Abstract
OBJECTIVES
Numerous studies support an association between depression and increased risk of dementia. Because few studies have directly examined the temporal ordering of depression and memory decline in late life, it is not clear whether depressive symptoms typically precede and/or follow memory declines.
DESIGN
An autoregressive latent trajectory model examined the direction of the relationship between depressive symptoms and memory decline observed over 12 years.
SETTING
Washington/Hamilton Heights Inwood Columbia Aging Project, a community-based longitudinal study of aging and dementia in Northern Manhattan.
PARTICIPANTS
2,425 initially non-demented older adults.
MEASUREMENTS
Memory composite scores were computed from three subscores of the Selective Reminding Test. Depressive symptoms were assessed with a 10-item version of the Center for Epidemiological Studies Depression Scale. Analyses controlled for age, sex, recruitment wave, education, Black race and Hispanic ethnicity measured at baseline, and chronic disease burden measured at each study visit.
RESULTS
Initial depressive symptoms predicted worse memory scores at the second study visit (B weight=−0.03; P=.003) as well as accelerated memory decline over the entire study period (B weight=−0.02; P=.03). Memory scores did not predict subsequent depressive symptoms.
CONCLUSION
These findings suggest that depressive symptoms precede memory decline, but not vice versa, in late life. This pattern of results is in line with hypotheses that depression is a prodrome of dementia and/or a causal contributor to memory decline. Clinicians should be aware that depressive symptoms may represent an early indicator not only of dementia, as reported previously, but also of memory decline more generally.
Keywords: Depression, episodic memory, statistical modeling
INTRODUCTION
Recent meta-analyses demonstrated that depression is a major risk factor for mild cognitive impairment (MCI) and dementia (1-2). Depressive symptoms also increased dementia risk in the Washington/Hamilton Heights Inwood Columbia Aging Project (WHICAP) (3). However, because depressive symptoms did not predict greater risk of MCI among cognitively normal older adults, it was concluded that depression accompanies, but does not precede, cognitive impairment (3). However, this study was not explicitly designed to test for leading and lagging relationships between depressive symptoms and cognitive impairment that would clearly demonstrate which occurs first. The present study sought to elucidate the temporal ordering of depressive symptoms and memory decline in WHICAP.
The question of whether depressive symptoms precede the development of memory impairment is of both theoretical and practice importance. Several hypotheses have been proposed. Depression may reflect psychological reaction to the perception of memory decline (4). Memory dysfunction may represent a risk factor for late-onset psychiatric illness (5). Depression may represent a prodrome of dementia (6). Depression may be a causal contributor to memory decline (7). Depression may lower the threshold for clinical detection of dementia without directly influencing brain pathology (8). Some evoke a “reciprocal relationship” (9). Each hypothesis provides specific predictions regarding the directionality of the relationship between depressive symptoms and memory decline. These predictions can only be tested in a longitudinal framework allowing for estimation of both potential outcomes simultaneously.
The current study uses the autoregressive latent trajectory (ALT) framework to test the direction of the relationship between depressive symptoms and memory decline among older adults. ALT is a structural equation model that combines two well-developed approaches: multivariate latent growth curve modeling and autoregressive cross-lagged panel analysis (10). ALT tests whether the initial level of one outcome influences the subsequent trajectory of another outcome while simultaneously testing for lagged relationships at individual occasions. The current study examines whether depressive symptoms precede memory impairment or vice versa over the course of 12 years in a sample of 2,425 initially non-demented older adults.
METHODS
Participants and Procedures
The 2,425 older adults were participants in WHICAP, a prospective, community-based longitudinal study of aging and dementia in a racially and ethnically diverse sample. Full descriptions of study procedures have been reported (11-12). Participants within the geographic area of Northern Manhattan were identified from Medicare records and recruited in two waves: 1992 (N=478) and 1999 (N=1,947). Ongoing follow-up at 18-24 month interval includes a battery of cognitive, functional, and health measures administered in the participant’s preferred language (English or Spanish). This study complies with the ethical rules for human experimentation that are stated in the Declaration of Helsinki, including approval of the local institutional review board and informed consent. Race and ethnicity is determined via self-report using the format of the 2000 U.S. Census. Baseline characteristics of the sample are shown in Table 1.
