SUMMARY
There is growing evidence that alterations in metabolism may contribute to tumorigenesis. Here, we report on members of families with the Li–Fraumeni syndrome who carry germline mutations in TP53, the gene encoding the tumor-suppressor protein p53. As compared with family members who are not carriers and with healthy volunteers, family members with these mutations have increased oxidative phosphorylation of skeletal muscle. Basic experimental studies of tissue samples from patients with the Li–Fraumeni syndrome and a mouse model of the syndrome support this in vivo finding of increased mitochondrial function. These results suggest that p53 regulates bioenergetic homeostasis in humans. (Funded by the National Heart, Lung, and Blood Institute and the National Institutes of Health; ClinicalTrials.gov number, NCT00406445.)
CASE REPORT
The Li–Fraumeni syndrome is caused by the transmission of germline TP53 mutations that result in a variety of early-onset sarcomas and carcinomas — there are about 600 such mutations, but not all are known to cause the Li–Fraumeni syndrome (International Agency for Research on Cancer [IARC] TP53 Database, version R15).1,2 It is well known that p53 plays an essential role in regulating various cellular activities that are directly related to tumor suppression, such as cell-cycle control and DNA repair, but there is accumulating evidence that p53 also regulates other activities, such as mitochondrial respiration and glycolysis.3,4 This protein can regulate mitochondrial function by means of both transcriptional activation of mitochondrial biogenesis genes and post-translational protein–protein interactions.5,6 A recent study elegantly showed that the retention of the metabolic activities of p53 (but not its cell-cycle and apoptotic activities) is sufficient to suppress tumors in mice, highlighting the critical role of these less-studied metabolic functions of p53 in tumorigenesis.7 On the basis of these observations, we hypothesized that members of families with the Li–Fraumeni syndrome who carry germline mutations in p53 may have evidence of altered mitochondrial function.
Although the results of treadmill exercise testing can be used as an indirect marker of mitochondrial function, many factors can contribute to aerobic fitness. To avoid the effects of variables such as physical fitness, lifestyle, diet, and even motivation, we used a more sensitive, noninvasive technique to directly measure mitochondrial function in the skeletal muscle of study participants.8 This method relies on the regeneration of phosphocreatine, which normally shuttles high-energy phosphate from the mitochondria to the cytosol in order to maintain ATP levels in skeletal muscle during physical activity. The measurement of phosphocreatine regeneration after exercise-induced depletion with phosphorus-31 magnetic resonance spectroscopy (31P-MRS) can therefore provide a unique and sensitive gauge of in vivo oxidative phosphorylation capacity.9 To support these in vivo findings, we concurrently examined mitochondrial function and biogenesis in cells isolated from family members with the Li–Fraumeni syndrome and in an established mouse model of the syndrome.
Methods
Study Participants
We enrolled both noncarriers and carriers of the TP53 mutation from families with the Li–Fraumeni syndrome, as well as healthy volunteers who served as additional controls in our pilot clinical study. The study was approved by the National Institutes of Health internal review board, and all participants were enrolled after providing written informed consent. Before enrollment in our clinical protocol, the members of families subject to inheritance of the Li–Fraumeni syndrome underwent TP53 genotyping in consultation with their physicians and genetic counselors (a list of genetic testing sites appears in the Supplementary Appendix, available with the full text of this article at NEJM.org). A medical history was obtained and a physical examination and basic laboratory tests were performed to ensure that all study participants were in good health. None of the participants with the Li–Fraumeni syndrome who carried the TP53 mutation had received a diagnosis of cancer within 1 year before enrollment in the study. (Table S1 in the Supplementary Appendix lists the following characteristics of TP53 mutation carriers: type of mutation, family identification number, age at enrollment, type of cancer, age at diagnosis, and chemotherapy history.)
