Abstract
Introduction
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are the acute onset of non-cardiac respiratory insufficiency associated with bilateral lung infiltrations. During the past decade, mechanical ventilation strategies using low tidal volumes have reduced the mortality of ALI/ARDS to around 20-40%. However, ALI/ARDS continues to be a major factor in global burden of diseases, with no pharmacologic agents currently available.
Areas covered
In this review we discuss several inflammatory proteins involved in the molecular pathogenesis of ALI/ARDS. The complement cleavage product, C5a, is a peptide acting as a potent anaphylatoxin. C5a may trigger the formation of neutrophil extracellular traps (NETs) and release of histone proteins to the extracellular compartment during ALI/ARDS.NETs may activate platelets to release TGFβ which is involved in tissue remodeling during the later phases of ALI/ARDS. Interception of C5a signaling or blockade of extracellular histones has recently shown promising beneficial effects in small animal models of ALI/ARDS.
Expert opinion
Novel protein-based strategies for the treatment of ALI/ARDS may inspire the hopes of scientists, clinicians and patients. While neutralization of extracellular histones / NETs, C5a and TGFβ is effective in experimental models of ALI/ARDS, controlled clinical trials will be necessary for further evaluation in future.
Keywords: Neutrophil extracellular traps, extracellular histones, inflammation, antibody, C5a, TGFβ
1. Introducing the problem
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are defined by the following diagnostic criteria: acute onset, bilateral lung infiltrates on chest X-rays and respiratory failure with exclusion of cardiac etiologies. Respiratory failure is due to pulmonary edema, resulting in diminished gas diffusion capacity. This is detectable by reduced ratios of arterial oxygen tension (paO2) and fraction of inspired oxygen (FiO2). In this nomenclature, ALI (paO2/FiO2 < 300 mmHg) is defined as the less severe form of respiratory insufficiency as compared to ARDS (paO2/FiO2 < 200 mmHg). In 2012, the novel ‘Berlin definition’ of ARDS was introduced, which classifies three categories of ARDS depending on the degree of hypoxemia: mild (paO2/FiO2 < 300 mmHg), moderate (paO2/FiO2 < 200 mmHg), and severe (paO2/FiO2 < 100 mmHg) 1.
It is estimated that 200,000 cases of ALI/ARDS occur annually in the United States 2, 3. The overall survival rates of ALI/ARDS are around 20-40% 4-7. The prognosis of survival is better for mild ARDS as compared to severe ARDS with an intermediate risk for moderate ARDS (according to the ‘Berlin definition’) 1. During the past two decades improved survival rates of ALI/ARDS have been accomplished 8. Most likely this is attributable to advances in critical care medicine. In particular, novel mechanical ventilation schemes using low tidal volumes (e.g. 6 ml/kg body weight) have reduced the risk of ventilation-induced pulmonary barotrauma and significantly increased survival rates and ventilator-free days in patients with ALI/ARDS 4.
A long list of predisposing factors may raise the risk for the development of ALI/ARDS. Clinical conditions that frequently precipitate ALI/ARDS include pneumonia, sepsis, fluids aspiration, severe trauma, massive transfusions and inhalation burns.
The pathophysiology of ALI/ARDS is characterized by initial injury (infection, chemical, mechanical) of the small airways (alveolar epithelium, lung endothelium) 8. Lung tissue injury initiates acute inflammation of the alveoli and bronchioles 9. This includes activation of Toll-like receptors, the complement system, plasmatic coagulation pathways as well as activation of platelets, alveolar macrophages, adjacent endothelial cells and type II alveolar epithelial cells 10, 11. The activation of coagulation pathways creates intra-alveolar fibrin depositions (‘hyaline membranes’). Chemotactic migration of innate immune cells to these sites results in the accumulation of polymorphonuclear neutrophils (PMNs) in the alveolar spaces within a few hours, while adaptive immune cells such as T lymphocytes mainly participate during the later phases of ALI/ARDS 12, 13. PMNs are phagocytic cells and clear infectious microorganisms and debris from the alveolar spaces. Furthermore, PMNs release cytotoxic granules and reactive oxygen species 14, 15.
Immune cells (PMNs, macrophages, T cells) and resident lung cells (epithelium, endothelium, fibroblasts) communicate by the release of a plethora of cytokines / chemokines. IL-1β, TNFα, Interferon-γ and IL-6 orchestrate and perpetuate lung inflammation during ALI/ARDS 16-18. These inflammatory cytokines also promote plasmatic coagulation in the small airways, for example via up-regulation of tissue factor in alveolar epithelial cells 19.
While these inflammatory responses are designed to provide pathogen clearance and restore homeostasis of the lung, they may also cause additional injury in the setting of ALI/ARDS. Loss of barrier function of the epithelial-endothelial interface in alveoli is considered a hallmark of ALI/ARDS. Apoptosis, necrosis and necroptosis of endothelial cells and type I alveolar epithelial cells results in disintegration of the alveolar lining (e.g. disruption of vascular-endothelial cadherin bonds), which is followed by influx of alveolar edema fluid and hemorrhage 11, 20, 21. Subsequently, pulmonary gas diffusion capacity is compromised leading to hypoxemia and respiratory failure. The later phases (>7 days) of ALI/ARDS may be dominated by proliferative and fibrotic responses. The mechanisms that distinguish healing and full recovery from ALI/ARDS versus progression to interstitial inflammation and fibrotic lung disease are currently not completely known, but may involve mesenchymal stem cells 22, 23.
To date clinical trials using pharmacologic approaches for the treatment of ALI/ARDS have largely failed to demonstrate beneficial effects. The primary endpoints of most studies have been survival at 28 days (or longer) and reduction of days of mechanical ventilation (ventilator-free days) in ALI/ARDS patients. Protein-based strategies have tested the administration of recombinant human proteins such as activated protein C (drotrecogin α), granulocyte macrophage colony stimulating factor (GM-CSF) and surfactant protein C based agents to ALI/ARDS patients 24-26. Additional trials have used methylprednisolone, nitric oxide, β2-adrenergic receptor agonists (albuterol, salbutamol), and antioxidants (N-acetylcysteine) 7, 27-30. All of these pharmacologic interventions were unable to demonstrate significant clinical efficacy (Table 1). Therefore, there remains a desperate need for better understanding of the pathophysiology of ALI/ARDS. Such knowledge will be helpful to direct future clinical trials testing novel strategies to ameliorate the adverse outcomes of ALI/ARDS. In this review, we will discuss recent progress in identifying potential target proteins, which may be key factors during the unfavorable course of ALI/ARDS.
TABLE 1.
Selected clinical trials without benefit in patients with ALI/ARDS
| References | ARDS patients | Pharmacologic intervention | Result |
|---|---|---|---|
| 25 | n = 130 | Recombinant GM-CSF | No differences in 28-day survival or ventilator-free days |
| 24 | n = 75 | Recombinant Activated Protein C | No differences in 60-day survival or ventilator-free days |
| 26 | n = 448 | Recombinant Surfactant Protein C | No differences in 28-day survival or ventilator-free days |
| 7 | n = 282 | β2-Agonists (inhalation) | No difference in ventilator-free days or survival before hospital discharge |
| 27 | n = 324 | β2-Agonists (intravenous) | No differences in 28-day survival |
| 116 | n = 385 | Nitric oxide (inhalation) | No differences in survival and ventilator-free days |
| 117 | n = 348 | Prostaglandin E1 (Liposomes) | No differences in 28-day survival or ventilator-free days |
| 28 / 30 | n = 99 / n = 180 | Methylprednisolone | No differences in 45-day survival / No differences in 180-day survival |
| 6 | n = 272 | Omega-3-fatty acids | No differences in 60-day survival |
| 29 | n = 46 | N-Acetylcysteine / Procysteine | No differences in survival |
2. Potential protein targets in Acute Lung Injury
2.1. Blocking of complement component C5a in acute lung injury
The complement system is an ancient part of the innate immune system, which is found in all vertebrates and many invertebrate species 31. In analogy to the coagulation system, complement proteins constitute a cascade of proteases with the capacity for specific cleavage of its factors during activation. The classical, alternative, MB-lectin and ficolin pathways may all initiate activation of complement during ALI/ARDS 32. The major effector functions of complement are direct lysis (membrane-attack complex) and opsonization (C3b) to facilitate phagocytosis of targeted cells (e.g. microbes). In addition, an abundant generation of chemotactic complement anaphylatoxins (C3a, C4a, C5a) occurs, which subsequently activate innate and adaptive immunity. C5a and C3a activate PMNs, macrophages and promote chemotactic migration of those cells to the alveolar spaces during ALI/ARDS 17, 33. C5a and C3a are substrates for rapid enzymatic cleavage of the C-terminal arginine residue by plasma carboxypeptidase R and carboxypeptidase N 34. The cleavage product, C5adesArg, displays a 10-fold lower biological activity as compared to C5a, while C3adesArg is regarded as completely inactive 35. C5a has the strongest biological potency as compared to C3a and C4a. C5a binds with high affinity to its two receptors, which have been termed C5aR (CD88; C5aR1) and C5L2 (GPR77; C5aR2) 36, 37. Both receptors, C5aR and C5L2, are encoded in vicinity on chromosome 19 in humans (mouse: chromosome 7), contain the structural motifs of seven-transmembrane spanning domains and display an amino acid homology of around 38% 38.
