Abstract
Background
Preclinical studies showed a Chinese botanical formula, PHY906, has synergistic anti-tumor activity with capecitabine. Our phase I study determined maximal tolerated dose of capecitabine 1500mg/m2 BID day 1–7 and PHY906 800mg BID day 1–4 every two weeks. We conducted this phase II study to explore the efficacy of capecitabine and PHY906 in patients with advanced pancreatic cancer who were previously treated with gemcitabine-based regimens.
Methods
Patients with pancreatic cancer and an ECOG performance status of 0 to 2 received PHY906 and capecitabine. Toxicity was assessed per NCI-CTCAE v3.0 and response per RECIST q 6 wks. Correlative studies of cytokines, chemokines and growth factors were tested using a cytometric bead array. Quality of life was assessed by utilizing Edmonton Symptom Assessment System. The primary objective was overall survival.
Results
The study enrolled 25 patients. Median progression-free survival (mPFS) was 10.1 weeks (range: 0.4–54.1) and median overall survival (mOS) was 21.6 weeks (range: 0.4–84.1). 18 patients received at least 2 cycles, achieved mPFS of 12.3 weeks and mOS of 28 weeks. Six-month survival rate was 44% (11/25). Unsupervised clustering of patients grouped those with shortened survival together by their cytokine profile showed that only IL-6 had a significant difference (p<.001) between short and long term survivors.
Conclusions
Capecitabine plus PHY906 provides a safe and feasible salvage therapy after gemcitabine failure for APC. Role of IL-6 in tumor progression and tumor cachexia needs to be investigated with respect to its relation to pathophysiology of pancreatic cancer and development of anti-IL-6 therapeutics.
Keywords: Capecitabine, PHY906, herbal medicines, pancreatic cancer, diarrhea, Hand-Foot syndrome (HFS)
Introduction
The prognosis of patients with advanced pancreatic carcinoma (APC) is extremely poor despite numerous trials with palliative chemotherapy or radiotherapy [1]. Gemcitabine has been the standard of care in both adjuvant setting and metastatic settings while combination treatment carries more toxicity [2]. Currently there is no standard second-line chemotherapeutic drug in cases refractory to or recurring following gemcitabine. The median survival rate with best supportive care in patients who have failed gemcitabine is approximately two months. Nearly half of patients with gemcitabine-pretreated disease may be candidates for further treatment. There is lack of data supporting the use of second-line therapy compared with best supportive care. The most acceptable approach for patients who have already received gemcitabine-based chemotherapy is fluopyrimidine-based chemotherapy and more specifically capecitabine, 5FU/leucovorin/oxaliplatin (OFF), and capecitabine plus oxaliplatin (CapeOx). However, the only established therapeutic choice is OFF regimen according to the Charité Onkologie (CONKO)-003 trial [3]. Therefore, there is a continuing need for clinical trials with a new agent for advanced pancreatic cancer in cases of gemcitabine failure.
Capecitabine, an oral fluoropyrimidine carbamate designed to generate 5-FU preferentially in tumor tissue through exploitation of high intratumoral concentrations of thymidine phosphorylase [4] has been investigated in patients with pancreatic cancer as a single agent [5,6] or in combination with chemotherapy and radiotherapy [4,7]. Cartwright et al. demonstrated that capecitabine 1250mg/m2 BID administered in a 14/7 schedule had clinically significant beneficial effects in chemotherapy-naïve APC patients, and was relatively well-tolerated [5]. Boeck et al also showed capecitabine 1250mg/m2 BID in a 14/7 schedule to be effective in controlling disease in gemcitabine-pretreated patients [6]. Scheithauer et al found that a 7/7 intermittent dosing (1750mg/m2 BID = total daily dose of 3500 mg/m2) was just as active as a 14/7 dosing when used in combination with oxaliplatin in CRC patients [8]. The National Comprehensive Cancer Network guidelines for APC recommends capecitabine as second-line treatment [9].
PHY906 is a botanical formulation composed of four distinct herbs: Scutellaria baicalensis Georgi, Glycyrrhiza uralensis Fisch., Ziziphus jujuba Mill., and Paeonia lactiflora Pall (Table 1) [10,11]. This herbal formula has been used in Asia to treat a variety of ailments such as abdominal cramps, fever, headache, vomiting, thirst, and diarrhea for over 1,700 years [12,13]. Anti-diarrheal activity was demonstrated in our previous clinical studies of PHY906 and irinotecan, PHY906 and5-FU/leucovorin in colorectal cancer (CRC), PHY906 and capecitabine in hepatocellular carcinoma (HCC), and phase I study of PHY906 and capecitabine in pancreatic cancer [11–13]. PHY906 was well tolerated up to 2.4 g/day and the frequency of diarrhea and vomiting was significantly lower with PHY906 than with placebo treatment.
