Summary
In monocytes and macrophages, the interaction of Porphyromonas gingivalis with Toll-like receptor 2 (TLR2) leads to the activation of a MyD88-dependent antimicrobial pathway and a phosphatidylinositol-3 kinase (PI3K)-dependent proadhesive pathway, which activates the β2 integrin complement receptor 3 (CR3). By means of its fimbriae, P. gingivalis binds CXC-chemokine receptor 4 (CXCR4) and induces crosstalk with TLR2 that inhibits the MyD88-dependent antimicrobial pathway. In this paper, we investigated the impact of the P. gingivalis-CXCR4 interaction on the proadhesive pathway. Using human monocytes, mouse macrophages, or receptor-transfected cell lines, we showed that the binding of P. gingivalis fimbriae to CXCR4 induces CR3 activation via PI3K, albeit in a TLR2-independent manner. An isogenic strain of P. gingivalis expressing mutant fimbriae that do not interact with CXCR4 failed to efficiently activate CR3, leading to enhanced susceptibility to killing in vivo as compared with the wild-type organism. This in vivo observation is consistent with previous findings that activated CR3 mediates safe entry of P. gingivalis into macrophages. Taken together with our previous work, these results indicate that the interaction of P. gingivalis with CXCR4 leads to inhibition of antimicrobial responses and enhancement of proadhesive responses, thereby maximizing its adaptive fitness in the mammalian host.
Keywords: P. gingivalis, CXCR4, TLR2, integrin, immune evasion, periodontitis
Introduction
Pattern-recognition receptors expressed on sentinel immune cells detect microbial pathogens and initiate a complex set of signaling pathways (Kawai & Akira, 2010). The host-cell response represents the integration of activated signaling pathways that may result in synergistic or antagonistic effects (Hajishengallis & Lambris, 2010; Natarajan et al., 2006). Therefore, signaling crosstalk may potentiate the host response or regulate it to prevent unwarranted inflammation. However, pathogens may also instigate crosstalk signaling for subverting or skewing the host response in ways that promote their virulence and survival (Hajishengallis & Lambris, 2011).
We have previously shown that Porphyromonas gingivalis, a keystone pathogen in periodontal disease (Hajishengallis & Lamont, 2012; Hajishengallis et al., 2011), induces crosstalk between the CXC-chemokine receptor 4 (CXCR4) and Toll-like receptor 2 (TLR2) that undermines the killing function of human monocytes or mouse macrophages (Hajishengallis et al., 2008). Specifically, P. gingivalis induces co-association and activation of CXCR4 and TLR2 in membrane lipid rafts resulting in enhanced cAMP-dependent protein kinase A (PKA) signaling, which in turn inhibits the production of nitric oxide, a potent antimicrobial molecule (Hajishengallis et al., 2008). Consistent with this, treatment of mice with a CXCR4 antagonist, the bicyclam drug AMD3100 (Donzella et al., 1998), confers protection against periodontal tissue colonization by P. gingivalis and development of periodontitis (McIntosh & Hajishengallis, 2012).
The P. gingivalis virulence factor responsible for CXCR4 exploitation is its surface fimbriae, which comprise polymerized fimbrilin (FimA) associated with a complex of accessory proteins (FimCDE) (Hajishengallis et al., 2008; Nishiyama et al., 2007; Pierce et al., 2009). Specifically, the binding of P. gingivalis fimbriae to CXCR4 is mediated by the FimCDE complex, whereas mutant fimbriae devoid of these accessory proteins (dubbed DAP fimbriae) fail to interact with CXCR4 (Pierce et al., 2009).
In monocytes and macrophages, TLR2 activation by P. gingivalis induces two distinct signaling pathways, a MyD88-dependent antimicrobial pathway and phosphatidylinositol-3 kinase (PI3K)-dependent proadhesive pathway (Hajishengallis et al., 2009). The proadhesive pathway involves TLR2 inside-out signaling via Rac1, PI3K, and cytohesin-1, leading to activation of complement receptor 3 (CR3; a β2 integrin consisting of CD11b and CD18 subunits), which thereby assumes its high-affinity binding state (Hajishengallis et al., 2009; Harokopakis et al., 2006). Although the P. gingivalis-induced CXCR4-TLR2 crosstalk inhibits the TLR2/MyD88-dependent pathway (Hajishengallis et al., 2008), its effect on the TLR2/CR3 proadhesive pathway is yet to be determined.
CR3 is not linked to strong microbicidal mechanisms (Caron & Hall, 1998; Lowell, 2006; Ricklin et al., 2010) and is exploited by P. gingivalis and other pathogens as a safe portal of entry that permits enhanced intracellular survival (Hajishengallis & Lambris, 2011; Oliva et al., 2009; Wang et al., 2007). The objective of this study was to determine whether the interaction of P. gingivalis with CXCR4 exerts an impact on the proadhesive pathway. If so, this could constitute a second mechanism by which P. gingivalis exploits CXCR4 to promote its adaptive fitness.
Methods
Reagents
Monoclonal antibodies (mAbs) to human CD11b (clone CBRM1/5, FITC-labeled; IgG1), to human/mouse CD11b (clone M1/70; IgG2b), or to mouse CXCR4 (clone 247506; IgG2b) and isotype controls were from R&D Systems. Immunoglobulin isotype controls were purchased from eBioscience. AMD3100 (CXCR4 inhibitor), phorbol myristate acetate (PMA), wortmannin (irreversible inhibitor of PI3K), LY294002 (reversible inhibitor of PI3K), LY30351 (inactive analog of LY294002), H89 (PKA inhibitor; blocks the ATP site of the enzyme), and GF109203X (inhibitor of protein kinase C; PKC) were from Sigma-Aldrich, and PKI 6-22 (PKA inhibitor; blocks the substrate site) from Calbiochem. Recombinant human or mouse Intercellular Adhesion Molecule-1 (ICAM-1) was purchased from the R&D Systems. The small-molecule inhibitor XVA143 (m.w. 585.35), which antagonizes CR3 (Harokopakis et al., 2006; Shimaoka et al., 2002), was kindly provided by N. Fotouhi (Roche, Nutley, NJ). P. gingivalis ATCC 33277 and its isogenic mutant OZ5001C were grown anaerobically at 37°C in hemin- and menadione-containing Gifu anaerobic medium (GAM) medium (Nissui Pharmaceutical) (Wang et al., 2007) and their fimbriae were extracted and chromatographically purified as previously described (Yoshimura et al., 1984). The final fimbrial preparations were free of any contaminating substances on silver-stained SDS-PAGE, and tested negative for endotoxin (<0.7 ng/mg protein) according to quantitative Limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD). Moreover, the purity of the fimbriae was confirmed using an Applied Biosystems 4800 MALDI TOF/TOF analyzer. All reagents were used at effective concentrations determined in preliminary experiments or in previous publications (Hajishengallis et al., 2008; Harokopakis et al., 2006; Pierce et al., 2009).