The first evidence that C5a is involved in the development and progression of ALI/ARDS was uncovered several decades ago 39, 40. Administration of cobra venom factor (CVF), which activates complement in circulation, resulted in PMN-dependent ALI 41. CVF is a three chain protein and functional analog of C3b, which is purified from cobra venom or recombinantly expressed 42. In plasma, CVF assembles with other complement proteins to form a C3/C5 convertase complex resulting in uncontrolled complement activation (including generation of C3a and C5a) and, ultimately, consumption of complement components.
The effects of C5a are partly mediated by the production of reactive oxygen species in PMNs (respiratory burst) 41. Administration of blocking anti-C5a antibody protects non-human primates from sepsis-induced ALI and mortality following live E. coli challenge 43. The complement degradation products, C5adesArg and C3adesArg, are detectable in blood of human patients with ALI/ARDS and sepsis, indicating complement activation 44. Gene-targeted disruption of the C5aR receptor reduces the severity of immune-complex-induced ALI in mice 45. Vitamin D binding protein deficient mice are protected against C5a-induced ALI and influx of inflammatory cells 46, while serum Vitamin D concentrations appear not to influence the severity of LPS-ALI 47.
C5a-signaling in alveolar macrophages is modulated by urokinase-type plasminogen activator and its receptor 48. C5 may play a profibrotic role in chronic stages of ALI, as studied in the bleomycin-induced lung injury model using C5-deficient mice 49. While in the acute phase of ALI the C5aR receptor clearly promotes inflammation 17, 45, the function of the C5L2 receptor is still controversial. We have recently reported that the C5L2 receptor is involved in promoting inflammation and disruption of the alveolar/epithelial barrier during ALI, while an earlier report has described an anti-inflammatory role of C5L2 during immune-complex ALI 17, 50.
In summary, C5a (via C5aR and C5L2) recruits PMNs and other inflammatory cells to the alveolar spaces during ALI 32. The intracellular effects of C5a are related to the activation of Ca2+-currents as well as activation of PI3K/Akt and MAPK signaling pathways 32, 51. Only recently, the down-stream effects of C5a-induced tissue injury have been associated with the appearance of neutrophil extracellular traps (NETs) 17. These findings may suggest a close pathophysiological link of C5a and NETs in the setting of ALI.
2.2 Targeting neutrophil extracellular traps (NETs) in acute lung injury
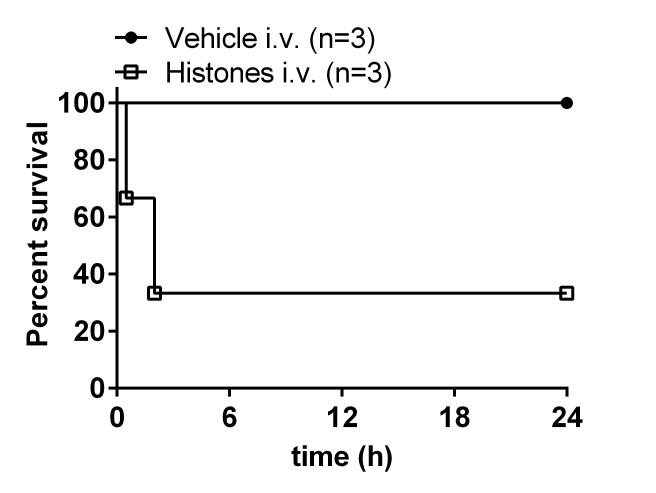
Accumulating evidence suggests the biological relevance of neutrophil extracellular traps (NETs) for the pathogenesis of inflammatory diseases. NETs are structures of nuclear chromatin, which contain DNA, histone proteins and other microbicidal / nuclear proteins. NETs are an effector defense mechanism of innate immunity 52, 53. PMNs may undergo programmed cell death and actively release their nucleus to create NETs 54. The process of formation of NETs may occur either in blood circulation or following chemotactic migration of PMNs to the local site of inflammation. NETs and their major component, extracellular histones, ensnare and kill bacteria in septic blood 55. Similarly, NETs are capable of killing Candida species 56. PMNs invading tissues form NETs following encountering Aspergillus fumigatus in vitro and during lung infection 57, 58. Furthermore, certain lung pathogens such as Streptococcus pneumonia have evolved counter strategies such as genes encoding for endonucleases to escape killing by NETs 59. This demonstrates that NETs are a part of the complex host-pathogen interactions, which have formed during evolution. While NETs may have been evolved to clear infectious pathogens, NETs may also cause adverse tissue injury to the host. Extracellular histones (the major components of NETs) are highly toxic and induce respiratory failure when infused intravenously into healthy research animals (Figure 1) 60. The cytotoxic activity of extracellular histones / NETs is in line with the fact, that several other intra-cellular proteins (e.g. HMGB1, hemoglobin) have detrimental effects following release to the extracellular compartment 61, 62.
Figure 1.
Infusion of extracellular histones (75 mg/kg body weight i.v.) purified from calf thymus mediates lethality in C57BL/6J mice. Death of mice was preceded by clinical signs of respiratory failure. This figure shows data by Bosmann and Ward, which are consistent with reported findings by Xu et al. 60.
Many factors typically present during ALI/ARDS have the potential do induce NET-formation. For instance, live bacteria, LPS, IL-8 or reactive oxygen species (ROS) may all trigger the appearance of NETs 54. The generation of NETs is an active process and requires an intra-cellular signaling program. Engagement of Raf-MEKERK kinase pathways occurs during NET formation 63. In addition, mammalian target of rapamycin (MTOR) and hypoxia inducible factor 1 (HIF-1) regulate the formation of NETs 64. The down-stream events of the aforementioned signaling pathways include chromatin decondensation, which is a prerequisite for NET generation. This is accomplished by enzymatic hypercitrullination of core histone proteins 65, 66.
When NETs derived from activated human PMNs are incubated with mouse or human cell lines of lung epithelial cells, NETs induce cell death of such epithelial cells 67. NETs are also cytotoxic for lung endothelial cells 60, 67. Components of NETs (MPO/DNA/histones) are detectable in broncho-alveolar lavage fluids (BALF) and lung sections by immunofluorescence microscopy of mice following LPS-induced ALI 67.
The major studies which have investigated the role of NETs (DNA/histones) during ALI/ARDS are summarized in Table 2. In experimental transfusion-related acute lung injury (TRALI), NETs are detectable in the lung microcirculation by immunofluorescence microscopy 68. In this study, NET formation in TRALI lungs was prevented by inhibition of platelet aggregation using acetylsalicylic acid 68. The blockade of NETs by administration of neutralizing antibodies directed against extracellular histones reduced lung vascular permeability and the volume of extravascular lung water during TRALI 68. Similarly, in vivo degradation of NET-derived DNA structures using DNAse1 reduced the severity of lung injury and mortality in the murine TRALI model 68. Moreover, myeloperoxidase (MPO)/DNA aggregates as makers of NETs were elevated in plasma samples of human patients with TRALI as compared to healthy controls 68. In addition, extracellular histones were co-localized with MPO and DNA in lung tissue sections of TRALI patients 68.
