Abstract
Background:
Prognosis of patients with spinal cord glioblastoma is poor, with an average survival of 18 months. There are reports in the literature describing cordectomy as a treatment option for patients with spinal cord tumors.
Case Description:
This is a case report of a patient with spinal cord glioblastoma who, in addition to radiation and chemotherapy, was treated with cordectomy. Outcome of treatment resulted in 12-year survival.
Conclusion:
Cordectomy in spinal cord glioblastoma can result in prolonged and meaningful survival.
Keywords: Cordectomy, ependymoma, neuro-oncology, spinal cord glioblastoma
INTRODUCTION
Primary glial tumors including astrocytomas, ependymomas, gangliogliomas, oligodendrogliomas, and subependymomas account for about 80% of intramedullary spinal cord tumors. Of these, spinal cord glioblastoma multiforme (GBM) is rare, representing approximately 7.5% of all spinal cord gliomas.[4,6,12,14,18,20,22,23] Treatment for spinal gliomas typically includes surgery with postoperative radiation and chemotherapy. In cases of spinal GBM, however, the prognosis is poor, with an average survival of 18 months.[2,4,6,8,13,15,18,19,20,23]
Cordectomy has generally been performed to treat syringomyelia, spasticity, and central pain.[10,12,16,17,18,21] There are few reports in the literature describing cordectomy as a treatment option for patients with spinal cord tumors.[12,17,18] The argument for cordectomy has been a longer survival than would otherwise be achievable with more radiation or the addition of chemotherapy. Herein, we report the case of a patient with spinal cord glioblastoma treated with cordectomy surviving nearly 12 years.
CASE REPORT
In February 2002, a 47-year-old man presented to our institution with a 2-year history of worsening low back pain, difficulty urinating, weakness, and lower extremity paresthesias. His physical examination demonstrated paresis of the lower extremities with hypoesthesia and hypoalgesia and a sensory level of T8. He was hyperreflexic with upgoing toes bilaterally. Magnetic resonance imaging (MRI) with enhancement revealed an enhancing mass of the spinal cord at T8-9, with edema extending to the mid T6 region rostrally and to T12 caudally [Figure 1a]. As part of his workup, the patient also underwent MRI of the brain [Figure 1b, c], showing no intracranial pathology. Continued progressive neurological decline prompted surgical intervention. On 23 February 2002, the patient underwent a T8, T9, and T10 laminectomy and intramedullary tumor resection. The pathology showed a high degree of nuclear atypia and mitotic activity. In regions of high cellularity, there was vascular proliferation, with occasional glomeruloid vascular structures. Some vessels were also thrombosed, and small regions of necrosis were identified. On the basis of this histopathology, a diagnosis of GBM was made [Figure 1d]. Postoperatively, his neurological exam remained unchanged. After discharge, he received 30 cycles of radiation therapy and one cycle of procarbazine, lomustine, vincristine (PCV) combination therapy, which was poorly tolerated, followed by one cycle of temozolomide.
Figure 1.