Cell culture
Monocytes were purified from human peripheral blood upon centrifugation over NycoPrep™1.068 (Axis-Shield, Oslo, Norway) as previously described (Harokopakis & Hajishengallis, 2005). Incidental non-monocytes were removed by magnetic depletion using a cocktail of biotin-conjugated mAbs and magnetic microbeads coupled to anti-biotin mAb (Monocyte isolation kit II; Miltenyi Biotec, Auburn, CA). Purified monocytes were cultured at 37°C and 5% CO2 atmosphere, in RPMI 1640 (InVitrogen/Gibco, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 10 mM HEPES, 100 units/ml penicillin G, 100 μg/ml streptomycin, and 0.05 mM 2-mercaptoethanol (complete RPMI). Human blood collections were conducted under institutional review board (IRB) approval and in compliance with established federal guidelines. Chinese hamster ovary (CHO) cells stably transfected with human CR3 (CHO-CR3 cells) were kindly provided by Dr. D.T. Golenbock (University of Massachusetts Medical School, Worcester, MA) (Levitz et al., 1997). These cells were cultured in Ham's F-12 nutrient mixture (InVitrogen/Gibco) supplemented with 2 mM L-glutamine, 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin. CHO-CR3 cells were transiently transfected with TLR2 and/or CXCR4 using the PolyFect transfection reagent (Qiagen), as we previously described (Hajishengallis et al., 2006; Hajishengallis et al., 2008; Pierce et al., 2009). Briefly, CHO-CR3 cells were transfected with human TLR2 and CD14, using a single plasmid (pDUO-hCD14/TLR2; InVivogen), with or without cotransfection with human CXCR4 (pORF-hCXCR4; InVivogen). The total amount of plasmid DNA per well was kept constant by supplementing with empty control vector. The cells were used in functional assays 48h post-transfection. Thioglycollate-elicited macrophages were isolated from the peritoneal cavity of C57BL/6 mice (The Jackson Laboratory), as we previously described (Hajishengallis et al., 2005b). Briefly, mice were intraperitoneally injected with 1 ml of sterile 3% thioglycollate, and cells were harvested 5 days later by flushing the peritoneal cavity with 10 ml of ice-cold PBS three times. Isolated cells were then subjected to density gradient centrifugation (Histopaque 1.083) to remove dead cells and red blood cell contamination. The purity of macrophage preparations (>90%) was confirmed by flow cytometry using phycoerythrin-labeled anti-F4/80 (clone BM8; eBioscience). The macrophages were rested overnight (at 37°C and 5% CO2 atmosphere in complete RPMI) prior to use in experiments. All animal procedures were approved by the Institutional Animal Care and Use Committee, in compliance with established Federal and State policies. Cell viability was monitored using the CellTiter-Blue™ assay kit (Promega, Madison, WI). None of the experimental treatments affected cell viability compared to medium-only control treatments.
Binding assay for P. gingivalis fimbriae
Biotinylated wild-type or DAP fimbriae (1 μg/ml) were allowed to bind to human monocytes or human cell lines for 30 min at 37°C, as previously described (Harokopakis & Hajishengallis, 2005). Subsequently, the cells were washed and incubated on ice with FITC-labeled streptavidin. After washing, binding was determined by measuring cell-associated fluorescence (in relative fluorescence units) on a microplate fluorescence reader (FL600, Bio-Tek Instruments) with excitation/emission wavelength settings of 485/530 nm. Background fluorescence was determined in cells treated with medium only and FITC-streptavidin.
CR3 activation assays
The CBRM1/5 epitope induction assay was used to monitor the activation state of human CR3 (CD11b/CD18), as we have previously described (Harokopakis & Hajishengallis, 2005). The assay is based on the property of the CBRM1/5 mAb to detect a conformational change on human CD11b that signifies the high-affinity binding state of CR3 (Diamond & Springer, 1993). Activation of mouse CR3 was assessed by monitoring its binding activity for soluble ICAM-1, a ligand that binds activated but not resting CR3 (Diamond et al., 1993; Harokopakis et al., 2006). Specifically, biotinylated sICAM-1 was allowed to bind to mouse macrophages for 30 min at 37°C. Subsequently, the cells were washed and incubated on ice with FITC-labeled streptavidin. After washing, binding was determined by measuring cell-associated fluorescence (in relative fluorescence units) on a microplate fluorescence reader (FL600, Bio-Tek) with excitation/emission wavelength settings of 485/530 nm. Background fluorescence was determined in cells treated with medium only and FITC-streptavidin.
Intraperitoneal infection model
Specific pathogen-free BALB/cByJ mice (8–10 wk old; The Jackson Laboratory) were pretreated with AMD3100 (i.p., 25 μg in 0.1 ml PBS) or PBS alone. After 1h, the mice were infected i.p. with P. gingivalis 33277 or OZ5001C (5×107 colony forming units; CFU). Peritoneal lavage was performed 24h postinfection. Serial 10-fold dilutions of peritoneal fluid were plated onto blood agar plates and cultured anaerobically at 37°C for enumerating recovered peritoneal CFU. All animal procedures were approved by the Institutional Animal Care and Use Committee and performed in compliance with established Federal and State policies
Statistical analysis
Data were evaluated by analysis of variance and the Dunnett multiple-comparison test using the InStat program (GraphPad Software, San Diego, CA). Where appropriate (comparison of two groups only), two-tailed t tests were also performed. P < 0.05 was taken as the level of significance. All experiments were performed at least twice for verification.
