Abstract
Background:
Malignant middle cerebral artery (MCA) infarction is a devastating clinical entity affecting about 10% of stroke patients. Decompressive craniectomy has been found to reduce mortality rates and improve outcome in patients.
Methods:
A retrospective case review study was conducted to compare patients treated with medical therapy and decompressive surgery for malignant MCA infarction in Hospital Kuala Lumpur over a period of 5 years (from January 2007 to December 2012). A total of 125 patients were included in this study; 90 (72%) patients were treated with surgery, while 35 (28%) patients were treated with medical therapy. Outcome was assessed in terms of mortality rate at 30 days, Glasgow Outcome Score (GOS) on discharge, and modified Rankin scale (mRS) at 3 and 6 months.
Results:
Decompressive craniectomy resulted in a significant reduction in mortality rate at 30 days (P < 0.05) and favorable GOS outcome at discharge (P < 0.05). Good functional outcome based on mRS was seen in 48.9% of patients at 3 months and in 64.4% of patients at 6 months (P < 0.05). Factors associated with good outcome include infarct volume of less than 250 ml, midline shift of less than 10 mm, absence of additional vascular territory involvement, good preoperative Glasgow Coma Scale (GCS) score, and early surgical intervention (within 24 h) (P < 0.05). Age and dominant hemisphere infarction had no significant association with functional outcome.
Conclusion:
Decompressive craniectomy achieves good functional outcome in, young patients with good preoperative GCS score and favorable radiological findings treated with surgery within 24 h of ictus.
Keywords: Decompressive craniectomy, malignant cerebral infarction, middle cerebral artery infarction
INTRODUCTION
Malignant middle cerebral artery (MCA) infarction is a clinical entity affecting up to 10% of all patients diagnosed with ischemic stroke. It is defined as an infarction involving an area encompassing at least two thirds of that supplied by the MCA.[7] Cerebral edema associated with infarcted brain tissue is responsible for the devastating effects of this condition. This edema results in mass effect which causes displacement of brain tissue and an increase in intracranial pressure (ICP). The initial presenting features include symptoms and signs of MCA occlusion, such as hemiparesis, hemiplegia, gaze preferences, and altered consciousness.[4,8] These patients, however, deteriorate rapidly within the first 48 h, heralding the presence of mass effect that can have potentially fatal consequences.[15] Intensive medical therapy with mechanical ventilation, osmotic diuretics, hypothermia, sedation, and hyperventilation have so far been ineffective, with reported mortality rates being as high as 80% despite optimum medical management.[3,7,8,22]
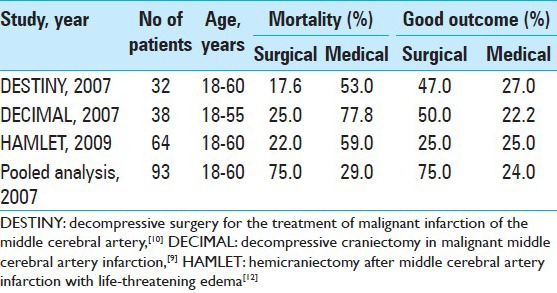
Decompressive craniectomy has been proposed as a treatment option for large hemispheric infarctions with cerebral edema.[9,17,21] The rationale of the treatment is to create space to accommodate for expansion of the swollen brain, thus allowing for normalization of intracranial pressure, preventing brain tissue herniation, and preserving cerebral blood flow to prevent secondary brain damage.[9,11] Decompressive craniectomy was initially proposed as a treatment option for malignant MCA infarction in 1956 by Scarcella.[2] Since then, various nonrandomized trials and reports have been published, citing its beneficial effect in reducing mortality in patients with malignant MCA infarction. Early critics cited the poor functional outcome, particularly with dominant hemisphere involvement, resulting in higher percentage of surviving patients who were dependent on caregivers, increasing their burden. Furthermore, the complication from surgery as well as the cosmetic deformity was deemed unacceptable. Various unresolved issues remained in these early trials, which included timing of surgery, age limit, the limits of acceptable outcome and patient selection. Results from three European randomized controlled trials for decompressive craniectomy in malignant MCA territory infarction, the DECIMAL,[9] HAMLET,[10] and DESTINY[11] trials published in 2007, demonstrated a significant reduction in mortality rates and improvement in functional outcome in patients treated with decompressive craniectomy as compared to medical therapy [Table 1]. Key factors associated with favorable outcome include younger age and early surgical treatment.