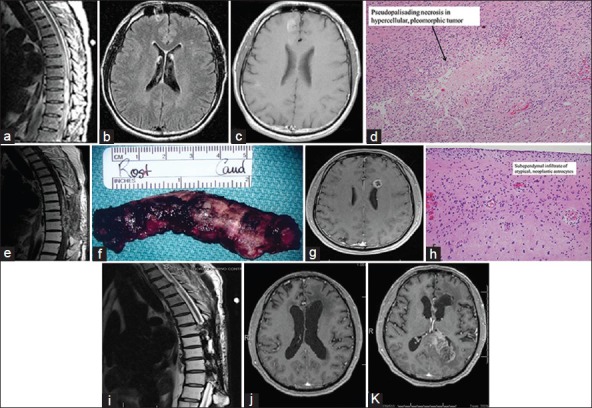
A 47-year-old man with a 2-year history of back pain developed progressive paraparesis 1 week prior to admission. His weakness rapidly progressed to paraplegia. MRI showed an enhancing intramedullary tumor at T8-9 with extensive edema (a). As part of his workup, the patient also underwent MRI of the brain (flair b and enhanced c), which showed no intracranial pathology. Two days later, the patient underwent a T8, T9, and T10 laminectomy and intramedullary tumor resection. Histopathology showed a high degree of nuclear atypia, mitotic activity, and vascular proliferation with thrombosis in regions of necrosis. The pathology was consistent with a glioblastoma (d). Postoperatively, the patient received 30 cycles of radiation therapy. Seven months later, an MRI with enhancement revealed tumor recurrence (e), extending to and including T7-T10. On 11 October 2002, based on the MRI imaging, the patient underwent T6-T11 laminectomy and transdural cordectomy. The illustration shows the excised cord within the dura (f). Histopathology on the resected cord again showed glioblastoma with marked atypia, mitosis, vascular proliferation, and necrosis. In August 2008, he presented with dizziness and diplopia of several months duration. MRI of the brain identified an enhancing mass in the left frontal periventricular white matter (g). Biopsy of this mass showed hypercellular brain parenchyma infiltrated by a population of atypical cells. There was a subependymal accumulation of these cells with mitotic activity (h). A diagnosis of grade III astrocytoma was made. Follow-up thoracic MRI (i) showed a stable exam with postsurgical and postradiation changes from T7 through T11 without gross evidence of progression of disease. Brain MRI in May 2013 (j) showed postradiation effects with ventriculomegaly, but no evidence of tumor recurrence in the frontal lobe. Latest MRI of the brain, taken in November 2013, showed tumor recurrence in the left occipital lobe with extension into the splenium of the corpus callosum, but no evidence of tumor in the frontal lobe (k)
An MRI taken 7 months after surgery revealed tumor recurrence [Figure 1e]. At that time, the patient's neurological exam remained unchanged; he was wheelchair-bound with a T8 sensory level and an American Spinal Injury Association (ASIA) score of C. The risks and benefits of a cordectomy were discussed with the patient and his family, and it was decided to proceed with tumor resection and cordectomy. On 11 October 2002, he underwent a T6, T7, and T11 laminectomy and transdural cordectomy [Figure 1f]. The proximal and distal dural stumps were oversewn in a watertight fashion. He tolerated the operation well and had a sensory level at T6 with flaccid paraplegia. Following the second surgery, he was started on carboplatin for seven cycles. He was placed on daily thalidomide from April 2004 to June 2007.
For nearly 6 years, he remained stable in regards to his physical examination, and surveillance MRIs of the thoracic cord were performed every 2 months. In August 2008, he presented with dizziness and diplopia of several months duration. MRI of the brain identified a left frontal mass [Figure 1g]. On 16 September 2008, he underwent a stealth-guided biopsy of the left frontal mass, which revealed grade III astrocytoma [Figure 1h]. Subsequently, he received 59.4 Gy in 33 fractions of radiation and was restarted on monthly cycles of temozolomide. Thoracic spine MRIs continue to show stable postoperative change and enhancement [Figure 1i]. Most recently, brain MR taken on 14 May 2013 showed interval resolution of the left frontal mass [Figure 1j]. When last seen, on 19 November 2013, he was becoming increasingly fatigued and felt that he was not tolerating his chemotherapy particularly well. His neurological exam remained stable. MRI taken on 12 November 2013 [Figure 1k] showed a left occipital mass with extension into the corpus callosum. The left frontal lobe appeared stable. The patient missed his last appointment on 17 January 2013 as he was too ill to travel, and passed away on 29 January 2014, 12 years following his initial presentation.