Table 1.
Patients’ demographic characteristics
| Characteristics | Number of patients |
|---|---|
|
| |
| Number of patients | 25 |
|
| |
| Number of patients received ≥2 cycles | 20 |
|
| |
| Median age (year) | 64 (45–84) |
|
| |
| Male: female | 15:10 |
|
| |
| ECOG PS, 0/1:2 | 24:1 |
|
| |
| Pancreatic adenocarcinoma | |
| LA, unresectable/recurrent | 4 (16%) |
| Metastatic | 21 (84%) |
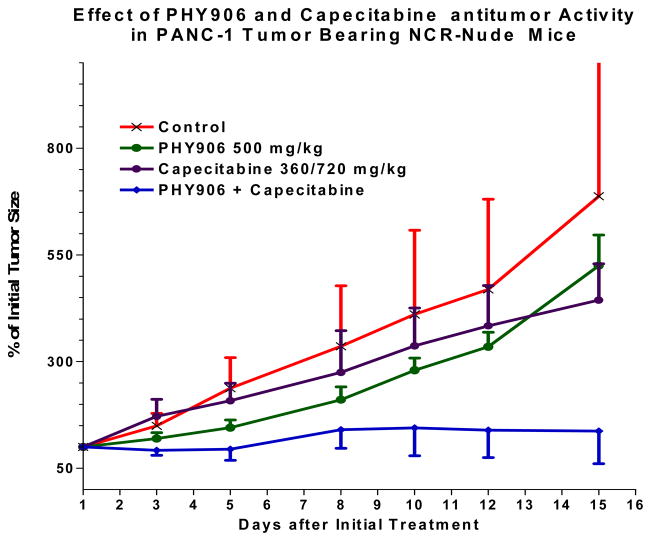
Beyond the cytoprotective benefit of PHY906, PHY906 also potentiates the effect of chemotherapy in preclinical models [14]. In a preclinical tumor-bearing mouse model using PANC-1 tumors, PHY906 alone has little, if any, cytotoxic anti-tumor activity, but it potentiates the action of capecitabine when given in combination [15]. There were no different in mouse bodyweight change or antitumor activity between the daily and the intermitted schedules of PHY906 when co-administrated with capecitabine. However, we observed one mortality in our mouse liver cancer model (N=5) after 14 consecutive day of PHY906 administration with capecitabine. Therefore, the proposed schedule was used in the clinical study. Our phase I study of PHY906 and capecitabine in patients with APC and other GI malignancies suggested that PHY906 could increase the therapeutic index of capecitabine in patients by reducing side effects such as diarrhea, and resulted in a disease control rate of 58% with one PR and thirteen SD out of 24 patients [16]. These previous results were sufficiently compelling to lead us to conduct a phase II study to evaluate the efficacy of PHY906 in combination with capecitabine (PHY906 800mg BID days 1–4 and capecitabine 1500mg/m2 BID days 1–7 of a 14-day cycle) in gemcitabine-pretreated patients with advanced pancreatic cancer.
Patients and Methods
Patient Selection
The inclusion criteria for this study were: i) histologically or cytologically proven pancreatic adenocarcinoma and unresectable locally advanced or metastatic disease; ii) at least one measurable lesion according to Response Evaluation Criteria in Solid Tumors (RECIST) (iii) prior chemotherapy with gemcitabine-based chemotherapy; iv) the ability to take oral medications; v) age, >18 years; vi) an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2; vii) adequate bone marrow function (neutrophil count ≥2,000/mm3 and platelet count ≥100,000/mm3); viii) adequate renal function [serum creatinine level ≤1.5 mg/dl; ix) adequate liver function [total bilirubin ≤2× UNL; aspartate transaminase (AST) and/or alanine transaminase (ALT) ≤2.5× UNL (if liver function abnormalities were due to underlying liver metastasis, then AST and/or ALT may be ≤5× UNL)].
The exclusion criteria for this study were patients who: i) had received chemotherapy or radiotherapy within 3 weeks; ii) had previously received an oral fluoropyrimidine except as a radiosensitizer; iii) had central nervous system metastases; iv) had an active infection or uncontrolled concurrent medical illness; v) had a history of other malignancies; vi) were pregnant or lactating; vii) had severe neurological impairment, a mental disorder or any severe drug-induced allergy.
The protocol and associated Informed Consent Forms were reviewed by the Investigational Review Boards (IRB), and approved prior to study initiation. This study was conducted in accordance with Good Clinical Practice guidelines of the International Conference on Harmonization, and the Declaration of Helsinki.
Treatment plan
Based on our phase I results, we chose PHY906 800mg BID days 1–4 and capecitabine 1500mg/m2 BID days 1–7 of a 14-day cycle to assess the clinical activity and tolerability of the combination in patients with gemcitabine-refractory pancreatic cancer [16].
