Abstract
The Th17 pathway has recently been shown to play a critical role in host defense, allergic responses and autoimmune inflammation. Th17 cells predominantly produce IL-17 and IL-22, which are two cytokines with broad effects in the lung and other tissues. This review summarizes not only what is currently known about the molecular regulation of this pathway and Th17-related cytokine signaling, but also the roles of these cytokines in pathogen immunity and asthma. In the last 5 years, the Th17 field has rapidly grown and research has revealed that the Th17 pathway is essential in lung pathogenesis in response to exogenous stimuli. As work in the field continues, it is expected that many exciting therapeutic advances will be made for a broad range of diseases.
Keywords: allergy, cytokines, lung, pneumonia, T cells
CD4+ T helper (Th) lymphocytes are essential regulators of immune responses and inflammatory diseases by facilitating B-cell responses, modulating innate immunity and regulating CD8+ T-cell expansion [1-4]. When naïve CD4+ Th cells encounter antigen, their response varies based on the site of interaction, antigen type and inflammatory milieu present. Further, this antigen-induced response influences Th cell differentiation into effector cells with specific functional characteristics. Th cells are subdivided into lineages on the basis of the cytokines that they secrete, the specific transcription factors they express and the immunological function that they meditate.
In the mid-1980s, Th cells were first characterized and divided into two distinct lineages: Th1 and Th2 [5-9]. Th1 cells are driven by IFN-γ and IL-12 to express the transcription factor T-box 21 (tbx21: T-bet) through STAT1 and STAT4 signaling [10-12]. These cells secrete IFN-γ and IL-12 and are known to protect the host against intracellular pathogens [13]. In contrast, Th2 cells, whose differentiation is driven by IL-4-induced STAT6 activation, express GATA-binding protein 3 (GATA-3) and secrete IL-4, IL-5 and IL-13 to mediate allergy, asthma and host defense against parasitic infections [14]. Th1 and Th2 cells are opposing cell types as the expression of IL-12, IFN-γ and T-bet, inhibits Th2 differentiation; and IL-4 and GATA3 expression antagonizes Th1 polarization [15-19]. More recently, Th17 cells producing IL-17A, IL-17F and IL-22 emerged as a distinct population of CD4+ T cells [20-23]. These cells are characterized by high expression of the transcription factors retinoic acid (RA)-related orphan receptor γ thymus (RORγT) and RA-related orphan receptor α (RORα), that are critical for Th17 differentiation. Th17 cells are not only critical for host defense against extracellular pathogens, but have been implicated in the pathogenesis of a number of inflammatory diseases, including autoimmune disorders and allergic asthma [24-28].
Regulation of Th17 differentiation
Antigen presenting cells drive CD4+ T cell differentiation through cytokines and costimulation [29]. Specifically, IL-23 is comprised of the IL-23-specific p19 subunit and the IL-12 p40 subunit, and is mainly produced by myeloid cells, such as dendritic cells and macrophages [25,30]. Although IL-23 was initially thought to induce Th17 polarization, naïve Th cells do not express IL-23 receptor (IL-23R) and therefore IL-23 is not required for early Th17 differentiation [31,32]. During Th17 development, IL-6 and TGF-β1 activates IL-23R expression; and IL-23 induces STAT3 and Th17 proliferation and production of cytokines, such as IL-17 and IL-22 [27,28]. In addition to IL-17 and IL-22, Th17 cells produce IL-21 which promotes Th17 cell lineage differentiation and potentially acts as an auto feedback mechanism [33]. IL-23 is also capable of inducing production of IL-17 from Th17 cells which are independent of T-cell receptor (TCR) ligation [34]. Overall, IL-23 is not required for the initiation of Th17 polarization, but instead is thought to stabilize and expand these cells, perhaps directing IL-17 and IL-22 production.
IL-6 and TGF-β are cytokines that participate in the induction and expansion of Th17 cells. These cytokines are known to be critical regulators of Th17 differentiation via signaling through the STAT3 and similar to mothers against decapentaplegic (SMAD) pathways, respectively [35]. Activation of STAT3 also increases expression of hypoxia inducible factor (HIF) 1α, which inhibits FoxP3 expression and promotes Th17 polarization [36]. TGF-β signaling promotes Th17 differentiation by activating RORγT and RORα and limiting T-bet expression. At low concentrations, TGF-β1 synergizes with IL-6 to promote Th17 differentiation [37,38]. However at high levels, TGF-β can induce FoxP3 expression and inhibit T-bet to promote Treg development [37]. Foxp3+ Treg cells are essential for maintaining immune homeostasis and play a critical role in limiting excessive immune and inflammatory responses. Although there are many types of Treg cells, naturally occurring Treg and inducible Treg cells are the best characterized. Specifically, the balance between Th17 and Tregs is known to be crucial for immune homeostasis and several mechanisms have been found to control the balance between Treg and Th17 cells. As mentioned, TGF-β is required for both Th17 and Treg differentiation and it can induce both Foxp3 and RORγT expression in these cells [39]. Further, TGF-β in conjunction with RA and STAT5 activation via IL-2 can increase FoxP3 expression to promote Treg formation. Both FoxP3 and RORγT form complexes with Runx1 transcription factor and therefore regulate each other [40]. Specifically, FoxP3 is able to associate with RORγT and inhibit its transcriptional activation [39]. However, in the presence of IL-6, FoxP3-mediated inhibition is abrogated and Th17 differentiation is initiated. The reciprocal relationship between these cells, both an excess in Th17 function or increased number of Th17 cells or a defect in Treg function or reduced numbers of Treg, may promote inflammation and disease pathogenesis. Both TGF-β and IL-6 are capable of controlling the balance between Th17 and Treg differentiation; however, the regulation of Th17 and Treg polarization is multifactorial and is still emerging.
Many other cytokines and factors are known to influence TH17 differentiation. IL-1β promotes TH17 polarization through p38 MAPK and serine-threonine protein kinase AKT/mTOR pathway. IL-1β also induces interferon regulated factor (IRF)-4, which regulates the autocrine function of the IL-21 [41-43]. Aryl hydrocarbon receptors are cytosolic receptors with transcriptional activator activity that are known to promote Th17 polarization when stimulated with exogenous ligand [44]. High levels of aryl hydrocarbon receptors induce early Th17 expression and animals deficient in aryl hydrocarbon receptors are partly protected from experimental autoimmune encephalomyelitis, a Th17-cell mediated disease [45].
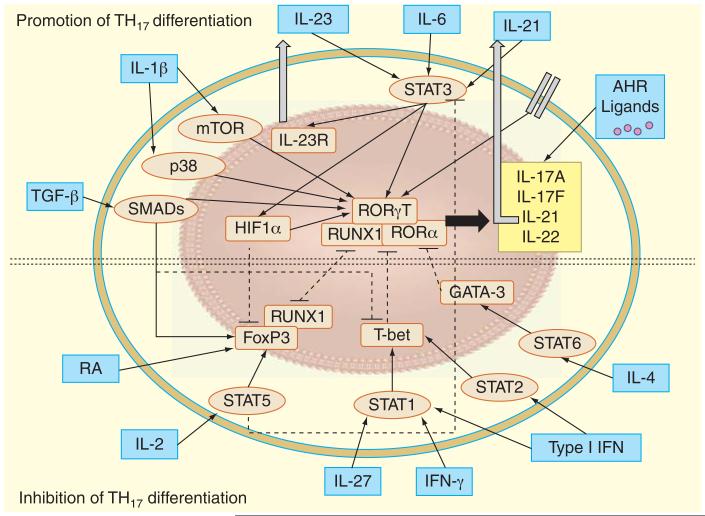
Several cytokines oppose Th17 polarization by inhibiting RORγT and RORα expression. For instance, Type 1 interferon, Type 2 interferon and IL-27, negatively regulate Th17 cell polarization and cytokine production and promote T-bet expression [46,47]. Similarly, IL-4 activates STAT6 to promote the transcription factor GATA3 expression that inhibits Th17 polarization. Further, IL-2 signaling via STAT5 attenuates Th17 differentiation and STAT5 is known to directly limit IL-17A gene expression [48,49]. Overall, the regulation of Th17 cell differentiation and proliferation is complex and requires multiple factors (Figure 1).
Figure 1. Regulation of Th17 differentiation.
Th17 cells are induced upon TCR ligation with presented antigen from dendritic cells in the presence of the cytokines, IL-6, IL-23 and IL-21, which activate signal STAT3. The transcription factors RORγT and RORα activate expression of gene encoding Th17-related cytokine, IL-17A, IL-17F, IL-21 and IL-22. Once secreted, IL-21 acts in an autocrine fashion to further promote Th17 differentiation and proliferation. Further, STAT3 activation via IL-6 increases the expression of IL-23R, thus causing the Th cell to be more sensitive to the polarizing effects of IL-23. Activation of STAT3 also increases expression of HIF1α, which inhibits FoxP3 expression and promotes Th17 polarization. IL-1β promotes Th17 differentiation by activating the AKT/ mTOR and p38 MAPK. TGF-β signals through SMADs to promote Th17 differentiation by activating RORγT and RORα and limiting T-box transcription factor TBX21 (T-bet) expression. Further, TGF-β in conjunction with RA and STAT5 activation via IL-2 can increase FoxP3 expression to promote Treg formation. When stimulated with exogenous ligand, aryl hydrocarbon receptors in the cytosol cause Th17 cells to produce IL-17 and also may negatively regulate STAT1 and STAT5 to enhance Th17 differentiation (not shown). Several cytokines that promote Th1 and Th2 cell polarization oppose Th17 polarization by inhibiting RORγT and RORα expression. For instance, Type 1 interferon signals through STAT1 and 2 signaling and IFN-γ and IL-27 via STAT1 to promote T-bet expression, which inhibits Th17 differentiation. Similarly, IL-4 activates STAT6 to promote the transcription factor GATA-3 expression that inhibits Th17 polarization. Both FoxP3 and RORγT form complexes with Runx1 and therefore regulate each other. RA is known to limit Th17 polarization. Arrowed lines (solid) indicate activation or production, while square lines (dotted) illustrate inhibition. IL-23R: IL-23 receptor; RA: Retinoic acid; RORα: RA-related orphan receptor α; RORγT: RA-related orphan receptor γ thymus; TCR: T-cell receptor; Th: T helper.
Emerging influences on Th differentiation
Despite T cell fate determination, recent discoveries have emphasized that T cells are phenotypically unstable. For instance, it is apparent from both in vitro and in vivo studies that some T cells can produce both IFN-γ and IL-17 [50]. In addition, Th17 cells can produce IL-21 and/or IL-22 (proposed Th22 cells) in the absence of IL-17 [20,32,51]. It is also recognized that Th17 cells as well as other T cell subsets produce the immunosuppressive cytokine IL-10; thus, not all Th17 cells may be pathogenic [52]. The plasticity of T cell differentiation is thought to be cytokine-regulated and also is suggested to play a role in modulating inflammatory diseases [21]. This phenomenon is also thought to occur in humans. Human circulating memory Th cells co-expressing IL-17/IL-4 have been identified in asthmatics [53]. Further, circulating Th cells from healthy controls and asthmatics were found to express multiple transcription factors [54]. Together, these data support the concept that individual Th cells may have a flexible phenotype and multiple functions in vivo.
