Abstract
Background
Prostate cancer treatment is often accompanied by untoward side effects. Therefore, chemoprevention to reduce the risk and inhibit the progression of prostate cancer may be an effective approach to reducing disease burden. We investigated the safety and efficacy of Polyphenon E, a green tea extract, in reducing the progression of prostate cancer in TRAMP mice.
Methods
119 male TRAMP and 119 C57BL/6J mice were treated orally with one of three doses of Polyphenon E (200, 500, 1,000 mg/kg/day) in drinking water ad libitum replicating human achievable doses. Baseline assessments were performed prior to treatments. Safety and efficacy assessments during treatments were performed when mice were 12, 22 and 32 weeks old.
Results
The number and size of tumors in treated TRAMP mice were significantly decreased compared to untreated animals. In untreated 32 weeks old TRAMP mice, prostate carcinoma metastasis to distant sites was observed in 100% of mice (8/8), compared to 13% of mice (2/16) treated with high dose Polyphenon E during the same period. Further, Polyphenon E treatment significantly inhibited metastasis in TRAMP mice in a dose-dependent manner (P=0.0003). Long-term (32 weeks) treatment with Polyphenon E was safe and well tolerated with no evidence of toxicity in C57BL/6J mice.
Conclusion
Polyphenon E is an effective chemopreventive agent in preventing the progression of prostate cancer to metastasis in TRAMP mice. Polyphenon E showed no toxicity in these mouse models.
Impact
Our findings provide additional evidence for the safety and chemopreventive effect of Polyphenon E in preventing metastatic progression of PCa.
Keywords: Polyphenon E, prostate cancer, TRAMP mice
Introduction
Prostate cancer (PCa) is the most common non-melanoma malignancy among men in the Unites States. In 2013, 238,590 new cases and 29,720 deaths related to PCa were estimated.(1) Although early screening and detection has been used historically as strategies for PCa prevention, recently these recommendations have been a subject of much debate.(2-4) The features of PCa, namely, its high prevalence and the uncertainty with regard to the effectiveness and value of early screening provide an excellent opportunity and need to develop alternate PCa control strategies. Chemoprevention to reduce the risk and inhibit the progression of PCa may be an effective approach to reduce the burden of the disease.(5)
Botanicals have been shown to influence multiple biochemical and molecular cascades that inhibit mutagenesis and proliferation, induce apoptosis, and suppress the formation and growth of human cancers, thus modulating several hallmarks of carcinogenesis. Additionally, these agents appear promising in their potential to make a dramatic impact in cancer chemoprevention, with a significantly superior safety profile than most agents evaluated to date.(6-9) Several epidemiological studies have demonstrated a protective effect of tea consumption against human cancers including PCa.(10-13) In contrast, a few studies have associated an increased risk potentially attributed to confounding factors that include consumption of salted or very hot tea, geographical location, tobacco and alcohol use, and other dietary differences.(10, 12-15) Of all the tea produced worldwide, about 20% of green tea is consumed in Asian countries such as China, Japan, Korea and India. Interestingly, these populations consistently demonstrate lower risk of PCa.(16-21)
Several published preclinical studies using green tea, green tea leaves, green tea extracts (GTEs), green tea polyphenol mixtures, green tea catechin (GTC) mixtures, and individual catechins have demonstrated chemopreventive efficacy in PCa.(22-31) Tea and tea compounds reduce growth and/or induce apoptosis in several human cancer cell lines in vitro, including the prostate. Among the constituents of GTEs, laboratory studies have identified epigallocatechin gallate (EGCG) as the most potent chemopreventive agent which appears to affect a number of molecular processes including induction of apoptosis and inhibition of tumor growth and angiogenesis.(32-35) EGCG has been found to affect several cancer-related proteins including p27, Bcl-2 or Bcr-Abl oncoproteins, Bax, matrix metalloproteinases (MMP-2 and MMP-9),(36) the androgen receptor, epidermal growth factor (EGF) receptor, activator proteins 1 (AP1), and some cell cycle regulators.(37-40) By using various proteasome inhibitors, including a standardized mixture of GTCs (Polyphenon E), we and others have demonstrated that the ubiquitin/proteasome pathway plays an essential role in the regulation of apoptosis, and activation of the cellular apoptotic program and may be used as a strategy for PCa chemoprevention.(40)
The safety of tea and tea compounds is supported by centuries of consumption by the human population. In four Phase I single-dose and multi-dose studies of catechins-Polyphenon E containing a dose range of 200–1200 mg EGCG has been well tolerated.(41-44) In recent years, oral use of GTEs has been associated with several instances of hepatotoxicity.(45-48) Most affected patients were women, and many were consuming GTEs for the purpose of weight loss. Although hepatotoxicity in most cases resolved within four months of stopping GTE, there have been cases of positive GTE rechallenge and liver failure requiring liver transplantation.(48) Histological changes in liver, mesenteric lymph nodes and thymus of rodents have also been reported.(49) Elevations in prostate weight, mild liver enzyme elevations, moderate anemia, mild hematopoiesis alterations, and transient ocular symptoms have been noted in some animals administered Polyphenon E.(50) Although previous studies of safety had been established using other compositions of GTCs, to date, there have been no specific reports of the safety and effectiveness of Polyphenon E in reducing the progression of PCa. Although several early clinical trials have been initiated using Polyphenon E, including our clinical trial in progress, based on the available data from preclinical studies, restrictions to eligibility imposed by the FDA continue to place a significant challenge on recruitment of subjects to these trials.(5, 51)
Therefore, the goal of the current study was to examine the safety and efficacy of the specific GTC mixture, Polyphenon E in reducing the progression of PCa in the Transgenic Adenocarcinoma of the Mouse Prostate (TRAMP) mice, an established preclinical model that has been used in PCa chemoprevention studies.
Materials and Methods
Polyphenon E
Polyphenon E study drug is supplied by Mitsui Norin Co., Ltd, Japan. Polyphenon E® is a botanical drug substance containing a mixture of catechins originating from the leaves of green tea (Camellia sinensis). To manufacture Polyphenon E®, a hot water extract of green tea is extracted further with ethyl acetate. The resulting crude extract is dissolved in methanol and purified by affinity column fractionation. Once dried, the final product contains 85–95% total catechins; the main component is EGCG, which comprises 55–72% of the material. Other catechins present in Polyphenon E include epicatechin, epicatechin gallate, epigallocatechin, gallocatechin gallate, gallocatechin, catechin gallate and catechin. Polyphenon E may also contain small quantities of caffeine (<1.0%), theobromine (<1.0%), gallic acid (<0.5%) and other green tea-derived components.
Animals and Tissues
One hundred and nineteen (119) C57BL/6J male mice and 119 TRAMP male mice (The Jackson Laboratory, Bar Harbor, ME), each 5 weeks of age were randomly assigned to one of four experimental groups, each maintained under constant environmental conditions (20-22°C, 40-60% relative humidity, 12 hour light/12 hour dark diurnal cycle), in specific pathogen-free and viral antibody-free (SPF/VAF) conditions fed AIN-76A diet (Harlan Teklad Global Diets, Wilmington, DE) ad libitum, acclimated for two weeks, and used in accordance with an Institutional Animal Care and Use Committee approved protocol.