Table 1.
Sample Characteristics at Baseline
| Mean | Standard deviation | Range | |
|---|---|---|---|
| Age (years) | 77.3 | 6.6 | [65.8, 101.8] |
| Education (years) | 10.3 | 4.8 | [0, 20] |
| Sex (% female) | 67.2 | - | - |
| Race (% Black) | 32.2 | - | - |
| Ethnicity (% Hispanic) | 37.7 | - | - |
| Illness burdena | 2.4 | 1.5 | [0, 8] |
| CESDb | 1.8 | 2.0 | [0, 10] |
| Memory composite scorec | 0.0 | 0.8 | [−3.3, 2.2] |
| Number of CESD assessmentsd | 2.7 | 1.2 | [0, 4] |
| Number of memory assessmentse | 2.6 | 1.2 | [0, 4] |
One point was assigned for the presence of each chronic condition listed in the text; range [0, 15].
CESD=Centers for Epidemiological Studies Depression Scale; range [0, 10].
Memory composite scores are averaged z-scores from immediate recall, delayed recall, and delayed recognition scores from the Selective Reminding Test.
Number of assessments during the study period; three individuals did not complete the CESD but had memory testing.
Number of assessments during the study period; 24 individuals had no memory testing but had been administered the CESD.
Because the depression instrument used in these analyses was not included in the original WHICAP battery, the first visit was defined as the first at which each participant was administered this instrument. The current sample included only participants who did not meet DSM-III criteria for dementia at this first visit, according to consensus conference. A total of four visits (follow-up of 12 years) were analyzed in the current analyses to maximize covariance coverage.
Outcomes
Episodic memory was assessed with the Selective Reminding Test (SRT) (13). This test was chosen because of its sensitivity to longitudinal change and dementia conversion in this population. Participants are given six trials to learn 12 words. Following each trial, participants are only reminded of words they failed to recall. Total learning is the number of words recalled after six learning trials. Delayed recall is the number of words recalled after a 15-minute delay. Delayed recognition is the number of words recognized immediately following delayed recall. Total learning, delayed recall, and delayed recognition scores at each occasion were standardized to z-score metric using the sample’s means and standard deviations at the initial occasion. Memory composite scores were computed by averaging the three z-scores at each occasion. Higher scores indicate better memory.
Depressive symptoms were assessed with a 10-item version of the Center for Epidemiological Studies Depression Scale (CESD) (14). Participants self-report responses to 10 dichotomous items. Total scores range from 0 to 10, with higher scores indicating worse depression.
Statistical Analysis
Descriptive statistics were computed using SPSS version 19 (IBM Corp., Armonk, NY). Growth curve and ALT analyses were conducted in Mplus version 7 (Muthén & Muthén, Los Angeles, CA) using maximum likelihood estimation. Time was parameters as years from study entry, accommodating differing intervals between visits. The average number of years between visits was 2.6 (SD=0.9). Missing data were managed with full information maximum likelihood (FIML), which accumulates and maximizes casewise likelihood functions computed using all available data for each participant. FIML produces less biased estimates than alternative methods (15). FIML does not assume that data are missing completely at random and can therefore accommodate missingness related to previous scores. This feature of FIML is desirable because participants lost to follow-up are often those who scored lower at earlier occasions.
First, unconditional latent growth curve models (LGC) were estimated separately for the two outcomes (i.e., episodic memory and depressive symptoms) to characterize their trajectories. Models allowing only linear change were statistically compared to models allowing both linear and quadratic change with the chi square test.