Aside from cytokine and immune cells influences, the host environment can also affect Th cell differentiation. The microbiome has recently been shown to influence various aspects of host innate and adaptive immunity, such as the development and differentiation of Th17 cells [55-57]. Intestinal lymphocytes are thought to be regulated by commensal microbiota [55]. Indeed, Th17 cells are present in the lamina propria of the small intestines and the number of Th17 cells is reduced in germ free or antibiotic-treated mice [58-60]. Atarashi and colleagues further elucidated this relationship as they found that extracellular adenosine 5′-triphosphate from microbiota activates dendritic cells in the lamina propria thereby inducing Th17 cells [60]. Mice housed in different facilities had different compositions of intestinal bacteria and were also found to have marked difference in the number of Th17 cells in their gastrointestinal tract [59]. Further, segmented filamentous bacteria (SFB) were identified as the first microbiota known to induce Th17 cell accumulation in the small intestines [61]. This induction of Th17 cells via mono-colonization of SFB in the gastrointestinal tract protected against the pathogenic bacterium, Citrobacter rodentium [61]. Although much is known about the influence of the microbiome on immunity in the gastrointestinal tract, the relationship between commensal microbiota and the host immune system in the lung is still poorly understood.
In addition to the microbiota, recent studies have shown that other environmental factors may affect Th17 responses, such as a high salt diet. Recent findings by Wu et al. and Kleinewietfeld et al. elucidate novel mechanisms by which sodium chloride (NaCl) induces pathogenic Th17 development and exacerbates Th17-mediated inflammatory and autoimmune disease [62,63]. Using transcriptional profiling, Wu and colleagues identified serum glucocorticoid kinase 1 (SGK1), a serine/threonine kinase known to influence cellular Na+ transportation and NaCl homeostasis, as a downstream regulator of IL-23 signaling [62]. Further, they found that IL-23 signaling is critical to maintain SGK1 expression during Th17 cell differentiation and to stabilize Th17 cell phenotype by deactivating mouse Forkhead box protein O1 (FoxO1), a direct repressor of IL-23R expression [62]. Kleinewietfeld and colleagues also observed that high salt conditions activate SGK1, nuclear factor of activated T cells 5 and p38/MAPK during Th17 polarization [63]. Further, silencing these factors inhibited the high salt-induced Th17 cell development [63]. More significantly, both studies demonstrate via a mouse model of multiple sclerosis that a high-salt diet accelerates neuropathology in experimental autoimmune encephalomyelitis [62,63]. Other molecules related to sodium homeostasis, such as aldosterone and the mediators of the renin-angiotensin pathway are also known to influence Th17-mediated responses [64,65]. Further investigations are ultimately required to understand the role of these factors as well as other dietary and environmental factors in Th differentiation and the regulation of other immune cells and pathways influential in disease pathogenesis, completely.
Th17 cytokines
The canonical Th17 cytokine, IL-17, is a proinflammatory cytokine that was originally identified as cytotoxic T lymphocyte antigen (CTLA)-8 [66]. IL-17 is now recognized as a family of cytokines including IL-17A, IL-17B, IL-17C, IL-17D, IL-17E (IL-25) and IL-17F [67]. To date, IL-17A and IL-17F have been the most extensively studied and are localized to the same chromosome in both humans and mice (chromosomes 6 and 1, respectively) [68,69]. There is also a 62% sequence homology in IL-17A between mice and humans [68,69]. IL-17A is secreted as a disulfide-linked homodimeric glycoprotein [68]. IL-17A promotes inflammation through induction of cytokines and chemokines: CXCL1 (KC), CXCL2 (MIP-2), CXCL5 (LIX), CXCL8 (IL-8), CXCL9 (MIG), CXCL10 (IP-10), G-CSF and GMCSF [68,70,71]. On the other hand, IL-17F has weaker biological activity than IL-17A [72,73]. Although IL-17F is known to be secreted at higher concentrations than IL-17A, it weakly associates the IL-17 receptor (IL-17R) [74]. IL-17F, like IL-17A, also forms a homodimer, but can also form a heterodimer with IL-17A. Functionally, both IL-17A and IL-17F are known to recruit, activate and regulate the migration of neutrophils [67]. Distinct actions of IL-17F have been described, but the precise function of this cytokine is yet to be determined [35]. In addition to IL-17, TH17 cells also produce IL-22, a member of the IL-10 cytokine family, which is thought to play an influential role in inflammation and tissue homeostasis [75]. Although IL-10 and IL-22 have limited homology, IL-22 is structurally similar as it is composed of bundles of α-helices [76] and is located on chromosome 12q15 in humans [77]. The biological role of IL-22 is the subject of many current studies.
Cellular sources of TH17 cytokines
IL-17 and IL-22 were originally thought to be produced exclusively by αβ T lymphocytes, however many other immune cells are now known to secrete these cytokines. Other cellular sources of IL-17 include γδ T cells, cytotoxic T cells, invariant NK T cells, NK cells, innate lymphoid cells (ILCs) and lymphoid tissue inducer cells [78,79]. Recently, much work has focused on the identification and characterization of IL-17-producing γδ T cells and ILCs, which will be further discussed.
γδ T cells account for a small percentage of lymphocytes (<5% of total lymphocytes) and are most prevalent in mucosal and epithelial sites, such as in the gut and lung. Although these cells are not abundant, γδ T cells are known to be a more potent source of IL-17 than αβ T cells following Myobacterium tuberculosis infection [80]. Although γδ T cells are primarily activated through their TCR, activation of γδ T cells for the induction of IL-17 is known to involve various cytokines, chemokines and pattern recognition receptors. Indeed, IL-1, IL-6, IL-18, IL-23 and TGF-β1 expression as well as activation of Toll-like receptor 2, DC-associated C-type lectin 1 and aryl hydrocarbon receptor, have all been implicated with IL-17 production by γδ T cells [81]. Specifically, Lalor and colleagues found that IL-18 synergizes with IL-23 to promote IL-17 production by γδT cells [82]. Further, these authors also demonstrate that the processing of IL-1β and IL-18 via inflammasome-triggered pathways is important for the generation of IL-17-secreting γδ T cells [82]. Similar to αβ T cells, RORγT and STAT3 expression have also been associated with activated IL-17 producing γδ T cells [58]. However, γδ T cells are known to have the high constitute expression when compared to the other T lymphocytes [83].
ILCs are a heterogeneous population of innate effector cells that originate from hematopoietic progenitors in the bone marrow. ILCs lack expression of specific antigen receptors but are further divided into three functional groups based on the transcription factors they express and the cytokines they secrete [84]. Although some types of ILCs are known to have a protective role in inflammatory disease and infection [85], recent studies suggest that some Group 3 ILCs (ILC3), which produce the cytokines IL-17A or IL-22, promote infection, inflammation and autoimmune disease [86]. One of the main challenges in understanding the role of ILCs in disease pathogenesis is that different nomenclature used to describe these populations. However, these main subsets of ILCs and their expression profiles, functional characteristics and disease roles have been recently extensively reviewed elsewhere [84,87]. Other than Th17 cells [88], many cell types can produce IL-22, including but not limited to activated Th1 and CD8+ T cells [76,89,90], NK cells [90-92], CD11c+ myeloid cells [76,93], IL-22-producing CD4+ T cells (Th22) [94], lymphoid tissue inducer-like cells [91] and IL-22 producing ILC3s [95]. Overall, the discovery of these novel sources of IL-17 and IL-22 have led to the re-examination of Th17-related diseases, where these cytokines, such as infection, autoimmune disease, allergic airway disease and other inflammatory conditions.
Th17 cytokine signaling
IL-17 signaling pathways are implicated in the pathogenesis of a number of autoimmune and inflammatory diseases. IL-17 induces inflammation through binding to the IL-17 receptor (IL-17R) family, which includes IL-17RA, IL-17RB, IL-17RC, IL-17RD and IL-17RE [96]. IL-17R is widely expressed by T and B lymphocytes, neutrophils, epithelial, endothelial, fibroblast, monocytes and mesenchymal stromal cells as well as keratinocytes [97]. Although IL-17R is ubiquitously expressed, the majority of its effects in the lung are thought to be on epithelial, endothelial and fibroblast cells [78].
IL-17RA and IL-17RC bind both IL-17A and IL-17F [97]. In fact, IL-17RA binds to IL-17A with a higher affinity than IL-17F in humans, while IL-17F has a higher affinity for IL-17RC than IL-17A [98]. In mice, IL-17RA can bind both IL-17A and IL-17F but murine IL-17RC may only bind IL-17F [98]. IL-17RA complexes with IL-17RB to form the IL-25 receptor and thus IL-17 and IL-25 share the IL-17RA subunit. IL-17RB is also known to be a receptor for IL-17B [97]. The orphan receptor IL-17RD can also differentially regulate IL-17A-dependent pathways [99]. Lastly, recent studies reveal IL-17RE can form a receptor complex with IL-17RA to bind IL-17C and may have a role in intestinal immunity [72,100]. Additional studies are required to clarify the roles of IL-17RD and IL-17RE along with the functions of IL-17B, IL-17C and IL-17D in the lung.
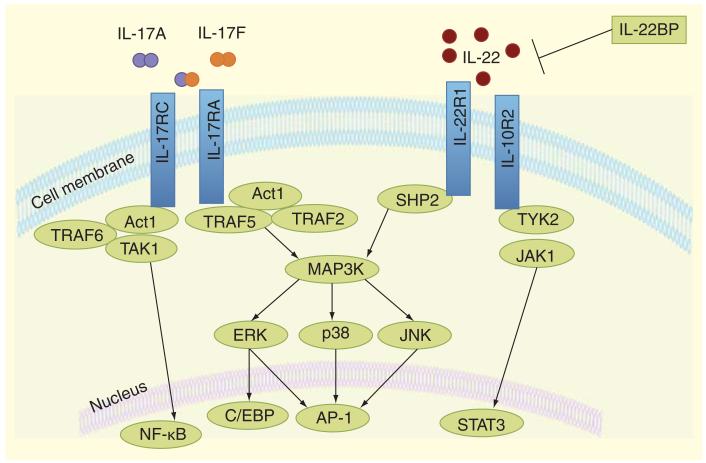
Members of the IL-17R family possess an intracellular SEF/IL-17R (SEFIR) domain that is important for triggering down-stream signaling [101]. IL-17 signaling results in regulation of gene expression by activating the transcription factor NF-κB and MAPK pathways (Figure 2) [102]. Stimulation of IL-17RA leads to an association with the adaptor protein Act1, via SEFIR-SEFIR interactions, followed by recruitment and polyubiquitination of the scaffold protein TRAF6, thus initiating downstream activation of NF-κB and MAPKs [103,104]. Specifically, IL-17A and IL-17F have also been shown to activate MAPK, extracellular signal-regulated kinase (ERK), p38 and c-Jun N-terminal kinase (JNK) [105]. Recently, JNK signaling was shown to be required for IL-17 driven inflammatory cytokine and antimicrobial peptide production in epithelial cells and the lung [106]. IL-17 may also impact mRNA stability pathways downstream of Act1 that are independent of TRAF6 [107]. In addition, IL-17RD deficiency results in enhanced IL-17A-induced activation of NF-κB and IL-6 and keratinocyte chemoattractant (KC) expression [99]. IL-17RD disrupts the interaction of Act1 and TRAF6 causing differential regulation of NF-κB and p38 mitogen-activated protein kinase signaling pathways [99]. Deletion of the IL-17R SEFIR domain or Act1 results in impaired IL-17 signaling and activation of NF-κB [108]. Many IL-17 target genes contain AP-1 DNA binding motifs that may be sites for direct regulation of transcription [109]. IL-17 also activates the CCAAT/enhancer binding protein (C/EBP) transcription factors. The IL-17 target genes IL-6 and lipocalin-2 both require C-EBP activation for transcription [110].