Polyphenon E treatment protocol
Polyphenon E (Polyphenon Pharma, Inc. Japan) was provided ad libitum in the drinking water to three cohorts each of C57BL/6J and TRAMP mice at one of three doses (200, 500 and 1,000 mg Polyphenon E/kg body weight/day). A fourth cohort each of C57BL/6J and TRAMP mice served as untreated controls. C57BL/6J mice were used to assess safety and for potential toxicities associated with Polyphenon E consumption. TRAMP mice were used to assess efficacy of Polyphenon E consumption to prevent prostate tumor progression to metastasis. Eight C57BL/6J and eight TRAMP mice were euthanatized after acclimation at 7 weeks of age to establish baseline clinical and histopathological findings prior to treatment, after which Polyphenon E or vehicle treatments were initiated in all other mice. Assessments during Polyphenon E treatment were performed when mice were 12, 22 and 32 weeks of age, at which times 11-16 mice of each cohort were evaluated. The volume of Polyphenon E consumed mimicked the consumption of 6-8 cups of green tea per day by an average adult human,(52) as mice were monitored to ensure consumption of ≥0.2 ml drinking water/g body weight/day.
Assessment of Polyphenon E safety
Mice were monitored daily, including for food and water consumption, and were weighed weekly. When mice were 12, 22 and 32 weeks of age, 11-16 mice from each cohort were subjected to phlebotomy and comprehensive systematic necropsy.
The reproductive tract and associated glands including all four lobes of the prostate were weighed and whole blood was collected. Major organs, including the lungs, heart, liver, gall bladder, spleen, pancreas, kidneys, adrenal glands, and the entire alimentary tract, including the esophagus, stomach, duodenum, jejunum, ileum, cecum, colon and the associated mesentery, and mesenteric lymph nodes were evaluated. Tissues were fixed in 10% neutral buffered formalin, dehydrated, embedded in paraffin, sectioned at 5 μm and stained with H&E.
The safety of the three doses (i.e, 200, 500, and 1,000 mg/kg/day) of Polyphenon E was assessed based on clinical and anatomic pathology, including microscopic evaluation of tissues. Blood samples were taken for complete blood counts (CBC) with leukocyte differentials and serum biochemistry indicative of multi-organ functions including total protein, albumin, globulin, glucose, total bilirubin, alanine transaminase (ALT), alkaline phosphatase (ALP), gamma glutamyl transferase (GGT), blood urea nitrogen (BUN), creatinine, calcium and phosphorus levels. Liver function was monitored by levels of ALT, ALP, total bilirubin, and GGT. Kidney function was monitored by levels of BUN and creatinine.
Assessment of Polyphenon E effect on PCa progression
Efficacy of whether three different doses of Polyphenon E modulate PCa development and progression to metastasis was evaluated in TRAMP mice by magnetic resonance imaging (MRI), ultrasound (US), systematic comprehensive necropsy and histopathology. Efficacy of treatment was determined by weight of the prostate, quantifying prostate epithelial pathology, and assessing incidence of tumor progression to metastasis at distant organ sites.
MRI and US analysis
Six TRAMP mice from each group (treated and untreated) were randomly selected and monitored for tumor growth and volume by MRI or US at 32 weeks of age. Animals were induced under 3.5% isoflurane and maintained sedated during MRI and US image acquisition using 2% isoflurane. The animal’s temperature was continuously monitored using a fiber optic probe and controlled using warm air. MRI was completed on a 7 T Varian MRI spectrometer ASR310 (Agilent Life Sciences Technologies, Santa Clara, CA) with a 30 cm bore using VNMRJ 3.1, using a Varian 72 mm quadrature coil. Axial T1 weighted images were obtained to determine the orientation of the coronal slices (using the both kidneys as a plane of reference); these images were obtained using an FSEMS (multi-slice fast spin echo) sequence with TR=600 ms, TE=8.92 ms, field of view=40 mm×40 mm, 20 slices and a matrix size of 128×128. The coronal images were obtained using an FSEMS sequence with TR 2400, 3500 or 4000 ms (depending on number of slices), effective TE=48 ms, field of view=90 mm×40 mm, 21, 28 or 35 slices (depending on the size of the mouse) and a matrix size of 256×192. The coronal images were obtained using respiration gaiting with seven forced block slices to minimize motion artifacts. Images were archived as DICOM, and central slices including a cross section of the bladder were chosen for comparison of resultant gross anatomy. The US images were completed using a Visual Sonics Vevo 2100 high-resolution small animal US. Animal hair in the area of interest was removed via shaving and chemical depilatory (Nair, Church & Dwight Co. Inc. Lakewood, NJ). Mice were placed supine on a thermo regulated platform. Pre-warmed gel was applied to cover the abdomen, and the area was manually surveyed to determine approximate size and location of the prostate. Three dimensional images were then obtained using the 3D functionality of the VEVO2100. Post-processing of the images, including calculation of prostate volume, was completed using the VEVO2100 1.1.1 software.
Measurements and images for figures were taken from images which displayed the bladder to ensure comparable anatomy and measurement sites between animals. All MRIs and US images were taken with the same acquisition parameters between animals (to ensure same scale comparisons/measurements).
Histopathological analysis
To assess effect of Polyphenon E on PCa progression and metastasis, each lobe of the prostate, the liver, lungs, and kidneys were subjected to histological analysis. Each lobe of prostate was evaluated separately (i.e., anterior, dorsal, lateral, and ventral lobes) since lesions have been demonstrated to progress differently in each lobe. Prostates collected at necropsy were assessed and scored for epithelial pathology as previously described.(23) Briefly, scoring of proliferative epithelial lesions in the prostate was based on histological growth patterns observed in each individual lobe. Severity of proliferative lesions was divided into 6 principle categories, 3 levels of hyperplasia, 2 levels of adenoma, and adenocarcinoma, each of which were classified as either focal, multifocal or diffuse. Prostate epithelial hyperplasia of increasing severity presented as simple, flat epithelial lesions with increased basophilia and crowding (grades 1-3), the presence of papillary or cribriform structures and epithelial piling (grades 4-6), or protruding papillary or cribriform structures projecting into the lumen with or without smooth muscle proliferation (grades 7-9). Prostate adenoma presented as papillary or cribriform structures occupying a significant portion of the acinus lumen (grades 10-12), or papillary or cribriform structures filling and/or expanding the acinus lumen (grades 13-15). Prostate adenocarcinoma presented as poorly differentiated epithelia locally invasive beyond the acinus capsule with or without metastasis (grades 16-18). The grade of the most advanced lesion was identified for each lobe and its distribution estimated semi-quantitatively. A numerical score combining grade and distribution of the most advanced lesion in each lobe was assigned and termed the “distribution-adjusted lesion grade”; the mean of these scores was calculated for each treatment group. The presence or absence of metastatic prostate cancerous cells in distant organ sites including the lymph nodes, liver, lung, or kidney was determined.
Statistical analysis
Mean distribution-adjusted lesion grades for treated and untreated mice were compared using a Student’s t-test or ANOVA and proportions were compared using a Chi-squared test. All analyses were done using Statistical Analysis System version 9.3 (SAS Institute, Inc., Cary, NC) and a P<0.05 was considered statistically significant.
Results
Safety of Polyphenon E
There was no noticeable toxicity with regards to mouse appearance, behavior or changes in prostate and body weights after 32 weeks of treatment for all the three doses of Polyphenon E in C57BL6J mice. As expected, the prostate and body weight of C57BL/6J mice in all three groups increased with age. However, there was no statistically significant difference of body and prostate weights between control and treated groups (Supplemental Fig. 1A and 1B). Further, no discernible histopathological changes were observed in the liver, lung, or any prostate lobe of C57BL/6J mice treated with the three different doses of Polyphenon E (Fig. 1A, 1B, 1C and 1D).In hematology and serum biochemistry, none of the variables were changed by long term treatment of Polyphenon E except hemoglobin. The hemoglobin levels in the treated group were increased 3-6% as compared to the control (Table 1).
