The inability of the adult human heart to regenerate in response to injury, disease and aging stands as a central challenge in cardiovascular medicine. Following myocardial infarction (MI), billions of cardiomyocytes are lost and replaced with an avascular fibrotic scar. While various medical interventions have augmented survival rates following MI, the fibrotic myocardium mitigates cardiac contractility, leading to a poor long-term prognosis in these patients. Thus, there is an immense need for innovative approaches to repopulate lost cardiomyocytes following cardiac injury.
In principle, several biological approaches to heart regeneration can be envisioned, including stem cell therapies, reprogramming of cardiac fibroblasts into cardiomyocytes, activation of cardiomyocyte proliferation, and suppression of fibrosis. However, each of these approaches faces uncertainties and challenges that have yet to be overcome. For example, while harnessing the potential of ostensible cardiac stem cells (CSCs) represents an attractive approach to repopulate lost cardiomyocytes, the process has proven to be, thus far, inefficient and tenuous due to the inability of stem cells to fully adopt a contractile phenotype, incomplete electro-physical integration into the myocardium and inefficient long-term retention of transplanted cells1–3. What is irrefutable is that the level of turnover of cardiomyocytes in adult mammals is inadequate to account for significant regeneration or functional restoration of the heart following severe injury.
Another approach, in principle, to replenish myocytes following injury would be to convert resident cardiac fibroblasts directly into cardiomyocytes. Recently, it has been demonstrated that fibroblasts can be converted into cardiomyocytes in vitro with the viral addition of a cocktail of cardiac transcription factors4. In related studies, forced expression of only three or four cardiac transcription factors was shown to be sufficient to induce cardiac gene expression in cardiac fibroblasts in vivo, which enhanced cardiac function and attenuated ventricular remodeling following MI5, 6. Targeting fibroblasts for cellular reprogramming would potentially circumvent some of the obstacles usually associated with transplantation techniques, although different challenges will be faced with such a reprogramming approach, such as the quantity of myocytes needed to restore cardiac function and the necessity to generate mature, adult myocytes that integrate seamlessly with the injured myocardium.
In contrast to the resistance of the adult mammalian heart to regeneration, the neonatal heart displays remarkable regenerative potential. Regeneration of the neonatal mouse heart in response to apical amputation or MI appears to occur primarily through proliferation of cardiomyocytes rather than activation of a stem cell population7. Similar conclusions have been reached in studies of zebrafish heart regeneration8, 9. Thus, enhancing cardiomyocyte proliferation by exploiting the young heart’s innate ability to regenerate during later stages of adulthood seems particularly attractive as an approach for cardiac repair.
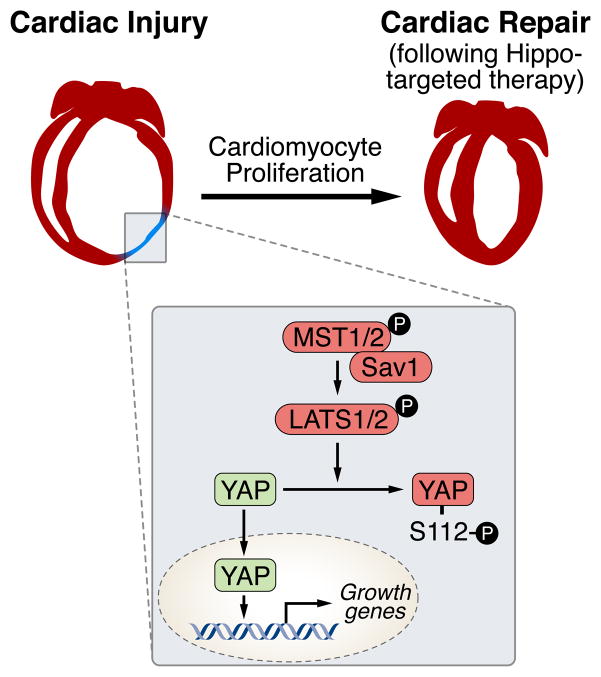
Multiple signaling molecules have been shown to positively regulate cardiomyocyte proliferation, including neuregulin, fibroblast growth factor, IGF1, and periostin10–13. More recently, the Hippo-Yap signaling pathway has been shown to exert powerful growth regulatory activity in cardiomyocytes14, 15. Highly conserved between mammals and Drosophila melanogaster, the Hippo kinase cascade restrains cell proliferation in response to extracellular cues16. This pathway consists of a series of adapter proteins and kinases, which culminate in the phosphorylation and inactivation of YES-associated protein (YAP) and the related protein TAZ, preventing their nuclear translocation (see Figure).