Toxicity was assessed per NCI-CTCAE v3.0 [17]. Dose reduction and omission criteria for both agents were defined for hematologic and non-hematologic toxicity. Doses of capecitabine were omitted if the ANC was <500/μL or platelets were <25,000/μL, and then reduced by 250mg/m2 if the ANC recovered to ≥1,000/μL or if the platelet count was ≥50,000/μL. There was no replacement of missed doses of capecitabine. Whenever capecitabine was omitted, PHY906 was also held during that period. Treatment was also omitted if any non-hematologic toxicity of grade 2 or higher emerged and had not resolved to baseline or ≤ grade 1 by scheduled start of treatment. Oral prophylactic antiemetics were administered 30 minutes prior to each dose of capecitabine. Pyridoxine and emollients were routinely prescribed for all patients who entered the study.
Assessment of Efficacy
All patients were included in efficacy measurements on an intent-to-treat basis. Tumor responses were evaluated according to the RECIST Criteria [18]. QOL was assessed by utilizing a validated system called as Edmonton Symptom Assessment System, a clinical assessment scale commonly used in palliative care settings, at baseline and then before each cycle [19]. A tenth scale was included for any other symptom of particular importance to the patient.
Investigational studies/laboratory correlates
Research bloods (one 5 ml heparinized tubes; green) were collected by vena puncture prior to each of the treatments (between day −7 to day 1), day 8 and before the start of cycle 2. Blood samples were centrifuged to obtain plasma. Plasma collected from patients was stored at −80°C, and then analyzed for factors using the BD™CBA flex kits from BD with the BD FACSArray™. Cytometric Bead Array (CBA), commonly referred to as a multiplexed bead assay is a flow cytometric based assay for the detection of human cytokines from cell supernatants or serum samples as previously published [20]. Cytokines measured included interleukins (IL): IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, interferon gamma (IFNγ), RANTES (also known as CCL5 and SISd), granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF or GCSF), monocyte chemoattractant protein-1 (MCP-1), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), interferon gamma-induced protein 10 (IP-10), and angiogenin (Ang). Data was generated using the FCAPArray™ software
Statistical analysis
The Primary objective for the Phase II portion of this trial was to determine the overall survival time (OS) for patients with gemcitabine-refractory APC treated with PHY906 + capecitabine. The Phase II portion of this study follows a Simon two-stage design with an early stopping rule. The study was powered to improve median survival of these patients from 2 months to 4 months. The primary outcome was the number of patients who survived to three-months. A two-month median survival would result in 35% patients surviving until three months. This calculation assumes an exponential survival distribution. We took this 35% rate to be our null hypothesis. If the median survival could be improved in this protocol to 4 months then approximately 60% of patients would survive until three months. The 60% three-month survival rate was the alternative hypothesis. The design of the Phase II portion of this protocol called for enrolling up to 24 patients. If 4 or more of the first 12 patients survived at least three months then we planned to enroll an additional 12 patients for a total of 24. If 12 or more of the 24 survived at least three months then we rejected the 35% survival rate in favor of the 60% rate. This design has significance level .087 and power .86. There is probability .58 of stopping early under the 35% three-month survival rate. Overall survival was measured as the time from start of treatment to the date of death or the last date the patient was known to be alive, and analyzed by using Kaplan-Meier analysis. All patients who receive any study treatment were included in the final summaries and listing of safety data.
Results
Patient Characteristics
A total of 25 patients were enrolled in the study from December 2005 to December, 2008 at Yale Cancer Center. All patients received treatment, three patients did not finish the first cycle. All patients were evaluated for toxicity and survival, 20 patients received 2 cycles or above were evaluated for response. Among the 25 enrolled on the study, 15 were men, 10 were women; 24 patients with ECOG PS of 0 to 1, and the median age was 64 years (range, 45–84 years). All patients in this study were previously treated with gemcitabine single agent or in combinations, five (20%) patients had prior adjuvant gemcitabine after surgery (Whipple), two out of these five patients also received local radiotherapy with capecitabine as a radiosensitizer. Among 25 patients, 21 had metastatic disease upon enrolling, 4 had locally advanced, unresectable or recurrent disease. The characteristics of the 25 patients are presented in Table 1.
Treatment Compliance and Toxicities
A total of 137 cycles were delivered with a median of 5 cycles per patient (range, 1–19 cycles). Twenty patients completed at least 2 cycles of treatment. The most common reason for discontinuation was disease progression (14 patients; 56%) followed by drug-related toxicity (8 patients; 32%). Table 2 lists the common hematologic and nonhematologic treatment-related toxicities. Eight patients discontinued treatment due to toxicities, two due to non-treatment related infections (cholangitis, pneumonia) and one patient died (unrelated; significant cardiac history).
Table 2.
