Abstract
Background:
Although caffeine enhances respiratory control and decreases the need for mechanical ventilation and resultant bronchopulmonary dysplasia, it may also have anti-inflammatory properties in protecting lung function.
Objective:
We hypothesized that caffeine improves respiratory function via an anti-inflammatory effect in lungs of a lipopolysaccharide (LPS)-induced pro-inflammatory amnionitis rat pup model.
Methods:
Caffeine was given orally (10 mg/kg/d) from postnatal days (p) 1-14 to pups exposed to intra-amniotic LPS or normal saline (NS). Expression of IL-1β was assessed in lung homogenates at p8 and p14, and respiratory system resistance (Rrs) and compliance (Crs) as well as CD68 cell counts and radial alveolar counts (RACs) were assessed at p8.
Results:
In LPS-exposed rats IL-1β and CD68 cell counts both increased at p8 compared to NS controls. These increases in proinflammatory markers were no longer present in caffeinetreated LPS-exposed pups. Rrs was higher in LPS-exposed pups (4.7±0.9 cmH2O/ml.s) at p8 vs controls (1.6±0.3 cmH2O/ml.s, p<0.01). LPS-exposed pups no longer exhibited a significant increase in Rrs (2.8±0.5 cmH2O/ml.s) after caffeine. Crs did not differ significantly between groups, although radial alveolar counts were lower in both groups of LPS-exposed pups.
Conclusions:
Caffeine promotes anti-inflammatory effects in the immature lung of prenatal LPS-exposed rat pups associated with improvement of Rrs and suggesting a protective effect of caffeine on respiratory function via an anti-inflammatory mechanism.
Keywords: caffeine, inflammation, amnionitis, lung function, newborn
Introduction
Bronchopulmonary dysplasia (BPD) is the most prevalent chronic lung disease secondary to preterm birth and a major cause of morbidity. Inflammation, cellular-endothelial interaction, chemotaxis of neutrophils, increased pro-inflammatory cytokines, proteolytic and oxidative damage, increased alveolar-capillary permeability and tissue remodeling involving fibrosis and vessel growth are all considered to be involved in the pathogenesis of BPD [1]. These inflammatory mechanisms that have important roles in the pathogenesis of BPD may result from chorioamnionitis in the antenatal period with superimposed mechanical ventilation, oxygen therapy, and nosocomial infections in the postnatal period [2-4]. In addition to amplifying the inflammatory response of the preterm lung to further interventions in the early neonatal period, chorioamnionitis and BPD both predispose to increased airway reactivity [4-6].
The main interventions for preventing or ameliorating BPD comprise protecting previously injured lung from further injury and controlling the inflammatory process [7]. Caffeine has been widely used for apnea of prematurity and, apart from significantly decreasing the incidence of BPD, has significant beneficial effects on various neonatal morbidities [8-10]. Although the exact mechanism of lung protective effects of caffeine has not yet been elucidated, it has been proposed that it is due to decreased need for mechanical ventilation by enhancing respiratory control [11]. Additionally, caffeine may also have anti-inflammatory effects, as recently reported in a model of hyperoxia-mediated pulmonary inflammation in rat pups [12]. We, therefore, sought to test the hypothesis that caffeine contributes to improved respiratory function via an anti-inflammatory effect in lungs of a lipopolysaccharide (LPS)-induced pro-inflammatory rat pup model of amnionitis.