DISCUSSION
Cordectomy is a surgical option for patients with spinal cord tumors in the setting of a complete deficit. There are several reports in the literature of spinal cord tumors treated with cordectomy. Kyoshima et al.[17] reported a case of a 48-year-old man with fibrillary astrocytoma of the conus medullaris treated initially with surgical resection followed by radiation. The patient underwent three more surgeries for tumor recurrence before undergoing cordectomy. Pathological examination was that of an anaplastic astrocytoma. The patient underwent three additional cordectomies for tumor recurrence and survived 15 months following the first cordectomy. Marchan et al.[18] reported the case of a 50-year-old man who was diagnosed with a spinal cord GBM initially treated with radiation and chemotherapy. Within 3 months, the patient had a complete deficit and underwent cordectomy. He had a 6-year disease-free interval after his cordectomy before developing metastasis to the brain and died 1 month later. The authors concluded that cordectomy may improve survival in patients with high-grade spinal glioma who were paraplegic. Ewelt et al.[12] reported the case of a 27-year-old woman diagnosed with a spinal cord anaplastic astrocytoma at T12. In 2004, she was treated with surgical resection and chemotherapy. In August 2007, because of progressive neurological deficit, she underwent a T9 cordectomy. Follow-up MRI taken 15 months later revealed no evidence of tumor recurrence. Raza et al.[21] reported a 65-year-old woman who presented with a recurrent thoracic meningioma (WHO grade 1) extending from T2 to T6. At the time of presentation, she was paraplegic and complained of upper extremity paresthesias. She had undergone three previous resections, radiation therapy, and chemotherapy. She then underwent a T2-8 cordectomy. Postoperatively, the patient developed bilateral upper extremity hyperesthesia and new interossei and grip weakness bilaterally. Two-month postoperative MRI showed a right-sided enhancing mass at the T10-11 spinal foramina, as well as a mass along the posterior meninges at T11. She survived 15 months following cordectomy.
The present cases illustrate cordectomy as an option for patients with high-grade intramedullary spinal cord tumors and a complete neurological deficit. The goal of surgery is resection of the infiltrating tumor and cord while preserving function in the cervical and upper thoracic cord. Preservation of the upper thoracic cord is important for maintaining autonomic function. Autonomic dysreflexia resulting in hemodynamic instability is a known complication of resection of the thoracic cord; therefore, if at all possible, the upper thoracic cord should remain intact.[9]
Estimates of spinal cord metastases from intracranial GBM have been reported as between 1 and 2%.[24] Extension of intracranial dissemination of spinal cord glioma, though less frequent, is well-described in the literature for both low- and high-grade lesions.[1,3,4,5,7,8,11,14] In the report by Johnson et al.[14] 17 cases of intracranial metastases from spinal cord malignant astrocytoma were reported in addition to their own. Diagnosis of intracranial extension was based on radiological studies, mostly computed tomography (CT), and not histopathology. The development of intracranial disease occurred from 1 to 11 months, with an average of 6 months, from diagnosis of the spinal cord tumor. Metastases occurred after surgery for the spinal tumor in 13. Asano et al.[4] presented their own case of spinal cord GBM that developed intracranial metastases in spite of localized radiation. The patient succumbed to intracranial metastases 26 months later. In their paper, Asano et al. reviewed eight other cases of spinal cord glioblastomas from the literature with intracranial metastasis, for an average survival of 12 months range 1-1 26 months. Six of the total nine received localized radiation before developing intracranial disease. In our case, the patient survived 12 years from the diagnosis of spinal cord GBM and 11 years from his cordectomy. He was able to witness the graduation of his daughter from high school and completion of her training in Physical Therapy and Rehabilitation. We believe that cordectomy contributed substantially to the meaningful survival of this patient.
CONCLUSION
In the absence of brain and distant metastases, cordectomy in a patient with spinal cord glioblastoma and paraplegia is a treatment option. Our case and other similar case reports suggest that cordectomy may delay intracranial extension and prolong survival.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2014/5/1/101/135305
Contributor Information
Stephanus Viljoen, Email: stephanus.viljoen@uiowa.edu.