Table 1.
Summary of randomized clinical trials
This study was conducted to compare the difference in outcome in terms of mortality rate at 30 days and functional outcome at discharge and 3 and 6 months following decompressive craniectomy for treatment of malignant MCA infarction and medical treatment, as well as to study the association of factors influencing outcome in patients treated with decompressive craniectomy.
METHODS
A retrospective case review of patients diagnosed with malignant MCA territory infarction admitted to the neurosurgery department of Hospital Kuala Lumpur between January 2007 and January 2012 was performed. Data were collected from patients’ medical records, surgical records, and radiological images. A total of 125 patients between the ages of 18 and 65 years were included in this study. Malignant MCA territory infarction was defined as an infarction of at least two-thirds of MCA territory with evidence of space-occupying edema and mass effect on non-contrasted computed tomographic (CT) imaging of the brain. Volume of infarct was calculated using the ABC/2 method. This method has shown to rapidly and accurately estimate infarct volume size on CT scan, and has high intra-rater and inter-rater variability (71-99%).[12,13,18] Pretreatment clinical evaluation was based on the Glasgow Coma Scale (GCS).[19] Surgical treatment consisted of standardized decompressive craniectomy measuring 10 × 12 cm in diameter with fascio-duroplasty. Those receiving medical therapy included treatment with osmotic diuretics, sedation, and mechanical ventilation without any surgery. All patients underwent cerebral protection in the neurosurgery intensive care unit (ICU) for duration of 24-48 h, and weaning of therapy was done based on clinical and serial CT scan findings. Outcome measured was assessed based on mortality rate at 30 days, Glasgow Outcome Score (GOS) on discharge,[20] and modified Rankin scale (mRS) at 3 and 6 months. Patients with a GCS score of 6 and below or those with evidence of absent brain stem reflexes were excluded from this study.
Statistical analysis
Sample size calculation was done using Power and Sample Size Calculation (PS) Software,[5] with a level of significance (α) of 0.05 and a power of 0.80. Based on the mortality rate in the study center and considering a dropout rate of 20%, the calculated sample size was 127 patients, with 90 patients for treatment with surgery and 37 patients for treatment with medical therapy. Data entry and analysis were done using the Statistical Package for Social Sciences (SPSS) Software, version 19. Pearson's Chi-square test and Fisher's exact test were used to determine significant differences in outcome based on mortality rate at 30 days, GOS at discharge, and mRS at 3 and 6 months between patients treated with surgery and medical treatment, as well as to determine significant difference in factors influencing outcome in patients treated with surgery. The GOS was dichotomized as favorable outcome (GOS 4 and 5) and unfavorable outcome (GOS ≤3), and the mRS as good outcome (mRS 4-6) and poor outcome (mRS ≤3). Variables with P values less than 0.05 and clinically relevant variables (P > 0.05) were subjected to multivariable logistic regression analysis to determine independent associative factors for long-term outcome based on mRS at 6 months. For this purpose, the GOS was dichotomized as favorable (GOS 1-2) and unfavorable (GOS 4 and 5), and the mRS as good outcome (mRS 0-4) and poor outcome (mRS 5 and 6).
