Abstract
Background:
The underlying pathophysiology leading to syringomyelia is elusive with multiple flow-related theories constituting our current limited understanding of the disease process. Syringomyelia is associated with pathologies related to the disturbance of cerebral spinal fluid flow found in conditions such as Chiari I malformations, spinal malignancy, spinal cord tethering, trauma, or arachnoid adhesions. Our aim is to describe a unique surgical shunting technique used to treat refractory cases of idiopathic syringomyelia.
Methods:
Five patients, aged 22-50, presented with progressive neurologic symptoms associated with an idiopathic syrinx. All underwent decompressive laminectomy surgery with syringosubarachnoid shunting using the silastic wedge technique.
Results:
In five cases of idiopathic syringomyelia, clinical and radiographic follow up ranges from 3 to 36 months. Three patients have radiographic and clinical follow up greater than 24 months. All patients improved clinically and their symptoms have been stable.
Conclusions:
Shunting procedures for the syringomyelia disease spectrum have been criticized due to the inconsistent long-term outcomes. This surgical technique used to treat symptomatic idiopathic syringomyelia has been devised based on our intraoperative experience, surgical outcomes, and evaluation of the literature. The purpose of the wedges is to preserve patency of the communication between the syrinx cavity and the expanded subarachnoid space by preventing healing of the myelotomy edges and by maintaining an artificial conduit between the syrinx cavity and the subarachnoid space. Although short-term results are promising, continued long-term follow up is needed to determine the ultimate success of the silastic wedge shunting procedure.
Keywords: Idiopathic syringomyelia, silastic wedge technique, syringosubarachnoid shunt
INTRODUCTION
Surgical intervention for syringomyelia is considered when conservative medical treatment fails and neurological symptoms progress.[5,6,7,8,9] The underlying pathophysiology leading to syringomyelia is elusive with multiple flow-related theories constituting our current limited understanding of the disease process.[4,5,12] Syringomyelia is associated with pathologies related to the disturbance of cerebral spinal fluid flow found in conditions such as Chiari I malformations, spinal malignancy, spinal cord tethering, trauma, or arachnoid adhesions.[5,6] In these conditions, surgical intervention involves directly removing the source causing the syrinx formation. Malignancy resection, decompressive, and untethering procedures with adhesion removal and duraplasty have been successful in removing offending pathologies creating the syringomyelia condition.[1,3,11] In cases where these methods have failed, the use of syrinx shunting, whether syringoperitoneal, syringopleural, or syringosubarachnoid, has been advocated as a procedure of last resort. Many shunting procedures have been described and the results have been erratic. Complications include fibrosis with subsequent obstruction, shunt migration, and shunt infections, which minimize the success of this surgical treatment.[10,11] Data from long-term studies on shunting procedures for syringomyelia suggest 12-53% of patients improve, 10-56% unchanged, and 12-32% regress.[2,11]
Idiopathic syringomyelia is an entity not associated with any of the previously mentioned conditions.[2] With no overt etiology, surgical decision making and treatment can be challenging. This report describes a unique surgical shunting technique used to treat refractory cases of idiopathic syringomyelia.
MATERIALS AND METHODS
Institutional review board approval was obtained and all patients with refractory idiopathic syringomyelia who underwent a syringosubarachnoid shunt using the silastic wedge technique at a single institution were analyzed.
Patients
Five patients, aged 22-50, presented with progressive neurologic symptoms associated with an idiopathic syrinx [Table 1]. One patient (Case 1) had a history of multiple sclerosis with new onset left sided chest pain in a band-like distribution and exacerbation of her gait abnormalities and urinary retention. There was no proceeding traumatic event prior to the onset of symptoms. Two patients (Cases 2 and 3) presented with intractable neck pain and headaches following motor vehicle accidents. They were conservatively treated without relief of their symptoms. One patient (Cases 4) presented with intractable thoracic radiculopathy and no evidence of trauma preceding the symptoms.
Table 1.