Next, best-fitting univariate LGCs were combined into the ALT model shown in Figure 1. In the latent part of the model, initial levels (i.e., intercepts), rates of change (i.e., linear slopes), and changes in rates of change (i.e., quadratic slopes) were estimated as latent variables using information from all four occasions. To determine whether initial levels of depressive symptoms and memory impairment were uniquely associated with subsequent changes in either domain over the entire study period, rates of change in each symptom type were regressed on initial levels of each symptom type. All other relationships between latent variables were estimated as correlations.
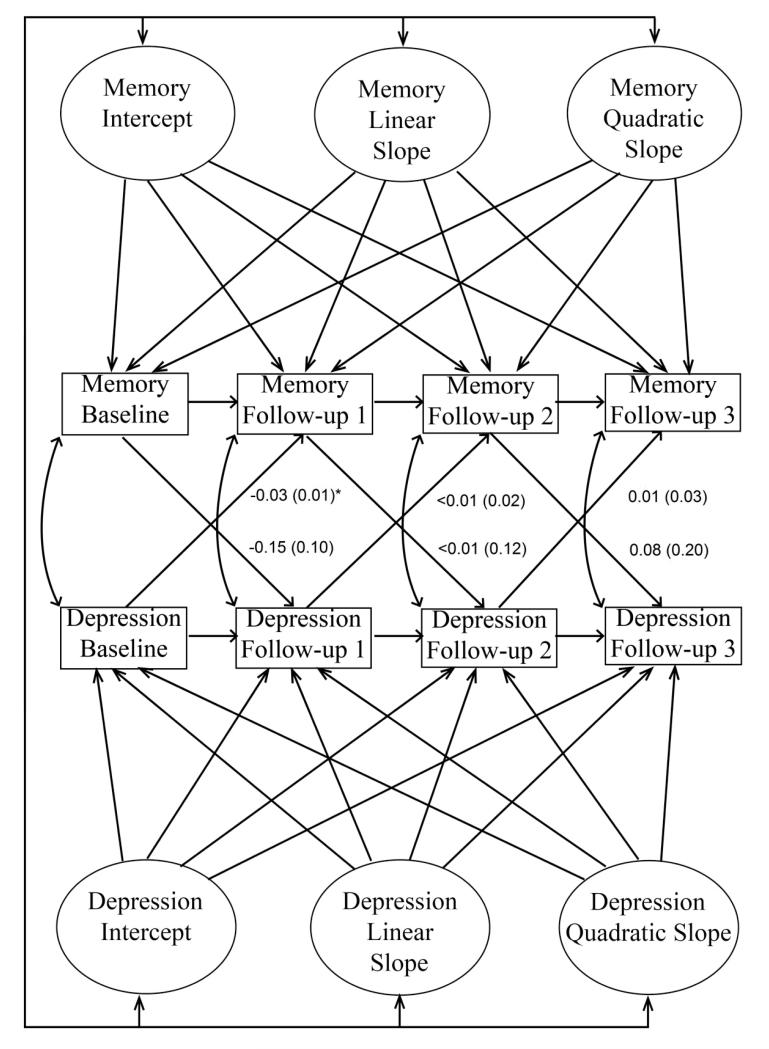
Figure 1.
Schematic of the autoregressive latent trajectory (ALT) model. For simplicity, covariates are not shown. Values represent unstandardized B weights for the cross-lagged paths, adjusted for age, education, recruitment year, race, ethnicity and illness burden, followed by standard errors in parentheses. As shown, all associations between the latent variables (i.e., intercepts and slopes) were estimated. Lower initial memory score (Memory Intercept) and higher initial level of depressive symptoms (Depression Intercept) were each independently associated with faster subsequent memory decline (Memory Linear Slope). * P=.003
In the autoregressive cross-lagged part of the model, within-domain autoregressive paths, cross-lagged regression paths, and occasion-specific correlations across domains were estimated. Type 1 error was controlled by applying a Bonferroni correction to these parameter estimates (i.e., 0.05/16=0.003125). Importantly, cross-lagged paths tested whether depressive symptoms at one study visit predicted memory scores at the next visit, and vice versa.