Measurement of Phosphocreatine Levels and Other Tests
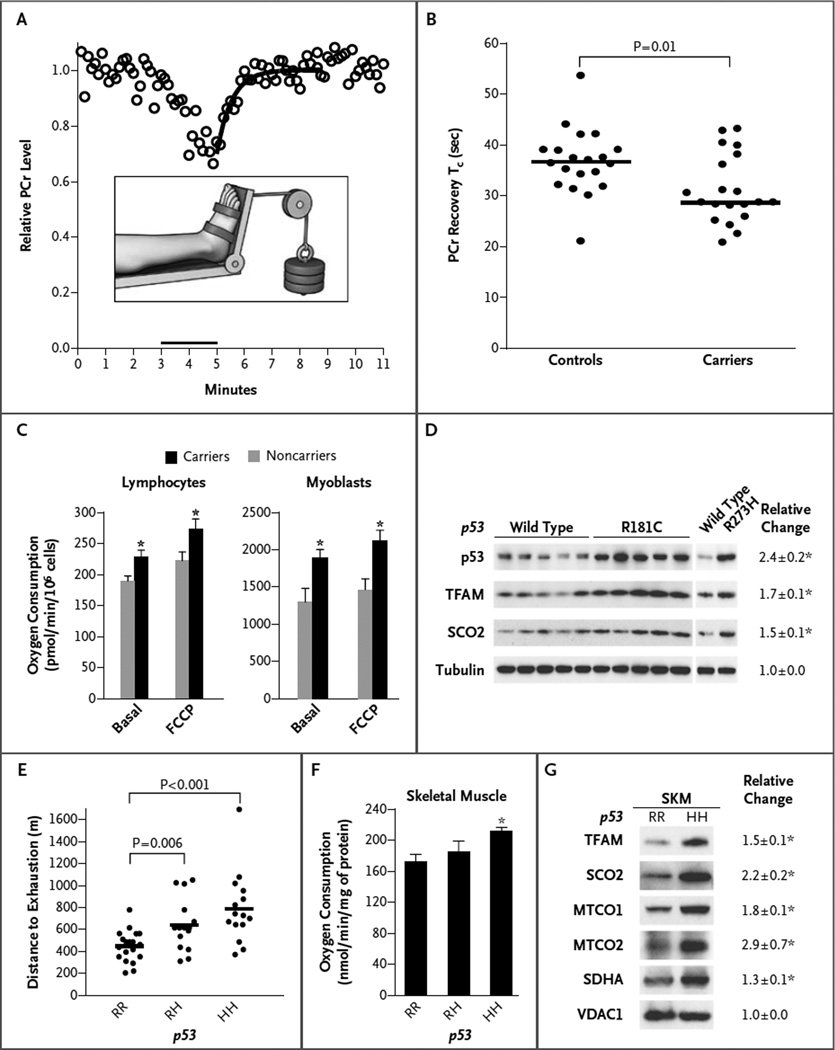
We developed a foot-exercise apparatus (Fig. 1A) that, when used, would deplete phosphocreatine levels in the tibialis anterior, a muscle in the superficial anterior lateral aspect of the leg mainly composed of oxidative type I and type IIA fibers enriched in mitochondria (Fig. 1A).9 Each participant engaged in submaximal exercise by dorsiflexing one foot against 30% of the maximum weight lifted before testing. The phosphocreatine level was measured with the use of 31PMRS during a 3minute rest period, a 2minute exercise period, and a 6minute recovery period, from which the single exponential recovery time constant (Tc) was calculated with data obtained during the postexercise recovery period (Fig. 1A). In accordance with the protocol, 31P spectra were obtained at rest and during exercise and recovery, and the results were analyzed with the use of SAGE 7 (GE Healthcare) and IDL, version 6.4 (Exelis Visual Information Solutions), software (see the Supplementary Appendix).10 The study timeline for testing (i.e., the total number of months to complete testing of all participants) did not differ significantly between the control group and the participants carrying a TP53 mutation (Fig. S1 in the Supplementary Appendix).
Figure 1. Oxidative Metabolic Capacity in Study Participants with TP53 Mutations and Controls, and in Mice with the Li–Fraumeni Syndrome.