Figure 1.
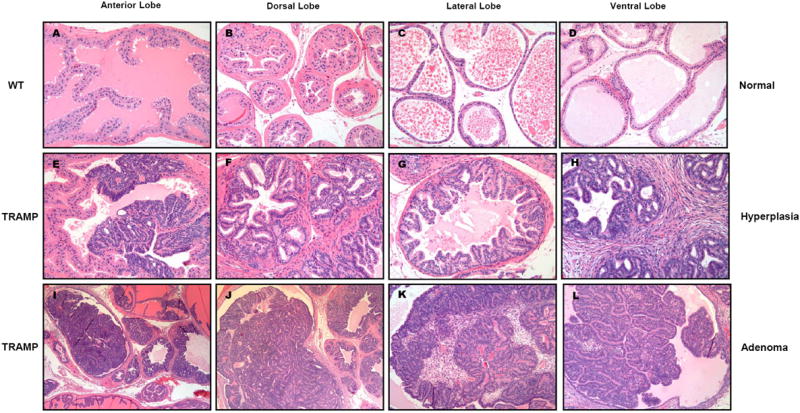
Contrasting histological appearance of the (A) anterior, (B) dorsal, (C) lateral, and (D) ventral lobes of the prostate gland from 32 week old C57BL/6J mice administered high dose treatment of 1000 mg Polyphenon E/kg body weight/day throughout life beginning at 7 weeks of age, and corresponding prostate lobes of age-matched TRAMP mice (E-L) administered the same life-long high dose of Polyphenon E. Prostates of high dose Polyphenon E treated C57BL/6J mice were without significant lesions (A-D), while prostates of high dose Polyphenon E treated 32 week old TRAMP mice developed either epithelial hyperplasia (E-H) or adenoma (I-L), which did not progress to metastatic carcinoma as frequently as untreated TRAMP control mice.
Table 1.
Hematology and serum biochemistry results for C57BL/6J male mice treated with Polyphenon E
| Variables | Unit | Control (n=3) | Low (n=3) | Medium (n=3) | High (n=3) | P value |
|---|---|---|---|---|---|---|
| WBC | 109/L | 5.83±0.88 | 9.8±1.85 | 6.97±4.22 | 4.67±1.69 | 0.27 |
| Lymphocyte | % | 81.4±0.68 | 82.9±1.06 | 77.5±4.63 | 77.1±3.09 | 0.19 |
| Monocyte | % | 6.06±0.12 | 5.03±0.45 | 5.43±0.34 | 5.97±1.02 | 0.32 |
| Granulocyte | % | 12.5±0.57 | 12.1±0.74 | 17.1±4.74 | 16.9±2.08 | 0.17 |
| Hematocrit | % | 36.96±1.22 | 38.43±0.46 | 37.93±0.70 | 38.6±0.21 | 0.21 |
| Hemoglobin | g/dL | 12.66±0.31 | 13.27±0.21 | 13.1±0.16 | 13.4±0.08 | 0.04 |
| Platelet | 109/L | 656.33±33.2 | 567.33±33.87 | 473.33±117.03 | 474.67±156.2 | 0.28 |
| BUN | mg/dL | 26.1±1.80 | 27.1±2.40 | 24.23±0.69 | 24.13±2.17 | 0.37 |
| Creatinine | mg/dL | <0.2 | <0.2 | <0.2 | <0.2 | |
| Phosphorus | mg/dL | 7.2±0.36 | 7.33±0.58 | 8.03±1.35 | 7.73±0.61 | 0.73 |
| Calcium | mg/dL | 10.3±0.0 | 10.17±0.05 | 10.17±0.12 | 10.27±0.17 | 0.33 |
| Total protein | g/dL | 5.6±0.14 | 5.53±0.19 | 5.43±0.09 | 5.5±0.08 | 0.67 |
| Albumin | g/dL | 2.97±0.05 | 2.97±0.17 | 2.87±0.12 | 2.86±0.09 | 0.71 |
| Globulin | g/dL | 2.63±0.09 | 2.57±0.05 | 2.57±0.05 | 2.63±0.05 | 0.55 |
| Glucose | mg/dL | 194.33±9.03 | 185.33±7.13 | 188.67±4.50 | 191.67±15.86 | 0.83 |
| ALT | IU/L | 16.67±0.47 | 19.67±1.70 | 37.0±21.92 | 20.0±0.82 | 0.22 |
| ALP | IU/L | 64.33±2.87 | 63.67±5.31 | 60.33±1.89 | 68.33±2.62 | 0.31 |
| GGT | IU/L | <10 | <10 | <10 | <10 | |
| Total bilirubin | mg/dL | 0.1±0.0 | 0.4±0.14 | 0.17±0.09 | 0.23±0.09 | 0.07 |
Effect of Polyphenon E on PCa development and progression
To determine the effect of Polyphenon E on the progression of PCa, seven week old TRAMP mice were treated with three doses (i.e, 200, 500, and 1000 mg/kg/day) of Polyphenon E until 12, 22 or 32 weeks old. Similar to C57BL/6J mice, there was no statistically significant change of body and prostate weights among treated and untreated TRAMP mice (Supplemental Fig. 1C and 1D). However, we observed a significant difference of prostate weight between untreated C57BL/6J and TRAMP mice at 32 weeks of age (P<0.001)
To determine whether metastasis of the primary tumor had occurred, we examined the tissue for the presence or absence of cancerous cells in the liver, lungs, kidneys, and lymph nodes. All the untreated TRAMP controls (100%, 8/8) developed metastasis after 32 weeks of age compared to only 13% of mice (2/16) treated with high dose Polyphenon E during the same period (Fig. 2A). In addition, we observed a significant dose dependant inhibition of metastasis development with Polyphenon E treatment (P=0.0003).
Figure 2.
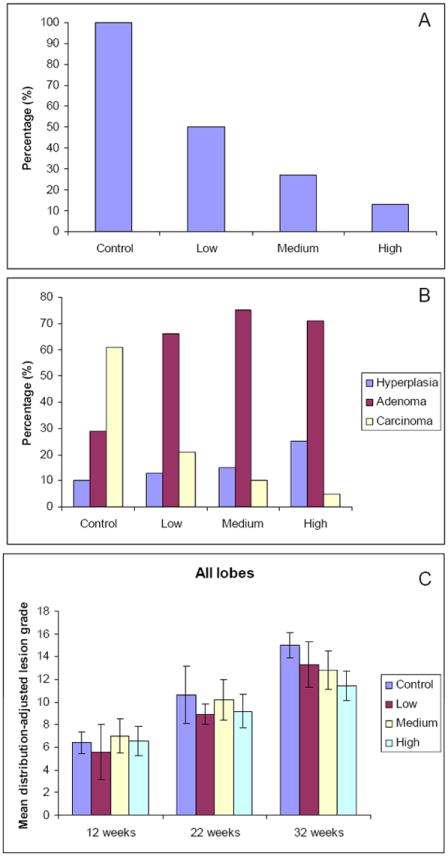
A. Proportion of mice with metastasis: Polyphenon E treatment at low (200 mg/kg/day), medium (500 mg/kg/day), and high (1000 mg/kg/day) doses reduced metastasis. Controls were administered drinking water with no Polyphenon E. Metastasis inhibition by Polyphenon E treatment showed a dose-dependent response (P=0.0003). B. Incidence of hyperplasia, adenoma, and high grade carcinoma in 32 week old TRAMP mice administered low, medium, and high doses of Polyphenon E or tap water (control) (*P<0.05 compared to controls that developed carcinoma). C. Mean distribution-adjusted lesion grade of total and different lobes (anterior, dorsal, lateral and ventral) of the prostate in control TRAMP mice and mice treated with low, medium and high Polyphenon E.