RESULTS
A total of 125 patients with malignant MCA territory infarction during the study period were included in this study. The mean (±SD) age of patients was 53.8 (±7.17) years. The age range was between 34 and 65 years, with a majority of them being within 46-55 years of age (43%). Non-dominant hemisphere was involved in 66 patients (52.8%), while dominant hemisphere involvement was seen in 59 patients (47.2%). All patients had at least one scan done within 24 h of stroke onset, and a repeat scan done within the following 24-48 h. Mean time from stroke onset to brain CT scan was 10.5 h. Infarct volume ranged between 200 ml and 340 ml, and was classified as either less than or exceeding 250 ml. Infarct volume exceeding 250 ml was seen in 57% of the total population sample (72 patients). Median midline shift in this cohort was 7.34 mm, and a cut-off of 10 mm was used to stratify patients. Midline shift exceeding 10 mm was noted in 52% of the patient population.
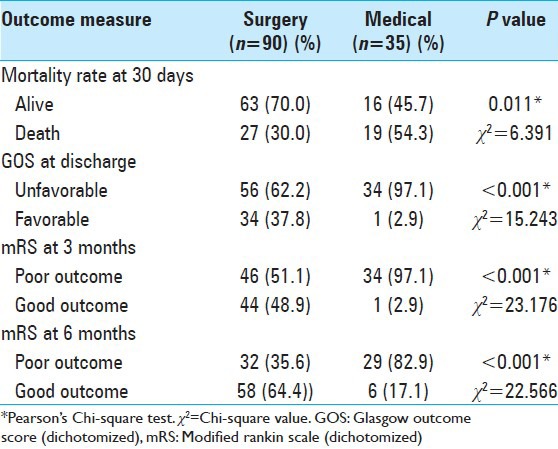
A total of 90 patients (72%) were treated with surgery, while 35 patients (28%) were managed with medical therapy. Majority of the patients (87%) had a GCS score of between 6 and 9 before surgery. Mean time (±SD) between stroke onset and surgery was 26.8 h (±8.3). A significant difference was noted between patients treated with decompressive surgery and those treated with medical therapy in terms of mortality rate at 30 days, GOS at discharge, and mRS at 3 months and 6 months [Table 2]. Patients treated with surgery had a mortality rate of 30, ascompared to 54.3% in patients treated with medical therapy (P < 0.05). Favorable outcome based on GOS at discharge was noted in 37.8% of the patients treated with surgery, as compared to 2.9% of the patients treated with medical treatment (P < 0.05). Good outcome based on dichotomized mRS (mRS <4) was seen in 48.9% and 64.4% of patients treated with surgery at 3 months and 6 months, respectively, compared to 2.9% and 17.1% of patients receiving medical treatment at 3 months and 6 months, respectively (P < 0.05).
Table 2.
Comparison of outcome in patients with malignant MCA territory infarction treated with surgery and medical therapy
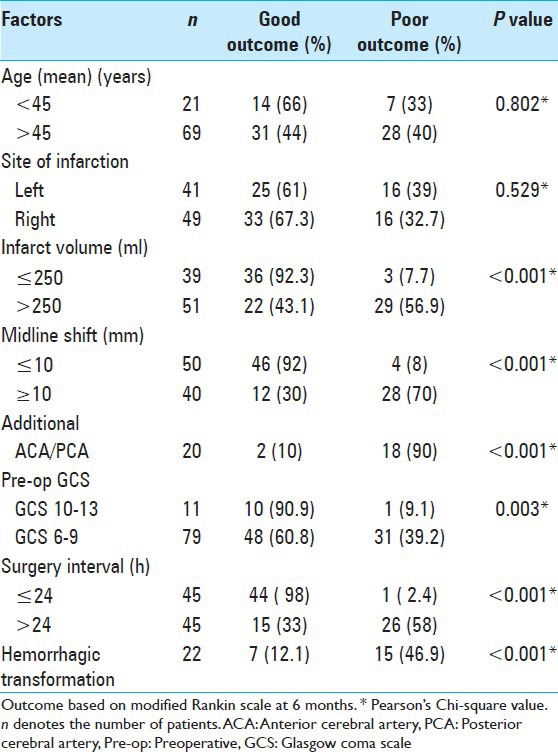
In patients treated with surgery, the factors significantly associated with good outcome at 6 months [Table 3] were volume of infarct less than 250 ml (P < 0.001), midline shift less than 10 mm (P < 0.001), absence of additional vascular territory involvement (P < 0.001), preoperative GCS of 10-13 (P = 0.003), and time interval to surgery within 24 h (P < 0.001). Age and dominant hemisphere involvement were not significantly associated with outcome at 6 months (P > 0.05). Postoperative complication of hemorrhagic transformation was significantly associated with poor outcome at 6 months (P < 0.001).
