Abstract
Purpose of Review
To summarize evidence characterizing the interactions between adrenal- and calcium-regulating hormones, and the relevance of these interactions to human cardiovascular and skeletal health.
Recent Findings
Human studies support the regulation of parathyroid hormone (PTH) by the renin-angiotensin-aldosterone system (RAAS): angiotensin II may stimulate PTH secretion via an acute and direct mechanism, whereas aldosterone may exert a chronic stimulation of PTH secretion. Studies in primary aldosteronism, congestive heart failure, and chronic kidney disease have identified associations between hyperaldosteronism, hyperparathyroidism, and bone loss, which appear to improve when inhibiting the RAAS. Conversely, elevated PTH and insufficient vitamin D status have been associated with adverse cardiovascular outcomes, which may be mediated by the RAAS. Studies of primary hyperparathyroidism implicate PTH-mediated stimulation of the RAAS, and recent evidence shows that the vitamin D-vitamin D receptor (VDR) complex may negatively regulate renin expression and RAAS activity. Ongoing human interventional studies are evaluating the influence of RAAS inhibition on PTH and the influence of VDR agonists on RAAS activity.
Summary
While previously considered independent endocrine systems, emerging evidence supports a complex web of interactions between adrenal and calcium-regulating hormones, with implications for human cardiovascular and skeletal health.
Keywords: renin-angiotensin-aldosterone system, parathyroid hormone, vitamin D
INTRODUCTION
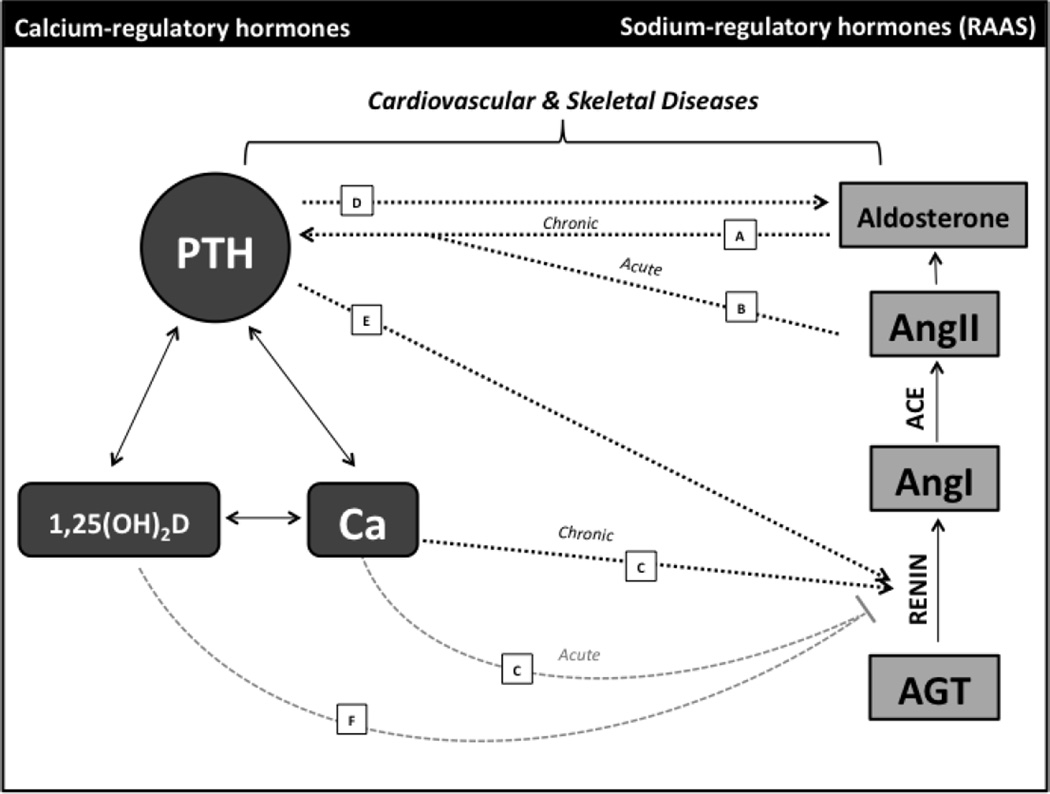
Beyond its known roles in the control of blood pressure and intravascular volume, the renin-angiotensin-aldosterone system (RAAS), has been implicated as a major factor in the pathogenesis of cardiovascular disease [1, 2], and clinical use of medications targeting the RAAS has a proven benefit for cardiovascular morbidity and mortality [3–6]. New evidence suggests that inhibiting the RAAS may also play a role in improving skeletal health [7]. Conversely, components of the calcium-regulatory system, including parathyroid hormone (PTH), vitamin D metabolites, and the vitamin D receptor (VDR), have been increasingly implicated in the pathogenesis of cardiovascular disease [8*,9*,10] in addition to their known roles in bone mineral metabolism [11–13]. A growing body of evidence identifies bidirectional relationships between these adrenal- and calcium-regulating systems that might underlie their shared associations with cardiovascular and bone health. A model summarizing the interactions described below is shown in Figure 1.
Figure 1. Interactions between Calcium-Regulatory Hormones and the Renin-Angiotensin-Aldosterone System.
Increasing evidence supports multiple, complex interactions between the calcium-regulatory system and the RAAS, and these interactions are relevant to both cardiovascular and skeletal disease. Shown on the left is a simplified diagram of the key components of the calcium-regulating system, and on the right, from bottom to top, is a simplified schematic of the RAAS. Solid arrows denote established interactions within the calcium- and adrenal-regulatory hormone systems, respectively. Dashed arrows indicate newly appreciated interactions between the two systems. Black arrows indicate a stimulatory relationship, whereas grey lines terminating in a bar indicate an inhibitory relationship. Arrows A and B depict RAAS-mediated stimulation of the calcium-regulatory system. (A) Chronic exposure to aldosterone stimulates PTH, possibly secondary to urinary calcium loss, though the mineralocorticoid receptor is expressed in the parathyroid [14**,15**,16*]. (B) AngII acutely stimulates PTH, and the angiotensin type 1 receptor is expressed in parathyroid tissue [17**]. Arrows C-F show Ca-, PTH-, and Vitamin D-mediated control of the RAAS. (C) Ca acutely inhibits renin release, but chronic elevations in Ca may stimulate renin production [18]. (D) PTH augments the aldosterone response to AngII [19], and PTH receptors are expressed in aldosterone-producing cells [20]. (E) PTH directly stimulates renin in vitro [21], and PTH infusion results in increased AngII levels [22]. (F) The 1,25(OH)2D-Vitamin D Receptor complex inhibits renin expression in vitro [23**], insufficient Vitamin D status has been associated with increased plasma renin activity [24], and Vitamin D supplementation can downregulate the RAAS [25**]. RAAS, renin-angiotensin-aldosterone system; PTH, parathyroid hormone; 1,25(OH)2D, 1,25-dihydroxyvitamin D; Ca, calcium; AGT, angiotensinogen; AngI, angiotensin I; ACE, angiotensin converting enzyme; AngII, angiotensin II.