Histopathological evaluation of each lobe of the prostate gland revealed contrasting differences in appearance. Histological morphology of the anterior, dorsal, lateral, and ventral lobes of the prostate gland from 32 week old C57BL/6J mice with high dose Polyphenon E treatment was normal, but significantly different compared to corresponding prostate lobes of age-matched TRAMP mice (Fig. 1E-1L) administered the same life-long high dose of Polyphenon E. Among 59 lobes histologically evaluated in 32 week old TRAMP mice with high dose Polyphenon E treatment, prostate carcinoma were found in only 5% (3/59), while most lobes (96%, 53/59) contained either prostate epithelial hyperplasia (Fig. 1E, 1F, 1G and 1H), or prostate adenoma (Fig. 1J, 1J, 1K and 1L). Hyperplastic prostate epithelium (Fig. 1E-1H) of TRAMP mice was crowded, basophilic, stratified, and formed cribriform or papillary structures that projected into the lumens. Hyperplastic prostate lobes were often surrounded by proliferating smooth muscle (Fig. 1F) or fibrous connective tissue (Fig. 1H). The prostate adenoma (Fig. 1I-1L) in TRAMP mice occupied a significant portion of the prostate acinus lumen as a distinct mass causing distortion or compression of the surrounding glandular tissue.
Prostate cancers of untreated TRAMP mice presented as either an expansile mass of proliferating cells that invaded beyond the acinus basement membrane into the interstitium (Fig. 3A, arrow) or as a cribriform, papillary, tubular mass of well differentiated epithelial cells with supporting stroma that expanded well beyond the acinus capsule and effaced normal glandular structures (Fig. 3B), or as a sheet of pleomorphic epithelial cells that invaded and surrounded glandular acini (Fig. 3C). Further, the tumors were often comprised of anaplastic, pleomorphic cells (Fig. 3D, 3E), with one or multiple metastases to distant foci, including the lymph node (Fig. 3F), lung (Fig. 3G), kidney (Fig. 3H), or liver (Fig. 3I).
Figure 3.
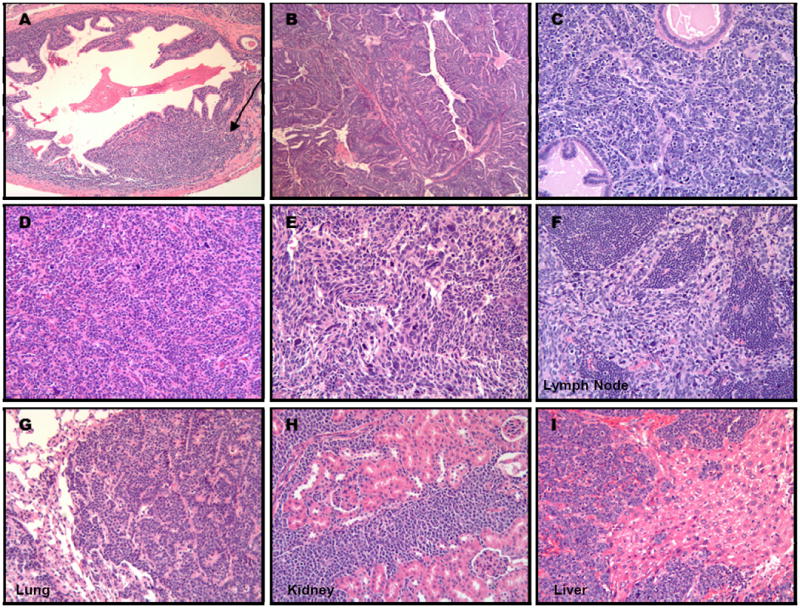
Metastatic prostate adenocarcinoma was abrogated by high dose Polyphenon E consumption, but developed invariably in all 32 week old untreated control TRAMP mice provided drinking water without Polyphenon E treatment (A-I). PCa of TRAMP mice presented as either an expansile mass of proliferating cells that invaded beyond the acinus basement membrane into the interstitium (A, arrow), as a cribriform, papillary, tubular mass of well differentiated epithelial cells with supporting stroma that expanded well beyond the acinus capsule and effaced normal glandular structures (B), or as a sheet of pleomorphic epithelial cells that invaded and surrounded glandular acini (C). PCa of vehicle-treated control TRAMP mice were often comprised of anaplastic, pleomorphic cells (D, E), with one or multiple metastases to distant foci, including the lymph node (F), lung (G), kidney (H), or liver (I).
We compared the mean distribution-adjusted lesion grades of each lobe of the prostate gland among the control and low, medium and high Polyphenon E treatment groups of TRAMP mice. At 12 or 22 weeks of age, mean distribution-adjusted lesion grades of control and Polyphenon E treatment groups of TRAMP mice were not significantly different except within the lateral lobe of the 22 week old Polyphenon E treatment groups (Supplemental Fig. 2C). A significant reduction of lesion progression in the lateral lobe of 22 week old Polyphenon E-treated TRAMP mice was observed (control vs treatment; 11.8±2.6 vs 8.8±2.0, P=0.004). At 32 weeks of age, the mean lesion grades of anterior, dorsal, and lateral lobes were significantly reduced in each of the low, medium, and high dose Polyphenon E-treated cohorts compared to untreated TRAMP controls, P=0.004, P=0.004, P=0.001, respectively (Supplemental Fig. 2A, 2B, and 2C).
Polyphenon E treatment clearly inhibited lesion progression to carcinoma when mean distribution-adjusted lesion grades of all prostatic lobes were considered together after 25 weeks of treatment from 7 to 32 weeks of age (Fig. 2B). In addition, we observed a dose-dependent response in the mean distribution-adjusted lesion grades of the anterior and dorsal lobes, as well as the total prostate in TRAMP mice at this 32 week of age interval (Fig. 2B & Supplemental Fig. 2A & 2B). These trends are evidenced by differences in the mean distribution adjusted grades between groups and by percentage of affected prostatic lobes. Sixty-one percent (17/28) of the prostate lobes of control TRAMP mice developed high grade carcinoma (grades 16-18) as compared to 21% (8/38), 10% (4/40) and 5% (3/59) of the prostate lobes of TRAMP mice treated with low, medium and high Polyphenon E, respectively (Fig. 2C).
In hematology and serum biochemistry, the only differences noted between cohorts were platelet and albumin which were decreased 7-33% and 4-12%, respectively in the TRAMP mice administered a life-long high dose treatment of Polyphenon E as compared to the controls. Means of hemoglobin and serum calcium were also different among cohorts, but there was no discernable trend (Table 2).
Table 2.