Results
The interaction of P. gingivalis fimbriae with CXCR4 induces CR3 activation
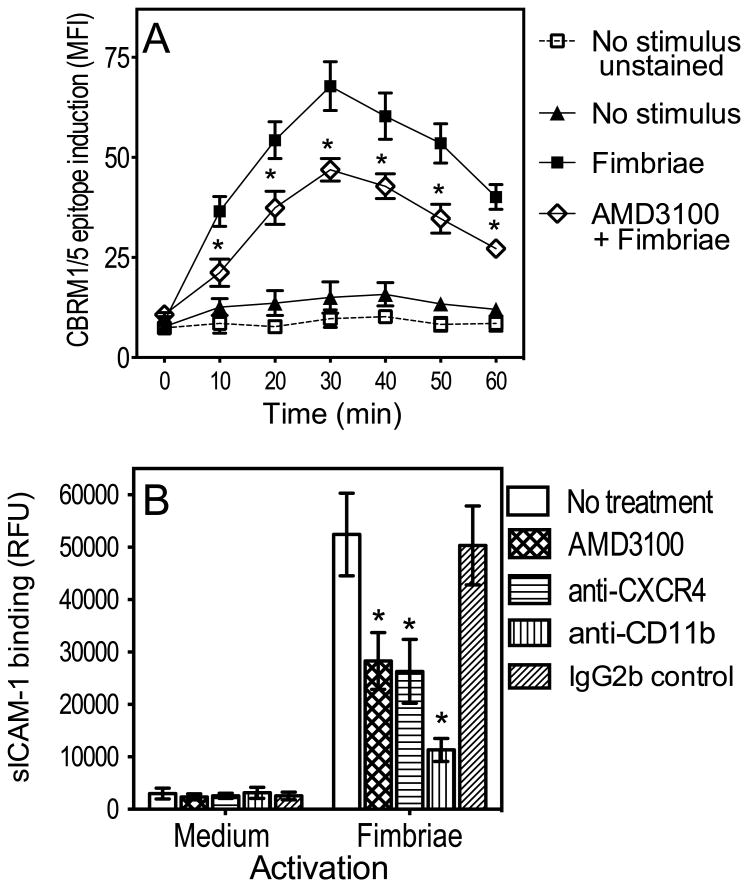
To determine the role of CXCR4 in P. gingivalis fimbria-induced activation of CR3 in human monocytes, we examined whether the CXCR4 antagonist AMD3100 inhibits the ability of fimbriae to induce an activation-specific epitope (CBRM1/5) (Diamond & Springer, 1993; Harokopakis & Hajishengallis, 2005). AMD3100 was used at 1 μg/ml, which completely inhibits the interaction of fimbriae with CXCR4 (Hajishengallis et al., 2008). Induction of the CBRM1/5 epitope was evident at 10 min after stimulation with fimbriae, peaked at 30 min, and slowly declined thereafter, consistent with the transient nature of CR3 activation (Harokopakis & Hajishengallis, 2005; Shimaoka et al., 2002). However, the induction of the CBRM1/5 epitope was suppressed in the presence of AMD3100 (P < 0.01; Fig. 1A), suggesting that CXCR4 contributes to proadhesive signaling for CR3 activation.
Figure 1. CXCR4 is involved in P. gingivalis fimbria-induced CR3 activation.
(A) Human monocytes were stimulated or not with fimbriae (1μg/ml) for the indicated times, with or without AMD3100 (1μg/ml), and assayed for CBRM1/5 epitope induction using FITC-labeled CBRM1/5 mAb and flow cytometry. FITC-labeled IgG1 control was used to assess background fluorescence (“unstained” group). CBRM1/5 induction is reported in mean fluorescence intensity (MFI) values. (B) Mouse peritoneal macrophages were activated with fimbriae (1 μg/ml), with or without AMD3100 (1 μg/ml) or anti-CXCR4 (5 μg/ml), and assayed for CR3-dependent binding of FITC-labeled sICAM-1 at 30 min following activation. CR3 dependence was confirmed by including groups treated with anti-CR3 (anti-CD11b) mAb or isotype control. sICAM-1 binding is reported in relative fluorescent units (RFU). Data are means ± SD (n = 3). *, statistically significant (P < 0.01) inhibition of CBRM1/5 epitope induction (A) or of sICAM-1 binding (B).
The notion that CXCR4 activates CR3 was further substantiated by experiments in mouse macrophages: CXCR4 blockade with AMD3100 or a blocking anti-CXCR4 monoclonal antibody (mAb) suppressed the ability of P. gingivalis fimbria-activated mouse macrophages to bind soluble ICAM-1 (sICAM-1) (Fig. 1B), a ligand that binds activated but not resting CR3 (Diamond et al., 1993; Hajishengallis et al., 2009). Indeed, as expected, the binding of sICAM-1 to medium-treated macrophages was negligible (Fig. 1B). Moreover, a mAb to the CD11b subunit of CR3 inhibited the binding of sICAM-1 to fimbria-activated macrophages confirming the CR3 dependence of sICAM-1 binding (Fig. 1B). Taken together, these data show that P. gingivalis fimbriae induce CXCR4-dependent CR3 activation.