The ALT model also controlled for covariates. Associations between the following variables, measured at baseline, and the latent variables for each outcome were estimated: age, sex, recruitment year, education, Black race, and Hispanic ethnicity. These time-invariant covariates were centered to facilitate parameter interpretation. Specifically, values of zero correspond to age 77, male sex, recruitment in 1992, and 10 years of education. Finally, a time-varying covariate reflecting overall illness burden was allowed to correlate with both depressive symptoms and memory scores at each occasion. One point was assigned for the presence of each of the following conditions: heart disease, hypertension, stroke, diabetes, pulmonary disease, thyroid disease, liver disease, renal insufficiency, peptic ulcer disease, peripheral vascular disease, cancer, Parkinson’s disease, Essential Tremor, Multiple Sclerosis, and arthritis. Points were summed to reflect illness burden at each occasion as previously described (16-17). All reported associations from the ALT model are independent of all covariates.
RESULTS
Unconditional Univariate Models
Characterizing the Trajectories
Trajectories of change for each outcome were characterized with unconditional univariate growth models. Compared to models allowing only linear change, fit was improved in models allowing for curvilinear change in both memory (Δχ2(4)=−64.03, p<.001) and depressive symptoms (Δχ2(4)=−35.55, p<.001). Therefore, models estimating both linear and quadratic slopes were retained.
Descriptions of the Trajectories in the Best-Fitting Univariate Models
In these unconditional univariate models, memory scores declined by 0.13 points per year (P<.001). This rate of change decelerated by 0.01 points per year (P<.001). Participants with higher initial memory scores exhibited slower subsequent decline (covariance =0.04, P=.003). Scores on the CESD improved by 0.12 points per year (P<.001). This rate of change decelerated by 0.02 points per year (P<.001). Initial level of depressive symptoms was not related to subsequent changes (covariance =−0.21, P=.08).
Autoregressive Latent Trajectory Model
Best-fitting univariate models were combined, and all covariates were added, as shown in Figure 1. All reported associations are independent of all other associations in the model, including covariate effects.
Associations between the Latent Variables
Initial level of depressive symptoms was not associated with subsequent changes in depressive symptoms (P=.22). Initial level of depressive symptoms was not associated with initial memory score (covariance=0.06; P=.35). Rates of change in depressive symptoms and memory were not correlated (covariance=0.01; P=.52).
Higher initial memory scores were associated with slower subsequent memory decline (B=0.07; P=.01). Independent of this relationship, higher initial level of depressive symptoms was associated with accelerated memory decline (B =−0.02; beta=−0.26; P=.03). Specifically, each additional depressive symptom was associated with 0.02 more points of annual decline on the memory composite scores, independent of initial memory score and all covariates. Initial memory score was not associated with subsequent changes in depressive symptoms (B =−0.06; beta=−0.15; P=.08). This pattern of results was unchanged after exclusion of individuals with MCI at baseline (12).
Autoregressive and Cross-Lagged Paths
No within-domain autoregressive paths were significant. Cross-lagged paths are shown in Figure 1. Memory scores did not predict depressive symptoms at the next visit. Greater depressive symptoms at the first visit significantly predicted worse memory scores at the second visit (P=.003) after Bonferroni correction. No other cross-lagged paths were significant. This pattern of results was unchanged after excluding individuals with MCI at baseline.
Covariate Effects
Effects of the time-invariant covariates are shown in Table 2. Greater illness burden was associated with more depressive symptoms at each occasion (all p’s<.001). Illness burden was not associated with memory scores at any occasion.
Table 2.