Patient clinical and operative data
Technique
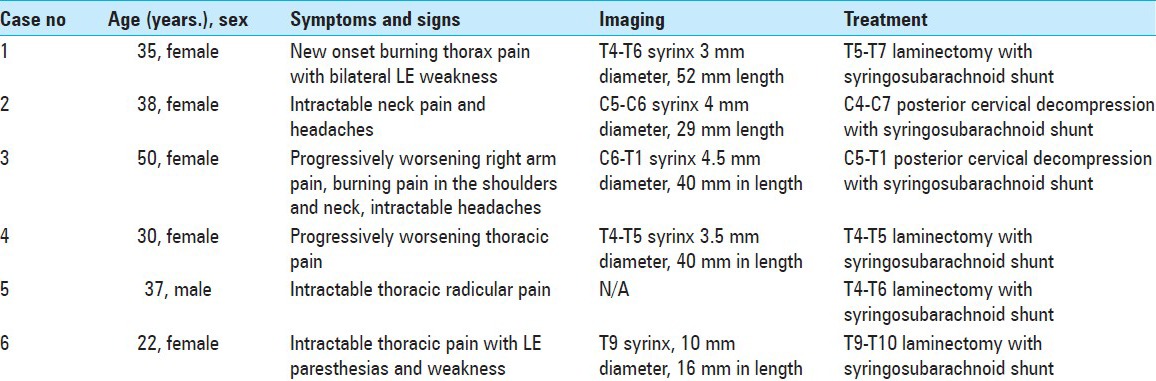
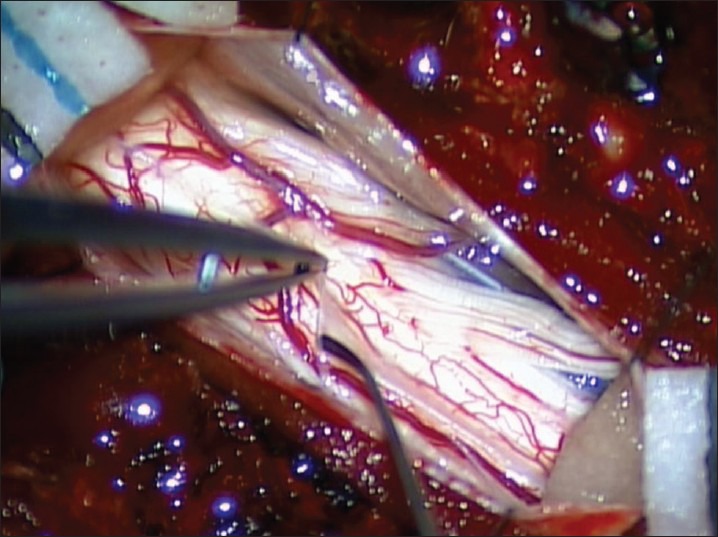
A decompressive laminectomy extending rostral and caudal to the syrinx cavity is performed. The dura is opened and reflected laterally and secured with 4-0 sutures. Arachnoid inspection is undertaken with assistance of an operating microscope to identify any possible constricting/flow diverting lesions [Figure 1]. Intraoperative ultrasound is employed to identify the syrinx cavity and a midline myelotomy is performed [Figure 2]. Once the syrinx cavity has been entered, two silastic wedges are contoured from a 0.13 mm silastic sheet to fit the myelotomy dimensions [Figure 3]. The two wedges placed side-by-side and sutured to the pia using 9-0 prolene [Figure 4]. If adhesions are encountered, a duraplasty with Gortex is performed. Otherwise closure is performed in a typical layered fashion.
Figure 1.
Opening the arachnoid membrane
Figure 2.

Contoured silastic wedges
Figure 3.
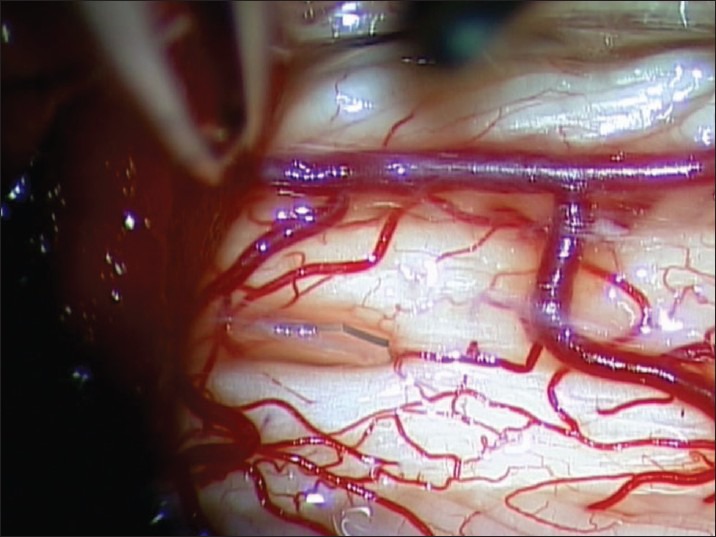
Initial placement of contoured silastic wedge
Figure 4.