TABLE 2.
Studies on the role of NETs / extracellular histones during ALI
| Reference | Species | Disease Model | Major findings |
|---|---|---|---|
| Caudrillier et al. 68 |
M. musculus, H.
sapiens |
Transfusion- induced ALI (TRALI), clinical samples |
-Detection of NETs in lungs and plasma of ALI patients -Blockade of extracellular histones is protective in TRALI -Activated platelets induce NETs during TRALI |
| Abrams et al. 69 |
H. sapiens, M.
musculus |
Clinical samples | -Circulating histones in patients with non-thoracic trauma increase the risk for ALI -Histones induce cytokine release -Histones are toxic for endothelial cells |
| Bosmann et al. 17 |
M. musculus, H.
sapiens |
C5a-induced ALI, clinical samples |
-C5a mediates appearance of extracellular histones in lungs during ALI -Blockade of histones is protective in complement-induced ALI -Intra-tracheal administration of purified histones precipitates symptoms of ALI -Detection of extracellular histones in BALF of patients with ALI |
| Saffarzadeh et al. 67 |
H. sapiens, M.
musculus |
LPS-induced ALI, cell cultures |
-NETs are cytotoxic for human alveolar epithelial cells and endothelial cells |
Trauma-associated ALI/ARDS has been recently associated with extracellular histones in blood circulation 69. In patients with severe nonthoracic blunt trauma, levels of circulating nucleosomes and extracellular histones increase with injury severity scores 69. When purified histones are infused intravenously in mice, the lung is the most susceptible organ, showing histological signs of inflammation and microvascular thrombi 17, 69. This observation may be explained by the fact, that the lungs contain the first capillary bed following intravenous administration of any substance. In response to severe trauma, extracellular histones may be released by dying cells other than PMNs. Circulating histones may be transported via the venous blood flow to the lung endothelium, resulting in extensive lung injury. In fact, it has been described, that extracellular histones derived from dying parenchymal cells rather than NETs can aggravate organ dysfunction 70. Neutralizing anti-histone single chain variable fragments (scFv) have been used to suppress histone-induced toxicity and reverse coagulation activation 69.
Treatment of C57BL/6J mice with antibodies targeting histone H4 and H2A is protective in a model of C5a-induced ALI (Figure 2) 17. Extracellular histones are released in BALF during ALI, when induced by LPS, recombinant C5a or IgG immune-complexes 17. In all three models, the appearance of extracellular histones requires the presence of the C5a receptors, C5aR and C5L2 17. Extracellular histones and nucleosomes are also detectable in BALF of around 50% of human patients suffering von ALI/ARDS 17. Extracellular histones mediate the intracellular influx of Ca2+ in type II alveolar epithelial cell lines, most likely related to provoking cell death 17. In rats, the direct administration of purified histones to the airways results in severe respiratory acidosis, compromised respiratory excursions, pulmonary edema and acute lung inflammation 17. Histones induce the release of a wide spectrum of inflammatory mediators such as IL-1β, TNFα, IL-6, Eotaxin, G-CSF, KC, MCP-1, MIP-1α, MIP-1β and RANTES 17, 69. Extracellular histones may directly or indirectly recruit the activity of TLR2 and TLR4 receptors for promoting inflammation 71. In influenza A virus mediated ALI, the accumulation of PMNs, NETs and lung epithelial injury was reduced by a potent arthropod-derived C5-binding inhibitor of complement activation (OmCI) 72.
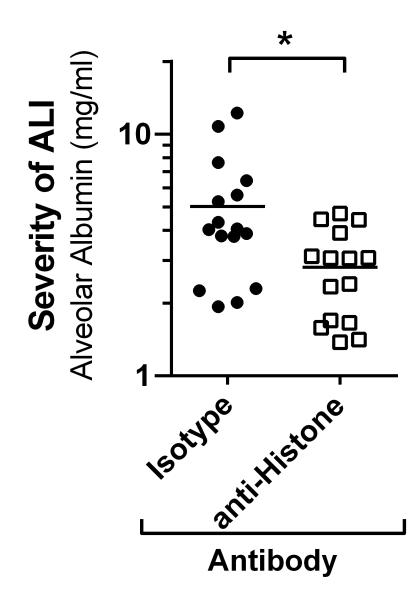
Figure 2.
Neutralization of extracellular histones using monoclonal anti-H4 antibody reduces the severity of ALI in C57BL/6J mice. ALI was induced by intra-tracheal administration of recombinant mouse C5a (500 ng/mouse). Groups of mice were treated with either anti-Histone antibody (300 μg/mouse) or non-specific matched isotype IgG1κ antibody (300 μg/mouse). Severity of ALI was determined by quantification of albumin (ELISA) in broncho-alveolar lavage fluids after 8 h. Data taken from 17).
Intra-alveolar hemorrhage is a typical histologic finding of ALI/ARDS. A growing list of evidence suggests the interactions of NETs / extracellular histones with the coagulation system. The major components of NETs, extracellular histones and DNA, are detectable in venous thrombus formations 73. In detail, NETs can activate the intrinsic plasmatic coagulation pathway by direct interaction with coagulation factor XII, resulting in generation of the fibrin clot 74. During severe sepsis, platelets are activated via their TLR4 receptor and bind to PMNs, which triggers the formation of NETs in pulmonary capillaries 55. On the other hand, platelets can bind to preformed NETs. In fact, NETs provide a scaffold for platelet aggregation under shear stress, thereby promoting thrombosis 73. Extracellular DNA and histones are detectable during acute thrombotic microangiopathies and may potentially precipitate the formation of microthrombi 75. In co-incubation studies of human PMNs with platelets, the formation of NETs was induced, when platelets were pre-activated using proteinase-activated receptor (PAR-1) agonist or thrombin receptor-activating peptide (TRAP) 68.
Platelets are a major source of TGFβ in plasma. NET-induced platelet activation would be expected to promote the release of TGFβ from the α-granules of platelets. As highlighted below, TGFβ is considered a critical factor especially during the later stages of ALI that determine tissue repair and remodeling.
2.3 Manipulation of transforming growth factor-beta in acute lung injury
Transforming growth factor-beta (TGFβ) is a highly conserved cytokine with several existing isoforms (TGFβ1, TGFβ2, TGFβ3) 76. TGFβ typically forms homodimers.
The release of TGFβ occurs as a large latent protein complex (LLC) consisting of TGFβ, latency-associated peptide (LAP) and latent TGFβ-binding protein (LTBP) 77. The LLC binds to components of the extracellular matrix. Latent TGF can also bind to glycoprotein A repetitions predominant (GARP) expressed on regulatory T cells 78.
The multiple molecular events transforming latent TGFβ into active TGFβ are not completely understood. One mechanism of activation is dependent on integrins such as αVβ6 integrin, which is expressed by lung epithelial cells 79. In addition, integrin-independent activation steps may include reactive oxygen species, pH reduction (acidification), metallo-proteases (e.g. MMP-2, MMP-9, MMP-14) and thrombospondin-1 80-82.
TGFβ initiates intracellular signaling by binding to the tetrameric TGFβ receptor complex. This complex is composed of two homodimers of TGFβ receptor I (TGFbRI) and two homodimers of TGFβ receptor II (TGFbRII) 83. Initially, TGFβ binds to TGFbRII and subsequently TGFbRI is recruited. Both receptors have intracellular domains with serine/threonine kinase activity. In addition, a TGFbRIII is required for optimal signaling of the TGFβ2 isoform 84. For intracellular signaling, TGFβ recruits activin receptor-like kinases (ALK1, ALK5), that subsequently phosphorylate SMAD proteins 85, 86. In addition, non-Smad-dependent signaling pathways such as p38 MAPK and DAXX are activated 87, 88.