Figure 2. IL-17 and IL-22 signaling pathways.
IL-17 and IL-22 bind to transmembrane heterodimeric cell surface receptors to induce cellular signaling pathways. Specifically, IL-17A or IL-17F binds to the IL-17RA and IL-17RC, respectively as homodimers or heterodimers. The binding of IL-17 to its receptor leads to the recruitment of adaptor protein and E3 ligase, Act1. Scaffold proteins TRAF6 and TAK1 interact with Act1 to activate NF-κB expression, which leads to subsequent induction of proinflammatory genes. IL-17 signaling through its receptor also induces ERK, p38, and JNK activation via Act1 binding with TRAF2 and TRAF5. Further, this MAP kinase activation can induce downstream signaling to activate AP-1 and CCAAT/enhancer binding protein (C/EBP). Receptor signaling of IL-22 through its heterodimer receptor of IL-10R2 and IL-22R1 induces phosphorylation of tyrosine kinases Jak1 and Tyk2, which activate the transcription factor STATs. In some cases, MAP kinases (ERK, p38, and JNK) are also activated through a distinct pathway following IL-22R activation. IL-22 binding protein (IL-22BP) acts as a soluble antagonist.
To activate target cells, IL-22 signals through a heterodimeric receptor complex comprised of IL-22R1 and IL-10R2. Although IL-10R2 is present on the many immune cells, IL-22R1 is expressed predominantly on endothelial and epithelial cells [111]. Savan et al. demonstrated that the restriction of IL-22R1 to non-hematopoietic cells is biologically significant as transgenic mice that artificially expressed IL-22R1 on lymphocytes developed normally, but acquired lethal multi-organ inflammation 2-3 months after birth [112]. Similar to IL-10, IL-22 signaling activates STAT3 [90,113], that induces STAT3 signaling and the suppressor of the cytokine signaling (SOCS)-3 [114-116]. Upon binding of IL-22 to its receptor tyrosine kinases Jak1 and Tyk2 are phosphorylated, which in turn activates STAT1, STAT3 and STAT5 [111,117]. Similar to IL-17, IL-22 also activates the JAK/STAT, ERK, JNK and p38 MAPK pathways [117]. An endogenous antagonist of IL-22, IL-22 binding protein (IL-22BP), regulates the bioavailability of IL-22 thereby inhibiting IL-22-driven STAT3 activation [118]. The immune role of IL-22BP in the lung is currently unclear.
Role of IL-17 in asthma
Asthma is a common respiratory disease affecting approximately 300 million people worldwide with no broadly effective preventions or cures. Atopic asthma is driven by the development and recruitment of CD4+ T lymphocytes to the lungs and is characterized by pulmonary inflammation, mucus hypersecretion and airway hyperresponsiveness (AHR). Asthma is traditionally viewed as an eosinophilic airway disorder and a great deal of research has focused on the role of Th 2 cells and cytokines (IL-4, IL-5 and IL-13) in promoting disease pathogenesis. However, Th17 cells, which mediate neutrophil recruitment, are known to play an influential role in asthma pathogenesis, especially in asthmatics who have severe disease and fail to respond to glucocorticoid therapy. Studies have also indicated IL-23-induced Th17 cell effector function, which was impaired in gene variant carriers of the protective allele IL-23R R381Q resulting in significantly reduced IL-17A production and STAT3 phosphorylation, which supports a critical role for the IL-23/IL-23R signaling in generating pathogenic Th17 responses [119].
IL-17 is known to be strongly upregulated in asthma [120] as serum and airway IL-17 mRNA and protein levels are found to be elevated in asthmatics [121-124]. The increased level of IL-17A and IL-17F in the lung directly correlates with disease severity (i.e., increased AHR to methacholine) [125,126]. In addition, Zhao et al. showed that the percentage of Th17 cells and the IL-17 and IL-22 levels correlated with increased disease severity [127]. The antigenic drivers of the IL-17 response in asthma are currently unclear. A recent study suggests that IL-17 levels are higher in asthmatics with elevated IgE compared to asthmatics with low IgE [128]. Another study showed that IL-17 production by T cells in response to Dermatophagoides farinae extract was significantly induced in atopic asthmatics when compared to nonatopic asthmatics and normal controls [120]. Together, these results suggest that IL-17 may play a larger role in atopic asthma. The association of an IL-17F polymorphism (His161Arg) with asthma suggests that IL-17F plays an important role in asthma pathogenesis [129]. Further, IL-17F was also reported in cells from the bronchoalveolar lavage following antigen stimulation in patients with asthma [130].
IL-17 plays a critical role in driving neutrophil influx into the airways [122]. Specifically, IL-17A drives the production of neutrophil growth factors and chemokines, such as IL-6, granulocyte-colony stimulating factor (G-CSF), KC and macrophage inflammatory protein (MIP)-2/IL-8 that are known to be elevated in some asthmatics [131]. Overexpression of IL-17F in the lung also results in increased neutrophilic inflammation in the airspaces [132]. Further, IL-17A and IL-17F induce human fibroblasts to produce IL-6 and IL-8, which serve to activate and promote the expansion of these cells [74]. Several studies have shown that the extent of airway neutrophilia correlates to asthma severity [133-135]. Indeed, asthmatics with neutrophilic inflammation exhibit decreased improvement in forced expiratory volume in one second (FEV1) and airway responsiveness following glucocorticoid treatment [136]. Furthermore, neutrophils are known to be largely steroid-insensitive and glucocorticoids are known to inhibit neutrophil apoptosis [137,138], which may account for the increase in neutrophils observed in the lungs.
Animal models of the allergic airway disease have also established a causative link between Th17 cells and glucocorticoid-insensitive allergic airway disease in mice [139]. The model described by McKinley et al., is characterized by elevated neutrophil chemokines, growth factors and neutrophilic inflammation in the lung. Transfer of ovalbumin (OVA)-specific Th17 cells to donor mice resulted in neutrophilic inflammation, AHR and mucus metaplasia following OVA challenge, which could not be attenuated by glucocorticoid treatment [139]. This study showed that Th17 cells are sufficient to promote many of the hallmark characteristics of asthma in vivo and that this response was steroid insensitive, unlike many Th2-dependent models utilized in asthma research.
IL-17 has been shown to play a regulatory role in allergic airway disease in animal models, although many findings are somewhat contradictory. IL-17 was shown to synergize with IL-4 and IL-13 to enhance Th2 cytokines and CCL11 in a murine OVA model [140,141]. However, neutralization of IL-17A prior to OVA challenge increased airway eosinophilia and Th2 cytokines levels in the lungs, while decreasing neutrophilia [142,143]. IL-17R−/− mice displayed decreased eosinophilia in the airways, IgE, and Th2 cytokines following OVA [144]. Consistent with this, studies have shown that IL-17 neutralization or downregulation inhibit TH2-driven disease [145,146]. Although, anti-IL-17 monoclonal antibodies (mAb), given repeatedly after OVA challenge in OVA-sensitized mice, reduced bronchial neutrophilic influx but aggravated allergen-induced bronchial eosinophilia [143]. Exogenous IL-17A treatment also decreased eosinophil recruitment and bronchial hyperreactivity in mice [144]. Overall, these studies suggest that IL-17 may both promote and inhibit Th2 driven airway inflammation and AHR depending on the context and conditions of the study. Additional work is necessary to define the role of IL-17 in asthma and will require distinct modeling of asthma phenotypes.
Besides its role in inflammation, IL-17 is implicated in airway remodeling, which is more prominent in chronic steroid-resistant disease [125,143]. Indeed, IL-17 induces gene expression of mucins (Muc5ac and Muc5b) in human bronchial and murine epithelial cells [147]. Oda et al. found that overexpression of IL-17F increased goblet cell hyperplasia and mucin expression in mice [132]. Further, IL-17A was reported to increase IL-6 and IL-11 production in human bronchial fibroblasts and thus may promote remodeling [148]. IL-17 can directly induce steroid insensitivity in bronchial epithelial cells [149]. In murine models of the allergic airway disease, IL-17 has been shown to control bronchial hyperresponsiveness and airway remodeling. Further, IL-17A has been shown to have direct effects on bronchial smooth muscle cell contractility [150]. Together, these findings show that IL-17 may contribute to asthma pathogenesis by inducing airway remodeling in addition to inflammatory effects.
IL-23 is also thought to have an important role in severe asthma as it is a key molecule for Th17 cell propagation. In a murine asthma model, IL-23 levels were elevated in lung homogenates and IL-23p19 mRNA was induced upon antigen inhalation in the lung of antigen-sensitized mice [151]. In this study, IL-23 also enhanced antigen-induced activation of both Th17 and Th2 cells and administration of anti-IL-23p19 antibodies lessened airway inflammation and Th2 cytokine production [151]. Specific roles for IL-23 in asthma are currently emerging.
IL-22 in asthma
IL-22 is thought to play a dual role in the allergic airway disease, as recent studies have shown that this Th17-related cytokine exhibits both pro-inflammatory and anti-inflammatory properties. The pro-inflammatory properties of IL-22 are demonstrated as IL-22 is detected at the sites of allergic airway inflammation [152]. In fact, it was reported that serum levels of IL-22 are higher in patients with severe asthma than those seen in patients with mild asthma and healthy control subjects [127]. In addition, blocking IL-22 during Th2 sensitization significantly decreased eosinophilic inflammation, Th2 cytokine production, AHR and mucus hyperplasia in a mouse model of asthma. On the other hand, studies showed that IL-22 neutralization during allergen challenge increased airway inflammation and Th2 cytokine production and that treatment with recombinant IL-22 during allergen challenge attenuated it [153,154]. These somewhat contradictory data illustrate the critical importance of study design when modeling human asthma. IL-22 is also thought to promote epithelial repairs through the production of antimicrobial peptides (AMP) and by suppressing production of pro-inflammatory cytokines and chemokines. These findings suggest that the functions of IL-22 are influenced by the inflammatory milieu present in the lung. Indeed, IL-17A influences the proinflammatory and pathological versus protective role of IL-22 in the lung [155]. Sonnenberg and colleagues found that in the absence of IL-17A, IL-22 serves a tissue-protective role, while when IL-17A is present IL-22 contributes to the disease pathogenesis in a model of airway inflammation [155].
Role of IL-17 in host defense
In addition to its role in asthma, IL-17 plays a key role in pulmonary host defense by inducing chemokine production for neutrophil emigration into infected tissues and regulating the production of AMPs (Table 1). It protects against pathogenic microorganisms at the mucosa, although may contribute to tissue injury in chronic biofilm infections that occur in patients with cystic fibrosis and bronchiectasis [156].