Methods
Animal model
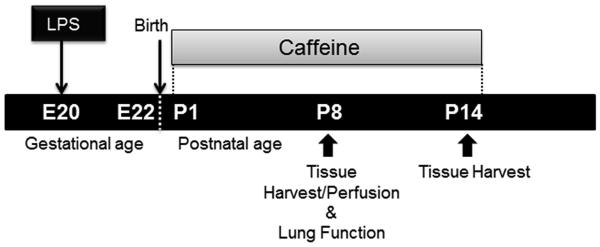
Experiments were performed on timed-pregnancy Sprague Dawley rats (Charles River Laboratories, Portage, MI). All experimental procedures were conducted in accordance with NIH guidelines and institutional approval. Maternal animals were anesthetized, and after exposing the rat uterus by laparotomy, LPS (E. coli 055:B5, Sigma) or NS control solution was injected into each amniotic sac (1 μg/amniotic sac) on day 20 in a volume of 10 μl. After returning the uterus to the abdomen, animals recovered in individual cages under close observation. Pups were delivered spontaneously two days after LPS or NS injections. A total of 3-4 dams were employed per study protocol, and survival of pups was 75-90%, which did not differ between antenatal LPS or NS exposure. Caffeine base (10 mg/kg/d), or water placebo, was given orally for 14 days starting from postnatal day one (p1) to subgroups of pups exposed to LPS or NS (Figure 1). Caffeine blood levels were obtained via cardiac puncture and evaluated by high-performance liquid chromatography at p2, p7 and p14 in an additional 4-5 pups at each age.
Figure 1.
The experimental protocol employed in this study.
Cytokine protein measurements in lung tissue
Lung harvests from rat pups for cytokine analysis were performed after lethal injection. Lungs were homogenized in RIPA cell lysis buffer containing phenylmethylsulfonyl fluoride (2 mM), and 1 μg/ml aprotinin, leupeptin, and pepstatin A and were centrifuged for 30 minutes at 12,000 g at 4°C. Supernatants were collected and stored at −80°C and total protein content was measured. LPS-induced cytokine protein level was measured by commercially available Milliplex rat cytokine panels using Luminex technology [13] at day 8 for three major proinflammatory cytokines: interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNFα) (Millipore Corporation, Temecula, CA) in response to antenatal LPS or NS exposure. Expression of IL-1β was additionally measured at days 8 and 14 in NS and LPS-exposed pups after oral caffeine or water placebo administration (n=4, all groups). Quantification of relative cytokine expression for different age groups was normalized with BSA protein assay and expressed as pg of cytokine/μg of protein.
CD68 Immunohistochemistry
Following euthanasia at day 8, pups’ lungs from four groups (NS, LPS, LPS+caffeine and NS+caffeine, n=6-7, all groups) were perfused with PBS/heparin and then fixed in situ with 10% neutral buffered formalin solution. The fixed left lung tissue was embedded in paraffin wax after tissue processing, sectioned at 5 μm thickness and then processed for immunohistochemistry. The tissues were stained with rabbit polyclonal CD68 antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) using a standard immunostaining protocol. Blinded inflammatory scoring was performed by counting positive CD68 cells in nine comparable non-overlapping high-power fields from each animal.
Respiratory system compliance and resistance measurements
Respiratory system compliance and resistance were measured in 8-day old pups using a pneumotach and a custom-made head-out plethysmography system. Data were obtained in the four groups of pups exposed to antenatal LPS or NS followed by postnatal caffeine or placebo (n>8/group). Rat pups were anesthetized with an intraperitoneal injection of ketamine/xylazine (5 mg and 3 mg/100 g body weight). A custom-made latex mask was sealed over the face of the rat, and the body was then placed inside a small plethysmograph with its head protruding into a separate compartment. A pneumotach (8430 Series, Hans Rudolph Inc., Shawnee, KS) was attached to the mask for the measurement of airflow associated with breathing; a bias flow of 100% O2 was also delivered to the mask, digitally converted (PowerLab 8/30, ADInstruments, Colorado Springs, CO) and electronically offset for integration of tidal volume. Rectal and chamber temperature were monitored and respiratory system compliance (Crs) and resistance (Rrs) determined as described previously by ourselves [14]. Briefly, a vacuum applied to the body compartment enabled negative inflation pressure (PI) to be measured continuously. A small adjustable leak allowed fine-control of individual inflation pressures. During inflation the vacuum was then released at the occurrence of the first breath, resulting in a passive decay in lung volume. Using the inflation volume (VL), Crs was calculated (Crs = VL/PI). Rrs was calculated by determining the time constant of the passive decay in lung volume (Rrs=τrs/Crs). One initial maneuver was performed to minimize lung hysteresis and the values for Crs and Rrs were determined from the subsequent 3-4 consecutive inflations.