Toxicity
| Toxicities (CTCAE v.3) | G1 | G2 | G3 | G4 | G2/3 |
|---|---|---|---|---|---|
|
| |||||
| Hematological toxicities | |||||
| Anemia | 14 | 2 | 0 | 0 | 2 (8%) |
| Neutropenia | 4 | 3 | 0 | 0 | 3 (12%) |
| Thrombocytopenia | 3 | 3 | 0 | 0 | 3 (12%) |
|
| |||||
| Non-hematological toxicities | |||||
| Mucositis | 0 | 3 | 1 | 0 | 4 (16%) |
| Anorexia | 4 | 6 | 2 | 0 | 8 (32%) |
| Dry mouth/taste alteration | 5 | 2 | 0 | 0 | 2 (8%) |
| Nausea/vomiting | 10 | 2 | 1 | 0 | 3 (12%) |
| Diarrhea | 7 | 1 | 3 | 0 | 4 (16%) |
| Constipation | 9 | 3 | 0 | 0 | 3 (12%) |
| Fatigue | 11 | 4 | 3 | 0 | 7 (28%) |
| Hand-foot syndrome | 4 | 4 | 1 | 0 | 5 (20%) |
| Hyperpigmentation | 4 | 3 | 0 | 0 | 3 (12%) |
| Elevated LFTs | 6 | 0 | 2 | 0 | 2 (8%) |
| Electrolytes imbalance | 4 | 0 | 2 | 1 | 2 (8%) |
Efficacy
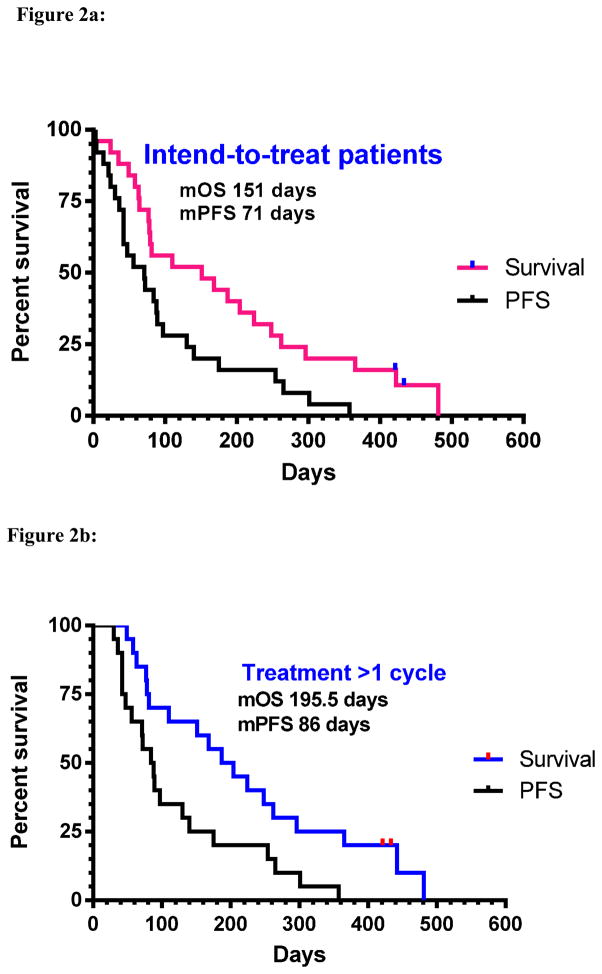
Two of 20 evaluable patients (10%) had a partial response (confirmed) as the best response during treatment period, 11 patients (55%) had stable disease. For all intended to treat patients, median progression-free survival was 10.1 weeks (range 0.4–54.1 wks); median overall survival was 21.6 weeks (range 0.4–84.1 wks) (Table 3 and Figure 2). For patients who tolerated 2 cycles or above, the mPFS was 12.3 weeks (range 4.2–51wks), and the mOS was 28 weeks (range 7–84.1wks) (Figure 2). Three-month survival was reached in 68% patients (17/25). Six-month survival rate was 44% (11/25) and nine-month survival rate reached 22%.
Table 3.
Efficacy data
| Best Response | Number of Patients (%) | Historical data |
|---|---|---|
| # of evaluable patients | 20 | 35 |
| Complete response (CR) | 0 | 0 |
| Partial response (PR) | 2 (10%) | 0 |
| Stable disease (SD) | 11 (55%) | 13 (37%) |
| Disease control rate (CR+PR+SD) | 65% | 37% |
| Progressive disease (PD) | 7 (35%) | N.R. |
| Survival (intent-to-treat patients) | ||
| mPFS (weeks) | 10.1 (0.4–54.1) | 9.4 (TTP) |
| mOS (weeks) | 21.6 (0.4–84.1) | 32.1 |
| Survival (patients tolerated 2 cycles or above) | ||
| mPFS (weeks) | 12.3 (4.2–51) | NR |
| mOS (weeks) | 28 (7–84.1) | NR |
Figure 2.