Table 1. The role of IL- 17 and IL- 22 in host defense.
| Pathogen | Overall | Experimental approaches | Ref. |
|---|---|---|---|
| Gram-negative bacteria | |||
|
| |||
|
Klebsiella
pneumoniae |
Protective | IL17RA−/− mice have increased mortality and bacterial dissemination | [158] |
| IL17RA−/− mice have reduced production of G-CSF and MIP- 2 and delayed neutrophil recruitment | [158] | ||
| Exogenous IL- 17 improves clearance | [157] | ||
| IL- 23p19−/− mice have increased mortality | [159] | ||
| IL- 17 induces heterologous antibody immunity | [160] | ||
| WT mice in which IL- 22 is neutralized have increased morbidity | [175] | ||
|
| |||
|
Francisella
tularensis |
Protective | WT mice have increased IL- 17 producing cells in the lung and bronchoalveolar lavage fluid | [161] |
| IL17RA−/− and IL- 23a−/− mice have increased bacterial burden | [162] | ||
|
| |||
|
Bordatella
pertussis |
Protective Vaccine response |
B. pertussis drives Th17 polarization in human epithelial kidney cells | [164] |
| WT mice in which IL- 17 is neutralized have higher bacterial burden | [165] | ||
| Baboons with B. pertussis infection have higher levels of Th17 axis cytokines | [166] | ||
| Baboons following B. pertussis infection have circulating B. pertussis-specific Th17 cells 2 years post infection | [166] | ||
|
| |||
|
Pseudomonas
aeruginosa |
Protective | WT mice have increased IL- 17 levels in response to acute P. aeruginosa infection | [167] |
| WT mice in which IL- 17 is neutralized have higher bacterial burden | [168] | ||
| IL- 23p19−/− mice similar bacterial burden, reduced airway inflammation | [156] | ||
|
| |||
| Gram-positive bacteria | |||
|
| |||
|
Staphylococcal
aureus |
Protective | IL17R−/− mice have decreased bacterial clearance and decreased G-CSF | [170] |
| γδTCR−/− mice have decreased IL- 17 associated chemokine production and decreased neutrophil recruitment | [171] | ||
| IL- 22 mice have decreased bacterial clearance | [170] | ||
|
| |||
|
Streptococcus
pneumoniae |
Protective | WT mice in which IL- 17 is neutralized have decreased bacterial clearance and macrophage recruitment | [172] |
| WT mice have increased levels of IL- 17 during infection and γδTCR−/− mice have decreased levels of IL- 17 during infection |
[173] | ||
|
| |||
|
Listeria
monocytogenes |
? | IL- 17R−/− mice have normal host defense | [175] |
|
| |||
| Chlamydia muridarum | Protective | WT mice in which IL- 17 is neutralized have increased bacterial growth, dissemination and mortality | [174] |
|
| |||
| Acid-fast bacteria | |||
|
| |||
|
Mycobacterium
tuberculosis |
Vaccine response | IL17R−/− mice have no change in bacterial growth | [175] |
| IL- 23p19−/− mice have no change in bacterial growth although there are decreased CD4+ IL- 17-producing cells | [176] | ||
| Vaccinated WT mice in which IL- 17 is neutralized produce less antigen-specific IFN- γ-producing T cells | [178] | ||
| IL- 22 is produced in humans exposed to M. tuberculosis | [195] | ||
|
| |||
|
Mycobacterium
bovis |
? | IL- 17−/− mice have no change in bacterial growth but decreased granuloma formation | [177] |
|
| |||
| Viruses | |||
| Influenza | ? | WT mice in which IL- 17 is neutralized have increased weight loss and increased mortality (H1N1) | [180] |
| IL- 17−/− mice have increased weight loss and increased mortality (H5N1) | [181] | ||
| IL- 17R−/− mice have decreased weight loss and increased survival (H1N1) | [179,198] | ||
| WT mice in which IL- 22 is neutralized have no change in morbidity, mortality (H1N1 and H3N2) | [200] | ||
| IL- 22 from iNKT cells prevents lung epithelial injury (H3N2) | [201] | ||
| IL- 22 from conventional NK cells promotes epithelial repair (H1N1) | [202] | ||
| IL- 22−/− mice have increased lung epithelial injury and decreased lung function (H1N1) | |||
|
| |||
| Rhinovirus | ? | IL- 17 syngergizes with HRV in epithelial cells to induce IL- 8 | [182] |
|
| |||
| Viral/bacterial co-infection |
Protective | WT mice co-infected with influenza AIS. aureus have increased bacterial burden, but decreased levels of IL- 17 and associated chemokines |
[170] |
| WT mice co-infected with influenza AIS. pneumoniae have increased mortality and bacterial burden, but decreased levels of IL- 17 |
[183] | ||
| IL- 22−/− mice co-infected with influenza AIS. pneumoniae have increased bacterial burden following sublethal influenza infection |
[203] | ||
|
| |||
| Fungi | |||
|
| |||
|
Pneumocystis
carinii |
Protective | WT mice in which IL- 17 is neutralized have increased fungal burden | [185] |
|
| |||
|
Aspergillus
fumigatus |
? | WT mice in which IL- 17 is neutralized have increased fungal burden | [184] |
| IL- 17−/− mice have improved fungal clearance | [192] | ||
| IL- 17 promotes lung pathology and impedes clearance | [186] | ||
| WT mice in which IL- 22 is neutralized have improved lung function | [196] | ||
| WT mice in which IL- 22 is neutralized have increased fungal burden | [197] | ||
| IL- 22−/− mice have increased fungal burden | [197] | ||
|
| |||
|
Blastomyces
dermatitidis |
Vaccine
response |
Vaccinated WT mice in which IL- 17 is neutralized have increased fungal burden | [187] |
| Vaccinated IL- 17RA−/− mice have increased fungal burden | [187] | ||
|
| |||
|
Coccidioides
posadasii |
Vaccine response |
Vaccinated IL- 17RA−/− mice have increased fungal burden | [187] |
|
| |||
|
Histoplasma
capsu latum |
Vaccine response |
Vaccinated IL- 17RA−/− mice have increased fungal burden | [187] |
G-CSF: Granulocyte colony stimulating factor; MIP: Macrophage inflammatory protein; TCR: T cell receptor; WT: Wild-type.
IL-17 is protective in several models of gram-negative bacterial infection including extracellular Klebsiella pneumoniae [157,158]. Mice with homozygous deletion of IL-17RA have increased mortality and bacterial dissemination compared to wild-type controls following challenge with K. pneumoniae. They have reduced production of G-CSF and MIP-2 with a delay in neutrophil recruitment to the alveolar space [158]. Mice that receive exogenous IL-17A prior to K. pneumoniae have increased production of G-CSF and MIP-2 with improved survival and bacterial clearance [157]. Mice that produce decreased amounts of IL-17A and IL-17F due to a homozygous deletion in IL-23p19 have increased mortality from K. pneumoniae [159]. Recently, a role for IL-17 in promoting serotype-independent antibody immunity against K. pneumoniae has been demonstrated [160]. It has also been shown that mice infected with the intracellular pathogen, Francisella tularensis, have increased numbers of IL-17 producing cells in the lung and increased IL-17 in the bronchoalveolar lavage fluid [161]. IL-17 is critical to the clearance of F. tularensis and helps mediate the Th1 response against tularemia [162]. IL-17 is elevated in response to Bordatella pertussis infection and IL-17 helps mediate vaccine-induced cellular immunity protection against B. pertussis [163-166]. In acute Pseudomonas aeruginosa infection, IL-17 levels are increased and the late recruitment of neutrophils is dependent on IL-17 [167,168]. Vaccine induced protection against acute P. aeruginosa pneumonia is thought to be IL-17 dependent [169].
In addition to gram-negative bacteria, IL-17 is protective in several models of gram-positive infection. Multiple cells make IL-17 in response to Staphylococcus aureus pneumonia, including Th17 and γδ T cells [170]. IL-17R−/− challenged with S. aureus have decreased bacterial clearance, decreased G-CSF and decreased IL-6 production compared to wild-type controls [170]. Mice that have a homozygous deletion for the T cell receptor δ chain and lack γδT cell receptor expression have decreased production of KC, MIP-2, GM-CSF, IL-6 and TNF-α in response to S. aureus pneumonia. As expected, they have decreased neutrophil recruitment and increased bacterial burden of S. aureus in the lung [171]. Studies have also shown that IL-17 is required for the recruitment of neutrophils that facilitate the clearance of colonized S. pneumoniae from the mucosa of the nasopharynx [172]. In addition, IL-17 levels are increased in mice during acute S. pneumoniae pneumonia [173]. In the murine model of chlamydial pneumonia, IL-17 was critical for preventing bacterial clearance and dissemination as well as decreasing mortality [174]. Interestingly, the intracellular bacterium Mycobacterium tuberculosis and Mycobacterium bovis do not require IL-17 for clearance, although IL-17−/− mice have an altered inflammatory response to mycobacterial challenge [175-177]. Studies show that IL-17 plays a role in vaccine-induced immunity against M. tuberculosis by prompting the release of chemokines that recruit IFN-γ producing T cells [178].
IL-17 also plays a key role in host defense against viral and fungal pathogens. Crowe and colleagues found that IL-17R−/− mice had decreased lung inflammation, weight loss and mortality without attenuation of viral clearance from the lungs compared to wild-type mice after lethal influenza A infection [179]. These data show that IL-17 is required for acute lung injury during influenza A infection. However, others have shown that IL-17 deletion of neutralization resulted in worsened influenza-induced weight loss and mortality [180,181]. In humans, rhinovirus synergizes with IL-17 to enhance IL-8 production by epithelial cells, which may help to promote the recruitment of immune cells to the airway [182]. Although these data suggest that neutralization of IL-17 may be beneficial in viral pneumonia, bacterial pneumonia following viral infection is associated with increased mortality. Mice that are co-infected with influenza and S. aureus have decreased levels of IL-17, decreased chemokines G-SCF and KC, and increased bacterial burden compared to mice that receive S. aureus alone [170]. Similarly, mice infected with influenza followed by S. pneumoniae have decreased levels of IL-17, increase bacterial burden and increased mortality compared to mice that received S. pneumoniae alone [183]. In both studies, it is suggested that Type 1 IFN induction by influenza results in suppression of IL-17 and IL-22 production in the lung. These data suggest a mechanism by which influenza may promote subsequent bacterial infection. Regarding fungal pathogens, IL-17 neutralization in mice leads to impaired fungal clearance in both Pneumocystis carinii and Aspergillus fumigatus models [184,185]. Authors have also suggested that IL-17 interferes with antifungal immunity against A. fumigatus [186]. Interestingly, IL-17 has been found to play an integral part in vaccine induced immunity against multiple fungal pathogens that cause disease in North America including Blastomyces dermatitidis, Coccidioides posadasii and Histoplasma capsulatum [187].
Cystic fibrosis (CF) is a disorder caused by abnormalities in the CF transmembrane conductance regulator protein, which results in failure of transcellular chloride absorption and subsequent elevation of sodium and chloride in the airway surface liquid. Patients with CF have chronic airway infection and inflammation, which leads to a progressive decline in lung function. Common infectious pathogens in CF patients include P. aeruginosa and A. fumigatus. P. aeruginosa most often becomes a chronic biofilm infection in patients with CF and those colonized with P. aeruginosa have a more rapid decline in lung function compared to others. IL-17 has been linked to CF: patients colonized with P. aeruginosa have elevated levels of IL-17 and IL-23 in sputum during a pulmonary exacerbation [188]. Analysis of the airway submucosa has revealed that IL-17 producing cells are present [189]. In a recent study by Chan et al., explanted lungs from patients with CF undergoing transplantation were found to have antigen (P. aeruginosa and A. fumigatus)-responsive IL-17 and IL-22 positive cells [190]. Further, Tiringer and colleagues found that elevated IL-17 levels negatively correlated with FEV1 in patients with CF, proposing that IL-17 may contribute to a decline in the lung function. Elevated IL-17 levels were also predictive of future P. aeruginosa acquisition in this study [191]. In a murine model of mucoid P. aeruginosa, mice with a homozygous deletion in IL-23p19 produce decreased amounts of IL-17. They have similar bacterial burden but reduced airway inflammation compared to controls [156]. In a murine pulmonary aspergillosis model, IL-17−/− mice had improved fungal clearance and reduced airway inflammation compared to controls [192]. Although the specific role of IL-17 in CF remains unclear, the IL-23/Th17 axis has proved important. IL-17 may promote tissue injury and even make patients more susceptible to primary acquisition of chronic biofilm infections. Further, recent studies have shown that salt promotes the differentiation of CD4+ T lymphocytes to Th17 cells [62,63]. The role of environmental factors on this axis is still largely unexplored, but these findings suggest that high-salt concentration of the airway surface liquid of CF patients may contribute to disease by promoting tissue inflammation and Th17-mediated disease.