RAAS-MEDIATED CONTROL OF CALCIUM-REGULATORY HORMONES
Observational studies of patients with primary aldosteronism (PA) have shed light on a close interaction between the RAAS and the calcium-regulating hormone system. Early studies by Resnick and others first described alterations in calcium metabolism and elevated PTH levels in patients with PA [26, 27]; however, levels of vitamin D, a possible confounder, were not measured. More recently, Pilz et al. described 10 patients with PA with significantly higher PTH levels than 182 essential hypertensive controls despite similar 25-hydroxyvitamin D (25[OH]D) levels [14**]. In a larger observational study of 44 patients with PA, Maniero et al. found elevated PTH levels (+31%), while 25(OH)D, 1,25-dihydroxyvitamin D (1,25[OH]2D), serum and urinary calcium measurements were equivalent to a group of 61 essential hypertensive controls [15**]. Increased PTH levels are so much a feature of aldosteronism that Rossi et al. demonstrated that elevated PTH levels could even serve as a helpful marker in distinguishing unilateral aldosterone-producing adenoma from bilateral adrenal hyperplasia as causes of PA in hypertensive patients [28*], potentially due to an increased severity of hyperaldosteronism in aldosterone-producing adenoma. In each case, treatment of PA with either adrenalectomy or a mineralocorticoid receptor (MR) antagonist significantly reduced PTH to levels comparable with essential hypertensive controls [14**, 15**, 27, 28*]. These studies identify a reversible state of hyperparathyroidism associated with autonomous aldosterone hypersecretion.
Given the evidence of elevated PTH in PA and animal studies describing bone loss in hyperaldosteronism [29, 30], Salcuni et al. recently demonstrated lower bone mineral density (BMD) in 11 patients with PA compared with 15 non-PA controls, with accompanying increased PTH levels [31*]. PA was associated with osteoporosis (OR 15.4; 95% CI 1.83–130) and with vertebral fractures (OR 30.4; 95% CI 1.07–862) in this population [31*]. Furthermore, in the subset who underwent surgery or medical MR blockade, treatment was associated with reduced PTH levels and increased lumbar spine BMD at 6- and 12-month follow-up, respectively [31*], suggesting a potential PTH-mediated mechanism of reversible bone loss. In a larger prospective study, Ceccoli et al. evaluated PTH and calcium parameters in 116 patients with PA and 110 essential hypertensive controls, and assayed BMD and bone markers in a subset of 40 patients before and after medical or surgical treatment of PA [32*]. Again, patients with PA were found to have elevated PTH levels compared with controls. At 18–36 months after treatment of PA, Z-scores of BMD were improved at the lumbar spine, femoral neck, and total hip, and C-telopeptide, a marker of bone turnover, showed a non-significant trend toward improvement [32*]. In both studies, PA subjects had similar 25(OH)D levels compared with their non-PA controls. These studies have identified reversible loss of bone mass in PA and suggest a possible PTH-mediated mechanism of bone metabolism. However, additional mechanisms have been proposed, including a direct effect of aldosterone on bone, as MR expression has been identified in osteoblasts and osteoclasts [33]. Studies are needed to explore in more depth the skeletal consequences of primary aldosterone excess.
In addition to PA, observational evidence supporting a relationship between aldosterone and skeletal outcomes has extended to other states of maladaptive RAAS activity, including congestive heart failure (CHF). In a case-control design involving 167 CHF patients with non-traumatic fracture and 668 age- and race-matched controls, Carbone et al. found an inverse association between the use of spironolactone, an MR antagonist, and fractures (OR 0.575; 95% CI 0.346–0.955) [7]. While this was an uncontrolled, retrospective study in which levels of aldosterone, PTH, and other relevant calcium-related parameters were not available, the finding of an association between MR blockade and reduced fracture rates supports the possibility of a clinically meaningful interplay between the RAAS and calcium-regulatory hormones. In addition, in a study of patients with advanced chronic kidney disease (CKD), in which secondary hyperparathyroidism is common and these elevations in PTH are known to associate with adverse clinical outcomes [34–36], Koiwa et al. found that clinical use of RAAS-inhibitor medications was associated with lower PTH levels [37*]. Serum calcium levels did not differ with RAAS inhibitor medication use [37*], suggesting that blockade of the RAAS may have a direct effect on PTH levels in this population, though data on skeletal outcomes were not available.
Given the observational nature of these studies and the often limited availability of a complete assessment of calcium-regulatory parameters, the pathway by which aldosterone, or the RAAS, stimulates PTH is not clearly defined. However, investigators have hypothesized an indirect mechanism whereby chronic aldosterone excess induces hypercalciuria and hypocalcemia causing a reactive secondary hyperparathyroidism [38, 39]. Though not perfectly uniform between studies, various calcium metrics were affected in the studies in PA described above, consistent with this proposed mechanism. Depending on the study, ionized calcium levels [27] or serum calcium levels [14**, 32*] were lower in PA compared with essential hypertension, and serum calcium levels rose following treatment of PA [15**, 27, 31*], all of which support a secondary rise in PTH. Furthermore, urinary calcium was higher in some studies of PA [31*, 32*], with a nonsignificant trend toward higher urinary calcium in others [14**, 27], implicating a possible aldosterone-induced calciuric effect. However, despite these changes in calcium, a more direct effect of aldosterone on PTH is also plausible, as MR expression has been described in the parathyroid [16*, 17**]. Regardless of mechanism, chronic exposure to aldosterone excess appears to drive higher levels of PTH and/or reduced BMD, at least in observational studies of pathologic states of PA, CHF, and CKD (Figure 1A).