Lung morphology
Five μm-thick sections from the paraffin block of four groups (NS, LPS, LPS+caffeine and NS+caffeine, n=6-7, all groups) of 8 day old pups’ lungs were cut and stained with hematoxylin and eosin for tissue morphology. Three sections from each pup were analyzed using research-based digital image analysis software (Image-pro Plus 7.0, Media Cybernetics, Silver Spring, MD). The radial alveolar counts (RACs) were estimated by the method of Cooney and Thurlbeck [15], and were performed by a technician unaware of the treatment groups.
Statistical analysis
The effect of antenatal LPS vs NS on postnatal IL-1β, TNFα, and IL-6 cytokine protein levels at day 8 was compared via unpaired t-tests. All other comparisons were via one way ANOVA with post-hoc comparisons. Data are expressed as mean±SEM.
Results
Caffeine levels
We sought to ensure caffeine levels were comparable to therapeutic levels observed in neonates. Caffeine plasma levels were 13.74±1.04, 9.31±1.43, and 9.67±1.50 μg/mL at p2, p7, and p14, respectively (n=4-5/group).
Cytokine protein measurement and CD68 counts in lung tissue
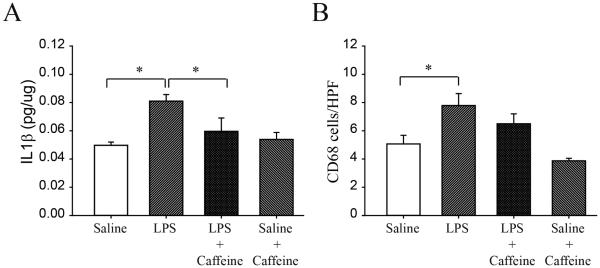
We initially sought to document an effect of prior LPS exposure on proinflammatory cytokine expression at day 8. IL-1β increased by 65% in LPS-exposed pups (0.049±0.002 vs 0.081±0.004 pg/μg, p<0.01, for NS vs LPS, respectively, n=4/group, Figure 2A). There was no effect of antenatal LPS on TNFα (0.09±0.04 vs 0.08±0.03 NS vs LPS) or on IL-6 (0.13±0.008 vs 0.13±0.01 pg/μg tissue, NS vs LPS), n=4, all groups. We, therefore, focused on the effect of postnatal caffeine on IL-1β expression. There was a significant effect of caffeine on IL-1β levels at day 8 which fell from 0.08±0.004 to 0.06±0.01 pg/μg, p<0.05 in LPS-exposed pups, Figure 2A. There was no effect of caffeine in NS treated controls not exposed to LPS (n=4, all groups). At day 14 IL-1β levels between groups no longer differed significantly (0.07±0.01, 0.10±0.01, 0.07±0.01 and 0.09±0.02 for NS control, LPS, LPS + caffeine, and NS control + caffeine groups, respectively, n=4, all groups). At day 14, TNFα and IL6 levels also did not differ between groups.
Figure 2.
IL-1β levels in pups of LPS and NS rats at day 8 with or without caffeine treatment (A). There was a significant increase in IL-1β after antenatal LPS exposure which decreased after postnatal caffeine treatment. Caffeine did not affect saline-exposed control pups. Parallel data for CD68 counts in the same groups (B). The significant increase after antenatal LPS exposure was no longer observed after caffeine. Mean±SEM (*p<.05)
As an additional proinflammatory marker, we employed CD68 counts which reflect macrophage activity. Intraamniotic LPS exposure significantly increased CD68 counts (5.10±0.62 vs 7.80±0.85 for NS controls vs LPS-exposed, respectively, p<0.05). In parallel to the IL-1β data, this increase in CD68 counts was no longer significant after caffeine treatment (Figure 2B).
