ||
| Negative | 1 | 18 |
| Positive | 0 | 3 |
| Biochemical recurrence | ||
| No | 1 | 20 |
| Yes | 0 | 1 |
DISCUSSION
The Cancer Genome Atlas program is a multi-institutional effort to accelerate our understanding of the genetics of cancer using innovative genome analysis technology, including deep sequencing (http://cancergenome.nih.gov/). In order to include prostate cancer in the next phase of TCGA, an efficient method of obtaining high-quality prostate cancer tissue is required without compromising clinical care. The first step required modification of our existing informed consent form to include purpose of genetic studies, storage of de-identified biospecimens and medical information outside WCMC, and public access to genetic analyses. Secondly, we had to optimize a prostate biobanking protocol in order to meet TCGA tissue collection requirements. TCGA collects tumor and matched normal sample for control, and requires the tumor sample to consist of more than 70% tumor nuclei and less than 20% necrotic area16. It is time and resource efficient to collect high-quality biospecimens as tissue samples undergo independent quality control measures at TCGA program. Our protocol generates sufficient tissue to make clinical diagnoses of prostate cancer without compromising patient care, while simultaneously providing fresh, high-quality prostate tissue suitable for genomic studies.
The protocol for partial sampling and procurement of prostate tissue was developed to perform expression profiling of prostate cancer samples17, but concern was raised regarding possible detrimental effects on the pathological diagnostic parameters. We initially reported that partial sampling (50% versus whole mount RP specimens) did not alter diagnostic accuracy, leading to the conclusion that entire sampling offered no significant advantages over the partial sampling technique in detecting adverse pathological features7. Grossfeld et al18 concluded that prostate sampling methods did not predict clinical outcomes of either secondary treatment or PSA recurrence. Desai et al19 found higher frequency of extra-prostatic extension and seminal vesicle invasion in the complete sampling method but no differences were apparent in terms of survival rates. A recent study by Salem et al20 found that whole mount and systematic sampling produced similar pathological data except for estimated tumor volume and multiple margins and similar prognosis for biochemical recurrence. Previous studies compared partial sampling to whole mounting of RP specimen, and others proposed different methods for prostate sampling7, 14, 18, 19, 21–28. However, inconsistent results in these studies reinforced a need for standardized method and protocol. This study presents our standardized partial sampling protocol to rapidly process and store RP specimens for pathological diagnosis and research utility.
After routine histopathological evaluation of FFPE and frozen H&E slides within the same case by multiple pathologists, no significant differences were found regarding the ability to detect adverse pathological features. Frozen samples are not accessible to researchers until final pathological diagnosis is made, and frozen H&E slides and blocks are always available to be returned to assist the surgical pathologist. As an additional measure, inconsistent findings from frozen slides are reported to the attending pathologist. Our findings confirm that a partial sampling method for biobanking does not affect the final diagnosis, prognostic information, or relevant clinical care. Our protocol also proves its efficiency in acquiring nucleic acid samples with excellent quality suitable for next-generation sequencing; 81.8% of cases with nucleic acid extraction were RNA-sequenced and sent to the Broad Institute for DNA-sequencing. Thus, the samples collected through this protocol are likely to meet the standards imposed by TCGA.
Access to high-quality prostate tissue provided by our biobanking protocol permits not only genome-wide discovery using platforms like next-generation sequencing, but also provides the raw material for critical validation studies. For instance, individual tumor RNA can be studied for gene expression using standard techniques like reverse transcriptase polymerase chain reaction (RT-PCR). Rearrangements or somatic copy number aberrations can be investigated with FISH assays or quantitative PCR. Discovered mutations can be validated by conventional Sanger sequencing on expanded numbers of samples. Matching paraffin material allows IHC studies of genes of interest at the protein level. Overall, the tissue collection procedure outlined here provides the material for comprehensive investigation of alterations in prostate cancer tissue at DNA, RNA, and protein levels.