PA, CHF, and CKD share common maladaptive RAAS activation, in particular aldosterone excess, with apparent effects on PTH and/or bone. However, the above observational studies in these conditions are limited in how definitively they can describe regulation of PTH by the RAAS due to observational design and variable dietary conditions, medication use, and renal function. We recently reported the combined results of four small, controlled human interventional studies examining control of PTH by the RAAS in relatively healthy subjects without PA, CHF, or CKD [17**]. Over two decades ago, Grant et al. reported a dose-dependent increase in PTH due to infused angiotensin II in a controlled study of healthy volunteers [22]. We extended this finding, showing that an infusion of angiotensin II acutely raised circulating PTH, and a short-acting oral ACE inhibitor (captopril) acutely lowered PTH to levels below baseline [17**]. Angiotensin II infusion raises both angiotensin II and aldosterone, and an ACE inhibitor similarly lowers both angiotensin II and aldosterone, meaning that changes in either angiotensin II or aldosterone might be suspected to drive the observed acute effect on PTH. To disaggregate the effects of each RAAS component, we showed that an infusion of aldosterone, which would presumably have a neutral or negative effect on angiotensin II levels, had no acute effect on circulating PTH [17**]. This finding, in contrast to observational evidence from pathologic states of chronic hyperaldosteronism, supports the conclusion that acute stimulation of PTH by the RAAS is due to angiotensin II, and not aldosterone, and is further supported by the detection of angiotensin type 1 receptor (AT1R) expression in both normal and adenomatous parathyroid tissue [17**]. It appears that under physiologic conditions, angiotensin II acutely raises PTH levels, possibly via a direct action on the parathyroid (Figure 1B). Whether targeting angiotensin II can alter PTH in states of PTH excess remains to be shown and is the subject of the ongoing Renin-Angiotensin-Aldosterone-System and Parathyroid Hormone Control (RAAS-PARC) Study (NCT01691781), an interventional study evaluating the effect of angiotensin converting enzyme inhibition on PTH levels in primary hyperparathyroidism.
Though the acute interaction between angiotensin II and PTH represents a novel aspect of physiology, uncovering the impact of chronic RAAS activation or inhibition on PTH may have more meaningful clinical implications. Under randomized, placebo-controlled conditions, we demonstrated that six weeks of mineralocorticoid blockade with spironolactone (50mg daily) resulted in a relative decrease in PTH compared to placebo [17**]. Though the effect was modest, it parallels what has been shown in PA, where MR antagonist use and/or surgical adrenalectomy resulted in lower PTH levels [14**, 15**, 27]. In this case, an MR-dependent effect on PTH was observed in relatively healthy subjects despite presumably normal aldosterone levels and minimal MR activation at baseline. Together with the above observational evidence in PA, CHF, and CKD, this evidence from human interventions implicates aldosterone in the control of PTH under chronic conditions and highlights MR blockade as a potential means of targeting PTH and mineral metabolism that is worthy of further investigation. The ongoing study Effects of Eplerenone in Patients with Primary Hyperparathyroidism (EPATH) will evaluate the impact of eplerenone, an MR antagonist, on circulating PTH levels, as well as skeletal and cardiovascular parameters in patients with primary hyperparathyroidism [40**].
In sum, interventional studies demonstrate acute stimulation of PTH by angiotensin II that is not achieved with aldosterone alone. Chronically, increased aldosterone appears to induce hyperparathyroidism, possibly via direct effects on the parathyroid itself, or indirectly via induction of hypercalciuria. Treatment that inhibits aldosterone action correlates with lower PTH levels (or improved bone outcomes) in PA, CHF, CKD, and, to a lesser degree, in normal volunteers with a healthy RAAS. These interactions highlight a new paradigm of RAAS-mediated control of PTH, summarized in Figure 1A–B.
CALCIUM-, PTH-, & VITAMIN D-MEDIATED CONTROL OF THE RAAS
In support of a bidirectional relationship between the RAAS and calcium-regulatory hormones, accruing evidence describes calcium ion-, PTH-, and vitamin D-mediated regulation of the RAAS, shown in Figure 1C–F.
Primary hyperparathyroidism (PHPT) has been linked to multiple cardiovascular outcomes though some newer studies have had conflicting results [41]. PHPT has been associated with hypertension [42, 43], coronary artery disease [44, 45], left ventricular hypertrophy [46, 47], and mortality [48–50]. Given the challenge of demonstrating a reversible effect on cardiovascular mortality, controlled interventional studies have assessed several intermediate cardiovascular phenotypes, including hypertension [51, 52] and arterial stiffness, and demonstrated improvement following parathyroidectomy [53, 54]. Aortic stiffness measured by pulse-wave velocity was increased in 44 patients with PHPT compared with 46 controls, despite adjustment for age and blood pressure [53]. In those treated with parathyroidectomy, pulse-wave velocity (i.e. stiffness) and systolic blood pressure were reduced at 6-month follow-up [53]. Using another measure of vascular function that serves as a marker of cardiovascular risk, Rubin et al. showed elevated arterial stiffness in 39 patients with mild PHPT, independent of typical cardiovascular risk factors [54]. As with hypertension, arterial stiffness has been previously linked to maladaptive RAAS activity and can be improved with RAAS blockade [55, 56], implicating the RAAS as one of several potential mediators of the adverse vascular phenotypes observed in PHPT. Other proposed mechanisms include indirect effects via hypertension and renal dysfunction, as well as direct effects of elevated PTH levels and/or the accompanying hypercalcemia [53, 57].
Calcium has been associated with vascular calcification and atherosclerotic coronary artery disease [58–60]. Furthermore, calcium has been identified as a regulator of the RAAS at the level of renin [18]. As shown in Figure 1C, calcium acutely inhibits juxtaglomerular cell release of renin via the calcium-sensing receptor (CaSR) [61*], whereas chronic hypercalcemia [62] and chronic stimulation of the CaSR [63] appear to increase plasma renin activity. These interactions highlight a key element of the complex crosstalk between the calcium- and sodium-regulating systems and may contribute to the cardiovascular features of PHPT.
Over and above the effect on calcium, PTH itself has been implicated as a regulator of the RAAS. Even in populations without PHPT or hypercalcemia, PTH levels have been linked in both cross-sectional and prospective studies to multiple cardiovascular outcomes, including hypertension [64], cardiovascular function [65*, 66*], sudden cardiac death [67], and cardiovascular and all-cause mortality [8*, 68*, 69]. In support of an effect of PTH on the RAAS is evidence from both PHPT and healthy volunteers, based initially on a case report describing hyperaldosteronism in a patient with PHPT [70]. In this case, a patient presenting with hypercalcemia, hypertension, and hypokalemia was found to have both a parathyroid adenoma and PA, diagnosed by an elevated and nonsuppressible aldosterone level and suppressed renin activity, though no adrenal adenoma was identified. At 1 year after parathyroidectomy, both PHPT and PA had resolved, for the first time suggesting a direct connection between PTH and the adrenal axis. In an observational study of 134 patients with PHPT undergoing parathyroidectomy, Brunaud et al. found that the pre-operative PTH, but not calcium, level predicted the aldosterone level, with a stronger correlation found among those patients not taking antihypertensive medications, which might be expected to confound aldosterone levels [71*]. At 3 month follow-up after parathyroidectomy, the correlation between PTH and aldosterone levels was lost [71*]. The lack of a relationship in the post-operative setting could be because upon the resolution of pathologically high PTH levels, a relationship between PTH and aldosterone does not exist or is more difficult to detect under uncontrolled conditions given the numerous environmental inputs on the RAAS.