Figure 2a: Survival data for all intended to treat patients (N=25): median progression free survival (mPFS) 71 days (10.1 weeks), median overall survival (mOS): 151 days (21.6 wks)
Figure 2b: Survival data for all patients received 2 cycles and above (n=20), mPFS: 86 days (12.3 wks), mOS: 196 days (28 wks)
Baseline CA 19-9 data were available for all 20 evaluable patients and the median CA 19-9 U/mL level was 727.5 (range, 4–19,012). Univariate regression analysis was performed and there was no correlation noted between baseline CA 19-9 and progression-free survival.
QOL
The Edmonton Symptom Assessment scale (ESAS) was used. Twenty patients reported significantly impaired QOL on nine of 9 scales/items (80% response) at the beginning of cycle 2 (median 1; range: 0 – 4) and at the beginning of cycle 3 (median 1; range: 0 – 5) compared to baseline (median 2; range: 0 – 8). Fatigue, loss of appetite, nausea and impaired sense of well-being were the most improved items noticed improved by 4+ points. The patients who remained on the study longer continued to maintain their improvement in QOL: at cycle 6 (median 0; range: 0 – 3), cycle 9 (median 0; range: 0 – 3), cycle 12 (median 0; range: 0 – 5), cycle 15 (median 0; range: 0 – 3) and cycle 18 (median 1; range: 0 – 2). Scores were not available for most of the patients at the time of death and therefore, no formal analysis was performed. About twelve patients have improved pain control either defined by pain scale or amount of analgesics resulting in a response rate of 48%. Only two patients had diarrhea while 11 patients noticed either improvement in diarrhea or no need to take anti-diarrheal medications (44%). Depression was improved in four patients without use of antidepressants (16%).
Correlative cytokine study
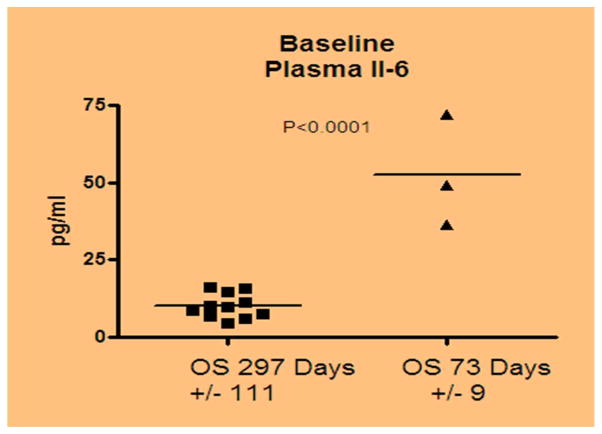
Correlative studies showed that plasma markers varied among patients and each one, by itself, did not appear to be correlated with response, survival, or toxicity. Preliminary unsupervised clustering of patients grouped those with shortened survival versus prolonged survival together by their cytokine profile was performed. Cytokine cluster analysis demonstrated the level of IL-6 is negatively correlative with the overall survival, the higher level correlates with a shorter length of survival (p <.001). Figure 3 showed the IL-6 level is negatively correlative with the overall survival.
Figure 3.
Relationship of serum IL-6 levels with the survival in patients with pancreatic cancer (Cytokine/chemokine cluster analysis). Detection limit of the assay was 3 pg/ml.
Discussion
Our study is the first-in-human clinical study that evaluated the combination of botanical formulation PHY906 and capecitabine in patients with gemcitabine refractory APC and met its end point. In the majority of patients, pancreatic cancer remains a chemoresistant cancer and doublet combination regimens have produced, at best, marginal results over single-agent gemcitabine but at the cost of more toxicity. The median survival with best supportive care in patients who have failed gemcitabine therapy is approximately 2 months [21]. Approximately half of the patients with gemcitabine-pretreated disease may be candidates for further treatment. Several clinical trials have evaluated the efficacy of salvage chemotherapeutic regimens in gemcitabine-pretreated patients and the survival duration is in the range of 3 to 8 months.
Preclinical models demonstrated synergistic effect of PHY906 on cytotoxic chemotherapy such as irinotecan, capecitabine, 5-FU etc [15,16,22]. Our efficacy data suggest that PHY906 potentiates the anti-tumor effect of capecitabine. All patients in this study were previously treated with gemcitabine single agent or in combinations. Overall this combination was well tolerated and resulted in a median overall survival of 22 weeks for all intended-to-treat patients, 28 weeks for patients received ≥2 cycles. The two patients who derived partial response had surprisingly long overall survival with persistent improved QOL, survival length of 69 and 84 weeks.