Patrick W. Hitchon, Email: patrick.hitchon@uiowa.edu.
Raheel Ahmed, Email: raheel.ahmed@uiowa.edu.
Patricia A. Kirby, Email: patricia.kirby@uiowa.edu.
REFERENCES
- 1.Abel TJ, Chowdhary A, Thapa M, Rutledge JC, Geyer JR, Ojemann J, et al. Spinal cord pilocytic astrocytoma with leptomeningeal dissemination to the brain. Case report and review of the literature. J Neurosurg. 2006;105(6 Suppl):S508–14. doi: 10.3171/ped.2006.105.6.508. [DOI] [PubMed] [Google Scholar]
- 2.Alvisi C, Cerisoli M, Giulioni M. Intramedullary spinal gliomas: Long-term results of surgical treatments. Acta Neurochir (Wien) 1984;70:169–79. doi: 10.1007/BF01406647. [DOI] [PubMed] [Google Scholar]
- 3.Andrews AA, Enriques L, Renaudin J, Tomiyasu U. Spinal intramedullary glioblastoma with intracranial seeding. Report of a case. Arch Neurol. 1978;35:244–5. doi: 10.1001/archneur.1978.00500280062013. [DOI] [PubMed] [Google Scholar]
- 4.Asano N, Kitamura K, Seo Y, Mukai K, Soga T, Hondo H, et al. Spinal cord glioblastoma multiforme with intracranial dissemination--case report. Neurol Med Chir (Tokyo) 1990;30:489–94. doi: 10.2176/nmc.30.489. [DOI] [PubMed] [Google Scholar]
- 5.Bell WO, Packer RJ, Seigel KR, Rorke LB, Sutton LN, Bruce DA, et al. Leptomeningeal spread of intramedullary spinal cord tumors. Report of three cases. J Neurosurg. 1988;69:295–300. doi: 10.3171/jns.1988.69.2.0295. [DOI] [PubMed] [Google Scholar]
- 6.Ciappetta P, Salvati M, Capoccia G, Artico M, Raco A, Fortuna A. Spinal glioblastomas: Report of seven cases and review of the literature. Neurosurgery. 1991;28:302–6. [PubMed] [Google Scholar]
- 7.Claus D, Sieber E, Engelhardt A, Rechlin T, Neubauer U, Volk B. Ascending central nervous spreading of a spinal astrocytoma. J Neurooncol. 1995;25:245–50. doi: 10.1007/BF01053158. [DOI] [PubMed] [Google Scholar]
- 8.Cohen AR, Wisoff JH, Allen JC, Epstein F. Malignant astrocytomas of the spinal cord. J Neurosurg. 1989;70:50–4. doi: 10.3171/jns.1989.70.1.0050. [DOI] [PubMed] [Google Scholar]
- 9.Constantini S, Miller DC, Allen JC, Rorke LB, Freed D, Epstein FJ. Radical excision of intramedullary spinal cord tumors: Surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg. 2000;93(2 Suppl):183–93. doi: 10.3171/spi.2000.93.2.0183. [DOI] [PubMed] [Google Scholar]
- 10.Durward QJ, Rice GP, Ball MJ, Gilbert JJ, Kaufmann JC. Selective spinal cordectomy: Clinicopathological correlation. J Neurosurg. 1982;56:359–67. doi: 10.3171/jns.1982.56.3.0359. [DOI] [PubMed] [Google Scholar]
- 11.Eden K. Dissemination of a glioma of the spinal cord in the leptomeninges. Brain. 1938;61:298–310. [Google Scholar]
- 12.Ewelt C, Stummer W, Klink B, Felsberg J, Steiger HJ, Sabel M. Cordectomy as final treatment option for diffuse intramedullary malignant glioma using 5-ALA fluorescence-guided resection. Clin Neurol Neurosurg. 2010;112:357–61. doi: 10.1016/j.clineuro.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Guidetti B, Mercuri S, Vagnozzi R. Long-term results of the surgical treatment of 129 intramedullary spinal gliomas. J Neurosurg. 1981;54:323–30. doi: 10.3171/jns.1981.54.3.0323. [DOI] [PubMed] [Google Scholar]