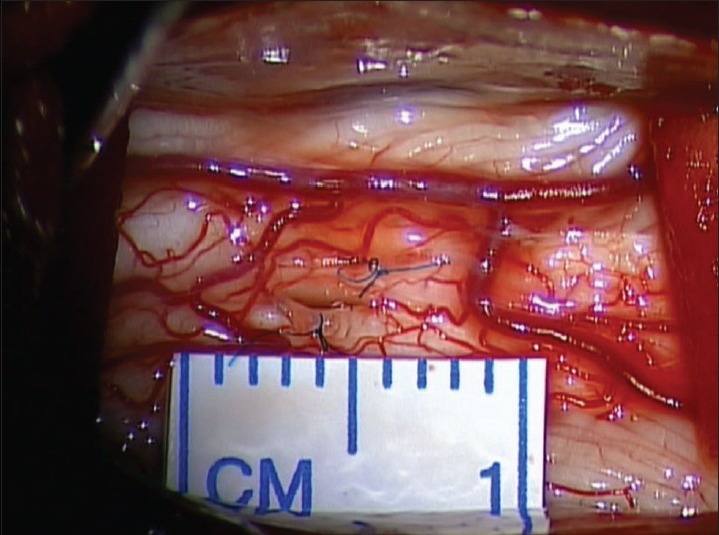
Final position of silastic wedges
RESULTS
In the five cases of idiopathic syringomyelia, clinical and magnetic resonance imaging (MRI) follow-up ranged from 3 to 36 months. Three patients have MRI and clinical follow up greater than 24 months. All patients improved clinically and symptoms have been stable. Cases 4 and 5 have limited follow up radiographic data. Case 4 has improved clinically but refuses follow up imaging. All shunted syrinx cavities have shown a decrease in size [Table 1] and have been stable from 3 to 36 months [Figures 5–8] in terms of MRI images.
Figure 5.
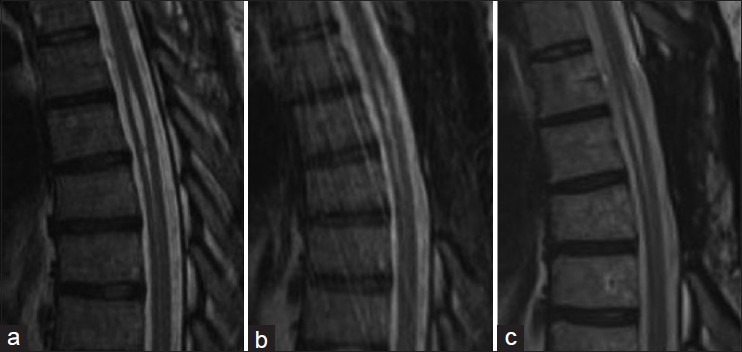
Case 1. (a) preoperative syrinx state. (b) 12 months postoperative. (c) 24 months postoperative
Figure 8.
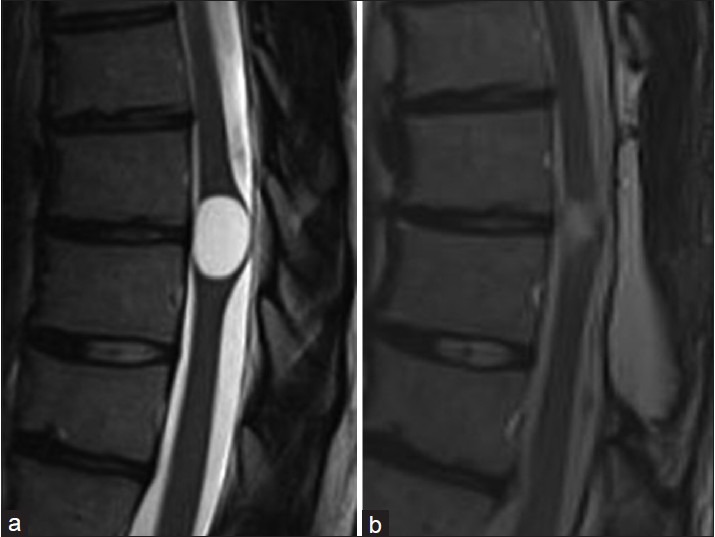
Case 4. (a) preoperative syrinx state. (b) 3 months postoperative
Figure 6.