Respiratory system compliance and resistance measurements
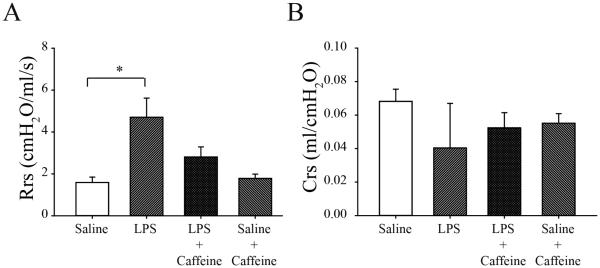
We then sought to correlate the decrease in IL-1β expression after caffeine exposure with an effect on respiratory system mechanics. As seen in Figure 3A, intra-amniotic LPS significantly increased Rrs (4.7±0.9 cmH2O/ml.s) compared to NS (1.6±0.3 cmH2O/ml.s) treated rats (n>8/group; p<.05). Rrs for intra-amniotic LPS-treated rats was not different from NS rats following daily caffeine treatment (LPS+caffeine, 2.8±0.5 cmH2O/ml.s), indicating that caffeine prevented the LPS-induced increase in Rrs. Caffeine had no effect on Rrs in NS control pups (Figure 3B). Respiratory system compliance (Crs) was not significantly affected by LPS or caffeine exposure (Figure 3B).
Figure 3.
Respiratory system resistance (Rrs) (A) and compliance (Crs) (B) in 8 day old rat pups with or without caffeine treatment following intra-amniotic LPS or normal saline. There was a significant (*p<.05) increase in Rrs after LPS exposure which was eliminated by caffeine treatment. In saline-exposed controls there was no effect of caffeine on Rrs. There was no difference in Crs (B) between treatment groups. Mean±SEM (*p<.05)
Lung morphology
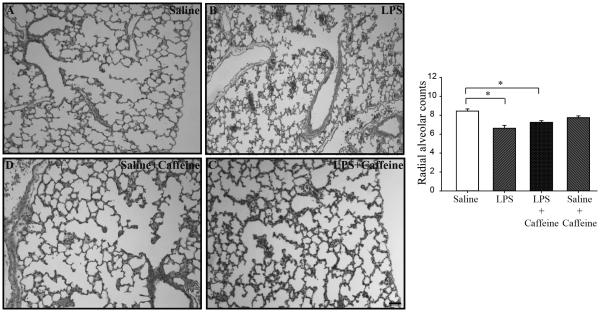
In order to assess alveolar morphology, radial alveolar counts were assessed in four pup groups at day 8: NS, LPS-exposed, and LPS+caffeine, and NS+caffeine (n=6-7/group). After antenatal LPS there was a significant decrease in radial alveolar counts (8.44±0.23 vs 6.63±0.31, p<0.05 for NS controls vs LPS-exposed, respectively). Radial alveolar counts in the LPS+caffeine pups were also lower when compared to the NS control pups (8.44±0.23 vs 7.23±0.18 for NS controls and LPS-caffeine, respectively, p<0.05, Figure 4).
Figure 4.
Representative lung micrographs for NS control (A), LPS-exposed (B), LPS+caffeine (C), and NS+caffeine (D) pups. Scale bar = 50 μm. Graphical representation of radial alveolar counts (RACs) demonstrates a significant decrease in both antenatal LPS-exposed groups. Mean±SEM (*p<.05)
Discussion
We have shown that intra-amniotic LPS injection resulted in a marked increase in levels of IL-1β accompanied by inflammatory change in the lungs of the offspring at postnatal day 8. This was associated with an increase in Rrs in LPS-exposed pups. These molecular and functional changes demonstrated in our pro-inflammatory model were both reversed by postnatal caffeine administration at plasma levels similar to therapeutic neonatal levels. We, therefore, speculate that caffeine may serve a protective effect on neonatal lung function via anti-inflammatory mechanisms.