Our patients also tolerated a much higher dose than an average American patient. Recent data using mathematical modeling suggests that drug delivery beyond seven days contributes to toxicity with diminishing anticancer effects [23]. Traina and colleagues achieved an MTD of capecitabine of 2000mg BID fixed-dosing for seven consecutive days followed by a seven-day rest period in breast cancer patients. In this study, we used BSA-based dosing of capecitabine and have shown the MTD to be 1500mg/m2 BID in a 7/7 dosing schedule. Compared to the fixed-dose 7/7 used by Traina, et al, the average BSA in our patients was 1.88mg/m2 yielding an median starting dose of 2725mg BID at the MTD and for the whole study, significantly more than the 2000mg BID fixed-dosing. Compared to conventional 14/7 capecitabine dosing of 1250mg/m2 BID, our 7/7 schedule of 1500mg/m2 BID achieves a 90% relative dose intensity (1000mg/m2 less per week), and may be a more tolerable schedule, especially when used in conjunction with PHY906.
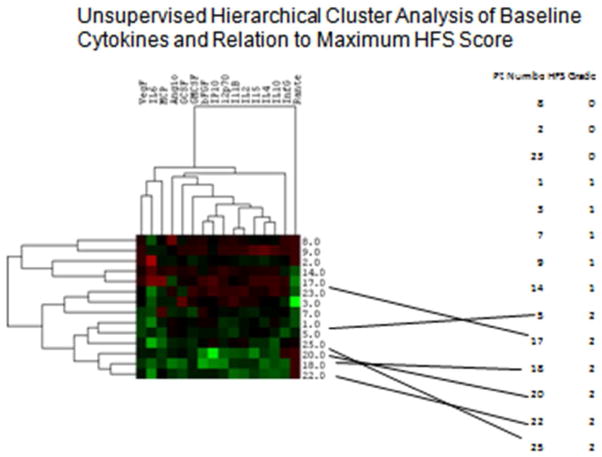
In traditional eastern herbal medicine, multiple herbs are used together either to induce a synergistic effect, enhance the efficacy, or reduce the side effects of the formulation. This combination had demonstrated some anti-diarrhea effects in phase I study in several GI malignancies [16]. In this phase II study, we also observed a lower incidence of grade ≥ 2 HFS and diarrhea. This triggered us to further analyze the cytokine data which revealed less expression of several cytokines in patients with higher HFS scores (Figure 4).
Figure 4.
Cytokine data appears to show less expression of several cytokines in patients with higher HFS scores
Biochemical studies reveal that the PHY906 formulation possesses a wide range of pharmacological activities. The potential mechanism(s) of action of PHY906 include (1) enhancement of cellular uptake of chemotherapeutic agents via inhibition of MDR (multi-drug resistance) mechanisms; (2) modulation of NF-kB activity; (3) inhibition of MMP (matrix metalloproteinase) activity; and (4) inhibition of angiogenesis [12,13,16]. Therefore, correlative studies in this study measured chemokines such as IL-2, TNF-a, etc as surrogates for NF-kB expression, to further elucidate the effects of PHY906. The cytokine/chemokine cluster analysis demonstrated that IL-6 could be used as a prognostic marker. IL-6 is a multipotent cytokine exerting numerous biological activities and its serum levels are elevated in many solid tumors, including lung cancer, renal cell carcinoma and ovarian cancer [24,25]. In our study, unsupervised clustering of patients grouped those with shortened survival versus prolonged survival together by their cytokine profile demonstrated the level of IL-6 is negatively correlated with the overall survival, the higher level correlates with a shorter length of survival (p <.001) between short and long term survivors. It is not yet clear whether the increased serum IL-6 levels in PC patients were produced by the cancer cells or tumor-associated host cells such as fibroblasts and macrophages [26]. Excessive amounts of IL-6 in serum may play various roles in tumor progression, including metastasis and clinical manifestations such as weight loss [27]. Anti-IL-6 or anti-IL-6 receptor antibodies could be useful in treating patients with IL-6-producing tumors [28].
The options for metastatic pancreatic cancer p[patients are changing over the last two years with the positive results of FOLFIRINOX and gemcitabine-nab-paclitaxel studies [29]. However, FOLFIRINOX regimen is suitable for selected fit patients and nab-paclitaxel is associated with neuropathy, which will not make OFF regimen (oxaliplatin-based) an ideal therapy for those with existing nerve damage. Therefore, capecitabine alone and its combinations, such as the one tested in our study offer an acceptable option for gemcitabine-refractory patients.
In conclusion, this is the first phase II study that evaluated an herbal drug in combination with chemotherapy for the treatment of APC. Although the number of patients is small, this study proved the hypothesis generated from preclinical as well as phase I studies. We believe that given the manageable toxicity, capecitabine-PHY906 has a role in patients with gemcitabine-pretreated APC and warrant further investigation. Moreover, studies are required to establish whether IL-6, either alone or in concert with other cytokines, is involved in the pathophysiology of pancreatic cancer, and finally anti-IL-6 therapeutics could serve as a useful tool for improving symptoms in these patients with high IL-6 levels.