Mice with targeted genetic disruption of TGFβ1 die prematurely from spontaneous generalized inflammation 89. TGFβ is a central player of acute lung injury 79, 90. A soluble chimeric TGFβ type II receptor has the activity of reducing pulmonary edema fluid following LPS-induced ALI 79. In monolayers of type II alveolar epithelial cells, recombinant TGFβ decreases the transepithelial resistance via depletion of the intracellular amounts of the reduced tripeptide, glutathione 79. A genetic defect in TGFβ activation, as present in mice with deficiency of αVβ6 integrin, results in reduced pulmonary edema and lung epithelial permeability during ALI 79. Furthermore, TGFβ1 directly regulates the transport of ions and water in type II alveolar epithelial cells by MAPK-dependent and ERK1/2-dependent suppression of ion transporter proteins such as the epithelial sodium channel alpha subunit (alphaENaC) 91. In addition, wounded epithelial cell monolayers release HMGB1, that appears to accelerates healing of the alveolar lung epithelium via αvβ6-dependent activation of TGFβ1 92.
TGFβ is a prototypic cytokine for promoting pulmonary fibrosis 93, 94. TGFβ facilitates the proliferation and chemotaxis of fibroblasts 95. The local transformation of fibroblasts or lung epithelial cells into myofibroblasts is promoted, while the apoptosis of myofibroblasts is limited by TGFβ 96-98. Myofibroblasts are important for the production of extracellular matrix components and produce additional TGFβ in a positive feedback loop 99. TGFβ reduces the activity of matrix metallo-proteases and other matrix-degrading proteases 100. In addition, TGFβ facilitates the production of several other profibrotic cytokines such as platelet-derived growth factor (PDGF) in vascular endothelial cells 101.
In summary, TGFβ may be especially important during the later profibrotic stages of ALI/ARDS, although there is also the view that TGFβ might be active early in ALI 102. Blockade of TGFβ activation (e.g. anti-αvβ6 integrin antibody) or modulation of TGFβ signaling (e.g. ALK5 inhibitors) are currently evaluated as therapeutic strategies to prevent adverse tissue remodeling and pulmonary fibrosis 103, 104.
3. Conclusion
In this review, we have discussed three potential protein targets for future therapies of ALI/ARDS (Figure 3). The complement fragment, C5a, NETs and TGFβ orchestrate the development and progression of ALI/ARDS at different stages of disease. C5a is considered an early proinflammatory mediator of the innate immune system. Accumulating evidence suggest, that NETs are an integral effector mechanism of immunity but may mediate adverse outcomes of ALI/ARDS. Extracellular histones and DNA are major components of NETs, but may also be passively released by lung epithelial or endothelial cells. Finally, the later profibrotic phases of ALI/ARDS may involve TGFβ, resulting in healing (restoration of homeostasis) or progression to fibrosis with impaired organ function. Therapeutic modulation of the activity of C5a, NETs or TGFβ (e.g. by specific neutralizing antibodies) may be helpful in future for patients with ALI/ARDS. However, the clinical efficacy and feasibility of such strategies remains to be seen.
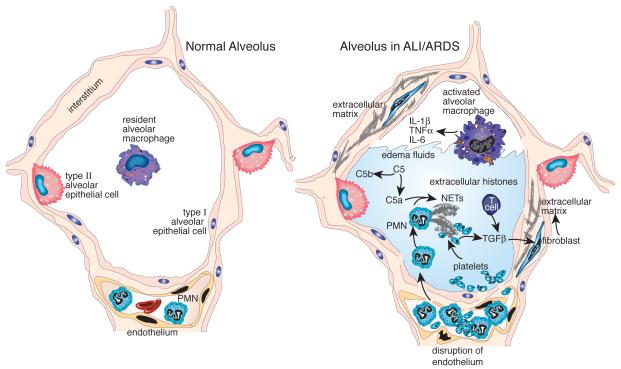
Figure 3.
Current concepts on the pathophysiologic mechanisms of ALI/ARDS involving extracellular histones / NETs, C5a and TGFβ. The frame on the left shows the architecture of the alveolus, which is composed of type I and type II alveolar epithelial cells, resident intra-alveolar macrophages and adjacent lung capillaries with intact endothelial lining. The frame on the right displays the injured alveolus in ALI/ARDS: Complement activation products (C5a) and inflammatory mediators released by activated macrophages orchestrate the influx of PMNs, monocytes and adaptive immune cells to the alveolar compartment. C5a promotes release of NETs and extracellular histones, thereby resulting in tissue damage and disruption of the epithelial/endothelial barrier. Intra-alveolar hemorrhage includes the presence of platelets, which interact with NETs and release TGFβ. The later phases of ALI/ARDS may include TGFβ-mediated fibro-proliferative responses and accumulation of extracellular matrix.
4. Expert opinion
The improvement in survival rates of ALI/ARDS which has been observed during the past 1-2 decades are mainly explained by advances in supportive critical care medicine 8. The optimization of mechanical ventilation strategies has been clearly beneficial. On the other hand, even with best supportive care, death occurs in at least 20% of patients with ALI/ARDS. To date all clinical trials investigating novel or traditional pharmacologic substances have failed to show an advantage in the treatment of lung injury. Despite these sobering results, emerging experimental studies have recently raised new enthusiasm to achieve a better pathophysiologic understanding of ALI/ARDS in future.
The role of NETs and extracellular histones during the development and perpetuation of ALI/ARDS may turn out to be an important aspect for this disease. However, it should also be mentioned that the reports on NETs have been faced with some degree of skepticim. 105 Some scientists have questioned that the presence of extracellular DNA and histones on microscopic slides of activated PMNs may represent laboratory artefacts due to mechanical disruption of cells during isolation and staining procedures rather than a phenomenon of biological significance. On the other hand, several studies have successfully employed intra-vital imaging of NETs or strategies to antagonize NETs in vivo (e.g. treatment with DNAse or anti-histone antibodies). The controversy extends to the level of importance that NET-formation may play for innate host defense. While it is recognized that certain bacteria encode DNAses for degradation of NETs as a strategy of immune evasion 106, it is not entirely clear how essential NETs are to achieve pathogen clearance.
In analogy to NETs and appearance of histones, the release of other intracellular proteins such as myoglobin (e.g. during rhabdomyolysis) or hemoglobin (e.g. during destruction of red blood cells) has long been recognized as a risk factor for tissue damage and organ dysfunction 107. In the area following the discovery of penicillin many substances had been evaluated for their bactericidal activity. Indeed, it was already in the 1940s when extracts of purifies histone proteins were demonstrated to effectively kill bacteria in culture media 108. It is somewhat tragic, that it took modern science until the 21st century to appreciate that the endogenous release of histone/DNA in the form of NETs may constitute an innate defense mechanism 52. As true for several immune effector mechanisms, NETs may not only be beneficial for clearance of infectious pathogens, but may rather cause collateral damage to endothelial and epithelial cells during ALI/ARDS. In our opinion, extracellular histones / NETs clearly display adverse effects in the setting of ALI/ARDS. The bactericidal effects of extracellular histones / NETs may not be needed in patients with ALI/ARDS, as long as control of pathogen growth can effectively be achieved by the administration of antibiotics. Hence, selective suppression of certain host immune defense systems (NETs, C5a), which cause tissue injury, may be save to exploit as a pharmacologic principle. It was a key finding that C5a via C5aR and C5L2 promotes the appearance of extracellular histones, which can subsequently mediate cell death of epithelial cells and production of inflammatory mediators in the alveolar compartment during ALI/ARDS 17.
Histone H4 appears to be the histone protein with the highest cytotoxic activity. Blockade of H4 using antibodies reduced the severity of ALI by around 50% in several studies 17, 68. An important development in future would be the identification of antibodies with higher avidity for the cytotoxic domains of several core histones in order to fully maintain normal alveolar/endothelial barrier function during ALI/ARDS.