In Panel A, the inset shows the apparatus used to assist 20 TP53 mutation carriers and 20 controls in exercising the highly oxidative tibialis anterior muscle for 2 minutes in order to produce data on the phosphocreatine (PCr) recovery time constant (Tc), shown in the graph (circles indicate raw data). The y-axis shows PCr levels relative to the pre-exercise baseline. Panel B shows the PCr recovery Tc in controls (36.7 seconds) and carriers (28.7 seconds). The horizontal lines indicate the median values. The P value was determined with the use of the Mann–Whitney test. Panel C shows the rates of oxygen consumption by lymphocytes (from 10 controls and 14 carriers with seven different TP53 mutations) and skeletal- muscle myoblasts (from 4 controls and 7 carriers with seven different TP53 mutations). Basal and uncoupled respiration rates are shown. Panel D shows the levels of two mitochondrial biogenesis regulators — mitochondrial transcription factor A (TFAM) and synthesis of cytochrome c oxidase 2 (SCO2) — in myoblasts obtained from 5 male carriers of the TP53 codon R181C mutation and 5 male noncarriers (wild type) in a family with the Li–Fraumeni syndrome (Family 1). Data are also shown for 2 female members of Family 2 who had the R273H hot-spot mutation. Quantification of the relative change in expression (the ratio of the value in carriers to the value in noncarriers) was performed by means of densitometry (6). As expected, mutated p53 protein was overexpressed as compared with wild-type protein. Tubulin is shown as a loading control. Panel E shows the endurance capacity (measured with treadmill exercise) of male mice with the p53 R172H mutation (which is homologous to the human TP53 R175H hot spot mutation in patients with the Li–Fraumeni syndrome). Results are shown for heterozygous mice (RH), homozygous mice (HH), and wild-type mice (RR). The horizontal lines indicate the mean value (number of mice, ≥15). Panel F shows oxygen consumption of mitochondria purified from mouse skeletal muscle, measured with glutamate, malate, and adenosine diphosphate (state 3 respiration) (number of mice, ≥3). The ratio of state 3 to state 4 respiration (respiratory control rate, approximately 8) did not significantly differ among the three genotypes (RR, RH, and HH). Panel G shows levels of mitochondrial protein expression in Western blots and the relative change in expression (the ratio of expression in HH mice to expression in RR mice), with quantification obtained by densitometry (number of mice, ≥4). MTCO1 and MTCO2 denote mitochondrial cytochrome c oxidase subunits 1 and 2, respectively; SDHA denotes succinate dehydrogenase A, and VDAC1 voltage-dependent anion channel 1 (the protein-loading control). Values for relative change in Panels D and G and the T bars in Panels C and F are means ±SE, and the asterisks indicate significant differences (P<0.05).
The validity of our technical protocol was confirmed as follows. In the control group, recovery Tcs for phosphocreatine levels were tightly clustered around previously reported values for healthy subjects.8 In the group of participants with a TP53 mutation, there was a significant correlation between the recovery Tc for phosphocreatine levels and oxygen consumption at the ventilatory threshold (i.e., the point during exercise at which significant anaerobic metabolism ensues), an independent measure of oxidative metabolic capacity (r = −0.73, P = 0.01) (Fig. S2A in the Supplementary Appendix).11
Detailed descriptions of myoblast preparations from biopsy specimens of skeletal muscle, tissue culture, and studies in the mouse model of the Li–Fraumeni syndrome are available in the Supplementary Appendix. Standard techniques were used for purifying mitochondria from tissue samples and for measuring mitochondrial oxygen consumption, as detailed in the Supplementary Appendix. Information on the antibodies used and on sample preparation and Western blotting techniques is provided in the Supplementary Appendix.
Statistical Analysis
Data were analyzed with the use of a two-tailed Student’s t-test. The normality of the phosphocreatine recovery Tc data obtained with 31P-MRS was not assumed, and the unpaired, nonparametric Mann–Whitney test was used to analyze these data (GraphPad InStat, version 3.06).
Results
Study Families and Controls
The control group comprised 9 healthy volunteers and 11 members of families with the Li–Fraumeni syndrome who did not have TP53 mutations. The carrier group comprised 20 members of families with the Li–Fraumeni syndrome who carried a variety of TP53 mutations but were otherwise healthy, without a diagnosis of active cancer (Table S1 in the Supplementary Appendix). Controls were approximately matched with the TP53 mutation carriers with respect to age, sex, body-mass index, and physical activity level (Table 1).
Table 1.