Table 3.
Factors influencing outcome at 6 months in patients treated with surgery
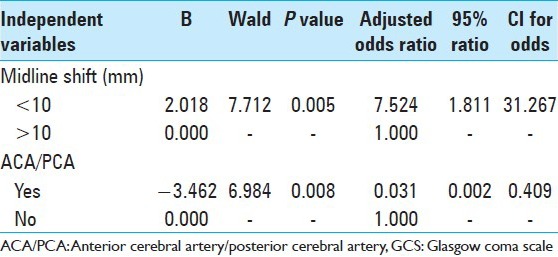
Significant predictors of good outcome at 6 months based on multivariate logistic regression analysis [Table 4] were absence of additional vascular territory involvement [odds ratio (OR) 0.031; 95% confidence interval (CI) 0.002-0.409)] and midline shift of less than 10 mm (OR 7.524; 95% CI 0.002-0.409).
Table 4.
Multivariable binary logistic regression analysis of outcome at 6 months in patients treated with surgery
DISCUSSION
The results from this study reaffirm the beneficial role of decompressive craniectomy in reducing mortality rate and improving functional outcome in patients with malignant MCA territory infarction. Decompressive craniectomy reduced the 30-day mortality rate by 24.3% and the proportion of patients with unfavorable outcome at discharge by 34.9%. Good functional outcome (mRS 0-4) was seen in 48.9% and 64.4% at 3 months and 6 months, respectively, in patients treated with surgery, compared to 2.9% and 17.1%, respectively, in patients treated with medical treatment. Percentage of patients treated with surgery and with moderate to slight disability (mRS 0-3) was 27.8% at 3 months and 50% at 6 months, while no patients treated with medical therapy achieved an mRS score of 0-3 at 3 months and 6 months. Percentage of patients left bedridden with severe disability at the end 6 months was 4.4% with surgical treatment and 25.7% with medical treatment. At the end of the follow-up period, 67% of patients treated with surgery and 43% of patients treated with medical therapy were alive. An increase of 15% in good functional outcome from 3 to 6 months was noted in both treatment groups, similar to the observations reported by various authors. This is probably a reflection of the natural healing process by neuromodulation over time.[1] Additional contributing factors include aggressive rehabilitation measures, physiotherapy, management of secondary complications, and control of underlying risk factors. The percentage of good outcome at 3 months and 6 months in this series is comparable with that reported in earlier studies,[9,6,16] but is lower than the reported figures in three randomized trials.[10,11,21] A possible explanation for this is the inclusion of patients beyond the age of 60 years in this study population, compared to the randomized trials, where the patient age limit was set at 60 years.
Patients with a mean age of 50 years had significantly better outcome at discharge and at 3 months and 6 months, regardless of the treatment modalities (P = 0.002, P = 0.001, and P = 0.015, respectively). In patients treated with surgery, no significant association was noted between age groups and patient outcome at 6 months. The percentage of patients achieving good outcome (mRS 0-4) was comparable between patients younger than 55 years and older than 56 years. When analyzed based on mRS of 0-3, good outcome was seen in 54% of patients younger than 55 years at 6 months, as compared to 46% in patients older than 56 years (P > 0.05). A trend of improved functional outcome was noted with younger age, with 76% of patients younger than 45 years achieving good outcome at 6 months (mRS 0-4), and 68% having mRS of 0-3 at 6 months. An interesting finding in this study is an improved outcome in patients between 56 and 65 years of age compared to patients between 46 and 55 years of age, who were treated with surgery. Similar findings were also noted in the HAMLET trial.[10] It can be postulated that older patients may be protected from the effects of cerebral edema due to cerebral atrophy and they deteriorate over a longer time interval.[1,2] Conversely, younger patients who have not suffered the effects of cerebral atrophy may deteriorate faster, and present with a lower preoperative GCS score. In this study, preoperative GCS score was found to be significantly associated with patient outcome at 6 months, and this factor may explain the comparable outcomes observed in this study at 6 months between younger and older patients.
