Abstract
Purpose:
To describe the limitations of Fourier-domain optical coherence tomography (OCT) in imaging common conjunctival and corneal pathology.
Materials and Methods:
Retrospective, single-center case series of 40 patients with conjunctival and cornea pathology.
Results:
Fourier-domain OCT imaged laser in situ keratomileusis (LASIK) flaps in detail, including its relation to other corneal structures and abnormalities. Similarly, in infectious or degenerative corneal disorders, Fourier-domain OCT successfully showed the extent of infiltration or material deposition, which appeared as hyper-reflective areas. In cases with pterygium, the underlying cornea could not be imaged. All cases of common conjunctival pathologies, such as nevus or pinguecula, were successfully imaged in detail. Nevi, scleritis, pterygium, pinguecula, and subconjunctival hemorrhage were hyper-reflective lesions, while cysts and lymphangiectasia were hyporeflective. The details of the underlying sclera were not uniformly imaged in conjunctival pathologies. Fourier-domain OCT imaged the trabeculectomy bleb in detail, whereas the details of structures of the anterior chamber angle were not routinely visualized in all cases.
Conclusions:
Light scatter through vascularized, densely inflamed, or thick lesions limits the imaging capabilities of Fourier-domain anterior segment OCT.
Keywords: Anterior Segment Optical Coherence Tomography, Conjunctiva, Cornea, Optical Coherence Tomography
INTRODUCTION
Optical coherence tomography (OCT) is a non-contact, non-invasive diagnostic imaging device that provides high resolution, real-time and in situ visualization of tissue microstructure.1,2 OCT technologies are based on the scanning methods which are either time-domain OCT (Stratus and Visante, Carl Zeiss Meditec Inc., Dublin, CA, USA) or Fourier/spectral-domain (RTVue, Optovue Inc., Fremont, CA, USA).2,3,4 Time-domain OCT uses 2000 axial scans per second and Fourier or spectral-domain OCT uses 26,000 axial scans.1 Time-domain OCT can image anterior segment structures with long wavelength light (1310 nm) and the retina with short wavelength light (820 nm). Fourier or spectral-domain OCT provides high-resolution images of retina and is available at most major ophthalmology centers.3 The development of a corneal adaptor module (CAM) for Fourier or spectral-domain OCT enables imaging of the anterior segment structures. Time-domain anterior segment OCT can provide images with a tissue resolution ranging from 5 to 17 μm with an imaging depth varying from 2.3 to 6 mm, while the Fourier-domain OCT with the corneal adaptor module provides images with a tissue resolution of 5 μm and an imaging depth of 2.3 mm.1,2 In the literature, Fourier or spectral-domain OCT with CAM has been used mostly to evaluate corneal thickness, LASIK flaps and anterior chamber angle structures.3,5,6,7,8,9,10,11,12,13,14,15 There is relative paucity of information on the limitations of imaging common corneal and conjunctival pathologies with Fourier-domain OCT with CAM. In this study, we evaluated common corneal and conjunctival pathologies with Fourier-domain OCT equipped with CAM and described the limitations of the imaging technique.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board. Patients with common conjunctival and corneal pathologies who presented to the Department of Ophthalmology, Henry Ford Hospital who underwent anterior segment Fourier-domain OCT were included in this study. Data were collected on age, gender, the diagnosis of corneal and conjunctival pathology. The anterior segment Fourier-domain OCT was performed by the same examiner with CAM attached to the RTVue OCT unit. CAM consists of 2 adaptor lenses: The wide angle lens (CAM-L) and high magnification lens (CAM-S). Lens selection is based on the extent of the pathology. Common corneal and conjunctival pathologies were scanned by using both wide angle and high magnification lenses. All imaging data were correlated to patient data. The slit lamp photography was performed for common corneal and conjunctival pathologies.
RESULTS
Forty patients with various conjunctival and corneal pathologies were included in this study. Of 40 patients, 4 patients had a history of previous refractive surgery, 6 patients had corneal dystrophy, 7 patients had corneal infectious or inflammatory infiltrates, 10 patients had corneal opacity, 8 patients had conjunctival lesion and 5 patients had scleritis/episcleritis.