PI3K, but not TLR2, is involved in CXCR4-dependent CR3 activation by P. gingivalis fimbriae
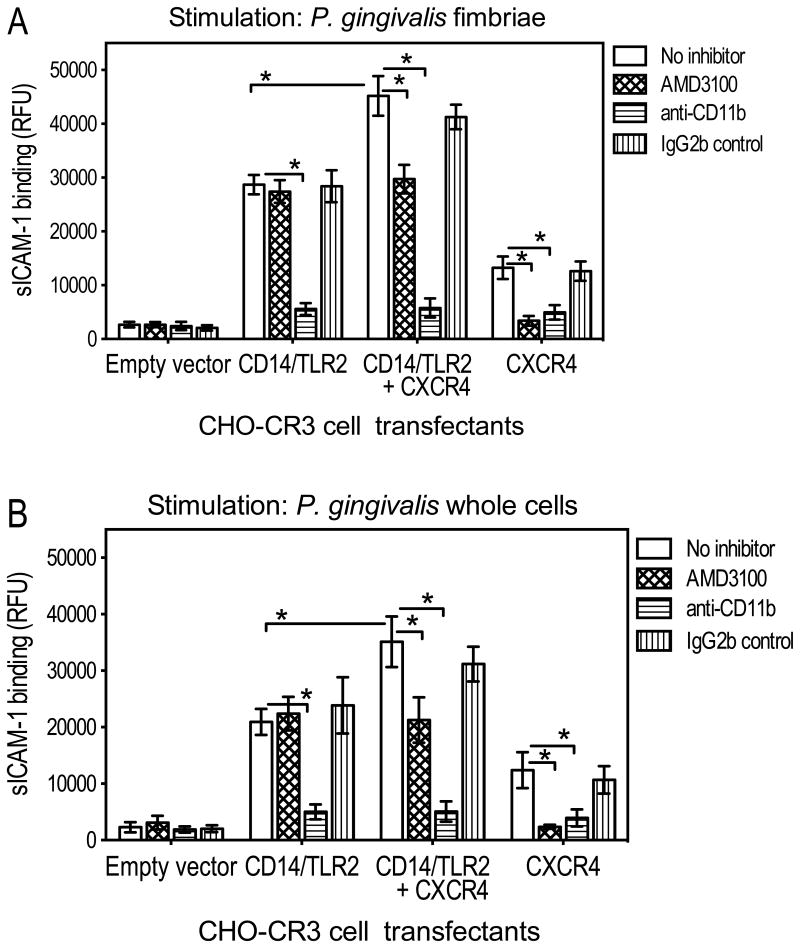
We next examined whether the observed activating effect of CXCR4 on CR3 involved crosstalk with TLR2, as is the case for the inhibitory effect of CXCR4 on the MyD88-dependent antimicrobial response (Hajishengallis et al., 2009; Hajishengallis et al., 2008). For this purpose, we used a recombinant TLR2 inside-out proadhesive signaling system developed in CHO cells stably transfected with human CR3 (CHO-CR3 cells) (Hajishengallis et al., 2006). CHO cells lack functional endogenous TLR2 but express TLR1 and TLR6, either of which is capable of cooperative signaling with TLR2 (Heine et al., 1999; Henneke et al., 2001; Ozinsky et al., 2000). Therefore, transfection of exogenous TLR2 in CHO-CR3 cells permits reconstitution of the TLR2 proadhesive signaling for CR3 activation (Hajishengallis et al., 2006). Similarly, in these experiments, CHO-CR3 cells transfected with human TLR2 and its CD14 coreceptor acquired the capacity for CR3-dependent binding of sICAM-1, upon stimulation with purified fimbriae or whole cells of P. gingivalis (Fig. 2, A and B, respectively). Consistent with the involvement of CXCR4 in CR3 activation (Fig. 1), cotransfection of CD14/TLR2 with CXCR4 further enhanced the ability of CHO-CR3 cells to bind sICAM-1 (Fig. 2). As expected, the CXCR4 effect was reversed by AMD3100 (Fig. 2). However, significant CR3-dependent binding of sICAM-1 was also seen in CHO-CR3 cells transfected with CXCR4 alone, i.e., without CD14/TLR2 contransfection (Fig. 2). It therefore appeared that CXCR4 exerted an additive effect on CR3 activation independent of TLR2, ruling out the possibility that CXCR4 activates CR3 by potentiating TLR2 inside-out signaling.
Figure 2. CR3-dependent binding activities of CHO-CR3 cells transfected with TLR2 and/or CXCR4.
CHO-CR3 cells, transiently transfected with the indicated receptors or empty vector control, were activated with P. gingivalis fimbriae (1 μg/ml) (A) or whole cells of P. gingivalis (MOI = 10:1) (B) and assayed for CR3-dependent binding of FITC-labeled sICAM-1 at 30 min following activation. CR3 dependence was confirmed by including groups treated with an anti-CR3 (anti-CD11b) mAb or isotype control. sICAM-1 binding is reported in relative fluorescent units (RFU). Data are means ± SD (n = 3). *, statistically significant (P < 0.01) differences between the indicated groups.
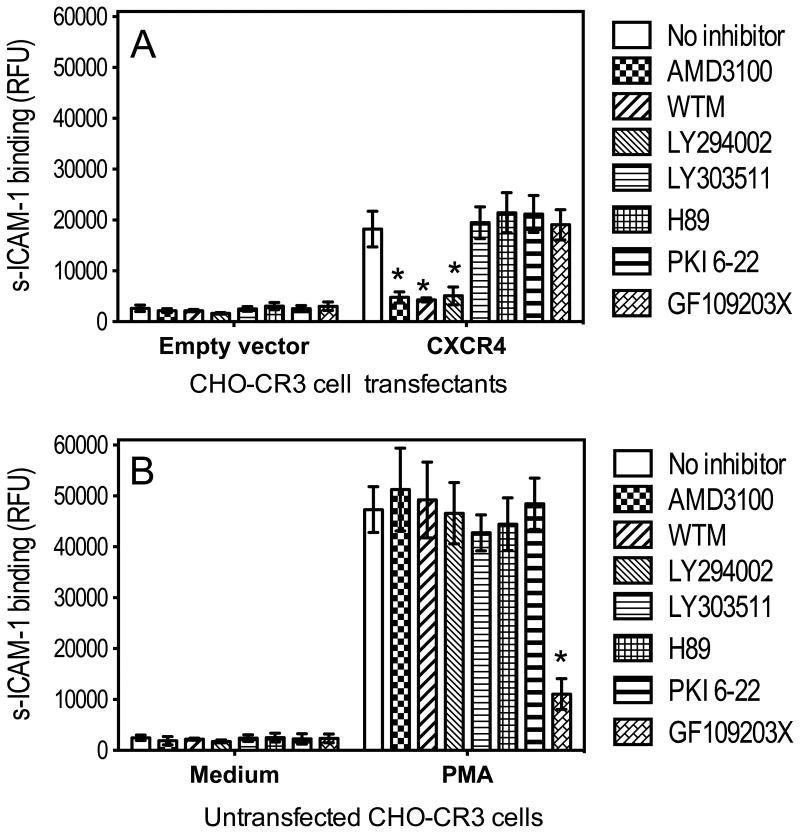
Several kinases, such as PI3K, PKA, and PKC, have been implicated in integrin activation (Luo et al., 2007; Nagel et al., 1998). The ability of P. gingivalis fimbriae to induce CXCR4-dependent activation of CR3 was not reversed by inhibitors of PKA (H89 and PKI 6-22) or PKC (GF109203X), but was significantly (P < 0.01) inhibited by inhibitors of PI3K, specifically wortmannin and LY294002 (but not by the inactive analog LY303511) (Fig. 3A). The inhibitory effects of wortmannin and LY294002 could not be attributed to nonspecific toxic effects, because both compounds failed to affect PMA-induced CR3 activation, which- as expected- was inhibited by the PKC inhibitor GF109203X (P < 0.01; Fig. 3B). In summary, the data from figures 1-3 show that P. gingivalis fimbriae induce CXCR4-dependent and PI3K-mediated activation of CR3.