Good functional outcome at 6 months was seen in patients aged 56-65 years and treated with surgery (64.4% good outcome), compared to patients treated with medical therapy (25% good outcome), which suggests a potential benefit for surgery in this age group. In a recent study,[24,25] analysis of outcome following surgery for malignant cerebral infarction in 131 patients aged 70 years or older showed a lower mortality rate in the group treated with surgery compared to the medically treated group. There is still ongoing debate with regards to the exact age limit that influences outcome in patients. Patient's health status, functional and cognitive status, daily living index, comorbidities, neurological condition on presentation and extent of infarction, social and employment situation, as well as patient's and family expectations should be taken into account in treatment decisions.
The GCS score was used as the primary tool of assessment in this study. GCS score has a strong predictive value for early mortality and functional outcome in patients with malignant infarction.[23] Favorable outcome on discharge was noted in 81% of patients with preoperative GCS score 10-13, compared to 31.6% of patients with a preoperative GCS of 6-9. Good functional outcome was noted in patients with preoperative GCS of 10-13 at 3 months (81.8%) and 6 months (90.9%), compared to those with a preoperative GCS score of 6-9 (45.6% and 60.8%, respectively). In keeping with the findings from previously published papers, the GCS score is accurate in predicting early mortality and outcome in patients with malignant MCA territory infarction.[23]
Results from the European trials,[10,11,21] showed that outcome form dominant hemisphere involvement was comparable with that from non - dominant hemispheric infarction. Similar findings were noted in this study. Dominant hemisphere infarction was significantly associated with unfavorable outcome at discharge and at 3 months follow - up. However, at 6 months follow-up, good outcome was noted in 61% of patients with dominant hemisphere involvement and 67% of patients with non-dominant hemispheric involvement. It can be postulated that dominant hemispheric infarction may impair the initial phase of recovery, and cause biasness in outcome assessment. With neuromodulation and rehabilitation, an improvement in deficits over time may result in improved long-term outcome in patients.[1]
Infarct volume has been shown to be significantly associated with patient outcome. An infarct volume of more than 250 ml in patients treated with surgery was associated with poor outcome at 6 months, a significant difference when compared to an infarct volume of less than 250 ml (P < 0.05). An infarct volume of more than 240 ml was predictive of malignant transformation with 76% accuracy, and an infarct volume of more than 280 ml was associated with poor outcome.[14] A similar association was noted between the extent of midline shift and additional vascular territory involvement with patient outcome in the surgical group. A significant difference between the extent of midline shift and outcome was noted, with good outcome at 6 months seen in patients with midline shift of less than 10 mm (P < 0.05). Additional vascular territory involvement was also significantly associated with outcome in patients in the surgical group, with poor outcome at 6 months noted with additional vascular territory involvement (P < 0.05). A larger infarct volume, midline shift, and additional vascular territory involvement led to increased mass effect and brain herniation, resulting in compression of brainstem structures and a decrease in potentially viable ischemic brain tissue. This finding is in keeping with known predictors of outcome in MCA territory infarction.