Fourier-domain OCT was successful in identifying the borders and thickness of LASIK and DSEK flaps [Figure 1a, b, d]. In a patient who developed corneal haze beneath the LASIK flap, Fourier-domain OCT showed corneal haze as a hyper-reflective reflex without any shadowing between the LASIK flap and corneal stroma [Figure 1a]. In another patient who developed infiltration beneath the LASIK flap following blunt trauma, Fourier-domain OCT imaged the infiltrate as a thick hyper-reflective reflex with shadowing in the corneal stroma beneath the LASIK flap [Figure 1b]. Fourier-domain OCT successfully imaged the location of corneal deposits throughout the thickness of a cornea with corneal dystrophy. In a patient with central Schnyder crystalline corneal dystrophy, Fourier-domain OCT showed multiple hyper-reflective deposits without shadowing in the anterior stroma of the central cornea [Figure 1c]. In a patient with Fuchs endothelial dystrophy, irregular endothelial layer with intermittent thin areas were observed [Figure 1e]. In a patient with Thygeson's superficial punctate keratopathy, the OCT image revealed hyper-reflective deposits within the epithelium and the basal membrane.
Figure 1 (a-k).
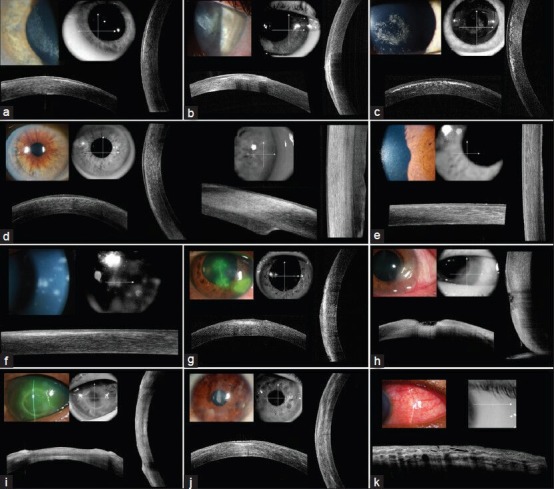
Fourier-domain optical coherence tomography imaging of common corneal pathologies (a) 61-year-old female developed haze under her laser in situ keratomileusis (LASIK) flap in the right eye at 9 months postoperatively (wide angle lens) (b) A 35-year-old female developed infiltration under the LASIK flap following blunt trauma 5 years after surgery (wide angle lens) (c) An 8-year-old male with central crystalline dystrophy of Schnyder (wide angle lens) (d) A 74-year-old male who underwent DSEK for Fuchs' endothelial dystrophy (wide angle lens (left side), and high magnification lens (right side)) (e) A 65-year-old female with Fuchs' endothelial dystrophy (high magnification lens) (f) A 61-year-old man with subepithelial infiltrates secondary to epidemic viral keratoconjunctivitis (high magnification lens) (g) A 50-year-old male with herpes simplex keratitis (wide angle lens) (h) A 59-year-old female with peripheral corneal ulcer (wide angle lens) (i) A 47-year-old male with neurotrophic ulcer secondary to severe diabetes mellitus (wide angle lens) (j) A 20-year-old female with bilateral interstitial keratitis secondary to hidradenitis suppurativa (wide angle lens) (k) A 52-year-old male with scleritis secondary to sarcoidosis (wide angle lens)
The extent of corneal involvement in corneal infectious infiltrates was successfully imaged with Fourier-domain OCT. In a patient with adenoviral keratoconjunctivitis, a line of hyper-reflective deposits without shadowing was observed under normal thickness corneal epithelium [Figure 1f]. Similarly, in a patient with corneal dendritic ulcer from herpes simplex virus infection, Fourier-domain OCT showed a band area of hyper-reflective deposits without shadowing under the irregularly thickened corneal epithelium [Figure 1g]. In a patient with infectious peripheral corneal ulcer, the center of the peripheral ulcer was thinned with increased reflectivity in the underlying stroma [Figure 1h]. The surrounding peripheral epithelium was thickened and intraepithelial reflectivity was increased, causing underlying shadowing in the corneal stroma. In a patient with central sterile neurotrophic corneal ulcer, thinned central cornea without hyper-reflectivity was observed [Figure 1i].