Additionally, molecular studies on urine and blood specimens enable an exhaustive characterization of germline and somatic changes present in the samples. A potential application for blood samples is detection of circulating prostate tumor cells, while urine samples can be used in PCR-based assays to detect fusion transcripts (i.e urine measurement of TMPRSS2-ERG29–31 for the early detection of significant prostate cancer), validating the effort in securing these samples within the biobank.
Potential limitations of this protocol are related to non-tumor cell contamination and a bias toward larger tumors. The more than expected non-tumor cell contamination is most likely due to two main factors. First, there tends to be an overestimation of tumor nuclei even in dense areas. Second, the histologic section does not represent the whole tissue block. One way to lower the requirements for using all samples would require either a more careful tumor isolation process such as laser capture microdissection or a more focused genomics approach. For instance, if selected molecular mutations were being investigated, admixture of benign tissues would not be as critical. Using more sensitive assays could allow for deeper sequencing of known mutations.
In conclusion, we describe a prostate cancer protocol that allows for high-throughput biomedical research without compromising clinical care. Genomic, transcriptomic, as well as proteomic data extracted from high-quality fresh tissue could serve as a valuable resource for precise diagnosis, prognosis, and future personalized treatment of prostate cancer, and also lead to an increased understanding of tumor biology and the molecular events involved in disease progression.
Supplementary Material
Acknowledgments
Supported by Early Detection Research Network RFA-CA-09-017, STARR I4-A424, and DOD Synergy PC101020.
Footnotes
Disclosure/Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 4.Berger MF, Lawrence MS, Demichelis F, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470(7333):214–20. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pflueger D, Terry S, Sboner A, et al. Discovery of non-ETS gene fusions in human prostate cancer using next-generation RNA sequencing. Genome Res. 2011;21(1):56–67. doi: 10.1101/gr.110684.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sboner A, Habegger L, Pflueger D, et al. FusionSeq: a modular framework for finding gene fusions by analyzing paired-end RNA-sequencing data. Genome Biol. 2010;11(10):R104. doi: 10.1186/gb-2010-11-10-r104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollenbeck BK, Bassily N, Wei JT, et al. Whole mounted radical prostatectomy specimens do not increase detection of adverse pathological features. J Urol. 2000;164(5):1583–6. [PubMed] [Google Scholar]
- 8.Dhir R. Prostate cancer biobanking. Curr Opin Urol. 2008;18(3):309–14. doi: 10.1097/MOU.0b013e3282fb7cbe. [DOI] [PubMed] [Google Scholar]
- 9.Menon M, Tewari A, Peabody J. Vattikuti Institute prostatectomy: technique. J Urol. 2003;169(6):2289–92. doi: 10.1097/01.ju.0000067464.53313.dd. [DOI] [PubMed] [Google Scholar]
- 10.Tewari A, Peabody J, Sarle R, et al. Technique of da Vinci robot-assisted anatomic radical prostatectomy. Urology. 2002;60(4):569–72. doi: 10.1016/s0090-4295(02)01852-6. [DOI] [PubMed] [Google Scholar]
- 11.Tewari AK, Patel ND, Leung RA, et al. Visual cues as a surrogate for tactile feedback during robotic-assisted laparoscopic prostatectomy: posterolateral margin rates in 1340 consecutive patients. BJU Int. 2010;106(4):528–36. doi: 10.1111/j.1464-410X.2009.09176.x. [DOI] [PubMed] [Google Scholar]
- 12.Tuck MK, Chan DW, Chia D, et al. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res. 2009;8(1):113–7. doi: 10.1021/pr800545q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yau C, Holmes CC. CNV discovery using SNP genotyping arrays. Cytogenet Genome Res. 2008;123(1–4):307–12. doi: 10.1159/000184722. [DOI] [PubMed] [Google Scholar]