IL-22 & host defense
IL-22 is critical for maintaining the integrity of the epithelium in the lung upon exposure to injurious and infectious agents and is believed to be mainly protective and regenerative [75,193,194]. IL-22 also acts synergistically with IL-17 to induce the production of multiple AMPs, including but not limited to β-defensins, S100 proteins, regenerating islet-derived protein (Reg) 3 β and γ and lipocalin 2, suggesting a role in bacterial clearance [20,175]. Similar to IL-17, deletion of IL-22 results in impaired host defense against gram-negative bacteria. IL-22 levels are increased during infection with K. pneumonia and the neutralization of IL-22 results in increased morbidity and mortality [175]. In a model of gram-positive S. aureus pneumonia, the deletion of IL-22 results in decreased bacterial clearance [170]. IL-22 protein levels are increased in human bronchoalveolar lavage fluid from patients with M. tuberculosis disease and peripheral blood from healthy adults that have been exposed to M. tuberculosis have IL-22 producing CD4+ T cells [195]. Dectin-1-dependent interleukin-22 production contributes to early innate lung defense against A. fumigatus and lung inflammation and immunopathology associated with persistent fungal exposure [196,197].
The role of IL-22 in anti-viral host defense and virus-associated inflammation has also been studied. Although neutralization of IL-22 in mice does not affect disease mortality resulting from H1N1 influenza A infection [198,199], IL-22 has been shown to protect bronchial epithelial cells against damage cause by influenza A virus [200] and thought to participate in airway epithelial cell regeneration 7–18 days after H1N1 influenza A virus infection [201]. Recently, IL-22 was observed to play a critical role in lung repair following H1N1 influenza A infection in mice. At 21st day following infection, IL-22 null mice (compared to wild-type) had increased lung injury, decreased lung function, decreased epithelial metaplasia and altered gene expression of genes known to be involved in epithelial repair [202]. In addition, during sublethal H3N2 influenza A infection, IL-22 functions to protect against respiratory tissue damage and decreases susceptibility to secondary bacterial infections [203]. In influenza, S. aureus co-infection models that display severe lung injury, IL-22 levels are suppressed (similar to IL-17) compared to S. aureus alone [170]. These data suggest that a primary role of IL-22 during lung injury is maintenance of epithelial integrity and promotion of repair.
Conclusion
In this review, the primary pathways that regulate Th17 immunity in disease have been discussed. Many discoveries have been made regarding the molecular regulation of this subset of T cells. The primary secretory cytokines, IL-17 and IL-22, have been the focus of many recent studies in both mouse and man. Herein, the current role of these cytokines in asthma and host defense was described (Figure 3). It is now clear that these pathways are critical immune regulators in many diseases ranging from infectious to allergic to autoimmune disorders.
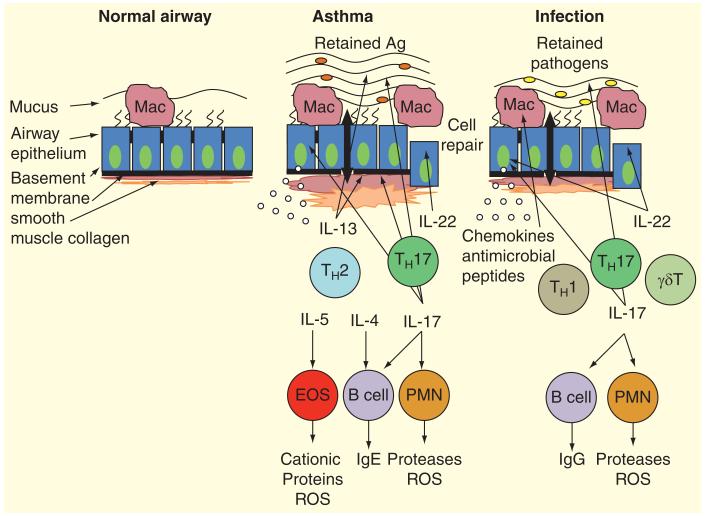
Figure 3. The role of Th17 cytokines in asthma and infection.
The normal airway (left panel) is characterized by low or undetectable levels of Th17 cytokines. In the context of allergy (center panel), Th17 and Th2 cells likely interact to drive the airway changes characteristic of disease; mucus metaplasia, smooth muscle hypertrophy, airway wall thickening, and inflammation. IL-17 orchestrates neutrophilic inflammation via the airway epithelium production of chemokines and growth factors. IL-22 functions to regulate epithelial injury. The precise mechanisms by which Th17 cytokines interact with Th2 pathways remain unclear. During infection (right panel) Th17 cells and other sources of IL-17 and IL-22 drive inflammation and pathogen clearance. Many of these functions involve activation of the airway epithelium to produce chemokines and AMPs. It is likely that there is additional crosstalk with Th1 and IFN-γ regulated pathways in many contexts.
Ag: Antigen; AMPs: Antimicrobial peptides; EOS: Eosinophils; Ig: Immunoglobulin; Mac: Alveolar macrophage; PMN: Polymorphonuclear cells; ROS: Reactive oxygen species.
Expert commentary
Th17 immunity encompasses a broad range of functions and roles in the lung. In addition to these, Th17 cells regulate autoimmunity in many organ systems, which has been reviewed elsewhere [28]. Th17 cells and IL-17 and IL-22 also play critical host defense roles in the intestine and other mucosal sites. The pharmaceutical potential in the Th17 pathway has been targeted in diseases such as psoriasis, Crohn’s disease and rheumatoid arthritis. Antibodies against IL-17 and IL-23 are the subject of several completed and ongoing clinical trials. Recently, anti-IL-17 therapy has begun to be tested in asthma. Data that show that the Th17 pathway may be critical in steroid insensitive, refractory asthma is enticing. Disease processes that are predominantly characterized by inflammatory (neutrophilic) pathologies present the highest probability of efficacy for drugs against the Th17 pathway. The therapeutic potential of targeting IL-17, IL-22 and/or IL-23 in acute lung injury is largely unknown. Data from several animal models suggest that lung injury can be alleviated by blocking Th17-driven inflammation. Further, it may be possible to separate the inflammatory damage induced by Th17 cytokine activation from the host defense mechanisms in order to preserve pathogen clearance and lung integrity. Due the fact that the Th17 pathway has broad action in many disease states, it will likely be difficult to target the detrimental roles of these cytokines without attenuating the beneficial functions. Therapeutic design will have to show great care to achieve improved patient outcome. As we have seen thus far, blocking the autoimmune inflammation promoting activity of IL-17 has not resulted in serious host defense side effects however, diligence is necessary as we move forward.
Five-year view
The Th17 lineage of T cells has yet to see its 10th birthday, however much has been discovered. Despite this, there are many critical issues to be resolved as the field moves forward. It is now clear that many immune cell types produce IL-17 and IL-22 in disease models. Over the next few years, the field must define which cell types are critical in each circumstance. This information is essential to understand the mechanisms of immunity, allergy and autoimmunity. Much of the literature is well stocked with contradictory findings regarding cellular sources and even IL-17 and IL-22 function. It is a significant challenge to design better animal models and to translate current findings into human disease to better resolve these uncertainties. While the pro-inflammatory functions of IL-17 are fairly well known, additional roles of IL-17 are rapidly emerging. It is likely that neutrophil recruitment will only comprise one area of IL-17 biology. For IL-22, the epithelial protective/repair role has just recently been described. IL-22 may very well represent the yin to IL-17’s yang during the normal host defense response. To better understand these cytokines’ action, the field will need to better resolve which lung cells are responding to the stimuli and the downstream networks that are essential to pathogen clearance. In the last 5 years, our understanding about Th17 cells, IL-17 and IL-22 has moved forward tremendously. The field has grown substantially in terms of publications and focused research grants. If the current pace is maintained, we should expect to have better answers to many of these questions when the calendar turns to 2018.
Key issues.
The Th17 lineage of T cells was first described in 2006 and is characterized by expression of retinoic acid-related orphan receptor α (RORα), retinoic acid-related orphan receptor γ thymus (RORγT) and STAT3.
Several cytokines, such as IL-6, IL-1β, TGF-β, IL-21 and IL-23 positively regulate Th17 polarization and function.
Th17 cells are opposed by the functions of Type 1 and 2 interferons, IL-27 and IL-4.
Segmented filamentous bacteria in the gut microbiome regulate Th17 (and Treg) priming and likely impact lung immunity.
Th17 cells (and several additional lymphocytes) secrete IL-17 and IL-22 as effector cytokines.
IL-17 primarily enhances inflammation by inducing chemokine and growth factor production by the lung epithelium. IL-17 and IL-22 stimulate antimicrobial peptide production and IL-22 regulates epithelial repair.
IL-17, IL-22 and IL-23 all play emerging roles in asthma and allergy which may be both beneficial and detrimental depending on context.
In host defense, IL-17, IL-22 and IL-23 promote pathogen clearance, drive inflammation and orchestrate epithelial repair.