Hematology and serum biochemistry results for TRAMP male mice treated with Polyphenon E
| Variables | Unit | Control (n=3) | Low (n=5) | Medium (n=5) | High (n=6) | P value |
|---|---|---|---|---|---|---|
| WBC | 109/L | 7.83±1.21 | 7.48±2.66 | 7.80±2.22 | 8.36±3.48 | 0.97 |
| Lymphocyte | % | 77.4±3.80 | 77.2±3.86 | 77.6±4.93 | 71.4±5.53 | 0.20 |
| Monocyte | % | 6.60±1.06 | 6.04±1.01 | 5.86±0.37 | 6.73±0.67 | 0.37 |
| Granulocyte | % | 16.03±2.74 | 16.72±3.04 | 16.5±4.73 | 21.83±5.05 | 0.20 |
| Hematocrit | % | 38.87±1.55 | 38.4±0.95 | 36.7±1.03 | 38.8±0.88 | 0.05 |
| Hemoglobin | g/dL | 13.1±0.33 | 13.08±0.24 | 12.5±0.34 | 13.1±0.30 | 0.03 |
| Platelet | 109/L | 713.3±14.0 | 663.0±97.2 | 523.8±87.9 | 477.7±114.6 | 0.02 |
| BUN | mg/dL | 18.1±0.19 | 20.3±4.2 | 17.28±2.63 | 19.9±2.18 | 0.42 |
| Creatinine | mg/dL | <0.2 | <0.2 | <0.2 | <0.2 | |
| Phosphorus | mg/dL | 6.9±0.41 | 6.54±0.80 | 7.20±0.65 | 7.77±0.68 | 0.09 |
| Calcium | mg/dL | 10.6±0.19 | 9.94±0.37 | 10.18±0.19 | 10.45±0.05 | 0.01 |
| Total protein | g/dL | 5.8±0.12 | 5.46±0.22 | 5.40±0.09 | 5.61±0.18 | 0.06 |
| Albumin | g/dL | 3.03±0.09 | 2.84±0.12 | 2.68±0.04 | 2.90±0.10 | 0.002 |
| Globulin | g/dL | 2.73±0.21 | 2.62±0.12 | 2.72±0.07 | 2.72±0.18 | 0.71 |
| Glucose | mg/dL | 220.7±5.79 | 215.6±21.3 | 229.6±21.18 | 211.0±19.63 | 0.55 |
| ALT | IU/L | 32.70±4.19 | 29.6±19.4 | 33.6±15.49 | 22.5±4.03 | 0.61 |
| ALP | IU/L | 66.7±2.05 | 71.8±7.14 | 57.4±8.48 | 67.33±6.39 | 0.06 |
| GGT | IU/L | <10 | <10 | <10 | <10 | |
| Total bilirubin | mg/dL | 0.10±0.0 | 0.14±0.08 | 0.18±0.10 | 0.12±0.04 | 0.47 |
MRI and US analysis
MRI and US were used as a measure of tumor progression (Fig. 4A and 4B). The images were taken in sample mice at 32 weeks from each cohort: untreated controls (1) or mice treated with low (2), medium (3) or high (4) doses of Polyphenon E. The MRI shows the high resolution in vivo gross anatomy of the prostate, seminal vesicles and bladder in each of the animals. The white arrows indicate the long axis of the prostate/seminal vesicle tumors showing a decrease in the size from untreated compared to treated (low, medium or high) animals (Fig. 4A). The US images demonstrate decrease in the prostate tumor in the treated group (Fig. 4B), which was significant between control and all treated mice (P<0.04) (Table 3).
Figure 4.
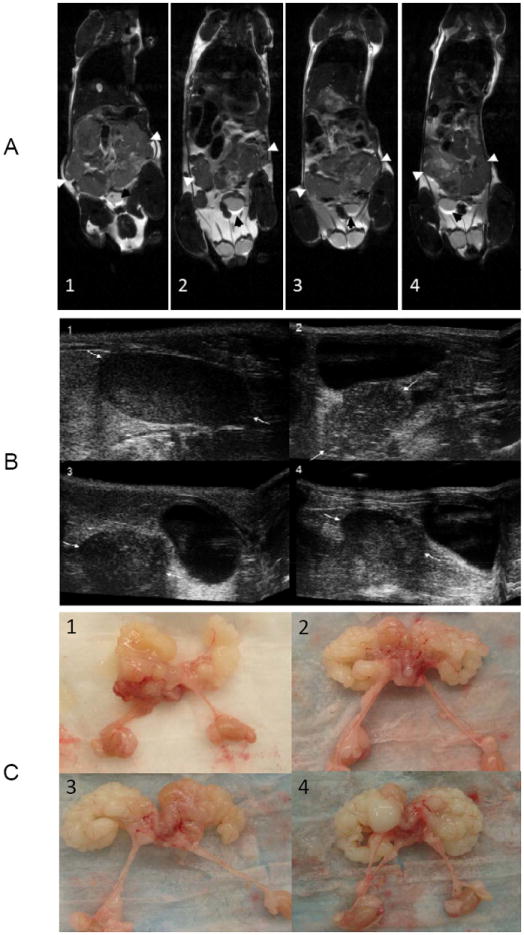
Demonstration of MRI (A), US (B) and gross pathology (C) of prostate tumors in the 32 week group of TRAMP mice. In the MRI, white arrows point to the prostate/seminal vesicles and black arrows point to the bladder for reference. In the US images, white arrows point to prostate tumor. 1, Control; 2, Low; 3, Medium; 4, High
Table 3.
Results from imaging analysis for TRAMP male mice treated with Polyphenon E
| Variables | Unit | Control (n=7) |
Low (n=6) |
Medium (n=6) |
High (n=7) |
P value1 | All treated (n=19) |
P value2 |
|---|---|---|---|---|---|---|---|---|
| # tumor | ea | 1.57±0.98 | 0.83±0.41 | 1.0±0.0 | 0.86±0.38 | 0.09 | 0.89±0.32 | 0.01 |
| Unidimension | 9.67±4.91 | 6.02±3.41 | 6.60±1.33 | 6.53±3.01 | 0.23 | 6.39±2.61 | 0.04 | |
| Bidimension | 30.84±16.6 | 21.7±11.6 | 25.7±8.06 | 26.3±12.4 | 0.65 | 24.7±10.5 | 0.27 | |
| Volume | mm3 | 107.5±60.1 | 90.0±76.9 | 92.5±53.6 | 80.2±36.3 | 0.85 | 87.2±54.0 | 0.42 |
P values were estimated from ANOVA
P values were estimated from t test comparing control vs. treated
During necropsy, ex vivo images of the prostate, seminal vesicles and kidneys were taken to show the final stage of the tumors in each treatment group. These images illustrate seminal vesicle involvement in each of the groups (Fig. 4C1-4).
Discussion
In this study we investigated the safety of Polyphenon E in C57BL/6J mice and its ability to inhibit the progression of PCa in the TRAMP mouse model. We showed that long-term treatment with Polyphenon E is safe and well tolerated with no evidence of toxicity to major organs, such as the lungs, liver and kidneys. Treatment with Polyphenon E at any dose significantly inhibited the progression of prostate lesion formation. Our findings provide additional evidence of the safety and chemopreventive effect of green tea on PCa and most significantly, the inhibition of PCa development and progression to distant organ metastasis.