Hemorrhagic transformation was seen in 24% of patients treated with surgery, and this was significantly associated with outcome at 6 months (P < 0.001). Development of hemorrhagic transformation can be due to shearing injury of the brain tissue which herniates beyond the craniectomy margins, or an increase in cerebral perfusion pressure following surgery leads to reperfusion injury of ischemic or infracted brain tissue with altered autoregulation and microvascular structural defects. Postoperative hydrocephalus occurred in 21% of patients treated with surgery, and was significantly associated with poor outcome at discharge and at 3 months, but there was no significant association with outcome at 6 months. This is possibly due to treatment for hydrocephalus with CSF diversion procedures or early cranioplasty in these patients.
CONCLUSION
Decompressive craniectomy significantly reduces mortality rate and improves functional outcome. Patient selection plays a key role in translating the beneficial effects of surgery. Factors significantly influencing outcome in this study were age, GCS on admission and preoperatively, extent of infarction and mass effect, and time interval from onset to surgery. Additional vascular territory involvement and midline shift of more than 10 mm were significantly associated with poor outcome at 6 months. Dominant hemisphere involvement had no significant effect on outcome in this study. The issue of age limit remains unclear as older patients subjected to surgery had lower mortality rate compared to older patients who were treated medically in this study.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2014/5/1/102/135342
Contributor Information
Mohammad Azman Mohammed Raffiq, Email: deshum77@yahoo.com.
Mohammed Saffari Mohammad Haspani, Email: saffarineuro@yahoo.com.
Regunath Kandasamy, Email: kandasamy.regunath@gmail.com.
Jafri Malin Abdullah, Email: brainsciences@gmail.com.
REFERENCES
- 1.Arac A, Blanchard V, Lee M, Steinberg GK. Assessment of outcome following decompressive craniectomy for malignant middle cerebral artery infarction in patients older than 60 years of age. Neurosurg Focus. 2009;26:E3. doi: 10.3171/2009.3.FOCUS0958. [DOI] [PubMed] [Google Scholar]
- 2.Berrouschot J, Sterker M, Bettin S, Koster J, Schneider D. Mortality of space-occupying (malignant) middle cerebral artery infarction under conservative intensive care. Int Care Med. 1998;24:620–3. doi: 10.1007/s001340050625. [DOI] [PubMed] [Google Scholar]
- 3.Cheung A, Telaghani CK, Wang J, Yang Q, Mosher TJ, Reichwein RK, et al. Neurological recovery after decompressive craniectomy for massive ischemic stroke. Neurocrit Care. 2005;3:216–23. doi: 10.1385/NCC:3:3:216. [DOI] [PubMed] [Google Scholar]
- 4.Demchuk AM, Krieger DW. Mass effect with cerebral infarction. Curr Treat Options Neurol. 1999;1:189–99. doi: 10.1007/s11940-999-0003-y. [DOI] [PubMed] [Google Scholar]
- 5.Dupont WD, Plummer WD., Jr Power and sample size calculation; a review and computer program. Controlled Clin Trials. 1998;11:116–28. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 6.Gupta R, Connolly ES, Mayer S, Elkind MS. Hemicraniectomy for massive middle cerebral artery territory infarction. A systematic review. Stroke. 2004;35:539–43. doi: 10.1161/01.STR.0000109772.64650.18. [DOI] [PubMed] [Google Scholar]
- 7.Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. Malignant middle cerebral artery territory infarction: Clinical course and prognostic signs. Arch Neurol. 1996;53:309–15. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- 8.Heinsius T, Bogousslavsky J, Van Melle G. Large infarcts in the middle cerebral artery territory: Etiology and outcome patterns. Neurology. 1998;50:341–50. doi: 10.1212/wnl.50.2.341. [DOI] [PubMed] [Google Scholar]