Baseline Characteristics of Controls and TP53 Mutation Carriers.*
| Variable | Controls (N = 20) |
Carriers (N = 20) |
P Value |
|---|---|---|---|
| Age (yr) | 40±15 | 38±13 | 0.57 |
| Female sex (%) | 55 | 60 | 1.00 |
| Body-mass index† | 26.9±4.2 | 27.2±5.3 | 0.83 |
| Physical-activity level‡ | 3.1±1.2 | 3.3±1.2 | 0.59 |
| Weight lifted in foot exercise (kg)§ | 3.4±1.2 | 3.3±1.5 | 0.88 |
Plus–minus values are means ±SD. The control group consisted of 9 healthy volunteers and 11 members of families with the Li–Fraumeni syndrome who were not carriers of the TP53 mutation.
Body-mass index is the weight in kilograms divided by the square of the height in meters.
Physical-activity level was self-rated by each study participant and ranged from 1 (inactive, with no regular physical activity) to 5 (vigorously active, with physical activity at least 4 days a week for 60 minutes or more each time).
The weight lifted represented 30% of the maximum weight against which a participant could dorsiflex one foot before testing.
Recovery of Phosphocreatine
During exercise, the amount of weight lifted by the two groups was similar (Table 1), but the median recovery Tc for phosphocreatine levels in the carrier group was 28.7 seconds (mean [±SD], 31.1±6.7 seconds), which was significantly shorter than that for the noncarrier group, at 36.7 seconds (mean, 36.7±6.6 seconds) (P = 0.01 by the Mann–Whitney test) (Fig. 1B).
Mitochondrial Function
Mitochondrial function was examined in cultured lymphocytes and myoblasts from the members of families with the Li–Fraumeni syndrome. As compared with noncarriers (who had wild-type TP53), carriers of TP53 mutations had increased oxygen consumption (Fig. 1C). In one family with the Li–Fraumeni syndrome, multiple members had the same mutation (TP53 codon R181C); mitochondrial respiration was significantly increased in R181C myoblasts and was associated with increased levels of mitochondrial respiratory complex proteins (Fig. S3 in the Supplementary Appendix). In other studies, p53 has been shown to regulate mitochondrial biogenesis by means of several factors, including mitochondrial transcription factor A (TFAM) and the synthesis of cytochrome c oxidase 2 (SCO2).5,6 Notably, both TFAM and SCO2 were detected at consistently higher levels in myoblasts from family members who carried the TP53 R181C mutation or the hotspot R273H mutation than in myoblasts from noncarrier family members (Fig. 1D).
Mouse Model
Although the data from the study participants indicated that the TP53 mutations associated with the Li–Fraumeni syndrome can promote oxidative metabolism by means of increased mitochondrial biogenesis in a cell-autonomous manner, we sought to substantiate the metabolic phenotype in a homogeneous genetic background, using an established mouse model of the syndrome.12 Treadmill testing of mice that were heterozygous (RH) or homozygous (HH) for the p53 R172H mutation (which is homologous to the human hotspot R175H mutation in the Li–Fraumeni syndrome) revealed a gene dose-dependent increase in endurance during exercise as compared with wild-type (RR) mice with a similar body-mass composition (Fig. 1E, and Fig. S4A in the Supplementary Appendix). Basal metabolic measurements revealed a lower respiratory exchange ratio during periods of increased activity in the homozygous mice (Fig. S4B in the Supplementary Appendix), indicating a propensity for metabolizing fatty acids that not only contributes to endurance during exercise but is also thought to play a role in tumorigenesis.13–15 Mitochondrial oxygen consumption and levels of respiratory-complex proteins in skeletal muscle were increased in the mice with the p53 R172H mutation, as compared with the wild-type mice (Fig.1F and 1G), findings that were consistent with those in the study participants. The messenger RNA levels of the mitochondrial biogenesis genes TFAM and SCO2 were also increased in cells from mice with the Li– Fraumeni syndrome, suggesting the presence of a transcriptional regulatory mechanism (Fig. S5 in the Supplementary Appendix).