The ultimate goal in the field of research on ALI/ARDS would be the availability of an easy-to-administer recombinant protein / enzyme to efficiently antagonize the unfavorable effects of certain endogenous proteins (NETs/extracellular histones, C5a, TGFβ). Protein-based therapies for ALI/ARDS may hold the promise to reduce mortality rates and prevent the need for invasive mechanical ventilation, which can be a traumatizing event for patients and their relatives. Current treatment strategies with supportive critical care treatment result in substantial costs for the national health systems. It is unclear, if a cost benefit of protein-based anti-ALI/ARDS drugs would be accomplished, especially when such agents may require de novo pharmacologic development and extensive clinical testing.
The humanized monoclonal anti-C5 antibody, eculizumab, would have the advantage that it has already been FDA-approved for the treatment of atypical hemolytic uremic syndrome (aHUS) and paroxysmal nocturnal hemoglobinuria (PNH) 109. It is tempting to speculate that eculizumab may also block C5a-induced release of NETs / extracellular histones in the setting of ALI/ARDS. At least, this could be easily tested in experimental models of ALI.
We may at least expect a good amount of scientific progress in the coming years. In particular, the area of experimental research on the role of NETs / extracellular histones during ALI/ARDS may provide additional insights how NETs are situated in the immunologic networks.
A weakness of the current data on NETs is that it was mainly (but not exclusively) accumulated in animal models of ALI/ARDS. It should be cautioned that a certain degree of disconnect between experimental and clinical studies of ALI/ARDS is likely to exist. Experimental studies typically use rodents (M. musculus, R. norvegicus) with defined induction of ALI by a single insult. For example, lipopolysaccharide (LPS) is frequently used to induce ALI in rodents. It should be mentioned that rodents are much more resistant against the adverse effects of LPS as compared to humans, which is partly explained by a high level of genetic variability in the extracellular domain of their respective TLR4 receptors 110. Experimental TRALI may involve a two hit model starting with LPS injections followed by administration of mouse MHC class I antibodies 68. This experimental approach in rodents provides a more standardized insult as compared to the complex etiology of TRALI in humans 111. In most small animal studies measurements of paO2 are not routinely performed and the term ALI is used, therefore, discrimination between ALI and ARDS would be difficult. In fact, the severity of many ALI/ARDS models in rodents is less as compared to ALI/ARDS observed in human patients. More severe experimental models of ALI/ARDS in rodents would require the use of small animal mechanical ventilation, which is not feasible in most laboratories.
Consequently, the relevance of experimental results obtained by studies with small animal models for the situation in human patients with ALI/ARDS is not entirely clear 112. For example, the use of β2-adrenoceptor agonists or glucocorticoids has shown some efficacy in experimental ALI using rodents 113, 114, but such agents have failed to show protective results in clinical trials in humans 28, 30, 115. These observations may suggest the existence of considerable discrepancies in the molecular pathophysiology of experimental ALI in small animals as compared to ALI/ARDS in humans. Unlike experimental ALI, most clinical studies with humans have employed the evaluation of 30-60-day mortality and ventilator-free days. These primary endpoints are often supplemented by collection of BALF and plasma at serial time points. Clinical studies typically require high numbers of patients given the heterogeneity of underlying causes of ALI/ARDS. In addition, study populations may vary because of different definitions of ALI/ARDS (e.g. severity of disease according to the traditional definition versus the novel ‘Berlin definition’) making the comparison of study results sometimes difficult.
In future, the use of novel experimental models such as ‘humanized mice’ may have some potential to overcome the limited relevance of current animal models for human diseases. ‘Humanized mice’ are generated by transplantation of human CD34+ hematopoietic stem cells into immunocompromised mouse strains (e.g. NSG mice) resulting in the co-presence of a human immune system in a mouse lung during experimental ALI/ARDS. Additional progress may be achieved by the utilization of novel methodologies such as next-generation-sequencing approaches, the use of flow cytometry based high-throughput immunocytofluorescence (e.g. ImageStreamX Mark II, Amnis) and single-cell protein biomarker analysis (e.g. chipcytometry technology, Zellkraftwerk).
In conclusion, subsequent decades may be full of scientific advancement as far as the understanding of the pathophysiology and drug development for the treatment of ALI/ARDS is concerned.
Article highlights.
Acute lung injury (ALI) is among the leading causes of morbidity and mortality worldwide.
While advances in critical care medicine (e.g. low-tidal volume ventilation) have somewhat improved the outcomes of ALI/ARDS, no specific pharmacologic therapies are currently available to ameliorate the adverse consequences of ALI.
Many studies support the idea of an essential role for the complement cleavage product, C5a, during ALI. The knowledge on the precise molecular effector functions of how C5a-modulated inflammation is translated into organ dysfunction have been recently expanded.
It has recently been uncovered that C5a mediates the appearance of extracellular histones in broncho-alveolar lavage fluids (BALF) during experimental ALI.
Extracellular histones, which are major components of neutrophil extracellular traps (NETs), are detectable in plasma and BALF of human patients with ALI/ARDS in humans.
Extracellular histones / NETs compromise lung function and alveolar gas exchange by displaying cytotoxic activity for alveolar epithelial cells / endothelial cells, and promoting acute inflammation and the accumulation of lung edema fluids.
Neutralization of extracellular histones using anti-H4 antibodies reduces the severity of experimental ALI as induced by C5a or transfusions (TRALI).
Extracellular histones are a substrate for enzymatic degradation by activated protein C (APC), but this inactivation process may not be sufficient to protect from ALI/ARDS in clinical settings.
NETs interact with platelets, the latter being major sources of TGFβ. TGFβ may dominate the fibro-proliferative stages of ALI and may determine the conditional transition to fibrotic conversions in lung architecture.
Interception of NETs / extracellular histone using neutralizing antibodies or degradation by site specific enzymes may evolve as novel protein-based strategies to cope with ALI/ARDS. Alternatively, interference with up-stream mechanisms (anti-C5a strategies) or down-stream effector proteins (anti-TGFβ strategies) could prove helpful for certain patients with ALI/ARDS.
This box summarizes key points contained in the article.
Acknowledgments
Peter Ward was supported by grants from the National Institutes of Health, USA (GM-29507, GM-61656,), Markus Bosmann was supported by the Federal Ministry of Education and Research (BMBF, 01EO1003), Deutsche Forschungsgemeinschaft (BO 3482/3-1), B. Braun Foundation, MAIFOR Program of the University Medical Center Mainz, Marie Curie Career Integration Grant of the European Union (project 334486) and a Clinical Research Fellowship of the European Hematology Association. The authors are responsible for the scientific content of this publication.
Footnotes
Financial and competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012 Jun 20;307(23):2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 2.Goss CH, Brower RG, Hudson LD, et al. Incidence of acute lung injury in the United States. Critical care medicine. 2003;31(6):1607–11. doi: 10.1097/01.CCM.0000063475.65751.1D. [DOI] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005 Oct 20;353(16):1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 4.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000 May 4;342(18):1301–8. doi: 10.1056/NEJM200005043421801. NoAuthorsListed. * This clinical study was a milestone to improve the clinical management of ALI/ARDS by simple reduction of tidal volumes.