Acknowledgments
This work was supported by a Research Advisory Committee Fellowship from Children’s Hospital of Pittsburgh of UPMC (ML Manni), NIH T32 HL007563 (KR Robinson) and NIH NHLBI 1R01HL107380 (JF Alcorn).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112(5):1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bevan MJ. Helping the CD8(+) T-cell response. Nat. Rev. Immunol. 2004;4(8):595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 3.Swain SL, Mckinstry KK, Strutt TM. Expanding roles for CD4(+) T cells in immunity to viruses. Nat. Rev. Immunol. 2012;12(2):136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4(+) T cells. Curr. Opin. Immunol. 2009;21(2):200–208. doi: 10.1016/j.coi.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986;136(7):2348–2357. [PubMed] [Google Scholar]
- 6.Coffman RL. Origins of the T(H)1-T(H) 2 model: a personal perspective. Nat. Immunol. 2006;7(6):539–541. doi: 10.1038/ni0606-539. [DOI] [PubMed] [Google Scholar]
- 7.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 9.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J. Exp. Med. 1989;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Afkarian M, Sedy JR, Yang J, et al. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat. Immunol. 2002;3(6):549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 11.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100(6):655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 12.Oestreich KJ, Weinmann AS. Transcriptional mechanisms that regulate T helper 1 cell differentiation. Curr. Opin. Immunol. 2012;24(2):191–195. doi: 10.1016/j.coi.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agnello D, Lankford CS, Bream J, et al. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J. Clin. Immunol. 2003;23(3):147–161. doi: 10.1023/a:1023381027062. [DOI] [PubMed] [Google Scholar]
- 14.Mowen KA, Glimcher LH. Signaling pathways in Th2 development. Immunol. Rev. 2004;202:203–222. doi: 10.1111/j.0105-2896.2004.00209.x. [DOI] [PubMed] [Google Scholar]
- 15.Szabo SJ, Jacobson NG, Dighe AS, Gubler U, Murphy KM. Developmental commitment to the Th2 lineage by extinction of IL-12 signaling. Immunity. 1995;2(6):665–675. doi: 10.1016/1074-7613(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 16.Ouyang W, Ranganath SH, Weindel K, et al. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9(5):745–755. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 17.Usui T, Preiss JC, Kanno Y, et al. T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J. Exp. Med. 2006;203(3):755–766. doi: 10.1084/jem.20052165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307(5708):430–433. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 19.Oestreich KJ, Weinmann AS. T-bet employs diverse regulatory mechanisms to repress transcription. Trends Immunol. 2012;33(2):78–83. doi: 10.1016/j.it.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006;203(10):2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 22.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 23.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y, Danilenko DM, Valdez P, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445(7128):648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 25.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13(5):715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 26.Murphy CA, Langrish CL, Chen Y, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 2003;198(12):1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yen D, Cheung J, Scheerens H, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 2006;116(5):1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson KM, Manni ML, Biswas PS, Alcorn JF. Clinical consequences of targeting IL-17 and T17 in autoimmune and allergic disorders. Curr. Allergy Asthma Rep. 2013 doi: 10.1007/s11882-013-0361-0. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy KM, Ouyang W, Farrar JD, et al. Signaling and transcription in T helper development. Annu. Rev. Immunol. 2000;18:451–494. doi: 10.1146/annurev.immunol.18.1.451. [DOI] [PubMed] [Google Scholar]
- 30.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 2005;5(7):521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 31.Yang XO, Panopoulos AD, Nurieva R, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007;282(13):9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 32.Zhou L, Ivanov Ii, Spolski R, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 2007;8(9):967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 33.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448(7152):484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aggarwal S, Ghilardi N, Xie MH, De Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2003;278(3):1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 35.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 2008;8(5):337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 36.Shi LZ, Wang R, Huang G, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 2011;208(7):1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 38.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Zhou L, Lopes JE, Chong MM, et al. TGF-beta-induced Foxp3 inhibits T(H) 17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453(7192):236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat. Immunol. 2008;9(11):1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gulen MF, Kang Z, Bulek K, et al. The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation. Immunity. 2010;32(1):54–66. doi: 10.1016/j.immuni.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung Y, Chang SH, Martinez GJ, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30(4):576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Q, Yang W, Gupta S, et al. IRF-4-binding protein inhibits interleukin-17 and interleukin-21 production by controlling the activity of IRF-4 transcription factor. Immunity. 2008;29(6):899–911. doi: 10.1016/j.immuni.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veldhoen M, Hirota K, Westendorf AM, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453(7191):106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 45.Stockinger B, Hirota K, Duarte J, Veldhoen M. External influences on the immune system via activation of the aryl hydrocarbon receptor. Sem. Immunol. 2011;23(2):99–105. doi: 10.1016/j.smim.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Diveu C, Mcgeachy MJ, Boniface K, et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J. Immunol. 2009;182(9):5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 47.Ramgolam VS, Sha Y, Jin J, Zhang X, Markovic-Plese S. IFN-beta inhibits human Th17 cell differentiation. J. Immunol. 2009;183(8):5418–5427. doi: 10.4049/jimmunol.0803227. [DOI] [PubMed] [Google Scholar]
- 48.Yang XP, Ghoreschi K, Steward-Tharp SM, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat. Immunol. 2011;12(3):247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laurence A, Tato CM, Davidson TS, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26(3):371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 50.Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007;8(6):639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 51.Nurieva R, Yang XO, Martinez G, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448(7152):480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 52.Mcgeachy MJ, Bak-Jensen KS, Chen Y, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T (H)-17 cell-mediated pathology. Nat. Immunol. 2007;8(12):1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 53.Cosmi L, Maggi L, Santarlasci V, et al. Identification of a novel subset of human circulating memory CD4(+) T cells that produce both IL-17A and IL-4. J. Allergy Clin. Immunol. 2010;125(1):222–230. e221–e224. doi: 10.1016/j.jaci.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 54.Malmhall C, Bossios A, Radinger M, et al. Immunophenotyping of circulating T helper cells argues for multiple functions and plasticity of T cells in vivo in humans–possible role in asthma. PloS ONE. 2012;7(6):e40012. doi: 10.1371/journal.pone.0040012. • This study examined expression of the transcription factors, T-bet, GATA-3, RORγT and FOXP3 in T cells from asthmatics and found the majority expressed multiple transcription factors potentially indicating T cell plasticity in vivo.
- 55.Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu. Rev. Immunol. 2012;30:759–795. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hooper LV, Stappenbeck TS, Hong CV, Gordon JI. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat. Immunol. 2003;4(3):269–273. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- 57.Peterson DA, Mcnulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2(5):328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 58.Ivanov Ii, Mckenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 59.Ivanov Ii, Frutos Rde L, Manel N, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4(4):337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Atarashi K, Nishimura J, Shima T, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455(7214):808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 61.Ivanov Ii, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu C, Yosef N, Thalhamer T, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496(7446):513–517. doi: 10.1038/nature11984. •• This recent work identifies The control of Th17 differentiation by salt concentrations are far reaching effects on infection, cystic fibrosis and many other autoimmune disease.
- 63.Kleinewietfeld M, Manzel A, Titze J, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496(7446):518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herrada AA, Contreras FJ, Marini NP, et al. Aldosterone promotes autoimmune damage by enhancing Th17-mediated immunity. J. Immunol. 2010;184(1):191–202. doi: 10.4049/jimmunol.0802886. [DOI] [PubMed] [Google Scholar]
- 65.Stegbauer J, Lee DH, Seubert S, et al. Role of the renin-angiotensin system in autoimmune inflammation of the central nervous system. Proc. Natl Acad. Sci. USA. 2009;106(35):14942–14947. doi: 10.1073/pnas.0903602106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J. Immunol. 1993;150(12):5445–5456. [PubMed] [Google Scholar]
- 67.Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J. Leukoc. Biol. 2002;71(1):1–8. [PubMed] [Google Scholar]
- 68.Fossiez F, Djossou O, Chomarat P, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 1996;183(6):2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kennedy J, Rossi DL, Zurawski SM, et al. Mouse IL-17: a cytokine preferentially expressed by alpha beta TCR + CD4-CD8-T cells. J. Interferon Cytokine Res. 1996;16(8):611–617. doi: 10.1089/jir.1996.16.611. [DOI] [PubMed] [Google Scholar]
- 70.Laan M, Cui ZH, Hoshino H, et al. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 1999;162(4):2347–2352. [PubMed] [Google Scholar]
- 71.Laan M, Prause O, Miyamoto M, et al. A role of GM-CSF in the accumulation of neutrophils in the airways caused by IL-17 and TNF-alpha. Eur. Respir. J. 2003;21(3):387–393. doi: 10.1183/09031936.03.00303503. [DOI] [PubMed] [Google Scholar]
- 72.Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17Fregulates inflammatory responses. Cell Res. 2007;17(5):435–440. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- 73.Wright JF, Guo Y, Quazi A, et al. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J. Biol. Chem. 2007;282(18):13447–13455. doi: 10.1074/jbc.M700499200. [DOI] [PubMed] [Google Scholar]
- 74.Hymowitz SG, Filvaroff EH, Yin JP, et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 2001;20(19):5332–5341. doi: 10.1093/emboj/20.19.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 2011;12(5):383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 76.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 77.Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J. Immunol. 2000;164(4):1814–1819. doi: 10.4049/jimmunol.164.4.1814. [DOI] [PubMed] [Google Scholar]
- 78.Aujla SJ, Alcorn JF. T(H)17 cells in asthma and inflammation. Biochim. Biophys. Acta. 2011;1810(11):1066–1079. doi: 10.1016/j.bbagen.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 79.Simonian PL, Wehrmann F, Roark CL, Born WK, O’brien RL, Fontenot AP. gammadelta T cells protect against lung fibrosis via IL-22. J. Exp. Med. 2010;207(10):2239–2253. doi: 10.1084/jem.20100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 2006;177(7):4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 81.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31(2):321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 82.Lalor SJ, Dungan LS, Sutton CE, Basdeo SA, Fletcher JM, Mills KH. Caspase-1-processed cytokines IL-1beta and IL-18 promote IL-17 production by gammadelta and CD4 T cells that mediate autoimmunity. J. Immunol. 2011;186(10):5738–5748. doi: 10.4049/jimmunol.1003597. [DOI] [PubMed] [Google Scholar]
- 83.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31(2):331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 84.Spits H, Artis D, Colonna M, et al. Innate lymphoid cells-a proposal for uniform nomenclature. Nat. Rev. Immunol. 2013;13(2):145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 85.Zheng Y, Valdez PA, Danilenko DM, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008;14(3):282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 86.Buonocore S, Ahern PP, Uhlig HH, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464(7293):1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang H, Li Y, Qi X. Cytokine signaling in the differentiation of innate effector cells. Jak. Stat. 2013;2(1):e23531. doi: 10.4161/jkst.23531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. New Engl J. Med. 2009;361(9):888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 89.Bachmann M, Ulziibat S, Hardle L, Pfeilschifter J, Muhl H. IFNalpha converts IL-22 into a cytokine efficiently activating STAT1 and its downstream targets. Biochem. Pharmacol. 2013;85(3):396–403. doi: 10.1016/j.bcp.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 90.Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin. Immunopathol. 2010;32(1):17–31. doi: 10.1007/s00281-009-0188-x. [DOI] [PubMed] [Google Scholar]