- 14.Kim K, Pak PJ, Ro JY, et al. Limited sampling of radical prostatectomy specimens with excellent preservation of prognostic parameters of prostate cancer. Arch Pathol Lab Med. 2009;133(8):1278–84. doi: 10.5858/133.8.1278. [DOI] [PubMed] [Google Scholar]
- 15.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56(6):899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 16.Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhanasekaran SM, Barrette TR, Ghosh D, et al. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412(6849):822–6. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- 18.Grossfeld GD, Chang JJ, Broering JM, et al. Does the completeness of prostate sampling predict outcome for patients undergoing radical prostatectomy?: data from the CAPSURE database. Urology. 2000;56(3):430–5. doi: 10.1016/s0090-4295(00)00705-6. [DOI] [PubMed] [Google Scholar]
- 19.Desai A, Wu H, Sun L, et al. Complete embedding and close step-sectioning of radical prostatectomy specimens both increase detection of extra-prostatic extension, and correlate with increased disease-free survival by stage of prostate cancer patients. Prostate Cancer Prostatic Dis. 2002;5(3):212–8. doi: 10.1038/sj.pcan.4500600. [DOI] [PubMed] [Google Scholar]
- 20.Salem S, Chang SS, Clark PE, et al. Comparative analysis of whole mount processing and systematic sampling of radical prostatectomy specimens: pathological outcomes and risk of biochemical recurrence. J Urol. 2010;184(4):1334–40. doi: 10.1016/j.juro.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 21.Bova GS, Fox WM, Epstein JI. Methods of radical prostatectomy specimen processing: a novel technique for harvesting fresh prostate cancer tissue and review of processing techniques. Mod Pathol. 1993;6(2):201–7. [PubMed] [Google Scholar]
- 22.Cohen MB, Soloway MS, Murphy WM. Sampling of radical prostatectomy specimens. How much is adequate? Am J Clin Pathol. 1994;101(3):250–2. doi: 10.1093/ajcp/101.3.250. [DOI] [PubMed] [Google Scholar]
- 23.Haggman M, Norberg M, de la Torre M, Fritjofsson A, Busch C. Characterization of localized prostatic cancer: distribution, grading and pT-staging in radical prostatectomy specimens. Scand J Urol Nephrol. 1993;27(1):7–13. doi: 10.3109/00365599309180407. [DOI] [PubMed] [Google Scholar]
- 24.Hall GS, Kramer CE, Epstein JI. Evaluation of radical prostatectomy specimens. A comparative analysis of sampling methods. Am J Surg Pathol. 1992;16(4):315–24. doi: 10.1097/00000478-199204000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Jhavar SG, Fisher C, Jackson A, et al. Processing of radical prostatectomy specimens for correlation of data from histopathological, molecular biological, and radiological studies: a new whole organ technique. J Clin Pathol. 2005;58(5):504–8. doi: 10.1136/jcp.2004.021808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison C, Cheney R, Johnson CS, Smith G, Mohler JL. Central quadrant procurement of radical prostatectomy specimens. Prostate. 2009;69(7):770–3. doi: 10.1002/pros.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riddick AC, Barker C, Sheriffs I, et al. Banking of fresh-frozen prostate tissue: methods, validation and use. BJU Int. 2003;91(4):315–23. doi: 10.1046/j.1464-410x.2003.03041.x. discussion 23–4. [DOI] [PubMed] [Google Scholar]
- 28.Sehdev AE, Pan CC, Epstein JI. Comparative analysis of sampling methods for grossing radical prostatectomy specimens performed for nonpalpable (stage T1c) prostatic adenocarcinoma. Hum Pathol. 2001;32(5):494–9. doi: 10.1053/hupa.2001.24322. [DOI] [PubMed] [Google Scholar]
- 29.Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310(5748):644–8. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 30.Salami SS, Schmidt F, Laxman B, et al. Combining urinary detection of TMPRSS2:ERG and CaP3 with serum PSA to predict diagnosis of prostate cancer. Urol Oncol. 2011 doi: 10.1016/j.urolonc.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laxman B, Morris DS, Yu J, et al. A first-generation multiplex biomarker analysis of urine for the early detection of prostate cancer. Cancer Res. 2008;68(3):645–9. doi: 10.1158/0008-5472.CAN-07-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