Effects of the Time-Invariant Covariates Measured at Baseline
| Depressive symptoms |
Memory |
|||
|---|---|---|---|---|
| Initial level | Rate of change | Initial level | Rate of change | |
| Age (years) | 0.008 | −0.002 | −0.037** | −0.007* |
| Female sex | 0.530** | 0.021 | 0.254** | 0.024 |
| Black | −0.498** | −0.022 | −0.308** | −0.029 |
| Hispanic | 0.262* | −0.071 | −0.300** | 0.048 |
| Education (years) | −0.015 | −0.001 | 0.049** | 0.003 |
| Recruitment yeara | 0.177 | −0.115 | −0.109* | −0.109** |
1992 or 1999
P<.05
P<.001
DISCUSSION
The results of this study indicate that depressive symptoms preceded memory decline in this sample of 2,425 initially non-demented older adults followed up to 12 years. Specifically, higher scores on the depression measure at the first study visit predicted lower scores on a memory composite at the next study visit. Further, higher initial level of depression predicted faster rates of memory decline measured over the entire study period. This is the first study to directly examine the temporal ordering of depressive symptoms and memory impairment in older adults using the autoregressive latent trajectory (ALT) framework. These results have implications for both memory prognosis and theories regarding how depressive symptoms relate to memory decline in late life.
These findings add to those of a recent study that used survival analyses to reveal an association between depressive symptoms and increased dementia risk in this sample (3). That study did not find an association between baseline depressive symptoms and conversion from a normal cognitive state to MCI. In contrast, the present study found that depressive symptoms predicted steeper cognitive decline even among individuals who were cognitive normal at baseline. These discrepancies likely relate to the earlier study’s focus on conversion rather than the rate of cognitive decline and the dichotomization of the CESD de-emphasizing subsyndromal depressive symptoms.
Results are in line with two prominent hypotheses regarding the nature of the relationship between depressive symptoms and memory impairment in late life. Specifically, depression may be a prodrome of dementia (6) and/or a causal contributor to memory decline (7). Our finding that memory scores did not predict subsequent changes on a depression instrument do not support hypotheses that depression reflects a psychological reaction to the perception of memory decline (4) or that memory dysfunction is a risk factor for late-onset depression (5). However, it should be noted that neither participants’ perceptions of memory impairment nor formal psychiatric diagnoses were analyzed in this study. In addition, the results of this large-scale study do not imply that such relationships do not exist for individual cases.
The finding that depressive symptoms predicted worse subsequent memory decline was independent of age, sex, education, race, ethnicity, recruitment year and illness burden, including vascular disease. It has been suggested that the link between depression and dementia risk may be mediated by vascular health (18). However, such a link is not strongly supported by epidemiologic data (19). Further, a previous report from our group showed that vascular risk factors and stroke did not explain the prospective relation between depressive symptoms and Alzheimer’s disease (AD) (20).
If depression represents a prodrome of dementia, one might predict an association between depressive symptoms and dementia neuropathology. Increased AD pathology (e.g., plaques and tangles) has been reported among AD patients with a history of major depression (21). However, recent studies found greater subcortical Lewy bodies, hippocampal neuronal loss and white matter lesions, but not AD pathology, in the brains of non-demented older adults with depression (22-23).
If depression is a causal contributor to dementia, one might expect depressive symptoms to predict subsequent changes in brain integrity. For example, depression may cause hippocampal damage through a glucocorticoid cascade (24). A recent longitudinal study of 334 older adults with mild cognitive impairment (MCI) found that depressive symptoms predicted accelerated cortical thinning in the prefrontal cortex, where glucorticoid receptors are highly expressed (25). However, it is also possible that a separate pathology (e.g., hippocampal sclerosis) causes both depressive symptoms and memory loss in older adults.
The present results complement and clarify those from a recent study that used ALT to show reciprocal relationships between depressive symptoms and disability among community-dwelling adults in Taiwan (26). That study concluded that disability is a stronger predictor of depressive symptoms than vice versa. Together, these studies support depression as a negative prognostic indicator for both cognition and disability. However, the lack of a relationship between memory and subsequent depressive symptoms in the present study suggests that the previously-described link between disability and changes in depressive symptoms (26) is likely not mediated by memory decline. Rather, physical changes that limit the performance of activities of daily living are more likely to lead to depressive symptoms than is memory decline. A future study that includes measures of depression, memory, and disability is needed to directly test this hypothesis.