The slow progression of PCa through initial hyperplasia to adenoma to adenocarcinoma and subsequently to metastatic disease provides an opportunity to intervene in the progression of the disease by chemoprevention.(53-57) Intensive research efforts have been pursued to identify chemopreventive agents for PCa and several substances have been evaluated in preclinical models,(32, 55, 58-61) and human trials.(62-65) Among all the agents examined to date, GTCs have been consistently promising. Since chemopreventive agents are intended for healthy individuals or patients, a high level of safety and anticancer benefits of green tea needs to be established. Our present findings together with previous findings(41-44, 66, 67) show that long term use of a standardized GTC mixture such as Polyphenon E is safe and well tolerated with no adverse effect on major organs of the body, including the prostate.
Our finding that Polyphenon E inhibits the progression and metastasis of PCa in TRAMP mice is similar to that observed in previous studies using other GTC mixtures.(55, 58) These findings are promising and suggest that Polyphenon E is a potential chemoprevention agent for preventing metastatic progression of PCa in humans, despite anatomical differences between the human and mouse prostate. Indeed, as previously stated, early clinical trials of green tea polyphenols involving human PCa patients have shown promising results; although results from these studies have been inconsistent. In some studies, minimal clinical activity or limited anti-neoplastic activity has been observed among hormone-refractory PCa patients treated with GTEs.(64, 65) In other studies, among men with high-grade prostatic intraepithelial neoplasia, tumor incidence was significantly lower in green tea-treated men compared with placebo-treated men and the effect lasted for at least one year.(62, 63) Previous studies in TRAMP mice have also shown that treatment with green tea at an early stage of PCa is more effective than treatment at a later stage of the disease.(32, 58-61) Taken together, these results suggest that intervention with green tea is more beneficial at an earlier stage of the disease. In our study, we started treatment when the TRAMP mice were seven weeks old and had no signs of lesions. Unfortunately, we did not examine the effect of green tea at other stages of cancer development in the present study. Nevertheless, our findings suggest that treatment at an early stage prevents the progression and metastasis of PCa in TRAMP mice, confirming previous findings.(32, 58-61)
Several mechanisms have been proposed for the anti-cancer properties of green tea on PCa. Previous studies suggest that GTCs exert anti-cancer effects on PCa cells by causing apoptosis and cell cycle arrest, and inhibiting angiogenesis and metastasis.(25, 36, 68-70) Our group has shown that GTCs, particularly EGCG, induced prostate epithelial cell apoptosis by inhibiting the proteasome pathway.(33-35) As a result of proteasome inhibition, proteasome targets, p27 and IκBα accumulated in treated cells, leading to cell cycle arrest and apoptosis.(33, 35) We have proposed a novel cumulative model in which GTCs cause proteasome inhibition, which results in cell cycle arrest, inhibition of cell proliferation, apoptosis, and inhibition of angiogenesis and metastasis. We hypothesize that these mechanisms work in concert and largely through the NFκB pathway.(70) We are currently testing this model in our laboratory with Polyphenon E. Davalli et al.(71) showed that Polyphenon E administration inhibited the condensing and secretory activities of the endoplastic reticulum-golgi apparatus, suggesting that the endoplastic reticulum-golgi apparatus could also be the target of GTEs. There is a great need to conduct more studies to gain insight into the GTC-induced chemopreventive mechanisms against PCa. These data are critical to identifying the most appropriate clinical settings to utilize green tea for PCa chemoprevention.
In summary, our results suggest that a standardized mixture of GTCs-Polyphenon E inhibits the growth and progression of PCa in TRAMP mice. Based on our observations, we have shown the safety and efficacy of Polyphenon E in inhibiting the progression of PCa and its metastasis to distant sites in TRAMP mice. Our data support the epidemiologic evidence that green tea may reduce PCa risk in humans. Our findings provide additional evidence for the safety and chemopreventive effect of Polyphenon E in preventing early as well as metastatic progression of PCa.
Supplementary Material
Acknowledgments
The research study was funded by Polyphenon Pharma. Mitsui Norin Co., Japan.
Footnotes
All authors have no conflict of interest, thus no direct or indirect commercial financial incentive associated with this study.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Ilic D, O’Connor D, Green S, Wilt TJ. Screening for prostate cancer: an updated Cochrane systematic review. BJU Int. 2011;107:882–91. doi: 10.1111/j.1464-410X.2010.10032.x. [DOI] [PubMed] [Google Scholar]
- 3.Andriole GL, Crawford ED, Grubb RL, 3rd, Buys SS, Chia D, Church TR, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moyer VA. Force USPST. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–34. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 5.Kumar N, Crocker T, Smith T, Pow-Sang J, Spiess PE, Connors S, et al. Prostate Cancer Chemoprevention Targeting High Risk Populations: Model for Trial Design and Outcome Measures. Journal of cancer science & therapy. 2012;2011 doi: 10.4172/1948-5956.s3-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia L. Cancer complementary and alternative medicine research at the US National Cancer Institute. Chinese journal of integrative medicine. 2012;18:325–32. doi: 10.1007/s11655-011-0950-5. [DOI] [PubMed] [Google Scholar]
- 7.Gogtay NJ, Bhatt HA, Dalvi SS, Kshirsagar NA. The use and safety of non-allopathic Indian medicines. Drug safety : an international journal of medical toxicology and drug experience. 2002;25:1005–19. doi: 10.2165/00002018-200225140-00003. [DOI] [PubMed] [Google Scholar]
- 8.Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. J Clin Oncol. 2009;27:2712–25. doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy BS. Chemoprevention of colon cancer by minor dietary constituents and their synthetic analogues. Prev Med. 1996;25:48–50. doi: 10.1006/pmed.1996.0017. [DOI] [PubMed] [Google Scholar]
- 10.Bushman JL. Green tea and cancer in humans: a review of the literature. Nutr Cancer. 1998;31:151–9. doi: 10.1080/01635589809514697. [DOI] [PubMed] [Google Scholar]
- 11.Kelloff GJ, Crowell JA, Hawk ET, Steele VE, Lubet RA, Boone CW, et al. Strategy and planning for chemopreventive drug development: clinical development plans II. J Cell Biochem Suppl. 1996;26:54–71. doi: 10.1002/jcb.240630705. [DOI] [PubMed] [Google Scholar]