- 5.National Heart L. Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N. Wiedemann HP, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006 Jun 15;354(24):2564–75. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 6.Rice TW, Wheeler AP, Thompson BT, et al. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011 Oct 12;306(14):1574–81. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Heart L. Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N. Matthay MA, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta(2)-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011 Sep 1;184(5):561–8. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012 Aug 1;122(8):2731–40. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward PA. Acute lung injury: how the lung inflammatory response works. Eur Respir J Suppl. 2003 Sep;44:22s–23s. doi: 10.1183/09031936.03.00000703a. [DOI] [PubMed] [Google Scholar]
- 10.Gao H, Neff T, Ward PA. Regulation of lung inflammation in the model of IgG immune-complex injury. Annual review of pathology. 2006;1:215–42. doi: 10.1146/annurev.pathol.1.110304.100155. [DOI] [PubMed] [Google Scholar]
- 11.Corada M, Mariotti M, Thurston G, et al. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci U S A. 1999 Aug 17;96(17):9815–20. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw JO, Henson PM. Pulmonary intravascular sequestration of activated neutrophils: failure to induce light-microscopic evidence of lung injury in rabbits. Am J Pathol. 1982 Jul;108(1):17–23. [PMC free article] [PubMed] [Google Scholar]
- 13.Garibaldi BT, D’Alessio FR, Mock JR, et al. Regulatory T cells reduce acute lung injury fibroproliferation by decreasing fibrocyte recruitment. Am J Respir Cell Mol Biol. 2013 Jan;48(1):35–43. doi: 10.1165/rcmb.2012-0198OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imai Y, Kuba K, Neely GG, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008 Apr 18;133(2):235–49. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chabot F, Mitchell JA, Gutteridge JM, et al. Reactive oxygen species in acute lung injury. Eur Respir J. 1998 Mar;11(3):745–57. [PubMed] [Google Scholar]
- 16.Warren JS, Yabroff KR, Remick DG, et al. Tumor necrosis factor participates in the pathogenesis of acute immune complex alveolitis in the rat. The Journal of clinical investigation. 1989 Dec;84(6):1873–82. doi: 10.1172/JCI114374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosmann M, Grailer JJ, Ruemmler R, et al. Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J. 2013 Aug 27; doi: 10.1096/fj.13-236380. * In this report, the intra-tracheal administration of histone extracts to healthy rodents resulted in ALI/ARDS-like symptoms.
- 18.Chen ES, Greenlee BM, Wills-Karp M, et al. Attenuation of lung inflammation and fibrosis in interferon-gamma-deficient mice after intratracheal bleomycin. Am J Respir Cell Mol Biol. 2001 May;24(5):545–55. doi: 10.1165/ajrcmb.24.5.4064. [DOI] [PubMed] [Google Scholar]
- 19.Bastarache JA, Sebag SC, Grove BS, et al. Interferon-gamma and tumor necrosis factor-alpha act synergistically to up-regulate tissue factor in alveolar epithelial cells. Exp Lung Res. 2011 Oct;37(8):509–17. doi: 10.3109/01902148.2011.605512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bardales RH, Xie SS, Schaefer RF, et al. Apoptosis is a major pathway responsible for the resolution of type II pneumocytes in acute lung injury. Am J Pathol. 1996 Sep;149(3):845–52. [PMC free article] [PubMed] [Google Scholar]
- 21.Polunovsky VA, Chen B, Henke C, et al. Role of mesenchymal cell death in lung remodeling after injury. J Clin Invest. 1993 Jul;92(1):388–97. doi: 10.1172/JCI116578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curley GF, Hayes M, Ansari B, et al. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax. 2012 Jun;67(6):496–501. doi: 10.1136/thoraxjnl-2011-201059. [DOI] [PubMed] [Google Scholar]
- 23.Gotts JE, Matthay MA. Mesenchymal stem cells and acute lung injury. Crit Care Clin. 2011 Jul;27(3):719–33. doi: 10.1016/j.ccc.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu KD, Levitt J, Zhuo H, et al. Randomized clinical trial of activated protein C for the treatment of acute lung injury. Am J Respir Crit Care Med. 2008 Sep 15;178(6):618–23. doi: 10.1164/rccm.200803-419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paine R, 3rd, Standiford TJ, Dechert RE, et al. A randomized trial of recombinant human granulocyte-macrophage colony stimulating factor for patients with acute lung injury. Crit Care Med. 2012 Jan;40(1):90–7. doi: 10.1097/CCM.0b013e31822d7bf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spragg RG, Lewis JF, Walmrath HD, et al. Effect of recombinant surfactant protein C-based surfactant on the acute respiratory distress syndrome. N Engl J Med. 2004 Aug 26;351(9):884–92. doi: 10.1056/NEJMoa033181. [DOI] [PubMed] [Google Scholar]
- 27.Gao Smith F, Perkins GD, Gates S, et al. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet. 2012 Jan 21;379(9812):229–35. doi: 10.1016/S0140-6736(11)61623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernard GR, Luce JM, Sprung CL, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med. 1987 Dec 17;317(25):1565–70. doi: 10.1056/NEJM198712173172504. [DOI] [PubMed] [Google Scholar]
- 29.Bernard GR, Wheeler AP, Arons MM, et al. A trial of antioxidants N-acetylcysteine and procysteine in ARDS. The Antioxidant in ARDS Study Group. Chest. 1997 Jul;112(1):164–72. doi: 10.1378/chest.112.1.164. [DOI] [PubMed] [Google Scholar]
- 30.Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006 Apr 20;354(16):1671–84. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 31.Nonaka M, Yoshizaki F. Evolution of the complement system. Molecular immunology. 2004;40(12):897–902. doi: 10.1016/j.molimm.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Bosmann M, Ward PA. Role of C3, C5 and anaphylatoxin receptors in acute lung injury and in sepsis. Adv Exp Med Biol. 2012;946:147–59. doi: 10.1007/978-1-4614-0106-3_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulligan MS, Schmid E, Beck-Schimmer B, et al. Requirement and role of C5a in acute lung inflammatory injury in rats. J Clin Invest. 1996 Jul 15;98(2):503–12. doi: 10.1172/JCI118818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell WD, Lazoura E, Okada N, et al. Inactivation of C3a and C5a octapeptides by carboxypeptidase R and carboxypeptidase N. Microbiol Immunol. 2002;46(2):131–4. doi: 10.1111/j.1348-0421.2002.tb02669.x. [DOI] [PubMed] [Google Scholar]
- 35.Zwirner J, Gotze O, Sieber A, et al. The human mast cell line HMC-1 binds and responds to C3a but not C3a(desArg) Scand J Immunol. 1998 Jan;47(1):19–24. doi: 10.1046/j.1365-3083.1998.00250.x. [DOI] [PubMed] [Google Scholar]
- 36.Gerard NP, Gerard C. The chemotactic receptor for human C5a anaphylatoxin. Nature. 1991;349(6310):614–7. doi: 10.1038/349614a0. [DOI] [PubMed] [Google Scholar]
- 37.Okinaga S, Slattery D, Humbles A, et al. C5L2, a nonsignaling C5A binding protein. Biochemistry. 2003 Aug 12;42(31):9406–15. doi: 10.1021/bi034489v. [DOI] [PubMed] [Google Scholar]
- 38.Li R, Coulthard LG, Wu MC, et al. C5L2: a controversial receptor of complement anaphylatoxin, C5a. FASEB J. 2013 Mar;27(3):855–64. doi: 10.1096/fj.12-220509. [DOI] [PubMed] [Google Scholar]
- 39.Ward PA. Immune complex injury of the lung. Am J Pathol. 1979 Oct;97(1):85–92. [PMC free article] [PubMed] [Google Scholar]
- 40.Larsen GL, McCarthy K, Webster RO, et al. A differential effect of C5a and C5a des Arg in the induction of pulmonary inflammation. Am J Pathol. 1980 Jul;100(1):179–92. [PMC free article] [PubMed] [Google Scholar]
- 41.Till GO, Johnson KJ, Kunkel R, et al. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J Clin Invest. 1982 May;69(5):1126–35. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vogel CW, Fritzinger DC, Hew BE, et al. Recombinant cobra venom factor. Molecular immunology. 2004 Jun;41(2-3):191–9. doi: 10.1016/j.molimm.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 43.Stevens JH, O’Hanley P, Shapiro JM, et al. Effects of anti-C5a antibodies on the adult respiratory distress syndrome in septic primates. J Clin Invest. 1986 Jun;77(6):1812–6. doi: 10.1172/JCI112506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinberg PF, Matthay MA, Webster RO, et al. Biologically active products of complement and acute lung injury in patients with the sepsis syndrome. Am Rev Respir Dis. 1984 Nov;130(5):791–6. doi: 10.1164/arrd.1984.130.5.791. [DOI] [PubMed] [Google Scholar]
- 45.Bozic CR, Lu B, Hopken UE, et al. Neurogenic amplification of immune complex inflammation. Science. 1996 Sep 20;273(5282):1722–5. doi: 10.1126/science.273.5282.1722. [DOI] [PubMed] [Google Scholar]
- 46.Trujillo G, Habiel DM, Ge L, et al. Neutrophil recruitment to the lung in both C5a- and CXCL1-induced alveolitis is impaired in vitamin D-binding protein-deficient mice. J Immunol. 2013 Jul 15;191(2):848–56. doi: 10.4049/jimmunol.1202941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klaff LS, Gill SE, Wisse BE, et al. Lipopolysaccharide-induced lung injury is independent of serum vitamin D concentration. PLoS One. 2012;7(11):e49076. doi: 10.1371/journal.pone.0049076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shushakova N, Eden G, Dangers M, et al. The urokinase/urokinase receptor system mediates the IgG immune complex-induced inflammation in lung. J Immunol. 2005 Sep 15;175(6):4060–8. doi: 10.4049/jimmunol.175.6.4060. [DOI] [PubMed] [Google Scholar]
- 49.Addis-Lieser E, Kohl J, Chiaramonte MG. Opposing regulatory roles of complement factor 5 in the development of bleomycin-induced pulmonary fibrosis. J Immunol. 2005 Aug 1;175(3):1894–902. doi: 10.4049/jimmunol.175.3.1894. [DOI] [PubMed] [Google Scholar]
- 50.Gerard NP, Lu B, Liu P, et al. An anti-inflammatory function for the complement anaphylatoxin C5a-binding protein, C5L2. Journal of Biological Chemistry. 2005;280(48):39677–80. doi: 10.1074/jbc.C500287200. [DOI] [PubMed] [Google Scholar]
- 51.Bosmann M, Haggadone MD, Hemmila MR, et al. Complement activation product C5a is a selective suppressor of TLR4-induced, but not TLR3-induced, production of IL-27(p28) from macrophages. J Immunol. 2012 May 15;188(10):5086–93. doi: 10.4049/jimmunol.1102914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004 Mar 5;303(5663):1532–5. doi: 10.1126/science.1092385. ** This is the initial report introducing the concept of neutrophil extracellular traps.