Strengths of this study include its large sample size, long follow-up period, and statistical framework. Limitations include the relatively brief measure of depressive symptoms and the use of a single memory measure. While the association between initial memory performance and the rate of change in depressive symptoms was not significant in this study of 2,425 older adults followed over 12 years (P=.08), it is possible that this association could reach significance in a larger study. It should also be noted that depressive symptoms are less common in this and other community-based samples, compared with clinic-based samples. Future studies should determine whether these findings differ in clinic-based samples and investigate whether temporal relationships between depressive symptoms and cognitive status differ for different cognitive domains.
CONCLUSION
Depressive symptoms precede memory decline, but not vice versa, in this sample of initially non-demented older adults followed over 12 years. Clinicians should be aware that depressive symptoms may represent an early indicator not only of dementia, as reported previously, but also of memory decline more generally. This pattern of results is in line with hypotheses that depression is a prodrome of dementia and/or a causal contributor to memory decline.
ACKNOWLEDGMENTS
Funding sources: National Institute on Aging (grant numbers AG037212, AG034189, AG000261).
Sponsor’s Role
This work was supported by the National Institute on Aging (grant numbers AG037212, AG034189, AG000261). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The sponsors had no role in the study design, methods, subject recruitment, data collection, analysis, preparation of article, or decision to submit the article for publication.
Footnotes
Author Contributions
LBZ: Conception and design, analysis and interpretation of data, drafting the article, final approval of the version to be published
YS: Conception and design, interpretation of data, critical revision, final approval of the version to be published
JJM: Conception and design, interpretation of data, critical revision, final approval of the version to be published
Conflict of Interest Checklist:
| LBZ | YS | JJM | ||||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | |
| Employment/Affiliation | X | X | X | |||
| Grants/Funds | X | X | X | |||
| Honoraria | X | X | X | |||
| Speaker Forum | X | X | X | |||
| Consultant | X | X | X | |||
| Stocks | X | X | X | |||
| Royalties | X | X | X | |||
| Expert Testimony | X | X | X | |||
| Board Member | X | X | X | |||
| Patents | X | X | X | |||
| Personal Relationship | X | X | X | |||
REFERENCES
- 1.Gao Y, Huang C, Zhao K, et al. Depression as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Int J Geriatr Psychiatry. 2013;28:441–449. doi: 10.1002/gps.3845. [DOI] [PubMed] [Google Scholar]
- 2.Diniz BS, Butters MA, Albert SM, et al. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and emta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202:329–335. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richard E, Reitz C, Honig LH, et al. Late-life depression, mild cognitive impairment, and dementia. Arch Neurol. 2012 doi: 10.1001/jamaneurol.2013.603. 000-000. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassuk SS, Berkman LF, Wypij D. Depressive symptomatology and incident cognitive decline in an elderly community sample. Arch Gen Psychiatry. 1998;55:1073–1081. doi: 10.1001/archpsyc.55.12.1073. [DOI] [PubMed] [Google Scholar]
- 5.Teng E, Tassniyam K, Lu PH. Reduced quality of life ratings in mild cognitive impairment: analyses of subject and informant responses. Am J Geriatr Psychiatry. 2010;20:1016–1025. doi: 10.1097/JGP.0b013e31826ce640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reding M, Haycox J, Blass J. Depression in patients referred to a dementia clinic. Arch Neurol. 1985;42:894–896. doi: 10.1001/archneur.1985.04060080080019. [DOI] [PubMed] [Google Scholar]