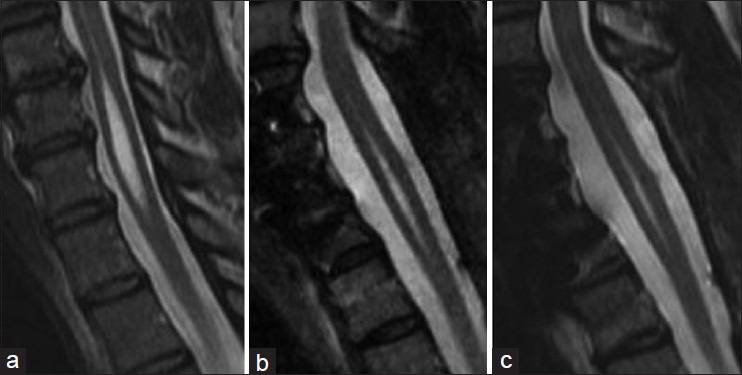
Case 2. (a) preoperative syrinx state. (b) 12 months post perative. (c) 24 months postoperative
Figure 7.
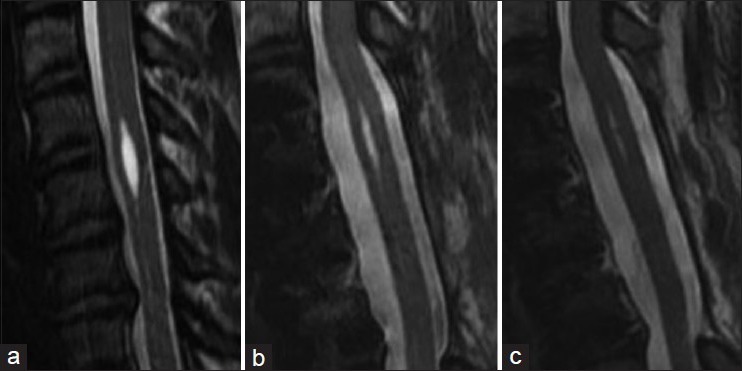
Case 3. (a) preoperative syrinx state. (b) 12 months postoperative. (c) 36 months postoperative
DISCUSSION
Shunting procedures for the syringomyelia disease spectrum have been criticized due to the inconsistent long-term outcomes. This is largely the result of small volume flow at a very low-pressure profile leading to occlusion or malfunction of the shunts. This surgical technique used to treat symptomatic idiopathic syringomyelia has been devised based on our intraoperative experience, surgical outcomes, and evaluation of the literature. The purpose of the wedges is to preserve patency of the communication between the syrinx cavity and the expanded subarachnoid space by preventing healing of the myelotomy edges and by maintaining an artificial conduit between the syrinx cavity and the subarachnoid space. The goal is to enable small volume flow more consistently. The silastic wedges allow for the passage of cerebral spinal fluid through capillary action under very small pressures without collapse. This technique allows for insertion of two wedges. Capillary action will allow for the fluid to travel along the space between the two wedges. The benefit to this method is that even after the syrinx cavity is drained and pressures within the cavity diminish, the patency of the conduit and the mechanism of the capillary action will still be maintained. For traditional shunt procedures with diversionary tubing, a higher pressure must be maintained to achieve continued flow. As fluid is drained, the pressure decreases and the cavity collapses around the perforations in the shunt tubing. Flow through the tube becomes stagnant and the susceptibility to obstruction, a well-recognized complication with shunting procedures, increases. With the silastic wedges, a potential space is created between the two surfaces allowing a constant channel for fluid to pass under changes in pressure and cavity size. The low profile of the silastic wedges within the subarachnoid space may also help reduce subarachnoid adhesions that have previously been reported in cases using tubing to shunt the syrinx cavity. When securing the traditional tubing into place, the round opening of the tubing makes it difficult to be sutured to the pia. The flexible silastic wedge allows for a simple stitch that can be placed under direct microscopic visualization to avoid damage to any surface vessels. The triangular shape of the wedge allows for direct visualization and safe placement into the syrinx cavity without blind advancement.