Several small interventional studies further support stimulation of the RAAS by PTH alone or with calcium, though a consensus has not yet been reached on the specific mechanism. In a controlled interventional study of 5 subjects with PHPT, angiotensin II infusion resulted in an enhanced aldosterone response compared with matched controls [19], and in a study of healthy volunteers, PTH infusion led to increased levels of urinary tetrahydroaldosterone [72]. These studies emphasize an action at the level of adrenocortical aldosterone synthesis, where there is in vitro evidence of PTH receptor expression [16*, 20] and of a stimulatory effect of intracellular calcium [73] and PTH [74] on adrenal aldosterone-producing cells, providing a plausible direct effect of the calcium system on aldosterone (Figure 1D).
However, other interventional studies suggest a distinct relationship. Kovacs et al. studied 16 patients with PHPT, in whom basal aldosterone and plasma renin activity (PRA) levels correlated positively with PTH levels [75]. Following parathyroidectomy, aldosterone and PRA levels were reduced both at baseline and after stimulation with upright posture and furosemide to activate the RAAS [75]. In addition to such findings in patients with pathologic PTH excess, studies of healthy volunteers have also supported a more proximal action of PTH on the RAAS; PTH infusion in healthy adults resulted in an increase in angiotensin II [22]. These human studies corroborate in vitro evidence of direct stimulation of renin by PTH [21, 76] (Figure 1E), an alternative or potentially complementary mechanism of PTH action on the RAAS. Thus, evidence from human studies supports multiple pleiotropic effects of PTH and calcium on the RAAS, with possible sites of action at renin, angiotensin II, and/or aldosterone.
It should be noted that studies have not been universally positive regarding stimulation of the RAAS by PTH. Bernini et al. evaluated the RAAS in 20 subjects with PHPT and found no difference in PRA, serum aldosterone, or urinary aldosterone compared with normotensive or hypertensive controls, or at 1- and 6-months after parathyroidectomy [77]. However, these negative findings may have been due to incomplete control of posture, interfering medications, and sodium intake, all of which can dramatically alter measurements of the RAAS. Furthermore, the interventional studies described above are limited by the lack of a complete assessment of the calcium-regulating apparatus, in particular levels of 25(OH)D and 1,25(OH)2D, which might allow more definitive conclusions to be drawn.
Like calcium and PTH, vitamin D has also been implicated in control of the RAAS, with potential clinical consequences. In observational analyses, vitamin D deficiency has been associated with hypertension and cardiovascular disease [78], a relationship that may depend on the RAAS. In animal studies, the vitamin D receptor (VDR) bound to its ligand, 1,25(OH)2D, has been shown to negatively regulate renin [23**, 79, 80] (Figure 1F). In humans, several cross-sectional studies have also demonstrated an association between vitamin D metabolites and components of the RAAS: 25(OH)D levels have been shown to inversely correlate with PRA [24] as well as with elevated circulating levels of angiotensin II [81], signifying increased RAAS activation in states of low 25(OH)D. In a large cohort of 3296 patients presenting for coronary angiogram, both 25(OH)D and 1,25(OH)2D were independently associated with renin and angiotensin II concentrations [82*]. Further, a functional polymorphism in the VDR, when combined with 25(OH)D levels, predicted PRA in hypertensive and normotensive individuals [83]. This cross-sectional evidence suggests a role for the vitamin D-VDR complex in negatively regulating the RAAS, which may underlie epidemiologic associations between 25(OH)D deficiency and cardiovascular disease.
In interventional studies, sensitivity to angiotensin II infusion, manifested by adrenal aldosterone production and vascular responses to angiotensin II, has been negatively correlated with activity of the local tissue-RAAS [84, 85]. Lower 25(OH)D levels have been associated with blunted sensitivity to angiotensin II [81], a state of local RAAS excess implicated in adverse vascular outcomes [84, 86, 87]. Furthermore, we have previously shown that high-dose vitamin D3 supplementation can reduce local tissue-RAAS over-activity, restoring sensitivity to angiotensin II in obese hypertensive adults with 25(OH)D deficiency [25**]. The ongoing Evaluating Hormonal Mechanisms for Vitamin D Receptor Agonist Therapy in Diabetes (VALIDATE-D) Study is a randomized, placebo-controlled study that seeks to evaluate the ability of the direct VDR ligand, 1,25(OH)2D, to downregulate circulating and local RAAS activity and possibly improve vascular health in diabetes [88]. A demonstration of downregulation of the RAAS by 1,25(OH)2D could provide additional evidence of a clinically relevant and complex interplay between calcium-regulating hormones and the RAAS.
CONCLUSION
Excess RAAS activity, excess PTH and calcium, and insufficient vitamin D status have all been associated with adverse clinical outcomes, both cardiovascular and skeletal. Recent observational and interventional studies in both pathologic states and normal physiology describe the existence of meaningful bidirectional crosstalk between the RAAS and calcium-regulating hormone systems. Additional investigation is needed to further clarify the extent of these complex endocrine interactions and evaluate the clinical impacts of targeting these systems on cardiovascular and bone health.
KEY POINTS.
There is increasing evidence of bidirectional interactions between the RAAS and calcium-regulating hormones, with possible implications for cardiovascular and skeletal disease.
Primary aldosteronism is associated with reversible elevation in parathyroid hormone and reduction in bone mineral density.
Recent interventional evidence suggests that angiotensin II stimulates PTH acutely, while aldosterone may promote increased PTH chronically.
Primary hyperparathyroidism is associated with adverse cardiovascular risk, which may be mediated by the RAAS.
The 1,25(OH)2D-VDR complex can suppress the RAAS by inhibiting renin expression, and whether this interaction has cardiovascular consequences is under current study.
Future studies should evaluate these bidirectional interactions and their clinical consequences in more detail.
ACKNOWLEDGEMENTS
The authors acknowledge and thank their funding sources: The National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K23HL111771 (AV); the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. They also thank the William Randolph Hearst Foundation and Brigham and Women’s Hospital for awarding funding from the Hearst Young Investigator Award (AV) and recognize support from the Harvard Medical School Research Fellowship (JMB).