In interstitial keratitis, Fourier-domain OCT showed areas of patchy stromal hyper-reflectivity [Figure 1j]. In a patient with granulomatous uveitis secondary to sarcoidosis, mutton-fat keratic precipitates were observed as hyper-reflective deposits over the endothelium [Figure 1l]. In cases with pterygium over the cornea, the imaging was limited to the fibrovascular tissue of pterygium and did not extend through to the underlying cornea [Figure 1m]. Corneal scar secondary to a previous trauma was imaged as a hyper-reflective reflex over the cornea without imaging the corneal stroma due to scatter of light [Figure 1n].
Figure 1 (l-s).
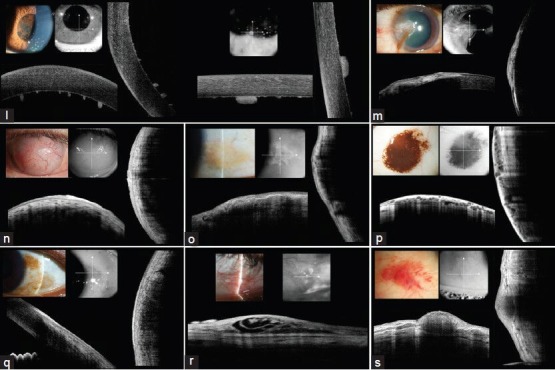
(l) A 44-year-old female with granulomatous uveitis secondary to sarcoidosis (wide angle lens (left side), and high magnification lens (right side)) (m) A 78-year-old male with pterygium (wide angle lens) (n) A 42-year-old male with vascularized corneal scar secondary to trauma occurred 35 years ago (wide angle lens) (o) A 49-year-old female with pinguecula (high magnification lens) (p) A 47-year-old male with conjunctival nevus (high magnification lens) (q) A 75-year-old female with conjunctival primary acquired melanosis (high magnification lens) (r) A 45-year-old female with lymphangiectasia (high magnification lens) (s) A 70-year-old female with subconjunctival hemorrhage (high magnification lens)
In conjunctival lesions, Fourier-domain OCT showed the conjunctival epithelium, but failed to image the episclera and sclera. In a patient with pinguecula, the lesion was imaged for the entire extent of the lesion but not the underlying sclera due to light scatter [Figure 1o]. Similarly, in pigmented conjunctival nevus, the entire extent of the lesion with multiple cysts was observed but some areas of underlying sclera were not visible [Figure 1p]. The pigmented portion of the conjunctival nevus prevented the imaging of the underlying episclera and sclera. Similarly, the entire extent of conjunctival primary acquired melanosis was visible, but some areas of the underlying sclera were missing due to light scatter [Figure 1q]. Interestingly, in a patient with lymphangiectasia, the underlying sclera as well as the details of the lesion were imaged successfully [Figure 1r]. In a patient with subconjunctival hemorrhage, the entire area of hemorrhage was imaged including its posterior borders [Figure 1s]. In patients with scleritis and episcleritis, hyporeflectivity consistent with edema was observed within the superficial layers of sclera under the conjunctival epithelium [Figure 1k]. However, the extent of imaging was limited to the superficial scleral layers. The trabeculectomy bleb was visualized in detail, while anterior chamber angle structures were not routinely visualized in all cases.