- 12.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Critical reviews in food science and nutrition. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 13.Ahn WS, Yoo J, Huh SW, Kim CK, Lee JM, Namkoong SE, et al. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. Eur J Cancer Prev. 2003;12:383–90. doi: 10.1097/00008469-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Kelloff GJ, Crowell JA, Hawk ET, Steele VE, Lubet RA, Boone CW, Covey JM, Doody LA, Omenn GS, Greenwald P, Hong WK, Parkinson DR, Bagheri D, Baxter GT, Blunden M, Doeltz MK, Eisenhauer KM, Johnson K, Knapp GG, Longfellow DG, Malone WF, Nayfield SG, Seifried HE, Swall LM, Sigman CC. Clinical Development Plan: Tea extracts. green tea polyphenols, epigallocatechin gallate. J Cell Biochem Suppl. 1996;26:236–57. doi: 10.1002/jcb.240630705. [DOI] [PubMed] [Google Scholar]
- 15.Montague JA, Butler LM, Wu AH, Genkinger JM, Koh WP, Wong AS, et al. Green and black tea intake in relation to prostate cancer risk among Singapore Chinese. Cancer Causes Control. 2012;23:1635–41. doi: 10.1007/s10552-012-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson WG. Agents in development for prostate cancer prevention. Expert opinion on investigational drugs. 2004;13:1541–54. doi: 10.1517/13543784.13.12.1541. [DOI] [PubMed] [Google Scholar]
- 17.Jian L, Xie LP, Lee AH, Binns CW. Protective effect of green tea against prostate cancer: a case-control study in southeast China. Int J Cancer. 2004;108:130–5. doi: 10.1002/ijc.11550. [DOI] [PubMed] [Google Scholar]
- 18.Wu AH, Yu MC, Tseng CC, Hankin J, Pike MC. Green tea and risk of breast cancer in Asian Americans. Int J Cancer. 2003;106:574–9. doi: 10.1002/ijc.11259. [DOI] [PubMed] [Google Scholar]
- 19.Denis L, Morton MS, Griffiths K. Diet and its preventive role in prostatic disease. European urology. 1999;35:377–87. doi: 10.1159/000019912. [DOI] [PubMed] [Google Scholar]
- 20.Gupta S, Ahmad N, Mukhtar H. Prostate cancer chemoprevention by green tea. Seminars in urologic oncology. 1999;17:70–6. [PubMed] [Google Scholar]
- 21.Li J, Mercer E, Gou X, Lu YJ. Ethnical disparities of prostate cancer predisposition: genetic polymorphisms in androgen-related genes. American journal of cancer research. 2013;3:127–51. [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10350–5. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suttie A, Nyska A, Haseman JK, Moser GJ, Hackett TR, Goldsworthy TL. A grading scheme for the assessment of proliferative lesions of the mouse prostate in the TRAMP model. Toxicol Pathol. 2003;31:31–8. doi: 10.1080/01926230390173842. [DOI] [PubMed] [Google Scholar]
- 24.Khan N, Mukhtar H. Cancer and metastasis: prevention and treatment by green tea. Cancer Metastasis Rev. 2010;29:435–45. doi: 10.1007/s10555-010-9236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan N, Adhami VM, Mukhtar H. Review: green tea polyphenols in chemoprevention of prostate cancer: preclinical and clinical studies. Nutr Cancer. 2009;61:836–41. doi: 10.1080/01635580903285056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Inst. 1997;89:1881–6. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- 27.Baliga MS, Meleth S, Katiyar SK. Growth inhibitory and antimetastatic effect of green tea polyphenols on metastasis-specific mouse mammary carcinoma 4T1 cells in vitro and in vivo systems. Clin Cancer Res. 2005;11:1918–27. doi: 10.1158/1078-0432.CCR-04-1976. [DOI] [PubMed] [Google Scholar]
- 28.Thangapazham RL, Singh AK, Sharma A, Warren J, Gaddipati JP, Maheshwari RK. Green tea polyphenols and its constituent epigallocatechin gallate inhibits proliferation of human breast cancer cells in vitro and in vivo. Cancer Lett. 2007;245:232–41. doi: 10.1016/j.canlet.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 29.Yang GY, Liao J, Kim K, Yurkow EJ, Yang CS. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis. 1998;19:611–6. doi: 10.1093/carcin/19.4.611. [DOI] [PubMed] [Google Scholar]
- 30.Adhami VM, Mukhtar H. Human cancer chemoprevention: hurdles and challenges. Topics in current chemistry. 2013;329:203–20. doi: 10.1007/128_2012_342. [DOI] [PubMed] [Google Scholar]
- 31.Lee SC, Chan WK, Lee TW, Lam WH, Wang X, Chan TH, et al. Effect of a prodrug of the green tea polyphenol (-)-epigallocatechin-3-gallate on the growth of androgen-independent prostate cancer in vivo. Nutr Cancer. 2008;60:483–91. doi: 10.1080/01635580801947674. [DOI] [PubMed] [Google Scholar]
- 32.Caporali A, Davalli P, Astancolle S, D’Arca D, Brausi M, Bettuzzi S, et al. The chemopreventive action of catechins in the TRAMP mouse model of prostate carcinogenesis is accompanied by clusterin over-expression. Carcinogenesis. 2004;25:2217–24. doi: 10.1093/carcin/bgh235. [DOI] [PubMed] [Google Scholar]
- 33.Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. The Journal of biological chemistry. 2001;276:13322–30. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- 34.Kazi A, Wang Z, Kumar N, Falsetti SC, Chan TH, Dou QP. Structure-activity relationships of synthetic analogs of (-)-epigallocatechin-3-gallate as proteasome inhibitors. Anticancer research. 2004;24:943–54. [PubMed] [Google Scholar]
- 35.Smith DM, Wang Z, Kazi A, Li LH, Chan TH, Dou QP. Synthetic analogs of green tea polyphenols as proteasome inhibitors. Mol Med. 2002;8:382–92. [PMC free article] [PubMed] [Google Scholar]
- 36.Adhami VM, Ahmad N, Mukhtar H. Molecular targets for green tea in prostate cancer prevention. J Nutr. 2003;133:2417S–24S. doi: 10.1093/jn/133.7.2417S. [DOI] [PubMed] [Google Scholar]
- 37.Liang YC, Lin-shiau SY, Chen CF, Lin JK. Suppression of extracellular signals and cell proliferation through EGF receptor binding by (-)-epigallocatechin gallate in human A431 epidermoid carcinoma cells. Journal of cellular biochemistry. 1997;67:55–65. doi: 10.1002/(sici)1097-4644(19971001)67:1<55::aid-jcb6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 38.Chung JY, Huang C, Meng X, Dong Z, Yang CS. Inhibition of activator protein 1 activity and cell growth by purified green tea and black tea polyphenols in H-ras-transformed cells: structure-activity relationship and mechanisms involved. Cancer Res. 1999;59:4610–7. [PubMed] [Google Scholar]
- 39.Liang YC, Lin-Shiau SY, Chen CF, Lin JK. Inhibition of cyclin-dependent kinases 2 and 4 activities as well as induction of Cdk inhibitors p21 and p27 during growth arrest of human breast carcinoma cells by (-)-epigallocatechin-3-gallate. Journal of cellular biochemistry. 1999;75:1–12. [PubMed] [Google Scholar]
- 40.Kazi A, Daniel KG, Smith DM, Kumar NB, Dou QP. Inhibition of the proteasome activity, a novel mechanism associated with the tumor cell apoptosis-inducing ability of genistein. Biochem Pharmacol. 2003;66:965–76. doi: 10.1016/s0006-2952(03)00414-3. [DOI] [PubMed] [Google Scholar]
- 41.Chow HH, Cai Y, Alberts DS, Hakim I, Dorr R, Shahi F, et al. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol Biomarkers Prev. 2001;10:53–8. [PubMed] [Google Scholar]
- 42.Chow HH, Hakim IA, Vining DR, Crowell JA, Ranger-Moore J, Chew WM, et al. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin Cancer Res. 2005;11:4627–33. doi: 10.1158/1078-0432.CCR-04-2549. [DOI] [PubMed] [Google Scholar]
- 43.Pisters KM, Newman RA, Coldman B, Shin DM, Khuri FR, Hong WK, et al. Phase I trial of oral green tea extract in adult patients with solid tumors. J Clin Oncol. 2001;19:1830–8. doi: 10.1200/JCO.2001.19.6.1830. [DOI] [PubMed] [Google Scholar]