Footnotes
CONFLICTS OF INTEREST: None
REFERENCES
- 1.Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98:121–128. doi: 10.1016/j.amjcard.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 2.Probstfield JL, O'Brien KD. Progression of cardiovascular damage: the role of renin-angiotensin system blockade. Am J Cardiol. 2010;105:10A–20A. doi: 10.1016/j.amjcard.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 4.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 5.Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 6.Pitt B, Reichek N, Willenbrock R, et al. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation. 2003;108:1831–1838. doi: 10.1161/01.CIR.0000091405.00772.6E. [DOI] [PubMed] [Google Scholar]
- 7.Carbone LD, Cross JD, Raza SH, et al. Fracture risk in men with congestive heart failure risk reduction with spironolactone. J Am Coll Cardiol. 2008;52:135–138. doi: 10.1016/j.jacc.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 8. van Ballegooijen AJ, Reinders I, Visser M, et al. Serum parathyroid hormone in relation to all-cause and cardiovascular mortality: the Hoorn study. J Clin Endocrinol Metab. 2013;98:E638–E645. doi: 10.1210/jc.2012-4007. In this analysis of 633 participants from the prospective, population-based Hoorn cohort study, all-cause and cardiovascular mortality increased with increasing quartiles of PTH. Higher PTH levels were associated with all-cause mortality in multivariate modeling, including after adjustment for renal function.
- 9. Tomaschitz A, Ritz E, Pieske B, et al. Aldosterone and parathyroid hormone interactions as mediators of metabolic and cardiovascular disease. Metabolism. 2014;63:20–31. doi: 10.1016/j.metabol.2013.08.016. This in-depth review provides a detailed discussion of the interactions between aldosterone and PTH, including in vitro and animal evidence of the underlying molecular and cellular mechanisms. Additional mediators, including klotho and dietary sodium, are described.
- 10.Vaidya A, Forman JP. Vitamin D and vascular disease: the current and future status of vitamin D therapy in hypertension and kidney disease. Curr Hypertens Rep. 2012;14:111–119. doi: 10.1007/s11906-012-0248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 12.Silverberg SJ, Shane E, de la Cruz L, et al. Skeletal disease in primary hyperparathyroidism. J Bone Miner Res. 1989;4:283–291. doi: 10.1002/jbmr.5650040302. [DOI] [PubMed] [Google Scholar]
- 13.Vestergaard P, Mollerup CL, Frøkjaer VG, et al. Cohort study of risk of fracture before and after surgery for primary hyperparathyroidism. BMJ. 2000;321:598–602. doi: 10.1136/bmj.321.7261.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pilz S, Kienreich K, Drechsler C, et al. Hyperparathyroidism in patients with primary aldosteronism: cross-sectional and interventional data from the GECOH study. J Clin Endocrinol Metab. 2012;97:E75–E79. doi: 10.1210/jc.2011-2183. This study demonstrated significantly higher PTH levels and lower calcium levels in patients with primary aldosteronism (PA) compared to those with essential hypertension, despite equivalent 25-hydroxyvitamin D levels. After treatment with adrenalectomy or mineralocorticoid receptor blockade, PTH levels decreased significantly to levels comparable with essential hypertensives. The authors conclude a reversible secondary hyperparathyroidism due to PA.
- 15. Maniero C, Fassina A, Seccia TM, et al. Mild hyperparathyroidism: a novel surgically correctable feature of primary aldosteronism. J Hypertens. 2012;30:390–395. doi: 10.1097/HJH.0b013e32834f0451. In this observational study of 105 hypertensive patients, patients with aldosterone-producing adenoma (n=44) had significantly higher PTH levels (+31%). In patients treated with adrenalectomy, PTH levels normalized. PTH receptor expression was detected in excised adenomatous and adjacent normal adrenal tissue, leading the authors to conclude that PTH might play a role in maintaining hyperaldosteronism.
- 16.Maniero C, Fassina A, Guzzardo V, et al. Primary hyperparathyroidism with concurrent primary aldosteronism. Hypertension. 2011;58:341–346. doi: 10.1161/HYPERTENSIONAHA.111.173948. [DOI] [PubMed] [Google Scholar]
- 17. Brown JM, Williams JS, Luther JM, et al. Human interventions to characterize novel relationships between the renin-angiotensin-aldosterone system and parathyroid hormone. Hypertension. 2013 doi: 10.1161/HYPERTENSIONAHA.113.01910. Epub ahead of print. This paper combines secondary analyses of four controlled, interventional studies that demonstrated that angiotensin II acutely raises PTH levels while aldosterone may provide a chronic PTH stimulus in volunteers without hyperaldosteronism. ACE inhibition and mineralocorticoid receptor antagonism were both shown to reduce PTH levels. Preliminary in vitro studies also detected expression of the angiotensin type 1 receptor as well as the mineralocorticoid receptor in the parathyroid gland, consistent with multiple interactions.
- 18.Atchison DK, Beierwaltes WH. The influence of extracellular and intracellular calcium on the secretion of renin. Pflugers Arch. 2013;465:59–69. doi: 10.1007/s00424-012-1107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fallo F, Rocco S, Pagotto U, et al. Aldosterone and pressor responses to angiotensin II in primary hyperparathyroidism. J Hypertens Suppl. 1989;7:S192–S193. doi: 10.1097/00004872-198900076-00092. [DOI] [PubMed] [Google Scholar]
- 20.Kitazawa S, Fukase M, Kitazawa R, et al. Immunohistologic evaluation of parathyroid hormone-related protein in human lung cancer and normal tissue with newly developed monoclonal antibody. Cancer. 1991;67:984–989. doi: 10.1002/1097-0142(19910215)67:4<984::aid-cncr2820670421>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Saussine C, Judes C, Massfelder T, et al. Stimulatory action of parathyroid hormone on renin secretion in vitro: a study using isolated rat kidney, isolated rabbit glomeruli and superfused dispersed rat juxtaglomerular cells. Clin Sci (Lond) 1993;84:11–19. doi: 10.1042/cs0840011. [DOI] [PubMed] [Google Scholar]
- 22.Grant FD, Mandel SJ, Brown EM, et al. Interrelationships between the renin-angiotensin-aldosterone and calcium homeostatic systems. J Clin Endocrinol Metab. 1992;75:988–992. doi: 10.1210/jcem.75.4.1400892. [DOI] [PubMed] [Google Scholar]
- 23.Li YC, Kong J, Wei M, et al. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaidya A, Forman JP, Hopkins PN, et al. 25-Hydroxyvitamin D is associated with plasma renin activity and the pressor response to dietary sodium intake in Caucasians. J Renin Angiotensin Aldosterone Syst. 2011;12:311–319. doi: 10.1177/1470320310391922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vaidya A, Sun B, Larson C, et al. Vitamin D3 therapy corrects the tissue sensitivity to angiotensin ii akin to the action of a converting enzyme inhibitor in obese hypertensives: an interventional study. J Clin Endocrinol Metab. 2012;97:2456–2465. doi: 10.1210/jc.2012-1156. This controlled, interventional study evaluated the impact of high-dose vitamin D3 therapy on activity of the tissue-level RAAS in 14 obese adults with hypertension and 25-hydroxyvitamin D insufficiency. Vitamin D3 therapy successfully raised 25-hydroxyvitamin D levels and resulted in improved renal-vascular hemodynamics and an improved sensitivity to angiotensin II infusion, consistent with reduced tissue-RAAS activity and similar to the effect of an ACE inhibitor.