DISCUSSION
Currently, most of the knowledge on features of anterior segment OCT imaging is based on the studies using time-domain OCT.16,17,18,19,20,21,22 Information about imaging features of common corneal and conjunctival pathologies with Fourier-domain OCT technology is limited. Rosas Salaroli et al.10 reported that Fourier-domain OCT gave highly repeatable flap-thickness measurements in LASIK patients. Wylegala et al.3 compared Fourier-domain OCT with time-domain OCT for evaluating the anterior segment morphology including central corneal thickness, trabecular-iris angle, angle opening distance, and found that measurements for both OCT modalities were highly correlated. On morphologic analysis, time-domain OCT provided lower resolution, but all structures were visible in 1 image.3 Fourier-domain OCT provided high-resolution images of small areas of the anterior segment structure.3 Gumus et al.13 evaluated cross-sectional areas of conjunctivochalasis and tear meniscus in 12 eyes of 7 patients. They13 reported that anterior segment Fourier domain OCT is a useful and reproducible instrument to measure a cross-sectional area of conjunctiva prolapsing into the tear meniscus of patients with conjunctivochalasis. Recently, slit lamp-adapted Fourier domain OCT (SL-OCT) was developed to image the anterior segment structures.23,24,25,26 Sandler et al.23 reported that SL-OCT produced reproducible measurements for central corneal thickness and anterior chamber depth. These measurements were comparable to ultrasonic pachymetry and axial OCT biometry.23
In our study, Fourier-domain OCT successfully imaged the details of the clear cornea and related pathologies. The higher resolution of Fourier-domain OCT aided in imaging of the details of 53.4 μm-thick corneal epithelium and 5-10 μm-thick corneal endothelium and Descemet's membrane.27 The cholesterol and phospholipid depositions in central Schnyder crystalline corneal dystrophy, thickened Descemet's membrane with guttae in Fuchs' dystrophy, and infiltration under LASIK flap or in adenoviral keratoconjunctivitis were imaged in detail and provided information on the location and extent of the pathology. In cases with dense corneal infiltrate such as infectious corneal ulcer, light scatter prevented imaging the entire thickness of the infiltrate and cornea; however, it provided an estimate of how thin area the ulcerated cornea was. In cases with overlying light-scattering tissue such as fibrovascular tissue of pterygium, the short wavelength light of Fourier-domain OCT could not penetrate through the pterygium. The details of imaging were limited to the pterygium tissue and the details of underlying cornea could not be imaged.
Information about the OCT imaging of conjunctival lesions is limited to conjunctival nevus. Shields and co-workers28 reviewed time-domain OCT findings of conjunctival nevus and reported that time-domain OCT provided clear images of all nevus margins and visualized intralesional cyst in 77% of cases. Buchwald et al.29 reviewed 13 conjunctival lesions that underwent time-domain OCT and found that compared to ultrasound biomicroscopy, time-domain OCT was able to show small cystic structures more distinctly, while ultrasound biomicroscopy was better in assessing the margins of the tumor. There is no information on the imaging feature of Fourier-domain OCT. We observed that Fourier-domain OCT imaged conjunctival lesions, but did not uniformly provide a detailed image of the episcleral/scleral margin. We think that scattering of short wavelength light used in the Fourier-domain OCT through vascularized or pigmented conjunctival tissue is a factor in limiting the imaging of the posterior margin. Supporting this observation, the entire extent of trabeculectomy blebs or lymphangiectasia were imaged in detail. The shorter wavelength light of Fourier-domain OCT penetrated easily through the avascular bleb walls or thin lymphatic vessel walls and imaged up to the episcleral/scleral surface. Thickness of the conjunctival lesion is another limiting factor in imaging of the underlying sclera. Desjardins et al.30 reported that the mean thickness of conjunctival nevi is 2.3 mm, which is also the upper limit of the imaging depth of Fourier-domain OCT.
In summary, our study shows that Fourier-domain anterior segment OCT is a useful adjunct to conventional examination of the corneal and conjunctival pathologies. It successfully images the clear cornea, LASIK flaps and deposits throughout the cornea. When there is extensive infiltrate or overlying vascular tissue, it failed to show the underlying corneal tissue. It also successfully imaged conjunctival lesions, but it did not uniformly image the underlying sclera or show their relation to the sclera. The thickness, vascularity and pigmentation of conjunctival lesions caused limitations in imaging of underlying sclera.
Footnotes
Source of Support: Generous Gift from Mrs. and Mr. Witham
Conflict of Interest: None declared.