Figure 3. CXCR4-dependent activation of CR3 is mediated by PI3K.
(A) CHO-CR3 cells, transiently transfected with CXCR4 or empty vector control, were stimulated with P. gingivalis fimbriae (1 μg/ml) and assayed for binding FITC-labeled sICAM-1 at 30 min following activation. Prior to stimulation, the cells were pretreated for 30 min with AMD3100 (1 μg/ml), wortmannin (WTM; 50 nM), LY294002 (20 μM), LY30351 (20 μM), H89 (5 μM), PKI 6-22 (1 μM), or GF109203X (10 μM). (B) Similar experiment in untransfected CHO-CR3 cells which were stimulated with PMA (0.1 μg/ml) or medium-only control. sICAM-1 binding is reported in relative fluorescent units (RFU). Data are means ± SD (n = 3). *, statistically significant (P < 0.01) inhibition of of sICAM-1 binding.
Comparative interactions of wild-type and DAP fimbriae with CXCR4 and CR3
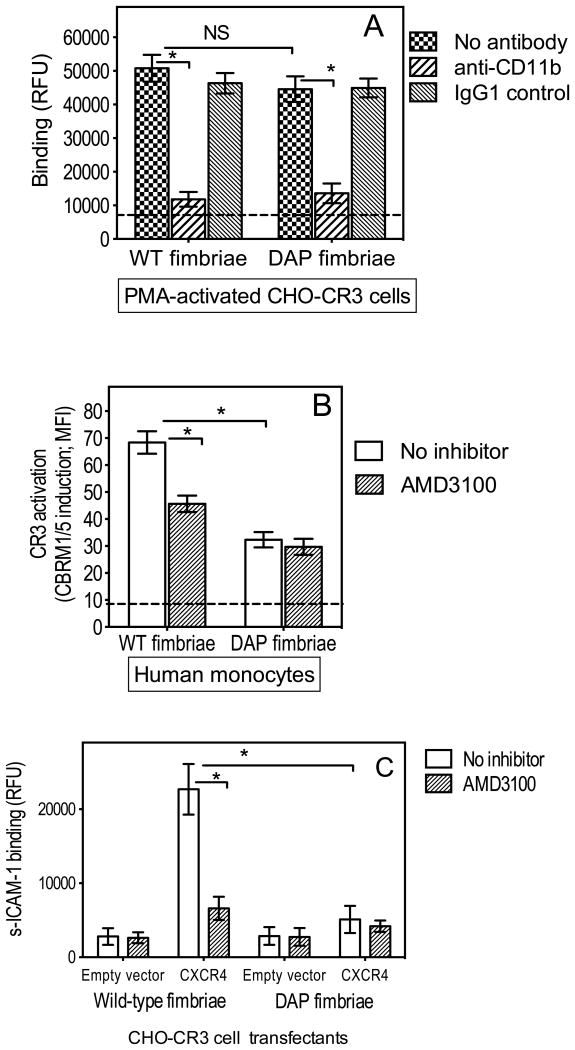
We previously showed that a mutant form of P. gingivalis fimbriae which lacks FimCDE (DAP fimbriae) fails to interact with CXCR4 (Pierce et al., 2009). Moreover, unlike wild-type fimbriae, DAP fimbriae interacted relatively poorly with CR3, although the molecular basis of this observation was not addressed (Wang et al., 2007). In view of the role of CXCR4 in fimbria-induced CR3 activation (Figs. 1 and 2), we hypothesized that the poor interaction of DAP fimbriae with CR3 could be attributed to their inability to bind CXCR4, rather than to an inherent defect preventing CR3 binding. To address this hypothesis, we first compared the abilities of wild-type and DAP fimbriae to bind PMA-treated CHO-CR3 cells. PMA activates CR3 independently of receptor interactions and inside-out signaling (not functional in untrasfected CHO-CR3 cells) (Hajishengallis et al., 2006; Harokopakis & Hajishengallis, 2005). Wild-type and DAP fimbriae bound comparably to PMA-activated CHO-CR3 cells in a CR3-dependent way (Fig. 4A), indicating that DAP fimbriae do not have any defect that prevents their binding to CR3.
Figure 4. DAP fimbriae bind pre-activated CR3 but do not efficiently activate CR3.
(A) CHO-CR3 cells were preactivated with PMA (0.1 μg/ml) and assayed for binding wild-type (WT) or DAP fimbriae (both at 1 μg/ml) after 30-min incubation, in the absence or presence of anti-CD11b mAb or IgG1 isotype control. (B) Human monocytes were stimulated with WT or DAP fimbriae (both at 1 μg/ml) with or without AMD3100 (1 μg/ml) and assayed for CBRM1/5 epitope induction at 30 min following activation. CBRM1/5 induction is reported in mean fluorescence intensity (MFI) values. The horizontal dashed lines indicate background binding (6943 ± 892 RFU) to empty vector-transfected cells (A) or baseline CBRM1/5 induction (8.8 ± 1.7 MFI) in unstimulated cells (B). (C) CHO-CR3 cells, transiently transfected with CXCR4 or empty vector control, were stimulated with WT or DAP fimbriae (both at 1 μg/ml) with or without AMD3100 (1 μg/ml) and after 30 min were assayed for binding of FITC-labeled sICAM-1. Data are means ± SD (n = 3). *, statistically significant (P < 0.01) differences between the indicated groups. NS, not significant.