- 26.Resnick LM, Laragh JH. Calcium metabolism and parathyroid function in primary aldosteronism. Am J Med. 1985;78:385–390. doi: 10.1016/0002-9343(85)90328-6. [DOI] [PubMed] [Google Scholar]
- 27.Rossi E, Sani C, Perazzoli F, et al. Alterations of calcium metabolism and of parathyroid function in primary aldosteronism, and their reversal by spironolactone or by surgical removal of aldosterone-producing adenomas. Am J Hypertens. 1995;8:884–893. doi: 10.1016/0895-7061(95)00182-O. [DOI] [PubMed] [Google Scholar]
- 28. Rossi GP, Ragazzo F, Seccia TM, et al. Hyperparathyroidism can be useful in the identification of primary aldosteronism due to aldosterone-producing adenoma. Hypertension. 2012;60:431–436. doi: 10.1161/HYPERTENSIONAHA.112.195891. This cross-sectional analysis of 46 patients with aldosterone-producing adenoma (APA), 12 patients with bilateral adrenal hyperplasia, and 74 essential hypertensive controls used receiver operator curves to demonstrate that serum PTH levels could be used distinguish between causes of primary aldosteronism. A PTH cut-off of 80ng/mL yielded a sensitivity of 74% and a specificity of 82% for APA.
- 29.Chhokar VS, Sun Y, Bhattacharya SK, et al. Loss of bone minerals and strength in rats with aldosteronism. Am J Physiol Heart Circ Physiol. 2004;287:H2023–H2026. doi: 10.1152/ajpheart.00477.2004. [DOI] [PubMed] [Google Scholar]
- 30.Runyan AL, Chhokar VS, Sun Y, et al. Bone loss in rats with aldosteronism. Am J Med Sci. 2005;330:1–7. doi: 10.1097/00000441-200507000-00001. [DOI] [PubMed] [Google Scholar]
- 31. Salcuni AS, Palmieri S, Carnevale V, et al. Bone involvement in aldosteronism. J Bone Miner Res. 2012;27:2217–2222. doi: 10.1002/jbmr.1660. This observational study of patients with an incidental adrenal mass found higher PTH levels, higher urinary calcium excretion, lower bone mineral density, and a higher prevalence of osteoporosis in patients with primary aldosteronism (PA) compared to those without. In a subset treated for PA, PTH was lower and bone density at the lumbar spine was higher at follow-up.
- 32. Ceccoli L, Ronconi V, Giovannini L, et al. Bone health and aldosterone excess. Osteoporos Int. 2013;24:2801–2807. doi: 10.1007/s00198-013-2399-1. In this observational study, patients with primary aldosteronism (PA) had increased PTH levels compared with essential hypertensive controls. The impact of PA treatment was prospectively assessed in a subset of 40 patients. These patients demonstrated reduced PTH levels and improved bone mineral density Z-scores at the lumbar spine, femoral neck, and total hip at post-treatment follow-up.
- 33.Beavan S, Horner A, Bord S, et al. Colocalization of glucocorticoid and mineralocorticoid receptors in human bone. J Bone Miner Res. 2001;16:1496–1504. doi: 10.1359/jbmr.2001.16.8.1496. [DOI] [PubMed] [Google Scholar]
- 34.Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52:519–530. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 35.Melamed ML, Eustace JA, Plantinga L, et al. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney Int. 2006;70:351–357. doi: 10.1038/sj.ki.5001542. [DOI] [PubMed] [Google Scholar]
- 36.Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 37. Koiwa F, Komukai D, Hirose M, et al. Influence of renin-angiotensin system on serum parathyroid hormone levels in uremic patients. Clin Exp Nephrol. 2012;16:130–135. doi: 10.1007/s10157-011-0534-x. This retrospective study evaluated factors associated with PTH levels in 1076 patients initiating hemodialysis. Significantly lower PTH levels were found in patients taking medications that inhibit the renin-angiotensin-aldosterone system, as well as in males and those with diabetes mellitus.
- 38.Rastegar A, Agus Z, Connor TB, Goldberg M. Renal handling of calcium and phosphate during mineralocorticoid "escape" in man. Kidney Int. 1972;2:279–286. doi: 10.1038/ki.1972.107. [DOI] [PubMed] [Google Scholar]
- 39.Rossi E, Perazzoli F, Negro A, et al. Acute effects of intravenous sodium chloride load on calcium metabolism and on parathyroid function in patients with primary aldosteronism compared with subjects with essential hypertension. Am J Hypertens. 1998;11:8–13. doi: 10.1016/s0895-7061(97)00366-x. [DOI] [PubMed] [Google Scholar]
- 40. Tomaschitz A, Fahrleitner-Pammer A, Amrein K, et al. Effect of eplerenone on parathyroid hormone levels in patients with primary hyperparathyroidism: a randomized, double-blind, placebo-controlled trial. BMC Endocr Disord. 2012;12:19. doi: 10.1186/1472-6823-12-19. This ongoing randomized, placebo-controlled study is examining the impact of 8 weeks of mineralocorticoid receptor blockade with eplerenone on PTH levels and on multiple secondary endpoints including bone markers and echocardiographic variables in 110 patients with primary hyperparathyroidism.