- 44.Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–9. [PubMed] [Google Scholar]
- 45.Bonkovsky HL. Hepatotoxicity associated with supplements containing Chinese green tea (Camellia sinensis) Annals of internal medicine. 2006;144:68–71. doi: 10.7326/0003-4819-144-1-200601030-00020. [DOI] [PubMed] [Google Scholar]
- 46.Molinari M, Watt KD, Kruszyna T, Nelson R, Walsh M, Huang WY, et al. Acute liver failure induced by green tea extracts: case report and review of the literature. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2006;12:1892–5. doi: 10.1002/lt.21021. [DOI] [PubMed] [Google Scholar]
- 47.Pedros C, Cereza G, Garcia N, Laporte JR. [Liver toxicity of Camellia sinensis dried etanolic extract] Medicina clinica. 2003;121:598–9. doi: 10.1016/s0025-7753(03)74026-3. [DOI] [PubMed] [Google Scholar]
- 48.Mazzanti G, Menniti-Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C, et al. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. European journal of clinical pharmacology. 2009;65:331–41. doi: 10.1007/s00228-008-0610-7. [DOI] [PubMed] [Google Scholar]
- 49.Chan PC, Ramot Y, Malarkey DE, Blackshear P, Kissling GE, Travlos G, et al. Fourteen-week toxicity study of green tea extract in rats and mice. Toxicol Pathol. 2010;38:1070–84. doi: 10.1177/0192623310382437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kapetanovic IM, Crowell JA, Krishnaraj R, Zakharov A, Lindeblad M, Lyubimov A. Exposure and toxicity of green tea polyphenols in fasted and non-fasted dogs. Toxicology. 2009;260:28–36. doi: 10.1016/j.tox.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar N, Crocker T, Smith T, Pow-Sang J, Spiess PE, Egan K, et al. Challenges and potential solutions to meeting accrual goals in a Phase II chemoprevention trial for prostate cancer. Contemporary clinical trials. 2012;33:279–85. doi: 10.1016/j.cct.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang ZY, Agarwal R, Bickers DR, Mukhtar H. Protection against ultraviolet B radiation-induced photocarcinogenesis in hairless mice by green tea polyphenols. Carcinogenesis. 1991;12:1527–30. doi: 10.1093/carcin/12.8.1527. [DOI] [PubMed] [Google Scholar]
- 53.Adhami VM, Mukhtar H. Anti-oxidants from green tea and pomegranate for chemoprevention of prostate cancer. Molecular biotechnology. 2007;37:52–7. doi: 10.1007/s12033-007-0047-8. [DOI] [PubMed] [Google Scholar]
- 54.Kelloff GJ, Lippman SM, Dannenberg AJ, Sigman CC, Pearce HL, Reid BJ, et al. Progress in chemoprevention drug development: the promise of molecular biomarkers for prevention of intraepithelial neoplasia and cancer--a plan to move forward. Clin Cancer Res. 2006;12:3661–97. doi: 10.1158/1078-0432.CCR-06-1104. [DOI] [PubMed] [Google Scholar]
- 55.Siddiqui IA, Malik A, Adhami VM, Asim M, Hafeez BB, Sarfaraz S, et al. Green tea polyphenol EGCG sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated apoptosis and synergistically inhibits biomarkers associated with angiogenesis and metastasis. Oncogene. 2008;27:2055–63. doi: 10.1038/sj.onc.1210840. [DOI] [PubMed] [Google Scholar]
- 56.Syed DN, Khan N, Afaq F, Mukhtar H. Chemoprevention of prostate cancer through dietary agents: progress and promise. Cancer Epidemiol Biomarkers Prev. 2007;16:2193–203. doi: 10.1158/1055-9965.EPI-06-0942. [DOI] [PubMed] [Google Scholar]
- 57.Syed DN, Suh Y, Afaq F, Mukhtar H. Dietary agents for chemoprevention of prostate cancer. Cancer Lett. 2008;265:167–76. doi: 10.1016/j.canlet.2008.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adhami VM, Siddiqui IA, Ahmad N, Gupta S, Mukhtar H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64:8715–22. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- 59.Harper CE, Patel BB, Wang J, Eltoum IA, Lamartiniere CA. Epigallocatechin-3-Gallate suppresses early stage, but not late stage prostate cancer in TRAMP mice: mechanisms of action. Prostate. 2007;67:1576–89. doi: 10.1002/pros.20643. [DOI] [PubMed] [Google Scholar]
- 60.McCarthy S, Caporali A, Enkemann S, Scaltriti M, Eschrich S, Davalli P, et al. Green tea catechins suppress the DNA synthesis marker MCM7 in the TRAMP model of prostate cancer. Mol Oncol. 2007;1:196–204. doi: 10.1016/j.molonc.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sartor L, Pezzato E, Dona M, Dell’Aica I, Calabrese F, Morini M, et al. Prostate carcinoma and green tea: (-) epigallocatechin-3-gallate inhibits inflammation-triggered MMP-2 activation and invasion in murine TRAMP model. Int J Cancer. 2004;112:823–9. doi: 10.1002/ijc.20496. [DOI] [PubMed] [Google Scholar]
- 62.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–40. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 63.Brausi M, Rizzi F, Bettuzzi S. Chemoprevention of human prostate cancer by green tea catechins: two years later. A follow-up update. European urology. 2008;54:472–3. doi: 10.1016/j.eururo.2008.03.100. [DOI] [PubMed] [Google Scholar]
- 64.Choan E, Segal R, Jonker D, Malone S, Reaume N, Eapen L, et al. A prospective clinical trial of green tea for hormone refractory prostate cancer: an evaluation of the complementary/alternative therapy approach. Urologic oncology. 2005;23:108–13. doi: 10.1016/j.urolonc.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 65.Jatoi A, Ellison N, Burch PA, Sloan JA, Dakhil SR, Novotny P, et al. A phase II trial of green tea in the treatment of patients with androgen independent metastatic prostate carcinoma. Cancer. 2003;97:1442–6. doi: 10.1002/cncr.11200. [DOI] [PubMed] [Google Scholar]
- 66.Adhami VM, Aziz MH, Reagan-Shaw SR, Nihal M, Mukhtar H, Ahmad N. Sanguinarine causes cell cycle blockade and apoptosis of human prostate carcinoma cells via modulation of cyclin kinase inhibitor-cyclin-cyclin-dependent kinase machinery. Mol Cancer Ther. 2004;3:933–40. [PubMed] [Google Scholar]
- 67.Ullmann U, Haller J, Decourt JP, Girault N, Girault J, Richard-Caudron AS, et al. A single ascending dose study of epigallocatechin gallate in healthy volunteers. J Int Med Res. 2003;31:88–101. doi: 10.1177/147323000303100205. [DOI] [PubMed] [Google Scholar]
- 68.Saleem M, Adhami VM, Siddiqui IA, Mukhtar H. Tea beverage in chemoprevention of prostate cancer: a mini-review. Nutr Cancer. 2003;47:13–23. doi: 10.1207/s15327914nc4701_2. [DOI] [PubMed] [Google Scholar]
- 69.Pandey M, Gupta S. Green tea and prostate cancer: from bench to clinic. Frontiers in bioscience. 2009;1:13–25. doi: 10.2741/s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Connors SK, Chornokur G, Kumar NB. New insights into the mechanisms of green tea catechins in the chemoprevention of prostate cancer. Nutr Cancer. 2012;64:4–22. doi: 10.1080/01635581.2012.630158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davalli P, Rizzi F, Caldara GF, Davoli S, Corti A, Silva A, et al. Chronic administration of green tea extract to TRAMP mice induces the collapse of Golgi apparatus in prostate secretory cells and results in alterations of protein post-translational processing. International journal of oncology. 2011;39:1521–7. doi: 10.3892/ijo.2011.1136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