To document a potential anti-inflammatory effect of caffeine therapy, we studied rat pups exposed to a pro-inflammatory intrauterine environment after intra-amniotic LPS (at the same dose employed by others [6]) or saline and observed a high survival rate in the pups which did not differ between groups. Our initial observation that IL-1β is markedly elevated on postnatal day 8 after intrauterine LPS exposure caused us to focus on this pro-inflammatory. We did not observe a response of TNFα or IL-6 to antenatal LPS in our model, however, an elevation in those cytokines may have resolved by postnatal day 8. Small group sizes may also have contributed to this observation as well as the failure of IL-1β levels to remain significantly elevated at p14. Future study, incorporating message and protein expression for a greater number of cytokines at different time points and in different models, should address this issue. Recent data, however, failed to show upregulation of IL-6 associated with inflammatory changes in bronchoalveolar lavage fluid of BPD infants [16].
Xanthines are used extensively for apnea of prematurity, and in recent years caffeine became the focus of interest with the newly discovered protective effects for BPD and cerebral palsy at 18-21 months of age [9,10]. This effect of caffeine on respiratory drive may result in less frequent intermittent hypoxic episodes and decrease oxidant stress which, in turn, may diminish inflammation [17]. However, a potential anti-inflammatory effect of caffeine has not been widely considered. Our current demonstration of a decrease in IL-1β protein expression after postnatal caffeine was limited to LPS-exposed pups. These data are supported by our additional observation that the increase in macrophage activity induced by LPS is no longer significant after caffeine exposure. We know of no comparable data in preterm infants relating the beneficial effect of caffeine to an upregulated proinflammatory environment.
Chorioamnionitis, preterm birth, and BPD are all well recognized precursors of later airway reactivity [5]. Prior studies have exposed pregnant rats or mice to intraperitoneal LPS and observed postnatal inflammation implicated in delayed lung maturation [18,19]. Choi et al did not observe an effect of intra-amniotic LPS on airway function in 14 day old rat pups in the absence of postnatal hyperoxia [6]. In contrast, Velten et al observed a significant increase in pulmonary resistance and a non-significant decrease in compliance in 14 day old mouse pups exposed to antenatal LPS and postnatal room air [19]. Their findings are consistent with our respiratory mechanics data, and suggest a unique vulnerability of the immature airways to perinatal inflammation.
Radial alveolar counts are a widely used measure of alveolar morphology [15] and were decreased after LPS exposure. There appeared to be a corresponding decrease in Crs after LPS, although this was not significant. Caffeine did not significantly improve alveolar morphology or Crs after LPS exposure, in contrast to the significant increase in Rrs after LPS which was no longer observed after caffeine. These data suggest that the effect of caffeine is not primarily on alveolar morphology as demonstrated by our Crs and RAC data, but rather on the Rrs of LPS-exposed pups. We do acknowledge that while caffeine-treated LPS-exposed pups no longer differed from controls, there was no significant difference between the two LPS-exposed pups. Future study of inflammatory markers should, therefore, focus on airway related structures and a possible anti-inflammatory effect of caffeine at this site, although beyond the scope of this study. We also acknowledge that future experiments should incorporate IL-1β blockade to clearly document a causal relationship between caffeine treatment and improved respiratory function.