- 41.Silverberg SJ, Lewiecki EM, Mosekilde L, et al. Presentation of asymptomatic primary hyperparathyroidism: proceedings of the third international workshop. J Clin Endocrinol Metab. 2009;94:351–365. doi: 10.1210/jc.2008-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lind L, Ridefelt P, Rastad J, et al. Relationship between abnormal regulation of cytoplasmic calcium and elevated blood pressure in patients with primary hyperparathyroidism. J Hum Hypertens. 1994;8:113–118. [PubMed] [Google Scholar]
- 43.Letizia C, Ferrari P, Cotesta D, et al. Ambulatory monitoring of blood pressure (AMBP) in patients with primary hyperparathyroidism. J Hum Hypertens. 2005;19:901–906. doi: 10.1038/sj.jhh.1001907. [DOI] [PubMed] [Google Scholar]
- 44.Nilsson IL, Aberg J, Rastad J, Lind L. Left ventricular systolic and diastolic function and exercise testing in primary hyperparathyroidism-effects of parathyroidectomy. Surgery. 2000;128:895–902. doi: 10.1067/msy.2000.110240. [DOI] [PubMed] [Google Scholar]
- 45.Vestergaard P, Mollerup CL, Frøkjaer VG, et al. Cardiovascular events before and after surgery for primary hyperparathyroidism. World J Surg. 2003;27:216–222. doi: 10.1007/s00268-002-6541-z. [DOI] [PubMed] [Google Scholar]
- 46.Piovesan A, Molineri N, Casasso F, et al. Left ventricular hypertrophy in primary hyperparathyroidism. Effects of successful parathyroidectomy. Clin Endocrinol (Oxf) 1999;50:321–328. doi: 10.1046/j.1365-2265.1999.00651.x. [DOI] [PubMed] [Google Scholar]
- 47.Stefenelli T, Abela C, Frank H, et al. Time course of regression of left ventricular hypertrophy after successful parathyroidectomy. Surgery. 1997;121:157–161. doi: 10.1016/s0039-6060(97)90285-3. [DOI] [PubMed] [Google Scholar]
- 48.Hedbäck G, Odén A. Increased risk of death from primary hyperparathyroidism--an update. Eur J Clin Invest. 1998;28:271–276. doi: 10.1046/j.1365-2362.1998.00289.x. [DOI] [PubMed] [Google Scholar]
- 49.Nilsson IL, Yin L, Lundgren E, et al. Clinical presentation of primary hyperparathyroidism in Europe--nationwide cohort analysis on mortality from nonmalignant causes. J Bone Miner Res. 2002;17(Suppl 2):N68–N74. [PubMed] [Google Scholar]
- 50.Yu N, Donnan PT, Leese GP. A record linkage study of outcomes in patients with mild primary hyperparathyroidism: the Parathyroid Epidemiology and Audit Research Study (PEARS) Clin Endocrinol (Oxf) 2011;75:169–176. doi: 10.1111/j.1365-2265.2010.03958.x. [DOI] [PubMed] [Google Scholar]
- 51.Nainby-Luxmoore JC, Langford HG, Nelson NC, et al. A case-comparison study of hypertension and hyperparathyroidism. J Clin Endocrinol Metab. 1982;55:303–306. doi: 10.1210/jcem-55-2-303. [DOI] [PubMed] [Google Scholar]
- 52.Ringe JD. Reversible hypertension in primary hyperparathyroidism--pre- and posteroperative blood pressure in 75 cases. Klin Wochenschr. 1984;62:465–469. doi: 10.1007/BF01726908. [DOI] [PubMed] [Google Scholar]
- 53.Rosa J, Raska I, Wichterle D, et al. Pulse wave velocity in primary hyperparathyroidism and effect of surgical therapy. Hypertens Res. 2011;34:296–300. doi: 10.1038/hr.2010.232. [DOI] [PubMed] [Google Scholar]
- 54.Rubin MR, Maurer MS, McMahon DJ, et al. Arterial stiffness in mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2005;90:3326–3330. doi: 10.1210/jc.2004-1400. [DOI] [PubMed] [Google Scholar]
- 55.Mahmud A, Feely J. Arterial stiffness and the renin-angiotensin-aldosterone system. J Renin Angiotensin Aldosterone Syst. 2004;5:102–108. doi: 10.3317/jraas.2004.025. [DOI] [PubMed] [Google Scholar]
- 56.Koumaras C, Tziomalos K, Stavrinou E, et al. Effects of renin-angiotensin-aldosterone system inhibitors and beta-blockers on markers of arterial stiffness. J Am Soc Hypertens. 2013 doi: 10.1016/j.jash.2013.09.001. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 57.Rossi GP. Hyperparathyroidism, arterial hypertension and aortic stiffness: a possible bidirectional link between the adrenal cortex and the parathyroid glands that causes vascular damage? Hypertens Res. 2011;34:286–288. doi: 10.1038/hr.2010.251. [DOI] [PubMed] [Google Scholar]
- 58.Jorde R, Sundsfjord J, Fitzgerald P, Bønaa KH. Serum calcium and cardiovascular risk factors and diseases: the Tromsø study. Hypertension. 1999;34:484–490. doi: 10.1161/01.hyp.34.3.484. [DOI] [PubMed] [Google Scholar]
- 59.Foley RN, Collins AJ, Ishani A, Kalra PA. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2008;156:556–563. doi: 10.1016/j.ahj.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 60.Lind L, Skarfors E, Berglund L, et al. Serum calcium: a new, independent, prospective risk factor for myocardial infarction in middle-aged men followed for 18 years. J Clin Epidemiol. 1997;50:967–973. doi: 10.1016/s0895-4356(97)00104-2. [DOI] [PubMed] [Google Scholar]
- 61.Atchison DK, Harding P, Beierwaltes WH. Hypercalcemia reduces plasma renin via parathyroid hormone, renal interstitial calcium, and the calcium-sensing receptor. Hypertension. 2011;58:604–610. doi: 10.1161/HYPERTENSIONAHA.111.172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spangler WL, Gribble DH, Lee TC. Vitamin D intoxication and the pathogenesis of vitamin D nephropathy in the dog. Am J Vet Res. 1979;40:73–83. [PubMed] [Google Scholar]
- 63.Watanabe S, Fukumoto S, Chang H, et al. Association between activating mutations of calcium-sensing receptor and Bartter's syndrome. Lancet. 2002;360:692–694. doi: 10.1016/S0140-6736(02)09842-2. [DOI] [PubMed] [Google Scholar]
- 64.Mateus-Hamdan L, Beauchet O, Bouvard B, et al. High parathyroid hormone, but not low vitamin D concentrations, expose elderly inpatients to hypertension. Geriatr Gerontol Int. 2013;13:783–791. doi: 10.1111/j.1447-0594.2012.00945.x. [DOI] [PubMed] [Google Scholar]
- 65. van Ballegooijen AJ, Visser M, Kestenbaum B, et al. Relation of vitamin D and parathyroid hormone to cardiac biomarkers and to left ventricular mass (from the Cardiovascular Health Study) Am J Cardiol. 2013;111:418–424. doi: 10.1016/j.amjcard.2012.10.021. In a large cohort study of patients referred for coronary angiography, PTH levels ≥65pg/mL were associated with increased cardiac biomarkers and ventricular mass in patients with chronic kidney disease, but not in patients with normal kidney function.