CONCLUSION
The inconsistent results that are reported with shunt tubing leads us to question whether drainage will continue once the initial drainage is achieved. Because a consistently high pressure would be required to maintain flow, there is most likely cerebral spinal fluid egress around the shunt tubing into the subarachnoid space rather than drainage through the tubing. We have found that the silastic wedges provide a similar conduit requiring less pressure to drain fluid from the cavity. The shape and pliability of the wedges lend to a one-step, safe insertion that does not require blind advancement or manipulation into the syrinx cavity. The wedge will also be able to maintain small volume flow more consistently even under a low-pressure profile. Although short-term results are promising, continued long-term follow up is needed to determine the ultimate success of the silastic wedge shunting procedure.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2014/5/1/114/137536
Contributor Information
Teck M. Soo, Email: tsoo111292MI@comcast.net.
Lee Sandquist, Email: sandql@hotmail.com.
Doris Tong, Email: Doris.Tong@michiganspineandbrainsurgeons.com.
Ryan Barrett, Email: Ryan.Barrett@michiganspineandbrainsurgeons.com.
REFERENCES
- 1.Bonfield CM, Levi AD, Arnold PM, Okonkwo DO. Surgical management of post-traumatic syringomyelia. Spine. 2010;35(21 Suppl):S245–58. doi: 10.1097/BRS.0b013e3181f32e9c. [DOI] [PubMed] [Google Scholar]
- 2.Brodbelt AR, Stoodley MA. Post-traumatic syringomyelia: A review. J Clin Neurosci. 2003;10:401–8. doi: 10.1016/s0967-5868(02)00326-0. [DOI] [PubMed] [Google Scholar]
- 3.Goel A, Desai K. Surgery for syringomyelia: An analysis based on 163 surgical cases. Acta Neurochir (Wien) 2000;142:293–301. doi: 10.1007/s007010050038. [DOI] [PubMed] [Google Scholar]
- 4.Heiss JD, Snyder K, Peterson MM, Patronas NJ, Butman JA, Smith RK, et al. Pathophysiology of primary spinal syringomyelia. J Neurosurg Spine. 2012;17:367–80. doi: 10.3171/2012.8.SPINE111059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klekamp J. Treatment of Syringomyelia related to nontraumatic arachnoid pathologies of the spinal cord. Neurosurgery. 2013;72:376–89. doi: 10.1227/NEU.0b013e31827fcc8f. [DOI] [PubMed] [Google Scholar]
- 6.Milhorat TH. Classification of syringomyelia. Neurosurg Focus. 2000;8:E1. doi: 10.3171/foc.2000.8.3.1. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura M, Ishii K, Watanabe K, Tsuji T, Matsumoto M, Toyama Y, et al. Clinical significance and prognosis of idiopathic syringomyelia. J Spinal Disord Tech. 2009;22:372–5. doi: 10.1097/BSD.0b013e3181761543. [DOI] [PubMed] [Google Scholar]
- 8.Porensky P, Muro K, Ganju A. Nontraumatic cervicothoracic syrinx as a cause of progressive neurologic dysfunction. J Spinal Cord Med. 2007;30:276–81. doi: 10.1080/10790268.2007.11753937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy AK, Slimack NP, Ganju A. Idiopathic syringomyelia: Retrospective case series, comprehensive review, and update on management. Neurosurg Focus. 2011;31:E15. doi: 10.3171/2011.9.FOCUS11198. [DOI] [PubMed] [Google Scholar]
- 10.Sgouros S, Williams B. A critical appraisal of drainage in syringomyelia. J Neurosurg. 1995;82:1–10. doi: 10.3171/jns.1995.82.1.0001. [DOI] [PubMed] [Google Scholar]
- 11.Ushewokunze SO, Gan YC, Phillips K, Thacker K, Flint G. Surgical treatment of post-traumatic syringomyelia. Spinal Cord. 2010;48:710–3. doi: 10.1038/sc.2010.17. [DOI] [PubMed] [Google Scholar]
- 12.Williams B. Pathogenesis of syringomyelia. Acta Neurochir (Wien) 1993;123:159–65. [PubMed] [Google Scholar]