REFERENCES
- 1.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254:1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramos JL, Li Y, Huang D. Clinical and research applications of anterior segment optical coherence tomography-a review. Clin Experiment Ophthalmol. 2009;37:81–9. doi: 10.1111/j.1442-9071.2008.01823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wylegała E, Teper S, Nowiñska AK, Milka M, Dobrowolski D. Anterior segment imaging: Fourier-domain optical coherence tomography versus time-domain optical coherence tomography. J Cataract Refract Surg. 2009;35:1410–4. doi: 10.1016/j.jcrs.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 4.Wojtkowski M, Leitgeb R, Kowalczyk A, Bajeaszewski T, Fercher AF. In vivo human retinal imaging by Fourier domain optical coherence tomography. J Biomed Opt. 2002;7:457–63. doi: 10.1117/1.1482379. [DOI] [PubMed] [Google Scholar]
- 5.Prakash G, Agarwal A, Jacob S, Kumar DA, Agarwal A, Banerjee R. Comparison of Fourier-domain and time-domain optical coherence tomography for assessment of corneal thickness and intersession repeatability. Am J Ophthalmol. 2009;148:282–90.e2. doi: 10.1016/j.ajo.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Zhou S, Li Y, Lu AT, Liu P, Tang M, Yiu SC, et al. Reproducibility of tear meniscus measurement by Fourier-domain optical coherence tomography: A pilot study. Ophthalmic Surg Lasers Imaging. 2009;40:442–7. doi: 10.3928/15428877-20090901-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SE, Yoon JS, Lee SY. Tear measurement in prosthetic eye users with Fourier-domain optical coherence tomography. Am J Ophthalmol. 2010;149:602–7.e1. doi: 10.1016/j.ajo.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Huang JY, Pekmezci M, Yaplee S, Lin S. Intra-examiner repeatability and agreement of corneal pachymetry map measurement by time-domain and Fourier-domain optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2010;248:1647–56. doi: 10.1007/s00417-010-1360-7. [DOI] [PubMed] [Google Scholar]
- 9.Knecht PB, Kaufmann C, Menke MN, Watson SL, Bosch MM. Use of intraoperative Fourier-domain anterior segment optical coherence tomography during descemet stripping endothelial keratoplasty. Am J Ophthalmol. 2010;150:360–5.e2. doi: 10.1016/j.ajo.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Rosas Salaroli CH, Li Y, Zhang X, Tang M, Branco Ramos JL, Allemann N, et al. Repeatability of laser in situ keratomileusis flap thickness measurement by Fourier-domain optical coherence tomography. J Cataract Refract Surg. 2011;37:649–54. doi: 10.1016/j.jcrs.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Can I, Bayhan HA, Celik H, Bostancý Ceran B. Anterior segment optical coherence tomography evaluation and comparison of main clear corneal incisions in microcoaxial and biaxial cataract surgery. J Cataract Refract Surg. 2011;37:490–500. doi: 10.1016/j.jcrs.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Tang M, Chen A, Li Y, Huang D. Corneal power measurement with Fourier-domain optical coherence tomography. J Cataract Refract Surg. 2010;36:2115–22. doi: 10.1016/j.jcrs.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gumus K, Crockett CH, Pflugfelder SC. Anterior segment optical coherence tomography: A diagnostic instrument for conjunctivochalasis. Am J Ophthalmol. 2010;150:798–806. doi: 10.1016/j.ajo.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tai MC, Chien KH, Lu DW, Chen JT. Angle changes before and after cataract surgery assessed by Fourier-domain anterior segment optical coherence tomography. J Cataract Refract Surg. 2010;36:1758–62. doi: 10.1016/j.jcrs.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Doors M, Berendschot TT, de Brabander J, Webers CA, Nuijts RM. Value of optical coherence tomography for anterior segment surgery. J Cataract Refract Surg. 2010;36:1213–29. doi: 10.1016/j.jcrs.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Singh M, Chew PT, Friedman DS, Nolan WP, See JL, Smith SD, et al. Imaging of trabeculectomy blebs using anterior segment optical coherence tomography. Ophthalmology. 2007;114:47–53. doi: 10.1016/j.ophtha.2006.05.078. [DOI] [PubMed] [Google Scholar]