Figure 1.
Effect of PHY906 with capecitabine in Panc-1 tumor bearing NCR-nude mice.
Acknowledgments
Funding: This study was approved and funded by the National Comprehensive Cancer Network (NCCN) from general research support provided by Roche Laboratories; PO1CA154295; and Dr. Cheng is a Fellow of the National Foundation for Cancer Research.
Footnotes
PRESENTED as a Poster at the American Society of Clinical Oncology Annual and Gastrointestinal Cancers Symposium, 2009 and 2010
References
- 1.American Cancer Society. Cancer Facts & Figures 2013. Atlanta, Ga: American Cancer Society; 2013. [Google Scholar]
- 2.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 3.Pelzer U, Schwaner I, Stieler J, Adler M, Seraphin J, Dörken B, Riess H, Oettle H. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer. 2011 Jul;47(11):1676–81. doi: 10.1016/j.ejca.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Saif MW, Eloubeidi MA, Russo S, Steg A, Thornton J, Fiveash J, Carpenter M, Blanquicett C, Diasio RB, Johnson MR. Phase I study of capecitabine with concomitant radiotherapy for patients with locally advanced pancreatic cancer: expression analysis of genes related to outcome. J Clin Oncol. 2005 Dec 1;23(34):8679–87. doi: 10.1200/JCO.2005.02.0628. [DOI] [PubMed] [Google Scholar]
- 5.Cartwright TH, Cohn A, Varkey JA, Chen YM, Szatrowski TP, Cox JV, Schulz JJ. Phase II study of oral capecitabine in patients with advanced or metastatic pancreatic cancer. J Clin Oncol. 2002 Jan 1;20(1):160–4. doi: 10.1200/JCO.2002.20.1.160. [DOI] [PubMed] [Google Scholar]
- 6.Boeck S, Wilkowski R, Bruns CJ, Issels RD, Schulz C, Moosmann N, Laessig D, Haas M, Golf A, Heinemann V. Oral capecitabine in gemcitabine-pretreated patients with advanced pancreatic cancer. Oncology. 2007;73(3–4):221–7. doi: 10.1159/000127413. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, Harper PG, Dunn J, Tudur-Smith C, West J, Falk S, Crellin A, Adab F, Thompson J, Leonard P, Ostrowski J, Eatock M, Scheithauer W, Herrmann R, Neoptolemos JP. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009 Nov 20;27(33):5513–8. doi: 10.1200/JCO.2009.24.2446. [DOI] [PubMed] [Google Scholar]
- 8.Scheithauer W, Kornek GV, Raderer M, Schull B, Schmid K, Kovats E, Schneeweiss B, Lang F, Lenauer A, Depisch D. Randomized multicenter phase II trial of two different schedules of capecitabine plus oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2003 Apr 1;21(7):1307–12. doi: 10.1200/JCO.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Tempero MA, Arnoletti JP, Behrman SW, Ben-Josef E, Benson AB, 3rd, Casper ES, Cohen SJ, Czito B, Ellenhorn JD, Hawkins WG, Herman J, Hoffman JP, Ko A, Komanduri S, Koong A, Ma WW, Malafa MP, Merchant NB, Mulvihill SJ, Muscarella P, 2nd, Nakakura EK, Obando J, Pitman MB, Sasson AR, Tally A, Thayer SP, Whiting S, Wolff RA, Wolpin BM, Freedman-Cass DA, Shead DA National Comprehensive Cancer Networks. Pancreatic Adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2012 Jun 1;10(6):703–13. doi: 10.6004/jnccn.2012.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rilton R, Paiva AA, Guan J, Marathe R, Jiang Z, van Eyndhoven W, Bjoraker J, Wang H, Liu SH, Cheng Y-C. PhytomicsQC: A Comprehensive Approach to Define Quality Control of Botanical Drugs - A Case Study of PHY906. Chin Med. 2010;20(5):30. doi: 10.1186/1749-8546-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang W, Saif MW, Dutschman GE, Li X, Lam W, Bussom S, Jiang Z, Ye M, Chu E, Cheng YC. Identification of chemicals and their metabolites from PHY906, a Chinese medicine formulation, in the plasma of a patient treated with irinotecan and PHY906 using liquid chromatography/tandem mass spectrometry (LC/MS/MS) J Chromatogr A. 2010 Sep 10;1217(37):5785–93. doi: 10.1016/j.chroma.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kummar S, Copur MS, Rose M, Wadler S, Stephenson J, O’Rourke M, Brenckman W, Tilton R, Liu SH, Jiang Z, Su T, Cheng YC, Chu E. A phase I study of the chinese herbal medicine PHY906 as a modulator of irinotecan-based chemotherapy in patients with advanced colorectal cancer. Clin Colorectal Cancer. 2011 Jun;10(2):85–96. doi: 10.1016/j.clcc.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Yen Y, So S, Rose M, Saif MW, Chu E, Liu SH, Foo A, Jiang Z, Su T, Cheng YC. Phase I/II study of PHY906/capecitabine in advanced hepatocellular carcinoma. Anticancer Res. 2009 Oct;29(10):4083–92. [PubMed] [Google Scholar]