This finding lent support to the hypothesis that the poor interaction of DAP with CR3 in normal cells (which have not been treated with PMA and CR3 activation would depend on inside-out signaling) reflects their inability to optimally activate CR3. Indeed, in human monocytes, DAP fimbriae showed significantly reduced ability to activate CR3 compared to wild-type fimbriae (P < 0.01; Fig. 4B). In the same cells, AMD3100 significantly (p < 0.01) suppressed CR3 activation by wild-type fimbriae but not by DAP fimbriae (Fig. 4B), further confirming that wild-type but not DAP fimbriae interact with CXCR4 to activate CR3. Moreover, in contrast to wild-type fimbriae, DAP fimbriae failed to induce CR3 activation in CXCR4-transfected CHO-CR3 cells (Fig. 4C). Therefore, the differential abilities of wild-type and DAP fimbriae to activate CR3 can be attributed, at least in large part, to the inability of DAP fimbriae to bind CXCR4 which contributes to CR3 activation.
Combined inhibition of CXCR4 or CR3 in vivo further promotes the killing of P. gingivalis
P. gingivalis exploits both CXCR4 (Hajishengallis et al., 2008) and CR3 (Hajishengallis et al., 2007) to resist killing in vivo, and therefore blockade of either CXCR4 or CR3 with specific receptor antagonists [AMD3100 (Hajishengallis et al., 2008) or XVA143 (Hajishengallis et al., 2007), respectively] promotes the ability of the mouse host to clear intraperitoneal infection with P. gingivalis. However, whether combined treatment with AMD3100 and XVA143 achieves a greater protective effect against P. gingivalis in this model was not previously examined. This question was addressed in parallel with experiments using an isogenic mutant (strain OZ5001C) expressing DAP fimbriae, which- as alluded to above- do not mediate efficient exploitation of the receptors under investigation.
At 24h postinfection, the recovery of the OZ5001C mutant (viable CFU counts) from the peritoneal cavity of PBS-pretreated mice was significantly (p <0.01) lower compared to wild-type P. gingivalis (Fig. 5), suggesting that OZ5001C is not as capable as the wild-type strain in resisting killing. As expected, pretreatment of mice with either AMD3100 or XVA143 promoted the killing of wild-type P. gingivalis (Fig. 5). Importantly, combined pretreatment with AMD3100 and XVA143 resulted in significantly (p < 0.01) enhanced killing of P. gingivalis compared to either antagonist alone (Fig. 5). None of these antagonist treatments could significantly affect the killing of the OZ5001C mutant (Fig. 5), reflecting the limited interactions of this strain with the receptors involved. In summary, the expression of fully functional (i.e., wild-type) fimbriae is important for the ability of P. gingivalis to productively exploit both CXCR4 and CR3 and thereby effectively promote its survival in the mammalian host.
Figure 5. P. gingivalis expressing DAP fimbriae does not exploit CXCR4 or CR3.
BALB/cByJ mice were i.p. pretreated with AMD3100 (25 μg in 0.1 ml PBS), XVA143 (15 μg in 0.1 ml PBS), both AMD3100 and XVA143, or PBS alone. After 1h, the mice were i.p. infected with 5×107 CFU P. gingivalis 33277 or OZ5001C (FimCDE-deficient isogenic mutant). Peritoneal lavage was performed 24h postinfection. Serial 10-fold dilutions of peritoneal fluid were plated for anaerobic growth and enumeration of recovered CFU. Horizontal lines show mean CFU counts. Asterisks indicate significant (P < 0.01) differences in P. gingivalis peritoneal CFU between PBS-treated mice and mice treated with receptor antagonists. The arrow sign shows significant (P < 0.01) difference between dual and single antagonist treatments. The difference between 33277 and OZ5001C CFU in PBS-pretreated mice is statistically significant (p < 0.01).
Discussion
An imbalance either in the composition of the periodontal microbiota (dysbiosis) or in local regulatory mechanisms that control inflammatory cell recruitment can precipitate pathologic periodontal inflammation (Eskan et al., 2012; Hajishengallis et al., 2012). P. gingivalis appears to be one of the causes of periodontal dysbiosis through its ability to subvert the host response in ways that may benefit also bystander bacterial species (Hajishengallis & Lamont, 2012). One of the receptors exploited by P. gingivalis to inhibit macrophage killing is CXCR4 (Hajishengallis et al., 2008), the pharmacologic inhibition of which blocks experimental periodontitis in a mouse model (McIntosh & Hajishengallis, 2012). In this paper we demonstrated a second CXCR4-dependent mechanism by which P. gingivalis undermines the host response to its own benefit. Specifically, in addition to hijacking CXCR4 to interfere with the TLR2 antimicrobial response (Hajishengallis et al., 2008), P. gingivalis was now shown to exploit CXCR4 to activate CR3, which mediates safe internalization of this pathogen by macrophages (Wang et al., 2007) (Fig. 6). Combined inhibition of CXCR4 and CR3 resulted in enhanced killing of P. gingivalis in vivo compared to single inhibition of either receptor.
Figure 6. Exploitation of CXCR4 by P. gingivalis.
In macrophages, P. gingivalis interacts with CD14 and the TLR2/TLR1 signaling complex resulting in inside-out signaling for activating and binding CR3, which leads to a relatively ‘safe’ uptake of these organisms by macrophages (Hajishengallis et al., 2007; Hajishengallis et al., 2006; Wang et al., 2007). The signaling pathway that activates the high-affinity state of CR3 is mediated by Rac1, PI3K and cytohesin 1 (Cyt1) (Hajishengallis et al., 2009; Harokopakis et al., 2006; Harokopakis & Hajishengallis, 2005). P. gingivalis-activated TLR2/TLR1 also induces a MyD88-dependnet pathway that can potentially promote the killing of this bacterium (Hajishengallis et al., 2009; Hajishengallis et al., 2008). However, by means of its fimbriae, P. gingivalis instigates a crosstalk between CXCR4 and TLR2 which interferes with this antimicrobial mechanism(Hajishengallis et al., 2008). In this study, P. gingivalis was shown to also utilize CXCR4 to induce PI3K-dependent activation of CR3, independently of TLR2, which further contributes to its capacity to evade killing. CXCR4 exploitation requires fully functional fimbriae, i.e., containing both the FimA and FimCDE components, which can directly bind CXCR4 (Pierce et al., 2009).