- 7.Meeks TW, Ropacki SA, Jeste DV. The neurobiology of neuropsychiatric symptoms in dementia. Curr Opin Psychiatry. 2006;19:581–586. doi: 10.1097/01.yco.0000245746.45384.0e. [DOI] [PubMed] [Google Scholar]
- 8.Mortimer JA. Do psychosocial risk factors contribute to Alzheimer’s disease? In: Henderson AS, Henderson JH, editors. Etiology of Dementia of Alzheimer’s Type. Wiley; Chichester: 1988. pp. 39–52. [Google Scholar]
- 9.O’Hara R. The reciprocal relationship of neurocognitive and neuropsychiatric function in late life. Am J Geriatr Psychiatry. 2010;20:1001–1005. doi: 10.1097/JGP.0b013e318275d60f. [DOI] [PubMed] [Google Scholar]
- 10.Bollen KA, Curran PJ. Autoregressive latent trajectory (ALT) models: a synthesis of two traditions. Sociol Method Res. 2004;32:336–383. [Google Scholar]
- 11.Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 12.Manly JJ, Bell-McGinty S, Tang MX, et al. Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Arch Neurol. 2005;62:1739–1746. doi: 10.1001/archneur.62.11.1739. [DOI] [PubMed] [Google Scholar]
- 13.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 14.Irwin M, Haydari K, Oxman MN. Screening for depression in the older adult: criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D) Arch Intern Med. 1999;159:1701–1704. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- 15.Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct Equ Modeling. 2001;8:430–457. [Google Scholar]
- 16.Grunebaum MF, Oquendo MA, Manly JJ. Depressive symptoms and antidepressant use in a random community sample of ethnically diverse, urban elder persons. J Affect Disord. 2008;105:273–277. doi: 10.1016/j.jad.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manly JJ, Schupf N, Stern Y, Brickman AM, Tang MX, Mayeux R. Telephone-based identification of MCI and dementia in a multicultural cohort. Arch Neurol. 2011;68:607–614. doi: 10.1001/archneurol.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexopoulos GS, Meyers BS, Young RC, et al. Clinically defined vascular depression. Am J Psychiatry. 1997;154:562–565. doi: 10.1176/ajp.154.4.562. [DOI] [PubMed] [Google Scholar]
- 19.Panza F, Frisardi V, Capurso C, et al. Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am J Geriatr Psychiatry. 2010;18:98–116. doi: 10.1097/JGP.0b013e3181b0fa13. [DOI] [PubMed] [Google Scholar]
- 20.Luchsinger JA, Honig LS, Tang M-X, et al. Depressive symptoms, vascular risk factors, and Alzheimer’s disease. Int J Geriatr Psychiatry. 2008;23:922–928. doi: 10.1002/gps.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rapp MA, Schnaider-Beeri M, Grossman HT, et al. Increased hippocampal plaques and tangles in patients with Alzheiemr’s disease with a lifetime history of major depression. Arch Gen Psychiatry. 2006;63:161–167. doi: 10.1001/archpsyc.63.2.161. [DOI] [PubMed] [Google Scholar]
- 22.Tsopelas C, Stewart R, Savva, et al. Neuropathological correlates of late-life depression in older people. Br J Psychiatry. 2011;198:109–114. doi: 10.1192/bjp.bp.110.078816. [DOI] [PubMed] [Google Scholar]
- 23.Madsen K, Hasselbalch BJ, Frederiksen KS, et al. Lack of association between prior depressive episodes and cerebral [11C]PiB binding. Neurobiol Aging. 2012;33:2334–2342. doi: 10.1016/j.neurobiolaging.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Sapolsky RM. Depression, antidepressants, and the shrinking hippocampus. Proc Natl Acad Sci USA. 2001;98:12320–12322. doi: 10.1073/pnas.231475998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zahodne LB, Gongvatana A, Cohen R, et al. Are apathy and depression independently associated with longitudinal trajectories of cortical atrophy in mild cognitive impairment? Am J Geriatr Psychiatry. doi: 10.1016/j.jagp.2013.01.043. 000-000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen C-M, Mullan J, Su Y-Y, et al. The longitudinal relationship between depressive symptoms and disability for older adults: a population-based study. J Gerontol A Biol Sci Med Sci. 2010;67:1059–1067. doi: 10.1093/gerona/gls074. [DOI] [PubMed] [Google Scholar]