Caffeine acts as a non-specific antagonist of adenosine receptors. However, there is no clear consensus on the mechanisms whereby xanthines might influence inflammation. In contrast to our findings, a protective effect of adenosine A2 receptor agonist has been observed in a mature rat lung injury model [20]. Li showed that chronic caffeine treatment at low dose and acute caffeine treatment at high dose attenuated lung damage and cytokine response in a mature mouse lung injury model, whereas acute caffeine treatment at low dose enhanced lung damage [21]. Their data illustrate the impact of caffeine dose on anti-inflammatory effects, and that this might be independent of A2A receptors.
An anti-inflammatory effect of caffeine in newborns has been suggested, via A1 receptor blockade that inhibits pre-transcriptional TNF-alpha production by cord blood monocytes [22]. Cytokine profiles have been measured in preterm infants and correlated with serum caffeine levels. Plasma IL-1β levels were not clearly influenced by serum caffeine levels, although a pro-inflammatory profile was observed at caffeine levels >20 μg/ml [23]. Recent data employing a neonatal rat pup have shown that in a model of hyperoxic lung injury, caffeine therapy reduced cellular and biochemical markers of pulmonary inflammation [12]. Clearly there is need for further investigation to evaluate the relative roles of adenosine receptor subtype, maturation and caffeine concentration in any anti-inflammatory effects of xanthine therapy in human infants.
In summary, we have provided evidence in a neonatal rat pup model that plasma concentrations of caffeine exert an anti-inflammatory effect that is associated with improvement in respiratory mechanics. Benefit was limited to rat pups exposed to a pro-inflammatory intrauterine environment. It is tempting to speculate that the beneficial effect of caffeine in preterm infants might be enhanced in the presence of chorioamnionitis, a known precipitant of preterm birth and longer term neonatal morbidity.
Acknowledgments
The authors acknowledge Drs. Jeffrey Blumer and Jacob Aranda for assistance with caffeine dosing and analysis, and Juliann Di Fiore for assistance with statistical analysis.
References
- 1.Chess PR, D'Angio CT, Pryhuber GS, Maniscalco WM. Pathogenesis of bronchopulmonary dysplasia. Semin Perinatol. 2006;30:171–178. doi: 10.1053/j.semperi.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Speer CP. Inflammation and bronchopulmonary dysplasia: a continuing story. Semin Fetal Neonatal Med. 2006;11:354–362. doi: 10.1016/j.siny.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Viscardi RM, Muhumuza CK, Rodriguez A, Fairchild KD, Sun CC, Gross GW, Campbell AB, Wilson PD, Hester L, Hasday JD. Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res. 2004;55:1009–1017. doi: 10.1203/01.pdr.0000127015.60185.8a. [DOI] [PubMed] [Google Scholar]
- 4.Jobe AH. Antenatal factors and the development of bronchopulmonary dysplasia. Semin Neonatol. 2003;8:9–17. doi: 10.1016/s1084-2756(02)00188-4. [DOI] [PubMed] [Google Scholar]
- 5.Reyburn B, Martin RJ, Prakash YS, MacFarlane PM. Mechanisms of injury to the preterm lung and airway: Implications for long-term pulmonary outcome. Neonatology. 2012;101:345–352. doi: 10.1159/000337355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi CW, Kim BI, Mason SN, Potts-Kant EN, Brahmajothi MV, Auten RL. Intra-amniotic LPS amplifies hyperoxia-induced airway hyperreactivity in neonatal rats. Pediatr Res. 2013;1:11–18. doi: 10.1038/pr.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Greenough A, Ahmed N. Perinatal prevention of bronchopulmonary dysplasia. J Perinat Med. 2013;41:119–126. doi: 10.1515/jpm-2012-0084. [DOI] [PubMed] [Google Scholar]