- 66. van Ballegooijen AJ, Visser M, Cotch MF, et al. Serum vitamin D and parathyroid hormone in relation to cardiac structure and function: the ICELAND-MI substudy of AGES-Reykjavik. J Clin Endocrinol Metab. 2013;98:2544–2552. doi: 10.1210/jc.2012-4252. In this cross-sectional analysis from an older population-based cohort study, a PTH level in highest quartile was significantly associated with increased left ventricular mass and lower ejection fraction compared to the lowest quartile, suggesting a link between PTH and cardiovascular function. No association was observed with 25-hydroxyvitamin D levels.
- 67.Deo R, Katz R, Shlipak MG, et al. Vitamin D, parathyroid hormone, and sudden cardiac death: results from the Cardiovascular Health Study. Hypertension. 2011;58:1021–1028. doi: 10.1161/HYPERTENSIONAHA.111.179135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pilz S, Tomaschitz A, Drechsler C, et al. Parathyroid hormone level is associated with mortality and cardiovascular events in patients undergoing coronary angiography. Eur Heart J. 2010;31:1591–1598. doi: 10.1093/eurheartj/ehq109. [DOI] [PubMed] [Google Scholar]
- 69.Hagström E, Hellman P, Larsson TE, et al. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation. 2009;119:2765–2771. doi: 10.1161/CIRCULATIONAHA.108.808733. [DOI] [PubMed] [Google Scholar]
- 70.Barkan A, Marilus R, Winkelsberg G, et al. Primary hyperparathyroidism: possible cause of primary hyperaldosteronism in a 60-year-old woman. J Clin Endocrinol Metab. 1980;51:144–147. doi: 10.1210/jcem-51-1-144. [DOI] [PubMed] [Google Scholar]
- 71.Brunaud L, Germain A, Zarnegar R, et al. Serum aldosterone is correlated positively to parathyroid hormone (PTH) levels in patients with primary hyperparathyroidism. Surgery. 2009;146:1035–1041. doi: 10.1016/j.surg.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 72.Hulter HN, Melby JC, Peterson JC, Cooke CR. Chronic continuous PTH infusion results in hypertension in normal subjects. J Clin Hypertens. 1986;2:360–370. [PubMed] [Google Scholar]
- 73.Tomaschitz A, Ritz E, Pieske B, et al. Aldosterone and parathyroid hormone: a precarious couple for cardiovascular disease. Cardiovasc Res. 2012;94:10–19. doi: 10.1093/cvr/cvs092. [DOI] [PubMed] [Google Scholar]
- 74.Mazzocchi G, Aragona F, Malendowicz LK, Nussdorfer GG. PTH and PTH-related peptide enhance steroid secretion from human adrenocortical cells. Am J Physiol Endocrinol Metab. 2001;280:E209–E213. doi: 10.1152/ajpendo.2001.280.2.E209. [DOI] [PubMed] [Google Scholar]
- 75.Kovács L, Góth MI, Szabolcs I, et al. The effect of surgical treatment on secondary hyperaldosteronism and relative hyperinsulinemia in primary hyperparathyroidism. Eur J Endocrinol. 1998;138:543–547. doi: 10.1530/eje.0.1380543. [DOI] [PubMed] [Google Scholar]
- 76.Helwig JJ, Musso MJ, Judes C, Nickols GA. Parathyroid hormone and calcium: interactions in the control of renin secretion in the isolated, nonfiltering rat kidney. Endocrinology. 1991;129:1233–1242. doi: 10.1210/endo-129-3-1233. [DOI] [PubMed] [Google Scholar]
- 77.Bernini G, Moretti A, Lonzi S, et al. Renin-angiotensin-aldosterone system in primary hyperparathyroidism before and after surgery. Metabolism. 1999;48:298–300. doi: 10.1016/s0026-0495(99)90075-6. [DOI] [PubMed] [Google Scholar]
- 78.Vaidya A, Williams JS. The relationship between vitamin D and the renin-angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metabolism. 2012;61:450–458. doi: 10.1016/j.metabol.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yuan W, Pan W, Kong J, et al. 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem. 2007;282:29821–29830. doi: 10.1074/jbc.M705495200. [DOI] [PubMed] [Google Scholar]
- 80.Li YC. Vitamin D regulation of the renin-angiotensin system. J Cell Biochem. 2003;88:327–331. doi: 10.1002/jcb.10343. [DOI] [PubMed] [Google Scholar]
- 81.Forman JP, Williams JS, Fisher ND. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension. 2010;55:1283–1288. doi: 10.1161/HYPERTENSIONAHA.109.148619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tomaschitz A, Pilz S, Ritz E, et al. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin Chim Acta. 2010;411:1354–1360. doi: 10.1016/j.cca.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 83.Vaidya A, Sun B, Forman JP, et al. The Fok1 vitamin D receptor gene polymorphism is associated with plasma renin activity in Caucasians. Clin Endocrinol (Oxf) 2011;74:783–790. doi: 10.1111/j.1365-2265.2011.03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shoback DM, Williams GH, Moore TJ, et al. Defect in the sodium-modulated tissue responsiveness to angiotensin II in essential hypertension. J Clin Invest. 1983;72:2115–2124. doi: 10.1172/JCI111176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Redgrave J, Rabinowe S, Hollenberg NK, Williams GH. Correction of abnormal renal blood flow response to angiotensin II by converting enzyme inhibition in essential hypertensives. J Clin Invest. 1985;75:1285–1290. doi: 10.1172/JCI111828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee MA, Böhm M, Paul M, Ganten D. Tissue renin-angiotensin systems. Their role in cardiovascular disease. Circulation. 1993;87:IV7–IV13. [PubMed] [Google Scholar]
- 87.Nguyen Dinh Cat A, Touyz RM. A new look at the renin-angiotensin system--focusing on the vascular system. Peptides. 2011;32:2141–2150. doi: 10.1016/j.peptides.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 88.Brown JM, Secinaro K, Williams JS, Vaidya A. Evaluating hormonal mechanisms of vitamin D receptor agonist therapy in diabetic kidney disease: the VALIDATE-D study. BMC Endocr Disord. 2013;13:33. doi: 10.1186/1472-6823-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]