- 53.Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol. 2007 Aug;5(8):577–82. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 54.Fuchs TA, Abed U, Goosmann C, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007 Jan 15;176(2):231–41. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clark SR, Ma AC, Tavener SA, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007 Apr;13(4):463–9. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 56.Urban CF, Reichard U, Brinkmann V, et al. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006 Apr;8(4):668–76. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 57.Bruns S, Kniemeyer O, Hasenberg M, et al. Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog. 2010 Apr;6(4):e1000873. doi: 10.1371/journal.ppat.1000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCormick A, Heesemann L, Wagener J, et al. NETs formed by human neutrophils inhibit growth of the pathogenic mold Aspergillus fumigatus. Microbes Infect. 2010 Nov;12(12-13):928–36. doi: 10.1016/j.micinf.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 59.Beiter K, Wartha F, Albiger B, et al. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol. 2006 Feb 21;16(4):401–7. doi: 10.1016/j.cub.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 60.Xu J, Zhang X, Pelayo R, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009 Nov;15(11):1318–21. doi: 10.1038/nm.2053. ** This article provides elegant experimental evidence for the cleavage of extracellular histones by activated protein C in sepsis.
- 61.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–62. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klune JR, Dhupar R, Cardinal J, et al. HMGB1: endogenous danger signaling. Mol Med. 2008 Jul-Aug;14(7-8):476–84. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hakkim A, Fuchs TA, Martinez NE, et al. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol. 2011 Feb;7(2):75–7. doi: 10.1038/nchembio.496. [DOI] [PubMed] [Google Scholar]
- 64.McInturff AM, Cody MJ, Elliott EA, et al. Mammalian target of rapamycin regulates neutrophil extracellular trap formation via induction of hypoxia-inducible factor 1 alpha. Blood. 2012 Oct 11;120(15):3118–25. doi: 10.1182/blood-2012-01-405993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li P, Li M, Lindberg MR, et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010 Aug 30;207(9):1853–62. doi: 10.1084/jem.20100239. * This article highlights histone hypercitrullination as a prerequisite for NET-formation.
- 66.Neeli I, Khan SN, Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol. 2008 Feb 1;180(3):1895–902. doi: 10.4049/jimmunol.180.3.1895. [DOI] [PubMed] [Google Scholar]
- 67.Saffarzadeh M, Juenemann C, Queisser MA, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7(2):e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caudrillier A, Kessenbrock K, Gilliss BM, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012 Jul 2;122(7):2661–71. doi: 10.1172/JCI61303. * This is the first report focusing on the role of NETs during ALI/ARDS.
- 69.Abrams ST, Zhang N, Manson J, et al. Circulating Histones Are Mediators of Trauma-associated Lung Injury. Am J Respir Crit Care Med. 2013 Jan 15;187(2):160–9. doi: 10.1164/rccm.201206-1037OC. * This article nicely combines experimental and clinical data suggesting that the lung is the most susceptible organ to trauma-induced release of histones.
- 70.Allam R, Scherbaum CR, Darisipudi MN, et al. Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol. 2012 Aug;23(8):1375–88. doi: 10.1681/ASN.2011111077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu J, Zhang X, Monestier M, et al. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol. 2011 Sep 1;187(5):2626–31. doi: 10.4049/jimmunol.1003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garcia CC, Weston-Davies W, Russo RC, et al. Complement C5 activation during influenza A infection in mice contributes to neutrophil recruitment and lung injury. PLoS One. 2013;8(5):e64443. doi: 10.1371/journal.pone.0064443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010 Sep 7;107(36):15880–5. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.von Bruhl ML, Stark K, Steinhart A, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012 Apr 9;209(4):819–35. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fuchs TA, Kremer Hovinga JA, Schatzberg D, et al. Circulating DNA and myeloperoxidase indicate disease activity in patients with thrombotic microangiopathies. Blood. 2012 Aug 9;120(6):1157–64. doi: 10.1182/blood-2012-02-412197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li MO, Wan YY, Sanjabi S, et al. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 77.Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem. 2005 Mar 4;280(9):7409–12. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- 78.Stockis J, Colau D, Coulie PG, et al. Membrane protein GARP is a receptor for latent TGF-beta on the surface of activated human Treg. Eur J Immunol. 2009 Dec;39(12):3315–22. doi: 10.1002/eji.200939684. [DOI] [PubMed] [Google Scholar]
- 79.Pittet JF, Griffiths MJ, Geiser T, et al. TGF-beta is a critical mediator of acute lung injury. J Clin Invest. 2001 Jun;107(12):1537–44. doi: 10.1172/JCI11963. * This article demonstrated that integrin αvβ6 activates latent TGFβ for promoting ALI.