- 8.Aranda JV, Beharry K, Valencia GB, Natarajan G, Davis J. Caffeine impact on neonatal morbidities. J Matern Fetal Neonatal Med. 2010;23(Suppl 3):20–23. doi: 10.3109/14767058.2010.517704. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W. Caffeine for Apnea of Prematurity Trial Group: Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354:2112–2121. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W. Caffeine for Apnea of Prematurity Trial Group: Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357:1893–1902. doi: 10.1056/NEJMoa073679. [DOI] [PubMed] [Google Scholar]
- 11.Bancalari E. Caffeine for apnea of prematurity. N Engl J Med. 2006;354:2179–81. doi: 10.1056/NEJMe068028. [DOI] [PubMed] [Google Scholar]
- 12.Weichelt U, Cay R, Schmitz T, Strauss E, Sifringer M, Bührer C, Endesfelder S. Prevention of hyperoxia-mediated pulmonary inflammation in neonatal rats by caffeine. Eur Respir J. 2013;41:966–973. doi: 10.1183/09031936.00012412. [DOI] [PubMed] [Google Scholar]
- 13.Tsuchida S, Engelberts D, Roth M, McKerlie C, Post M, Kavanagh BP. Continuous positive airway pressure causes lung injury in a model of sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;289:L554–L564. doi: 10.1152/ajplung.00143.2005. [DOI] [PubMed] [Google Scholar]
- 14.MacFarlane PM, Frappell PB, Mortola JP. Mechanics of the respiratory system in the newborn tammar wallaby. J Exp Biol. 2002;205:533–538. doi: 10.1242/jeb.205.4.533. [DOI] [PubMed] [Google Scholar]
- 15.Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 1 – Postnatal lung growth. Thorax. 1982;37:572–579. doi: 10.1136/thx.37.8.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakraborty M, McGreal EP, Davies PL, Nowell MA, Jones S, Kotecha S. Role of interleukin-6, its receptor and soluble gp130 in chronic lung disease of prematurity. Neonatology. 2013;104:161–167. doi: 10.1159/000351015. [DOI] [PubMed] [Google Scholar]
- 17.Martin R, Wang K, Köroğlu Ö, Di Fiore J, Kc P. Intermittent hypoxic episodes in preterm infants: Do they matter? Neonatology. 2011;100:303–310. doi: 10.1159/000329922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao L, Wang J, Tseu I, Luo D, Post M. Maternal exposure to endotoxin delays alveolarization during postnatal rat lung development. Am J Physiol. 2009;296:L726–L737. doi: 10.1152/ajplung.90405.2008. [DOI] [PubMed] [Google Scholar]
- 19.Velten M, Heyob KM, Rogers LK, Welty SE. Deficits in lung alveolarization and function after systemic maternal inflammation and neonatal hyperoxia exposure. J Appl Physiol. 2010;108:1347–1356. doi: 10.1152/japplphysiol.01392.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CM, Penuelas O, Quinn K, Cheng KC, Li CF, Zhang H, Slutsky AS. Protective effects of adenosine A2A receptor agonist in ventilator-induced lung injury in rats. Crit Care Med. 2009;37:2235–2241. doi: 10.1097/CCM.0b013e3181a55273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Li G, Hu JL, Fu XH, Zeng YJ, Zhou YG, Xiong G, Yang N, Dai SS, He FT. Chronic or high dose acute caffeine treatment protects mice against oleic acid-induced acute lung injury via an adenosine A2A receptor-independent mechanism. Eur J Pharmacol. 2011;654:295–303. doi: 10.1016/j.ejphar.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 22.Chavez-Valdez R, Wills-Karp M, Ahlawat R, Cristofalo EA, Nathan A, Gauda EB. Caffeine modulates TNF-alpha production by cord blood monocytes: the role of adenosine receptors. Pediatr Res. 2009;65:203–208. doi: 10.1203/PDR.0b013e31818d66b1. [DOI] [PubMed] [Google Scholar]
- 23.Chavez Valdez R, Ahlawat R, Wills-Karp M, Nathan A, Ezell T, Gauda EB. Correlation between serum caffeine levels and changes in cytokine profile in a cohort of preterm infants. J Pediatr. 2011;158:57–64. doi: 10.1016/j.jpeds.2010.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]