Discussion
The major finding of our study is that in persons with a germline mutation in TP53, the recovery Tc for phosphocreatine levels in skeletal muscle after exercise is significantly shorter than it is in controls. This finding is consistent with an increased capacity for oxidative phosphorylation in mutation carriers. Our in vivo data from members of families with the Li–Fraumeni syndrome are supported by mitochondrial studies of cells isolated from family members and studies in a mouse model of the syndrome. Our findings contrast with the slower recovery of phosphocreatine levels observed in patients with primary mitochondrial myopathies but are consistent with the role of p53 in regulating mitochondrial respiration.3,8 Alterations in normal p53 activity have been associated with increased oxidative stress, largely attributable to the mitochondria, which could result in oxidative DNA damage, possibly leading to genomic instability and, ultimately, the development of cancer.16–18 It has recently been reported that in human carriers of the TP53 R337H mutation who have the Li–Fraumeni syndrome or a similar syndrome, there is evidence of increased oxidative damage in plasma, which is thought to be due to deregulated cell bioenergetics, inflammation, or both.19
Whether this metabolic phenotype contributes to tumorigenesis in persons with the Li–Fraumeni syndrome remains to be determined. Our study was not designed to demonstrate a causal linkage between the gain of function in oxidative metabolism and tumorigenesis in the Li–Fraumeni syndrome. However, our study does show that TP53 mutations in persons with the Li–Fraumeni syndrome can increase oxidative metabolism through the mitochondria in a cell-autonomous manner. Recent studies of the role of oxidative substrates, TFAM, and other mitochondrial proteins in tumorigenesis have in fact highlighted the importance of mitochondrial metabolism.15,20–23 Thus, such a gain-of-function mutation could provide cancer cells with survival and proliferative advantages. Nonetheless, it will be challenging to isolate this metabolic effect of mutated p53 from its many other cellular activities to show that it has a direct effect on tumorigenesis, especially in light of the cellular complexities introduced by factors such as mitochondrial signaling through reactive oxygen species and the metabolic environment of cancer cells.20,24
In the group of study participants who carried the TP53 mutation, we were not able to detect a significant difference in the recovery Tc of phosphocreatine levels between persons with and those without a history of cancer, but the interpretation of this result could be limited by the small size of our exploratory study (Fig. S6 in the Supplementary Appendix). The small number of participants also precluded our ability to draw conclusions about a close correlation between the recovery Tc for phosphocreatine levels and TP53 mutations, since there were only two families with more than two carriers (Table S1 in the Supplementary Appendix). Larger studies examining a variety of mutations related to the Li–Fraumeni syndrome in multiple families with the same mutation are needed to address these important questions. In light of the expanding role of the mitochondria in tumorigenesis, it is tempting to speculate that patients with the Li–Fraumeni syndrome may have a good response to metabolic interventions because of their increased mitochondrial respiration. For example, metformin, which has been shown to prevent cancer, inhibits mitochondrial activity.25
The increase in oxidative metabolic capacity caused by germline mutations in TP53 has implications not only for tumorigenesis but also for normal physiology. The presence of p53-like sequences has been reported in simple life forms and in a number organisms that are normally not subject to tumor development.26 Thus, it has been proposed that before its co-option as a tumor suppressor, p53 may have served basic adaptive functions for cell survival. The abundance of different germline TP53 mutations reported to date makes it still more tempting to speculate that they may have provided thermogenic or bioenergetic advantages for survival in adverse climates or in environments with a limited supply of nutrients. Given the crucial role of p53-regulated metabolism in tumorigenesis, beyond its cell-cycle activities,7 the current finding of the metabolic gain of function in the Li–Fraumeni syndrome provides impetus for continuing to explore metabolic strategies directed at cancer prevention.
Supplementary Material
Acknowledgments
Supported by the Division of Intramural Research, National Heart, Lung, and Blood Institute and by a National Institutes of Health Bench-to-Bedside Award (to Drs. Hwang and Strong).