Figure. Signaling by the Hippo-Yap pathway in cardiac repair.
Inhibition of upstream targets in the Hippo pathway15, 26 or stimulation of YAP14, 21, 22 have proven to be an effective means to stimulate cardiomyocyte proliferation and enhance cardiac regeneration. Therefore, selective inhibitors of MST1/LATS1 or YAP agonists could be considered as therapeutics for cardiac repair.
The mammalian STE20-like protein kinase 1 (MST1) sits atop the Hippo pathway and interacts with Salvador (SAV), which phosphorylates and activates large tumor suppressor homologue 1 (LATS1). LATS1 then phosphorylates YAP, creating a binding site for the 14-3-3 chaperone protein, which prevents nuclear accumulation of YAP. In the absence of phosphorylation, YAP (or TAZ) enter the nucleus where they interact with TEAD transcription factors to activate genes involved in cell proliferation and organ growth.
The Hippo pathway is activated in response to cell-cell signaling. At low cell density, YAP becomes released from repression and dephosphorylated, enabling its entry into the nucleus and activation of growth-promoting genes. Conversely, at high cell density, Yap is retained in the cytoplasm and its growth-stimulatory activity is suppressed. Yap activity is highly sensitive to perturbation of the actin cytoskeleton and is regulated by association with α-catenin and members of the angiomotin family, which form a complex with phosphorylated Yap and 14-3-3 to restrain cell proliferation17, 18. It has been shown that Yap activity is enhanced through G-protein coupled receptor stimulation by lysophosphatidic acid and sphingosine-1 phosphate, which involves a Rho-GTPase signaling pathway and actin cytoskeleton organization19.
Manipulation of the upstream effectors of Hippo-Yap signaling dramatically perturbs cardiac growth. When Mst1 or its cofactor Sav is disrupted in the embryonic mouse heart, Yap phosphorylation is abolished and heart size increases 2.5 fold, a phenotype due to cardiomyocyte proliferation rather than hypertrophy15, 20. Moreover, manipulation of Hippo signaling in the adult heart through inactivation of Sav or Lats1/2 increases cardiomyocyte proliferation and survival and promotes myocardial regeneration in different models of cardiac injury.
Cardiac deletion of Yap during mouse embryogenesis results in cardiac hypoplasia and death at embryonic day 10.5, whereas deletion during the post-natal period causes impaired neonatal cardiac regeneration and death by 20 weeks of age due to loss of cardiomyocytes14, 21. Cardiac deletion of Taz does not evoke an obvious cardiac phenotype, but Yap and Taz exert dosage-sensitive effects on cardiac growth such that combined deletion of Yap with heterozygous deletion of Taz accelerates the onset of lethality from 20 weeks, as seen with Yap deletion, to 10 days. These findings likely reflect essential and redundant roles in maintaining cardiomyocyte proliferation and survival.
Overexpressing a phosphorylation-resistant, activated form of Yap (YapS112A) in the embryonic heart leads to an increased number of cardiomyocytes and larger hearts, and is sufficient to induce proliferation and cytokinesis in postnatal cardiomyocytes in vitro14, 20. Moreover, expression of YapS112A in adult mice under the control of the Myh6 promoter not only increases heart size in 4-month-old mice, but also enhances the regenerative response in adults following MI.
These findings also highlighted Yap as an integrator of IGF and PI3K-Akt signaling, pathways previously known for their roles in cardiac proliferation and embryonic growth14, 15. YapS112A-expressing cardiomyocytes display enhanced IGF signaling and phosphorylated GSK-3b, resulting in stabilization of β-catenin. It was further demonstrated that increased β-catenin is necessary for the pro-proliferative effects of YapS112A on cardiomyocytes.