- 80.Schultz-Cherry S, Murphy-Ullrich JE. Thrombospondin causes activation of latent transforming growth factor-beta secreted by endothelial cells by a novel mechanism. J Cell Biol. 1993 Aug;122(4):923–32. doi: 10.1083/jcb.122.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol. 1996 Sep;10(9):1077–83. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- 82.Karsdal MA, Larsen L, Engsig MT, et al. Matrix metalloproteinase-dependent activation of latent transforming growth factor-beta controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosis. J Biol Chem. 2002 Nov 15;277(46):44061–7. doi: 10.1074/jbc.M207205200. [DOI] [PubMed] [Google Scholar]
- 83.Wrana JL, Attisano L, Wieser R, et al. Mechanism of activation of the TGF-beta receptor. Nature. 1994 Aug 4;370(6488):341–7. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 84.Wang XF, Lin HY, Ng-Eaton E, et al. Expression cloning and characterization of the TGF-beta type III receptor. Cell. 1991 Nov 15;67(4):797–805. doi: 10.1016/0092-8674(91)90074-9. [DOI] [PubMed] [Google Scholar]
- 85.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997 Dec 4;390(6659):465–71. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 86.Birukova AA, Adyshev D, Gorshkov B, et al. ALK5 and Smad4 are involved in TGF-beta1-induced pulmonary endothelial permeability. FEBS Lett. 2005 Jul 18;579(18):4031–7. doi: 10.1016/j.febslet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 87.Perlman R, Schiemann WP, Brooks MW, et al. TGF-beta-induced apoptosis is mediated by the adapter protein Daxx that facilitates JNK activation. Nat Cell Biol. 2001 Aug;3(8):708–14. doi: 10.1038/35087019. [DOI] [PubMed] [Google Scholar]
- 88.Hannigan M, Zhan L, Ai Y, et al. The role of p38 MAP kinase in TGF-beta1-induced signal transduction in human neutrophils. Biochem Biophys Res Commun. 1998 May 8;246(1):55–8. doi: 10.1006/bbrc.1998.8570. [DOI] [PubMed] [Google Scholar]
- 89.Shull MM, Ormsby I, Kier AB, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992 Oct 22;359(6397):693–9. doi: 10.1038/359693a0. * This article showed that endogenous TGFβ is required to prevent lethal spontaneous inflammation in mice.
- 90.Murray LA, Chen Q, Kramer MS, et al. TGF-beta driven lung fibrosis is macrophage dependent and blocked by Serum amyloid P. Int J Biochem Cell Biol. 2011 Jan;43(1):154–62. doi: 10.1016/j.biocel.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 91.Frank J, Roux J, Kawakatsu H, et al. Transforming growth factor-beta1 decreases expression of the epithelial sodium channel alphaENaC and alveolar epithelial vectorial sodium and fluid transport via an ERK1/2-dependent mechanism. J Biol Chem. 2003 Nov 7;278(45):43939–50. doi: 10.1074/jbc.M304882200. [DOI] [PubMed] [Google Scholar]
- 92.Pittet JF, Koh H, Fang X, et al. HMGB1 accelerates alveolar epithelial repair via an IL-1beta-and alphavbeta6 integrin-dependent activation of TGF-beta1. PLoS One. 2013;8(5):e63907. doi: 10.1371/journal.pone.0063907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bartram U, Speer CP. The role of transforming growth factor beta in lung development and disease. Chest. 2004 Feb;125(2):754–65. doi: 10.1378/chest.125.2.754. [DOI] [PubMed] [Google Scholar]
- 94.Adamali HI, Maher TM. Current and novel drug therapies for idiopathic pulmonary fibrosis. Drug Des Devel Ther. 2012;6:261–72. doi: 10.2147/DDDT.S29928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Postlethwaite AE, Keski-Oja J, Moses HL, et al. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med. 1987 Jan 1;165(1):251–6. doi: 10.1084/jem.165.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Desmouliere A, Geinoz A, Gabbiani F, et al. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993 Jul;122(1):103–11. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Willis BC, Liebler JM, Luby-Phelps K, et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005 May;166(5):1321–32. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang HY, Phan SH. Inhibition of myofibroblast apoptosis by transforming growth factor beta(1) Am J Respir Cell Mol Biol. 1999 Dec;21(6):658–65. doi: 10.1165/ajrcmb.21.6.3720. [DOI] [PubMed] [Google Scholar]
- 99.Kramann R, DiRocco DP, Humphreys BD. Understanding the origin, activation and regulation of matrix-producing myofibroblasts for treatment of fibrotic disease. J Pathol. 2013 Nov;231(3):273–89. doi: 10.1002/path.4253. [DOI] [PubMed] [Google Scholar]
- 100.Garcia-Alvarez J, Ramirez R, Checa M, et al. Tissue inhibitor of metalloproteinase-3 is up-regulated by transforming growth factor-beta1 in vitro and expressed in fibroblastic foci in vivo in idiopathic pulmonary fibrosis. Exp Lung Res. 2006 May;32(5):201–14. doi: 10.1080/01902140600817481. [DOI] [PubMed] [Google Scholar]
- 101.Taylor LM, Khachigian LM. Induction of platelet-derived growth factor B-chain expression by transforming growth factor-beta involves transactivation by Smads. J Biol Chem. 2000 Jun 2;275(22):16709–16. doi: 10.1074/jbc.275.22.16709. [DOI] [PubMed] [Google Scholar]
- 102.Dhainaut JF, Charpentier J, Chiche JD. Transforming growth factor-beta: a mediator of cell regulation in acute respiratory distress syndrome. Crit Care Med. 2003 Apr;31(4 Suppl):S258–64. doi: 10.1097/01.CCM.0000057901.92381.75. [DOI] [PubMed] [Google Scholar]
- 103.Peng R, Sridhar S, Tyagi G, et al. Bleomycin induces molecular changes directly relevant to idiopathic pulmonary fibrosis: a model for “active” disease. PLoS One. 2013;8(4):e59348. doi: 10.1371/journal.pone.0059348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.STX-100 in Patients With Idiopathic Pulmonary Fibrosis (IPF) http://clinicaltrialsgov/ct2/show/study/NCT01371305.
- 105.Nauseef WM. Editorial: Nyet to NETs? A pause for healthy skepticism. J Leukoc Biol. 2012 Mar;91(3):353–5. doi: 10.1189/jlb.1011495. * This article provides a refreshing comment on the current field of research on NETs.
- 106.Buchanan JT, Simpson AJ, Aziz RK, et al. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Current biology : CB. 2006 Feb 21;16(4):396–400. doi: 10.1016/j.cub.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 107.Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009 Jul 2;361(1):62–72. doi: 10.1056/NEJMra0801327. [DOI] [PubMed] [Google Scholar]
- 108.Hirsch JG. Bactericidal action of histone. The Journal of experimental medicine. 1958 Dec 1;108(6):925–44. doi: 10.1084/jem.108.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006 Sep 21;355(12):1233–43. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 110.Smirnova I, Poltorak A, Chan EK, et al. Phylogenetic variation and polymorphism at the toll-like receptor 4 locus (TLR4) Genome biology. 2000;1(1) doi: 10.1186/gb-2000-1-1-research002. RESEARCH002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Looney MR, Gropper MA, Matthay MA. Transfusion-related acute lung injury: a review. Chest. 2004 Jul;126(1):249–58. doi: 10.1378/chest.126.1.249. [DOI] [PubMed] [Google Scholar]
- 112.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008 Sep;295(3):L379–99. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bosmann M, Grailer JJ, Zhu K, et al. Anti-inflammatory effects of beta2 adrenergic receptor agonists in experimental acute lung injury. FASEB J. 2012 May;26(5):2137–44. doi: 10.1096/fj.11-201640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Corbel M, Lagente V, Theret N, et al. Comparative effects of betamethasone, cyclosporin and nedocromil sodium in acute pulmonary inflammation and metalloproteinase activities in bronchoalveolar lavage fluid from mice exposed to lipopolysaccharide. Pulmonary pharmacology & therapeutics. 1999;12(3):165–71. doi: 10.1006/pupt.1999.0178. [DOI] [PubMed] [Google Scholar]
- 115.Singh B, Tiwari AK, Singh K, et al. beta2 Agonist for the Treatment of Acute Lung Injury: A Systematic Review and Meta-analysis. Respiratory care. 2014 Feb;59(2):288–96. doi: 10.4187/respcare.02571. [DOI] [PubMed] [Google Scholar]
- 116.Taylor RW, Zimmerman JL, Dellinger RP, et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004 Apr 7;291(13):1603–9. doi: 10.1001/jama.291.13.1603. [DOI] [PubMed] [Google Scholar]
- 117.Abraham E, Baughman R, Fletcher E, et al. Liposomal prostaglandin E1 (TLC C-53) in acute respiratory distress syndrome: a controlled, randomized, double-blind, multicenter clinical trial. TLC C-53 ARDS Study Group. Crit Care Med. 1999 Aug;27(8):1478–85. doi: 10.1097/00003246-199908000-00013. [DOI] [PubMed] [Google Scholar]