We thank the members of all the families with the Li–Fraumeni syndrome and other volunteers for making this clinical study possible; Jamie Grimes for clinical protocol administration; Brenda Holbrook, Jennifer Audibert, Suzanne McGehee, and Joyce Linderman for patient care; Kevin Smith for cardiopulmonary treadmill exercise testing; Renee Hill and Hellmut Merkle for assistance with MRS data acquisition and hardware, respectively; Audrey Noguchi and Danielle Springer of the National Heart, Lung, and Blood Institute Mouse Phenotyping Core for assistance with mouse metabolic characterization; Martin Pelletier and Richard M. Siegel for assistance and the use of their Seahorse XF96 analyzer; Myron A. Waclawiw for statistical advice; and Michael N. Sack and Toren Finkel for helpful advice and critical comments.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Malkin D, Li FP, Strong LC, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250:1233–1238. doi: 10.1126/science.1978757. [Erratum, Science 1993:259–878.] [DOI] [PubMed] [Google Scholar]
- 2.Schneider K, Garber J. Li-Fraumeni syndrome. In: Pagon RA, Bird TD, Dolan CR, Stephens K, Adam MP, editors. Gene Reviews. Seattle; University of Washington: 2010. (Internet). [Google Scholar]
- 3.Matoba S, Kang JG, Patino WD, et al. p53 Regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 4.Bensaad K, Tsuruta A, Selak MA, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 5.Gottlieb E, Vousden KH. p53 Regulation of metabolic pathways. Cold Spring Harb Perspect Biol. 2010;2(4):a001040. doi: 10.1101/cshperspect.a001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lago CU, Sung HJ, Ma W, Wang PY, Hwang PM. p53, Aerobic metabolism and cancer. Antioxid Redox Signal. 2011;15:1739–1748. doi: 10.1089/ars.2010.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li T, Kon N, Jiang L, et al. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012;149:1269–1283. doi: 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chance B, Im J, Nioka S, Kushmerick M. Skeletal muscle energetics with PNMR: personal views and historic perspectives. NMR Biomed. 2006;19:904–926. doi: 10.1002/nbm.1109. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa Y, Ratkevicius A, Mizuno M, Quistorff B. ATP economy of force maintenance in human tibialis anterior muscle. Med Sci Sports Exerc. 2005;37:937–943. [PubMed] [Google Scholar]
- 10.Argov Z, Löfberg M, Arnold DL. Insights into muscle diseases gained by phosphorus magnetic resonance spectroscopy. Muscle Nerve. 2000;23:1316–1334. doi: 10.1002/1097-4598(200009)23:9<1316::aid-mus2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 11.Balady GJ, Arena R, Sietsema K, et al. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 12.Lang GA, Iwakuma T, Suh YA, et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell. 2004;119:861–872. doi: 10.1016/j.cell.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Kiens B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev. 2006;86:205–243. doi: 10.1152/physrev.00023.2004. [DOI] [PubMed] [Google Scholar]
- 14.Nomura DK, Long JZ, Niessen S, Hoover HS, Ng SW, Cravatt BF. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nieman KM, Kenny HA, Penicka CV, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Cao L, Chen J, et al. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature. 2009;459:387–392. doi: 10.1038/nature08040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sung HJ, Ma W, Wang P-Y, et al. Mitochondrial respiration protects against oxygen-associated DNA damage. Nat Commun. 2010;1:5. doi: 10.1038/ncomms1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macedo GS, Lisbôa da Motta L, Giacomazzi J, et al. Increased oxidative damage in carriers of the germline TP53 p.R337H mutation. PLoS One. 2012;7(10):e47010. doi: 10.1371/journal.pone.0047010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinberg F, Hamanaka R, Wheaton WW, et al. Mitochondrial metabolism and ROS generation are essential for Krasmediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [Erratum, Nat Rev Cancer 2011:11–618.] [DOI] [PubMed] [Google Scholar]
- 22.Caro P, Kishan AU, Norberg E, et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell. 2012;22:547–560. doi: 10.1016/j.ccr.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marin-Valencia I, Yang C, Mashimo T, et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 2012;15:827–837. doi: 10.1016/j.cmet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ertel A, Tsirigos A, Whitaker-Menezes D, et al. Is cancer a metabolic rebellion against host aging? In the quest for immortality, tumor cells try to save themselves by boosting mitochondrial metabolism. Cell Cycle. 2012;11:253–263. doi: 10.4161/cc.11.2.19006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov. 2012;2:778–790. doi: 10.1158/2159-8290.CD-12-0263. [DOI] [PubMed] [Google Scholar]
- 26.Lu WJ, Amatruda JF, Abrams JM. p53 Ancestry: gazing through an evolutionary lens. Nat Rev Cancer. 2009;9:758–762. doi: 10.1038/nrc2732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.