- 17.Khurana RN, Li Y, Tang M, Lai MM, Huang D. High-speed optical coherence tomography of corneal opacities. Ophthalmology. 2007;114:1278–85. doi: 10.1016/j.ophtha.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Netto MV, Shekhar R, Kruger RR, Huang D. A longitudial study of LASIK flap and stromal thickness with high-speed optical coherence tomography. Ophthalmology. 2007;114:1124–32. doi: 10.1016/j.ophtha.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 19.Kohnen T, Thomala MC, Cichocki M, Strenger A. Internal anterior chamber diameter using optical coherence tomography compared with white-to-white distances using automated measurements. J Cataract Refract Surg. 2006;32:1809–13. doi: 10.1016/j.jcrs.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Meisler DM, Tang M, Lu AT, Thakrar V, Reiser BJ, et al. Keratoconus diagnosis with optical coherence tomography pachymetry mapping. Ophthalmology. 2008;115:2159–66. doi: 10.1016/j.ophtha.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Layanda R, Teo L, Friedman DS, Aung HT, Baskaran M, Gao H, et al. Comparison of anterior chamber depth measurements using the IOLMaster, scanning peripheral anterior chamber depth analyser and anterior segment optical tomography. Br J Ophthalmol. 2007;91:1023–6. doi: 10.1136/bjo.2006.113761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radhakrishan S, Goldsmith J, Huang D, Westphal V, Dueker DK, Rollins AM, et al. Comparison of optical coherence tomography and ultrasound biomicroscopy for detection of narrow anterior chamber angles. Arch Ophthalmol. 2005;123:1053–9. doi: 10.1001/archopht.123.8.1053. [DOI] [PubMed] [Google Scholar]
- 23.Sandler SF, Zelefsky JR, Dorairaj S, Arthur SN, Ritch R, Liebmann JM. Intra-observer and inter-observer reliability and reproducibility of slit-lamp-adapted optical coherence tomography for evaluation of anterior chamber depth and central corneal thickness. Ophthalmic Surg Lasers Imaging. 2008;39:299–303. doi: 10.3928/15428877-20080701-15. [DOI] [PubMed] [Google Scholar]
- 24.Yan PS, Lin HT, Wang QL, Zhang ZP. Anterior segment variations with age and accommodation demonstrated by slit-lamp-adapted optical coherence tomography. Ophthalmology. 2010;117:2301–7. doi: 10.1016/j.ophtha.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 25.Dinc U, Oncel B, Gorgun E, Alimgil L. Quantitative assessment of anterior chamber volume using slit-lamp OCT and Pentacam. Eur J Ophthalmol. 2009;19:411–5. doi: 10.1177/112067210901900314. [DOI] [PubMed] [Google Scholar]
- 26.Hoerauf H, Wirbelauer C, Scholz C, Engelhardt R, Koch P, Laqua H, et al. Slit-lamp-adapted optical coherence tomography of the anterior segment. Graefes Arch Clin Exp Ophthalmol. 2000;238:8–18. doi: 10.1007/s004170050002. [DOI] [PubMed] [Google Scholar]
- 27.Reinstein DZ, Archer TJ, Gobbe M, Silverman RH, Coleman DJ. Epithelial thickness in the normal cornea: Three dimensional display with Artemis very high frequency digital ultrasound. J Refract Surg. 2008;24:571–81. doi: 10.3928/1081597X-20080601-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shields CL, Belinsky I, Romanelli-Gobbi M, Guzman JM, Mazzuca D, Jr, Green WR, et al. Anterior segment optical coherence tomography of conjunctival nevus. Ophthalmology. 2011;118:915–9. doi: 10.1016/j.ophtha.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Buchwald HJ, Müller A, Kampmeier J, Lang GK. Optical coherence tomography versus ultrasound biomicroscopy of conjunctival and eyelid lesions. Klin Monbl Augenheilkd. 2003;220:822–9. doi: 10.1055/s-2003-812563. [DOI] [PubMed] [Google Scholar]
- 30.Desjardins L, Poncet P, Levy C, Schlienger P, Asselain B, Validire P. Prognostc factors in malignant melanoma of the conjunctiva. An anatomo-clinical study of 56 patients. [Article in French] J Fr Ophthalmol. 1999;22:315–21. [PubMed] [Google Scholar]