Rac1 is a key signaling component of the TLR2 proadhesive pathway leading to CR3 activation (Harokopakis et al., 2006). Since the signaling activity of Rac1 may be enhanced by PKA (O'Connor & Mercurio, 2001), the ability of P. gingivalis fimbriae to activate PKA through CXCR4 (Hajishengallis et al., 2008) could potentially lead to enhanced Rac1 signaling and thus potentiation of TLR2-induced CR3 activation. However, our results showed that the ability of CXCR4 to contribute to CR3 activation in cells exposed to purified fimbriae or whole cells of P. gingivalis, does not involve a crosstalk between CXCR4 and TLR2. Rather, CXCR4 independently activates CR3 and additively contributes to TLR2-induced CR3 activation (Fig. 6).
Stimulation of CXCR4 by its physiological ligand CXCL12 (also known as stromal cell-derived factor-1) can activate integrins and initiate firm adhesion of rolling leukocytes (Campbell et al., 1998; Constantin et al., 2000). Therefore, by activating the CR3 integrin through interaction with CXCR4, P. gingivalis actually mimics a physiological process, which thereby comes under the control of the pathogen.
Optimal CXCR4 and CR3 exploitation by P. gingivalis requires wild-type fimbriae, comprising FimA and the FimCDE accessory proteins. The relative inability of DAP fimbriae to interact with CR3 (Wang et al., 2007) could not be attributed to intrinsic defects of DAP fimbriae for CR3 binding, but rather to lack of interaction with CXCR4. Indeed, DAP fimbriae could bind pre-activated CR3, consistent with our previous identification of CR3-binding epitopes in the FimA peptide sequence (Hajishengallis et al., 2005a).
The inability of DAP fimbriae to interact with CXCR4 and thereby efficiently activate CR3 resulted in poor interactions with this integrin. Consequently, P. gingivalis expressing DAP fimbriae (OZ5001C) was more susceptible to in vivo killing being unable to exploit either CXCR4 or CR3, as shown by the experiments using antagonists of these receptors (AMD3100 and XVA143, respectively) alone or in combination. In contrast, wild-type P. gingivalis utilized both receptors to promote its in vivo survival. We previously showed that CXCR4 crosstalks with complement C5a receptor (C5aR) in P. gingivalis-challenged macrophages and P. gingivalis exploits this crosstalk to evade killing (Wang et al., 2010). Therefore, CXCR4 blockade would likely affect P. gingivalis' ability to crosstalk with both CR3 and C5aR. On the other hand, OZ5001C still expresses gingipains that are required for C5aR exploitation (Wang et al., 2010) and this could provide a degree of protection, consistent with the relatively modest persistence of this strain in the intraperitoneal infection model. Apparently, the ability of wild-type P. gingivalis to integrate subversive crosstalk signaling involving several receptors maximizes its adaptive fitness.
Taken together with our previous results (Hajishengallis et al., 2008), our current findings support that P. gingivalis exploits CXCR4 for inhibiting antimicrobial responses and promoting proadhesive activities, both of which lead to impaired host defense against this keystone pathogen (Darveau et al., 2012) (Fig. 6). These CXCR4-dependent evasive tactics can potentiate the impact of P. gingivalis on periodontal disease, a notion that is consistent with our recent report that CXCR4 blockade inhibits P. gingivalis-induced periodontitis in a mouse model (McIntosh & Hajishengallis, 2012).
Acknowledgments
This study was supported by U.S. Public Health Service Grants DE015254 and DE021685 (to G.H.).
References
- Burns E, Bachrach G, Shapira L, Nussbaum G. Cutting Edge: TLR2 is required for the innate response to Porphyromonas gingivalis: Activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J Immunol. 2006;177:8296–8300. doi: 10.4049/jimmunol.177.12.8296. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA, Butcher EC. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- Constantin G, Majeed M, Giagulli C, et al. Chemokines trigger immediate b2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity. 2000;13:759–769. doi: 10.1016/s1074-7613(00)00074-1. [DOI] [PubMed] [Google Scholar]
- Darveau RP, Hajishengallis G, Curtis MA. Porphyromonas gingivalis as a potential community activist for disease. J Dent Res. 2012;91:816–820. doi: 10.1177/0022034512453589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Garcia-Aguilar J, Bickford JK, Corbi AL, Springer TA. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J Cell Biol. 1993;120:1031–1043. doi: 10.1083/jcb.120.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Springer TA. A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J Cell Biol. 1993;120:545–556. doi: 10.1083/jcb.120.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzella GA, Schols D, Lin SW, et al. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- Eskan MA, Jotwani R, Abe T, et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 2012;13:465–473. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genco CA, Cutler CW, Kapczynski D, Maloney K, Arnold RR. A novel mouse model to study the virulence of and host response to Porphyromonas (Bacteroides) gingivalis. Infect Immun. 1991;59:1255–1263. doi: 10.1128/iai.59.4.1255-1263.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves DT, Fine D, Teng YT, Van Dyke TE, Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35:89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10:717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Lambris JD. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 2010;31:154–163. doi: 10.1016/j.it.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Lambris JD. Microbial manipulation of receptor crosstalk in innate immunity. Nat Rev Immunol. 2011;11:187–200. doi: 10.1038/nri2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: The Polymicrobial Synergy and Dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27 doi: 10.1111/j.2041-1014.2012.00663.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Liang S, Payne MA, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Ratti P, Harokopakis E. Peptide mapping of bacterial fimbrial epitopes interacting with pattern recognition receptors. J Biol Chem. 2005a;280:38902–38913. doi: 10.1074/jbc.M507326200. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Shakhatreh MA, Wang M, Liang S. Complement receptor 3 blockade promotes IL-12-mediated clearance of Porphyromonas gingivalis and negates its virulence in vivo. J Immunol. 