- 9.Hofmeijer J, Van der Worp HB, Kappelle LJ. Treatment of space occupying cerebral infarction. Crit Care Med. 2003;31:617–25. doi: 10.1097/01.CCM.0000050446.16158.80. [DOI] [PubMed] [Google Scholar]
- 10.Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB. HAMLET investigators. Surgical decompression for space occupying cerebral infarction the Hemicraniectomy after middle cerebral arteri infarction with life-threatening edema trial (HAMLET): A multicentre, open, randomised trial. Lancet Neurol. 2009;8:326–33. doi: 10.1016/S1474-4422(09)70047-X. [DOI] [PubMed] [Google Scholar]
- 11.Juttler E, Schwab S, Schmiedek P, Unterberg A, Hennerici M, Woitzik J, et al. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (DESTINY): A randomized controlled trial. Stroke. 2007;38:2518–25. doi: 10.1161/STROKEAHA.107.485649. [DOI] [PubMed] [Google Scholar]
- 12.Kasner SE, Demchuk AM, Berrouschot J, Schmutzhard E, Harms L, Verro P, et al. Predictors of fatal brain edema in massive hemispheric ischemic stroke. Stroke. 2001;32:2117–23. doi: 10.1161/hs0901.095719. [DOI] [PubMed] [Google Scholar]
- 13.Kunst M. Ischemic stroke. Radiol Clin N Am. 2011;49:1–26. doi: 10.1016/j.rcl.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Mori K, Nakao Y, Yamamoto T, Maeda M. Early external decompressive craniectomy with duroplasty improves functional recovery in patients with massive hemispheric embolic infarction; Timing and indications for surgery. Surg Neurol. 2004;62:420–30. doi: 10.1016/j.surneu.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Qureshi AI, Suarez JI, Yahia AM, Mohammad Y, Uzun G, Suri MF, et al. Timing of neurologic deterioration in massive middle cerebral artery infarction: A multicenter review. J Crit Care Med. 2003;31:272–7. doi: 10.1097/00003246-200301000-00043. [DOI] [PubMed] [Google Scholar]
- 16.Rieke K, Krieger D, Krieger D, von Kummer R, Aschoff A, Schuchardt V, et al. Decompressive surgery in space-occupying hemispheric infarction: Results of an open, prospective trial. J Crit Care Med. 1995;23:1576–87. doi: 10.1097/00003246-199509000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Schwab S, Steiner T, Aschoff A, Schwarz S, Steiner HH, Jansen O, et al. Early hemicraniectomy in patients with complete middle cerebral artery infarction. Stroke. 1998;29:1888–93. doi: 10.1161/01.str.29.9.1888. [DOI] [PubMed] [Google Scholar]
- 18.Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72:210410. doi: 10.1212/WNL.0b013e3181aa5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–4. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 20.Teasdale G, Jennett B. Assessment and prognosis of coma after head injury. Acta Neurochir (Wien) 1976;34:45–55. doi: 10.1007/BF01405862. [DOI] [PubMed] [Google Scholar]
- 21.Vahedi K, Vicaut E, Mateo J, Kurtz A, Orabi M, Guichard JP, et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial) Stroke. 2007;38:2506–17. doi: 10.1161/STROKEAHA.107.485235. [DOI] [PubMed] [Google Scholar]
- 22.Wartenberg KE. Malignant middle cerebral artery infarction. Curr Opin Crit Care. 2012;18:152–63. doi: 10.1097/MCC.0b013e32835075c5. [DOI] [PubMed] [Google Scholar]
- 23.Weir CJ, Bradford AP, Lees KR. The prognostic value of the components of the Glasgow Coma Scale following acute stroke. QJ M. 2003;96:67–74. doi: 10.1093/qjmed/hcg008. [DOI] [PubMed] [Google Scholar]
- 24.Yao Y, Liu W, Yang X, Hu W, Li G. Is decompressive craniectomy for malignant middle cerebral artery infarction of any benefit for elderly patients. Surg Neurol. 2005;64:165–9. doi: 10.1016/j.surneu.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 25.Yu JW, Choi JH, Kim DH, Cha JK, Huh JT. Outcome following decompressive craniectomy for patients older than 70 years old. J Cerebrovasc Endovasc Neurosurg. 2012;14:65–74. doi: 10.7461/jcen.2012.14.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]