- 14.Liu S-H, Jiang Z, Gao W, et al. PHY906, a Chinese herbal formulation enhances the therapeutic effect of cancer chemotherapy in human colorectal and liver cancer. Proc Am Soc Clin Oncol. 2003:Abstr #864. [Google Scholar]
- 15.Saif MW, Liu S, Elfiky A, Jiang Z, Cheng Y. Synergistic activity of PHY906 with capecitabine in pancreatic carcinoma. J Clin Oncol 2007; ASCO Annual Meeting Proceedings Part I; 2007. p. 15116. [Google Scholar]
- 16.Saif MW, Lansigan F, Ruta S, Lamb L, Mezes M, Elligers K, Grant N, Jiang ZL, Liu SH, Cheng YC. Phase I study of the botanical formulation PHY906 with capecitabine in advanced pancreatic and other gastrointestinal malignancies. Phytomedicine. 2010 Mar;17(3–4):161–9. doi: 10.1016/j.phymed.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 17.http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf
- 18.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Moro C, Brunelli C, Miccinesi G, Fallai M, Morino P, Piazza M, Labianca R, Ripamonti C, Moro C, Brunelli C, Miccinesi G, Fallai M, Morino P, Piazza M, Labianca R, Ripamonti C. Edmonton symptom assessment scale: Italian validation in two palliative care settings. Support Care Cancer. 2006 Jan;14(1):30–7. doi: 10.1007/s00520-005-0834-3. [DOI] [PubMed] [Google Scholar]
- 20.Morgan E, Varro R, Sepulveda H, Ember JA, Apgar J, Wilson J, Lowe L, Chen R, Shivraj L, Agadir A, Campos R, Ernst D, Gaur A. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin Immunol. 2004 Mar;110(3):252–66. doi: 10.1016/j.clim.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 21.Kang SP, Saif MW. Optimal second line treatment options for gemcitabine refractory advanced pancreatic cancer patients. Can we establish standard of care with available data? JOP. 2008 Mar 8;9(2):83–90. [PubMed] [Google Scholar]
- 22.Liu S-H, Jiang Z, Leung D, Lee Y, Cheng Y. PHY906: Enhancement of Cancer Chemotherapeutic Agents and Insights into Mechanism of Action. Proceedings of “Medicine in the 21st Century Tri-Conference and Bio-Forum; 2004; p. 77. [Google Scholar]
- 23.Traina TA, Theodoulou M, Feigin K, et al. Phase I study of a novel capecitabine schedule based on the Norton-Simon mathematical model in patients with metastatic breast cancer. J Clin Oncol. 2008 Apr 10;26(11):1797–1802. doi: 10.1200/JCO.2007.13.8388. [DOI] [PubMed] [Google Scholar]
- 24.Le J, Vilcek I. Interleukin-6: a multifunctional cytokine regulating immune reactions and the acute phase protein response. Lab Invest. 1989;61:588–602. [PubMed] [Google Scholar]
- 25.Berek JS, Chung C, Kaldi K, Watson JM, Knox RM, Martínez-Maza O. Serum interleukin-6 levels correlate with disease status in patients with epithelial ovarian cancer. Am J Obstet Gynecol. 1991 Apr;164(4):1038–42. doi: 10.1016/0002-9378(91)90582-c. discussion 1042–3. [DOI] [PubMed] [Google Scholar]
- 26.Martínez-Maza O, Berek JS. Interleukin 6 and cancer treatment. In Vivo. 1991 Nov-Dec;5(6):583–8. [PubMed] [Google Scholar]
- 27.Strassmann G, Fong M, Kenney JS, Jacob CO. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest. 1992 May;89(5):1681. doi: 10.1172/JCI115767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gougelet A, Mansuy A, Blay JY. Interleukin-6 and epithelial tumours: new convincing arguments in favour of the use of IL-6 targeted therapies. Med Sci (Paris) 2008 Aug-Sep;24(8–9):694–6. doi: 10.1051/medsci/20082489694. [DOI] [PubMed] [Google Scholar]
- 29.Jarboe J, Saif MW. First line therapy for metastatic pancreatic cancer. JOP. 2013 Jul 10;14(4):340–3. doi: 10.6092/1590-8577/1667. [DOI] [PubMed] [Google Scholar]