- 91.Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31(1):15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 92.Mehrotra PT, Donnelly RP, Wong S, et al. Production of IL-10 by human natural killer cells stimulated with IL-2 and/or IL-12. J. Immunol. 1998;160(6):2637–2644. [PubMed] [Google Scholar]
- 93.Pickert G, Neufert C, Leppkes M, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 2009;206(7):1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 2009;10(8):857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 95.Sonnenberg GF, Monticelli LA, Alenghat T, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336(6086):1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14(2):155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 97.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 2009;9(8):556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuestner RE, Taft DW, Haran A, et al. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J. Immunol. 2007;179(8):5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mellett M, Atzei P, Horgan A, et al. Orphan receptor IL-17RD tunes IL-17A signalling and is required for neutrophilia. Nat. Commun. 2012;3:1119. doi: 10.1038/ncomms2127. [DOI] [PubMed] [Google Scholar]
- 100.Song X, Zhu S, Shi P, et al. IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nat. Immunol. 2011;12(12):1151–1158. doi: 10.1038/ni.2155. [DOI] [PubMed] [Google Scholar]
- 101.Novatchkova M, Leibbrandt A, Werzowa J, Neubuser A, Eisenhaber F. The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem. Sci. 2003;28(5):226–229. doi: 10.1016/S0968-0004(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 102.Awane M, Andres PG, Li DJ, Reinecker HC. NF-kappa B-inducing kinase is a common mediator of IL-17-, TNF-alpha-, and IL-1 beta-induced chemokine promoter activation in intestinal epithelial cells. J. Immunol. 1999;162(9):5337–5344. [PubMed] [Google Scholar]
- 103.Qian Y, Liu C, Hartupee J, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat. Immunol. 2007;8(3):247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 104.Schwandner R, Yamaguchi K, Cao Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J. Exp. Med. 2000;191(7):1233–1240. doi: 10.1084/jem.191.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kawaguchi M, Onuchic LF, Huang SK. Activation of extracellular signal-regulated kinase (ERK)1/2, but not p38 and c-Jun N-terminal kinase, is involved in signaling of a novel cytokine, ML-1. J. Biol. Chem. 2002;277(18):15229–15232. doi: 10.1074/jbc.C100641200. [DOI] [PubMed] [Google Scholar]
- 106.Van Der Velden J, Janssen-Heininger YM, Mandalapu S, Scheller EV, Kolls JK, Alcorn JF. Differential requirement for c-Jun N-terminal kinase 1 in lung inflammation and host defense. PloS ONE. 2012;7(4):e34638. doi: 10.1371/journal.pone.0034638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sun D, Novotny M, Bulek K, Liu C, Li X, Hamilton T. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 andthe splicing-regulatory factor SF2 (ASF) Nat. Immunol. 2011;12(9):853–860. doi: 10.1038/ni.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maitra A, Shen F, Hanel W, et al. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc. Natl Acad. Sci. USA. 2007;104(18):7506–7511. doi: 10.1073/pnas.0611589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shen F, Hu Z, Goswami J, Gaffen SL. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J. Biolog. Chem. 2006;281(34):24138–24148. doi: 10.1074/jbc.M604597200. [DOI] [PubMed] [Google Scholar]
- 110.Ruddy MJ, Wong GC, Liu XK, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J. Biol. Chem. 2004;279(4):2559–2567. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- 111.Kotenko SV, Izotova LS, Mirochnitchenko OV, et al. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rbeta ) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J. Biol. Chem. 2001;276(4):2725–2732. doi: 10.1074/jbc.M007837200. [DOI] [PubMed] [Google Scholar]
- 112.Savan R, Mcfarland AP, Reynolds DA, et al. A novel role for IL-22R1 as a driver of inflammation. Blood. 2011;117(2):575–584. doi: 10.1182/blood-2010-05-285908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aujla SJ, Kolls JK. IL-22: a critical mediator in mucosal host defense. J. Mol. Med. (Berl.) 2009;87(5):451–454. doi: 10.1007/s00109-009-0448-1. [DOI] [PubMed] [Google Scholar]
- 114.Ito S, Ansari P, Sakatsume M, et al. Interleukin-10 inhibits expression of both interferon alpha- and interferon gamma-induced genes by suppressing tyrosine phosphorylation of STAT1. Blood. 1999;93(5):1456–1463. [PubMed] [Google Scholar]
- 115.Hoegl S, Bachmann M, Scheiermann P, et al. Protective properties of inhaled IL-22 in a model of ventilator-induced lung injury. Am. J. Respir. Cell Mol. Biol. 2011;44(3):369–376. doi: 10.1165/rcmb.2009-0440OC. [DOI] [PubMed] [Google Scholar]
- 116.Nagalakshmi ML, Rascle A, Zurawski S, Menon S, De Waal Malefyt R. Interleukin-22 activates STAT3 and induces IL-10 by colon epithelial cells. Int. Immunopharmacol. 2004;4(5):679–691. doi: 10.1016/j.intimp.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 117.Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, Renauld JC. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J. Biol. Chem. 2002;277(37):33676–33682. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]
- 118.Wei CC, Ho TW, Liang WG, Chen GY, Chang MS. Cloning and characterization of mouse IL-22 binding protein. Genes Immun. 2003;4(3):204–211. doi: 10.1038/sj.gene.6363947. [DOI] [PubMed] [Google Scholar]
- 119.Di Meglio P, Di Cesare A, Laggner U, et al. The IL23R R381Q gene variant protects against immune-mediated diseases by impairing IL-23-induced Th17 effector response in humans. PloS ONE. 2011;6(2):e17160. doi: 10.1371/journal.pone.0017160. • This study identifies the protective allele IL-23R R381Q with significantly reduced IL-17A production and STAT3 phosphorylation and illustrates a critical role for the IL-23/IL-23R signaling in generating pathogenic TH17 responses.
- 120.Hashimoto T, Akiyama K, Kobayashi N, Mori A. Comparison of IL-17 production by helper T cells among atopic and nonatopic asthmatics and control subjects. Int. Arch. Allergy Immunol. 2005;137(Suppl. 1):51–54. doi: 10.1159/000085432. [DOI] [PubMed] [Google Scholar]
- 121.Agache I, Ciobanu C, Agache C, Anghel M. Increased serum IL-17 is an independent risk factor for severe asthma. Respir. Med. 2010;104(8):1131–1137. doi: 10.1016/j.rmed.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 122.Bullens DM, Truyen E, Coteur L, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir. Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Molet S, Hamid Q, Davoine F, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J. Allergy Clin. Immunol. 2001;108(3):430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 124.Wong CK, Ho CY, Ko FW, et al. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-gamma, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin. Exp. Immunol. 2001;125(2):177–183. doi: 10.1046/j.1365-2249.2001.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chakir J, Shannon J, Molet S, et al. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J. Allergy Clin. Immunol. 2003;111(6):1293–1298. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 126.Barczyk A, Pierzchala W, Sozanska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir. Med. 2003;97(6):726–733. doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- 127.Zhao Y, Yang J, Gao YD, Guo W. Th17 immunity in patients with allergic asthma. Int. Arch. Allergy Immunol. 2010;151(4):297–307. doi: 10.1159/000250438. [DOI] [PubMed] [Google Scholar]
- 128.Manise M, Holtappels G, Van Crombruggen K, Schleich F, Bachert C, Louis R. Sputum IgE and cytokines in asthma: relationship with sputum cellular profile. PloS ONE. 2013;8(3):e58388. doi: 10.1371/journal.pone.0058388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kawaguchi M, Takahashi D, Hizawa N, et al. IL-17F sequence variant (His161Arg) is associated with protection against asthma and antagonizes wild-type IL-17F activity. J. Allergy Clin. Immunol. 2006;117(4):795–801. doi: 10.1016/j.jaci.2005.12.1346. [DOI] [PubMed] [Google Scholar]
- 130.Kawaguchi M, Onuchic LF, Li XD, et al. Identification of a novel cytokine, ML-1, and its expression in subjects with asthma. J. Immunol. 2001;167(8):4430–4435. doi: 10.4049/jimmunol.167.8.4430. [DOI] [PubMed] [Google Scholar]
- 131.Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu. Rev. Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 132.Oda N, Canelos PB, Essayan DM, Plunkett BA, Myers AC, Huang SK. Interleukin-17F induces pulmonary neutrophilia and amplifies antigen-induced allergic response. Am. J. Respir. Crit. Care Med. 2005;171(1):12–18. doi: 10.1164/rccm.200406-778OC. [DOI] [PubMed] [Google Scholar]
- 133.Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma : evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest. 2001;119(5):1329–1336. doi: 10.1378/chest.119.5.1329. [DOI] [PubMed] [Google Scholar]
- 134.Louis R, Lau LC, Bron AO, Roldaan AC, Radermecker M, Djukanovic R. The relationship between airways inflammation and asthma severity. Am J Respir Crit Care Med. 2000;161(1):9–16. doi: 10.1164/ajrccm.161.1.9802048. [DOI] [PubMed] [Google Scholar]
- 135.Wenzel SE, Balzar S, Cundall M, Chu HW. Subepithelial basement membrane immunoreactivity for matrix metalloproteinase 9: association with asthma severity, neutrophilic inflammation, and wound repair. J. Allergy Clin. Immunol. 2003;111(6):1345–1352. doi: 10.1067/mai.2003.1464. [DOI] [PubMed] [Google Scholar]
- 136.Green RH, Brightling CE, Woltmann G, Parker D, Wardlaw AJ, Pavord ID. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002;57(10):875–879. doi: 10.1136/thorax.57.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cox G. Glucocorticoid treatment inhibits apoptosis in human neutrophils. Separation of survival and activation outcomes. J. Immunol. 1995;154(9):4719–4725. [PubMed] [Google Scholar]
- 138.Schleimer RP, Freeland HS, Peters SP, Brown KE, Derse CP. An assessment of the effects of glucocorticoids on degranulation, chemotaxis, binding to vascular endothelium and formation of leukotriene B4 by purified human neutrophils. J. Pharmacol. Exp. Ther. 1989;250(2):598–605. [PubMed] [Google Scholar]
- 139.Mckinley L, Alcorn JF, Peterson A, et al. TH17 cells mediate steroid-resistant airway inflammation and airwayhyperresponsiveness in mice. J. Immunol. 2008;181(6):4089–4097. doi: 10.4049/jimmunol.181.6.4089. • This study reveals the novel role for TH17 cells in steroid-resistant, neutrophil-dominant asthma.
- 140.Rahman MS, Yamasaki A, Yang J, Shan L, Halayko AJ, Gounni AS. IL-17A induces eotaxin-1/CC chemokine ligand 11 expression in human airway smooth muscle cells: role of MAPK (Erk1/2, JNK, and p38) pathways. J. Immunol. 2006;177(6):4064–4071. doi: 10.4049/jimmunol.177.6.4064. [DOI] [PubMed] [Google Scholar]
- 141.Huang F, Wachi S, Thai P, et al. Potentiation of IL-19 expression in airway epithelia by IL-17A and IL-4/IL-13: important implications in asthma. J. Allergy Clin. Immunol. 2008;121(6):1415–1421. 1421 e1411–e1413. doi: 10.1016/j.jaci.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sergejeva S, Ivanov S, Lotvall J, Linden A. Interleukin-17 as a recruitment and survival factor for airway macrophages in allergic airway inflammation. Am. J. Respir. Cell Mol. Biol. 2005;33(3):248–253. doi: 10.1165/rcmb.2004-0213OC. [DOI] [PubMed] [Google Scholar]
- 143.Hellings PW, Kasran A, Liu Z, et al. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am. J. Respir. Cell Mol. Biol. 2003;28(1):42–50. doi: 10.1165/rcmb.4832. [DOI] [PubMed] [Google Scholar]
- 144.Schnyder-Candrian S, Togbe D, Couillin I, et al. Interleukin-17 is a negative regulator of established allergic asthma. J. Exp. Med. 2006;203(12):2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Park SJ, Lee KS, Kim SR, et al. Peroxisome proliferator-activated receptor gamma agonist down-regulates IL-17 expression in a murine model of allergic airway inflammation. J. Immunol. 2009;183(5):3259–3267. doi: 10.4049/jimmunol.0900231. [DOI] [PubMed] [Google Scholar]
- 146.Song C, Luo L, Lei Z, et al. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J. Immunol. 2008;181(9):6117–6124. doi: 10.4049/jimmunol.181.9.6117. [DOI] [PubMed] [Google Scholar]
- 147.Chen Y, Thai P, Zhao YH, Ho YS, Desouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J. Biol. Chem. 2003;278(19):17036–17043. doi: 10.1074/jbc.M210429200. [DOI] [PubMed] [Google Scholar]
- 148.Molet S, Hamid Q, Davoine F, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J. Allergy Clin. Immunol. 2001;108(3):430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 149.Zijlstra GJ, Ten Hacken NH, Hoffmann RF, Van Oosterhout AJ, Heijink IH. Interleukin-17A induces glucocorticoid insensitivity in human bronchial epithelial cells. Eur. Respir. J. 2012;39(2):439–445. doi: 10.1183/09031936.00017911. [DOI] [PubMed] [Google Scholar]
- 150.Kudo M, Melton AC, Chen C, et al. IL-17A produced by alphabeta T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat. Med. 2012;18(4):547–554. doi: 10.1038/nm.2684. •• In this study, IL-17A was shown to enhance contraction of airway smooth muscle through the IL-17 receptor A (IL-17RA)-IL-17RC complex via the ROCK2 signaling pathway.