In the current issue of Circulation Research, Lin et al generated mice that express the activated form of human YAP specifically in cardiomyocytes (YAPGOF) under the control of doxycycline (DOX)22. Consistent with previous studies, DOX treatment from 4–8 weeks of age resulted in increased numbers of cardiomyocytes in YAPGOF mice. However, while Xin et al observed larger hearts in Myh6-YapS112A mice at 4 months of age21, heart size was not apparently altered in DOX-treated YAPGOF mice at a 4.5-month time point. This could be due to the fact that the promoter elements of Myh6 express Yap much earlier and at a higher level than with DOX treatment at 4 weeks of age in the YAPGOF mice, and Yap might exert greater pro-growth effect in the embryonic and neonatal heart than the adult. Alternatively, the murine YapS112A that Xin et al used may have a greater stimulatory effect in mice than the human YAPGOF.
While markers for cytokinesis were not used, Lin et al assessed cardiomyocyte numbers following collagenase-perfusion of hearts. An in vivo clonal analysis of cardiomyocyte proliferation was also performed by expressing the human activated YAP in a fraction of cardiomyocytes while simultaneously labeling them with red fluorescent protein (RFP). In mice expressing the YAP transgene, there were significantly more clusters of RFP labeled cardiomyocytes, suggesting that individually labeled cardiomyocytes divided. The authors noted that the chance of independent Cre recombination events giving rise to a background of clusters could not be ruled out. Therefore, the authors turned to a multi-color clonal analysis, where each Cre recombination event triggers one of four reporters. The mice expressing the YAP transgene had significantly more monochromatic clusters, suggesting that YAP stimulated cardiomyocyte proliferation.
In response to MI, YAPGOF mice showed preservation of cardiac function and reduced infarct size, as seen in prior studies by Xin et al. However, it is noteworthy that Lin et al induced MI before activating the expression of YAP with DOX, while previous studies induced MI after Yap expression. That Lin et al saw enhanced cardiac regeneration following MI suggests that YAP expression is sufficient for cardiac repair, which may have significant clinical implications.
As a potential prelude to therapeutic applications, the authors tested the effects of adeno-associated virus (AAV9) delivery of activated human YAP, injected into three sites along the margin of the ischemic area, immediately following MI. Four weeks after MI, AAV9:hYAP injected mice displayed improved systolic function relative to control mice injected with AAV9:luciferase. At 23 weeks post-MI, AAV9:hYAP injected mice also showed improved survival, however, systolic function was not different between these mice and controls. The authors ascribe the latter findings to a survival bias in which the mice in the two groups with the lowest cardiac function may have died during the course of the study, thereby diminishing differences between the groups.
Consistent with previous reports of cardiac regeneration23, 24, microarray analysis revealed enrichment in expression of genes associated with the cell cycle and inflammation and reduced expression of genes involved in energy metabolism in AAV9:hYAP injected hearts. The gene expression profile of the latter hearts suggests the induction of an immature cardiac phenotype that is more proliferative. Given the importance of inflammatory responses in cardiac repair23, it will be of interest to identify specific inflammatory mediators of Yap activity and to define their contributions to the regenerative process downstream of Yap.
While the above studies suggest new opportunities for enhancing heart regeneration through regulation of Hippo-Yap signaling, numerous questions and technical challenges remain to be addressed before potential clinical application of this approach. For example, it will be of interest to determine what extracelluar signal(s) stimulates the Hippo pathway, how this is regulated pre- and postnatally and what receptors are involved. It will also be important to elucidate the precise Yap and β-catenin effector genes, which will likely provide further insight into the mechanisms of cardiomyocyte cell cycle control. It is also conceivable that a combinatorial approach using both Hippo kinase inhibitors and Yap stimulation, or other recently reported approaches to cardiac repair5, 6, 25, will yield a more robust effect in replacing damaged myocardium. The long-term consequences of YAP activation in the heart also remain to be determined. This would seem particularly important, given the immature cardiomyocyte phenotype observed in response to AAV9:hYAP expression. Moreover, YAP is a potent oncogene, so its possible expression in the heart would likely require tight control over the timing of expression to avoid possible oncogenicity in other cell types. Thus, much remains to be learned about potential delivery methods of Yap, the titration of therapeutic positive and negative regulators of the Hippo pathway for optimum outcome following MI, avoiding oncogenicity, and determining the cardiac-specific Yap effector genes. Finally, we should be mindful of the fact that a cardiac infarct in a mouse is relatively miniscule compared to that of a human heart, which can involve the loss of billions of cardiomyocytes. Thus, there will be unavoidable challenges of scale in translating these experimental observations into clinical practice. Nevertheless, these are exciting times in the field in which discoveries of fundamental biological regulatory mechanisms, often with key roles in developmental biology, are being brought to bear in a hypothesis-driven manner to solve some of the longstanding mysteries of the heart.