2007;179:2359–2367. doi: 10.4049/jimmunol.179.4.2359. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Tapping RI, Martin MH, et al. Toll-like receptor 2 mediates cellular activation by the B subunits of type II heat-labile enterotoxins. Infect Immun. 2005b;73:1343–1349. doi: 10.1128/IAI.73.3.1343-1349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Wang M, Harokopakis E, Triantafilou M, Triantafilou K. Porphyromonas gingivalis fimbriae proactively modulate β2 integrin adhesive activity and promote binding to and internalization by macrophages. Infect Immun. 2006;74:5658–5666. doi: 10.1128/IAI.00784-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Wang M, Liang S. Induction of distinct TLR2-mediated proinflammatory and proadhesive signaling pathways in response to Porphyromonas gingivalis fimbriae. J Immunol. 2009;182:6690–6696. doi: 10.4049/jimmunol.0900524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Wang M, Liang S, Triantafilou M, Triantafilou K. Pathogen induction of CXCR4/TLR2 cross-talk impairs host defense function. Proc Natl Acad Sci U S A. 2008;105:13532–13537. doi: 10.1073/pnas.0803852105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harokopakis E, Albzreh MH, Martin MH, Hajishengallis G. TLR2 transmodulates monocyte adhesion and transmigration via Rac1- and PI3K-mediated inside-out signaling in response to Porphyromonas gingivalis fimbriae. J Immunol. 2006;176:7645–7656. doi: 10.4049/jimmunol.176.12.7645. [DOI] [PubMed] [Google Scholar]
- Harokopakis E, Hajishengallis G. Integrin activation by bacterial fimbriae through a pathway involving CD14, Toll-like receptor 2, and phosphatidylinositol-3-kinase. Eur J Immunol. 2005;35:1201–1210. doi: 10.1002/eji.200425883. [DOI] [PubMed] [Google Scholar]
- Heine H, Kirschning CJ, Lien E, Monks BG, Rothe M, Golenbock DT. Cutting edge: cells that carry a null allele for toll-like receptor 2 are capable of responding to endotoxin. J Immunol. 1999;162:6971–6975. [PubMed] [Google Scholar]
- Henneke P, Takeuchi O, van Strijp JA, et al. Novel engagement of CD14 and multiple toll-like receptors by group B streptococci. J Immunol. 2001;167:7069–7076. doi: 10.4049/jimmunol.167.12.7069. [DOI] [PubMed] [Google Scholar]
- Issekutz AC, Rowter D, Springer TA. Role of ICAM-1 and ICAM-2 and alternate CD11/CD18 ligands in neutrophil transendothelial migration. J Leukoc Biol. 1999;65:117–126. doi: 10.1002/jlb.65.1.117. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Lee HM, Wysoczynski M, Liu R, et al. Mobilization studies in complement-deficient mice reveal that optimal AMD3100 mobilization of hematopoietic stem cells depends on complement cascade activation by AMD3100-stimulated granulocytes. Leukemia. 2009;24:573–582. doi: 10.1038/leu.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz SM, Tabuni A, Kozel TR, MacGill RS, Ingalls RR, Golenbock DT. Binding of Cryptococcus neoformans to heterologously expressed human complement receptors. Infect Immun. 1997;65:931–935. doi: 10.1128/iai.65.3.931-935.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell CA. Rewiring phagocytic signal transduction. Immunity. 2006;24:243–245. doi: 10.1016/j.immuni.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007 doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- McIntosh ML, Hajishengallis G. Inhibition of Porphyromonas gingivalis-induced periodontal bone loss by CXCR4 antagonist treatment. Mol Oral Microbiol. 2012;27:449–457. doi: 10.1111/j.2041-1014.2012.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerschaert J, Furie MB. The adhesion molecules used by monocytes for migration across endothelium include CD11a/CD18, CD11b/CD18, and VLA-4 on monocytes and ICAM-1, VCAM-1, and other ligands on endothelium. J Immunol. 1995;154:4099–4112. [PubMed] [Google Scholar]
- Nagel W, Zeitlmann L, Schilcher P, Geiger C, Kolanus J, Kolanus W. Phosphoinositide 3-OH kinase activates the b2 integrin adhesion pathway and induces membrane recruitment of cytohesin-1. J Biol Chem. 1998;273:14853–14861. doi: 10.1074/jbc.273.24.14853. [DOI] [PubMed] [Google Scholar]
- Natarajan M, Lin KM, Hsueh RC, Sternweis PC, Ranganathan R. A global analysis of cross-talk in a mammalian cellular signalling network. Nat Cell Biol. 2006;8:571–580. doi: 10.1038/ncb1418. [DOI] [PubMed] [Google Scholar]
- Nishiyama S, Murakami Y, Nagata H, Shizukuishi S, Kawagishi I, Yoshimura F. Involvement of minor components associated with the FimA fimbriae of Porphyromonas gingivalis in adhesive functions. Microbiology. 2007;153:1916–1925. doi: 10.1099/mic.0.2006/005561-0. [DOI] [PubMed] [Google Scholar]
- O'Connor KL, Mercurio AM. Protein kinase A regulates Rac and is required for the growth factor-stimulated migration of carcinoma cells. J Biol Chem. 2001;276:47895–47900. doi: 10.1074/jbc.M107235200. [DOI] [PubMed] [Google Scholar]
- Oliva C, Turnbough CL, Jr, Kearney JF. CD14-Mac-1 interactions in Bacillus anthracis spore internalization by macrophages. Proc Natl Acad Sci U S A. 2009;106:13957–13962. doi: 10.1073/pnas.0902392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozinsky A, Underhill DM, Fontenot JD, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce DL, Nishiyama S, Liang S, et al. Host adhesive activities and virulence of novel fimbrial proteins of Porphyromonas gingivalis. Infect Immun. 2009;77:3294–3301. doi: 10.1128/IAI.00262-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimaoka M, Takagi J, Springer TA. Conformational regulation of integrin structure and function. Annu Rev Biophys Biomol Struct. 2002;31:485–516. doi: 10.1146/annurev.biophys.31.101101.140922. [DOI] [PubMed] [Google Scholar]
- Wang M, Shakhatreh MA, James D, et al. Fimbrial proteins of Porphyromonas gingivalis mediate in vivo virulence and exploit TLR2 and complement receptor 3 to persist in macrophages. J Immunol. 2007;179:2349–2358. doi: 10.4049/jimmunol.179.4.2349. [DOI] [PubMed] [Google Scholar]
- Wang M, Krauss JL, Domon H, et al. Microbial hijacking of complement-toll-like receptor crosstalk. Sci Signal. 2010;3:ra11. doi: 10.1126/scisignal.2000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F, Takahashi K, Nodasaka Y, Suzuki T. Purification and characterization of a novel type of fimbriae from the oral anaerobe Bacteroides gingivalis. J Bacteriol. 1984;160:949–957. doi: 10.1128/jb.160.3.949-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]