- 151.Wakashin H, Hirose K, Maezawa Y, et al. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am. J. Respir. Crit. Care Med. 2008;178(10):1023–1032. doi: 10.1164/rccm.200801-086OC. [DOI] [PubMed] [Google Scholar]
- 152.Lajoie S, Lewkowich IP, Suzuki Y, et al. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat. Immunol. 2010;11(10):928–935. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Besnard AG, Sabat R, Dumoutier L, et al. Dual Role of IL-22 in allergic airway inflammation and its cross-talk with IL-17A. Am. J. Respir. Crit. Care Med. 2011;183(9):1153–1163. doi: 10.1164/rccm.201008-1383OC. • This study shows that IL-22 is required for the development of allergic airway inflammation, while lessens established allergic inflammation by regulating the proinflammatory properties of IL-17A in a murine allergic asthma model.
- 154.Takahashi K, Hirose K, Kawashima S, et al. IL-22 attenuates IL-25 production by lung epithelial cells and inhibits antigen-induced eosinophilic airway inflammation. J. Allergy Clin. Immunol. 2011;128(5):1067–1076. e1061–e1066. doi: 10.1016/j.jaci.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 155.Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J. Exp. Med. 2010;207(6):1293–1305. doi: 10.1084/jem.20092054. • This study was the first to identify a pathological role IL-22 in airway inflammation and that IL-17A influenced the proinflammatory and pathological versus protective role of IL-22 in the lung.
- 156.Dubin PJ, Kolls JK. IL-23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;292(2):L519–528. doi: 10.1152/ajplung.00312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ye P, Garvey PB, Zhang P, et al. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am. J. Respir. Cell. Mol. Biol. 2001;25(3):335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 158.Ye P, Rodriguez FH, Kanaly S, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 2001;194(4):519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Happel KI, Dubin PJ, Zheng M, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J. Exp. Med. 2005;202(6):761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen K, Mcaleer JP, Lin Y, et al. Th17 cells mediate clade-specific, serotype-independent mucosal immunity. Immunity. 2011;35(6):997–1009. doi: 10.1016/j.immuni.2011.10.018. • The authors show that the Th17 cell lineage confers an advantage to the host organism by providing heterologous mucosal immunity independent of serotype-specific antibody in response to Klebsiella pneumoniae.
- 161.Woolard MD, Hensley LL, Kawula TH, Frelinger JA. Respiratory Francisella tularensis live vaccine strain infection induces Th17 cells and prostaglandin E2, which inhibits generation of gamma interferon-positive T cells. Infect. Immun. 2008;76(6):2651–2659. doi: 10.1128/IAI.01412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lin Y, Ritchea S, Logar A, et al. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31(5):799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Carbonetti NH. Immunomodulation in the pathogenesis of Bordetella pertussis infection and disease. Curr. Opin. Pharmacol. 2007;7(3):272–278. doi: 10.1016/j.coph.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 164.Fedele G, Nasso M, Spensieri F, et al. Lipopolysaccharides from Bordetella pertussis and Bordetella parapertussis differently modulate human dendritic cell functions resulting in divergent prevalence of Th17-polarized responses. J. Immunol. 2008;181(1):208–216. doi: 10.4049/jimmunol.181.1.208. [DOI] [PubMed] [Google Scholar]
- 165.Higgins SC, Jarnicki AG, Lavelle EC, Mills KH. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J. Immunol. 2006;177(11):7980–7989. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- 166.Warfel JM, Merkel TJ. Bordetella pertussis infection induces a mucosal IL-17 response and long-lived Th17 and Th1 immune memory cells in nonhuman primates. Mucosal. Immunol. 2012;6(4):787–796. doi: 10.1038/mi.2012.117. [DOI] [PubMed] [Google Scholar]
- 167.Dubin PJ, Martz A, Eisenstatt JR, Fox MD, Logar A, Kolls JK. Interleukin-23-mediated inflammation in Pseudomonas aeruginosapulmonary infection. Infect. Immun. 2012;80(1):398–409. doi: 10.1128/IAI.05821-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liu J, Feng Y, Yang K, et al. Early production of IL-17 protects against acute pulmonary Pseudomonas aeruginosa infection in mice. FEMS Immunol. Med. Microbiol. 2011;61(2):179–188. doi: 10.1111/j.1574-695X.2010.00764.x. [DOI] [PubMed] [Google Scholar]
- 169.Wu W, Huang J, Duan B, et al. Th17-stimulating protein vaccines confer protection against Pseudomonas aeruginosa pneumonia. Am. J. Respir. Crit. Care Med. 2012;186(5):420–427. doi: 10.1164/rccm.201202-0182OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kudva A, Scheller EV, Robinson KM, et al. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J. Immunol. 2011;186(3):1666–1674. doi: 10.4049/jimmunol.1002194. • This study demonstrates viral suppression of subsequent TH17 immunity through type I interferons and increase susceptibility to secondary bacterial pneumonia for the first time.
- 171.Cheng P, Liu T, Zhou WY, et al. Role of gamma-delta T cells in host response against Staphylococcus aureus-induced pneumonia. BMC Immunol. 2012;13:38. doi: 10.1186/1471-2172-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J. Clin. Invest. 2009;119(7):1899–1909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ma J, Wang J, Wan J, et al. Morphine disrupts interleukin-23 (IL-23)/IL-17-mediated pulmonary mucosal host defense against Streptococcus pneumoniae infection. Infect. Immun. 2010;78(2):830–837. doi: 10.1128/IAI.00914-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang X, Gao L, Lei L, et al. A MyD88-dependent early IL-17 production protects mice against airway infection with the obligate intracellular pathogen Chlamydia muridarum. J. Immunol. 2009;183(2):1291–1300. doi: 10.4049/jimmunol.0803075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Aujla SJ, Chan YR, Zheng M, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 2008;14(3):275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Khader SA, Pearl JE, Sakamoto K, et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J. Immunol. 2005;175(2):788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 177.Umemura M, Yahagi A, Hamada S, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J. Immunol. 2007;178(6):3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 178.Khader SA, Bell GK, Pearl JE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 2007;8(4):369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 179.Crowe CR, Chen K, Pociask DA, et al. Critical role of IL-17RA in immunopathology of influenza infection. J. Immunol. 2009;183(8):5301–5310. doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hamada H, Garcia-Hernandez Mde L, Reome JB, et al. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J. Immunol. 2009;182(6):3469–3481. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang X, Chan CC, Yang M, et al. A critical role of IL-17 in modulating the B-cell response during H5N1 influenza virus infection. Cell Mol. Immunol. 2011;8(6):462–468. doi: 10.1038/cmi.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wiehler S, Proud D. Interleukin-17A modulates human airway epithelial responses to human rhinovirus infection. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;293(2):L505–L515. doi: 10.1152/ajplung.00066.2007. [DOI] [PubMed] [Google Scholar]
- 183.Li W, Moltedo B, Moran TM. Type I interferon induction during influenza virus infection increases susceptibility to secondary Streptococcus pneumoniae infection by negative regulation of gammadelta T cells. J. Virol. 2012;86(22):12304–12312. doi: 10.1128/JVI.01269-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Werner JL, Metz AE, Horn D, et al. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J. Immunol. 2009;182(8):4938–4946. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rudner XL, Happel KI, Young EA, Shellito JE. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect. Immun. 2007;75(6):3055–3061. doi: 10.1128/IAI.01329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Iannitti RG, Carvalho A, Cunha C, et al. Th17/Treg imbalance in murine cystic fibrosis is linked to indoleamine 2,3-dioxygenase deficiency but corrected by kynurenines. Am. J. Respir. Crit. Care Med. 2013;187(6):609–620. doi: 10.1164/rccm.201207-1346OC. [DOI] [PubMed] [Google Scholar]
- 187.Wuthrich M, Gern B, Hung CY, et al. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J. Clin. Invest. 2011;121(2):554–568. doi: 10.1172/JCI43984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mcallister F, Henry A, Kreindler JL, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J. Immunol. 2005;175(1):404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tan HL, Regamey N, Brown S, Bush A, Lloyd CM, Davies JC. The Th17 pathway in cystic fibrosis lung disease. Am. J. Respir. Crit. Care Med. 2011;184(2):252–258. doi: 10.1164/rccm.201102-0236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chan YR, Chen K, Duncan SR, et al. Patients with cystic fibrosis have inducible IL-17+IL-22+ memory cells in lung draining lymph nodes. J. Allergy Clin. Immunol. 2013;131(4):1117–1129. 1129 e1111–e1115. doi: 10.1016/j.jaci.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tiringer K, Treis A, Fucik P, et al. A Th17- and Th2-skewed cytokine profile in cystic fibrosis lungs represents a potential risk factor for Pseudomonas aeruginosa infection. Am. J. Respir. Crit. Care Med. 2013;187(6):621–629. doi: 10.1164/rccm.201206-1150OC. [DOI] [PubMed] [Google Scholar]
- 192.Murdock BJ, Falkowski NR, Shreiner AB, et al. Interleukin-17 drives pulmonary eosinophilia following repeated exposure to Aspergillus fumigatus conidia. Infect. Immun. 2012;80(4):1424–1436. doi: 10.1128/IAI.05529-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zenewicz LA, Flavell RA. IL-22 and inflammation: leukin’ through a glass onion. Eur. J. Immunol. 2008;38(12):3265–3268. doi: 10.1002/eji.200838655. [DOI] [PubMed] [Google Scholar]
- 194.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21(2):241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 195.Scriba TJ, Kalsdorf B, Abrahams DA, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J. Immunol. 2008;180(3):1962–1970. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lilly LM, Gessner MA, Dunaway CW, et al. The beta-glucan receptor dectin-1 promotes lung immunopathology during fungal allergy via IL-22. J. Immunol. 2012;189(7):3653–3660. doi: 10.4049/jimmunol.1201797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gessner MA, Werner JL, Lilly LM, et al. Dectin-1-dependent interleukin-22 contributes to early innate lung defense against Aspergillus fumigatus. Infect. Immun. 2012;80(1):410–417. doi: 10.1128/IAI.05939-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Guo H, Topham DJ. Interleukin-22 (IL-22) production by pulmonary Natural Killer cells and the potential role of IL-22 during primary influenza virus infection. J. Virol. 2010;84(15):7750–7759. doi: 10.1128/JVI.00187-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Monticelli LA, Sonnenberg GF, Abt MC, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection withinfluenza virus. Nat. Immunol. 2011;12(11):1045–1054. doi: 10.1031/ni.2131. •• This work identifies a novel role for lung innate lymphoid cells in promoting airway epithelial integrity and tissue homeostasis through the production of the epidermal growth factor family member amphiregulin.
- 200.Paget C, Ivanov S, Fontaine J, et al. Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damages. J. Biol. Chem. 2012;287(12):8816–8829. doi: 10.1074/jbc.M111.304758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kumar P, Thakar MS, Ouyang W, Malarkannan S. IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal. Immunol. 2013;6(1):69–82. doi: 10.1038/mi.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pociask DA, Scheller EV, Mandalapu S, et al. IL-22 is essential for lung epithelial repair following influenza infection. Am. J. Pathol. 2013;182(4):1286–1296. doi: 10.1016/j.ajpath.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ivanov S, Renneson J, Fontaine J, et al. Interleukin-22 reduces lung inflammation during influenza A virus infection and protects against secondary bacterial infection. J. Virol. 2013;87(12):6911–6924. doi: 10.1128/JVI.02943-12. • Demonstrates that IL-22 is beneficial during sublethal H3N2 influenza A virus infection as this cytokine limits inflammation and subsequent bacterial infection in the lung.