- 14.Johnson DL, Schwarz S. Intracranial metastases from malignant spinal-cord astrocytoma. Case report. J Neurosurg. 1987;66:621–5. doi: 10.3171/jns.1987.66.4.0621. [DOI] [PubMed] [Google Scholar]
- 15.Kim MS, Chung CK, Choe G, Kim IH, Kim HJ. Intramedullary spinal cord astrocytoma in adults: Postoperative outcome. J Neurooncol. 2001;52:85–94. doi: 10.1023/a:1010680924975. [DOI] [PubMed] [Google Scholar]
- 16.Klekamp J. Treatment of posttraumatic syringomyelia. J Neurosurg Spine. 2012;17:199–211. doi: 10.3171/2012.5.SPINE11904. [DOI] [PubMed] [Google Scholar]
- 17.Kyoshima K, Ito K, Tanabe A, Iwashita T, Goto T, Sato A, et al. Malignant astrocytoma of the conus medullaris treated by spinal cordectomy. J Clin Neurosci. 2002;9:211–6. doi: 10.1054/jocn.2001.0929. [DOI] [PubMed] [Google Scholar]
- 18.Marchan EM, Sekula RF, Jr, Jannetta PJ, Quigley MR. Long-term survival enhanced by cordectomy in a patient with a spinal glioblastoma multiforme and paraplegia. Case report. J Neurosurg Spine. 2007;7:656–9. doi: 10.3171/SPI-07/12/656. [DOI] [PubMed] [Google Scholar]
- 19.Mayer RR, Warmouth GM, Troxell M, Adesina AM, Kass JS. Glioblastoma multiforme of the conus medullaris in a 28-year-old female: A case report and review of the literature. Clin Neurol Neurosurg. 2012;114:275–7. doi: 10.1016/j.clineuro.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Raco A, Piccirilli M, Landi A, Lenzi J, Delfini R, Cantore G. High-grade intramedullary astrocytomas: 30 years’ experience at the Neurosurgery Department of the University of Rome “Sapienza”. J Neurosurg Spine. 2010;12:144–53. doi: 10.3171/2009.6.SPINE08910. [DOI] [PubMed] [Google Scholar]
- 21.Raza SM, Anderson WS, Eberhart CG, Wolinsky JP, Gokaslan ZL. The application of surgical cordectomy in the management of an intramedullary-extramedullary atypical meningioma: Case report and literature review. J Spinal Disord Tech. 2005;18:449–54. doi: 10.1097/01.bsd.0000155032.69394.23. [DOI] [PubMed] [Google Scholar]
- 22.Scarrow AM, Rajendran P, Welch WC. Glioblastoma multiforme of the conus medullaris. Clin Neurol Neurosurg. 2000;102:166–7. doi: 10.1016/s0303-8467(00)00086-x. [DOI] [PubMed] [Google Scholar]
- 23.Strik HM, Effenberger O, Schafer O, Risch U, Wickboldt J, Meyermann R. A case of spinal glioblastoma multiforme: Immunohistochemical study and review of the literature. J Neurooncol. 2000;50:239–43. doi: 10.1023/a:1006415703881. [DOI] [PubMed] [Google Scholar]
- 24.Tinchon A, Oberndorfer S, Marosi C, Ruda R, Sax C, Calabek B, et al. Malignant spinal cord compression in cerebral glioblastoma multiforme: A multicenter case series and review of the literature. J Neurooncol. 2012;110:221–6. doi: 10.1007/s11060-012-0955-8. [DOI] [PubMed] [Google Scholar]