Acknowledgments
Sources of Funding
Work in Eric Olson’s laboratory is supported by grants from the NIH, The Cancer Prevention and Research Institute of Texas, the Leducq Foundation, and the Robert A. Welch Foundation grant 1-0025.
Footnotes
Disclosures
None
References
- 1.Laflamme MA, Murry CE. Regenerating the heart. Nature biotechnology. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 2.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 3.Stadtfeld M, Hochedlinger K. Induced pluripotency: History, mechanisms, and applications. Genes & development. 2010;24:2239–2263. doi: 10.1101/gad.1963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jopling C, Sleep E, Raya M, Marti M, Raya A, Izpisua Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jopling C, Sune G, Morera C, Izpisua Belmonte JC. P38alpha mapk regulates myocardial regeneration in zebrafish. Cell cycle. 2012;11:1195–1201. doi: 10.4161/cc.11.6.19637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/erbb4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 11.Duerr RL, Huang S, Miraliakbar HR, Clark R, Chien KR, Ross J., Jr Insulin-like growth factor-1 enhances ventricular hypertrophy and function during the onset of experimental cardiac failure. The Journal of clinical investigation. 1995;95:619–627. doi: 10.1172/JCI117706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT. P38 map kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes & development. 2005;19:1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nature medicine. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 14.Xin M, Kim Y, Sutherland LB, Qi X, McAnally J, Schwartz RJ, Richardson JA, Bassel-Duby R, Olson EN. Regulation of insulin-like growth factor signaling by yap governs cardiomyocyte proliferation and embryonic heart size. Science signaling. 2011;4:ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan D. Hippo signaling in organ size control. Genes & development. 2007;21:886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- 17.Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, Camargo FD. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao B, Li L, Lu Q, Wang LH, Liu CY, Lei Q, Guan KL. Angiomotin is a novel hippo pathway component that inhibits yap oncoprotein. Genes & development. 2011;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL. Regulation of the hippo-yap pathway by g-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Gise A, Lin Z, Schlegelmilch K, Honor LB, Pan GM, Buck JN, Ma Q, Ishiwata T, Zhou B, Camargo FD, Pu WT. Yap1, the nuclear target of hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2394–2399. doi: 10.1073/pnas.1116136109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel-Duby R, Olson EN. Hippo pathway effector yap promotes cardiac regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:13839–13844. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Z, von Gise A, Zhou P, Gu F, Ma Q, Jiang J, Yau AL, Buck JN, Gouin KA, van Gorp PR, Zhou B, Chen J, Seidman JG, Wang DZ, Pu WT. Cardiac-specific yap activation improves cardiac function and survival in an experimental murine myocardial infarction model. Circulation research. 2014 doi: 10.1161/CIRCRESAHA.115.303632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. Macrophages are required for neonatal heart regeneration. The Journal of clinical investigation. 2014;124:1382–1392. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet S, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, Giacca M, Rabinovitch PS, Aroumougame A, Shah AM, Szweda LI, Sadek HA. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell. 2014;157:565–579. doi: 10.1016/j.cell.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, Giacca M. Functional screening identifies mirnas inducing cardiac regeneration. Nature. 2012;492:376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 26.Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, Johnson RL, Martin JF. Hippo signaling impedes adult heart regeneration. Development. 2013;140:4683–4690. doi: 10.1242/dev.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]